- 1Centre of Public Health Emergency Management, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- 2Felge Meles Primary Hospital, Addis Ababa Health Bureau, Addis Ababa, Ethiopia
- 3Health Financing Department, Clinton Health Access Initiative, Addis Ababa, Ethiopia
- 4Data Impact Program, Vital Strategies, New York, NY, United States
Background: Maternal near miss (MNM) is one of the newly adopted assessment parameters to gauge the quality of maternity care. In Ethiopia, several studies have been conducted to investigate the incidence, underlying causes, and determinants of MNM. However, the findings from those studies vary greatly and are largely inconsistent. Thus, this review aims to more robustly estimate the pooled prevalence, identify underlying causes, and single out determinants of MNM in Ethiopia.
Methods: Studies were searched from international databases (PubMed/ Medline, Cochrane Library, and Embase databases) and other potential sites. All observational studies were included. Heterogeneity between studies was checked using Cochrane Q test statistics and I2 test statistics and small study effects were checked using Egger’s statistical test at a 5% significance level. Outcome measures were overall and specific underlying causes (obstetrics hemorrhage, hypertensive disorder pregnancy, pregnancy-related infection) rates of MNMs per 10,000 live births.
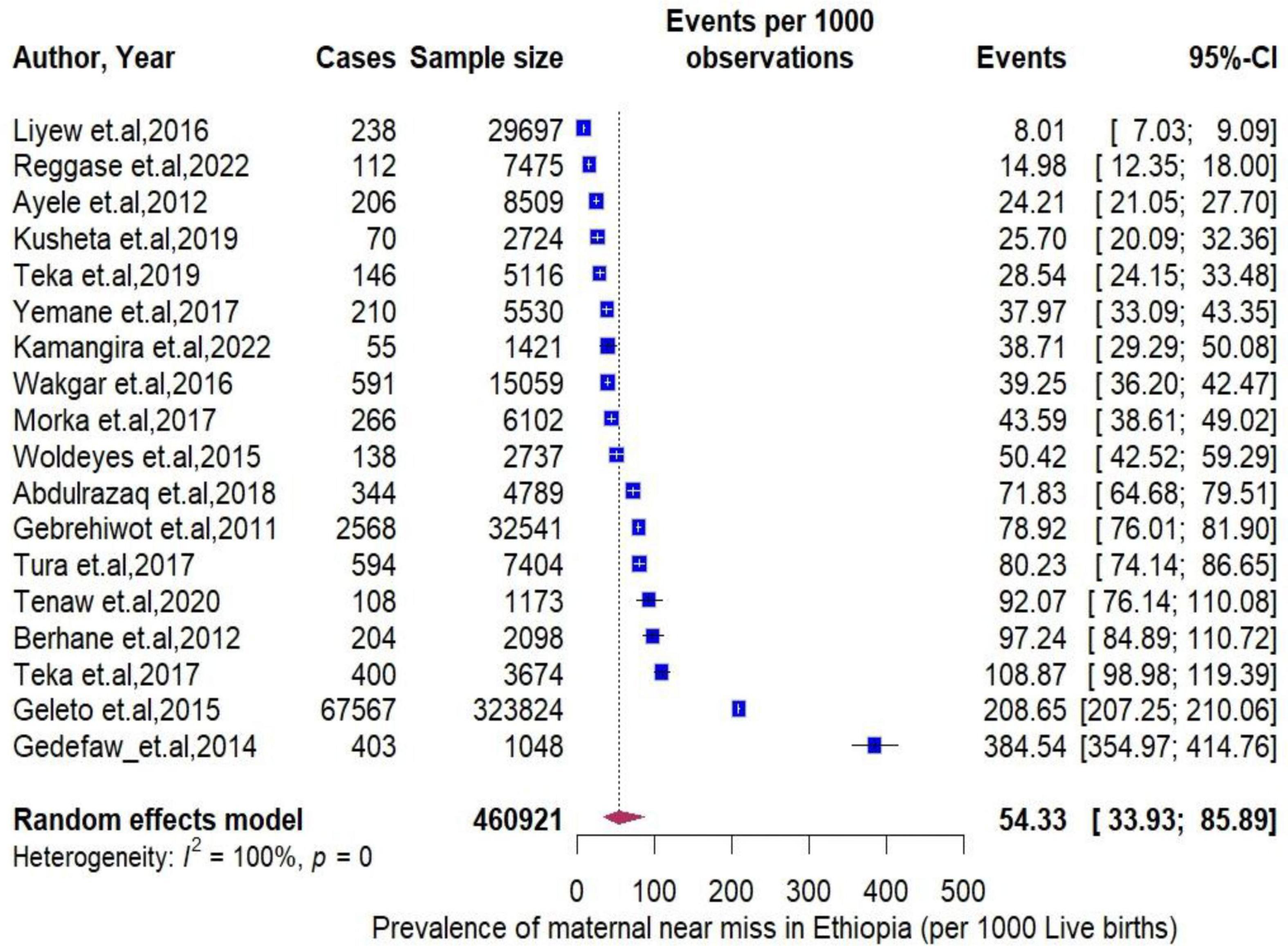
Result: The meta-analysis included 43 studies consisting of 77240 MNM cases. The pooled prevalence MNM per 1000 live births in Ethiopia was 54.33 (95% CI: 33.93 to 85.89). Between-study heterogeneity was high (I2 = 100%, P < 0.0001), with the highest rate observed in Amhara region (384.54 per 1000). The prevalence of obstetrics hemorrhage (14.56 per 1000) was higher than that of hypertensive disorder pregnancy (12.67 per 1000) and pregnancy-related infections (3.55 per 1000) were identified as underlying causes. Various factors, including socio demographic characteristics, previous medical and obstetrics history as well as access to and quality of care obtained, were associated with MNM.
Conclusion: Almost six women encounter near miss among a hundred live births in Ethiopia. Obstetric hemorrhage and hypertensive disorder pregnancy were the most common underlying causes of MNM. Both individual and facility level determinants were found to be associated with MNM. Considering the magnitude and identified factors, tailored measures should be taken at every stage of the continuum of care.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023395259.
Introduction
Maternal near miss (MNM) is a condition where a woman nearly died and survived complications that arise during pregnancy, childbirth, or within 42 days after the termination of pregnancy (1). It is a newly adopted parameter used to evaluate the quality of maternity service and provide additional insight into the overall level of the health system (2, 3).
Globally, similar to maternal deaths, the burden of maternal near miss (MNM) is more prominent in sub-Saharan African countries (4). To obtain comparable estimates, the World Health Organization (WHO) has developed identification criteria to capture MNM cases; however, these criteria have been criticized for underestimating the true burden of MNM in low-resource settings (5–7). Therefore, adapting and modifying the identification tool to the local context would help assess the continuum of care adequately and obtain comprehensive information to gain a clear view of the path to survival (8, 9). Additionally, conducting a review of MNM cases has far-reaching implications because it provides a live example of the quality of care rather than reviewing cases of women who died due to pregnancy-related complications (10). Moreover, routine review of MNM cases not only positively impacts efforts to reduce the burden of maternal mortality but also plays a role in improving prenatal outcomes and reducing treatment costs (11, 12).
Ethiopia is one of the countries with a very high burden of maternal mortality, with a rate of 401 per 100,000 live births in 2017, despite a decline in the last two decades (13, 14). Significant interventions, such as enhancing the health-seeking behaviors of the community, providing trained manpower, and expanding and equipping health facilities, have been implemented to improve maternal and child health outcomes in Ethiopia (15, 16). However, despite these measures, inadequate quality of care, lack of quantified evidence, and insufficient monitoring of the implemented plans have been major obstacles hindering the achievement of targets set to reduce maternal deaths at both the local and global levels (17–20).
Recognizing the gap in evidence generation, following the recommendation of the WHO, Ethiopia has established maternal and perinatal death surveillance and response (MPDSR) systems to prevent future deaths through a continuous action and surveillance cycle (21, 22). However, the system has not been fully implemented due to low community engagement, underreporting, and limited involvement of health professionals, who may avoid participation due to fear, blame, and accountability issues (23, 24). Additionally, the focus of MPDSR solely on mortality, without including severe morbidity, has made it challenging to estimate MNM using routine data (25).
As an alternative approach, the National Emergency Obstetric and Newborn Care (EmONC) assessment was used to measure the burden of MNM in Ethiopia. However, conducting this survey every five years has made it difficult to adapt to annual planning (26). Despite these challenges, the prevalence of MNM in Ethiopia ranges from 13% to 31%, which is higher than that in other sub-Saharan African countries (27, 28).
Similar to maternal death, MNM shares similar pathological and circumstantial factors (1). MNM is considered one of the inequalities within the multidimensional chain of causes that spans from macrosocial factors (such as quality of care and structural and societal factors) to micro clinical factors (including pathways from specific clinical precursors) and specific individual-level factors (29). The major underlying causes of MNM include hypertensive disorders of pregnancy (HDP), obstetric hemorrhage, uterine rupture, abortion-related complications, and malaria, all of which are associated with micro clinical factors (30–32). Individual-level factors related to MNM include maternal age, history of antenatal care (ANC), residence, income, educational status, unemployment, lack of awareness of pregnancy danger signs, and previous medical history [preexisting chronic illness and previous cesarean section (C/S)] (33–36). Macrosocial factors, such as delays in transportation and access to blood products, multiple referrals, and timely treatment after admission, are also significant contributors to MNM (37–40).
Several studies have been conducted on MNM in Ethiopia. Variations were observed in both prevalence and underlying causes across the studies, highlighting inconsistencies and uncertainties, primarily stemming from the failure to consider the most appropriate denominator, which is conspicuously lacking in most reviewed articles (41). This can lead to incorrect estimations and misinterpretations of the findings. Taking all of this into consideration, this review aimed to offer a comprehensive overview of the prevalence, underlying causes, and determinants of MNM in Ethiopia by carefully selecting the most suitable denominator and establishing a temporal trend of it.
Materials and methods
Protocol and registration
As depicted in Supplementary Material 1, the updated Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, along with and Meta-analysis of Observational Studies in Epidemiology (MOOSE), was used to report this review (42). The protocol has been registered on an International Prospective Register of Systematic Review (PROSPERO), University of York Center for Reviews and Dissemination1 (ref no ID: CRD42023395259).
Study design and search strategy
This review was designed to estimate the incidence, underlying causes, and determinants of MNM among women in Ethiopia. The PubMed/Medline, Cochrane Library, and Embase databases were systematically searched for relevant studies published online from January 2010 to December 2023. The gray literature was retrieved using Google Scholar searches with the inclusion of university repositories. These core search terms and phrases were considered interchangeably in different databases. The details are presented in Supplementary Material 2.
Eligible criteria
The PECO (population, exposure, comparison, and outcomes) technique, which primarily uses condition, context, and population (CoCoPop) questions, was employed by the authors to determine the inclusion and exclusion criteria for this systematic review and meta-analysis (43, 44) and the detail is annexed in Supplementary Material 3. Studies were included if they (1) were conducted in Ethiopia from January 2010 to December 2023; (2) were observational studies (cross-sectional, cohort, and case controls), (3) published in the English language, (4) defined the criteria of diagnosis of MNM either using WHO or the modified version of sub-Saharan African (SSA) as indicated in Supplementary Material 4 and (5) Both published and unpublished articles and reports were included. Anonymous reports, editorials, case reports, and articles without full access (after contacting the corresponding author two times through e-mail) were excluded from the review.
Outcome measurement
The primary goal of this review was to quantify the prevalence of MNM, calculated by dividing the number of MNM cases by the total number of live births, and then multiplying by 1000. The secondary objective was to determine the underlying medical causes leading to MNM, also computed by dividing the number of MNM cases with specific underlying causes by the total number of live births, and multiplying by 1000. The third outcome sought to analyze the determinants of MNM, encompassing factors ranging from individual-level to health facility or community-level factors.
Validity assessment
The quality of each study was assessed by the Joanna Briggs Institute (JBI) quality appraisal checklist designed for various study methodologies (45). These critical appraisal checklists were adapted for the prevalence, cross-sectional, case-control, and cohort studies. After selecting the appropriate checklists, two authors independently reviewed and evaluated the quality of each study using these tools. Disagreements between authors that arose while reviewing the quality were solved based on evidence-based discussions. With all quality assessment items of each study design, studies scores of 5 or more JBI critical appraise were considered as suitable for further analysis (46).
Data extraction and study selection
After retrieving all studies from the databases, they were imported into the reference manager, EndNote version 16 software, to remove duplicated studies. Afterwards, all eligible studies were examined by the authors based on the title and abstract for possible inclusion. The entire full-text articles were then reviewed to decide on articles to be included. All needed qualitative and quantitative data from the selected studies were extracted independently by the two authors using a predetermined standardized data extraction format. The data abstraction format included primary author, number of liver birth, year of publication, region of study, sample size, study design, study setting, study outcome, diagnosis criteria, and indicators to calculate the prevalence of MNM (MNM cases and the total number of live birth).
Statistical analysis
Data were extracted in Excel and exported to R software version 4.4.1 (R Project for Statistical Computing). for further analysis. The metaprop function from the R package meta was used to calculate the pooled effect estimates (47). Meta-analysis was performed with the DerSimonian-Laird random-effects model with Hartung-Knapp-Sidik-Jonkman variance correction (48). Individual and pooled estimates were graphically displayed using forest plots. A random-effects model was used to accommodate variation that stemming from study design, sample size, place of study and diagnosis criteria. Between studies heterogeneity was assessed using I2 test statistic values, expressed as % (low (25%), moderate (50%), and high (75%) and Cochrane’s Q statistic (significance level < 0.05) (49). To further identify the source of heterogeneity, multivariate meta-regression analysis was conducted. Moreover, a leave-one-out sensitivity analysis was done to point out the influence of a single study on the overall estimate. Finally, Egger’s test was used to assess publication bias objectively and publication bias was declared at a p-value of less than 0.05 (50).
Result
Study selection
A total of 542 articles were retrieved through electronic database searching, out of which 122 underwent full-text screening. Of the included studies, 43 articles met the inclusion criteria to be included in the final review (Figure 1).

Figure 1. Flow diagram showing the procedures of selecting studies for meta-analysis of the prevalence incidence underlying causes, and determinants of maternal near miss in Ethiopia, 2022 Characteristics of included studies.
Twenty-four cross-sectional, 14 case-control, and 5 cohort studies were included in the review. Regarding the geographical coverage of the review, two studies were conducted at a national level (defined as studies that included more than two regions of the country) (51, 52) and the remaining studies were conducted exclusively in ten regions and city administrations [Addis Ababa = 3 = [(53, 54)], Amhara = 8 (55–62), Central Ethiopia = 5 (63–67), Gambella = 1 (68), Harari = 4 (69–72), Oromia = 12 (73–84), Sidama = 2 (85, 86), South Ethiopia = 2 (87, 88), Southwest Ethiopia Peoples’ Region (SWEPR) = 1 (89), Tigray = 4 (90–93)]. A total of 77,240 cases of MNM were identified with a minimum of 31 cases from Tigray (91) and a maximum of 67,576 cases from a national study (52). Only five studies utilized SSA criteria for the identification of MNM cases while the remaining studies used the WHO criteria. The detail is annexed in Supplementary Material 5.
Pooled prevalence of maternal near miss
The pooled period prevalence of maternal near miss per 1000 live births was 54.33 (95% CI: 33.93 to 85.89). Between-study heterogeneity was observed (I2 = 100%, P < 0.0001, Figure 2). To assess regional differences in the pooled rates of MNMs, a subgroup meta-analysis was conducted stratified by region (Figure 3). The region of Amhara demonstrated the highest prevalence of MNMs at 384.54 (95% CI: 354.97 to 354.97, I2 = NA), while the lowest rate was observed in Addis Ababa at 8.01 (95% CI: 7.03 to 9.09, I2 = NA).
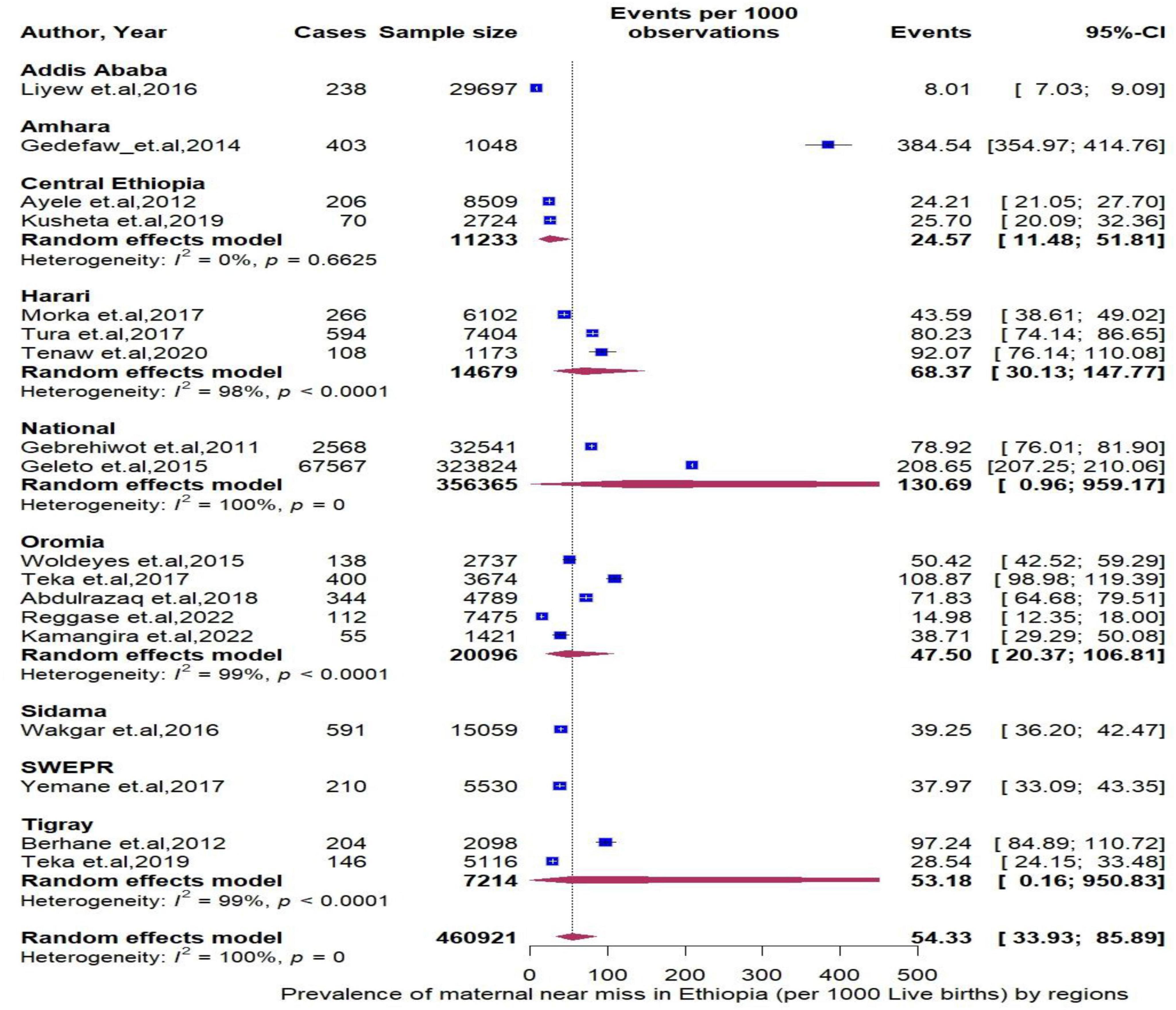
Figure 3. Forest plot showing the pooled prevalence of maternal near miss in Ethiopia stratified by region.
Additionally, MNM cases diagnosed through SSA criteria had a prevalence of 58.11 (95% CI: 13.75 to 214.51, I2 = 98%, Figure 4A), those captured by a cohort study design had a prevalence of 66.13 (95% CI: 0.47 to 914.41, I2 = 98%, P < 0.0001, Figure 4B), and those identified during the Millennium Development Goals (MDGs) era had a prevalence of 98.48 (95% CI: 17.20 to 405.39, I2 = 100%, P < 0.0001, Figure 4C), which indicate a higher prevalence of MNMs in these categories compared to their counterpart groups.

Figure 4. (A) The pooled prevalence of maternal near miss stratified by diagnostic criteria. (B) The pooled prevalence of maternal near miss stratified by study design. (C) The pooled prevalence of maternal near miss stratified by diagnostic year of study.
Underlying causes of maternal near miss
Next, we assess specific MNMs underline causes —obstetrics hemorrhage, hypertensive disorder of pregnancy, and pregnancy related infection. Among the MNMs, obstetrics hemorrhage rate was the highest, 14.56 per 1000 (95% CI: 9.16 to 23.06, I2 = 99%, P < 0.0001, Figure 5A) followed by hypertensive disorder of pregnancy; 12.96 (95% CI: 8.30 to 19.31, I2 = 98%, P < 0.0001, Figure 5B) and pregnancy related infection were reported less frequently, 3.55 per 1000 (95% CI: 1.76 to 7.18, I2 = 97%, P < 0.0001, Figure 5C). Using year of study, and starting from 2011, the rate of MNMs deceased by 0.9 % per year (P = 0.12) (Figure 6).
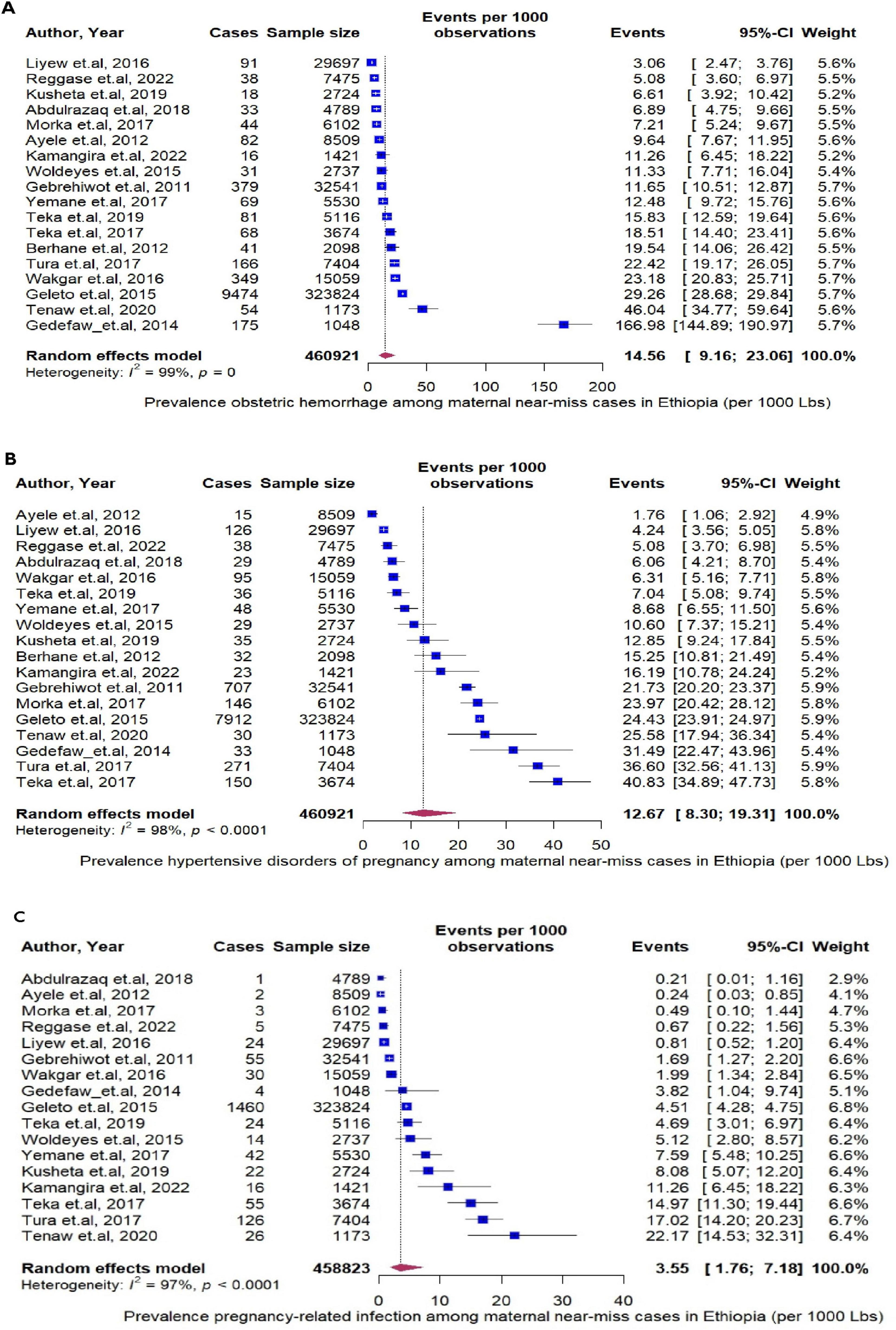
Figure 5. (A) The pooled prevalence of obstetrics hemorrhage per 1000 live births. (B) The pooled prevalence of by hypertensive disorder of pregnancy per 1000 live births. (C) The pooled prevalence of pregnancy related infection per 1000 live births.
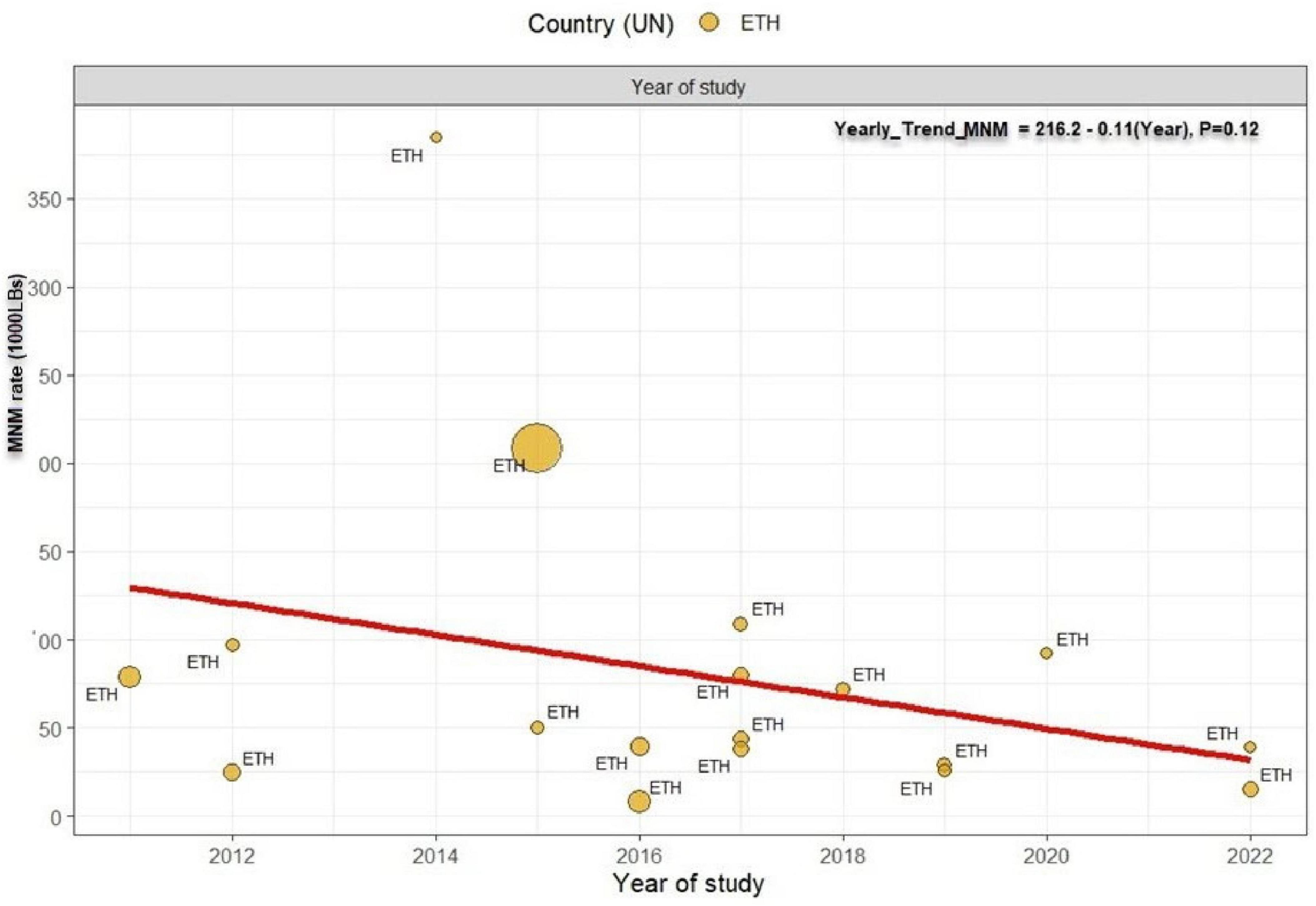
Figure 6. Temporal trend in the prevalence of maternal near miss in Ethiopia. The prevalence of MNM decreased at a rate of 0.9 per year from 2011 to 2022 with p-value 0.12. Linear fit from linear regression model. The size of the circle is proportional to the sample size of each study.
Sensitivity analysis and publication bias
To assess the potential for outlier and influential studies affecting the robustness of the pooled estimates, influence sensitivity analyses was conducted for the prevalence of MNMs (Supplementary Material 6), obstetrics hemorrhage (Supplementary Material 7), hypertensive disorder of pregnancy (Supplementary Material 8) and pregnancy related infection (Supplementary Material 9) separately (94). Trim and fill analysis was conducted to adjust for the potential publication bias. Analyses suggest that the adjusted effect estimates would fall in the range of 83 to 298 per 1000 live births, and 8 additional studies were added to the funnel plots (Supplementary Materials 10, 11). To further identify source of heterogeneity, multivariate meta-regression analysis was conducted. The only significant factor that remained was the study area. Geographically, when compared with a study from Addis Ababa, a high prevalence of maternal near miss was observed from studies conducted in Amhara region [β = 4.26, 95% CI (1.46–7.12)] and at national level [β = 2.88, 95% CI (0.70–5.06)] (Supplementary Material 12).
Determinants of maternal near miss
Various individual factors (i.e. socio demographic charactertics, previous medical and obstetrics history) were identified as determinants of MNM. These factors include lack of ANC follow-up [AOR = 2.76, 95% CI (1.75–5.06), I2 = 92.6%, P < 0.0001], rural residency [AOR = 1.96, 95% CI (1.39–2.78), I2 = 92.4%, P < 0.0001], lack of education [AOR = 2.39, 95% CI (1.69–3.39), I2 = 82.3%, P < 0.0001], being grand multipara women [AOR = 2.41, 95% CI (1.82–3.20), I2 = 66.9%, P < 0.0001], having a monthly income ≤ 60 USD [AOR = 1.81, 95% CI (1.07–3.04), I2 = 89.4%, P = 0.026], preexisting medical conditions [AOR = 6.17, 95% CI (3.77–10.11), I2 = 85.3%, P < 0.0001], advanced maternal age (above 35) [AOR = 1.90, 95% CI (1.44–2.52), I2 = 79.4%, P < 0.0001], previous history of C-section [AOR = 3.68, 95% CI (1.95–6.95), I2 = 84.8%, P < 0.0001], history of female genital mutilation (FGM) [AOR = 1.40, 95% CI (1.02–1.96), I2 = 78.5%, P = 0.032], and decide to seek care [AOR = 1.80, 95% CI (1.03–3.13), I2 = 90.9%, P = 0.039].
On the other hand, a delay in arrival at a health facility [AOR = 2.57, 95% CI (1.48–4.66), I2 = 94.8%, P < 0.0001] and obtaining inadequate care [AOR = 1.66, 95% CI (1.18–2.32), I2 = 48.4%, P = 0.003] were facility-level factors that increased the risk of developing MNM (Figure 7).
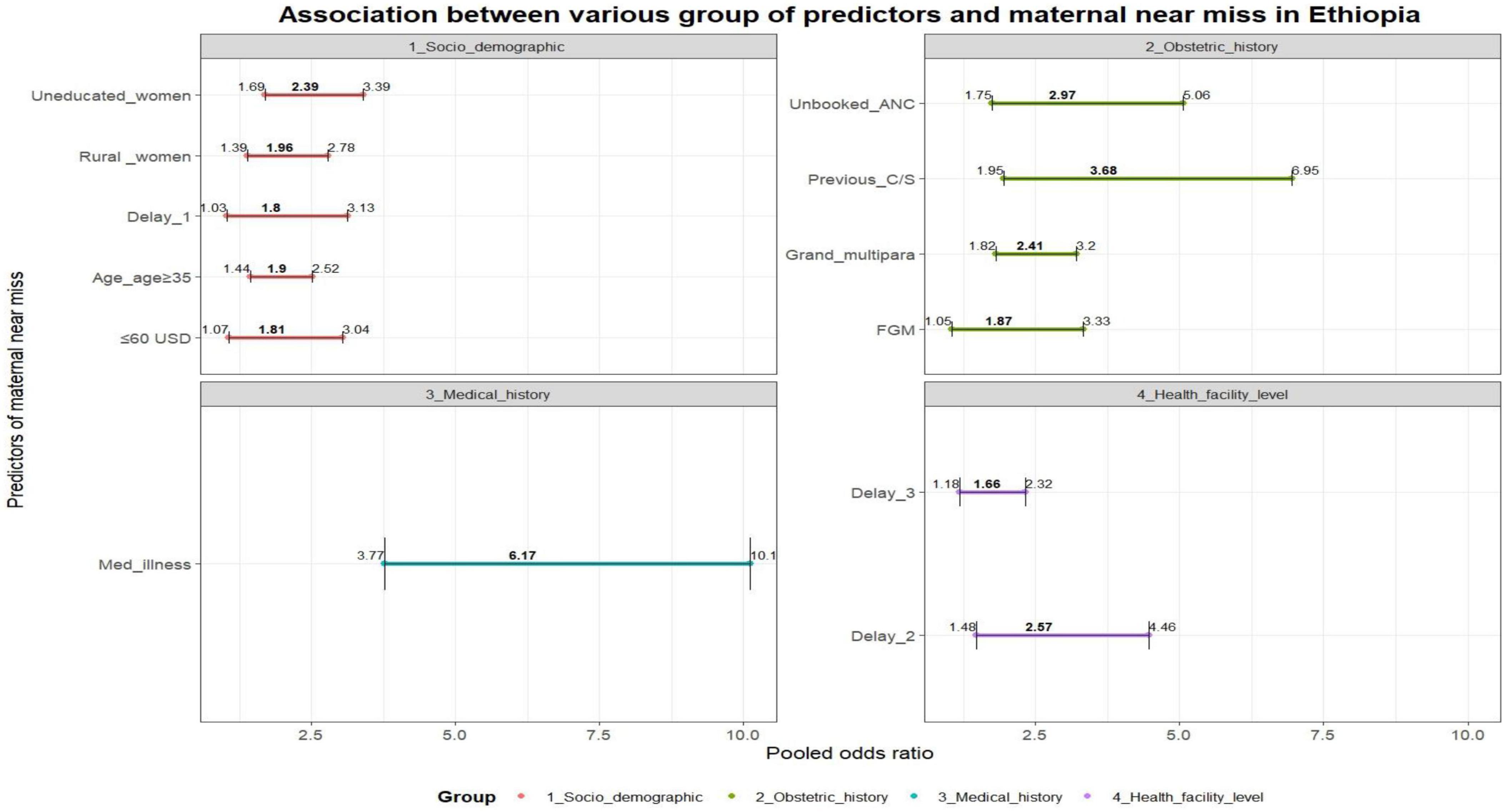
Figure 7. – Association between various group of predictors and maternal near miss in Ethiopia: Delay 1 (delay to decide to seek care), previous C/S (previous cesarian section), FGM (having female genital mutilation), med.illesss (previous chronic medical illness), Delay 2 (delay to reach care), delay 3 (delay to receive care).
Discussion
This review provides deep insight into the incidence of MNM and its underlying causes and determinants. The review also confirmed that the major causes of maternal death had a significant role in MNM. Furthermore, both individual factors (maternal age, maternal parity, educational status, monthly income, preexisting medical illness, delay in deciding to seek care, previous history of C/S, and ANC follow-up) and facility-level factors (delay in arriving at a health facility and delay in receiving care) played a decisive role in having MNM.
The pooled national estimate of MNM in Ethiopia was 54.33 cases per 1000 live births, which was higher than that reported in studies in Egypt (95), Tanzania (96), India (97), and Iraq (98) but lower than that reported in Pakistan (99). Variations in incidence may be due to differences in diagnostic criteria, study settings, and health status. The lack of globally agreed-upon diagnostic criteria complicates cross-country comparisons (100). Laboratory and management-based criteria vary, making comparisons difficult, particularly in resource-constrained countries (101). Significantly, MNM cases have declined during the Sustainable Development Goals (SDG) era compared to the Millennium Development Goals (MDG), reflecting progress made under the national health transformation plan. This underscores the necessity for comprehensive monitoring strategies, including workforce training, health facility upgrades, and heightened community engagement (25). Additionally, the review revealed noticeable regional discrepancies, with lower rates observed in Addis Ababa compared to higher rates in the Amhara region. These variations can be attributed to differences in the quality of care and accessibility, with the capital city often benefiting from superior infrastructure and skilled personnel (26). Therefore, concerted efforts should focus on reducing regional disparities by enhancing the quality of care and improving accessibility to healthcare services. Considering the establishment and integration of MNM surveillance into the existing MPDSR through setting suitable diagnosis criteria in consideration of the local context could facilitate and pave the way to gain a comprehensive picture of maternal severe morbidity from a live witness.
Obstetric hemorrhage, hypertensive disorders of pregnancy (HDP), and pregnancy-related infections are significant causes of MNM. Obstetric hemorrhage has been identified as the leading cause in various countries (102–106). In Ethiopia, factors such as advanced maternal age, lack of antenatal care follow-up, grand multiparity, and prolonged labor increase the risk of postpartum hemorrhage (107, 108). A shortage of blood and blood products in Ethiopia poses challenges in managing cases according to treatment protocols (109, 110), leading to the establishment of mini blood banks at Comprehensive Emergency Obstetric Care (CEmOC) facilities (111). However, the full implementation of these blood banks faces issues related to availability, safety, and quality (112, 113). In summary, targeting high-risk groups and improving access to blood products could help prevent complications from obstetric hemorrhage.
Hypertensive disorders of pregnancy (HDP) have emerged as a significant underlying cause of MNM, second to obstetric hemorrhage. This finding aligns with studies conducted in various countries, including Chad (114), and Brazil (115). In Ethiopia, individual-level factors such as advanced maternal age (> 35), family history of chronic illnesses (hypertension and diabetes mellitus), previous history of HDP, body mass index above 25, and lack of nutritional counseling were identified as risk factors for HDP (116–118). Additionally, health system readiness plays a role in determining outcomes (119), as the lack of essential supplies, medications, weak referral systems, and untrained health professionals hinder the control of HDPs (120). Overall, enhancing prenatal examinations, providing health education, strengthening referral systems, and improving the quality of care are essential measures to mitigate adverse outcomes associated with HDPs.
Pregnancy-related infections are cause of MNM in Ethiopia, which has also been revealed elsewhere (121, 122), with modifiable risk factors, including C/S delivery, premature rupture of membranes, anemia, multiple vaginal examinations, referral from other facilities, and home delivery (123–125). Incomplete monitoring and delayed antibiotic initiation exacerbated outcomes (126). Efforts to increase institutional delivery and health service uptake through local structures and a health extension program have led to a significant rise in institutional deliveries in the past two decades (127). Generally, best practices such as administering prophylactic antibiotics, closely monitoring vital signs, and timely care completion are crucial for managing pregnancy-related infections (128).
The risk of MNM in Ethiopia is increased by unattended ANC follow-up, aligning with studies in Nigeria (129), Sudan (130), and Brazil (131). This is attributed to the limited health information available to unbooked ANC women, hindering access to prenatal examinations for identifying maternal and fetal risk factors (132). Factors influencing ANC uptake in Ethiopia include limited media access, spouses’ education, proximity to health facilities, and region of residence (133–135). Additionally, challenges such as inadequate laboratory tests, long wait times, lack of confidentiality, insufficient counseling, and untrained professionals impede quality care (136–139). Efforts are underway to improve ANC uptake and quality in Ethiopia through initiatives such as behavioral change communication campaigns, creating a woman-friendly environment, and enhancing ANC provider capacity (140, 141). Thus, these efforts should be enhanced to improve the uptake of the service.
Residence, educational status, and monthly income were predictors of MNM in the present study. Women in rural areas with low education and income were more likely to experience MNM than were urban, educated, higher-income women. This aligns with studies in conducted elsewhere (142–146). Education, urban living, and higher income enhance women’s capacity to access and understand health information, leading to better decision-making in maternity care. In response to the socioeconomic context, Ethiopia has implemented a primary healthcare program involving community health workers and established health posts for basic services and education at the grassroot level (147). Therefore, strengthening the primary healthcare system is crucial for increasing access for disadvantaged women.
Older women and grand multiparas have a heightened risk of MNM, as supported by studies from South Africa (148) and Scotland (149). This increased risk is attributed to factors such as postpartum hemorrhage, gestational diabetes, and gestational hypertension due to reduced insulin sensitivity, vascular endothelial dysfunction, and decreased oxytocin receptors (150–152). The risks extend to the fetus, leading to perinatal mortality and preterm birth (153, 154). In Ethiopia, elderly women show low engagement in the continuum of care, with lower acceptance rates of contraceptives, institutional delivery, and ANC follow-up (127). Factors such as low education levels, early marriage, and polygamous marriage contribute to grand multiparity (155). Hence, mothers of advanced age and grand multiparas should be recognized as high-risk groups requiring comprehensive care throughout the continuum of care.
Women with preexisting chronic medical conditions and a history of C/S face a greater risk of MNM than do those without these factors, consistent with the findings of other studies (156–158). Conditions such as diabetes, cardiovascular disease, and previous C/S can complicate pregnancy by impacting hemostasis and increasing the likelihood of gestational diabetes, HDP, uterine rupture, and hemorrhage. In Ethiopia, cephalopelvic disproportion and non-reassuring fetal heart rate patterns are leading indications for C/S (159); however, unnecessary C/S procedures are being performed at private facilities beyond WHO recommendations (160). It is crucial to consider women with these risk factors as a high-risk group and provide specialized care throughout the pregnancy continuum. Governments should also ensure adherence to global C/S guidelines.
This study revealed that a history of FGM increased the risk of MNM, consistent with research in Tanzania and Gambia (161, 162). Long-term complications of FGM, such as scarring, which reduces birth canal elasticity, increase the likelihood of prolonged labor, obstetric issues, and difficult delivery (163, 164). In Ethiopia, FGM remains prevalent in regions such as Somalia and Afar (165, 166), where access to healthcare is limited. This practice is driven by beliefs about reducing sexual activity for marriage prospects (167, 168). Ethiopia has passed laws against FGM due to its health implications (169). In general, special attention during pregnancy can help prevent complications from FGM, and community awareness campaigns are essential.
The study demonstrated that delays in deciding to seek care were positively associated with MNM. This finding aligns with similar results from studies conducted in elsewhere (170). A possible explanation is that women faced severe complications that could have been easily treated early on but were hindered by limited awareness of pregnancy danger signs, decision-making power, and reliance on traditional birth attendants (171, 172). In Ethiopia, factors such as low education levels, residency in pastoralist regions, and lack of nearby health facilities contribute to delays in seeking care (173, 174). In conclusion, the results suggest the need to enhance community awareness of institutional delivery through health extension workers, with a particular focus on engaging male partners to facilitate timely decision-making.
In this study, delays in reaching care and receiving optimal care were found to be positively associated with MNM. Similar findings were reported in studies in similar settings (175, 176). The delay in reaching care is primarily attributed to ineffective referral communication, often compromised by a lack of essential ambulance equipment, infrastructure, governing documents, and trained manpower (177–179). In Ethiopia, the unavailability of skilled health providers, obstetric drugs, and a lack of respectful care at health facilities are the main factors contributing to delay three (180, 181). These findings suggest the need for improvement in the referral system and quality of care to enhance maternal health outcomes.
The main strengths of this review include the consideration of appropriate denominators for estimating prevalence through robust statistical techniques and an extensive literature search from various sources. While the review provides comprehensive insights on maternal near miss (MNM), there are limitations that should be acknowledged before interpreting the results. These limitations include: 1) all studies included were facility-based without any community-based studies, impacting the representativeness of the study; 2) not all regions of the country were covered, affecting the inclusiveness of the study; 3) most included studies were cross-sectional, potentially leading to the influence of confounding variables on the outcome variable; and 4) the presence of heterogeneity in the data obtained from the included studies could be considered another limitation of this study.
Conclusion
The overall prevalence of MNM in Ethiopia is very high, with obstetric hemorrhage, HDP, and pregnancy-related infections being the leading underlying causes. Sociodemographic, obstetric, and medical history, and facility-level factors all play a role in MNM development. Efforts should focus on improving community health-seeking behavior through ANC follow-up and other communication channels to reduce the burden of MNM. High-risk groups, including older women, grand multipara women, and those with a history of C/S and FGM, require special attention from preconception to postpartum care. Further improvements in the quality of care, including the referral system, provision of optimal care, training professionals, and ensuring the availability of essential drugs and supplies, are essential. Lastly, the establishment and integration of MNM surveillance into the existing MPDSR could have a positive impact on facilitating decision-making by providing robust information.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NT: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft, Writing – review and editing. GH: Conceptualization, Writing – original draft. DB: Conceptualization, Writing – original draft. MH: Conceptualization, Supervision, Writing – original draft. FT: Writing – original draft, Writing – review and editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. FW: Writing – original draft, Writing – review and editing. RJ: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge the authors of the included articles.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1393118/full#supplementary-material
Footnotes
References
1. World Health Organization. Evaluating the quality of care for severe pregnancy complications: the WHO near-miss approach for maternal health. Geneva: World Health Organization (2011).
2. Lewis G. Beyond the numbers: Reviewing maternal deaths and disabilities to make pregnancy safer. Matern Infant Deaths. (2010) 49:586. doi: 10.1016/j.bpobgyn.2007.10.007
3. Chhabra P. Maternal near miss: An indicator for maternal health and maternal care. Indian J Commun. (2014) 39:132.
4. Geller S, Koch A, Garland C, MacDonald E, Storey F, Lawton B. A global view of severe maternal morbidity: Moving beyond maternal mortality. Reprod Health. (2018) 15:31–43. doi: 10.1186/s12978-018-0527-2
5. Heemelaar S, Kabongo L, Ithindi T, Luboya C, Munetsi F, Bauer A, et al. Measuring maternal near-miss in a middle-income country: Assessing the use of WHO and sub-Saharan Africa maternal near-miss criteria in Namibia. Glob Health Act. (2019) 12:1646036. doi: 10.1080/16549716.2019.1646036
6. Tura A, Scherjon S, Stekelenburg J, Van Roosmalen J, Van Den Akker T, Zwart J. Severe hypertensive disorders of pregnancy in eastern Ethiopia: Comparing the original WHO and adapted sub-Saharan African maternal near-miss criteria. Int J Womens Health. (2020) 8:255–63. doi: 10.2147/IJWH.S240355
7. Verschueren K, Kodan L, Paidin R, Samijadi S, Paidin R, Rijken M, et al. Applicability of the WHO maternal near-miss tool: A nationwide surveillance study in Suriname. J Glob Health. (2020) 10:020429. doi: 10.7189/jogh.10.020429
8. Somer S, Sinkey R, Bryant A. Epidemiology of racial/ethnic disparities in severe maternal morbidity and mortality. Semin Perinatol. (2017) 41:258–65.
9. Tura A, Stekelenburg J, Scherjon S, Zwart J, van den Akker T, van Roosmalen J, et al. Adaptation of the WHO maternal near miss tool for use in sub–Saharan Africa: An international delphi study. BMC Pregnancy Childbirth. (2017) 17:445. doi: 10.1186/s12884-017-1640-x
10. Tunçalp Ö, Hindin MJ, Adu-Bonsaffoh K, Adanu R. Listening to women’s voices: The quality of care of women experiencing severe maternal morbidity, in Accra, Ghana. PLoS One. (2012) 7:e44536. doi: 10.1371/journal.pone.0044536
11. Mengistu T, Turner J, Flatley C, Fox J, Kumar S. The impact of severe maternal morbidity on perinatal outcomes in high income countries: Systematic review and meta-analysis. J Clin Med. (2020) 9:2035.
12. Juma K, Amo-Adjei J, Riley T, Muga W, Mutua M, Owolabi O, et al. Cost of maternal near miss and potentially life-threatening conditions, Kenya. Bull World Health Organ. (2021) 99:855. doi: 10.2471/BLT.20.283861
13. World Health Organization. Trends in maternal mortality 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World bank group and the United Nations population division. Geneva: World Health Organization (2017).
14. Central Statistical Agency [CSA], Ethiopia and ICF. Ethiopia demographic and health survey 2016. Rockville, MD: CSA and ICF (2016).
15. Ayele A, Tefera Y, East L. Ethiopia’s commitment towards achieving sustainable development goal on reduction of maternal mortality: There is a long way to go. Womens Health. (2021) 17:17455065211067073. doi: 10.1177/17455065211067073
16. Berhan Y, Berhan A. Commentary: Reasons for persistently high maternal and perinatal mortalities in Ethiopia: Part III–perspective of the “three delays” model. Ethiop J Health Sci. (2014) 24:137–48.
17. Alemayehu M, Yakob B, Khuzwayo N. Barriers and enablers to emergency obstetric and newborn care services use in Wolaita Zone, Southern Ethiopia: A qualitative case study. BMC Public Health. (2022) 22:1–3. doi: 10.1186/s12889-022-14504-y
18. Berhane B, Gebrehiwot H, Weldemariam S, Fisseha B, Kahsay S, Gebremariam A. Quality of basic emergency obstetric and newborn care (BEmONC) services from patients’ perspective in Adigrat town, Eastern zone of Tigray, Ethiopia. 2017: A cross sectional study. BMC Pregnancy Childbirth. (2019) 19:190. doi: 10.1186/s12884-019-2307-6
19. Melberg A, Teklemariam L, Moland K, Aasen H, Sisay M. Juridification of maternal deaths in Ethiopia: A study of the maternal and perinatal death surveillance and response (MPDSR) system. Health Policy Plann. (2020) 35:900–5. doi: 10.1093/heapol/czaa043
20. Rono J, Kamau L, Mangwana J, Waruguru J, Aluoch P, Njoroge MA. policy analysis of policies and strategic plans on maternal, newborn and child health in Ethiopia. Int J Equity Health. (2022) 21:73.
21. Tesfay N, Tariku R, Zenebe A, Habtetsion M, Woldeyohannes F. Place of death and associated factors among reviewed maternal deaths in Ethiopia: A generalised structural equation modelling. BMJ Open. (2023) 13:e060933. doi: 10.1136/bmjopen-2022-060933
22. Tesfay N, Tariku R, Zenebe A, Dejene Z, Woldeyohannes F. Cause and risk factors of early neonatal death in Ethiopia. PLoS One. (2022) 17:e0275475. doi: 10.1371/journal.pone.0275475
23. Cetin K, Worku D, Demtse A, Melberg A, Miljeteig I. “Death audit is a fight”–provider perspectives on the ethics of the maternal and perinatal death surveillance and response (MPDSR) system in Ethiopia. BMC Health Serv Res. (2022) 22:1214. doi: 10.1186/s12913-022-08568-0
24. Ayele B, Gebretnsae H, Hadgu T, Negash D, Gsilassie F, Alemu T, et al. Maternal and perinatal death surveillance and response in Ethiopia: Achievements, challenges and prospects. PLoS One. (2019) 14:e0223540. doi: 10.1371/journal.pone.0223540
25. Ethiopian Ministry of Health. Annual performance report 2013EFY (2020/2021). Addis Ababa: Ethiopian Ministry of Health (2022).
26. Keyes E, Haile-Mariam A, Belayneh NT, Gobezie WA, Pearson L, Abdullah M, et al. Ethiopia’s assessment of emergency obstetric and newborn care: Setting the gold standard for national facility-based assessments. Int J Gynecol Obstet. (2011) 115:94–100. doi: 10.1016/j.ijgo.2011.07.009
27. Mengist B, Desta M, Tura A, Habtewold T, Abajobir A. Maternal near miss in Ethiopia: Protective role of antenatal care and disparity in socioeconomic inequities: A systematic review and meta-analysis. Int J Africa Nurs Sci. (2021) 15:100332.
28. Yeshitila Y, Daniel B, Desta M, Kassa G. Obstructed labor and its effect on adverse maternal and fetal outcomes in Ethiopia: A systematic review and meta-analysis. PLoS One. (2022) 17:e0275400. doi: 10.1371/journal.pone.0275400
29. Carmichael S, Abrams B, El Ayadi A, Lee H, Liu C, Lyell D, et al. Ways forward in preventing severe maternal morbidity and maternal health inequities: Conceptual frameworks, definitions, and data, from a population health perspective. Womens Health Issues. (2022) 32:213–8. doi: 10.1016/j.whi.2021.11.006
30. Drechsel K, Adu-Bonsaffoh K, Loohuis K, Srofenyoh E, Boateng D, Browne J. Maternal near-miss and mortality associated with hypertensive disorders of pregnancy remote from term: A multicenter observational study in Ghana. AJOG Glob Rep. (2022) 2:100045. doi: 10.1016/j.xagr.2021.100045
31. Sayinzoga F, Bijlmakers L, van der Velden K, van Dillen J. Severe maternal outcomes and quality of care at district hospitals in Rwanda–a multicentre prospective case-control study. BMC Pregnancy Childbirth. (2017) 17:394. doi: 10.1186/s12884-017-1581-4
32. Vogel J, Fawole B, Adeniran A, Adegbola O, Oladapo O. The burden of severe maternal outcomes and indicators of quality of maternal care in Nigerian hospitals: A secondary analysis comparing two large facility-based surveys. BJOG. (2019) 126:49–57. doi: 10.1111/1471-0528.15698
33. Omona K, Babirye D. Maternal near misses (MNM) and their determinants among women who sought obstetric care from fort portal regional referral hospital, Western Uganda. Cogent Public Health. (2023) 10:2157996.
34. Mazhar S, Batool A, Emanuel A, Khan A, Bhutta S. Severe maternal outcomes and their predictors among Pakistani women in the WHO Multicountry survey on maternal and newborn health. Int J Gynecol Obstet. (2015) 129:30–3. doi: 10.1016/j.ijgo.2014.10.017
35. Assarag B, Dujardin B, Delamou A, Meski F, De Brouwere V. Determinants of maternal near-miss in Morocco: Too late, too far, too sloppy? PLoS One. (2015) 10:e0116675. doi: 10.1371/journal.pone.0116675
36. Dahie H. Determinants of maternal near miss events among women admitted to tertiary hospitals in Mogadishu, Somalia: A facility-based case–control study. BMC Pregnancy Childbirth. (2022) 22:658. doi: 10.1186/s12884-022-04987-3
37. Kiruja J, Osman F, Egal J, Essén B, Klingberg-Allvin M, Erlandsson K. Maternal near-miss and death incidences–frequencies, causes and the referral chain in Somaliland: A pilot study using the WHO near-miss approach. Sex Reprod Healthc. (2017) 12:30–6. doi: 10.1016/j.srhc.2017.02.003
38. Iwuh I, Fawcus S, Schoeman L. Maternal near-miss audit in the Metro West maternity service, Cape Town, South Africa: A retrospective observational study. South Afr Med J. (2018) 108:171–5. doi: 10.7196/SAMJ.2018.v108i3.12876
39. Haddad S, Cecatti J, Souza J, Sousa M, Parpinelli M, Costa M, et al. Applying the maternal near miss approach for the evaluation of quality of obstetric care: A worked example from a Multicenter Surveillance Study. BioMed Res Int. (2014) 4:2014. doi: 10.1155/2014/989815
40. Pacheco A, Katz L, Souza A, de Amorim M. Factors associated with severe maternal morbidity and near miss in the São Francisco Valley, Brazil: A retrospective, cohort study. BMC Pregnancy Childbirth. (2014) 14:1–8. doi: 10.1186/1471-2393-14-91
41. Negash A, Sertsu A, Mengistu D, Tamire A, Birhanu Weldesenbet A, Dechasa M, et al. Prevalence and determinants of maternal near miss in Ethiopia: A systematic review and meta-analysis, 2015–2023. BMC Womens Health. (2023) 23:380. doi: 10.1186/s12905-023-02523-9
42. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906.
43. Morgan R, Whaley P, Thayer K, Schünemann H. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. (2018) 121:1027. doi: 10.1016/j.envint.2018.07.015
44. Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. (2018) 18:1–9. doi: 10.1186/s12874-017-0468-4
45. Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. JBI Evid Implement. (2015) 13:163–9. doi: 10.1097/XEB.0000000000000064
46. Whittaker A, George R, O’Malley L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci Rep. (2022) 12:2135. doi: 10.1038/s41598-022-05682-1
48. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. (2007) 28:105–14.
49. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60.
51. Gebrehiwot Y, Tewolde B. Improving maternity care in Ethiopia through facility based review of maternal deaths and near misses. Int J Gynecol Obstet. (2014) 127:S29–34.
52. Geleto A, Chojenta C, Taddele T, Loxton D. Incidence of maternal near miss among women in labour admitted to hospitals in Ethiopia. Midwifery. (2020) 82:102597.
53. Liyew E, Yalew A, Afework M, Essen B. Incidence and causes of maternal near-miss in selected hospitals of Addis Ababa, Ethiopia. PLoS One. (2017) 12:e0179013. doi: 10.1371/journal.pone.0179013
54. Liyew E, Yalew A, Afework M, Essén B. Distant and proximate factors associated with maternal near-miss: A nested case-control study in selected public hospitals of Addis Ababa, Ethiopia. BMC Womens Health. (2018) 18:28. doi: 10.1186/s12905-018-0519-y
55. Erega B, Ferede WY. A cohort study of maternal near-miss events and its adverse perinatal outcomes: An obstetrical finding in Northwest Ethiopia. AJOG Glob Rep. (2024) 4:100311. doi: 10.1016/j.xagr.2024.100311
56. Gedefaw M, Gebrehana H, Gizachew A, Taddess F. Assessment of maternal near miss at Debre Markos referral hospital, Northwest Ethiopia: Five years experience. Open J Epidemiol. (2014) 4:199.
57. Rozina K. Maternal Near Miss and Associated Factors Among Mothers Who Gave Birth in Bahir Dar Public Hospitals, Bahir Dar, Northwest, Ethiopia. MPH [thesis]. Bahir Dar: Bahir Dar University (2022). Available online at: http://ir.bdu.edu.et/handle/123456789/14997
58. Fenta S, Nigussie A, Bante A, Asres E, Goedert M. Obstetric near miss in northwest ethiopia, has a pregnant woman still ‘one foot in the grave’? Clin Mother Child Health. (2020) 17:353. doi: 10.35248/2090-7214.20.17.353
59. Dile M, Abate T, Seyum T. Proportion of maternal near misses and associated factors in the referral hospitals of Amhara National Regional State, North-West Ethiopia: Institution-based cross-sectional study. Midirs Midwifery Digest. (2015) 25:517–25.
60. Asaye M. Proportion of maternal near-miss and its determinants among northwest Ethiopian women: A cross-sectional study. Int J Reprod Med. (2020) 2020:5257431. doi: 10.1155/2020/5257431
61. Gebremariam T, Demie T, Derseh B, Mruts K. Trends of and factors associated with maternal near-miss in selected hospitals in North Shewa Zone, central Ethiopia. J Pregnancy. (2022) 2022:2023652. doi: 10.1155/2022/2023652
62. Teshome H, Ayele E, Hailemeskel S, Yimer O, Mulu G, Tadese M. Determinants of maternal near-miss among women admitted to public hospitals in North Shewa Zone, Ethiopia: A case-control study. Front Public Health. (2022) 10:996885. doi: 10.3389/fpubh.2022.996885
63. Habte A, Wondimu M. Determinants of maternal near miss among women admitted to maternity wards of tertiary hospitals in Southern Ethiopia, 2020: A hospital-based case-control study. PLoS One. (2021) 16:e0251826. doi: 10.1371/journal.pone.0251826
64. Kusheta S, Tura G, Tadele A, Yesuf W. Determinants of maternal near-miss among women admitted to public hospitals in the Hadiya zone, central Ethiopia: A case-control study. Healthc Low Resourc Settings. (2024) 10:996885. doi: 10.4081/hls.2024.12474
65. Kusheta S, Tura G, Tadele A. The magnitude of maternal near-miss cases in public hospitals of Hadiya Zone, south-ern Ethiopia. J Clin Images Med Case Rep. (2023) 4:2455.
66. Kasahun A, Wako W. Predictors of maternal near miss among women admitted in Gurage zone hospitals, South Ethiopia, 2017: A case control study. BMC Pregnancy Childbirth. (2018) 18:260. doi: 10.1186/s12884-018-1903-1
67. Ayele B, Amenu D, Gurmessa A. Prevalence of maternal near miss and maternal death in Atat Hospital, Ethiopia. J Womens Health Issues Care. (2014) 3:2.
68. Demmen A, Bekele A, Goba G. Determinants of maternal near miss among women in public hospital maternity wards in Gambella region health facility, Gambella, Southwest of Ethiopia: a facility based unmatched case: control study, 2019. Int J Gynaecol Sci. (2020) 2:1–9. doi: 10.33545/26648393.2020.v2.i1a.8
69. Geze Tenaw S, Girma Fage S, Assefa N, Kenay Tura A. Determinants of maternal near-miss in private hospitals in eastern Ethiopia: A nested case–control study. Womens Health. (2021) 17:17455065211061949. doi: 10.1177/17455065211061949
70. Tenaw S, Assefa N, Mulatu T, Tura A. Maternal near miss among women admitted in major private hospitals in eastern Ethiopia: A retrospective study. BMC Pregnancy Childbirth. (2021) 21:181. doi: 10.1186/s12884-021-03677-w
71. Morka T. Determinants of Maternal Near Miss in Selected Hospitals in Harari Region, Eastern Ethiopia. MPH [thesis]. Haramaya: Haramaya University (2017). Available online at: http://localhost:8080/xmlui/handle/123456789/3311
72. Tura A, Zwart J, Van Roosmalen J, Stekelenburg J, Van Den Akker T, Scherjon S. Severe maternal outcomes in eastern Ethiopia: Application of the adapted maternal near miss tool. PLoS One. (2018) 13:e0207350. doi: 10.1371/journal.pone.0207350
73. Abdulrazaq B, Getahun M, Mohammed A, Kedir S, Nurahmed N, Abrha Y, et al. Determinant factors of maternal near miss in selected health facilities of Berak Woreda, Oromia national regional state, Ethiopia. Int J Sci Rep. (2020) 6:131–8.
74. Abdulrazak B, Alemseged F, Idossa Z, Mizana B. Maternal near-miss and its associated factors in governmental health centers in northern oromia regional state, Ethiopia. J Midwifery Reprod Health. (2021) 9:2859.
75. Kumela L, Tilahun T, Kifle D. Determinants of maternal near miss in Western Ethiopia. Ethiop J Health Sci. (2020) 30:161–8.
76. Aliyi A, Deyessa N, Dilnessie M. Effect of maternal near miss on neonatal mortality in selected hospitals: Prospective cohort study, Southeast Ethiopia. SAGE Open Med. (2021) 9:20503121211042219. doi: 10.1177/20503121211042219
77. Dessalegn F, Astawesegn F, Hankalo N. Factors associated with maternal near miss among women admitted in west Arsi Zone public hospitals, ethiopia: Unmatched case-control study. J Pregnancy. (2020) 2020:6029160. doi: 10.1155/2020/6029160
78. Mekonnen A, Fikadu G, Seyoum K, Ganfure G, Degno S, Lencha B. Factors associated with maternal near-miss at public hospitals of South-East Ethiopia: An institutional-based cross-sectional study. Womens Health. (2021) 17:17455065211060617. doi: 10.1177/17455065211060617
79. Woldeyes W, Asefa D, Muleta G. Incidence and determinants of severe maternal outcome in Jimma University teaching hospital, south-West Ethiopia: A prospective cross-sectional study. BMC Pregnancy Childbirth. (2018) 18:255. doi: 10.1186/s12884-018-1879-x
80. Morka W, Megersa G, Bekele E, Deksisa A. Incidence of adverse perinatal outcomes among women exposed to maternal near-misses in Arsi Zone in Ethiopia: Prospective cohort study in 2022. J Pregnancy. (2024) 2024:6560652. doi: 10.1155/2024/6560652
81. Regassa W, Gemeda G, Wakwoya E, Gelete B. Magnitude of maternal near-misses and associated factors in Arsi Zone public hospitals in Oromia, Ethiopia, 2022. Heliyon. (2024) 10:e24910. doi: 10.1016/j.heliyon.2024.e24910
82. Kamangira B, Ayele G, Dube P, Melaku K, Vushoma E. Maternal near miss hospitalizations in the Borana Zone, Ethiopia: A facility-based longitudinal cross-sectional study. J Public Health Res. (2024) 13:22799036241238665. doi: 10.1177/22799036241238665
83. Danusa K, Debelo B, Wakgari N, Seifu B, Kenasa K, Daba G, et al. Predictors of maternal Near miss in public hospitals of West Shoa Zone, Central Ethiopia: A case-control study. Front Med. (2022) 9:868992. doi: 10.3389/fmed.2022.868992
84. Bekelu T, Muluemebet A, Mahilet B. Quality of Maternal Health Care and Factors Associated With Maternal Death at Jimma University Medical Center, South West Ethiopia Retrospective Study Using Near-miss Approach. MPH [thesis]. Jimma: Jimma University (2018). Available online at: https://repository.ju.edu.et//handle/123456789/8018
85. Wakgar N, Dulla D, Daka D. Maternal near misses and death in southern Ethiopia: A retrospective study. Ethiop J Reprod Health. (2019) 11:9. doi: 10.69614/ejrh.v11i2.267
86. Tolesa D, Abera N, Worku M, Wassihun B. Prevalence and associated factors with maternal near-miss among pregnant women at Hawassa university comprehensive specialized Hospital, Sidama Region, Ethiopia. Int J Women Health Wellness. (2021) 7:127. doi: 10.23937/2474-1353/1510127
87. Kebede TT, Godana W, Utaile MM, Sebsibe YB. Effects of antenatal care service utilization on maternal near miss in Gamo Gofa zone, southern Ethiopia: Retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:209. doi: 10.1186/s12884-021-03683-y
88. Dana W, Dulla D, Astawesegn F, Koyira M. Predictors of maternal near miss among women receiving care at Wolaita Sodo university teaching hospital, southern Ethiopia: Institution based cross sectional study. Divers Equal Health Care. (2021) 18:438–46.
89. Yemane Y, Tiruneh F. Incidence-proportion of maternal near-misses and associated factors in Southwest Ethiopia: A prospective cross-sectional study. Int J Womens Health. (2020) 3:1125–34. doi: 10.2147/IJWH.S283122
90. Mekango D, Alemayehu M, Gebregergs G, Medhanyie A, Goba G. Determinants of maternal near miss among women in public hospital maternity wards in northern Ethiopia: A facility based case-control study. PLoS One. (2017) 12:e0183886. doi: 10.1371/journal.pone.0183886
91. Weldemariam M, Weldegeorges D, Angaw Y, Assefa N, Welay F, Werid W, et al. Magnitude and associated factors of maternal near miss in public hospitals of Tigrai, northern Ethiopia: A cross sectional study. Clin Nurs Res. (2024) 33:138–45. doi: 10.1177/10547738211029680
92. Teka H, Yemane A, Berhe Zelelow Y, Tadesse H, Hagos H. Maternal near-miss and mortality in a teaching hospital in Tigray region, Northern Ethiopia. Womens Health. (2022) 18:17455057221078739. doi: 10.1177/17455057221078739
93. Berhane G, Gessessew A, Van Roosmalen J, Van Den Akker T. Obstetric near-miss and maternal death: The case of Ayder Teaching Hospital, Mekelle, Ethiopia. Ethiop J Reprod Health. (2012) 6:2864.
94. Viechtbauer W, Cheung M. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. (2010) 1:112–25.
95. El-Agwany A. Severe maternal outcomes: World health organization maternal near-miss and maternal mortality criteria in university tertiary hospital Egypt. Apollo Med. (2019) 16:74.
96. Lilungulu A, Bintabara D, Mujungu S, Chiwanga E, Chetto P, Nassoro M. Incidence and predictors of maternal and perinatal mortality among women with severe maternal outcomes: A Tanzanian facility-based survey for improving maternal and newborn care. Obstet Gynecol Int. (2020) 0:2020. doi: 10.1155/2020/5390903
97. Kasliwal A, Kabra I, Yadav P. Maternal near miss: A single centric experience. Int J Clin Obstet Gynaecol. (2021) 5:34–7.
98. Jabir M, Abdul-Salam I, Suheil D, Al-Hilli W, Abul-Hassan S, Al-Zuheiri A, et al. Maternal near miss and quality of maternal health care in Baghdad, Iraq. BMC Pregnancy Childbirth. (2013) 13:11. doi: 10.1186/1471-2393-13-11
99. Zahoor F. Maternal health care in tertiary hospital in terms of maternal near misses (MNM) indicators. J Med Sci. (2021) 29:239–42.
100. Nelissen E, Mduma E, Broerse J, Ersdal H, Evjen-Olsen B, van Roosmalen J, et al. Applicability of the WHO maternal near miss criteria in a low-resource setting. PLoS One. (2013) 8:e61248. doi: 10.1371/journal.pone.0061248
101. van der Cammen O, Chobo S, Kasitu J, Mwampagatwa I, Mooij R, Hulsbergen M. Applicability and comparison of the sub-Saharan Africa and original WHO maternal near-miss criteria in a rural hospital in Western Tanzania. J Glob Health Rep. (2021) 5:e2021055.
102. Osaigbovoh J, Olukemi A, Inyang F, Obafemi J, Olumide A. Obstetric hemorrhage and adverse maternal outcomes: Experience of a private teaching hospital in Southwestern Nigeria. Afr J Health Sci. (2016) 29:105–18.
103. Cecatti J, Souza R, Pacagnella R, Leal M, Moura E, Santos L. Maternal near miss among women using the public health system in the Amazon and Northeast regions of Brazil. Rev Panam Salud Pública. (2015) 37:232–8.
104. Gupta D, Nandi A, Noor N, Joshi T, Bhargava M. Incidence of maternal near miss and mortality cases in central India tertiary care centre and evaluation of various causes. New Indian J OBGYN. (2018) 4:112–6.
105. Xiong L, Zeng M, Wang A, Xie D, Kong F, Liu Z. Analysis of the severe maternal outcomes between resource-poor and resource-rich hospitals in China’s Hunan Province from 2012 to 2018. BioMed Res Int. (2020) 2020:1–0.
106. Tesfay N, Tariku R, Zenebe A, Firde H, Woldeyohannes F. Target areas to reduce the burden of maternal death due to obstetric hemorrhage in Ethiopia. PLoS One. (2022) 17:e0274866. doi: 10.1371/journal.pone.0274866
107. Mesfin S, Dheresa M, Fage S, Tura A. Assessment of postpartum hemorrhage in a university hospital in Eastern Ethiopia: A cross-sectional study. Int J Womens Health. (2021) 6:663–9. doi: 10.2147/IJWH.S300143
108. Nigussie J, Girma B, Molla A, Tamir T, Tilahun R. Magnitude of postpartum hemorrhage and its associated factors in Ethiopia: A systematic review and meta-analysis. Reprod Health. (2022) 19:1–3.
109. Taye Makuria A, Gebremichael D, Demoz H, Hadush A, Abdella Y, Berhane Y, et al. Obstetric hemorrhage and safe blood for transfusion in Ethiopia: The challenges of bridging the gap. Transfusion. (2017) 57:2526–31. doi: 10.1111/trf.14219
110. Tesfay N, Tariku R, Zenebe A, Woldeyohannes F. Critical factors associated with postpartum maternal death in Ethiopia. PLoS One. (2022) 17:e0270495. doi: 10.1371/journal.pone.0270495
111. Germossa G, Wondie T, Gerbaba M, Mohammed E, Alemayehu W, Tekeste A, et al. Availability of comprehensive emergency obstetric and neonatal care in developing regions in Ethiopia: Lessons learned from the USAID transform health activity. BMC Health Serv Res. (2022) 22:1307. doi: 10.1186/s12913-022-08712-w
112. WHO. WHO African Region status report on blood availability, safety and quality. Brazzaville: WHO Regional Office for Africa (2022).
113. Getie A, Wondmieneh A, Bimerew M, Gedefaw G, Demis A. Blood donation practice and associated factors in Ethiopia: A systematic review and meta-analysis. BioMed Res Int. (2020) 6:2020. doi: 10.1155/2020/8852342
114. Madouea G, Abdelsalama S, Aliou D, Offi A, Emile S, Pallai E, et al. Maternal near-miss in N’Djamena Mother and Child Hospital, Chad. South Sudan Med J. (2017) 10:28–31.
115. Cecatti J, Souza J, Parpinelli M, Haddad S, Camargo R, Pacagnella R, et al. Brazilian network for the surveillance of maternal potentially life-threatening morbidity and maternal near-miss and a multidimensional evaluation of their long-term consequences. Reprod Health. (2009) 6:1–0. doi: 10.1186/1742-4755-6-15
116. Tesfa E, Nibret E, Gizaw S, Zenebe Y, Mekonnen Z, Assefa S, et al. Prevalence and determinants of hypertensive disorders of pregnancy in Ethiopia: A systematic review and meta-analysis. PLoS One. (2020) 15:e0239048. doi: 10.1371/journal.pone.0239048
117. Belayhun Y, Kassa Y, Mekonnen N, Binu W, Tenga M, Duko B. Determinants of pregnancy-induced hypertension among mothers attending public hospitals in Wolaita Zone, South Ethiopia: Findings from unmatched case-control study. Int J Hypertens. (2021) 8:2021. doi: 10.1155/2021/6947499
118. Katore F, Gurara A, Beyen T. Determinants of preeclampsia among pregnant women in Chiro referral hospital, Oromia regional state, Ethiopia: Unmatched case–control study. Integr Blood Pressure Control. (2021) 1:163–72. doi: 10.2147/IBPC.S336651
119. Meazaw M, Chojenta C, Taddele T, Loxton D. Audit of clinical care for women with preeclampsia or eclampsia and perinatal outcome in Ethiopia: Second national EmONC survey. Int J Womens Health. (2022) 8:297–310.
120. Meazaw M, Chojenta C, Forder P, Taddele T, Loxton D. health care readiness in management of preeclampsia/eclampsia in Ethiopia: Evidence from national facility-based survey. Risk Manag Healthc Policy. (2022) 16:1225–41. doi: 10.2147/RMHP.S366055
121. Dale M, Kajabwangu R, Mayengo H, Munyanderu B, Baluku A, Manyang A, et al. Factors associated with severe maternal outcomes at a regional referral hospital in south-western Uganda: a case-control study. Int J Womens Health Care. (2021) 5:11–7.
122. Goldenberg R, Saleem S, Ali S, Moore J, Lokangako A, Tshefu A, et al. Maternal near miss in low-resource areas. Int J Gynecol Obstet. (2017) 138:347–55.
123. Bishaw K, Sharew Y, Beka E, Aynalem B, Zeleke L, Desta M, et al. Incidence and predictors of puerperal sepsis among postpartum women at Debre Markos comprehensive specialized hospital, northwest Ethiopia: A prospective cohort study. Front Glob Womens Health. (2023) 4:966942. doi: 10.3389/fgwh.2023.966942
124. Melkie A, Dagnew E. Burden of puerperal sepsis and its associated factors in Ethiopia: A systematic review and meta-analysis. Arch Public Health. (2021) 79:1–1. doi: 10.1186/s13690-021-00732-y
125. Demisse G, Sifer S, Kedir B, Fekene D, Bulto G. Determinants of puerperal sepsis among post-partum women at public hospitals in west SHOA zone Oromia regional STATE, Ethiopia (institution BASEDCASE control study). BMC Pregnancy Childbirth. (2019) 19:95. doi: 10.1186/s12884-019-2230-x
126. Buddeberg B, Aveling W. Puerperal sepsis in the 21st century: Progress, new challenges and the situation worldwide. Postgrad Med J. (2015) 91:572–8. doi: 10.1136/postgradmedj-2015-133475
127. Ethiopian Public Health Institute [Ephi], Ethiopia and Icf. Ethiopia mini demographic and health survey 2019: Final report. Rockville, MD: EPHI and ICF (2021).
128. Bonet M, Brizuela V, Abalos E, Cuesta C, Baguiya A, Chamillard M, et al. Frequency and management of maternal infection in health facilities in 52 countries (GLOSS): A 1-week inception cohort study. Lancet Glob Health. (2020) 8:e661–71. doi: 10.1016/S2214-109X(20)30109-1
129. Ugwu G, Iyoke C, Ezugwu E, Ajah L, Onah H, Ozumba BC. Comparison of the characteristics of maternal near-misses and maternal deaths in Enugu, Southeast Nigeria: A 3-year prospective study. Int J Womens Health. (2020) 6:207–11. doi: 10.2147/IJWH.S237221
130. Ali A, Khojali A, Okud A, Adam G, Adam I. Maternal near-miss in a rural hospital in Sudan. BMC Pregnancy Childbirth. (2011) 11:1–4. doi: 10.1186/1471-2393-11-48
131. Galvão L, Alvim-Pereira F, de Mendonça C, Menezes F, Góis K, Ribeiro RF, et al. The prevalence of severe maternal morbidity and near miss and associated factors in Sergipe, Northeast Brazil. BMC Pregnancy Childbirth. (2014) 14:25. doi: 10.1186/1471-2393-14-25
132. Geltore T, Anore D. The impact of antenatal care in maternal and perinatal health. Empower Midwives Obstet Nurses. (2021) 16:107.
133. Belay A, Fenta S, Birhan Biresaw H, Abebaw Moyehodie Y, Melkam Yelam M, Mekie M. The magnitude of optimal antenatal care utilization and its associated factors among pregnant women in south gondar zone, northwest Ethiopia: A cross-sectional study. Int J Reprod Med. (2022) 2:2022. doi: 10.1155/2022/1415247
134. Suleman Hassen S, Mulatu Teshale B, Abate Adulo L. Identifying factors associated with barriers in the number of antenatal care service visits among pregnant women in rural parts of Ethiopia. Sci World J. (2021) 25:2021. doi: 10.1155/2021/7146452
135. Yemane G. The factors associated with antenatal care utilization in Ethiopia. Ann Med Surg. (2022) 79:104092.
136. Negash W, Fetene S, Shewarega E, Fentie E, Asmamaw D, Teklu R, et al. Multilevel analysis of quality of antenatal care and associated factors among pregnant women in Ethiopia: A community based cross-sectional study. BMJ Open. (2022) 12:e063426. doi: 10.1136/bmjopen-2022-063426
137. Nemomsa A, Wirtu D, Getachew M, Kejela G, Merdassa E, Diriba W, et al. Quality of antenatal care in selected public health facilities of West Ethiopia. Contracept Reprod Med. (2022) 7:18.
138. Hailu G, Weret Z, Adasho Z, Eshete B. Quality of antenatal care and associated factors in public health centers in Addis Ababa, Ethiopia, a cross-sectional study. PLoS One. (2022) 17:e0269710. doi: 10.1371/journal.pone.0269710
139. Tadesse Berehe T, Modibia L. Assessment of quality of antenatal care services and its determinant factors in public health facilities of Hossana town, Hadiya zone, southern Ethiopia: A longitudinal study. Adv Public Health. (2020) 2020:1–1.
140. Tesfay N, Hailu G, Woldeyohannes F. Effect of optimal antenatal care on maternal and perinatal health in Ethiopia. Front Pediatr. (2023) 11:1120979. doi: 10.3389/fped.2023.1120979
141. Mebratie A. Receipt of core antenatal care components and associated factors in Ethiopia: A multilevel analysis. Front Glob Womens Health. (2024) 5:1169347. doi: 10.3389/fgwh.2024.1169347
142. Adamu A, Okusanya B, Tukur J, Ashimi A, Oguntayo O, Tunau K, et al. Maternal near-miss and death among women with hypertensive disorders in pregnancy: A secondary analysis of the Nigeria Near-miss and Maternal Death Survey. BJOG. (2019) 126:12–8. doi: 10.1111/1471-0528.15427
143. Nakimuli A, Nakubulwa S, Kakaire O, Osinde M, Mbalinda S, Nabirye R, et al. Maternal near misses from two referral hospitals in Uganda: A prospective cohort study on incidence, determinants and prognostic factors. BMC Pregnancy Childbirth. (2016) 16:24. doi: 10.1186/s12884-016-0811-5
144. Kalisa R, Rulisa S, van den Akker T, van Roosmalen J. Maternal near miss and quality of care in a rural Rwandan hospital. BMC Pregnancy Childbirth. (2016) 16:324. doi: 10.1186/s12884-016-1119-1
145. Moudi Z, Arabnezhad L, Tabatabaei S. Severe maternal outcomes in a tertiary referral teaching hospital in sistan and baluchistan province, Iran: A cross sectional study. Med Surg Nurs J. (2018) 1:7.
146. Giordano J, Parpinelli M, Cecatti J, Haddad S, Costa M, Surita F, et al. The burden of eclampsia: Results from a multicenter study on surveillance of severe maternal morbidity in Brazil. PLoS One. (2014) 9:e97401. doi: 10.1371/journal.pone.0097401
147. Croke K. The origins of Ethiopia’s primary health care expansion: The politics of state building and health system strengthening. Health Policy Plann. (2020) 35:1318–27. doi: 10.1093/heapol/czaa095
148. Iputo R, Maswime S, Motshabi P. Perioperative management of caesarean section-related haemorrhage in a maternal near-miss population: A retrospective study. Southern Afr J Anaesth Analg. (2021) 27:286–91.
149. Masterson J, Adamestam I, Beatty M, Boardman J, Johnston P, Joss J, et al. Severe maternal morbidity in Scotland. Anaesthesia. (2022) 77:971–80.
150. Kahveci B, Melekoglu R, Evruke I, Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. (2018) 18:343. doi: 10.1186/s12884-018-1984-x
151. Braunthal S, Brateanu A. Hypertension in pregnancy: Pathophysiology and treatment. SAGE Open Med. (2019) 7:205031211 9843700.
152. Dasa T, Okunlola M, Dessie Y. Effect of grand multiparity on adverse maternal outcomes: A prospective cohort study. Front Public Health. (2022) 10:959633. doi: 10.3389/fpubh.2022.959633
153. Mersha A, Ayele G, Worku T, Zerdo Z, Shibiru S, Bante A, et al. Association between maternal age and adverse perinatal outcomes in Arba Minch zuria, and Gacho Baba district, southern Ethiopia: A prospective cohort study. BMC Pregnancy Childbirth. (2020) 20:590. doi: 10.1186/s12884-020-03285-0
154. Dasa T, Okunlola M, Dessie Y. Effect of grand multiparity on the adverse birth outcome: A hospital-based prospective cohort study in Sidama Region, Ethiopia. Int J Womens Health. (2022) 14:363–72. doi: 10.2147/IJWH.S350991
155. Dasa T, Okunlola M, Dessie Y. Multilevel analysis of grand multiparity: Trend and its determinants in the Sidama National Regional State of Ethiopia: A cross-sectional study design from demographic and health survey 2000–2016. BMJ Open. (2022) 12:e061697. doi: 10.1136/bmjopen-2022-061697
156. Akrawi V, Al-Hadithi T, Al-Tawil N. Major determinants of maternal near-miss and mortality at the maternity teaching hospital, Erbil city, Iraq. Oman Med J. (2017) 32:386. doi: 10.5001/omj.2017.74
157. Lindquist A, Knight M, Kurinczuk J. Variation in severe maternal morbidity according to socioeconomic position: A UK national case–control study. BMJ Open. (2013) 3:e002742. doi: 10.1136/bmjopen-2013-002742
158. Creanga A, Bateman B, Kuklina E, Callaghan W. Racial and ethnic disparities in severe maternal morbidity: A multistate analysis, 2008-2010. AM J Obstet Gynecol. (2014) 210:435–e1. doi: 10.1016/j.ajog.2013.11.039
159. Gedefaw G, Demis A, Alemnew B, Wondmieneh A, Getie A, Waltengus F. Prevalence, indications, and outcomes of caesarean section deliveries in Ethiopia: A systematic review and meta-analysis. Patient Saf Surg. (2020) 14:1–0. doi: 10.1186/s13037-020-00236-8
160. Bayou Y, Mashalla Y, Thupayagale-Tshweneagae G. Patterns of caesarean-section delivery in Addis Ababa, Ethiopia. Afr J Prim Health Care Fam Med. (2016) 8:1–6. doi: 10.4102/phcfm.v8i2.953
161. Suleiman I, Maro E, Shayo B, Alloyce J, Masenga G, Mahande M, et al. Trend in female genital mutilation and its associated adverse birth outcomes: A 10-year retrospective birth registry study in Northern Tanzania. PLoS One. (2021) 16:e0244888. doi: 10.1371/journal.pone.0244888
162. Kaplan A, Hechavarría S, Martín M, Bonhoure I. Health consequences of female genital mutilation/cutting in the Gambia, evidence into action. Reprod Health. (2011) 8:1–6. doi: 10.1186/1742-4755-8-26
163. Klein E, Helzner E, Shayowitz M, Kohlhoff S, Smith-Norowitz T. Female genital mutilation: Health consequences and complications–a short literature review. Obstet Gynecol Int. (2018) 0:2018. doi: 10.1155/2018/7365715
164. Berg R, Underland V. The obstetric consequences of female genital mutilation/cutting: A systematic review and meta-analysis. Obstet Gynecol Int. (2013) 1:2013.
165. Alemu A. Trends and determinants of female genital mutilation in Ethiopia: Multilevel analysis of 2000, 2005 and 2016 ethiopian demographic and health surveys. Int J Womens Health. (2021) 6:19–29. doi: 10.2147/IJWH.S287643
166. Azeze G, Williams A, Tweya H, Obsa M, Mokonnon T, Kanche Z, et al. Changing prevalence and factors associated with female genital mutilation in Ethiopia: Data from the 2000, 2005 and 2016 national demographic health surveys. PLoS One. (2020) 15:e0238495. doi: 10.1371/journal.pone.0238495
167. Anjulo B, Lambebo A. Prevalence and associated factors of Female genital mutilation among reproductive age women’s of Wolayita Zone, Southern Ethiopia: A cross-sectional study. Int J Sex Reprod Health Care. (2018) 4:91–8.
168. Abathun A, Sundby J, Gele A. Attitude toward female genital mutilation among Somali and Harari people, Eastern Ethiopia. Int J Womens Health. (2016) 6:557–69. doi: 10.2147/IJWH.S112226
169. Gudeta T, Regassa T, Gamtessa L. Female genital mutilation: Prevalence, associated factors and health consequences among reproductive age group women in Keffa Zone, Southwest, Ethiopia. Reprod Health. (2022) 19:1–9. doi: 10.1186/s12978-022-01364-3
170. Bagambe P, Umubyeyi A, Nyirazinyoye L, Luginaah I. Women’s experiences and perceptions on the impacts of maternal near miss and related complications in Rwanda: A qualitative study. Afr J Reprod Health. (2022) 26:63–71.
171. Shah B, Krishnan N, Kodish S, Yenokyan G, Fatema K, Uddin K, et al. Applying the Three Delays Model to understand emergency care seeking and delivery in rural Bangladesh: A qualitative study. BMJ Open. (2020) 10:e042690. doi: 10.1136/bmjopen-2020-042690
172. Kea A, Tulloch O, Datiko D, Theobald S, Kok M. Exploring barriers to the use of formal maternal health services and priority areas for action in Sidama zone, southern Ethiopia. BMC Pregnancy Childbirth. (2018) 18:95. doi: 10.1186/s12884-018-1721-5
173. Tiruneh G, Arega D, Kassa B, Bishaw K. Delay in making decision to seek care on institutional delivery and associated factors among postpartum mothers in South Gondar zone hospitals, 2020: A cross-sectional study. Heliyon. (2022) 8:e09056. doi: 10.1016/j.heliyon.2022.e09056
174. Tesfay N, Tariku R, Zenebe A, Mohammed F, Woldeyohannes F. Area of focus to handle delays related to maternal death in Ethiopia. PLoS One. (2022) 17:e0274909. doi: 10.1371/journal.pone.0274909
175. Alemu F, Fuchs M, Martin Vitale T, Abdalla Mohamed Salih M. Severe maternal morbidity (near-miss) and its correlates in the world’s newest nation: South Sudan. Int J Womens Health. (2019) 9:177–90. doi: 10.2147/IJWH.S160022
176. Worke M, Enyew H, Dagnew M. Magnitude of maternal near misses and the role of delays in Ethiopia: A hospital based cross-sectional study. BMC Res Notes. (2019) 12:585. doi: 10.1186/s13104-019-4628-y
177. Hailemichael A, Belayihun B, Asnake M, Lulu K, Desta B, Genene L, et al. Referral Service Barriers in Ethiopia: Experiences and perceptions of actors. Ethiop J Health Dev. (2021) 35:8597. doi: 10.1186/1471-2393-12-113
178. Guddu D, Demissie D. Patient satisfaction with referral service and associated factors among public hospitals in and around Addis Ababa, Central Ethiopia. SAGE Open Med. (2022) 10:20503121221089443. doi: 10.1177/20503121221089443
179. Yasin C, Geleto A, Berhane Y. Referral linkage among public health facilities in Ethiopia: A qualitative explanatory study of facilitators and barriers for emergency obstetric referral in Addis Ababa city administration. Midwifery. (2019) 79:102528. doi: 10.1016/j.midw.2019.08.010
180. Wanaka S, Hussen S, Alagaw A, Tolosie K, Boti N. Maternal delays for institutional delivery and associated factors among postnatal mothers at public health facilities of Gamo zone, Southern Ethiopia. Int J Womens Health. (2020) 4:127–38. doi: 10.2147/IJWH.S240608
Keywords: uterine hemorrhage, pregnancy induced hypertension, maternal near miss (MNM), puerperal infection, maternal death surveillance and response
Citation: Tesfay N, Hailu G, Begna D, Habtetsion M, Taye F, Woldeyohannes F and Jina R (2024) Prevalence, underlying causes, and determinants of maternal near miss in Ethiopia: a systematic review and meta-analysis. Front. Med. 11:1393118. doi: 10.3389/fmed.2024.1393118
Received: 28 February 2024; Accepted: 20 September 2024;
Published: 08 October 2024.
Edited by:
Sarah M. Cohen, Hadassah Medical Center, IsraelReviewed by:
Abraham Negash, College of Health and Medical Sciences Haramaya university, EthiopiaDrucilla Jane Roberts, Harvard Medical School, United States
Copyright © 2024 Tesfay, Hailu, Begna, Habtetsion, Taye, Woldeyohannes and Jina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neamin Tesfay, bmVhbWludGVzZmF5ZTIxMjNAZ21haWwuY29t
 Neamin Tesfay
Neamin Tesfay Girmay Hailu1
Girmay Hailu1 Fitsum Woldeyohannes
Fitsum Woldeyohannes