
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 18 April 2024
Sec. Dermatology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1384055
 Kerstin Wolk1,2
Kerstin Wolk1,2 Maximilian Schielein3
Maximilian Schielein3 Julia-Tatjana Maul4,5
Julia-Tatjana Maul4,5 Fontaine Widmayer6
Fontaine Widmayer6 Kerstin Wanke6
Kerstin Wanke6 Wolfgang Fischmann7
Wolfgang Fischmann7 Petra Nathan3*†
Petra Nathan3*† Robert Sabat1,2*†
Robert Sabat1,2*†Background: Chronic inflammatory skin diseases (CISDs) are among the most common diseases in the Western world. Current estimates of medical care for CISDs are primarily based on surveys among patients in medical care facilities and on health insurance data.
Aim: Survey-based examination to what extent CISD patients in health-aware environment consider their skin disease to be controlled.
Methods: The survey of CISD patients was carried out in 2022 among the employees of a pharmaceutical company located in Germany and Switzerland. Software-based, anonymous, self-reported questionnaires were used.
Results: The number of employees, who answered the questionnaire, was 905. Of these, 222 participants (24.5%) reported having at least one CISD. 28.7% of participants with CISD described their disease as being hardly or not controlled. Regarding the nature of disease, more than one third of participants suffering from hidradenitis suppurativa (HS) or psoriasis fell into the hardly/not controlled category. In contrast, the largest proportion of participants with chronic spontaneous urticaria (43%) or atopic dermatitis (42%) considered their CISD to be completely or well controlled. Only 35.5% of CISD sufferers stated that they were currently under medical care for their skin condition. Being under medical care, however, had no influence on the extent CISD sufferers considered their skin disease to be controlled. The number of active CISD episodes but not the total number of symptomatic days per year was negatively associated with poor disease control (p = 0.042 and p = 0.856, respectively). Poor disease control had a negative effect on the personal and professional lives of those affected, as deduced from its positive association with the extent of daily activity impairment and presenteeism (p = 0.005 and p = 0.005, respectively). Moreover, 41.4 and 20.7% of participants with hardly/not controlled disease stated that their CISD had a moderate and severe or very severe impact on their overall lives (p < 0.001), respectively. A severe or very severe impact of their CISD on their overall life was most commonly reported by participants with HS.
Conclusion: Medical care for CISDs, even in an environment with high socio-economic standard and high health-awareness, still appears to be limited and has a negative impact on individuals and society.
Chronic inflammatory skin diseases (CISDs) are among the most common diseases in the Western world. A recent survey conducted in a representative sample of the general population in 27 European countries estimated that ~25% of people aged ≥18 years suffered from CISDs during the previous 12 months (1). Atopic dermatitis (AD; 5.5%), psoriasis (PSO; 3.9%), chronic urticaria (1.0%), and hidradenitis suppurativa (HS; 0.6%) were among the most prevalent CISDs. The pathomechanisms and, consequently, the clinical pictures greatly vary among the CISDs (2). Impairment of the epidermal barrier function, Th2 cells (dominating the acute disease stage), and Th2/Th1/Th22 cells (dominating the chronic disease stage) play major roles in the pathogenesis of AD (3–5). Skin lesions of patients with AD are highly itchy and vary with disease stage and patient’s age. In acute stage, they are characterized by not clearly demarcated erythema with vesicles, excoriations, and serous exudate (4). In chronic stage, skin lesions are less erythematous, dry (xerosis), and thickened (lichenified) (4). The face and trunk are preferentially involved in small children while the flexor sides of the extremities are preferentially concerned in adults (4). In PSO, a Th17/Th22-cell mediated CISD, skin alterations in most patients manifest as sharply demarcated, erythematous plaques with silvery scales, preferentially located at the extensor sides of the extremities (6–9). Chronic urticaria manifests itself as itchy wheals and angioedema that occur spontaneously or as the result of trigger factors and that are primarily mediated by mast cell mediators (10–12). Skin alterations in HS develop in intertriginous sites in the form of deep-seated, painful nodules, abscesses, and pus-draining tunnels (13). The complex pathomechanisms in these patients include roles of neutrophils, Th1 and Th17 cells, and B cells (14–16). Not surprisingly then that due to the external visibility of the skin alterations and the association with itching, pain, scaling, exudation and/or infections, patients with those CISDs are not only physically but also psychologically burdened. If left untreated, CISDs lead to profound reduction in the quality of life (17, 18). Furthermore, patients with CISDs frequently suffer from systemic comorbidities (13, 19–21) that are associated with shortened life expectancy (22, 23). Owing to a substantial decrease in work ability and productivity of those affected, CISDs also have a significant negative socioeconomic dimension. In fact, HS alone leads to a 13 billion loss in gross value added in Germany (24). On the other hand, with the approval of biologics and high-efficacy oral compounds targeting pathogenetic immune mediators, the possibility of successfully treating many of these diseases has increased significantly over the past two decades (7, 25–27). Moreover, many European countries spend over a tenth of their gross domestic product on healthcare. This raises the question of how satisfied CSID patients are with the current care for their disease. In this study we performed a survey-based investigation to what extent CISD patients consider their skin disease to be controlled. The survey was carried out in a health-aware environment, namely among the employees of a pharmaceutical company, which itself has a high level of expertise in the field of CISDs. With Germany and Switzerland, the survey was conducted at two company locations with a high standard of medical care and with comparatively high levels of income.
This was a prospective anonymous survey conducted among the employees of Novartis Pharma at the company locations Nurnberg (Germany), Rotkreuz (Switzerland), and Basel (Switzerland). Software-based questionnaires were used to collect data on employees’ age and sex, socio-economic and professional aspects, whether they were suffering from AD, PSO, chronic spontaneous urticaria (CSU), lichen (ruber) planus (LP), and/or HS as well as details regarding the disease course. Patients were also asked to state if their disease was diagnosed by a physician. Furthermore, patients were asked whether they were currently under medical care for their skin disease. The extent to which the patients consider their CISD to be controlled/treated and the impact of their CISD on their overall life were asked based on five answer categories (not at all/hardly/ moderately/well/completely and very severe/severe/moderate/little/not at all, respectively) (self-designed questionnaire, can be provided by corresponding authors upon request). For the assessment of the impairment of work ability and productivity and the general daily activity during the last 7 days due to respective CISD, the work productivity and activity impairment (WPAI) questionnaire was used. The WPAI is a validated six-question questionnaire (24). Scores range between 0 and 100%, with higher scores indicating greater impairment. The survey lasted from June 2022 to July 2022 (Germany) and July 2022 to August 2022 (Switzerland).
Statistical calculations were made in SPSS Statistics software (IBM), version 27. Kruskall-Wallis test, Pearson Χ2 test, and Spearman correlation test were applied as indicated. Global alpha was set at 5%.
To investigate CISD patients’ perception of the control of their skin disease and to identify aspects associated with poor control, the employees of a pharmaceutical company in the locations in Germany and Switzerland were asked to provide anonymous information via a software-based questionnaire. The questionnaire was sent out via email and newsletter to about 15.000 employees – concrete numbers on read-through rate are unknown. Overall, 905 employees answered the questionnaire. Of these, 222 participants (24.5%) reported having at least one of the following CISDs: AD, PSO, CSU, HS, and LP. Among them, 23 stated having two CISDs, and two and one stated having three and four CISDs, respectively, resulting in a total of 252 cases. The prevalence of the investigated CISDs were: AD: 12.0%, PSO: 7.6%, CSU: 3.8%, HS: 1.7%, and LP: 0.5%. Of the 222 people having stated to suffer from investigated CISDs, 195 delivered a detailed report about at least one skin disease (211 cases). Of these 211 cases, 85.3% were diagnosed by a physician according to patient statements. The characteristics of these 195 participants are presented in Supplementary Table S1.
Participants suffering from CISD were asked about how well they consider their skin disease controlled. Figure 1A shows the proportions of participants considering their CISD as being: (i) completely or well controlled, (ii) moderately controlled, or (iii) hardly or not at all controlled. Only 39.6% described their skin disease as being completely or well controlled. The proportion of participants considering their CISD as being hardly or not at all controlled (in the following together referred to as poorly controlled) was 28.7%. When looking at the specific types of CISD, about one third of participants with PSO (35.9%) or HS (33.3%) reported poor disease control (Figure 1B). Participants suffering from CSU or AD showed the largest proportion of those reporting completely or well controlled CISD (42.9 and 41.6%, respectively), while this proportion was particularly small in case of HS (16.7%).
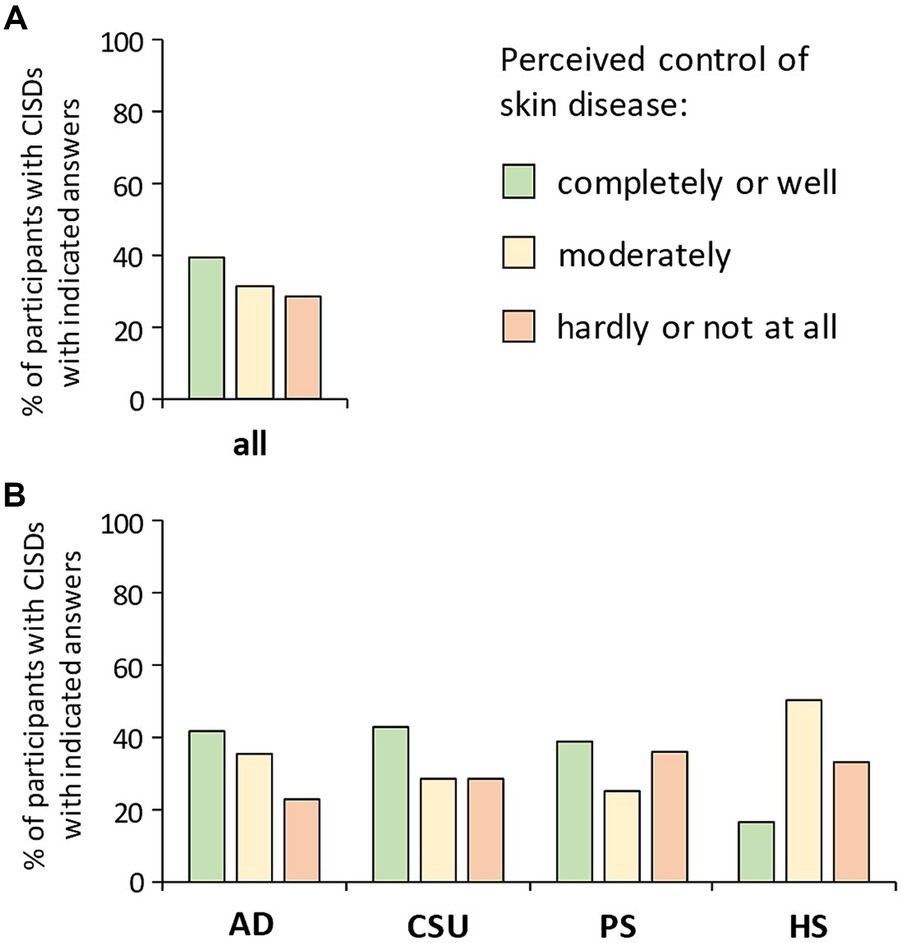
Figure 1. Patients’ perception of the control of their skin disease. Participants suffering from CISD were asked about how well they find their skin disease controlled. (A) The bar chart presents percentages of total cases (n = 202) with indicated answers. (B) The bar chart presents percentages of cases with indicated answers, broken down into the diseases most common among the participants [AD (n = 101), CSU (n = 28), PSO (n = 64), HS (n = 6)].
We then wondered why such a substantial proportion of participants with CISDs considered their disease as poorly controlled and inquired whether they were currently under medical care for their skin disease. As shown in Figure 2A, only 35.5% stated that they were. Interestingly, this proportion did not vary between the three disease-control categories (p = 0.549). When asking about the CISD course in the last 12 months, we found a negative association between perceived disease control and the number of acute CISD phases (p = 0.042) (Figure 2B), which was confirmed by correlation testing (rs = 0.16, p = 0.031). Surprisingly, there was no association between perceived disease control and the total number of symptomatic days per year (p = 0.856) (Figure 2C).
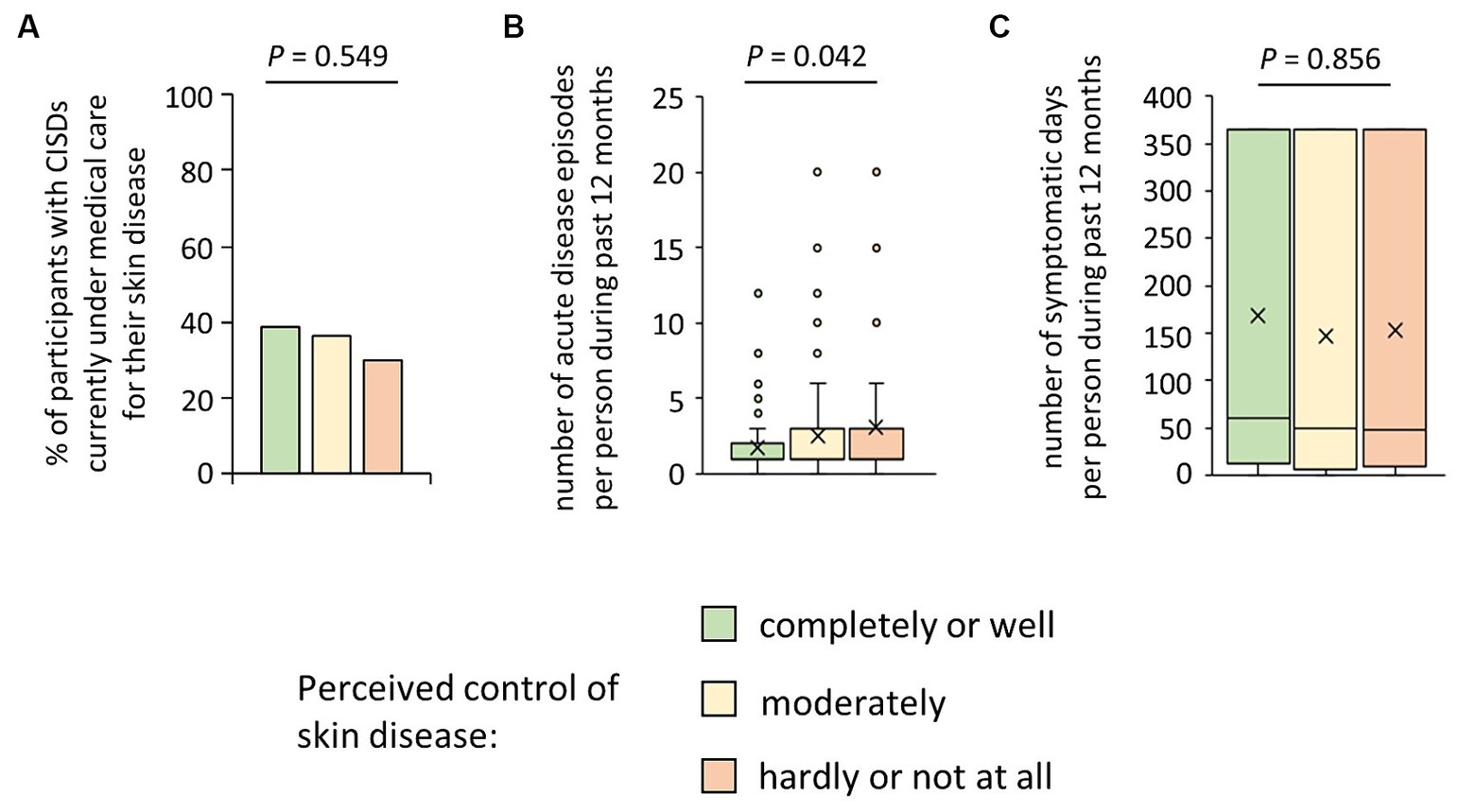
Figure 2. Possible reasons for patients’ perceived poor control of their skin disease. (A) Participants suffering from CISD were asked whether they were currently under medical care for their skin disease. The bar chart presents percentages of cases (n = 200) who answered the question with yes, broken down into their estimate of control of their skin disease. The p-value (Pearson Χ2 test) is presented. (B) Participants suffering from CISD were asked how many acute disease episodes they had in the last 12 months. Answers given by cases (n = 201), broken down into their estimate of control of their skin disease, are presented as box-and-whisker plots, with the maximum length of box whiskers corresponding to the most extreme values in the 1.5 fold interquartile range, outliers displayed as dots, and the “x” representing the mean of the data. The p-value (Kruskal Wallis test) is presented. (C) Participants suffering from CISD were asked how many acute disease episodes they had in the last 12 months and the average duration of these episodes, to calculate the total number of symptomatic days per year. Results (n = 201), broken down into their estimate of control of their skin disease, are presented as box-and-whisker plots, with the maximum length of box whiskers corresponding to the most extreme values in the 1.5 fold interquartile range, outliers displayed as dots, and the “x” representing the mean of the data. The p-value (Kruskal Wallis test) is presented.
Thirdly, we were interested in the effects of observed poor disease control on the general daily activity and the work productivity and ability of those affected. There was a clear positive association of poor disease control and the percent general daily activity impairment due to skin disease (mean ± SD; completely or well controlled disease: 16.2 ± 15.9% of general daily activity impairment; moderately controlled disease: 23.1 ± 23.9% of general daily activity impairment; hardly or not at all controlled disease: 28.3 ± 24.5% of general daily activity impairment; p = 0.005) (Figure 3A). Moreover, the percent loss in work productivity during working hours due to skin disease (presenteeism) increased dependent on the poorness of disease control (mean ± SD; completely or well controlled disease: 12.5 ± 12.1%; moderately controlled disease: 17.5 ± 17.0%; hardly or not at all controlled disease: 19.0 ± 15.9%; p = 0.005) (Figure 3B). Correlation analyses further confirmed the statistical link with both the general activity impairment (rs = 0.24, p = 0.001) and presenteeism (rs = 0.20, p = 0.001).
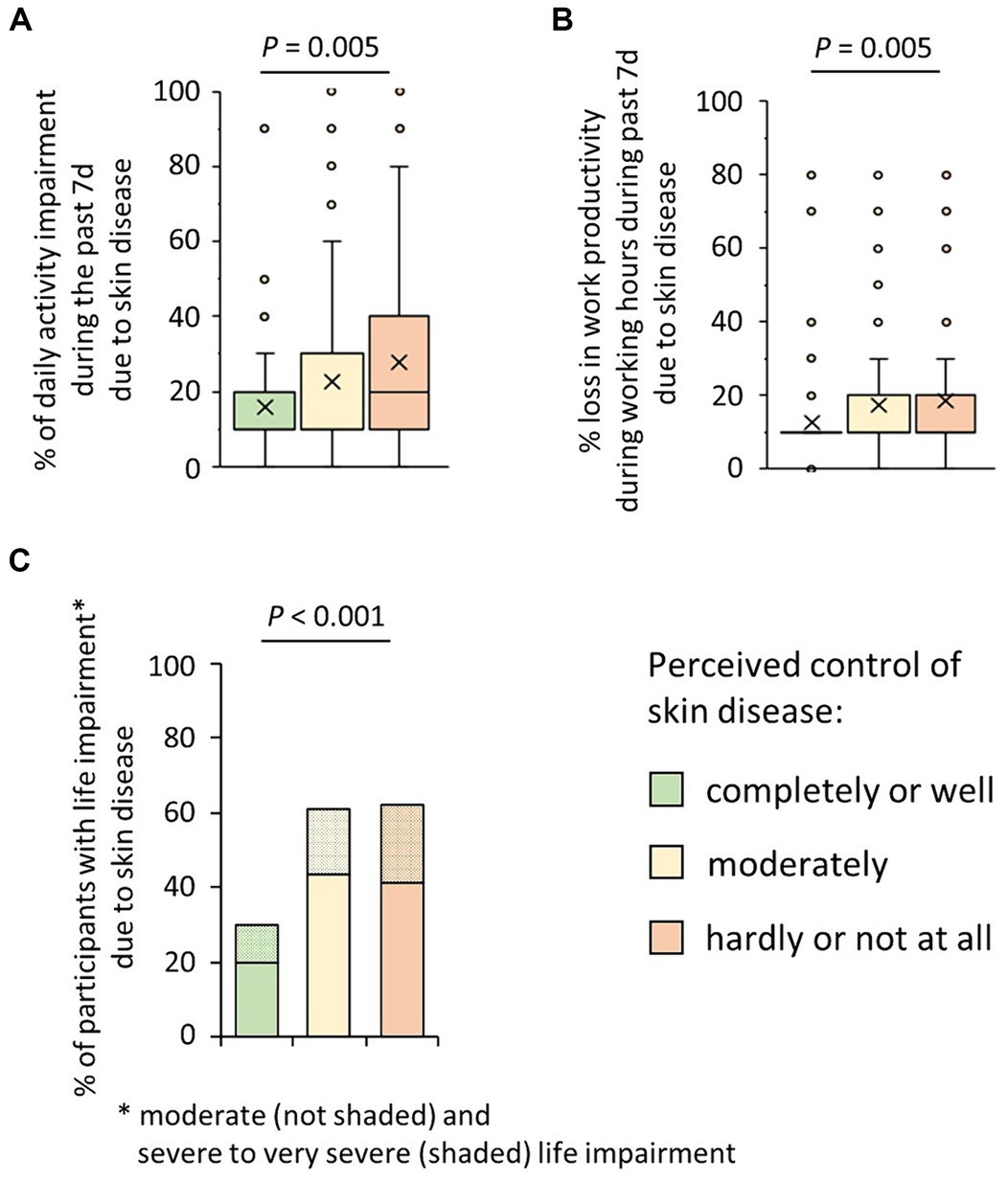
Figure 3. Possible consequences of patients’ perceived poor control of their skin disease. (A) Participants suffering from CISD were asked about their estimate of impairment of general daily activities (outside their professional activity) due to their skin disease in the past seven days (WPAI, on a 0–10 scale, corresponding to 0–100%). Answers given by the cases (n = 202), broken down into their estimate of control of their skin disease, are presented as box-and-whisker plots, with the maximum length of box whiskers corresponding to the most extreme values in the 1.5 fold interquartile range, outliers displayed as dots, and the “x” representing the mean of the data. The p-value (Kruskal Wallis test) is presented. (B) Participants suffering from CISD were asked about their estimate of impairment while working due to their skin disease in the past seven days (WPAI, presenteeism; on a 0–10 scale, corresponding to 0–100%). Answers given by the cases (n = 202), broken down into their estimate of control of their skin disease, are presented as box-and-whisker plots, with the maximum length of box whiskers corresponding to the most extreme values in the 1.5 fold interquartile range, outliers displayed as dots, and the “x” representing the mean of the data. The p-value (Kruskal Wallis test) is presented. (C) Participants suffering from CISD were asked how much their skin disease does impair their life. The bar chart presents percentages of cases (n = 202) who stated a moderate (not shaded) or a severe or very severe (shaded) life impairment, broken down into their estimate of control of their skin disease. p-value (Pearson Χ2 test) is presented.
Finally, when asking the participants about the impact of the CISDs on their overall life, 41.4 and 20.7% of participants with poor disease control stated a moderate or severe to very severe impact (P = <0.001) (Figure 3C). Correlation analysis also demonstrated the association between lower of disease control and the negative impact of disease on the overall lives of those affected (rs = 0.31, p < 0.001). Considering the type of skin diseases (CSU, AD, PSO, HS), participants with HS most often (33.3%) answered that the CISD had a severe or very severe impact on their lives (data not shown).
Intensive work by basic scientists, clinical researchers, and pharmaceutical company employees over the past two decades has led to the approval of highly effective drugs for certain CISDs (e.g., PSO) (7). To our surprise, in the investigated health-aware environment, almost 30% of the participants with CISDs rated their skin disease as hardly or non-controlled. The proportion of participants feeling this way was particularly high among participants with PSO, for whom highly effective drugs have been approved (7), and patients with HS, who were only moderately treatable with drugs at the time of the survey (13). We found significant general daily activity impairment, relevant presenteeism, and negative impact on the life as a whole as possible consequences of the inadequate disease control. Poor skin disease control therefore seems to have a significant impact on both those affected and the society. We have not found any study comparable to our work (internal company survey). Instead, numerous surveys conducted in medical institutions have been published, which, however, may not fully represent the total patient population, since only patients in medical care are represented. Relevant data come from some general population-based studies conducted online. Using an online questionnaire, a study run in 2016 in France, Germany, Italy, Spain, and UK documented 24.7% presenteeism for AD patients (28). A Japanese study from 2017 showed dissatisfaction with their health (25.5, 28.7, and 31.7%) and high presenteeism (22.4, 15.4, and 21.2%) among patients with AD, PSO, and CU, respectively (29). The value of 16% presenteeism determined in our study for all participants with skin diseases is slightly lower than the presenteeism in the studies described above. This could be related to the location of the survey (in our case in a company). According to an online study from Ireland published in 2021, 52% of AD patients considered their disease as being poorly controlled (30).
In contrast to the consequences, we found only vague hints for the reasons for the perceived poor disease control. There was an association with the number of acute disease phases, but not with the number of symptomatic days per year; thus, the disease course (phasic) certainly plays a role. Interestingly, no association with lacking current medical care was found, which was generally high.
We speculate that restrictions in medical care due to racial and ethnic aspects, which are relevant in other parts of the world (31), are not likely to have a relevant impact in our study. We rather suspect that the major cause for insufficient current medical care is a disappointment with medical care due to either delay in diagnosis [e.g., HS (32)], a limited efficacy of approved drugs [e.g., HS (13)], or the doctors’ hesitation to prescribe expensive, albeit highly effective drugs (33, 34). Regarding prescriptions, the most prevalent barriers to prescription of biologics reported by physicians in Germany in 2017 were the high cost, the low reimbursement and the fear of recourse (35). Unfortunately, this situation seems not to have relevantly changed (36). For example, only 3.7% of PSO patients were treated with biologics in Germany in 2020 (33). Data collected in ten western countries in 2019 showed that only 2.3% of patients with AD received biologics (37) and that in 75% of patients, treatment expectations were met only partially or not at all (37). With HS, the situation may be even worse. HS is a disease that, due to the specific pathomechanisms, leads to irreversible destruction of the normal skin architecture (38) and the classic treatment of HS does not improve patient’s quality of life in the long term (3). Patients consider a sustained therapeutic success as most important (39, 40) and treatment with biologics was associated with higher patients’ satisfaction (41). Nevertheless, data from Denmark indicated a mean time from the first systemic therapy to the start of a biological therapy of 15.3 years (42).
There are several limitations in our study. A relatively small proportion of employees answered the questions asked, which could create a potential bias. Moreover, the study design did not include assessment of skin disease severity and therapy recording by a dermatologist.
This study, unique in its focus on an environment with health-awareness and high socio-economic standard, highlights the inadequate disease control in individuals with CISDs, including psoriasis and HS. The correlation of the subjective perception of disease control by those affected with the disease flare frequency points to an actual existing issue, with limited efficacy of approved drugs and/or the physicians’ hesitation to prescribe highly effective, albeit costly, medications as potential contributors. The study further points out the substantial impact of inadequate CISD control on individuals and society.
Further data will be made available upon request according to the legal possibilities by the corresponding authors.
This survey was conducted in a blinded and voluntary way as patient-oriented program. All participants have agreed on the data collection and on possible publication of anonymous and aggregated data. The evaluations shown are secondary data evaluations of the original data sets. An ethics approval was not required for this study (Ethics commission of the Bavarian State Medical Association/Bayerischen Landesärztekammer, Germany).
KWo: Visualization, Writing – original draft, Writing – review & editing. MS: Writing – review & editing, Conceptualization, Investigation. J-TM: Writing – review & editing, Conceptualization. FW: Writing – review & editing, Investigation, Project administration. KWa: Writing – review & editing, Investigation, Project administration. WF: Visualization, Writing – review & editing, Data curation, Formal analysis, Methodology. PN: Writing – review & editing, Conceptualization, Funding acquisition. RS: Writing – review & editing, Conceptualization, Formal analysis.
The author(s) declare financial support was received for the publication of this article, as the publication fee was covered by Novartis Pharma GmbH. The authors did not receive financial support for the research and authorship. The survey described in this study was ordered from BMQ Evaluation & Consulting GbR Company and financed by Novartis Pharma. Novartis Pharma had a role in the initiation and the design of this study, but not its execution and the analysis of data. As coauthors, Novartis employees participated in the interpretation of data, the decision to submit the study results for publication, and the manuscript review.
KWo has received research grants, travel grants, consulting honoraria or lecturer’s honoraria from AbbVie, Biogen Idec, Celgene/Amgen, Celgen/Bristol Myers Squibb, Charité Research Organisation GmbH, European HS foundation, Flexopharm, Janssen-Cilag, Novartis Pharma, Rheinische Friedrich-Wilhelms-Universität Bonn, Sanofi-Aventis, TFS Trial Form Support, UCB Pharma, and University hospital Magdeburg. MS and PN are employees of Novartis Pharma GmbH, Germany and may own Novartis Pharma stocks or options. J-TM has served as advisor and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, BMS, Celgene, Eli Lilly, LEO Pharma, Incyte, Janssen-Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, UCB. FW and KWa are employees of Novartis Pharma AG and may own Novartis Pharma stocks or options. WF is the owner of BMQ Evaluation & Consulting GbR, contracted for the survey study implementation and data analysis, which was funded by Novartis Pharma. RS has received research grants, scientific awards, or honoraria for participation in advisory boards, clinical trials or as speaker for one or more of the following: AbbVie Inc., AbbVie Deutschland GmbH & Co. KG, Almirall Hermal GmbH, Amgen GmbH, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Bruno Bloch Stiftung, Celgene/Amgen, Celgene/Bristol Myers Squibb CSL Behring, Charité Research Organisation GmbH, CSL Behring GmbH, Flexopharm GmbH & Co. KG, ICON plc, ICON plc, IQVIA RDS GmbH, Incyte Corporation, Janssen-Cilag GmbH, MoonLake Immunotherapeutics AG, Novartis Pharma GmbH, Parexel International GmbH, Rheinischen Friedrich-Wilhelms-Universität Bonn, Sanofi–Aventis Deutschland GmbH, TFS GmbH, UCB Biopharma SPRL, Universitätsmedizin Greifswald, Wundnetz Berlin-Brandenburg e. V.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1384055/full#supplementary-material
1. Richard, MA, Paul, C, Nijsten, T, Gisondi, P, Salavastru, C, Taieb, C, et al. Prevalence of Most common skin diseases in Europe: a population-based study. J Eur Acad Dermatol Venereol. (2022) 36:1088–96. doi: 10.1111/jdv.18050
2. Sabat, R, Wolk, K, Loyal, L, Docke, WD, and Ghoreschi, K. T cell pathology in skin inflammation. Semin Immunopathol. (2019) 41:359–77. doi: 10.1007/s00281-019-00742-7
3. Zhang, B, Roesner, LM, Traidl, S, Koeken, V, Xu, CJ, Werfel, T, et al. Single-cell profiles reveal distinctive immune response in atopic dermatitis in contrast to psoriasis. Allergy. (2023) 78:439–53. doi: 10.1111/all.15486
4. Langan, SM, Irvine, AD, and Weidinger, S. Atopic dermatitis. Lancet. (2020) 396:345–60. doi: 10.1016/S0140-6736(20)31286-1
5. Rojahn, TB, Vorstandlechner, V, Krausgruber, T, Bauer, WM, Alkon, N, Bangert, C, et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J Allergy Clin Immunol. (2020) 146:1056–69. doi: 10.1016/j.jaci.2020.03.041
6. Wolk, K, Kunz, S, Witte, E, Friedrich, M, Asadullah, K, and Sabat, R. Il-22 increases the innate immunity of tissues. Immunity. (2004) 21:241–54. doi: 10.1016/j.immuni.2004.07.007
7. Ghoreschi, K, Balato, A, Enerback, C, and Sabat, R. Therapeutics targeting the Il-23 and Il-17 pathway in psoriasis. Lancet. (2021) 397:754–66. doi: 10.1016/S0140-6736(21)00184-7
8. Maeda, K, Tanioka, T, Takahashi, R, Watanabe, H, Sueki, H, Takimoto, M, et al. Mcam+Cd161-Th17 subset expressing Cd83 enhances Tc17 response in psoriasis. J Immunol. (2023) 210:1867–81. doi: 10.4049/jimmunol.2200530
9. Cole, S, Manghera, A, Burns, L, Barrett, J, Yager, N, Rhys, H, et al. Differential regulation of Il-17a and Il-17f via Stat5 contributes to psoriatic disease. J Allergy Clin Immunol. (2023) 152:783–98. doi: 10.1016/j.jaci.2023.03.035
10. Kolkhir, P, Gimenez-Arnau, AM, Kulthanan, K, Peter, J, Metz, M, and Maurer, M. Urticaria. Nat Rev Dis Primers. (2022) 8:61. doi: 10.1038/s41572-022-00389-z
11. Cildir, G, Toubia, J, Yip, KH, Zhou, M, Pant, H, Hissaria, P, et al. Genome-wide analyses of chromatin state in human mast cells reveal molecular drivers and mediators of allergic and inflammatory diseases. Immunity. (2019) 51:949–65 e6. doi: 10.1016/j.immuni.2019.09.021
12. Rutter, KJ, Peake, M, Hawkshaw, NJ, Scholey, R, Bulfone-Paus, S, Friedmann, PS, et al. Solar Urticaria involves rapid mast cell Stat3 activation and neutrophil recruitment, with Fcepsilonri as an upstream regulator. J Allergy Clin Immunol. (In press) (2024). doi: 10.1016/j.jaci.2023.12.021
13. Sabat, R, Jemec, GBE, Matusiak, L, Kimball, AB, Prens, E, and Wolk, K. Hidradenitis Suppurativa. Nat Rev Dis Primers. (2020) 6:18. doi: 10.1038/s41572-020-0149-1
14. Wolk, K, Warszawska, K, Hoeflich, C, Witte, E, Schneider-Burrus, S, Witte, K, et al. Deficiency of Il-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne Inversa. J Immunol. (2011) 186:1228–39. doi: 10.4049/jimmunol.0903907
15. Wolk, K, Brembach, TC, Šimaitė, D, Bartnik, E, Cucinotta, S, Pokrywka, A, et al. Activity and components of the granulocyte Colony-stimulating factor pathway in hidradenitis Suppurativa. Br J Dermatol. (2021) 185:164–76. doi: 10.1111/bjd.19795
16. Sabat, R, Šimaitė, D, Gudjonsson, JE, Brembach, TC, Witte, K, Krause, T, et al. Neutrophilic granulocyte-derived B-cell activating factor supports B cells in skin lesions in hidradenitis Suppurativa. J Allergy Clin Immunol. (2023) 151:1015–26. doi: 10.1016/j.jaci.2022.10.034
17. Ujiie, H, Rosmarin, D, Schön, MP, Ständer, S, Boch, K, Metz, M, et al. Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front Med (Lausanne). (2022) 9:875492. doi: 10.3389/fmed.2022.875492
18. Schneider-Burrus, S, Tsaousi, A, Barbus, S, Huss-Marp, J, Witte, K, Wolk, K, et al. Features associated with quality of life impairment in hidradenitis Suppurativa patients. Front Med (Lausanne). (2021) 8:676241. doi: 10.3389/fmed.2021.676241
19. Thyssen, JP, Halling, AS, Schmid-Grendelmeier, P, Guttman-Yassky, E, and Silverberg, JI. Comorbidities of atopic dermatitis-what does the evidence say? J Allergy Clin Immunol. (2023) 151:1155–62. doi: 10.1016/j.jaci.2022.12.002
20. Enos, CW, Ramos, VL, McLean, RR, Lin, TC, Foster, N, Dube, B, et al. Cardiometabolic multimorbidity is common among patients with psoriasis and is associated with poorer outcomes compared to those without comorbidity. J Dermatolog Treat. (2022) 33:2975–82. doi: 10.1080/09546634.2022.2089329
21. Papapostolou, N, Xepapadaki, P, Katoulis, A, and Makris, M. Comorbidities of chronic Urticaria: a glimpse into a complex relationship. Front Allergy. (2022) 3:1008145. doi: 10.3389/falgy.2022.1008145
22. Woo, YR, Cho, M, Do Han, K, Cho, SH, and Lee, JH. Atopic dermatitis and the risk of myocardial infarction and all-cause mortality: a Nationwide population-based cohort study. Allergy Asthma Immunol Res. (2023) 15:636–46. doi: 10.4168/aair.2023.15.5.636
23. Tiri, H, Jokelainen, J, Timonen, M, Tasanen, K, and Huilaja, L. Substantially reduced life expectancy in patients with hidradenitis Suppurativa: a Finnish Nationwide registry study. Br J Dermatol. (2019) 180:1543–4. doi: 10.1111/bjd.17578
24. Schneider-Burrus, S, Kalus, S, Fritz, B, Wolk, K, Gomis-Kleindienst, S, and Sabat, R. The impact of hidradenitis Suppurativa on professional life. Br J Dermatol. (2023) 188:122–30. doi: 10.1093/bjd/ljac027
25. Pandya, A, Adah, E, Jones, B, and Chevalier, R. The evolving landscape of immunotherapy for the treatment of allergic conditions. Clin Transl Sci. (2023) 16:1294–308. doi: 10.1111/cts.13546
26. Sabat, R, Gudjonsson, JE, Brembilla, NC, van Straalen, KR, and Wolk, K. Biology of Interleukin-17 and novel therapies for hidradenitis Suppurativa. J Interf Cytokine Res. (2023) 43:544–56. doi: 10.1089/jir.2023.0105
27. Casale, TB . Novel biologics for treatment of chronic spontaneous Urticaria. J Allergy Clin Immunol. (2022) 150:1256–9. doi: 10.1016/j.jaci.2022.06.027
28. Eckert, L, Gupta, S, Gadkari, A, Mahajan, P, and Gelfand, JM. Burden of illness in adults with atopic dermatitis: analysis of National Health and wellness survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol. (2019) 81:187–95. doi: 10.1016/j.jaad.2019.03.037
29. Itakura, A, Tani, Y, Kaneko, N, and Hide, M. Impact of chronic Urticaria on quality of life and work in Japan: results of a real-world study. J Dermatol. (2018) 45:963–70. doi: 10.1111/1346-8138.14502
30. Murray, G, O'Kane, M, Watson, R, and Tobin, AM. Psychosocial burden and out-of-pocket costs in patients with atopic dermatitis in Ireland. Clin Exp Dermatol. (2021) 46:157–61. doi: 10.1111/ced.14422
31. Nock, MR, Barbieri, JS, Krueger, LD, and Cohen, JM. Racial and ethnic differences in barriers to care among us adults with chronic inflammatory skin diseases: a cross-sectional study of the all of us research program. J Am Acad Dermatol. (2023) 88:568–76. doi: 10.1016/j.jaad.2022.09.054
32. Kokolakis, G, Wolk, K, Schneider-Burrus, S, Kalus, S, Barbus, S, Gomis-Kleindienst, S, et al. Delayed diagnosis of hidradenitis Suppurativa and its effect on patients and healthcare system. Dermatology. (2020) 236:421–30. doi: 10.1159/000508787
33. Heidbrede, T, Mevius, A, Kessel, S, Wilke, T, Maywald, U, and Thiem, A. Real-world systemic treatment of patients with psoriasis: a retrospective study based on German claims data. J Dtsch Dermatol Ges. (2023) 21:611–9. doi: 10.1111/ddg.15030_g
34. Schild, M, Weber, V, Thaçi, D, Kisser, A, Galetzka, W, Enders, D, et al. Treatment patterns and healthcare resource utilization among patients with atopic dermatitis: a retrospective cohort study using German health claims data. Dermatol Ther (Heidelb). (2022) 12:1925–45. doi: 10.1007/s13555-022-00773-3
35. Schielein, MC, Tizek, L, Rotter, M, Konstantinow, A, Biedermann, T, and Zink, A. Guideline-compliant prescription of biologicals and possible barriers in dermatological practices in Bavaria. J Eur Acad Dermatol Venereol. (2018) 32:978–84. doi: 10.1111/jdv.14811
36. Weiss, D, Nordhorn, I, Tizek, L, Werfel, T, Zink, A, Biedermann, T, et al. Prescription behaviour and barriers to prescription of biologicals for treatment of chronic inflammatory skin diseases in dermatological practice in two German Federal States. Acta Derm Venereol. (2021) 101:adv00560. doi: 10.2340/00015555-3901
37. Augustin, M, Costanzo, A, Pink, A, Seneschal, J, Schuster, C, Mert, C, et al. Real-world treatment patterns and treatment benefits among adult patients with atopic dermatitis: results from the atopic dermatitis patient satisfaction and unmet need survey. Acta Derm Venereol. (2022) 102:adv00830. doi: 10.2340/actadv.v102.3932
38. Wolk, K, Join-Lambert, O, and Sabat, R. Aetiology and pathogenesis of hidradenitis Suppurativa. Br J Dermatol. (2020) 183:999–1010. doi: 10.1111/bjd.19556
39. Willems, D, Hiligsmann, M, van der Zee, HH, Sayed, CJ, and Evers, S. Identifying unmet care needs and important treatment attributes in the Management of Hidradenitis Suppurativa: a qualitative interview study. Patient. (2022) 15:207–18. doi: 10.1007/s40271-021-00539-7
40. Faverio, K, Peitsch, WK, Gorig, T, Schneider-Burrus, S, Benzel, F, Goebeler, M, et al. Patient preferences in hidradenitis Suppurativa (approach-Hs): a discrete choice experiment. J Dtsch Dermatol Ges. (2022) 20:1441–52. doi: 10.1111/ddg.14886_g
41. Midgette, B, Strunk, A, Akilov, O, Alavi, A, Ardon, C, Bechara, FG, et al. Factors associated with treatment satisfaction in patients with hidradenitis Suppurativa: results from the global voice project. Br J Dermatol. (2022) 187:927–35. doi: 10.1111/bjd.21798
Keywords: skin inflammation, atopic dermatitis, psoriasis, chronic spontaneous urticaria, hidradenitis suppurativa, quality of life, work productivity, biologics
Citation: Wolk K, Schielein M, Maul J-T, Widmayer F, Wanke K, Fischmann W, Nathan P and Sabat R (2024) Patient-reported assessment of medical care for chronic inflammatory skin diseases: an enterprise-based survey. Front. Med. 11:1384055. doi: 10.3389/fmed.2024.1384055
Received: 08 February 2024; Accepted: 15 March 2024;
Published: 18 April 2024.
Edited by:
Andreas Recke, University of Lübeck, GermanyReviewed by:
Florica Sandru, Elias University Emergency Hospital, RomaniaCopyright © 2024 Wolk, Schielein, Maul, Widmayer, Wanke, Fischmann, Nathan and Sabat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Nathan, cGV0cmEubmF0aGFuQG5vdmFydGlzLmNvbQ==; Robert Sabat, cm9iZXJ0LnNhYmF0QGNoYXJpdGUuZGU=
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.