
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 02 September 2024
Sec. Gastroenterology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1393498
This article is part of the Research TopicGastrointestinal Tract Infections: A Global PerspectiveView all 19 articles
 Tian-Hang Yu1
Tian-Hang Yu1 Dan Bai2
Dan Bai2 Kai Liu1
Kai Liu1 Wei-Han Zhang1
Wei-Han Zhang1 Xin-Zu Chen1,3,4*
Xin-Zu Chen1,3,4* Jian-Kun Hu1 on behalf of the SIGES Research Group
Jian-Kun Hu1 on behalf of the SIGES Research GroupObjectives: A systematic review and meta-analysis was performed to evaluate the preventive effectiveness of Helicobacter pylori eradication against metachronous gastric cancer (MGC) or dysplasia following endoscopic resection (ER) for early gastric cancer (EGC) or dysplasia.
Methods: PubMed, Cochrane Library, MEDLINE, and EMBASE were searched until 31 October 2023, and randomized controlled trials or cohort studies were peer-reviewed. The incidence of metachronous gastric lesions (MGLs) including MGC or dysplasia was compared between Helicobacter pylori persistent and negative groups, eradicated and negative groups, and eradicated and persistent groups.
Results: Totally, 21 eligible studies including 82,256 observations were analyzed. Compared to those never infected, Helicobacter pylori persistent group (RR = 1.58, 95% CI = 0.98–2.53) trended to have a higher risk of MGLs and significantly in partial subgroups, while the post-ER eradicated group (RR = 0.79, 95% CI = 0.43–1.45) did not increase the risk of MGLs. Moreover, successful post-ER eradication could significantly decrease the risk of MGLs (RR = 0.54, 95% CI = 0.44–0.65) compared to those persistently infected. Sensitivity analysis obtained generally consistent results, and no significant publication bias was found.
Conclusion: The persistent Helicobacter pylori infection trends to increase the post-ER incidence of MGC or dysplasia, but post-ER eradication can decrease the risk correspondingly. Post-ER screening and eradication of Helicobacter pylori have preventive effectiveness on MGC, and the protocol should be recommended to all the post-ER patients.
Systematic review registration: The PROSPERO registration identification was CRD42024512101.
Gastric cancer is the fifth most common cancer and the fourth most common cause of cancer death globally, according to 768,793 deaths in 2020 (1, 2). Epidemiologic and clinical studies indicate that the hotspot of incidence and mortality events of gastric cancer exists in East Asia, probably due to the differences in population-specific genetic risk factors and infectious agents such as Helicobacter pylori (H. pylori) (3–7).
Gastrectomy with lymphadenectomy was regarded as the standard treatment for gastric cancer (8–10). In recent decades, endoscopic resection (ER) including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) has been widely accepted as curative therapy (8). Current clinical evidence suggests that long-term survival after ER for EGC is comparable to surgical resection (11–13). Additionally, patients who underwent ER might have a higher risk of metachronous gastric cancer (MGC) compared with those who underwent gastrectomy, but MGC after ER was successfully re-treated without affecting overall survival (14).
In 1975, Correa et al. first reported the astute observation that intestinal-type gastric adenocarcinoma was associated with an inflammatory process in the stomach, namely Correa’s cascade: normal gastric mucosa, non-atrophic gastritis, atrophic gastritis, intestinal metaplasia, intraepithelial neoplasia (dysplasia), and then gastric cancer (15). Later reports showed that H. pylori, as a particular bacterial species, could colonize the stomach and initiate an inflammatory response or atrophic gastritis, and therefore H. pylori infection was the most well-described risk factor for non-cardia gastric cancer (16–19).
However, the exact role of H. pylori infection in the development of post-ER metachronous gastric lesions (MGL) including late-stage precancerous lesion (dysplasia) or MGC has not been clearly elucidated. Some studies indicated that post-ER H. pylori eradication could reduce the risk of MGC, but a few suggested it was not worthy instead (20, 21). Thus, we aimed to conduct a systematic review and meta-analysis to evaluate the association between H. pylori status and the risk of MGLs. We hypothesized that (A) post-ER persistent infection of H. pylori might increase the risk of MGLs and then (B) successful post-ER eradication of H. pylori might decrease the risk of MGLs.
PubMed, Cochrane Library, MEDLINE, and EMBASE were searched until 31 October 2023. The search strategy combined the following MESH items: Helicobacter pylori; Endoscopy; Gastrointestinal, Neoplasms, Second Primary. The synonyms of these items were also included in the search strategy, such as Helicobacter nemestrinae, Campylobacter pylori, Endoscopic Gastrointestinal Surgery, Neoplasms, Metachronous, Second Malignancy. The search link in PubMed was shown in Supplementary materials. We mainly used PubMed, and the same search strategy was used in the Cochrane Library, MEDLINE, and EMBASE databases as supplements.
Either randomized controlled trials (RCTs) or cohort studies were potentially eligible. ERs were performed for EGC or dysplasia. The outcome of post-ER H. pylori eradication on the prevention of MGLs was compared to negative controls. The status of H. pylori infection was examined in all patients by any possible test, and they were classified into three categories: (A) H. pylori–negative group, (B) H. pylori-eradicated group, and (C) H. pylori-persistent group. The H. pylori-negative group consisted of patients who was never detected before the ER and were negative during follow-up. The H. pylori-eradicated group consisted of patients who were diagnosed with H. pylori infection at or before the time of ER and received eradication therapy, with no evidence of H. pylori infection after the re-examination during follow-up. The H. pylori-persistent group consisted of patients who remained H. pylori positive during follow-up regardless of eradication or not. Endoscopic follow-up was performed at the post-ER 1-year visit or later. The outcome measure was defined as the incidence of MGLs, including the subsets (A) MGC and dysplasia or (B) dysplasia only. There was no limitation on publication date, language, or country. If the outcome data were unextractable, the studies were excluded.
The eligibility of literature was peer-reviewed, and any disagreement was resolved through discussion between peer-reviewers or arbitration by a third party. Quality assessment was carried out using the Cochrane risk-of-bias tool for RCT and Newcastle–Ottawa scale for observational studies (22, 23). The general information of eligible studies was extracted including the first author, publication year, country, primary disease, endoscopic intervention, H. pylori test, eradication regimen, and follow-up duration. Besides, the number of observed participants and the number of MGL events were extracted or estimated in each group.
This meta-analysis was conducted using the R Studio software with the R package “meta.” The risk ratio (RR) and 95% confidence interval (CI) were estimated as the effect size. Between-study heterogeneity was assessed by I-square and Cochran’s Q. A fixed-effects model was used for those with I-square value of <50%, or a random-effects model was used instead. The forest plots were presented to display the meta-analysis. Subgroup analysis was conducted with regard to the study design and primary outcome. Publication bias was first estimated using the funnel plot and then confirmed using the Egger’s test, the AS-Thompson test, the Duval and Tweedie trim-and-fill method, the contour-enhanced meta-analysis funnel plot, and the Baujat plot, where applicable (24–26). A p-value of <0.05 was considered statistically significant.
Comparisons were conducted between persistent and negative groups for hypothesis A, as well as between eradicated and negative groups as secondary analyses. Comparisons were conducted between persistent and eradicated groups for hypothesis B. Subgroup analyses were carried out regarding the subsets of study designs, countries, primary diseases, and outcome measures. Sensitive analysis by the leave-one-out method was also performed to evaluate whether any study had excessive influence on the results of the pooled analysis.
This systematic review and meta-analysis was conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) 2000 statements (27), and a flow diagram was drawn in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (28).
The present meta-analysis was registered in the PROSPERO International Prospective Register of Systematic Reviews supported by the National Institute for Health Research of the National Health Service (NHS), UK (ID: CRD42024512101) (29).
Ethical approval was not required due to the nature of literature-based research.
The flow diagram of study selection is presented in Figure 1. Finally, 21 studies were included for the meta-analysis, including 3 RCTs (30–32) and 18 cohort studies (20, 21, 33–48). The brief characteristics of the included studies are summarized in Supplementary Table S1. Due to the lack of relevant research in European and American countries, all the included studies were from Japan and Korea. All the included studies were of high or moderate quality (Supplementary Table S2, S3). Among the 21 studies, MGLs developed in 1,233 out of 38,931 eradicated patients and 2,982 out of 41,969 persistent patients, compared with 92 out of 1,356 negative patients. Additionally, among those with dysplasia only receiving post-ER eradication therapy, 917 out of 34,494 eradicated patients and 2,469 out of 36,059 persistent patients found MGLs.
There were eight studies that compared the MGL risk between H. pylori-persistent and negative statuses, while the risk trended to be increased in the persistent group but not significantly yet (RR = 1.58, 95% CI = 0.98–2.53, I-square = 58%) (Table 1, Figure 2A). Subgroup analyses are shown in Table 1 and Supplementary Figures S1–S8. The majority of Korean studies obtained consistent results (RR = 1.61, 95% CI = 0.99–2.61, I-square = 63%). In the primary disease subsets of EGC combining dysplasia or not, higher risks of MGLs were found in the persistent group than those in the negative group (RR = 3.80, 95% CI = 1.31–10.97). Similarly, in the MGL outcome subsets of MGC combining dysplasia or not, the persistent group had a higher risk of MGL events (RR = 1.93, 95% CI = 1.17–3.17). Besides, the secondary analyses by comparing the eradicated group to the negative group demonstrated generally comparable risks of post-ER MGLs (Table 1, Figure 2B).
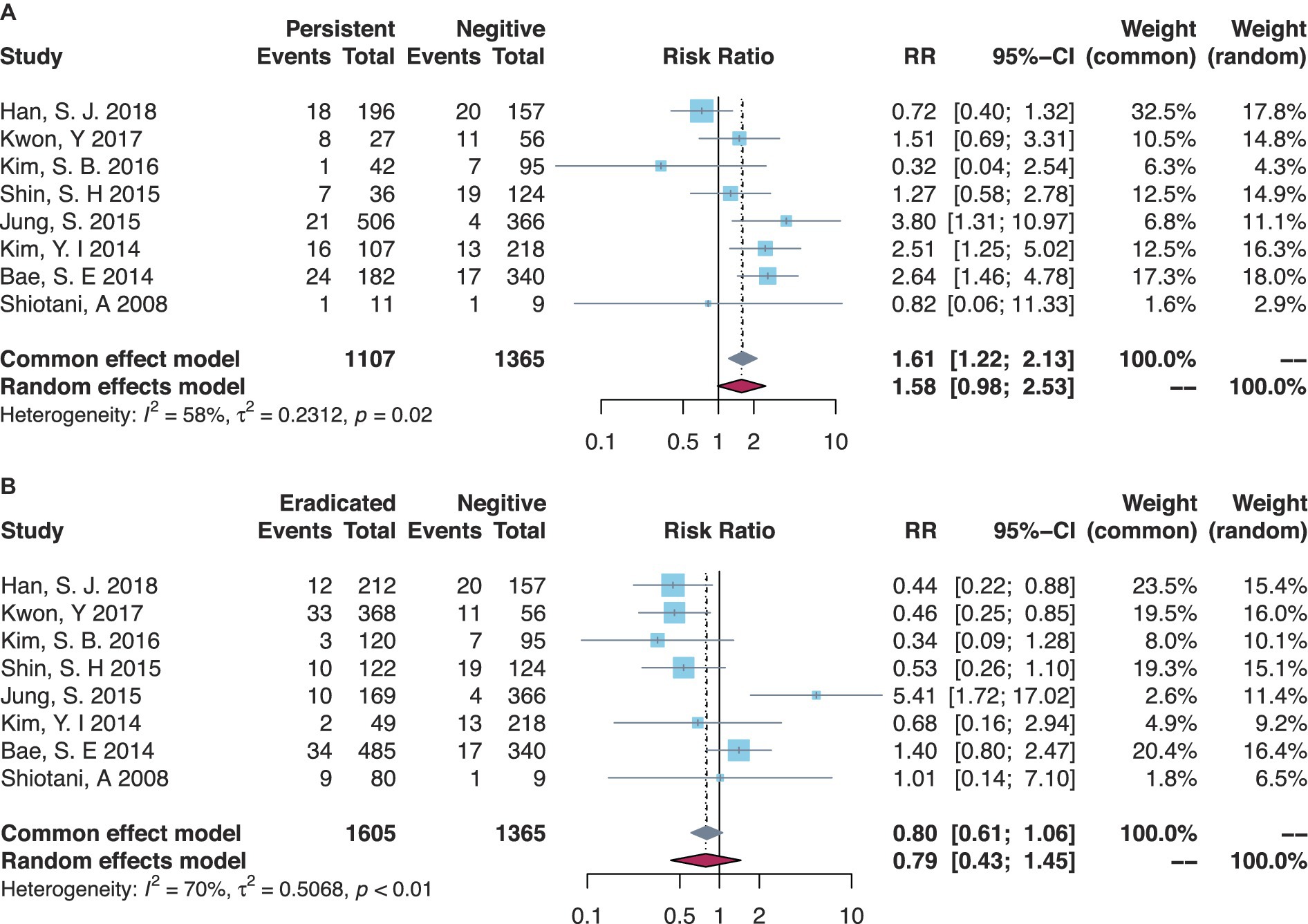
Figure 2. Forest plots of overall comparisons (A) between H. pylori-persistent and negative patients and (B) between eradicated and negative patients.
There were 21 studies that compared the MGL risk between H. pylori-eradicated and persistent statuses, and post-ER eradication had a significant protective effect by lowering the MGL risk (RR = 0.54, 95% CI = 0.44–0.65, I-square = 65%) (Table 2, Figure 3). In subgroup analysis (Table 2, Supplementary Figures S9–S12), meta-analyses on RCTs or cohort studies did not differ in results. Either Japanese or Korean studies obtained consistent results, respectively. In the primary disease subset of either EGC only or dysplasia only, similar protective effects against MGLs were found in the eradicated group compared to the persistent group. Additionally, in the MGL outcome subsets of MGC combining dysplasia or not, the eradicated group had a significantly lower risk of MGLs.
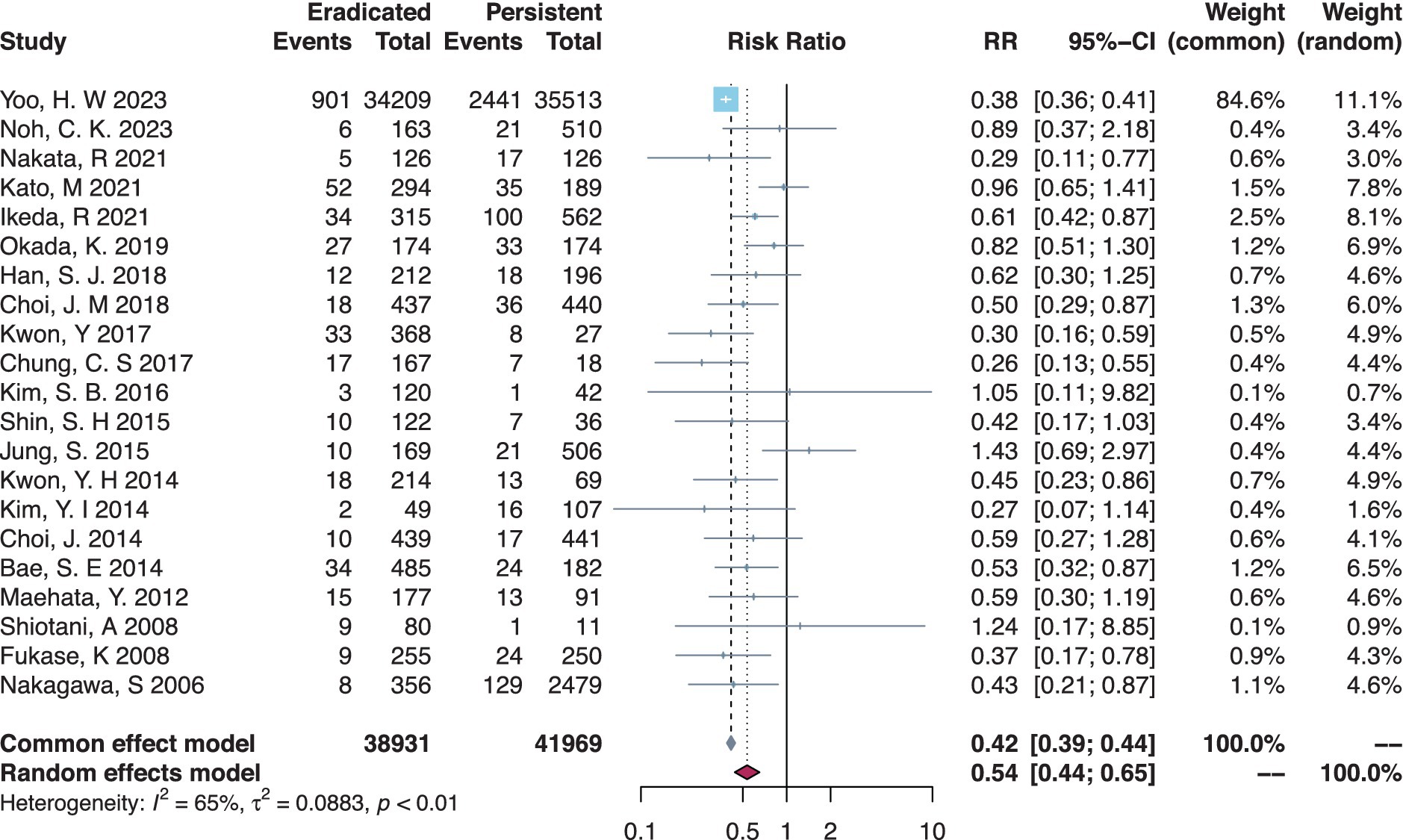
Figure 3. Forest plot of the overall comparison between H. pylori-eradicated and persistent patients.
Through leave-one-out analyses, consistent results were found in all the three principal meta-analyses, including the comparisons between H. pylori-persistent and negative groups, eradicated and negative groups, and eradicated and persistent groups (data not shown).
In the meta-analysis of H. pylori-persistent and negative groups, no significant publication bias was found using the funnel plot (Figure 4A) or Egger’s test (p = 0.63). Similarly, no significant publication bias was found in the meta-analysis of eradicated and negative groups using the funnel plot (Figure 4B) or Egger’s test (p = 0.78).
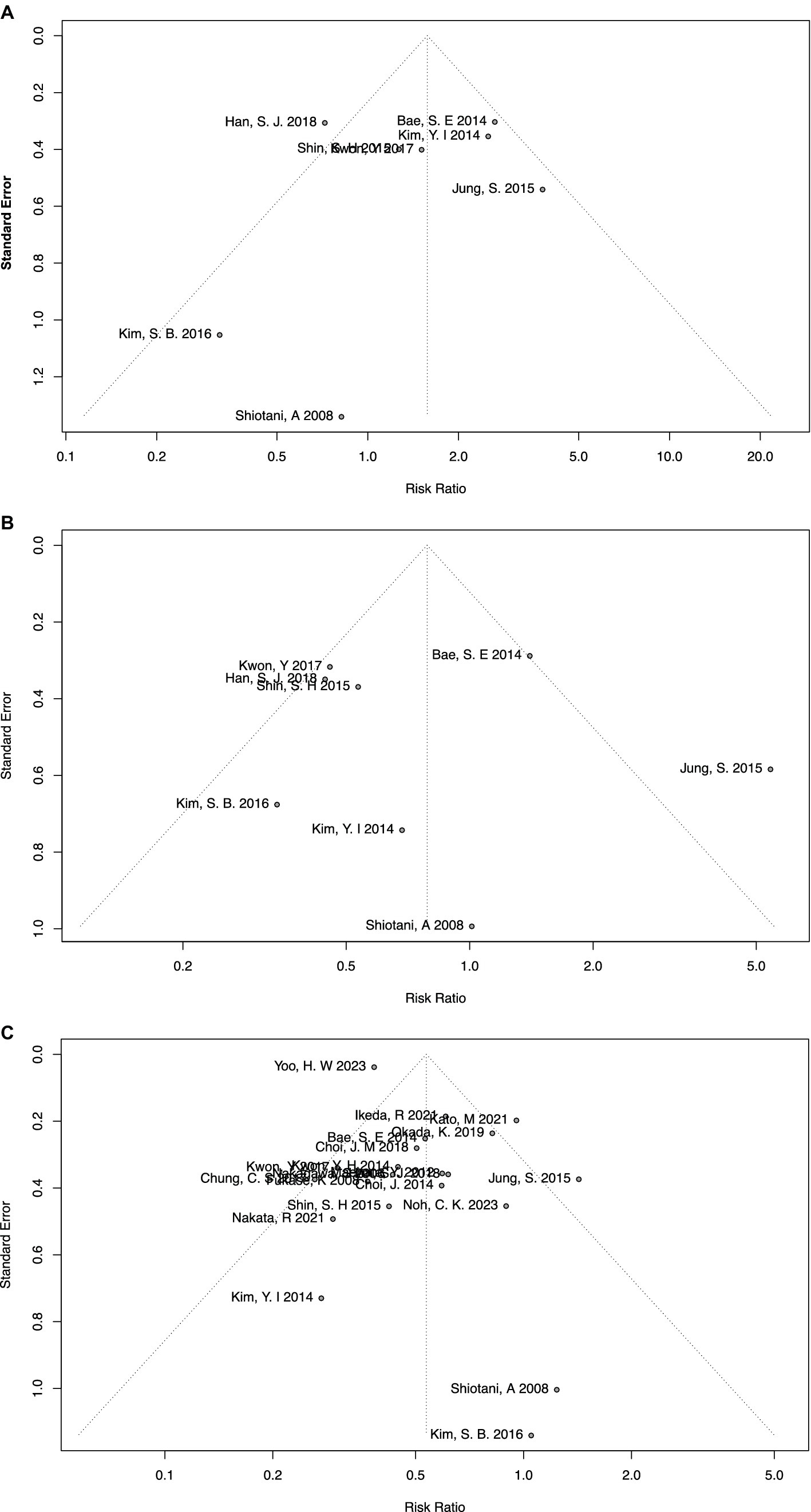
Figure 4. Funnel plots of meta-analyses on overall comparisons (A) between persistent and negative patients, (B) between eradicated and negative patients, and (C) between eradicated and persistent patients.
In particular, the meta-analysis of eradicated and persistent groups included more than 10 studies, and therefore the AS-Thompson test was used to test the potential sources of high heterogeneity. No significant publication bias was tested (p = 0.24), along with the funnel plot (Figure 4C). Additionally, the Duval and Tweedie trim-and-fill method and contour-enhanced meta-analysis funnel plots were also applied to visually depict publication bias. By using the Duval and Tweedie trim-and-fill method, seven more studies were included and all the added studies were located in the p < 0.05 area of the contour-enhanced meta-analysis funnel plots, namely, null publication bias (Supplementary Figure S13). Moreover, the Duval and Tweedie trim-and-fill method was also used as part of sensitivity analysis, which suggested consistent and reliable results (RR = 0.41, 95% CI = 0.32–0.53, I-square = 75%). Finally, the Baujat plot indicated that the study by Yoo et al. had the greatest impact on the overall result (33), and the study by Kato et al. had the greatest impact on heterogeneity (35).
This system review and meta-analysis analyzed 21 studies with 82,256 observations. H. pylori-persistent group trended to have a higher risk of MGLs compared to those never infected, but not in the post-ER eradicated group. Moreover, successful post-ER eradication could decrease the risk of MGLs compared to those persistently infected. Sensitivity analysis obtained generally consistent results, and no significant publication bias was found. However, heterogeneity commonly existed among meta-analyses, and a random-effects model was used accordingly. In the bibliographic view, the present report might be a most updated meta-analysis.
Gastric cancer is still one of the top malignancies in China, with a heavy burden of high population incidence (19, 49). H. pylori infection is one of the most important risk factors for gastric cancer in East Asia, particularly in China (50, 51). A multicenter prospective cohort study including 512,715 Chinese indicates that H. pylori infection accounted for 78.5% of non-cardia and 62.1% of cardia cancers, and up to 339,955 incident gastric cancers could be attributable to H. pylori infection (52). Due to consistent advocacy, health education, improved sanitary condition, and drinking water quality in China, the H. pylori infection rate among Chinese has slowly declined over the past 30 years, especially among the urban health check-up population (50, 53). Active H. pylori eradication within organized massive screening might simultaneously lower the incidence and improve population survival of gastric cancer (54).
Currently, a low proportion of EGC, usually no more than 20%, is the principal difficulty in improving the population survival of gastric cancer in China (55). In contrast, the proportions of EGC are fairly high in both Japan and Korea due to national organized massive screening for gastric cancer. Therefore, ER as a minimally invasive procedure is well-practiced for the early treatment of EGC, with a negligible risk of lymph node metastasis among high-selected candidates. According to Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition), the EMR or ESD absolute indications for EGC include a differentiated-type adenocarcinoma without ulcerative findings in which the depth of invasion is clinically diagnosed as T1a and the maximal size is ≤ 2 cm (56). The experiences of post-ER management in Japan and Korea should be much substantial, and thus the present meta-analysis pooled studies just available from Japan and Korea.
At the 1-year or later endoscopic follow-up after ER or gastrectomy for EGC, the MGC has been more prone to occur after ER instead of gastrectomy (57). It may be due to the fact that the stomach can be preserved after ER, while most of the stomach is removed postgastrectomy with a limited area of mucosa left. Meanwhile, some studies have shown that conventional mucosal resection has a higher incidence of post-ER MGC than expanded mucosal resection (58). However, providing repeated ER treatment, there is no significant difference in long-term survival outcomes between the expanded and conventional groups (58). Besides, the other potential risk factors for MGC after ER for EGC included male, older age, severe or corpus intestinal metaplasia, family history of gastric cancer, synchronous adenoma, and H. pylori infection (59–61). It is the rationale why the present meta-analysis hypothesizes H. pylori infection could increase MGC risk and post-ER eradication might benefit in preventing MGC.
Regarding H. pylori infection, some consider eradication is no longer meaningful since gastric cancer has already developed, but investigation has shown that after eradication, the richness and uniformity of the gastric microbiota could be restored to a state similar to that of negative subjects. From bench to bedside, our updated findings evidence the capacity of reducing MGL incidence by post-ER eradication with plausible reporting quality, consistent with previous studies (62–64). Some studies suggested that the effectiveness of eradication could be observed by decreasing MGC incidence after the 6th post-ER year, but not at 3-year follow-up (31). Specifically, adequate length of endoscopic surveillance must be quite important in common practice, and diverse follow-up situations might introduce the source of heterogeneity in the present meta-analysis. Additionally, the time interval between the first ER and H. pylori eradication needs to be taken into account. As reported in the study by Kato et al. (35), all the patients were required to undergo H. pylori eradication within no more than 1 year after ER. Moreover, the regimens of H. pylori eradication are unspecific to post-ER patients. Actually, the risk factors of MGLs and the best follow-up strategy are still underestimated up to the known knowledge.
Some limitations need attention yet. First, all the included studies came from Japan and Korea, and the extrapolation of results kept unclear to other high-risk areas. Second, although the quality of the included cohort studies was high or moderate, only three available RCTs indicated that selection and performance biases were inevitable. Third, in many studies, follow-up work was not long enough and without regular endoscopic surveillance, which could introduce between-study heterogeneity. Fourth, the time interval between H. pylori eradication and the first ER had not been detailed in several studies, and thus the prolonged infection status might potentially influence the preventive effectiveness. Finally, other confounders for gastric cancer risk such as infection-attributable oncoviruses, family history of gastric cancer, and ethnicity had not been considered in the present investigation (65–68).
In summary, the present meta-analysis can further evidence that the H. pylori infection might potentially increase the post-ER incidence of MGC or dysplasia, but post-ER eradication can decrease the risk of MGC or dysplasia. Specifically, post-ER screening and eradication of Helicobacter pylori have preventive effectiveness on MGC, and the protocol should be recommended to all the post-ER patients. Moreover, larger-scale RCTs with longer follow-up duration are still warranted to define the best cost-effective interval and length for endoscopic surveillance among H. pylori-infected individuals after ER.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
T-HY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. DB: Data curation, Investigation, Methodology, Writing – review & editing. KL: Data curation, Methodology, Writing – review & editing. W-HZ: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. X-ZC: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. J-KH: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Foundation of Science and Technology Department of Sichuan Province, China (No. 23ZDYF0839); The 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University, China (No. ZY2017304).
This study was a part of series of studies from the Sichuan Gastric Cancer Early Detection and Screening (SIGES) project. The authors thank the substantial work of the Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China. Additionally, Yibin Cancer Prevention and Control Center, Second People’s Hospital of Yibin—West China Yibin Hospital, Sichuan University, Yibin, China, supported the SIGES research project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1393498/full#supplementary-material
CI, Confidence interval; EGC, Early gastric cancer; EMR, Endoscopic mucosal resection; ER, Endoscopic resection; ESD, Endoscopic submucosal dissection; H. pylori, Helicobacter pylori; MGC, Metachronous gastric cancer; MGL, Metachronous gastric lesion; MGLs, Metachronous gastric lesions; MOOSE, Meta-analysis Of Observational Studies in Epidemiology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; RCTs, Randomized controlled trials; RR, Risk ratio.
1. Smyth, EC, Nilsson, M, Grabsch, HI, van Grieken, NCT, and Lordick, F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Li, Y, Choi, H, Leung, K, Jiang, F, Graham, DY, and Leung, WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2023) 8:553–64. doi: 10.1016/S2468-1253(23)00070-5
4. Arnold, M, Abnet, CC, Neale, RE, Vignat, J, Giovannucci, EL, McGlynn, KA, et al. Global burden of 5 major types of gastrointestinal Cancer. Gastroenterology. (2020) 159:335–49.e15. doi: 10.1053/j.gastro.2020.02.068
5. Bray, F, Ferlay, J, Laversanne, M, Brewster, DH, Gombe Mbalawa, C, Kohler, B, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. (2015) 137:2060–71. doi: 10.1002/ijc.29670
6. Chen, XZ, Schöttker, B, Castro, FA, Chen, H, Zhang, Y, Holleczek, B, et al. Association of helicobacter pylori infection and chronic atrophic gastritis with risk of colonic, pancreatic and gastric cancer: a ten-year follow-up of the ESTHER cohort study. Oncotarget. (2016) 7:17182–93. doi: 10.18632/oncotarget.7946
7. Wang, R, Zhang, MG, Chen, XZ, and Wu, H. Risk population of Helicobacter pylori infection among Han and Tibetan ethnicities in western China: a cross-sectional, longitudinal epidemiological study. Lancet. (2016) 388:S17. doi: 10.1016/S0140-6736(16)31944-4
8. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2020) 24:1–21. doi: 10.1007/s10120-020-01042-y
9. Chen, XZ, Hu, JK, Zhou, ZG, Rui, YY, Yang, K, Wang, L, et al. Meta-analysis of effectiveness and safety of D2 plus Para-aortic lymphadenectomy for resectable gastric cancer. J Am Coll Surg. (2010) 210:100–5. doi: 10.1016/j.jamcollsurg.2009.09.033
10. Zhang, WH, Chen, XZ, Liu, K, Chen, XL, Yang, K, Zhang, B, et al. Outcomes of surgical treatment for gastric cancer patients: 11-year experience of a Chinese high-volume hospital. Med Oncol. (2014) 31:150. doi: 10.1007/s12032-014-0150-1
11. Gotoda, T, and Ono, H. Stomach: endoscopic resection for early gastric cancer. Dig Endosc. (2022) 34:58–60. doi: 10.1111/den.14167
12. Suzuki, H, Ono, H, Hirasawa, T, Takeuchi, Y, Ishido, K, Hoteya, S, et al. Long-term survival after endoscopic resection for gastric Cancer: real-world evidence from a multicenter prospective cohort. Clin Gastroenterol Hepatol. (2023) 21:307–18.e2. doi: 10.1016/j.cgh.2022.07.029
13. Ono, H, Yao, K, Fujishiro, M, Oda, I, Uedo, N, Nimura, S, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. (2021) 33:4–20. doi: 10.1111/den.13883
14. Choi, KS, Jung, HY, Choi, KD, Lee, GH, Song, HJ, Kim, DH, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. (2011) 73:942–8. doi: 10.1016/j.gie.2010.12.032
15. Correa, P, Haenszel, W, Cuello, C, Tannenbaum, S, and Archer, M. A model for gastric cancer epidemiology. Lancet. (1975) 306:58–60. doi: 10.1016/S0140-6736(75)90498-5
16. Marshall, BJ, and Warren, JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. (1984) 1:1311–5.
17. Correa, P. Human gastric carcinogenesis: a multistep and multifactorial process--first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. (1992) 52:6735–40.
18. Zavros, Y, and Merchant, JL. The immune microenvironment in gastric adenocarcinoma. Nat Rev Gastroenterol Hepatol. (2022) 19:451–67. doi: 10.1038/s41575-022-00591-0
19. Wang, R, and Chen, XZ. High mortality from hepatic, gastric and esophageal cancers in mainland China: 40 years of experience and development. Clin Res Hepatol Gastroenterol. (2014) 38:751–6. doi: 10.1016/j.clinre.2014.04.014
20. Kim, SB, Lee, SH, Bae, SI, Jeong, YH, Sohn, SH, Kim, KO, et al. Association between Helicobacter pylori status and metachronous gastric cancer after endoscopic resection. World J Gastroenterol. (2016) 22:9794–802. doi: 10.3748/wjg.v22.i44.9794
21. Noh, CK, Lee, E, Park, B, Lim, SG, Shin, SJ, Lee, KM, et al. Effect of Helicobacter pylori eradication treatment on metachronous gastric neoplasm prevention following endoscopic submucosal dissection for gastric adenoma. J Clin Med. (2023) 12:1512. doi: 10.3390/jcm12041512
22. Jüni, P, Altman, DG, and Egger, M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. (2001) 323:42–6. doi: 10.1136/bmj.323.7303.42
23. Kim, SY, Park, JE, Lee, YJ, Seo, HJ, Sheen, SS, Hahn, S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
24. Bai, D, Liu, K, Wang, R, Zhang, WH, Chen, XZ, and Hu, JK. Prevalence difference of Helicobacter pylori infection between Tibetan and Han ethnics in China: a meta-analysis on epidemiologic studies (SIGES). Asia Pac J Public Health. (2023) 35:103–11. doi: 10.1177/10105395221134651
25. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
26. Peters, JL, Sutton, AJ, Jones, DR, Abrams, KR, and Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. (2008) 61:991–6. doi: 10.1016/j.jclinepi.2007.11.010
27. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
28. McInnes, MDF, Moher, D, Thombs, BD, McGrath, TA, and Bossuyt, PMand the PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and Meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
29. Yu, TH, and Chen, XZ. Helicobacter pylori eradication following endoscopic resection and metachronous gastric cancer: a systematic review and meta-analysis. PROSPERO (CRD42024512101) (2024). Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024512101
30. Choi, JM, Kim, SG, Choi, J, Park, JY, Oh, S, Yang, HJ, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc. (2018) 88:475–85.e2. doi: 10.1016/j.gie.2018.05.009
31. Choi, J, Kim, SG, Yoon, H, Im, JP, Kim, JS, Kim, WH, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. (2014) 12:793–800.e1. doi: 10.1016/j.cgh.2013.09.057
32. Fukase, K, Kato, M, Kikuchi, S, Inoue, K, Uemura, N, Okamoto, S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. (2008) 372:392–7. doi: 10.1016/S0140-6736(08)61159-9
33. Yoo, HW, Hong, SJ, and Kim, SH. Helicobacter pylori treatment and gastric cancer risk after endoscopic resection of dysplasia: a Nationwide Cohort Study. Gastroenterology. (2024) 166:313–22.e3. doi: 10.1053/j.gastro.2023.10.013
34. Nakata, R, Nagami, Y, Hashimoto, A, Sakai, T, Ominami, M, Fukunaga, S, et al. Successful eradication of Helicobacter pylori could prevent metachronous gastric cancer: a propensity matching analysis. Digestion. (2021) 102:236–45. doi: 10.1159/000504132
35. Kato, M, Hayashi, Y, Nishida, T, Oshita, M, Nakanishi, F, Yamaguchi, S, et al. Helicobacter pylori eradication prevents secondary gastric cancer in patients with mild-to-moderate atrophic gastritis. J Gastroenterol Hepatol. (2021) 36:2083–90. doi: 10.1111/jgh.15396
36. Ikeda, R, Hirasawa, K, Sato, C, Sawada, A, Nishio, M, Fukuchi, T, et al. Incidence of metachronous gastric cancer after endoscopic submucosal dissection associated with eradication status of Helicobacter pylori. Eur J Gastroenterol Hepatol. (2021) 33:17–24. doi: 10.1097/MEG.0000000000001788
37. Okada, K, Suzuki, S, Naito, S, Yamada, Y, Haruki, S, Kubota, M, et al. Incidence of metachronous gastric cancer in patients whose primary gastric neoplasms were discovered after Helicobacter pylori eradication. Gastrointest Endosc. (2019) 89:1152–9.e1. doi: 10.1016/j.gie.2019.02.026
38. Han, SJ, Kim, SG, Lim, JH, Choi, JM, Oh, S, Park, JY, et al. Long-term effects of Helicobacter pylori eradication on metachronous gastric cancer development. Gut Liver. (2018) 12:133–41. doi: 10.5009/gnl17073
39. Kwon, Y, Jeon, S, Nam, S, and Shin, I. Helicobacter pylori infection and serum level of pepsinogen are associated with the risk of metachronous gastric neoplasm after endoscopic resection. Aliment Pharmacol Ther. (2017) 46:758–67. doi: 10.1111/apt.14263
40. Chung, CS, Woo, HS, Chung, JW, Jeong, SH, Kwon, KA, Kim, YJ, et al. Risk factors for metachronous recurrence after endoscopic submucosal dissection of early gastric cancer. J Korean Med Sci. (2017) 32:421–6. doi: 10.3346/jkms.2017.32.3.421
41. Shin, SH, Jung, DH, Kim, JH, Chung, HS, Park, JC, Shin, SK, et al. Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS One. (2015) 10:e0143257. doi: 10.1371/journal.pone.0143257
42. Jung, S, Park, CH, Kim, EH, Shin, SJ, Chung, H, Lee, H, et al. Preventing metachronous gastric lesions after endoscopic submucosal dissection through Helicobacter pylori eradication. J Gastroenterol Hepatol. (2015) 30:75–81. doi: 10.1111/jgh.12687
43. Kwon, YH, Heo, J, Lee, HS, Cho, CM, and Jeon, SW. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients. Aliment Pharmacol Ther. (2014) 39:609–18. doi: 10.1111/apt.12633
44. Kim, YI, Choi, IJ, Kook, MC, Cho, SJ, Lee, JY, Kim, CG, et al. The association between Helicobacter pylori status and incidence of metachronous gastric cancer after endoscopic resection of early gastric cancer. Helicobacter. (2014) 19:194–201. doi: 10.1111/hel.12116
45. Bae, SE, Jung, HY, Kang, J, Park, YS, Baek, S, Jung, JH, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol. (2014) 109:60–7. doi: 10.1038/ajg.2013.404
46. Maehata, Y, Nakamura, S, Fujisawa, K, Esaki, M, Moriyama, T, Asano, K, et al. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. (2012) 75:39–46. doi: 10.1016/j.gie.2011.08.030
47. Shiotani, A, Uedo, N, Iishi, H, Yoshiyuki, Y, Ishii, M, Manabe, N, et al. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. (2008) 78:113–9. doi: 10.1159/000173719
48. Nakagawa, S, Asaka, M, Kato, M, Nakamura, T, Kato, C, Fujioka, T, et al. Helicobacter pylori eradication and metachronous gastric cancer after endoscopic mucosal resection of early gastric cancer. Aliment Pharmacol Ther. (2006) 24:214–8. doi: 10.1111/j.1365-2036.2006.00048.x
49. Chen, XZ, Liu, Y, Wang, R, Zhang, WH, and Hu, JK. Improvement of cancer control in mainland China: epidemiological profiles during the 2004–10 National Cancer Prevention and Control Program. Lancet. (2016) 388:S40. doi: 10.1016/S0140-6736(16)31967-5
50. Ding, SZ, Du, YQ, Lu, H, Wang, WH, Cheng, H, Chen, SY, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut. (2022) 71:238–53. doi: 10.1136/gutjnl-2021-325630
51. Huang, J, Lucero-Prisno, DE 3rd, Zhang, L, Xu, W, Wong, SH, Ng, SC, et al. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol. (2023) 20:271–87. doi: 10.1038/s41575-022-00726-3
52. Yang, L, Kartsonaki, C, Yao, P, de Martel, C, Plummer, M, Chapman, D, et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. (2021) 6:e888–96. doi: 10.1016/S2468-2667(21)00164-X
53. Zou, JC, Wen, MY, Huang, Y, Chen, XZ, and Hu, JK. Helicobacter pylori infection prevalence declined among an urban health check-up population in Chengdu, China: a longitudinal analysis of multiple cross-sectional studies. Front Public Health. (2023) 11:1128765. doi: 10.3389/fpubh.2023.1128765
54. Zou, JC, Yang, Y, and Chen, XZSichuan Gastric Cancer Early Detection and Screening Research Group. Active eradication of within organized massive screening might improve survival of gastric cancer patients. Gastroenterology. (2023) 164:162–3. doi: 10.1053/j.gastro.2022.05.009
55. Chen, XZ, Zhang, WH, and Hu, JK. A difficulty in improving population survival outcome of gastric cancer in mainland China: low proportion of early diseases. Med Oncol. (2014) 31:315. doi: 10.1007/s12032-014-0315-y
56. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
57. Ortigão, R, Figueirôa, G, Frazzoni, L, Pimentel-Nunes, P, Hassan, C, Dinis-Ribeiro, M, et al. Risk factors for gastric metachronous lesions after endoscopic or surgical resection: a systematic review and meta-analysis. Endoscopy. (2022) 54:892–901. doi: 10.1055/a-1724-7378
58. Probst, A, Schneider, A, Schaller, T, Anthuber, M, Ebigbo, A, and Messmann, H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. (2017) 49:855–65. doi: 10.1055/s-0043-110672
59. Lee, E, Kim, SG, Kim, B, Kim, JL, Kim, J, Chung, H, et al. Metachronous gastric neoplasm beyond 5 years after endoscopic resection for early gastric cancer. Surg Endosc. (2023) 37:3901–10. doi: 10.1007/s00464-023-09889-9
60. Rei, A, Ortigão, R, Pais, M, Afonso, LP, Pimentel-Nunes, P, Dinis-Ribeiro, M, et al. Metachronous lesions after gastric endoscopic submucosal dissection: first assessment of the FAMISH prediction score. Endoscopy. (2023) 55:909–17. doi: 10.1055/a-2089-6849
61. Na, YS, Kim, SG, and Cho, SJ. Risk assessment of metachronous gastric cancer development using OLGA and OLGIM systems after endoscopic submucosal dissection for early gastric cancer: a long-term follow-up study. Gastric Cancer. (2023) 26:298–306. doi: 10.1007/s10120-022-01361-2
62. Yoon, SB, Park, JM, Lim, CH, Cho, YK, and Choi, MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. (2014) 19:243–8. doi: 10.1111/hel.12146
63. Jung, DH, Kim, JH, Chung, HS, Park, JC, Shin, SK, Lee, SK, et al. Helicobacter pylori eradication on the prevention of metachronous lesions after endoscopic resection of gastric neoplasm: a meta-analysis. PLoS One. (2015) 10:e0124725. doi: 10.1371/journal.pone.0124725
64. Xiao, S, Li, S, Zhou, L, Jiang, W, and Liu, J. Helicobacter pylori status and risks of metachronous recurrence after endoscopic resection of early gastric cancer: a systematic review and meta-analysis. J Gastroenterol. (2019) 54:226–37. doi: 10.1007/s00535-018-1513-8
65. Chen, XZ, Chen, H, Castro, FA, Hu, JK, and Brenner, H. Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine. (2015) 94:e792. doi: 10.1097/MD.0000000000000792
66. Wang, H, Chen, XL, Liu, K, Bai, D, Zhang, WH, Chen, XZ, et al. Associations between gastric cancer risk and virus infection other than Epstein-Barr virus: a systematic review and meta-analysis based on epidemiological studies. Clin Transl Gastroenterol. (2020) 11:e00201. doi: 10.14309/ctg.0000000000000201
67. Wang, R, Bai, D, Xiang, W, and Chen, XZ. Tibetan ethnicity, birthplace, Helicobacter pylori infection, and gastric cancer risk. Am J Gastroenterol. (2022) 117:1010. doi: 10.14309/ajg.0000000000001757
Keywords: early gastric cancer, metachronous gastric cancer, Helicobacter pylori , endoscopic resection, eradication
Citation: Yu T-H, Bai D, Liu K, Zhang W-H, Chen X-Z and Hu J-K (2024) Helicobacterpylori eradication following endoscopic resection might prevent metachronous gastric cancer: a systematic review and meta-analysis of studies from Japan and Korea. Front. Med. 11:1393498. doi: 10.3389/fmed.2024.1393498
Received: 01 April 2024; Accepted: 25 July 2024;
Published: 02 September 2024.
Edited by:
Ponsiano Ocama, Makerere University, UgandaReviewed by:
Olivia Kituuka, Makerere University, UgandaCopyright © 2024 Yu, Bai, Liu, Zhang, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Zu Chen, Y2hlbnhpbnp1QHNjdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.