
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 22 May 2024
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1390049
This article is part of the Research Topic Treatment and Prognostic Assessment of Liver Cirrhosis and Its Complications, Volume II View all 21 articles
 Liwei Guo1,2
Liwei Guo1,2 Yong An2
Yong An2 Xu Huang2
Xu Huang2 Wenhua Liu2
Wenhua Liu2 Fangfang Chen3
Fangfang Chen3 Yuchen Fan4,5
Yuchen Fan4,5 Shuai Gao4,5
Shuai Gao4,5 Liyan Han4,5
Liyan Han4,5 Kai Wang1,4,5*
Kai Wang1,4,5*Background: Liver fibrosis significantly impacts public health globally. Untreated liver fibrosis eventually results in cirrhosis. Cigarette smoking is the main etiologic factor for various diseases. However, the causal effects of cigarette smoking on liver fibrosis and cirrhosis have yet to be fully elucidated.
Methods: In this study, Mendelian randomization (MR) analysis was performed to assess the association between cigarette smoking, liver fibrosis, and cirrhosis. Single-nucleotide polymorphisms (SNPs) were selected as instrumental variables from a genome-wide association study (GWAS) of European ancestry. Patients were divided into six exposure categories as follows: “ever smoked,” “pack years of smoking,” “age of smoking initiation,” “smoking status: never,” “smoking status: current,” and “smoking status: previous.” The outcomes of this study included liver fibrosis and cirrhosis. MR-Egger, weighted median, inverse variance weighted, simple mode, and weighted mode were selected as the analysis methods. Cochran’s Q and the MR-PRESSO tests were conducted to measure heterogeneity. The MR-Egger method was performed to evaluate horizontal pleiotropy, while the “leave-one-out” analysis was performed for sensitivity testing.
Results: The results of this study showed that having a smoking history increases the risk of liver fibrosis and cirrhosis [“ever smoked”: odds ratio (OR) = 5.704, 95% CI: 1.166–27.910, p = 0.032; “smoking status: previous”: OR = 99.783, 95% CI: 2.969–3.353e+03, p = 0.010]. A negative correlation was observed between patients who never smoked and liver fibrosis and cirrhosis (“smoking status: never”: OR = 0.171, 95% CI: 0.041–0.719, p = 0.016). However, there were no significant associations between “smoking status: current,” “pack years of smoking,” and “age of smoking initiation” and liver fibrosis and cirrhosis. Cigarette smoking did not have a significant horizontal pleiotropic effect on liver fibrosis and cirrhosis. The “Leave-one-out” sensitivity analysis indicated that the results were stable.
Conclusion: The study confirmed the causal effects of cigarette smoking on liver fibrosis and cirrhosis.
Liver disease is a long-standing challenge to global health (1). The etiological factors for chronic liver inflammation include viral hepatitis infections, alcohol consumption, drugs, metabolic factors, and autoimmune hepatitis. Untreated chronic liver inflammation causes liver fibrosis. Advanced liver fibrosis results in cirrhosis and hepatocellular carcinoma (HCC). Liver cirrhosis is the 11th most frequent cause of death worldwide, with two million deaths every year, due to complications such as chronic portal hypertension, bleeding events, and hepatic encephalopathy (2, 3).
Cigarette smoking is associated with an unhealthy lifestyle. The number of smokers is rapidly increasing, and more than one billion people worldwide have a pernicious smoking habit (4). Cigarette smoke contains more than 4,000 toxic substances that are etiological factors for various diseases (5–8). Cigarette smoking is primarily associated with respiratory diseases, and it is a major risk factor for cardiovascular diseases (9–12). Additionally, cigarette smoking increases the risk of gastrointestinal disorders (13). Cigarette smoking is associated with an increased incidence of tumors, including liver cancer (14). In addition, the incidence of liver cancer in current smokers is 1.5 times higher than that in non-smokers (15, 16). However, the effect of cigarette smoking on liver fibrosis and cirrhosis remains unclear.
Mendelian randomization (MR) is a novel tool that uses summary statistics from genome-wide association studies (GWAS) to investigate the causality between risk factors and diseases (17, 18). In MR studies, genetic variations are used as instrumental variables (IVs) (19, 20). Randomized controlled trials (RCTs) are widely accepted as the gold standard for assessing the association between exposures and outcomes. However, RCTs have many limitations, including high costs and difficulties in implementation (21, 22). MR is an analog method for RCTs (23). Additionally, MR can overcome the deficiencies of RCTs through the use of single-nucleotide polymorphisms (SNPs) as IVs (18, 24, 25). MR studies can assess the causal effects of various exposures of interest, including biological markers, daily behaviors, and disease exposures, on a range of diseases (26–28).
Previous studies estimated the causal effects of cigarette smoking on many diseases, such as stroke and cancer, using MR (7, 29–31). However, studies using MR to assess the effect of cigarette smoking on liver fibrosis and cirrhosis are limited. Hence, this MR study aimed to clarify the causal effect of cigarette smoking on liver fibrosis and cirrhosis.
A two-sample MR analysis was used to explore the association between exposure and outcome in two different samples. Compared to a one-sample MR, the sample size in a two-sample MR is larger and more precise (32). This study aimed to analyze the causal effects of cigarette smoking on liver fibrosis and cirrhosis. As such, six exposures related to cigarette smoking were selected, including “ever smoked,” “pack years of smoking,” “age of smoking initiation,” “non-smokers,” “current smokers,” and “former smokers.” The “ever smoked” group included participants who ever had smoking habits, regardless of whether they were currently smoking or not. Pack years of smoking were calculated based on the age of starting smoking and the age of quitting smoking, or the duration from starting smoking to participating in this program of the Integrative Epidemiology Unit GWAS database. The “non-smokers” group included participants who had never smoked. The “current smokers” group consists of participants who had the smoking habit and currently still smoke. The “former smokers” group consists of participants who used to smoke before but had completely quit smoking recently. Compared to non-smokers, anyone who has a smoking habit before or currently is considered to have a smoking history.
The exposed genetic variants were obtained from the Integrative Epidemiology Unit GWAS database. The sample sizes for “ever smoked,” “pack years of smoking,” “age of smoking initiation,” “smoking status: never,” “smoking status: current,” and “smoking status: previous” were 461,066, 142,387, 341,427, 359,706, 336,024, and 336,024, respectively. The study focused on outcomes such as liver fibrosis and cirrhosis, and the genetic variants obtained from the FinnGen consortium data (1,602 cases and 332,951 controls) were also included in this study. To avoid population stratification, the genetic variants used in this analysis were derived from European ancestries. The details of the data sources are listed in Table 1. In this MR study, genetic variants strongly correlated with exposure but failed to show associations with confounders. Therefore, genetic variants did not have an impact on the outcome, except through exposure (33).
SNPs with a p-value less than 5 × 10−8 and minor frequency > 1% as IVs, relating to “ever smoked,” “pack years,” “age of smoking initiation,” “non-smokers,” “current smokers,” and “former smokers,” were selected. Furthermore, the clumping method (r2 < 0.001, clumping distance = 10,000 kb) was used to avoid linkage disequilibrium. The F-statistic was used to evaluate the strength of the association between IVs and exposure. The general threshold of F in an MR study was 10 (31). SNPs with an F less than 10 were considered weak instruments and were eliminated from further MR analyses (32). In this study, F was calculated as β2/SE2 (β stands for the effect on the risk of exposure, and SE stands for the standard error) (34–36).
MR-Egger, weighted median, inverse variance weighted (IVW), simple mode, and weighted mode were selected as the methods of analysis. IVW is considered the most reliable method in MR analyses, and it was performed to assess the heterogeneity among the IVs (37). Cochran’s Q and the MR-PRESSO tests were used to measure the heterogeneity of individual SNPs (38). Heterogeneity existed if the p-value was less than 0.05 and the random-effects model was implemented; otherwise, the fixed-effects model was used. Funnel plots were used to show heterogeneity by drawing Wald ratios for the SNPs. The MR-Egger method was used to evaluate horizontal pleiotropy; if the intercept was significantly different from 0, with a p-value less than 0.05, horizontal pleiotropic effects existed (39). Finally, sensitivity analyses were conducted using the “leave-one-out” analysis.
In this study, the statistically significant level was set at p < 0.05. All analyses were performed in R (version 4.2.2) with the “TwoSampleMR” package (version 0.5.6).
The SNPs chosen for this MR analysis are presented in Supplementary File. In the final MR analysis, a total of 67 SNPs were related to “ever smoked,” 5 SNPs were related to “pack years of smoking,” 9 SNPs were related to “age of smoking initiation,” 61 SNPs were related to “smoking status: never,” 15 SNPs were related to “smoking status: current,” and 18 SNPs were related to “smoking status: previous.” The F statistics for these SNPs were more than 10, with mean F values of 40.63, 37.62, 81.23, 41.77, 40.79, and 35.30 for “ever smoked,” “pack years of smoking,” “age of smoking initiation,” “smoking status: never,” “smoking status: current,” and “smoking status: previous,” respectively. Furthermore, a weak instrumental variable bias was non-existent.
In this MR study, five methods were used to assess the causal effects of cigarette smoking on liver fibrosis and cirrhosis in the European population, and the IVW method was considered the most reliable method. As presented in Table 2, having a smoking history was correlated with liver fibrosis and cirrhosis (“ever smoked,” IVW: OR = 5.704, 95% CI: 1.166–27.910, p = 0.032). “Smoking status: previous” also had positive associations with liver fibrosis and cirrhosis (IVW: OR = 99.783, 95% CI: 2.969 – 3.353e + 03, p = 0.010). “Smoking status: never” had negative associations with liver fibrosis and cirrhosis (IVW: OR = 0.171, 95% CI: 0.04–0.719, p = 0.016). Additionally, “smoking status: current,” “pack years of smoking,” and “age of smoking initiation” were not associated with liver fibrosis and cirrhosis. The effect of each SNP on liver fibrosis and cirrhosis is shown in Figures 1, 2.
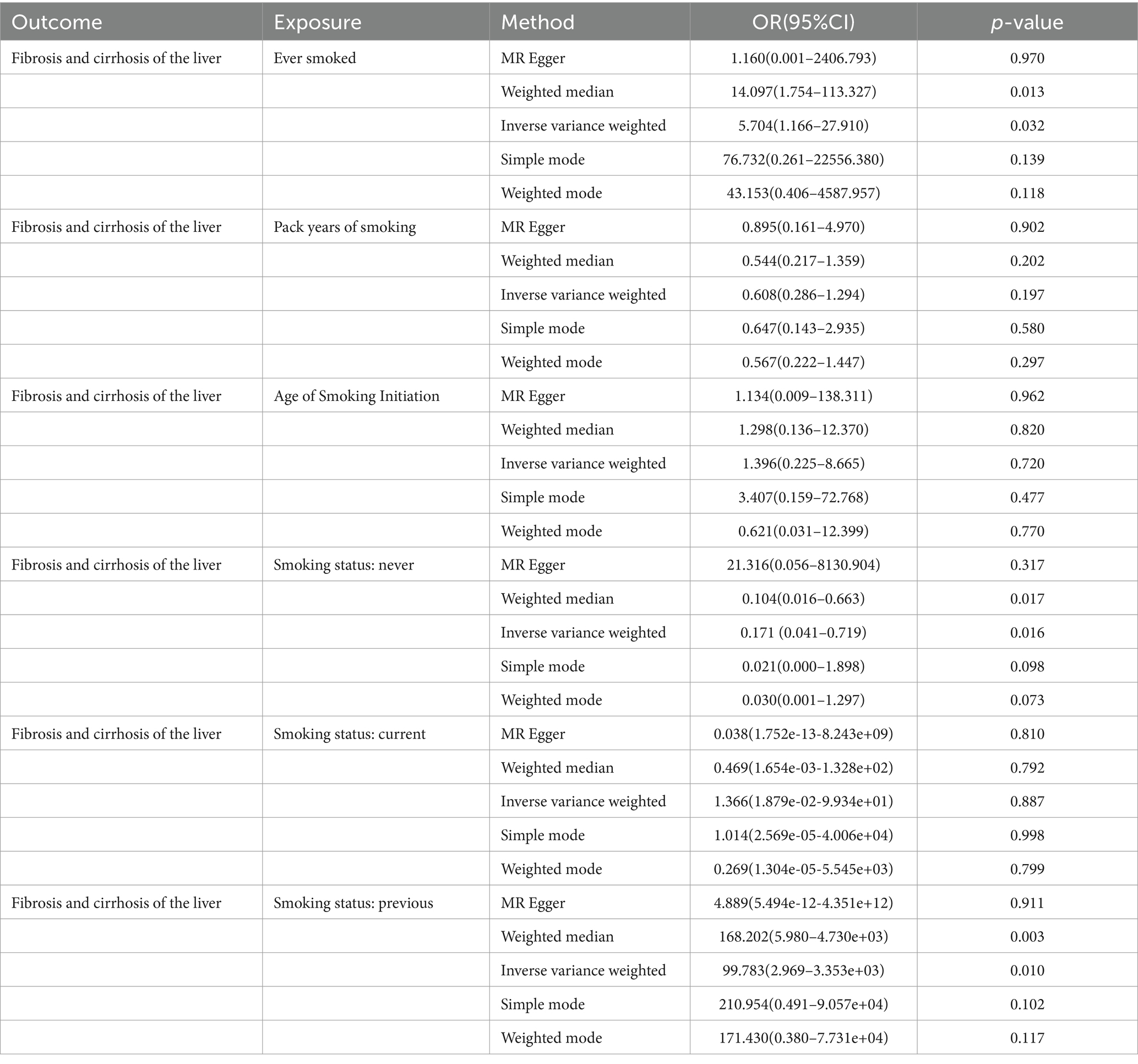
Table 2. MR analysis from each method assessing the causal effects of smoking on liver fibrosis and cirrhosis.
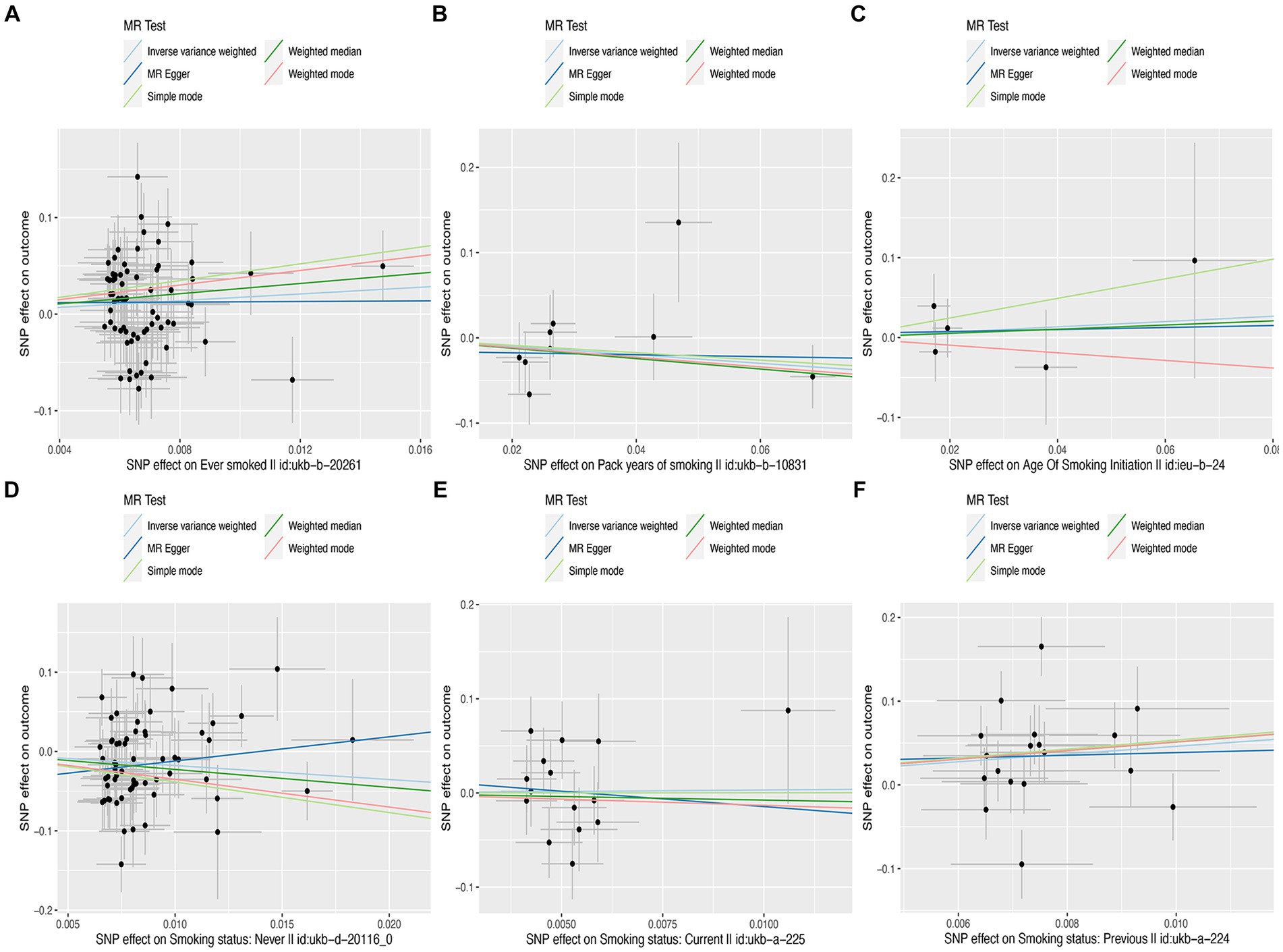
Figure 1. Scatter plots of effects of cigarette smoking-associated SNPs on liver fibrosis and cirrhosis. (A) “Ever smoked”; (B) “Pack years of smoking”; (C) “Age of smoking initiation”; (D) “Smoking status: never”; (E) “Smoking status: current”; and (F) “Smoking status: previous.”
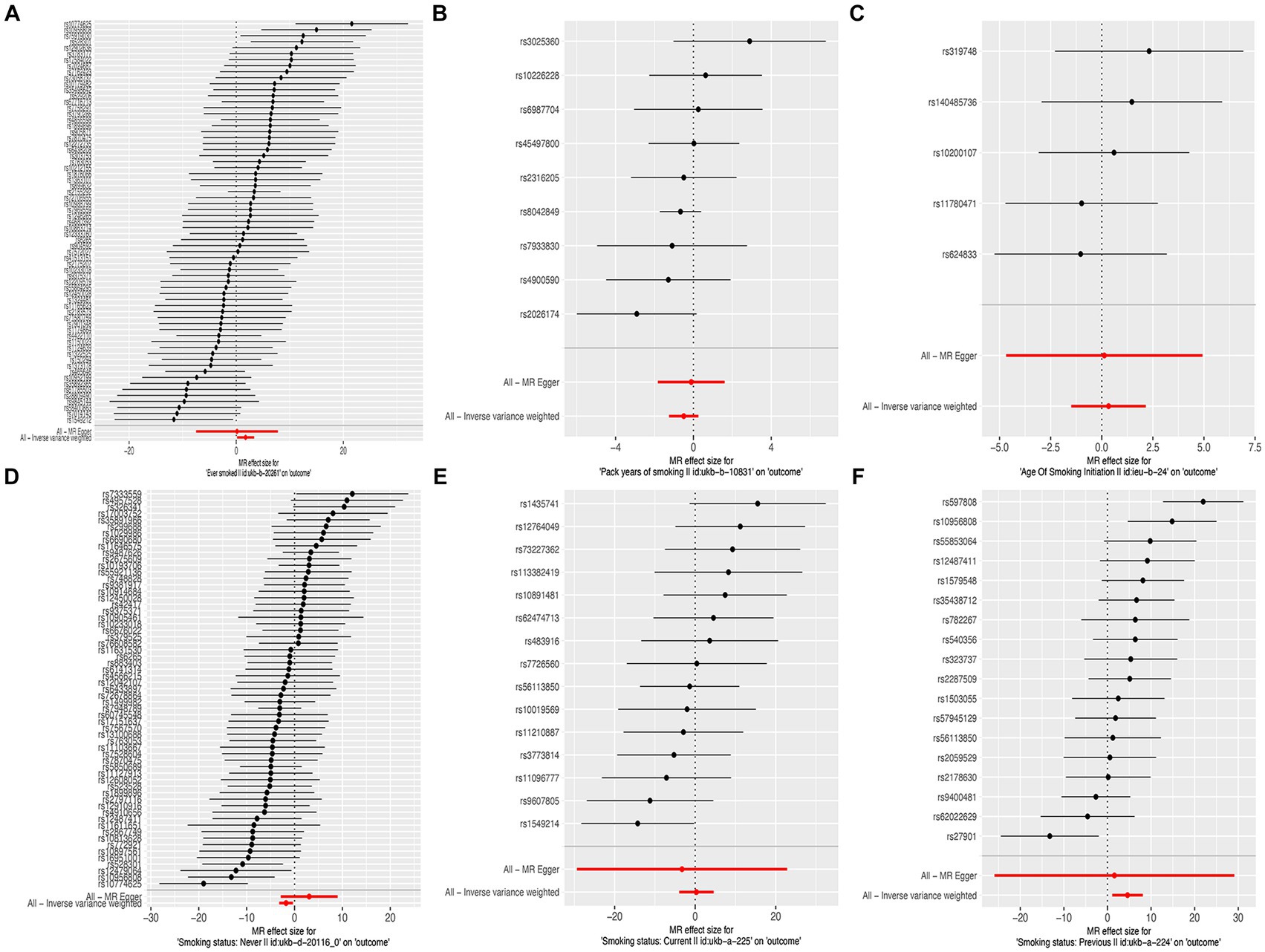
Figure 2. Forest plots of the effect size for each SNP for cigarette smoking on the risk of liver fibrosis and cirrhosis. (A) “Ever smoked”; (B) “Pack years of smoking”; (C) “Age of smoking initiation”; (D) “Smoking status: never”; (E) “Smoking status: current”; and (F) “Smoking status: previous.”
The results of the MR-Egger test indicated the absence of pleiotropy (p ˃0.05). In addition, all SNPs in this MR analysis did not affect liver fibrosis and cirrhosis via biological pathways independently (Table 3). Heterogeneity was tested using Cochran’s Q test and the MR-PRESSO test; the results are presented in Table 3. Funnel plots were used to visualize the heterogeneity of the effects of SNPs on liver fibrosis and cirrhosis; the results are presented in Supplementary Figure 1. The results of the leave-one-out analysis revealed reliable associations between exposures and outcomes (Figure 3).
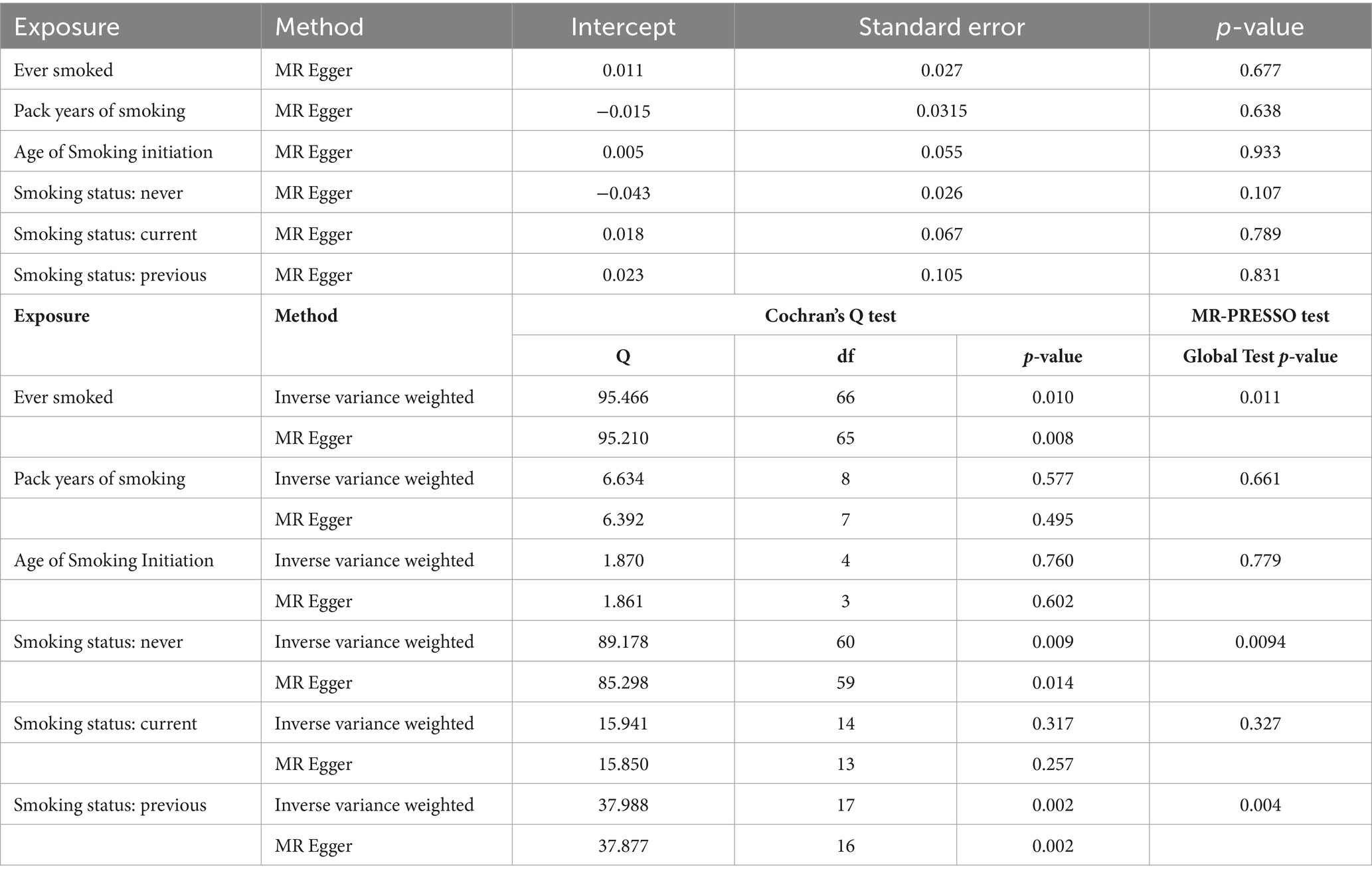
Table 3. Pleiotropy and heterogeneity testing of smoking associated with liver fibrosis and cirrhosis risk using the MR Egger method.
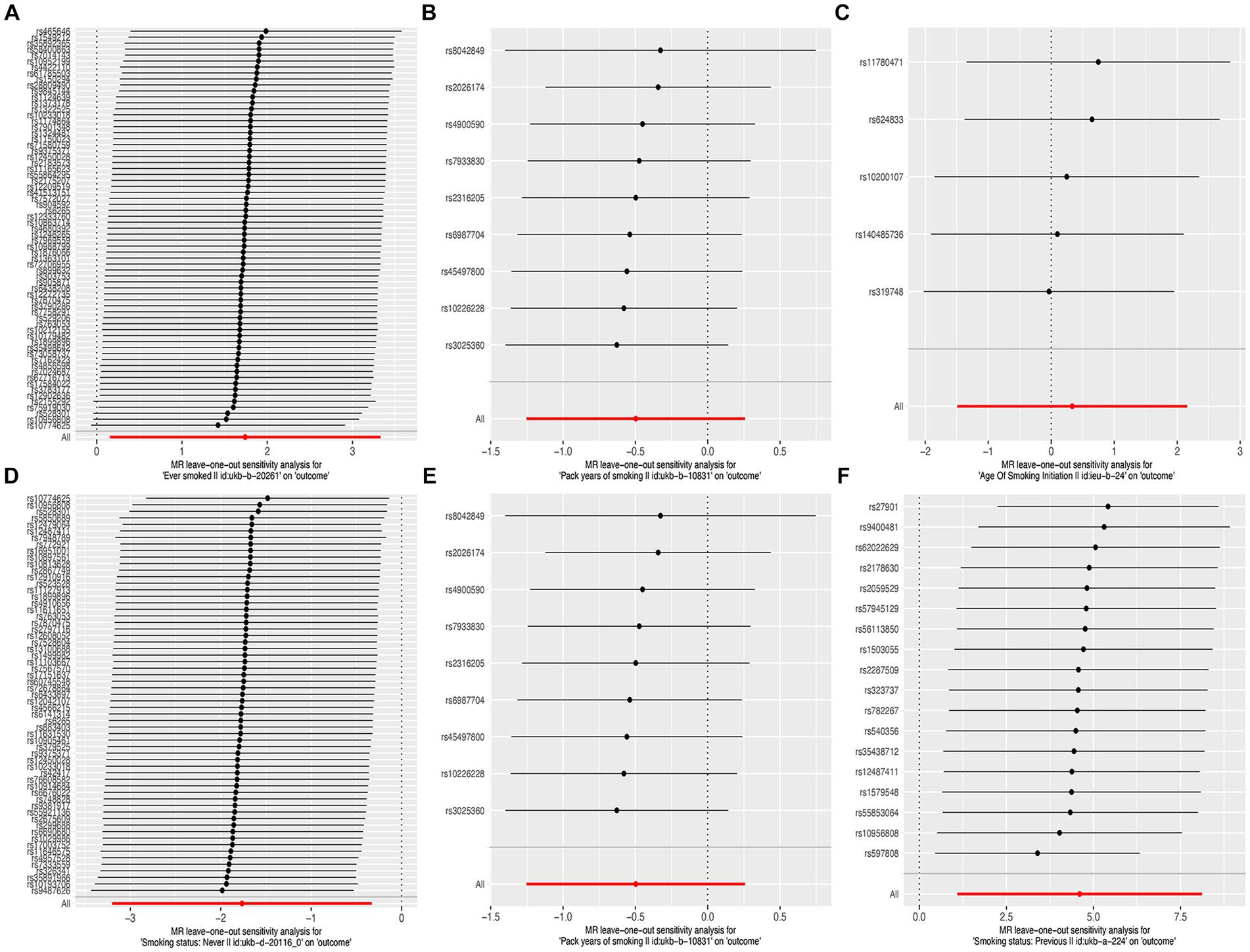
Figure 3. Leave-one-out sensitivity analysis of the causal effects of cigarette smoking on liver fibrosis and cirrhosis. (A) “Ever smoked”; (B) “Pack years of smoking”; (C) “Age of smoking initiation”; (D) “Smoking status: never”; (E) “Smoking status: current”; and (F) “Smoking status: previous.”
This is the first study to assess the causality of cigarette smoking on liver fibrosis and cirrhosis using the MR analysis and GWAS. In this study, five MR analysis methods were implemented. The results indicated that a smoking history increases the risk of liver fibrosis and cirrhosis, while a lack of a smoking history reduces this risk.
Liver fibrosis is a common liver disease that results in cirrhosis with the progression of fibrosis (40). The activation of hepatic stellate cells (HSCs) is a key etiological factor in liver fibrosis (41–43). Currently, liver fibrosis is assumed to be the result of pathological changes caused by an imbalance between extracellular matrix synthesis and degradation. In addition, liver tissues injured by viruses, alcohol, and other hazardous factors activate HSCs that secrete excessive extracellular matrix (ECM). The accumulation of ECM destroys the physiological architecture of the liver and leads to regression of fibrosis (44, 45).
Cigarette smoking is established as a harmful determinant of health that endangers almost all organ systems. However, the impact of cigarette smoking on the liver has been poorly studied. In recent years, the effect of cigarette smoking on the liver has attracted increasing attention (46). Long-term exposure to cigarette smoke increases the secretion of proinflammatory cytokines involved in liver cell injury (47). In addition, cigarette smoking is closely associated with non-alcoholic liver disease (NAFLD) (48). The cross-sectional study has found that increasing the daily cigarette quantity correlates with an increased incidence of fatty liver (49). A recent MR analysis identified that cigarette smoking is causally implicated in NAFLD (50). Cigarette smoking significantly increases the risk of liver fibrosis in NAFLD patients (51). Furthermore, second-hand smoking induces liver inflammation through the deregulation of genes and molecular pathways that regulate lipid metabolism (52).
This study aimed to establish a correlation between cigarette smoking and liver fibrosis and cirrhosis. To assess the causal effects, we selected six exposures, including “ever smoked,” “pack years of smoking,” “age of smoking initiation,” “smoking status: never,” “smoking status: current,” and “smoking status: previous.” Through strict statistical analysis, a positive correlation was identified between cigarette smoking and liver fibrosis and cirrhosis. “Ever smoked” and “smoking status: previous” were risk factors, whereas “smoking status: never” was seemingly protective. These results provide evidence supporting the adverse effects of cigarette smoking on liver fibrosis and cirrhosis. Cigarette smoke contains reactive oxygen species (ROS). Substantial evidence has demonstrated that ROS causes systemic oxidative damage to membrane lipids, proteins, and DNA in the human body. An imbalance between ROS and endogenous antioxidant defenses leads to oxidative stress (53–55). Several studies have shown that oxidative stress plays an important role in the development of liver fibrosis and cirrhosis (56–58). Cigarette smoking causes gut microbiota dysbiosis, which is closely associated with various diseases, including liver fibrosis (48). In this MR study, “ever smoked,” “smoking status: previous,” and “smoking status: never” rather than “smoking status: current,” “pack years of smoking,” or “age of smoking initiation” were found to be associated with liver fibrosis and cirrhosis. We speculate that it is related to the mechanisms mentioned above. This may be understood as the long-term effect of smoking on liver fibrosis and cirrhosis once it begins, and it will not stop due to changes in smoking status.
“Smoking status: previous” had positive associations with liver fibrosis and cirrhosis. The participants in the “Smoking status: previous” group had successfully quit smoking. The results indicated that smoking cessation could not reduce the risk of fibrosis and cirrhosis caused by smoking. This is an expected result because the smoking group included previous smokers who may had weight gain after smoking cessation. Both current smoking and weight gain after smoking cessation lead to a higher risk of NAFLD, which is a major cause of liver fibrosis and cirrhosis (59, 60).
The greatest advantage of this study is the use of a two-sample MR as a statistical method. Using a two-sample MR eliminates the bias caused by confounding and reverse causality issues (32). In addition, the population in this study was restricted to Europe; therefore, the bias resulting from population stratification was reduced. The exposures and outcomes were derived from different GWAS consortiums, and there was no sample overlap. Furthermore, the sample size in this study was sufficiently large to ensure the reliability of the results. Finally, five MR methods were used, and several sensitivity analyses were conducted to ensure the stability of the results.
However, limitations were present in this study. First, only the European population was assessed; therefore, the findings might not apply to other races. Second, the exposures focused on cigarette smoking habits and status, but the cigarette smoking propensity of patients was unavailable. Third, the causal effects of cigarette smoking on liver fibrosis and cirrhosis were assessed in this study, but the mechanisms underlying these effects are unknown. Fourth, cigarette filter types were not classified. Nowadays, many people have the habit of using electronic cigarettes, but this study could not observe the effects of electronic cigarettes on liver fibrosis and cirrhosis. Finally, numerous unhealthy lifestyle habits, such as drinking alcohol, lack of exercise, and unhealthy diet, are related to liver fibrosis and cirrhosis. However, the participants in this program of the Integrative Epidemiology Unit GWAS database were not grouped based on whether they had these bad habits or not, which led to our study only being able to observe the effects of smoking on liver fibrosis and cirrhosis and being unable to explore the mutual effects of these bad habits and smoking on liver fibrosis and cirrhosis. Future studies should strive to address these gaps.
This MR study provides evidence supporting the causal effects of cigarette smoking on liver fibrosis and cirrhosis. Cigarette smoking is a harmful determinant of health, and strict avoidance of cigarette smoking reduces the incidence of liver fibrosis and cirrhosis.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
LG: Writing – original draft, Formal analysis, Investigation, Validation. YA: Validation, Writing – review & editing. XH: Data curation, Formal analysis, Writing – review & editing. WL: Data curation, Formal analysis, Writing – review & editing. FC: Investigation, Writing – review & editing. YF: Writing – review & editing. SG: Writing – review & editing. LH: Writing – review & editing. KW: Investigation, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key Research and Development (R&D) Project of Shandong Province P. R. China (grant no. 2019GSF108129), National Key Research and Development Program of China (2021YFC2301801), National Natural Science Foundation of China (82272313), and Natural Science Foundation of Shandong Province (ZR2022MH006).
The authors acknowledge the support provided by the Integrative Epidemiology Unit (IEU) GWAS database and FinnGen consortium. We sincerely appreciate the great work of all investigators.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1390049/full#supplementary-material
1. Asrani, SK, Devarbhavi, H, Eaton, J, and Kamath, PS. Burden of liver diseases in the world. J Hepatol. (2019) 70:151–71. doi: 10.1016/j.jhep.2018.09.014
2. Llovet, JM, Zucman-Rossi, J, Pikarsky, E, Sangro, B, Schwartz, M, Sherman, M, et al. Hepatocellular Carcinoma. Nat Rev Dis Primers. (2016) 2:16018. doi: 10.1038/nrdp.2016.18
3. Roehlen, N, Crouchet, E, and Baumert, TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. (2020) 9:875. doi: 10.3390/cells9040875
4. Thomson, NC, Polosa, R, and Sin, DD. Cigarette smoking and asthma. J Allergy Clin Immunol Pract. (2022) 10:2783–97. doi: 10.1016/j.jaip.2022.04.034
5. Kim, NH, Jung, YS, Hong, HP, Park, JH, Kim, HJ, Park, DI, et al. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. (2018) 38:1487–94. doi: 10.1111/liv.13701
6. Bai, X, Wei, H, Liu, W, Coker, OO, Gou, H, Liu, C, et al. Cigarette smoke promotes colorectal Cancer through modulation of gut microbiota and related metabolites. Gut. (2022) 71:2439–50. doi: 10.1136/gutjnl-2021-325021
7. Larsson, SC, Burgess, S, and Michaëlsson, K. Smoking and stroke: a Mendelian randomization study. Ann Neurol. (2019) 86:468–71. doi: 10.1002/ana.25534
8. Bauer-Kemény, C, and Herth, F. Smoking-toxic substances and immunological consequences. Radiologie. (2022) 62:731–7. doi: 10.1007/s00117-022-01006-6
9. Sales, MPU, Araújo, AJ, Chatkin, JM, Godoy, I, Pereira, LFF, Castellano, MVCO, et al. Update on the approach to smoking in patients with respiratory diseases. J Bras Pneumol. (2019) 45:e20180314. doi: 10.1590/1806-3713/e20180314
10. Gan, H, Hou, X, Zhu, Z, Xue, M, Zhang, T, Huang, Z, et al. Smoking: a leading factor for the death of chronic respiratory diseases derived from global burden of disease study 2019. BMC Pulm Med. (2022) 22:1–11. doi: 10.1186/s12890-022-01944-w
11. DiGiacomo, SI, Jazayeri, M-A, Barua, RS, and Ambrose, JA. Environmental tobacco smoke and cardiovascular disease. Int J Environ Res Public Health. (2019) 16:96. doi: 10.3390/ijerph16010096
12. Kondo, T, Nakano, Y, Adachi, S, and Murohara, T. Effects of tobacco smoking on cardiovascular disease. Circ J. (2019) 83:1980–5. doi: 10.1253/circj.CJ-19-0323
13. Berkowitz, L, Schultz, BM, Salazar, GA, Pardo-Roa, C, Sebastián, VP, Álvarez-Lobos, MM, et al. Impact of cigarette smoking on the gastrointestinal tract inflammation: opposing effects in Crohn’s disease and ulcerative colitis. Front Immunol. (2018) 9:74. doi: 10.3389/fimmu.2018.00074
14. Hecht, SS, and Hatsukami, DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and Cancer prevention. Nat Rev Cancer. (2022) 22:143–55. doi: 10.1038/s41568-021-00423-4
15. Pang, Q, Qu, K, Zhang, J, Xu, X, Liu, S, Song, S, et al. Cigarette smoking increases the risk of mortality from liver Cancer: a clinical-based cohort and Meta-analysis. J Gastroenterol Hepatol. (2015) 30:1450–60. doi: 10.1111/jgh.12990
16. Lee, Y-CA, Cohet, C, Yang, Y-C, Stayner, L, Hashibe, M, and Straif, K. Meta-analysis of epidemiologic studies on cigarette smoking and liver Cancer. Int J Epidemiol. (2009) 38:1497–511. doi: 10.1093/ije/dyp280
17. Bowden, J, and Holmes, MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
18. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
19. Davies, NM, Holmes, MV, and Smith, GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
20. Emdin, CA, Khera, AV, and Kathiresan, S. Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
21. Jones, DS, and Podolsky, SH. The history and fate of the gold standard. Lancet. (2015) 385:1502–3. doi: 10.1016/S0140-6736(15)60742-5
22. Bothwell, LE, and Podolsky, SH. The emergence of the randomized, controlled trial. N Engl J Med. (2016) 375:501–4. doi: 10.1056/NEJMp1604635
23. Bennett, DA, and Holmes, MV. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. (2017) 103:1400–7. doi: 10.1136/heartjnl-2016-310605
24. Gupta, V, Walia, G, and Sachdeva, M. ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
25. Gala, H, and Tomlinson, I. The use of Mendelian randomisation to identify causal Cancer risk factors: promise and limitations. J Pathol. (2020) 250:541–54. doi: 10.1002/path.5421
26. Tin, A, and Köttgen, A. Mendelian Randomization Analysis as a Tool to Gain Insights into Causes of Diseases: A Primer. J Am Soc Nephrol. (2021) 32:2400–7. doi: 10.1681/ASN.2020121760
27. Richmond, RC. Davey Smith G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect Med. (2022) 12:a040501. doi: 10.1101/cshperspect.a040501
28. Ference, BA, Holmes, MV, and Smith, GD. Using Mendelian Randomization to Improve the Design of Randomized Trials. Cold Spring Harb Perspect Med. (2021) 11:a040980. doi: 10.1101/cshperspect.a040980
29. Zhou, W, Liu, G, Hung, RJ, Haycock, PC, Aldrich, MC, Andrew, AS, et al. Causal relationships between body mass index, smoking and lung Cancer: Univariable and multivariable Mendelian randomization. Int J Cancer. (2021) 148:1077–86. doi: 10.1002/ijc.33292
30. Tang, H, Yang, D, Han, C, and Mu, P. Smoking, DNA methylation, and breast Cancer: a Mendelian randomization study. Front Oncol. (2021) 11:745918. doi: 10.3389/fonc.2021.745918
31. Xiong, J, Yang, L, Deng, YQ, Yan, SY, Gu, JM, Li, BH, et al. The causal association between smoking, alcohol consumption and risk of bladder Cancer: a Univariable and multivariable Mendelian randomization study. Int J Cancer. (2022) 151:2136–43. doi: 10.1002/ijc.34228
32. Richmond, RC, and Davey, SG. Commentary: orienting causal relationships between two phenotypes using bidirectional Mendelian randomization. Int J Epidemiol. (2019) 48:907–11. doi: 10.1093/ije/dyz149
33. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and Bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
34. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, NA, and Thompson, JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using Mr-egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
35. Burgess, S, Small, DS, and Thompson, SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. (2017) 26:2333–55. doi: 10.1177/0962280215597579
36. Duan, C, Shi, J, Yuan, G, Shou, X, Chen, T, Zhu, X, et al. Causal association between heart failure and Alzheimer’s disease: a two-sample bidirectional Mendelian randomization study. Front Genet. (2022) 12:772343. doi: 10.3389/fgene.2021.772343
37. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
38. Verbanck, M, Chen, C-Y, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
39. Burgess, S, and Thompson, SG. Interpreting findings from Mendelian randomization using the Mr-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
40. Parola, M, and Pinzani, M. Liver fibrosis: pathophysiology, Pathogenetic targets and clinical issues. Mol Asp Med. (2019) 65:37–55. doi: 10.1016/j.mam.2018.09.002
41. Zhang, C-Y, Yuan, W-G, He, P, Lei, J-H, and Wang, C-X. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. (2016) 22:10512–22. doi: 10.3748/wjg.v22.i48.10512
42. Elpek, GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol. (2014) 20:7260–76. doi: 10.3748/wjg.v20.i23.7260
43. Nianan, L, Jiangbin, L, Yu, W, Jianguo, L, and Rui, D. Hepatic stellate cell: a double-edged sword in the liver. Physiol Res. (2021) 70:821–9. doi: 10.33549/physiolres.934755
44. Caligiuri, A, Gentilini, A, Pastore, M, Gitto, S, and Marra, F. Cellular and molecular mechanisms underlying liver fibrosis regression. Cells. (2021) 10:2759. doi: 10.3390/cells10102759
45. Dawood, RM, El-Meguid, MA, Salum, GM, and El Awady, MK. Key players of hepatic fibrosis. J Interf Cytokine Res. (2020) 40:472–89. doi: 10.1089/jir.2020.0059
46. Marti-Aguado, D, Clemente-Sanchez, A, and Bataller, R. Cigarette smoking and liver diseases. J Hepatol. (2022) 77:191–205. doi: 10.1016/j.jhep.2022.01.016
47. Moszczyński, P, Żabiński, Z, Moszczyński, P Jr, Rutowski, J, Słowiński, S, and Tabarowski, Z. Immunological findings in cigarette smokers. Toxicol Lett. (2001) 118:121–7. doi: 10.1016/s0378-4274(00)00270-8
48. Chen, B, Sun, L, Zeng, G, Shen, Z, Wang, K, Yin, L, et al. Gut Bacteria alleviate smoking-related Nash by degrading gut nicotine. Nature. (2022) 610:562–8. doi: 10.1038/s41586-022-05299-4
49. Jung, H-S, Chang, Y, Kwon, M-J, Sung, E, Yun, KE, Cho, YK, et al. Smoking and the risk of non-alcoholic fatty liver disease: a cohort study. Am J Gastroenterol. (2019) 114:453–63. doi: 10.1038/s41395-018-0283-5
50. Yuan, S, Chen, J, Li, X, Fan, R, Arsenault, B, Gill, D, et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur J Epidemiol. (2022) 37:723–33. doi: 10.1007/s10654-022-00868-3
51. Ou, H, Fu, Y, Liao, W, Zheng, C, and Wu, X. Association between smoking and liver fibrosis among patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. (2019) 2019:6028952–5. doi: 10.1155/2019/6028952
52. Tommasi, S, Yoon, J-I, and Besaratinia, A. Secondhand smoke induces liver steatosis through deregulation of genes involved in hepatic lipid metabolism. Int J Mol Sci. (2020) 21:1296. doi: 10.3390/ijms21041296
53. Caliri, AW, Tommasi, S, and Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and Cancer. Mutat Res Rev Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
54. Prasad, S, Gupta, SC, and Tyagi, AK. Reactive oxygen species (Ros) and Cancer: role of Antioxidative nutraceuticals. Cancer Lett. (2017) 387:95–105. doi: 10.1016/j.canlet.2016.03.042
55. Sahoo, BM, Banik, BK, Borah, P, and Jain, A. Reactive oxygen species (Ros): key components in Cancer therapies. Anti Cancer Agents Med Chem. (2022) 22:215–22. doi: 10.2174/1871520621666210608095512
56. Yi, J, Wu, S, Tan, S, Qin, Y, Wang, X, Jiang, J, et al. Berberine alleviates liver fibrosis through inducing ferrous redox to activate Ros-mediated hepatic stellate cells Ferroptosis. Cell Death Dis. (2021) 7:374. doi: 10.1038/s41420-021-00768-7
57. Xu, Y, Chen, J, Jiang, W, Zhao, Y, Yang, C, Wu, Y, et al. Multiplexing Nanodrug ameliorates liver fibrosis via Ros elimination and inflammation suppression. Small. (2022) 18:e2102848. doi: 10.1002/smll.202102848
58. Luangmonkong, T, Suriguga, S, Mutsaers, HA, Groothuis, GM, Olinga, P, and Boersema, M. Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol. (2018) 175:71–102. doi: 10.1007/112_2018_10
59. Jeong, S, Oh, YH, Choi, S, Chang, J, Kim, SM, Park, SJ, et al. Association of Change in smoking status and subsequent weight change with risk of nonalcoholic fatty liver disease. Gut Liver. (2023) 17:150–8. doi: 10.5009/gnl220038
Keywords: cigarette smoking, liver fibrosis, cirrhosis, Mendelian randomization, causality
Citation: Guo L, An Y, Huang X, Liu W, Chen F, Fan Y, Gao S, Han L and Wang K (2024) A Mendelian randomization study on the causal effects of cigarette smoking on liver fibrosis and cirrhosis. Front. Med. 11:1390049. doi: 10.3389/fmed.2024.1390049
Received: 22 February 2024; Accepted: 06 May 2024;
Published: 22 May 2024.
Edited by:
Xingshun Qi, General Hospital of Northern Theater Command, ChinaReviewed by:
Bin Wang, Huazhong University of Science and Technology, ChinaCopyright © 2024 Guo, An, Huang, Liu, Chen, Fan, Gao, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Wang, d2FuZ2RvYzIwMTBAMTYzLmNvbQ==; d2FuZ2RvYzg3NkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.