- 1Geriatrics Clinic, Department of Internal Medicine and Medical Specialties (DIMI), University of Genoa, Genoa, Italy
- 2IRCCS Ospedale Policlinico San Martino, Genoa, Italy
Aging is associated with an increased risk of developing pain, especially in the presence of concurrent chronic clinical conditions. Similarly, multimorbidity can affect the perception and ability of older adults to appropriately respond to and communicate pain, and there is a clinical heterogeneity in the processing of painful sensations in different neurological conditions. The present narrative review is aimed at assessing the prevalent diseases associated with poor communication and pain in older adults, together with the available diagnostic instruments for the clinical assessment of pain in such a vulnerable population. Dementia was the most described pathology identified in the current literature associated with poor communication in older adults affected by pain, along with Parkinson’s disease and stroke. Notably, a common pattern of pain behaviors in these neurological disorders also emerged, indicating potential similarities in the clinical presentation and appropriate diagnostic workout. At the same time, there are many differences in the way patients express their pain according to their main neurological pathology. In addition to this, although a plethora of observation-based tools for pain in patients with dementia have been developed, there is no gold standard, and the clinical utility of such measurements is still largely unaddressed. Meanwhile, there is substantially no standardized observation-based tool for pain in non-communicative patients with Parkinson’s disease, and only a few for stroke. Overall, the present narrative review provides an update on the prevalent diseases beyond dementia associated with a communicative disability and a painful condition in older adults.
Introduction
Aging is associated with an increased risk of developing pain, especially in the presence of multimorbidity and frailty (1). The high prevalence of chronic pain in both community-dwelling older persons and nursing home residents (2–4) is associated with unfavorable clinical outcomes, including poorer cognitive performance, reduced quality of life, depression, functional decline, disability, and social vulnerability (5, 6).
Similarly, with aging, there is a net increase in neurological conditions, especially in frail patients, that may impact communicative ability, with a higher likelihood of failure in the identification and appropriate management of pain in such vulnerable individuals (7, 8). Additionally, aging could bring changes in pain processing and communication, which can render pain assessment tools typically used for younger individuals unreliable (9). Finally, changes in pharmacokinetics and pharmacodynamics, coupled with the polypharmacotherapy often seen in older adult patients, increase the risk of adverse events from pain medications. This adds to the risk of underdiagnosis of pain, making its management even more complex (10).
To date, dementia is the key relevant clinical condition associated with communicative disabilities (11–15). Pain expressions in patients with dementia often take less obvious forms, such as confusion or social withdrawal, that are behavioral equivalents of pain in non-communicative patients. To overcome the limited diagnostic accuracy of pain self-report tools in non-verbal communicative persons, whose ability to respond to direct pain questioning is impacted, the American Geriatrics Society and the American Society for Pain Management Nursing (16–18) selected a list of behavioral pain indicators to develop reliable observational-based pain assessment tools. Namely, facial expressions, vocalizations, body movements, changes in interpersonal interactions, basal activities of daily living, and mental status have been reported to be the most sensitive indicators of pain in non-communicative older adults with dementia (19).
These effective measures have been implemented to recognize and treat pain in a timely manner for such patients. However, in an effort to build and refine clinical recommendations after two decades, no specific tool is considered the gold standard, and the assessment of pain or discomfort in non-communicative patients remains a major challenge. Pain communication in older adult patients can be complex owing to several factors, including cultural variables, apart from specific clinical morbid conditions (7).
Starting from this background, this narrative review is aimed at assessing the prevalent diseases associated with pain in non-communicative older adults, along with a brief overview of the available diagnostic instruments for the clinical assessment of pain in such a vulnerable population. Furthermore, by virtue of what was mentioned earlier, our review also aims, as an overall goal, to provide insights for further aspects to be researched or implemented from the current literature on the subject.
Materials and methods
This review was based on a search in the MEDLINE, Scopus, and PEDro databases for articles in English published from January 1, 1990, to June 22, 2024, regarding the presence of pain in non-communicative older adults.
The Scale for the Assessment of Narrative Review Articles (SANRA) was used as a methodological guideline in conducting the narrative review (20). Briefly, the six items that form the scale are rated from 0 to 2, with 1 as an intermediate score. The maximal sum score is 12. The sum score of the scale is intended to measure the quality of a narrative review and covers the following topics: justification of the article’s importance for the readership (item 1), statement of the aims or formulation of questions (item 2), description of the literature search (item 3), referencing (item 4), scientific reasoning (item 5), and appropriate presentation of data (item 6). It represents a scale developed especially for the evaluation of narrative reviews by editors and peer reviewers. It is also used, as in our case, in the drafting phase of the article in order to make it as organic and rigorous as possible.
Search terms
• Pain was defined as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage’ or described in terms of such damage, according to the International Association of Pain (21). Based on the standard definition, ‘chronic pain is one such common ailment reported in older adults that also poses a significant economic burden on health care.’ Chronic pain is defined as pain that lasts for 3 to 6 months or more than expected (22). Chronic, acute, and/or breakthrough, musculoskeletal, neuropathic, ischaemic, mixed, and cancer pains were included in the search.
• Non-communicative or non-verbal persons were referred to as older adults with impaired ability to perceive, express, or verbally communicate pain or discomfort.
• The older adult term used in our literature search referred to all studies that included patients aged 65 years or older. In geriatrics, the categorization of aging is based on the following stratifications: young old (age: 65–74 years), old (75–84 years), and oldest old (≥85 years).
• The settings of care were community dwellings, nursing homes, hospitals, and transitional care units.
Inclusion criteria
The inclusion criteria were all the above-mentioned keywords in all possible combinations. Retrospective, prospective cohort, observational, and interventional studies that evaluated at least 50 patients were included.
Exclusion criteria
Abstracts, editorials, case studies, score creation studies, pilot studies, studies with fewer than 50 patients, and studies without a specific focus on older adults (i.e., adults aged <65 years or no data including old age participants); older adults with pre-existing intellectual disability; patients admitted to intensive care units; and older adults with disorders of consciousness were excluded.
The initial phase of article selection was conducted by the two co-first authors, who reached a common agreement on the chosen articles. In the subsequent phase, rather than extraction, the two aforementioned authors independently undertook separate tasks: one focused on pathologies associated with communication issues, while the other concentrated on methods for pain assessment within this context. A comprehensive evaluation of the findings was then conducted, drawing from the articles selected by other contributors to the review.
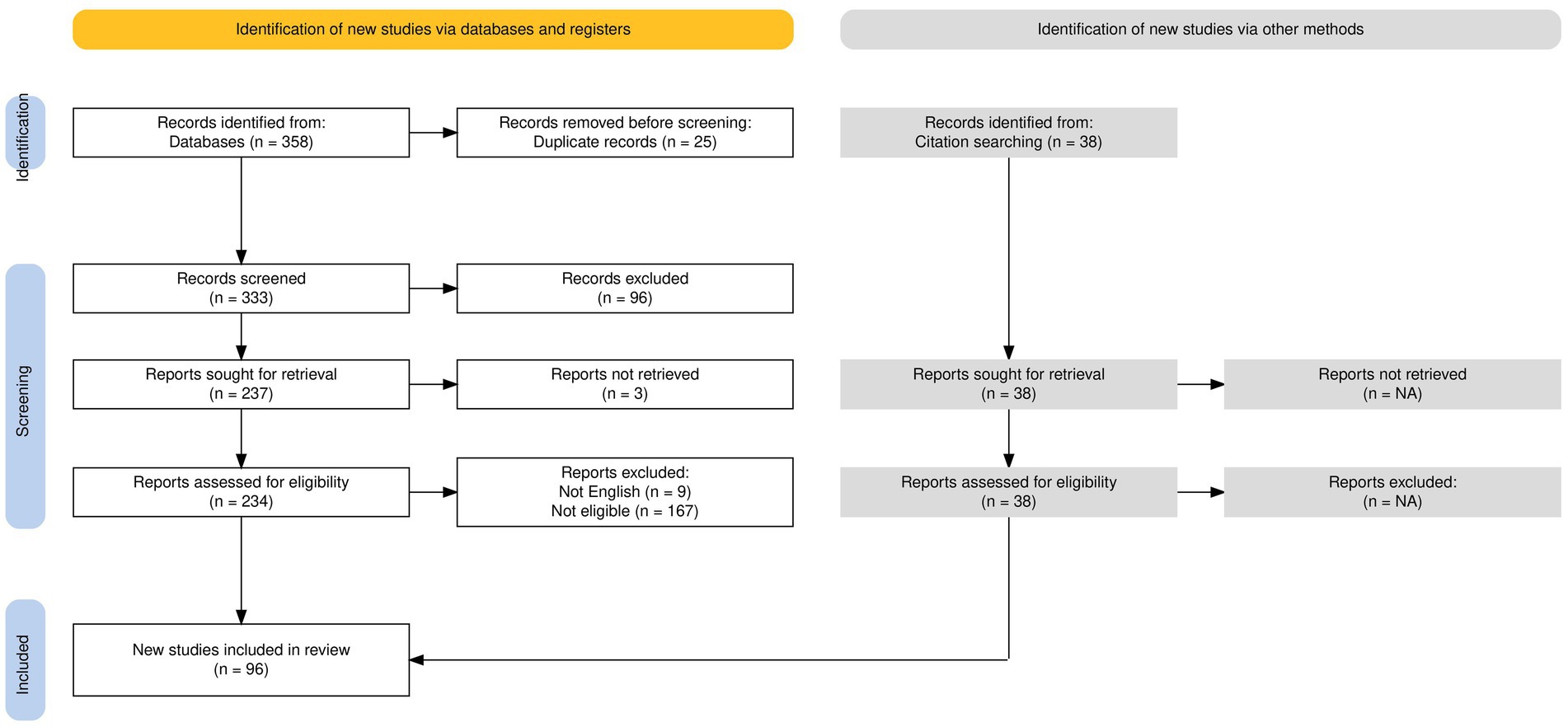
Figure 1 illustrates the selection process through a PRISMA flowchart (23). We included 58 suitable studies from 358 articles initially identified in the selected databases, as well as 38 articles not present in the chosen databases but included in the references of the identified articles. In particular, of the 358 initially identified articles, 25 were removed because they were duplicates; 96 were excluded according to the previously explained criteria based on the title and the abstract; and 179 articles were excluded after the evaluation of their full text.
Results
The most common chronic diseases associated with non-communicative older adults experiencing pain
Dementia was the most described disease associated with communicative inability in older adults experiencing pain or discomfort (2). Notably, orofacial pain was a highly prevalent type of pain, with an incidence ranging from 7.4 to 21.7%, especially in institutionalized older adult patients with dementia (48.8%) (24, 25). Impaired oral health care may be the result of executive cognitive dysfunction, motor apraxia, and/or abusing behavior such as neglect or resistance to care due to the patient’s behavioral disturbances (14, 25). Furthermore, suboptimal management of pain was found to be the catalyst for disruptive behavioral disturbances (26). In particular, verbalizations/vocalizations, sneezing, gasping, constrained facial expressions, restless or strained body expressions (e.g., raising the upper lip and guarding), impaired eating, agitation/aggressiveness, and resistance to care (2, 19, 27–30) were the most common disturbances displayed. As a result, the impaired ability to communicate made these vulnerable patients more likely to receive psychotropic medications than adequate pain medications (2).
Notably, verbalizations showed greater heterogeneity across different ethnic groups, potentially pointing out the socio-cultural background as a mediator of the overall pain experience (31).
While several articles demonstrate an improvement in pain recognition and its pharmacological management using observational scales in these patients, the clinical trial by Rostad and colleagues found that observational-based pain assessment in institutionalized patients with severe dementia did not result in an increase in analgesic drug administration (32–34).
Parkinson’s disease (PD) was the second most commonly reported disease associated with communicative inability and the experience of pain (35, 36). Although dementia was a comorbid condition in the advanced stage of PD, accounting for 30% of cases (37), poor communication was also associated with dysprosody, cognitive–linguistic impairment, alterations in social interactions, and pragmatics (37). Thus, an impaired ability to express pain through verbal and nonverbal communication (facial expression) was found in PD patients and casually linked to an impaired cognitive processing of painful sensations (35, 36).
Stroke was the third most described disease associated with poor communication of pain, with pain reported in these patients in a range of 42 to 72% (38, 39). In particular, 17.9% of patients had a co-occorrent diagnosis of dementia that was responsible for severe communicative inability, whereas aphasia or dysarthria were the main causes of non-communicative ability in those patients (40). In terminally ill patients with stroke, stroke-related pain was associated with central pain, shoulder–hand syndrome, and type 2 complex regional pain syndrome. Notably, pain behaviors were reported in 60% of dying patients, and wrinkled, contracted faces, moaning, and rubbing of the body were considered the most reliable pain indicators (39).
Figure 2 summarizes the prevalent pain equivalents associated with neurological disorders in older adults with communicative inability.
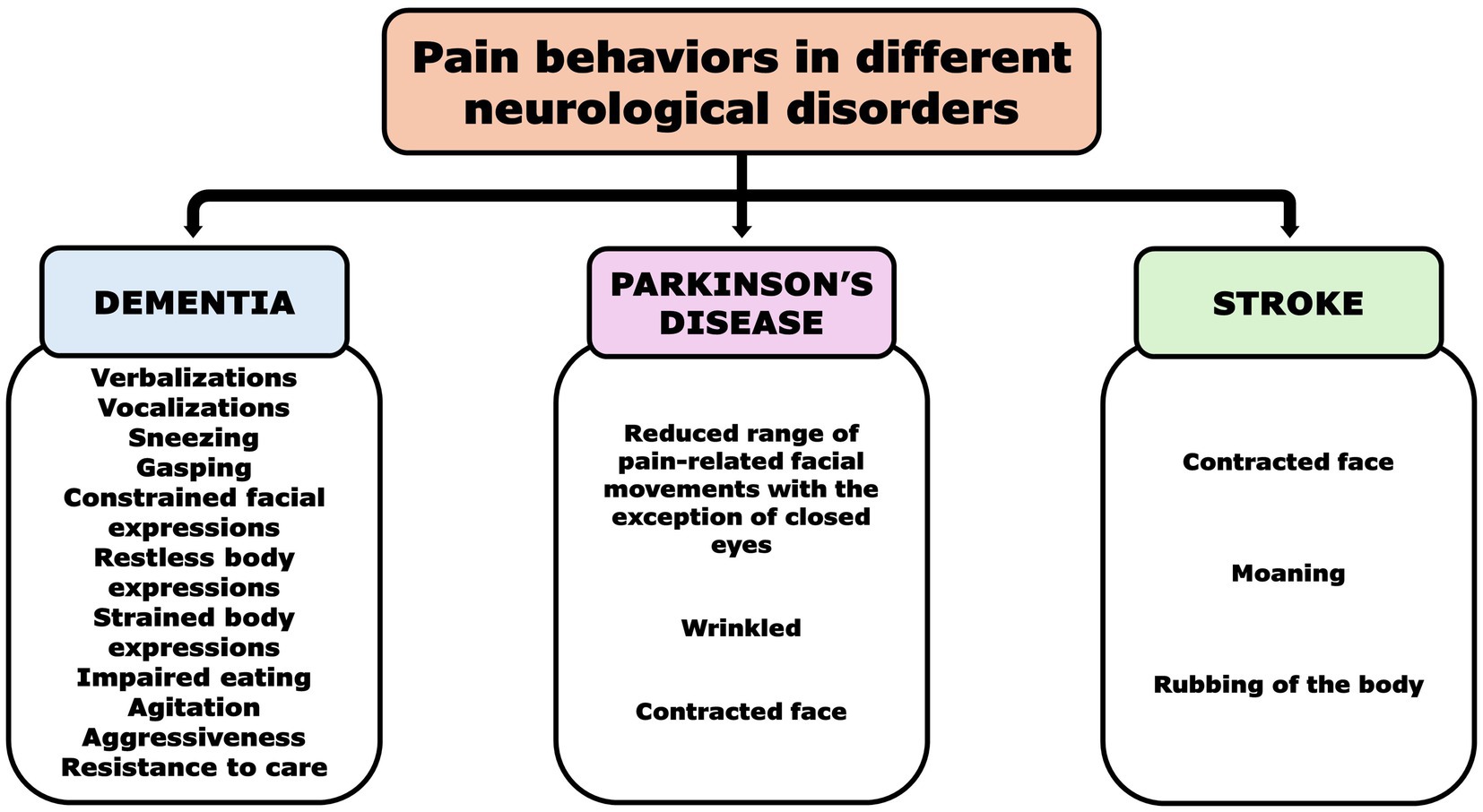
Figure 2. Pain behaviors in non-communicative patients with different neurological disorders [Dementia (2, 19, 27–30), Parkinson’s disease (36), and Stroke (39)].
Diagnostic pain assessment in non-communicative older adults
The following are the main assessment tools present in the literature for assessing pain in the non-communicating patient, which have been developed especially for patients with dementia.
We propose to classify pain assessment scales according to the degree of expertise of users (as indicated by the corresponding tool validation studies), including healthcare providers (for example, staff members, nurses, nurses assistants), medical professionals, and inexperienced observers or raters. There are no instruments in our data that are restricted to family members’ use.
Pain assessment tools administered by healthcare providers, including trained personnel
DOLOPLUS 2: the Doloplus 2 is a tool based on the Douleur Enfant Gustave Roussy scale for young children, adapted for use in older adults (11). It evaluates three distinct pain equivalents: facial expression, psychomotor behaviors, and psychosocial behaviors. Administered by nurses, it takes 6–12 min to complete and has a score range of 0–30. A score of 5 out of 30 suggests pain (11, 41). It has adequate clinometric properties for patients with dementia but lacks information on clinical phenotypes and stages of dementia (11). Validated in both acute and long-term care settings, it is a valuable tool for assessing pain in older adults.
ALGOPLUS: Algoplus is a five-item scale consisting of different facial, body, and movement-related behavioral indicators (42). It takes a few minutes to complete, and a cut-off score of 2 out of 5 indicates pain. It has been found to have good inter-rater reliability, with high sensitivity and specificity for identifying pain in patients with dementia, depression, or both, potentially including a wider set of non-communicative disorders. This scale, in its validation in multiple languages (English, Spanish, Italian, Portuguese, and Turkish), has also been successfully applied to stroke patients (43). The tool has been validated in emergency departments, acute care (geriatric and non-geriatric), rehabilitation, and long-term care settings. The tool may be administered by healthcare providers, including medical personnel.
PAINAD: the Pain Assessment in Advanced Dementia (PAINAD) scale is a modification of available pain assessment tools (FLACC and DS-DAT) (44) developed through expert consultations (45). It is composed of five behavioral indicators, resulting in a total score ranging from 0 to 10 (11, 45). The scale requires a 5-min observation period and can be administered by a nurse or nursing assistant (46), after a training session (45). The PAINAD scale has been validated in persons with advanced dementia in both long-term care and acute geriatric wards. However, the small sample used in developing the PAINAD limits its findings (45). A study by Mosele and colleagues confirmed the reliability and feasibility of the scale compared to self-reported pain assessment methods (47). Cross-culturally adapted versions of the tool, such as the Korean version (PAINAD-K) (48) and the Turkish version (PAINAD-T) (49), have shown promising results in different healthcare settings, such as long-term hospitals (PAINAD-K) and palliative or intensive care units (PAINAD-T) (48, 49). Additionally, Pinto and colleagues provided a Brazilian Portuguese version of the scale (PAINAD-Brazil), which showed good validity, reliability, and reproducibility (50). Indeed, PAINAD-Brazil was validated in a variety of clinical settings with a broad spectrum of patients (aged 20 to 104 years), yielding similar results when compared to the original and European Portuguese versions of the PAINAD scale (50).
PACSLAC and PACSLAC II: the Pain Assessment Checklist for Seniors with Severe Dementia (PACSLAC) is a 60-item checklist that measures pain behavior in older adults with dementia (45). It covers four subscales related to facial expressions, body movements, social interaction and mood, and physiological circadian rhythms as pain equivalents (11). It takes 5–8 min to complete by a healthcare provider (e.g., nurse), and no score threshold is currently available (45, 51). Although the PACSLAC has shown good clinometrics in patients with dementia, it may not be effective in acute care settings for patients with limited communicative abilities (52). The PACSLAC, similar to PAINAD, was regarded as one of the most clinically valuable and psychometrically strong tools (53). A revised version, PACSLAC-II (54, 55) consists of 31 items and six pain assessment domains, as recommended by the American Geriatrics Society, aiming to overcome the limitations of the original version. It takes a few minutes to complete by trained nurses and has been validated in patients with moderate-to-severe dementia in long-term care facilities. It has also shown meaningful correlations with the original PACSLAC scores and the ability to differentiate between different pain-related clinical conditions. The scoring procedure is the same as in the original version (sum of the scores for the single items, scoring 1 if observed).
NOPPAIN: the Non-Communicative Patient’s Pain Assessment Instrument (NOPPAIN) is a nursing assistant-administered instrument consisting of four main sections (activity chart checklist, pain behavior presence, pain behavior intensity, and pain intensity). Properly, it collects information about pain behaviors and the conditions under which they are observed. Pain intensity is scored using a 6-point Likert scale (4, 11, 56, 57). The NOPPAIN takes an average of 8 min to be completed, requiring minimal training (4, 56). The total NOPPAIN score is the sum of the scores for the four subitems (range = 0–55) (57). Pain intensity is scored on an 11-point numerical rating scale, with different levels indicating severity of pain observed in the patient (56). A cut-off is not clearly established (45). The tool has been validated in persons with dementia in various settings (57), but its validity and generalisability may be limited due to the use of a video approach to simulate a painful situation. In a training setting involving 142 hospital staff members, Novello and colleagues validated an Italian version of the tool using five videotapes showing varying degrees of pain intensity. The findings revealed that the Italian version of NOPPAIN achieved significant construct reliability and inter-rater reliability, accurately identifying the varied levels of pain intensity (58).
ABBEY PAIN SCALE: the Abbey Pain Scale by Abbey and colleagues (11, 59) includes six behavioral indicators (59): vocalization, facial expressions, change in body language, behavioral changes, and physiological and physical change. It requires 1 min of administration by a trained nurse and provides a score for each pain equivalent (a total possible score ranging from 0 to 18). A score of ≥3 indicates pain, with specific ranges for mild, moderate, and severe pain (45). The scale could discriminate between types of pain (28, 59) and has been validated in patients with moderate-to-severe dementia residing in home care settings (45). Gregersen and colleagues (28) performed a cross-cultural validation of the Danish version of the Abbey Pain Scale in a hospital setting and in a large population of older adults with dementia. Notably, the tool was more sensitive for the assessment of acute pain than chronic pain, with potential applications in the breakthrough pain assessment of patients with dementia (28).
CNPI: the Checklist of Nonverbal Pain Indicators (CNPI) includes a six-item scale designed to measure pain behaviors in the geriatric population with cognitive impairment. It includes non-verbal vocalizations, facial grimacing or wincing, bracing, rubbing, restlessness, and vocal complaints (60). Completing the CNPI requires 5–10 min (11) and the semiquantitative score is based on the sum of the scores for the subitems both in resting conditions and after movement. A clear cut-off has not been established (60). Attention to pain-related behaviors during transfers and patient care activities requires a nurse’s administration of the tool (60). However, the CNPI has a dichotomous scoring method, which may limit its clinical utility as it does not accurately score the severity of pain (11, 61).
REPOS: the Rotterdam Elderly Pain Observation Scale (REPOS) consists of 10 behavioral items relating to face expressions, emotional states, bodily behavior, and vocalizations, which the observer scores as present (1) or absent (0) after a 2-min observation period, preferably during a possible painful moment of care. Total scores range from 0 to 10. A cut-off of 3 or higher, along with a proxy NRS score of 4 or higher, suggests an elevated likelihood of pain (62). Although the REPOS does not assess pain intensity, it incorporates a decision-making tree to assist observers in assessing scores and managing pain (62). During the validation phase in nursing home residents, a large correlation emerged between 10-item REPOS and PAINAD, while correlations with NRS (resident self-report and nurse’s NRS) were limited (62). Originally developed for nursing home residents, the REPOS was shown to be a reliable and valid instrument for chronic and subacute pain assessment in different settings (nursing home, hospital, palliative care center) and populations (nursing home residents with various cognitive levels, institutionalized adults with cognitive impairment, palliative care patients, and non-communicative hospital patients) (62, 63).
PADE: the Pain Assessment for the Dementing Elderly (PADE) consists of 24 items and covers three dimensions: facial expressions, activities of daily living, and the overall healthcare provider’s judgment of pain symptoms. It takes 5–10 min to be completed by trained personnel. Scoring procedures are somewhat inconsistent and heterogeneous, and no cut-off is clearly established. It has adequate clinometric properties for assessing patients with advanced dementia (11). Despite some limitations in a study conducted with residents of long-term care facilities with advanced dementia (64), the PADE was found to be a reliable and valid tool for assessing pain in older adults with advanced dementia in such settings (45).
ADD: the Assessment for Discomfort in Dementia (ADD) evaluates six behavioral pain equivalents (facial expression, mood, body language, voice, behavior, and others) in older adults in long-term care facilities and includes treatment interventions for pain and emotional distress. The ADD protocol led to a significant increase in the use of pharmacologic and nonpharmacologic comfort interventions (65–67), due to its interactive assessment, which allows nurses to assess and manage the unmet needs of people with advanced stages of dementia (67). However, there is no available scoring or rating for pain intensity.
FLACC: the Faces, Legs, Activity, Cry, and Consolability (FLACC) Observational Tool is a behavioral scale (68) used in the assessment of older adults with cognitive impairment residing in long-term care settings (65, 68). It consists of five items evaluated on a 3-point scale for a total range score of 0–10. Observation is provided by trained nurses. However, this scale has not been shown to be a reliable pain assessment tool in patients with cognitive impairment due to limited data and a lack of cut-off scores or feasibility evaluation (65, 69).
MOBID-2: the MOBID-2 Pain Scale (70) is the nurse-administered version of the MOBID Pain Scale with added items to assess musculoskeletal and visceral pain. It has been validated (71) in non-communicative patients with severe dementia across different settings (dementia-assisted living groups, long-term care units, rehabilitation units, and palliative care units). The tool requires about 4 min to be completed, and it is also suitable for the virtual assessment of pain. In 2022, Scuteri and colleagues translated, adapted, and validated the Italian version of the MOBID-2 Pain Scale (I-MOBID2), using psychometric testing of the MOBID-2 for non-verbal and severely demented patients (72). Two groups of trained nurses conducted a validation study on a small sample (n = 11), with an average execution time of 5.38 min. The results confirmed the psychometric properties of the scale, demonstrating that the I-MOBID2 is a valuable tool that may be further refined and employed in community settings with healthcare provider administration. Interestingly, the I-MOBID2 was selected as the pain assessment tool in a randomized, double-blind, placebo-controlled trial evaluating the efficacy of NanoBEO, an engineered bergamot essential oil with proven analgesic and anxiolytic properties, in reducing agitation and pain in advanced dementia patients (72).
Pain assessment tools requiring professional expertise (e.g., medical or expert personnel)
DS-DAT: the Discomfort Scale in Dementia of the Alzheimer’s Type (DS-DAT/DS-DAT modified) (44) assesses discomfort in older adults with advanced dementia of the Alzheimer’s type. This scale consists of nine items, measured after a 5-min observation period based on frequency, intensity, and duration. The total score ranges from 0 to 27, with higher scores indicating a high level of discomfort (44). The tool, originally developed for research, has been tested in several settings (65) and can be completed in about 15 min by an expert rater (65).
OPS-NVI: the Orofacial Pain Scale for Non-Verbal Individuals (OPS-NVI) (14) was developed to diagnose orofacial pain in non-communicative persons. It is a meta-tool of the PAIC (13), which evaluates facial activities, body movements, vocalizations, and specific oral behaviors. The score ranges from 0 to 10, and a score of ≥1 is suggestive of pain. It takes prior training provided by a hygiene care specialist to utilize the tool (14). The tool has been validated in persons with dementia and other non-communicative disorders in different settings [outpatient memory clinics, geriatric outpatient clinics, hospital nursing homes (14), as well as acute hospitals (25)].
MOBID: the Orofacial MOBID Pain Scale assesses the presence of orofacial pain or discomfort-related behaviors based on pain noises, facial expression, and reaction to care, as well as the presence of dementia-related behaviors such as anxiety, aggression, and confusion. It requires about 1 h of training and less than 5 min to be completed. The pain intensity is evaluated at rest and during each rated movement using the NRS, and the overall pain intensity score is rated using the same NRS quantitative scoring for each item (73, 74). The MOBID Pain Scale has been validated in older individuals with dementia with the use of video uptakes (74) and with increased reliability in repeated assessments. In line with this, Husebo and colleagues reported moderate-to-excellent intra- and inter-rater reliabilities of the pain intensity for each item as well as the overall pain intensity score in the MOBID Pain Scale (75). Originally, the MOBID Pain Scale was validated in a nursing home setting, wherein pain was assessed by an expert senior dentist who rated the video uptake of patients undergoing oral health care. The teeth/mouth care item was not included in the initial draft of the MOBID Pain Scale due to its limited correlation with the overall score. Toxopeus and colleagues aimed to assess the reliability of this item by reviewing teeth and mouth care video fragments with elderly care dentists. Notably, their findings showed that all consistent scores pertained to dementia-related behaviors, not to orofacial pain or disability-related behaviors, supporting the decision of Husebo and colleagues to exclude the teeth/mouth care item from the original MOBID version (76).
Pain assessment tools without requested or indicated professional training
PAINE: the PAINE assessment tool is a caregiver or informant rating scale (53, 65, 77) that consists of 22 items divided into two parts: in the first, the tool evaluates the presence of physical repetitive movements, vocal repetitive behaviors, unusual behaviors, and any involvement in activities; in the second part, it explores physical signs of pain (in a dichotomous mode, yes/no) (77). The scale must be administered by an observer familiar with the patient, and in a study by Cohen and colleagues (77), data was collected from direct-care staff members. While it has been shown to be correlated with the PADE score, self-report, and observation (67), there is no information available on cut-off scores, rater training, or the feasibility and clinical utility of the tool (65).
PAIC: the Pain Assessment in Impaired Cognition (PAIC) scale was developed in a multidisciplinary effort to offer a scale that can be used by medical professionals (nurses, doctors). It is a meta-tool based on existing instruments with 36 consecutive items clustered into the domains of facial expression, vocalization, and body movements (13). This is an internationally agreed-upon tool to assess pain in individuals with cognitive impairment, especially dementia. All relevant pain-related observational items had been identified. However, existing scales include pain-irrelevant items or items of poor psychometric quality. Therefore, the main task was to reduce and refine the number of items (78). The PAIC scale has been validated in patients with dementia living in nursing homes. Each item is scored on a 4-point scale, and an observation time of at least 3 min in different settings is recommended (78). Observations can be conducted by healthcare professionals without receiving any special training (78). However, a standardized cut-off is unavailable. Four additional items (pained expression, raising the upper lip, pain-related words, and guarding) have been validated, and the item gasping has been identified as specific to pain (13).
The Observational Assessment of Pain or Distress tool has been used in post-acute care facilities with non-communicative older adults with dementia, showing an association between setting type and pain or distress (19). It is completed by staff observation in three steps: evaluating the patients’ ability to participate in a pain interview, the presence of potential pain indicators, and the response to pain treatment.
No standardized observation-based tool for the clinical assessment of pain in non-communicative patients with PD is available. Also, there is no observational tool specifically studied for the condition of stroke.
The Australian Pain Society’s Management Strategies of Pain in Residential Aged Care Facilities emphasizes the importance of using the observational pain measures we described both at rest and during movement (e.g., during transfers) to detect any exacerbation of the possible underlying pain condition (7, 8, 79, 80).
Lastly, we point out that NRS (or verbal descriptors) can be used by individuals with mild to moderate cognitive impairment and borderline communicative capacity, while observational scales are preferable in more advanced stages of cognitive impairment. The National Guidelines on the Assessment of Pain in Older People in the United Kingdom and the Australian Pain Society both support these recommendations (7, 79, 80). For situations relevant to our study, like limited indications of stroke and communication problems related to Parkinson’s disease, there is no specific guideline (7, 8, 79, 80). Table 1 summarizes the above-mentioned major findings.
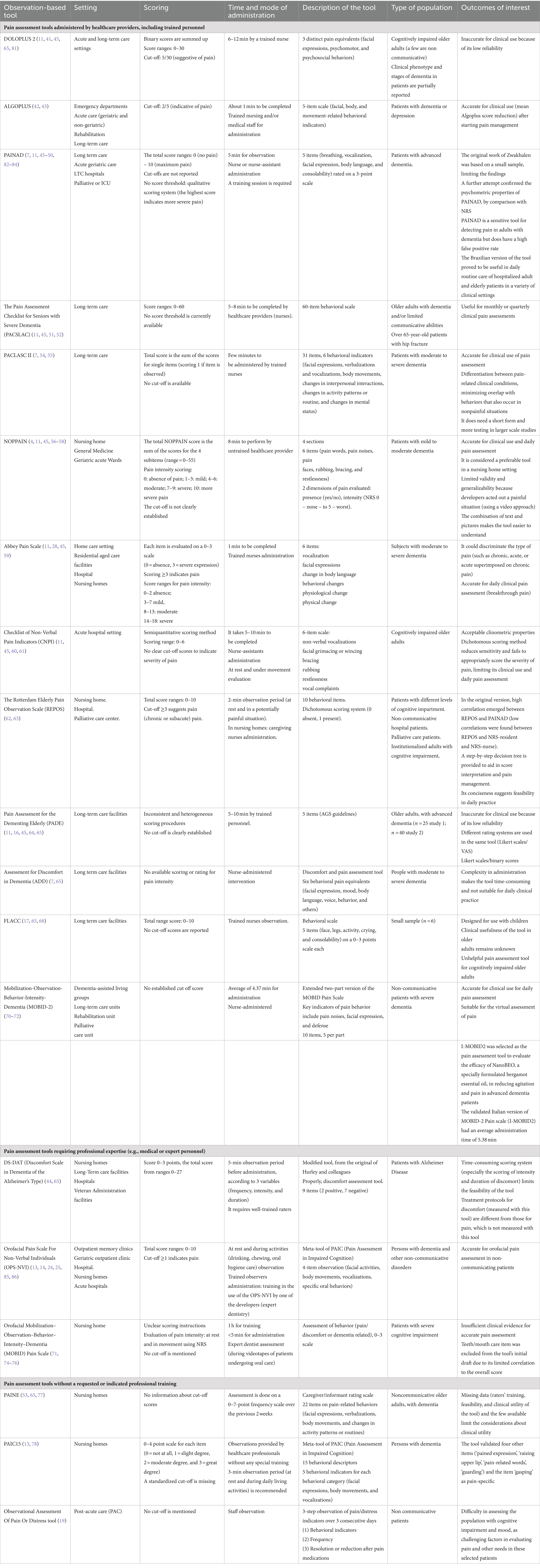
Table 1. Description of the main observational pain tools for non-communicating older adult patients (3).
High-technology tools for pain assessments
Recently, El Tallawy and colleagues (87) showed promising solutions offered by high-tech tools for pain assessment, especially in patients affected by moderate to severe dementia. The new technologies rely on various tools, including the detection of facial expressions, facial muscle movements, vocal cord responses, and behavioral changes caused by pain. The Automatic Pain Assessment with Video Systems is suitable for older patients with dementia and can complement other pain assessment methods. This tool primarily emphasizes the automatic analysis of facial expressions. Another promising high-tech tool is the smart wearable shirt, which is able to continuously monitor human physiological signs (heart rate, any changes in respiratory function, body movements) without impacting daily living. Finally, the authors refer to smart homes: residences equipped with Internet-connected devices used to collect, transfer, store, and analyze data over a network. Such tools would be able to minimize the possible bias found in other clinical methods and improve the quality of pain assessment.
Discussion
Our findings underscored that dementia, PD, and stroke were the most frequently described neurological diseases associated with communicative inability in older adults experiencing pain. To date, most evidence underscores that moderate-to-severe dementia stages have a major impact on the ability to express pain, increasing the risk of inappropriate medication prescriptions and poorer quality of care (2, 3, 48, 70, 88–91).
All these neurological conditions could affect not only pain communication but also its processing. In particular, it is noteworthy that pain processing undergoes substantial alterations in the presence of neurodegenerative diseases associated with dementia, and namely, incoherent pain-related facial expressions in response to pressure stimuli may be associated with structural changes in the prefrontal areas, the loss of inhibition of pain stimuli, and the amplification of the overall pain response in those patients (92). Similarly, these vulnerable patients experience altered descending endogenous pain modulation that impacts their ability to appropriately report pain (93–96).
Notably, alterations in the processing of painful stimuli have also been reported in different types of dementia, and, in particular, patients with vascular dementia were deemed to experience hyperpathia, whereas those with frontotemporal dementia seemed to experience reduced pain cognition (35, 97).
Our findings also originally emphasized that orofacial pain is one of the most painful conditions (24, 25), ranging from 7.4 to 60% in older people with dementia (98, 99), suggesting that the adoption of adequate oral health care may turn out to be a key relevant measure to prevent behavioral equivalents of pain in such vulnerable patients.
Moreover, on the basis of our results, a cluster of behavioral equivalents in patients with dementia experiencing pain was described, including verbalizations, gasping, constrained facial expressions, guarding, and restless or strained body expressions (13, 100).
Similarly, Ford and colleagues (31) have identified rubbing, bracing, restlessness, and pain vocalization as the most reliable behavioral equivalents of pain in patients with dementia.
However, the identification of pain in patients with communicative inability should go beyond the metrics of dementia, including different neurological conditions that may share similarities in terms of atypical presentation, underdiagnosis, and undertreatment.
Notably, regarding PD, pain is considered a relevant non-motor symptom in this condition, increasing the disease burden and affecting the quality of life (101). In particular, hypernociception may precede the development of the motor symptoms, and chronic pain may be considered the most prevalent non-motor symptoms of PD (102). Priebe and colleagues (36) have underscored that patients with PD experience a reduced range of pain-related facial movements in response to a pain trigger. However, facial movements with the eyes closed were unaltered, suggesting that ‘eye closure’ may be considered a reliable pain equivalent in those patients. Additionally, the overall frequency and intensity of facial movements in response to pain stimuli were reduced in patients with PD experiencing the ‘off phase’. This is a clinical phase of motor and non-motor downregulation due to long-term levodopa administration (36), suggesting that the dimension of pain may be associated with the extent of dopaminergic deficiency and related fluctuations (103).
As already underlined, the ‘eye closure’ could be a behavioral equivalent in patients affected by PD (36), although no specific tool has been validated in this type of neurodegenerative disease to estimate pain if there are communication issues, leaving a gap of knowledge (104). Relatively recent findings have implemented the classification and diagnosis based on the PD-Pain Classification System (103), which enables the differentiation of PD-related pain into nociceptive, neuropathic, and nociplastic types (103). Although the system has definitely improved the mechanistic understanding of pain in PD, pain assessment in patients with cognitive impairment and/or communicative inability is still largely unaddressed.
Stroke was the third most described clinical condition associated with communicative inability and pain. The presence of aphasia can affect speaking or auditory comprehension, and similarly, dysarthria may affect speech articulation for muscle coordination, making speech intelligibility possibly impaired. Although the multifaceted origin of the altered communication ability in patients with stroke is reported, evidence is conflicting regarding whether the assessment of patients with stroke and aphasia could rely on self-reported pain instruments. Mandysova and colleagues (105) concluded that a major concern that permeates several studies is the fact that stroke with severe communication problems fails to be appropriately diagnosed with self-report tools, and the majority of studies have focused mainly on mild-to-moderate aphasia. The use of the PACSLAC-II along with self-instruments for such a vulnerable population is then recommended (54, 106). Moreover, in their retrospective study on terminally ill patients with stroke, Mazzocato and colleagues (39) underscored a cluster of pain equivalents, such as wrinkled, contracted faces, moaning, and rubbing, which may be a preliminary platform for future studies to bridge the gap in knowledge.
Relative to the tools currently in the literature for pain assessment in nonverbal patients, it is beyond the scope of this review to give guidance on the most indicated one.
In a recent systematic review, strong and moderate evidence supported the use of the Facial Action Coding System, PACSLAC, PACSLAC-II, CNPI, Doloplus 2, Algoplus, MOBID Pain Scale, and MOBID-2 Scale for the assessment of pain among patients with dementia. However, insufficient time to use measurement tools, protracted time of administration and interpretation of results, undertreatment of pain in people with dementia, fear of side effects or drug interactions, limited evidence of the responsiveness, structural validity, and measurement error of the identified measures confine the use of most of the observational tools in research and point out the need for multidimensional tools (107).
Recently, the PAIC15 tool was developed to frame a multi-component pain assessment of patients to optimize the discrimination between normal and abnormal and/or noxious behaviors in patients with cognitive impairment and to differentiate acute from chronic pain in older adult patients with dementia (107). Hadjistavropoulos and colleagues (53) emphasize the need for a multi-component approach for pain assessment in non-communicative older adults, underlying the need to assess pain under movement and considering assessments before and after interventions.
However, the validity of the above-mentioned scales in the context of other neurological disorders remains a matter of debate, and further research is then needed to validate tools for clinical conditions other than dementia and in different clinical settings. In fact, as could be inferred, there are substantial differences in the types of pain that can be developed and the ways in which it is processed in subjects with these clinical conditions. By virtue of this, pain behaviors may be partially different in each condition, not completely allowing the rating scales for dementia to be generalized to other diseases as well. This is because most of the instruments were developed in patients with cognitive impairment, with some scales studied in patients with stroke but not specifically in patients with PD. This review can provide a starting point for the shared pain behaviors of the different diseases to begin with a tool that can be applied apart from dementia.
Another important point to highlight about the tools available for this issue is that they are mostly designed with dichotomous logic. This approach does not fully enable clinicians to understand the severity of the pain or hypothesize its nature. Moreover, this dichotomous logic complicates therapeutic management, as there are no standard criteria for initiating or revising therapy.
Furthermore, according to the main guidelines on this topic, the NRS, or verbal descriptors, can be used to assess pain in patients with mild-to-moderate neurocognitive disorders, as their ability to express pain through these methods is generally preserved (7, 8, 79, 80). Therefore, these tools can be a first choice in such conditions, while observational tools are mainly dedicated to patients with severe cognitive impairment. However, the complexity of daily clinical practice with these patients must be considered, given their possible fluctuations in cognitive status, especially in certain subtypes of neurodegenerative disorders, such as dementia with Lewy bodies, and possible incident cases of delirium (108, 109). Additionally, there is little evidence providing specific guidance in this sense for patients with communication problems associated with PD and stroke. Therefore, it is essential to conduct more studies on these topics with large sample sizes, considering all these issues, to make pain assessment and management for these patients increasingly systematic and effective.
To date, increasing education and research are still needed to minimize barriers and optimize a gold standard assessment tool for pain in non-communicative patients that should ideally include a multidimensional construct to address the complexity of this vulnerable and frail population (53, 107). Another opportunity to make the identification and management of pain in the non-communicating patient more cross-cutting and feasible is offered by new technologies. In particular, tools that take advantage of artificial intelligence may certainly be useful in the near future to make the assessment of indirect signs of pain more systematic and objective (87). However, these technologies still need much validation and optimization to enter everyday clinical practice. Furthermore, in order to overcome the current limitations of pain assessment in the non-communicating patient, it is crucial to ensure that this new field is not restricted solely to assessing pain in dementia but should also encompass the other neurological disorders discussed previously. New technologies, in the broader context of telemedicine, could make it possible to make the assessment of pain in neurological disorders described above more widespread, as is already happening, for example, with regard to telerehabilitation in entirely similar disease scenarios (110, 111). However, more research is needed even at this level.
The limitations of the present study include the lack of clinical phenotypes of patients, such as frailty or multimorbidity, which were not systematically investigated. Additionally, the heterogeneity of settings in terms of standardized requirements and facilities, as well as the limited population sample and the low number of prospective studies, were other sources of variability.
The strengths of the study are in its methodology, which is based on the SANRA and maintains a narrative approach to the presenting findings. In addition, the study presents evidence suggesting that non-communicative pain assessment may be applicable to other neurological diseases beyond dementia.
Conclusion
The present narrative review provides an update on the prevalent diseases beyond dementia associated with a communicative disability and a painful condition in older adults.
Standardizing methods for assessing pain in clinical settings is crucial, with a focus on using patient self-report tools whenever possible and observational scales when self-reporting is not feasible, as evidenced by multiple clinical recommendations (17, 18, 53).
The rapidly aging population carries a growing number of neurological conditions that share communicative disabilities; thus, the mandatory issue of early identification of pain in such a vulnerable population to constrain unfavorable clinical outcomes and reduced quality of life is a top priority. Alongside improving professionals’ training, education, and empowerment (38, 112, 113), the implementation of technology, such as specialized software capable of assessing pain levels concurrently, offers a promising integrated solution that warrants further exploration in the future (106, 114–119).
Author contributions
LT: Writing – original draft. GM: Writing – original draft. SO: Writing – review & editing. MM: Writing – review & editing. FR: Writing – review & editing. AN: Writing – review & editing. FM: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Elena Page for helping with the revision of the text from an English-language perspective.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnston, MC, Crilly, M, Black, C, Prescott, GJ, and Mercer, SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Pub Health. (2019) 29:182–9. doi: 10.1093/eurpub/cky098
2. Pieper, MJC, van Dalen-Kok, AH, Francke, AL, van der Steen, JT, Scherder, EJA, Husebø, BS, et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Res Rev. (2013) 12:1042–55. doi: 10.1016/j.arr.2013.05.002
3. While, C, and Jocelyn, A. Observational pain assessment scales for people with dementia: a review. Br J Community Nurs. (2009) 14:438–42. doi: 10.12968/bjcn.2009.14.10.44496
4. Horgas, AL, Nichols, AL, Schapson, CA, and Vietes, K. Assessing pain in persons with dementia: relationships among the non-communicative patient’s pain assessment instrument, self-report, and behavioral observations. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2007) 8:77–85. doi: 10.1016/j.pmn.2007.03.003
5. Pickering, G, Gibson, SJ, Serbouti, S, Odetti, P, Ferraz Gonçalves, J, Gambassi, G, et al. Reliability study in five languages of the translation of the pain behavioural scale Doloplus. Eur J Pain Lond Engl. (2010) 14:545.e1–545.e10. doi: 10.1016/j.ejpain.2009.08.004
6. Lysne, P, Cohen, R, Hoyos, L, Fillingim, RB, Riley, JL, and Cruz-Almeida, Y. Age and pain differences in non-verbal fluency performance: associations with cortical thickness and subcortical volumes. Exp Gerontol. (2019) 126:110708. doi: 10.1016/j.exger.2019.110708
7. Schofield, P, and Abdulla, A. Pain assessment in the older population: what the literature says. Age Ageing. (2018) 47:324–7. doi: 10.1093/ageing/afy018
8. Hadjistavropoulos, T, Craig, KD, Martin, N, Hadjistavropoulos, H, and McMurtry, B. Toward a research outcome measure of pain in frail elderly in chronic care. Pain Clin. (1997) 10:71–9.
9. Gagliese, L, and Katz, J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. (2003) 103:11–20. doi: 10.1016/S0304-3959(02)00327-5
10. Hall, T. Management of persistent pain in older people. J Pharm Pract Res. (2016) 46:60–7. doi: 10.1002/jppr.1194
11. Inelmen, EM, Mosele, M, Sergi, G, Toffanello, ED, Coin, A, and Manzato, E. Chronic pain in the elderly with advanced dementia. Are we doing our best for their suffering? Aging Clin Exp Res. (2012) 24:207–12. doi: 10.1007/BF03654801
12. Sengstaken, EA, and King, SA. The problems of pain and its detection among geriatric nursing home residents. J Am Geriatr Soc. (1993) 41:541–4. doi: 10.1111/j.1532-5415.1993.tb01892.x
13. Kappesser, J, Voit, S, Lautenbacher, S, and Hermann, C. Pain assessment for cognitively impaired older adults: do items of available observer tools reflect pain-specific responses? Eur J Pain Lond Engl. (2020) 24:851–62. doi: 10.1002/ejp.1536
14. Delwel, S, Perez, RSGM, Maier, AB, Hertogh, CMPM, de Vet, HCW, Lobbezoo, F, et al. Psychometric evaluation of the orofacial pain scale for non-verbal individuals as a screening tool for orofacial pain in people with dementia. Gerodontology. (2018) 35:200–13. doi: 10.1111/ger.12339
15. Romano, JM, Turner, JA, Jensen, MP, Friedman, LS, Bulcroft, RA, Hops, H, et al. Chronic pain patient-spouse behavioral interactions predict patient disability. Pain. (1995) 63:353–60.
16. AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. (2002) 50:S205–24. doi: 10.1046/j.1532-5415.50.6s.1.x
17. Herr, K, Coyne, PJ, McCaffery, M, Manworren, R, and Merkel, S. Pain assessment in the patient unable to self-report: position statement with clinical practice recommendations. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2011) 12:230–50. doi: 10.1016/j.pmn.2011.10.002
18. Herr, K, Coyne, PJ, Ely, E, Gélinas, C, and Manworren, RCB. ASPMN 2019 position statement: pain assessment in the patient unable to self-report. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2019) 20:402–3. doi: 10.1016/j.pmn.2019.07.007
19. Shier, V, Edelen, MO, McMullen, TL, Dunbar, MS, Bruckenthal, P, Ahluwalia, SC, et al. Standardized assessment of cognitive function, mood, and pain among patients who are unable to communicate. J Am Geriatr Soc. (2022) 70:1012–22. doi: 10.1111/jgs.17647
20. Baethge, C, Goldbeck-Wood, S, and Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. (2019) 4:5. doi: 10.1186/s41073-019-0064-8
21. Raja, SN, Carr, DB, Cohen, M, Finnerup, NB, Flor, H, Gibson, S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
22. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. (2009) 57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x
23. Haddaway, NR, Page, MJ, Pritchard, CC, and McGuinness, LA. PRISMA 2020: an R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. (2022) 18:e1230. doi: 10.1002/cl2.1230
24. van de Rijt, LJ, Feast, AR, Vickerstaff, V, Lobbezoo, F, and Sampson, EL. Prevalence and associations of orofacial pain and oral health factors in nursing home residents with and without dementia. Age Ageing. (2020) 49:418–24. doi: 10.1093/ageing/afz169
25. van de Rijt, LJM, Weijenberg, RAF, Feast, AR, Vickerstaff, V, Lobbezoo, F, and Sampson, EL. Oral health and orofacial pain in people with dementia admitted to acute hospital wards: observational cohort study. BMC Geriatr. (2018) 18:121. doi: 10.1186/s12877-018-0810-7
26. Manchola, EA. Alzheimer’s disease: Pathological and clinical findings, vol. 3. Blas Gil-Extremera, editor. Bentham Science Publishers (2019).
27. Helmer, LML, Weijenberg, RAF, de Vries, R, Achterberg, WP, Lautenbacher, S, Sampson, EL, et al. Crying out in pain-a systematic review into the validity of vocalization as an indicator for pain. Eur J Pain Lond Engl. (2020) 24:1703–15. doi: 10.1002/ejp.1623
28. Gregersen, M, Melin, AS, Nygaard, IS, Nielsen, CH, and Beedholm-Ebsen, M. Reliability of the Danish Abbey pain scale in severely demented and non-communicative older patients. Int J Palliat Nurs. (2016) 22:482–8. doi: 10.12968/ijpn.2016.22.10.482
29. Blomqvist, K, and Hallberg, IR. Recognising pain in older adults living in sheltered accommodation: the views of nurses and older adults. Int J Nurs Stud. (2001) 38:305–18. doi: 10.1016/S0020-7489(00)00078-X
30. Manfredi, PL, Breuer, B, Meier, DE, and Libow, L. Pain assessment in elderly patients with severe dementia. J Pain Symptom Manag. (2003) 25:48–52. doi: 10.1016/S0885-3924(02)00530-4
31. Ford, B, Snow, AL, Herr, K, and Tripp-Reimer, T. Ethnic differences in nonverbal pain behaviors observed in older adults with dementia. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2015) 16:692–700. doi: 10.1016/j.pmn.2015.03.003
32. Monacelli, F, Vasile Nurse, A, and Odetti, P. Doloplus-2 pain assessment: an effective tool in patients over 85 years with advanced dementia and persistent pain. Clin Ter. (2013) 164:e23–5. doi: 10.7417/CT.2013.1516
33. Ando, C, Ito, Y, Amemiya, S, Tamura, K, Kako, K, Tsuzura, S, et al. Effectiveness of the Japanese DOLOPLUS-2: a pain assessment scale for patients with moderate-to-severe dementia. Psychogeriatrics. (2016) 16:315–22. doi: 10.1111/psyg.12168
34. Rostad, HM, Utne, I, Grov, EK, Småstuen, MC, Puts, M, and Halvorsrud, L. The impact of a pain assessment intervention on pain score and analgesic use in older nursing home residents with severe dementia: a cluster randomised controlled trial. Int J Nurs Stud. (2018) 84:52–60. doi: 10.1016/j.ijnurstu.2018.04.017
35. Álvaro González, LC. The neurologist facing pain in dementia. Neurol Barc Spain. (2015) 30:574–85. doi: 10.1016/j.nrleng.2011.01.015
36. Priebe, JA, Kunz, M, Morcinek, C, Rieckmann, P, and Lautenbacher, S. Does Parkinson’s disease lead to alterations in the facial expression of pain? J Neurol Sci. (2015) 359:226–35. doi: 10.1016/j.jns.2015.10.056
37. Miller, N. Communication changes in Parkinson’s disease. Pract Neurol. (2017) 17:266–74. doi: 10.1136/practneurol-2017-001635
38. Muñoz-Narbona, L, Cabrera-Jaime, S, Lluch-Canut, T, Castaño, PB, and Roldán-Merino, J. E-learning course for nurses on pain assessment in patients unable to self-report. Nurse Educ Pract. (2020) 43:102728. doi: 10.1016/j.nepr.2020.102728
39. Mazzocato, C, Michel-Nemitz, J, Anwar, D, and Michel, P. The last days of dying stroke patients referred to a palliative care consult team in an acute hospital. Eur J Neurol. (2010) 17:73–7. doi: 10.1111/j.1468-1331.2009.02744.x
40. Schuster, J, Hoyer, C, Ebert, A, and Alonso, A. Use of analgesics in acute stroke patients with inability to self-report pain: a retrospective cohort study. BMC Neurol. (2020) 20:18. doi: 10.1186/s12883-020-1606-x
41. Ando, C, and Hishinuma, M. Development of the Japanese DOLOPLUS-2: a pain assessment scale for the elderly with Alzheimer’s disease. Psychogeriatr Off J Jpn Psychogeriatr Soc. (2010) 10:131–7. doi: 10.1111/j.1479-8301.2010.00324.x
42. Bonin-Guillaume, S, Jouve, E, Lauretta, R, Nalin, C, Truillet, R, Capriz, F, et al. Algoplus performance to detect pain in depressed and/or demented old patients. Eur J Pain Lond Engl. (2016) 20:1185–93. doi: 10.1002/ejp.844
43. Pickering, G, Monacelli, F, Pérez-Castejón Garrote, JM, Guarda, H, Batalha, L, Gibson, S, et al. Reliability study in five languages of the translation of the pain observational scale Algoplus. Pain Med. (2018) 19:252–61. doi: 10.1093/pm/pnw356
44. Hurley, AC, Volicer, BJ, Hanrahan, PA, Houde, S, and Volicer, L. Assessment of discomfort in advanced Alzheimer patients. Res Nurs Health. (1992) 15:369–77. doi: 10.1002/nur.4770150506
45. Zwakhalen, SMG, Hamers, JPH, Abu-Saad, HH, and Berger, MPF. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr. (2006) 6:3. doi: 10.1186/1471-2318-6-3
46. Warden, V, Hurley, AC, and Volicer, L. Development and psychometric evaluation of the pain assessment in advanced dementia (PAINAD) scale. J Am Med Dir Assoc. (2003) 4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7
47. Mosele, M, Inelmen, EM, Toffanello, ED, Girardi, A, Coin, A, Sergi, G, et al. Psychometric properties of the pain assessment in advanced dementia scale compared to self assessment of pain in elderly patients. Dement Geriatr Cogn Disord. (2012) 34:38–43. doi: 10.1159/000341582
48. Kwon, SH, Cho, YS, and Kim, H. Reliability and feasibility of the pain assessment in advanced dementia scale-Korean version (PAINAD-K). Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2021) 22:660–7. doi: 10.1016/j.pmn.2021.01.014
49. Büyükturan, Ö, Naharci, Mİ, Büyükturan, B, Kirdi, N, and Yetiş, A. The Turkish version of pain assessment in advanced dementia (PAINAD) scale. Noro Psikiyatr Ars. (2018) 55:271–5. doi: 10.29399/npa.22997
50. Pinto, MCM, Minson, FP, Lopes, ACB, and Laselva, CR. Cultural adaptation and reproducibility validation of the Brazilian Portuguese version of the pain assessment in advanced dementia (PAINAD-Brazil) scale in non-verbal adult patients. Einstein São Paulo. (2015) 13:14–9. doi: 10.1590/S1679-45082015AO3036
51. Fuchs-Lacelle, S, and Hadjistavropoulos, T. Development and preliminary validation of the pain assessment checklist for seniors with limited ability to communicate (PACSLAC). Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2004) 5:37–49. doi: 10.1016/j.pmn.2003.10.001
52. Beaupre, LA, Menon, MR, Almaazmi, K, Kang, SH, Dieleman, S, and Tsui, B. Preoperative nerve blocks for hip fracture patients: a pilot randomized trial. Injury. (2021) 52:548–53. doi: 10.1016/j.injury.2020.10.029
53. Hadjistavropoulos, T, Herr, K, Prkachin, KM, Craig, KD, Gibson, SJ, Lukas, A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. (2014) 13:1216–27. doi: 10.1016/S1474-4422(14)70103-6
54. Chan, S, Hadjistavropoulos, T, Williams, J, and Lints-Martindale, A. Evidence-based development and initial validation of the pain assessment checklist for seniors with limited ability to communicate-II (PACSLAC-II). Clin J Pain. (2014) 30:816–24. doi: 10.1097/AJP.0000000000000039
55. Ruest, M, Bourque, M, Laroche, S, Harvey, MP, Martel, M, Bergeron-Vézina, K, et al. Can we quickly and thoroughly assess pain with the PACSLAC-II? A convergent validity study in Long-term care residents suffering from dementia. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2017) 18:410–7. doi: 10.1016/j.pmn.2017.05.009
56. Snow, AL, Weber, JB, O’Malley, KJ, Cody, M, Beck, C, Bruera, E, et al. NOPPAIN: a nursing assistant-administered pain assessment instrument for use in dementia. Dement Geriatr Cogn Disord. (2004) 17:240–6. doi: 10.1159/000076446
57. Ferrari, R, Martini, M, Mondini, S, Novello, C, Palomba, D, Scacco, C, et al. Pain assessment in non-communicative patients: the Italian version of the non-communicative Patient’s pain assessment instrument (NOPPAIN). Aging Clin Exp Res. (2009) 21:298–306. doi: 10.1007/BF03324919
58. Novello, C, Ferrari, R, Scacco, C, and Visentin, M. The Italian version of the scale NOPPAIN: validation in a training context. Assist Inferm E Ric. (2009) 28:198–205.
59. Abbey, J, Piller, N, De Bellis, A, Esterman, A, Parker, D, Giles, L, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs. (2004) 10:6–13. doi: 10.12968/ijpn.2004.10.1.12013
60. Feldt, KS. The checklist of nonverbal pain indicators (CNPI). Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2000) 1:13–21. doi: 10.1053/jpmn.2000.5831
61. Scherder, E, and van Manen, F. Pain in Alzheimer’s disease: nursing assistants’ and patients’ evaluations. J Adv Nurs. (2005) 52:151–8. doi: 10.1111/j.1365-2648.2005.03577.x
62. van Herk, R, van Dijk, M, Tibboel, D, and Baar, F. The Rotterdam elderly pain observation scale (REPOS): a new behavioral pain scale for non-communicative adults and cognitively impaired elderly persons. J Pain Manag. (2009) 1:1.
63. Boerlage, AA, Sneep, L, Van Rosmalen, J, and Van Dijk, M. Validity of the Rotterdam elderly pain observation scale for institutionalised cognitively impaired Dutch adults. J Intellect Disabil Res. (2021) 65:675–87. doi: 10.1111/jir.12843
64. Villanueva, MR, Smith, TL, Erickson, JS, Lee, AC, and Singer, CM. Pain assessment for the dementing elderly (PADE): reliability and validity of a new measure. J Am Med Dir Assoc. (2003) 4:1–8. doi: 10.1097/01.JAM.0000043419.51772.A3
65. Lichtner, V, Dowding, D, Esterhuizen, P, Closs, SJ, Long, AF, Corbett, A, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. (2014) 14:138. doi: 10.1186/1471-2318-14-138
66. van Herk, R, van Dijk, M, Baar, FPM, Tibboel, D, and de Wit, R. Observation scales for pain assessment in older adults with cognitive impairments or communication difficulties. Nurs Res. (2007) 56:34–43. doi: 10.1097/00006199-200701000-00005
67. Corbett, A, Husebo, B, Malcangio, M, Staniland, A, Cohen-Mansfield, J, Aarsland, D, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. (2012) 8:264–74. doi: 10.1038/nrneurol.2012.53
68. Herr, K, Coyne, PJ, Key, T, Manworren, R, McCaffery, M, Merkel, S, et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manag Nurs Off J Am Soc Pain Manag Nurses. (2006) 7:44–52. doi: 10.1016/j.pmn.2006.02.003
69. Herr, K, Bjoro, K, and Decker, S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manag. (2006) 31:170–92. doi: 10.1016/j.jpainsymman.2005.07.001
70. Scuteri, D, Contrada, M, Tonin, P, Corasaniti, MT, Nicotera, P, and Bagetta, G. Dementia and COVID-19: a case report and literature review on pain management. Pharm Basel Switz. (2022) 15:199. doi: 10.3390/ph15020199
71. Husebo, BS, Strand, LI, Moe-Nilssen, R, Husebo, SB, and Ljunggren, AE. Pain in older persons with severe dementia. Psychometric properties of the mobilization-observation-behaviour-intensity-dementia (MOBID-2) pain scale in a clinical setting. Scand J Caring Sci. (2010) 24:380–91. doi: 10.1111/j.1471-6712.2009.00710.x
72. Scuteri, D, Contrada, M, Loria, T, Sturino, D, Cerasa, A, Tonin, P, et al. Pain and agitation treatment in severe dementia patients: the need for Italian mobilization–observation–behavior–intensity–dementia (I-MOBID2) pain scale translation, adaptation and validation with psychometric testing. Biomed Pharmacother. (2022) 150:113013. doi: 10.1016/j.biopha.2022.113013
73. Herr, K, Sefcik, JS, Neradilek, MB, Hilgeman, MM, Nash, P, and Ersek, M. Psychometric evaluation of the MOBID dementia pain scale in U.S. Nursing Homes Pain. Manag Nurs Off J Am Soc Pain Manag Nurses. (2019) 20:253–60. doi: 10.1016/j.pmn.2018.11.062
74. Husebo, BS, Strand, LI, Moe-Nilssen, R, Husebo, SB, Snow, AL, and Ljunggren, AE. Mobilization-observation-behavior-intensity-dementia pain scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. J Pain Symptom Manag. (2007) 34:67–80. doi: 10.1016/j.jpainsymman.2006.10.016
75. Husebo, BS, Strand, LI, Moe-Nilssen, R, Husebo, SB, and Ljunggren, AE. Pain behaviour and pain intensity in older persons with severe dementia: reliability of the MOBID pain scale by video uptake. Scand J Caring Sci. (2009) 23:180–9. doi: 10.1111/j.1471-6712.2008.00606.x
76. Toxopeus, AH, Husebo, BS, Strand, LI, Delwel, S, Van Wijk, AJ, Scherder, EJA, et al. The mouth care item of the MOBID pain scale: secondary analyses of unique video uptakes by dental professionals. Gerodontology. (2016) 33:61–8. doi: 10.1111/ger.12115
77. Cohen-Mansfield, J. Pain assessment in noncommunicative elderly persons—PAINE. Clin J Pain. (2006) 22:569–75. doi: 10.1097/01.ajp.0000210899.83096.0b
78. Kunz, M, de Waal, MWM, Achterberg, WP, Gimenez-Llort, L, Lobbezoo, F, Sampson, EL, et al. The pain assessment in impaired cognition scale (PAIC15): a multidisciplinary and international approach to develop and test a meta-tool for pain assessment in impaired cognition, especially dementia. Eur J Pain Lond Engl. (2020) 24:192–208. doi: 10.1002/ejp.1477
79. Scherer, S, Twigg, O, Wallace, M, Moore, N, Mantopoulos, S, Hogg, M, et al. In: R Goucke, editor. Pain in residential aged care facilities: Management strategies. 2nd ed. North Sydney, NSW: The Australian Pain Society (2019)
80. Schofield, P. The assessment of pain in older people: UK National Guidelines. Age Ageing. (2018) 47:i1–i22. doi: 10.1093/ageing/afx192
81. Hadjistavropoulos, T, Herr, K, Turk, DC, Fine, PG, Dworkin, RH, Helme, R, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. (2007) 23:S1–S43. doi: 10.1097/AJP.0b013e31802be869
82. Costardi, D, Rozzini, L, Costanzi, C, Ghianda, D, Franzoni, S, Padovani, A, et al. The Italian version of the pain assessment in advanced dementia (PAINAD) scale. Arch Gerontol Geriatr. (2007) 44:175–80. doi: 10.1016/j.archger.2006.04.008
83. Schuler, MS, Becker, S, Kaspar, R, Nikolaus, T, Kruse, A, and Basler, HD. Psychometric properties of the German ‘pain assessment in advanced dementia scale’ (PAINAD-G) in nursing home residents. J Am Med Dir Assoc. (2007) 8:388–95. doi: 10.1016/j.jamda.2007.03.002
84. Lin, PC, Lin, LC, Shyu, YIL, and Hua, MS. Chinese version of the pain assessment in advanced dementia scale: initial psychometric evaluation. J Adv Nurs. (2010) 66:2360–8. doi: 10.1111/j.1365-2648.2010.05405.x
85. de Vries, MW, Visscher, C, Delwel, S, van der Steen, JT, Pieper, MJC, Scherder, EJA, et al. Orofacial pain during mastication in people with dementia: reliability testing of the orofacial pain scale for non-verbal individuals. Behav Neurol. (2016) 2016:1–7. doi: 10.1155/2016/3123402
86. Delwel, S, Scherder, EJA, de Baat, C, Binnekade, TT, van der Wouden, JC, Hertogh, CMPM, et al. Orofacial pain and its potential oral causes in older people with mild cognitive impairment or dementia. J Oral Rehabil. (2019) 46:23–32. doi: 10.1111/joor.12724
87. El-Tallawy, SN, Ahmed, RS, Shabi, SM, Al-Zabidi, FZ, Zaidi, ARZ, Varrassi, G, et al. The challenges of pain assessment in geriatric patients with dementia: a review. Cureus. (2023) 15:e49639. doi: 10.7759/cureus.49639
88. Lukas, A, Barber, JB, Johnson, P, and Gibson, SJ. Observer-rated pain assessment instruments improve both the detection of pain and the evaluation of pain intensity in people with dementia. Eur J Pain Lond Engl. (2013) 17:1558–68. doi: 10.1002/j.1532-2149.2013.00336.x
89. Alexander, BJ, Plank, P, Carlson, MB, Hanson, P, Picken, K, and Schwebke, K. Methods of pain assessment in residents of long-term care facilities: a pilot study. J Am Med Dir Assoc. (2005) 6:137–43. doi: 10.1016/j.jamda.2004.12.024
90. Levintova-Romero, M, and Gotay, CC. Pain assessment in Hawaii nursing homes. Hawaii Med J. (2002) 61:121–3.
91. Hayes, R. Pain assessment in the elderly. Br J Nurs Mark Allen Publ. (1995) 4:1199–204. doi: 10.12968/bjon.1995.4.20.1199
92. Bunk, S, Zuidema, S, Koch, K, Lautenbacher, S, De Deyn, PP, and Kunz, M. Pain processing in older adults with dementia-related cognitive impairment is associated with frontal neurodegeneration. Neurobiol Aging. (2021) 106:139–52. doi: 10.1016/j.neurobiolaging.2021.06.009
93. Cole, LJ, Farrell, MJ, Duff, EP, Barber, JB, Egan, GF, and Gibson, SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer’s disease. Brain J Neurol. (2006) 129:2957–65. doi: 10.1093/brain/awl228
94. Monroe, TB, Beach, PA, Bruehl, SP, Dietrich, MS, Rogers, BP, Gore, JC, et al. The impact of Alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: a cross sectional study. J Alzheimers Dis JAD. (2017) 57:71–83. doi: 10.3233/JAD-161187
95. Anderson, AR, Iversen, WL, Carter, MA, Moss, KO, Cowan, RL, and Monroe, TB. Experimentally evoked pain in Alzheimer’s disease. J Am Assoc Nurse Pract. (2021) 34:18–25. doi: 10.1097/JXX.0000000000000580
96. Beach, PA, Huck, JT, Miranda, MM, and Bozoki, AC. Autonomic, behavioral, and subjective pain responses in Alzheimer’s disease. Pain Med Malden Mass. (2015) 16:1930–42. doi: 10.1111/pme.12769
97. Achterberg, W, Lautenbacher, S, Husebo, B, Erdal, A, and Herr, K. Pain in dementia. Pain Rep. (2020) 5:e803. doi: 10.1097/PR9.0000000000000803
98. Dagnino, APA, and Campos, MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. (2022) 16:736688. doi: 10.3389/fnhum.2022.736688
99. Delwel, S, Binnekade, TT, Perez, RSGM, Hertogh, CMPM, Scherder, EJA, and Lobbezoo, F. Oral health and orofacial pain in older people with dementia: a systematic review with focus on dental hard tissues. Clin Oral Investig. (2017) 21:17–32. doi: 10.1007/s00784-016-1934-9
100. Sampson, EL, White, N, Lord, K, Leurent, B, Vickerstaff, V, Scott, S, et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain. (2015) 156:675–83. doi: 10.1097/j.pain.0000000000000095
101. Ozturk, EA, Gundogdu, I, Kocer, B, Comoglu, S, and Cakci, A. Chronic pain in Parkinson’s disease: frequency, characteristics, independent factors, and relationship with health-related quality of life. J Back Musculoskelet Rehabil. (2016) 30:101–8. doi: 10.3233/BMR-160720
102. Tai, YC, and Lin, CH. An overview of pain in Parkinson’s disease. Clin Park Relat Disord. (2020) 2:1–8. doi: 10.1016/j.prdoa.2019.11.004
103. Mylius, V, Perez Lloret, S, Cury, RG, Teixeira, MJ, Barbosa, VR, Barbosa, ER, et al. The Parkinson disease pain classification system: results from an international mechanism-based classification approach. Pain. (2021) 162:1201–10. doi: 10.1097/j.pain.0000000000002107
104. Buhmann, C, Kassubek, J, and Jost, WH. Management of Pain in Parkinson’s disease. J Parkinsons Dis. (2020) 10:S37–48. doi: 10.3233/JPD-202069
105. Mandysova, P, Klugar, M, de Vries, NJC, and Matějková, I. Assessment instruments used for the self-report of pain by hospitalized stroke patients with communication problems: a scoping review protocol. JBI Evid Synth. (2020) 18:1731–7. doi: 10.11124/JBISRIR-D-19-00278
106. Soares, CD, Panuganti, PK, Shrivastava, A, Aroor, S, Keinath, KM, Bromagen, MC, et al. Experimental pain assessment in patients with poststroke aphasia. Neurology. (2018) 91:e793–9. doi: 10.1212/WNL.0000000000006081
107. Smith, TO, and Harvey, K. Psychometric properties of pain measurements for people living with dementia: a COSMIN systematic review. Eur Geriatr Med. (2022) 13:1029–45. doi: 10.1007/s41999-022-00655-z
108. Sampson, EL, West, E, and Fischer, T. Pain and delirium: mechanisms, assessment, and management. Eur Geriatr Med. (2020) 11:45–52. doi: 10.1007/s41999-019-00281-2
109. Matar, E, Shine, JM, Halliday, GM, and Lewis, SJG. Cognitive fluctuations in Lewy body dementia: towards a pathophysiological framework. Brain. (2020) 143:31–46. doi: 10.1093/brain/awz311
110. De Vitis, A, Battaglino, A, Sinatti, P, Sánchez Romero, EA, Bissolotti, L, Cotella, D, et al. Effects of telemedicine for postural instability in independent patients with Parkinson’s disease: a literature review. Top Geriatr Rehabil. (2023) 39:294–306. doi: 10.1097/TGR.0000000000000413
111. Bissolotti, L, Rota, M, Calza, S, Sanchez Romero, EA, Battaglino, A, and Villafañe, JH. Relationship between lower limbs performance and spinal alignment in Parkinson’s disease patients: an observational study with cross sectional design. J Clin Med. (2022) 11:3775. doi: 10.3390/jcm11133775
112. Vink, P, Torensma, B, Lucas, C, Hollmann, MW, van Schaik, IN, and Vermeulen, H. How incremental video training did not guarantee implementation due to fluctuating population prevalence. BMJ Open Qual. (2019) 8:e000447. doi: 10.1136/bmjoq-2018-000447
113. Savvas, SM, Toye, CM, Beattie, ERA, and Gibson, SJ. An evidence-based program to improve analgesic practice and pain outcomes in residential aged care facilities. J Am Geriatr Soc. (2014) 62:1583–9. doi: 10.1111/jgs.12935
114. Castillo, LI, Browne, ME, Hadjistavropoulos, T, Prkachin, KM, and Goubran, R. Automated vs. manual pain coding and heart rate estimations based on videos of older adults with and without dementia. J Rehabil Assist Technol Eng. (2020) 7:205566832095019. doi: 10.1177/2055668320950196
115. Atee, M, Hoti, K, Parsons, R, and Hughes, JD. Pain assessment in dementia: evaluation of a point-of-care technological solution. J Alzheimers Dis JAD. (2017) 60:137–50. doi: 10.3233/JAD-170375
116. Six, S, Laureys, S, Poelaert, J, Bilsen, J, Theuns, P, and Deschepper, R. Comfort in palliative sedation (Compas): a transdisciplinary mixed method study protocol for linking objective assessments to subjective experiences. BMC Palliat Care. (2018) 17:62. doi: 10.1186/s12904-018-0316-2
117. Bauschert, L, Prodhomme, C, Pierrat, M, Chevalier, L, Lesaffre, H, and Touzet, L. End-of-life comfort evaluation, is clinic enough? A retrospective cohort study of combined comfort evaluation with analgesia/nociception index and clinic in non-communicative patients. J Palliat Care. (2021) 39:122–8. doi: 10.1177/08258597211063687
118. Taylor, S, Allsop, MJ, Bennett, MI, and Bewick, BM. Usability testing of an electronic pain monitoring system for palliative cancer patients: a think-aloud study. Health Informatics J. (2019) 25:1133–47. doi: 10.1177/1460458217741754
Keywords: pain, older adults, non-verbal, non-communicative, dementia and neurodegenerative disorders
Citation: Tagliafico L, Maizza G, Ottaviani S, Muzyka M, Rovere FD, Nencioni A and Monacelli F (2024) Pain in non-communicative older adults beyond dementia: a narrative review. Front. Med. 11:1393367. doi: 10.3389/fmed.2024.1393367
Edited by:
Ann Horgas, University of Florida, United StatesReviewed by:
Eleuterio A. Sánchez Romero, European University of Madrid, SpainMing Zhang, Chinese Academy of Sciences (CAS), China
Copyright © 2024 Tagliafico, Maizza, Ottaviani, Muzyka, Rovere, Nencioni and Monacelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Tagliafico, dGFnbGlhZmljb2x1Y2ExOTkyQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Luca Tagliafico
Luca Tagliafico Giada Maizza
Giada Maizza Silvia Ottaviani1,2
Silvia Ottaviani1,2 Fiammetta Monacelli
Fiammetta Monacelli