
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 20 March 2024
Sec. Nuclear Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1382672
This article is part of the Research Topic Case Reports in PET Imaging 2023 View all 15 articles
Background: Non-gestational choriocarcinoma, also known as primary choriocarcinoma, is extremely rare in men, manifesting with specific signs such as breast feminization, testicular atrophy, and loss of libido. The presentation typically includes elevated serum β-hCG levels, widespread metastatic disease, and a rapid progression of the condition.
Case report: We present a rare case of a 41-year-old man diagnosed with choriocarcinoma, exhibiting a unique combination of multiple metastases, including lung, brain, bone, and retroperitoneal lymph node metastases, as confirmed by 18F-FDG PET/CT imaging. The patient was treated with aggressive chemotherapy and pembrolizumab, and the prognosis remained poor. The patient’s overall survival was a mere 5 months following diagnosis.
Conclusion: Non-gestational choriocarcinoma represents a rare entity in clinical practice and should be considered in young men presenting with gynaecomastia and elevated β-hCG levels alongside normal gonads. Thus, we advocate for a more comprehensive inquiry into medical history and a systematic examination. The 18F-FDG PET/CT examination not only visually delineates the lesion’s location and extent but also serves as a cornerstone for clinical tumor staging, providing valuable support for treatment monitoring and subsequent follow-up.
Choriocarcinoma, a rare trophoblastic tumor, exhibits high invasiveness and is characterized by the proliferation and interstitial transformation of abnormal chorionic trophoblast cells. It is distinguished by the absence of a chorionic structure, accompanied by hemorrhage and necrosis, and has the capability to secrete beta-human chorionic gonadotropin (β-hCG). The condition can be classified into two main categories: gestational choriocarcinoma and non-gestational choriocarcinoma (primary choriocarcinoma) (1–5). Gestational choriocarcinoma originates from the trophoblast of various gestational events, such as hydatidiform mole, spontaneous abortion, and normal pregnancy. On the other hand, non-gestational choriocarcinoma, also known as primary choriocarcinoma, is extremely rare in men, manifesting with specific signs such as breast feminization, testicular atrophy, and loss of libido (2, 6, 7). This type of choriocarcinoma can be further categorized into gonadal choriocarcinoma (testis) and extragonadal choriocarcinoma (such as mediastinum and retroperitoneum) based on its origin and primary site (8, 9). The presentation typically includes elevated serum β-hCG levels, widespread metastatic disease, and a rapid progression of the condition (10, 11).
In this report, we present a rare case of a 41-year-old man diagnosed with choriocarcinoma, exhibiting a unique combination of multiple metastases, including lung, brain, bone, and retroperitoneal lymph node metastases, as confirmed by 2-Deoxy-2-[fluorine-18]-fluoro-D-glucose (18F-FDG) positron emission tomography combined with computed tomography (PET/CT) imaging. Through an extensive literature search on the PubMed database, covering the period from 1996 to 2023 and utilizing keywords related to choriocarcinoma and CT, the search was carried out with and without the addition of filters, such as English language only, type of article, and subjects, excluding duplicate papers. We identified a total of 53 relevant publications. The summarized findings are presented in Table 1 (12–64). Previous studies have overwhelmingly reported cases of choriocarcinoma in pregnant females. The current case of primary choriocarcinoma occurring in a male is exceptionally rare.
A 41-year-old man presented with diminished appetite symptoms a year ago, but he did not undergo an examination. His condition deteriorated 2 months ago, marked by malaise, night sweats, and lower back pain, leading to a weight loss of 10.5 kg over 1 year. The patient was hospitalized for a physical examination, revealing bilateral breast development and a palpable mass measuring approximately 12 cm × 10 cm with poor mobility in the right abdomen. Laboratory tests indicated a white blood cell count of 12.00 × 109/L (normal range 3.5–9.5 × 109/L), D-dimer of 5.94 mg/L (normal range 0–0.5 mg/L), fibrinogen of 5.10 g/L (normal range 2–4 g/L), C-reactive protein of 99.90 mg/L (normal range 0–10 mg/L), and CA-reactive protein of 99.90 mg/L (normal range 0–10 mg/L). Additionally, CA-199 was elevated at 47.76 U/mL (normal range 0–37 U/mL), non-small cell lung cancer antigen 21–1 was elevated at 28.16 ng/mL (normal range 0–3 ng/mL), neuron-specific enolase was elevated at 67.66 ng/mL (normal range 0–16.3 ng/mL), prolactin was elevated at 35.62 ng/mL (normal range 2.1–17.7 ng/mL), estradiol was lower than normal at 26.33 pg./mL (normal range 35–245 pg./mL), testosterone was elevated at 179.32 ng/mL (normal range 1.75–7.81 ng/mL), beta-human chorionic gonadotropin (β-hCG) was markedly elevated at 937,268.00 mIU/mL (normal range 0–5 mIU/mL), and EBV antibody IgG was 4.06 (positively expressed).
The patient underwent an enhanced CT examination, revealing bilateral enlarged breasts (Figure 1A), empty scrotums bilaterally, with the testes not visualized (Figure 1B). A roundish mass with heterogeneous density was observed in the pelvis, post-enhancement, the mass exhibited heterogeneous mild enhancement (Figures 1C,D). Multiple enlarged lymph nodes were identified in the retroperitoneum, merging with one another, encircling the abdominal aorta and vessels, resulting in displacement of surrounding organs (Figure 1E). Additionally, multiple hypodense foci, characterized by ring-shaped and mild enhancement, were detected in the liver (Figure 1F). Furthermore, multiple rounded hyperdense nodules were observed in both lungs (Figure 1G). Following a thorough physical examination, laboratory tests, and CT imaging, the clinician initially suspected the presence of seminomas and metastases.
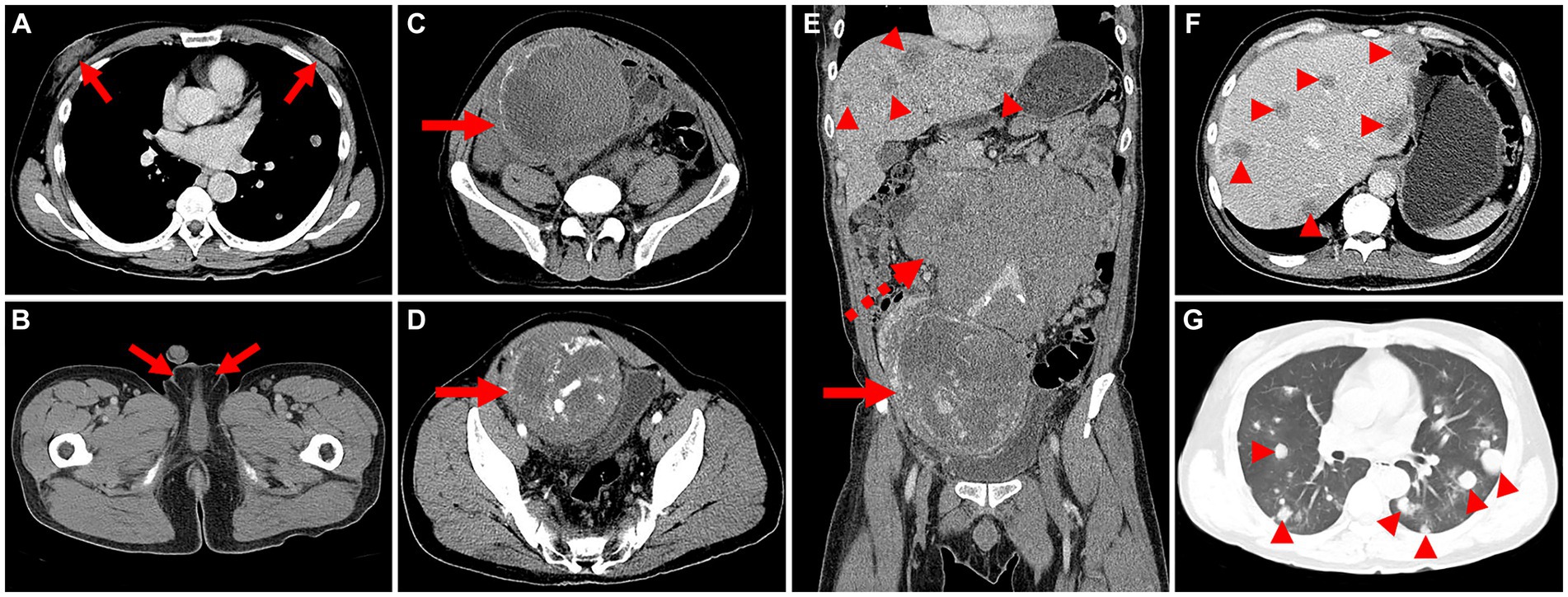
Figure 1. Computed tomography (CT) images of male choriocarcinoma with multiple systemic metastases (December 15, 2018). (A) The transverse image reveals bilaterally enlarged breasts (long arrows). (B) The transverse image displays empty scrotums bilaterally, with the testes not visualized (long arrows). (C) A roundish mass with heterogeneous density is observed in the pelvis, featuring eggshell-like calcifications at its margins (long arrows). (D) The transverse image in the arterial phase shows heterogeneous mild enhancement of the lesion, with evident thickening of blood-supplying arteries, areas of necrotic cystic degeneration in the interior, and tortuous, dilated veins in the surrounding area (long arrows). (E) The coronal image in the venous phase reveals multiple enlarged lymph nodes in the retroperitoneum, merging with one another (dashed arrows). (F) The transverse image in the venous phase exhibits multiple hypodense foci in the liver, characterized by ring-shaped and mild enhancement (arrowheads). (G) The transverse image depicts multiple rounded hyperdense nodules in both lungs (arrowheads).
For staging, the patient underwent further 18F-FDG PET/CT (Figures 2A,B). The 18F-FDG PET/CT scan, performed utilizing Siemens Biograph Truepoint 64 PET/CT machine, was conducted 60 min after the intravenous administration of 18F-FDG (6.1 mCi), revealing multiple soft tissue nodules in both lungs with significantly increased 18F-FDG uptake (SUVmax = 12.7, Figure 2C). The liver exhibited multiple slightly hypodense nodules and masses, characterized by markedly increased 18F-FDG uptake (SUVmax = 38.6). The larger liver mass measured approximately 3.6 cm × 2.1 cm (Figure 2D). In the retroperitoneum, numerous intermingled soft tissue masses with markedly increased 18F-FDG uptake (SUVmax = 13.1) were observed, with the largest dimension measuring about 16.2 cm × 16.3 cm. Additionally, patchy calcifications were evident within this area (Figure 2E). A soft tissue mass of irregular shape was identified in the right pelvis, displaying unevenly increased 18F-FDG uptake (SUVmax = 19.5). The maximum dimensions of this mass were approximately 12.0 cm × 15.2 cm, with areas of cystic necrosis and calcifications noted (Figure 2F). Markedly increased 18F-FDG uptake (SUVmax = 11.8) was detected at the thoracic 11 vertebral attachments (Figure 2G). Notably, no testes were visualized in the bilateral scrotum. The bilateral breast glands exhibited thickening with a slight increase in 18F-FDG uptake (SUVmax = 1.2, Figure 2H).
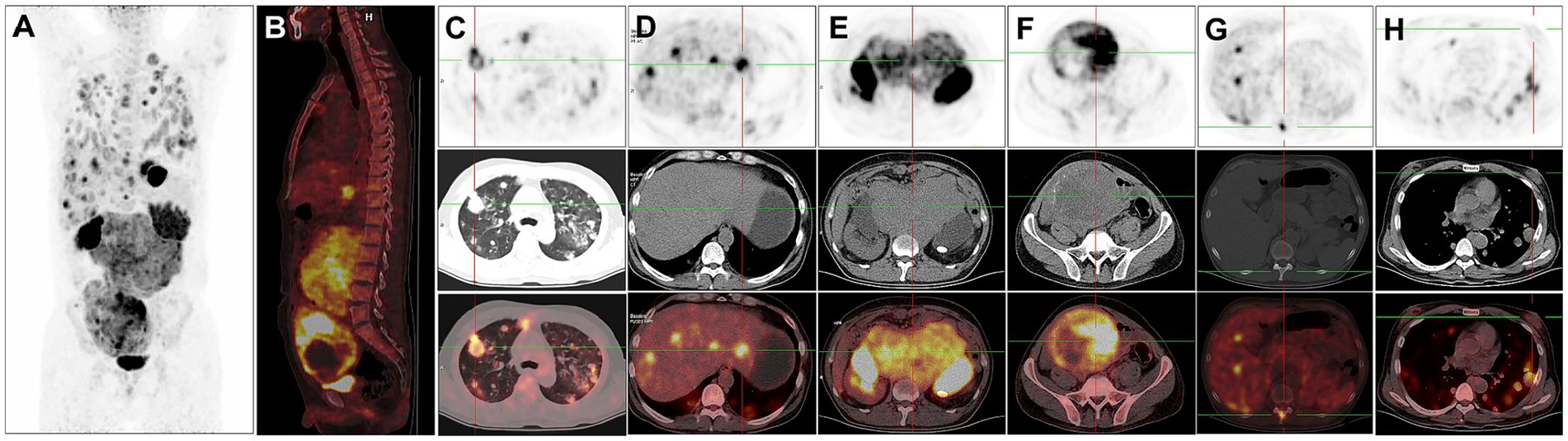
Figure 2. 18F-FDG PET/CT images of male choriocarcinoma with multiple systemic metastases (December 20, 2018). (A) The anteroposterior 3-dimensional maximum intensity projection (MIP) image demonstrates increased metabolic activity in the both lungs, abdominal cavity, and pelvic cavity. (B) The sagittal fusion image demonstrates increased metabolic activity in the liver, retroperitoneum and pelvis. (C) Transverse images reveal multiple soft tissue nodules in both lungs with significantly increased 18F-FDG uptake (SUVmax = 12.7). (D) Transverse images reveal the liver exhibiting multiple slightly hypodense nodules and masses, characterized by markedly increased 18F-FDG uptake (SUVmax = 38.6). (E) Transverse images depict, in the retroperitoneum, numerous intermingled soft tissue masses with markedly increased 18F-FDG uptake (SUVmax = 13.1). (F) Transverse images identify a soft tissue mass of irregular shape in the right pelvis, displaying unevenly increased 18F-FDG uptake (SUVmax = 19.5). The maximum dimensions of this mass are approximately 12.0 cm × 15.2 cm, with areas of cystic necrosis and calcifications noted. (G) Transverse images reveal markedly increased 18F-FDG uptake at the thoracic 11 vertebral attachments (SUVmax = 11.8). (H) Transverse images show the bilateral breast glands exhibiting thickening with a slight increase in 18F-FDG uptake (SUVmax = 1.2).
The patient underwent CT-guided puncture biopsy of a lesion on the liver, extensive hemorrhage was seen microscopically with typical features of choriocarcinoma. The tumor consists of mononuclear cytotrophoblasts and multinucleated syncytiotrophoblasts (Figures 3A,B). Immunohistochemistry showed positive expression of HPL (Figure 3C), β-HCG (Figure 3D), CD34 (vascular), CK7, CK19 and SALL-4. In addition, Ki-67 was observed to be positive in 90% of the tumor cells. The pathologic diagnosis confirmed choriocarcinoma metastasis.
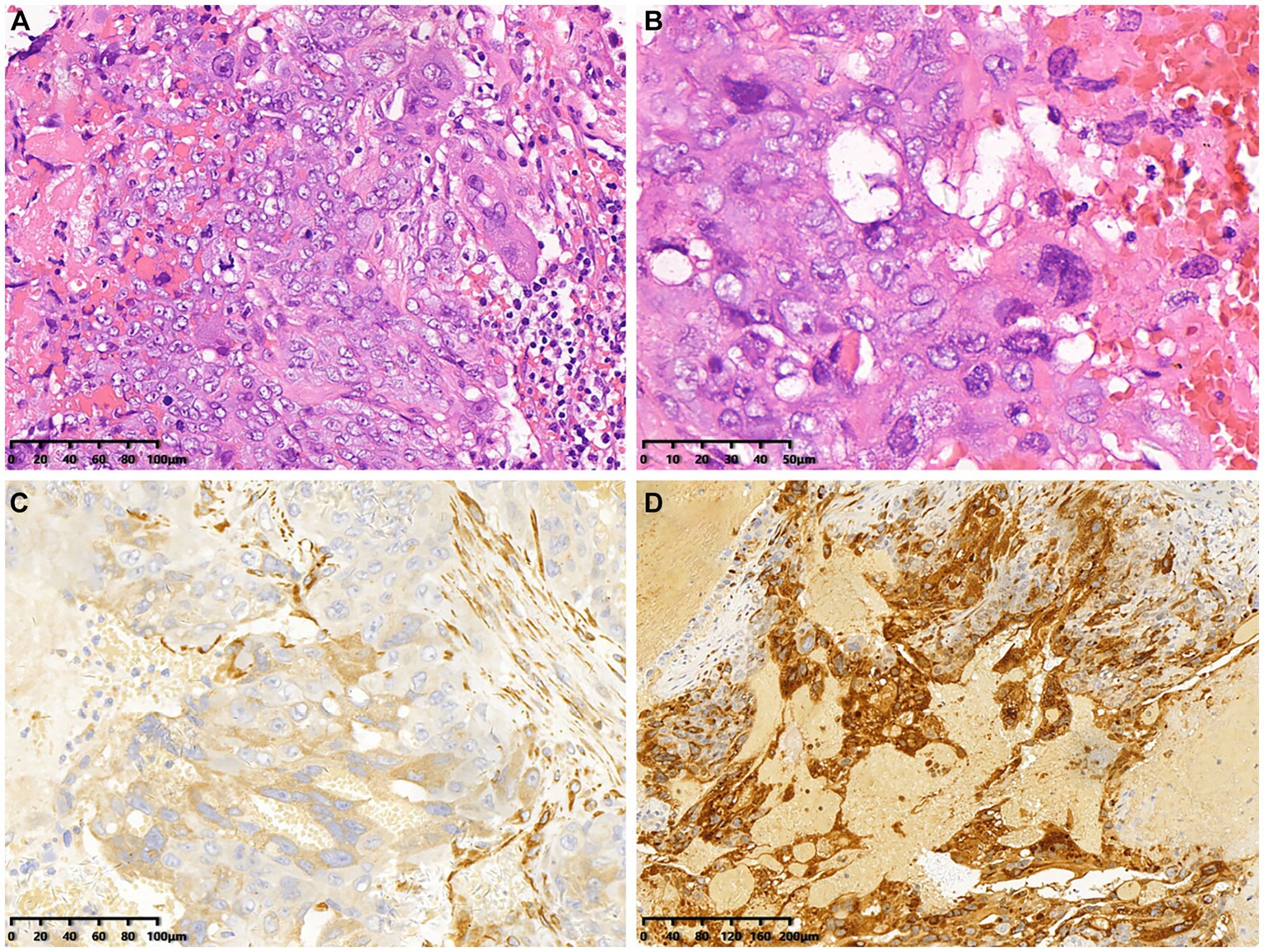
Figure 3. Histopathological and immunohistochemical images of choriocarcinoma (December 22, 2018). (A,B) Hematoxylin–eosin (HE) staining (magnification ×200 and 400) showed extensive hemorrhage and characteristic features indicative of choriocarcinoma. (C,D) Immunohistochemistry showed that the tumor cells were positive for HPL and β-HCG (magnification ×200).
After confirming the absence of contraindications to chemotherapy, the patient underwent treatment with an eight-cycle regimen consisting of etoposide (200 mg/d1–4), cisplatin (40 mg/d1–3), and bleomycin (30 U/d1,5). According to RECIST guidelines, subsequent CT examinations indicated stable disease (SD) in the patient’s status. Considering the patient’s chemotherapy tolerance, a collaborative decision was made to initiate a four-cycle course of anti-tumor therapy using the PD-1 antibody (pembrolizumab). Unfortunately, the patient experienced adverse effects of diarrhoea during this treatment phase. At the conclusion of the Avelumab treatment, follow-up CT scans revealed an increase in size of metastatic lesions in both lungs and liver, accompanied by the emergence of multiple metastases in the vertebral body (Figure 4A). Due to an inadequate response to pembrolizumab, the patient was subsequently treated with the regimen of “methotrexate (1.5 g) + actinomycin (0.4 mg).” Two weeks post-treatment initiation, the patient presented with dizziness and headache. A cranial MRI disclosed a rounded lesion in the left frontal lobe with high signal on T2WI (Figure 4B), measuring approximately 1.7 cm in diameter, and exhibiting inhomogeneous annular enhancement (Figure 4C). Nine days later, the patient’s secondary epileptic symptoms exacerbated, with a repeat MRI showing an enlarged frontal lobe lesion surpassing its previous size (Figure 4D). Additionally, new bilateral occipital lobe metastases were evident (Figure 4E). Despite medical recommendations, the patient declined further treatment and succumbed to the illness 2 weeks later. The patient’s overall survival was a mere 5 months following diagnosis.

Figure 4. CT images and magnetic resonance images after treatment for choriocarcinoma (May 5 & 14, 2020). (A) The sagittal bone window CT image reveals multiple metastases in the vertebral body. (B,C) The T2-weighted imaging (T2WI) and enhanced images indicate the development of brain metastases in the patient. (D,E) Subsequent scans after 9 days demonstrate an enlargement and increased size of the patient’s brain metastatic lesions compared to the previous images in both T2WI and enhanced sequences.
Germ cell tumors encompass various cell types, broadly categorized into seminoma and non-seminoma. Non-seminomatous germ cell tumors (NSGCTs) exhibit four subtypes: embryonal carcinoma, yolk sac tumor, teratoma, and choriocarcinoma, often displaying a combination of seminomatous and non-seminomatous components. Choriocarcinoma, the rarest subtype, lacks a clear etiology in males, potentially associated with various risk factors such as Klinefelter syndrome, cryptorchidism, exposure to radiation, and more. Cryptorchidism may be one of the significant factors contributing to testicular choriocarcinoma. It has been reported that the likelihood of choriocarcinoma in cryptorchidism patients is 20–40 times higher compared to individuals with normal testes (65, 66). The absence of the testicle in this particular case may be a significant factor contributing to the development of testicular choriocarcinoma.
Due to its pronounced invasiveness into blood vessels and tissues, extensive hemorrhage, necrosis, and lymphovascular invasion are common findings (67). Choriocarcinoma primarily metastasizes hematogenously, resulting in early and extensive dissemination to sites such as the lungs, liver, skin, retroperitoneal lymph nodes, gastrointestinal tract, and central nervous system (37, 68–71). In this case, the diagnosis of choriocarcinoma was established at an advanced stage, with multiple metastases already present throughout the body. A thorough medical history and examination are necessary to detect the primary lesion. Characteristics of the typical choriocarcinoma patient history include pregnancy-relatedness, elevated β-hCG levels, abnormal uterine bleeding, and vaginal bleeding. Patients with choriocarcinoma usually have a history of multiple pregnancies. Primary choriocarcinoma is extremely rare in men, manifesting with specific signs such as breast feminization, testicular atrophy, and loss of libido.
The determination of serum tumor markers, specifically serum β-hCG and AFP, proves beneficial in choriocarcinoma diagnosis as they are elevated in approximately 80% of cases. Our case report demonstrates markedly elevated serum β-hCG, produced by syncytiotrophoblasts, indicating its diagnostic relevance. Monitoring the serum concentration of β-hCG also aids in assessing treatment response. According to the International Cooperative Organization for Germ Cell Cancer, a β-hCG level exceeding 50,000 mIU/mL signifies a poor prognosis. Some patients may manifest hyperthyroidism or bilateral gynecomastia, attributed to markedly elevated serum β-hCG levels, often exceeding 50,000 mIU/mL (66, 72). In our case, elevated β-hCG levels stimulated supraphysiological testosterone secretion, subsequently aromatized to estradiol, resulting in gynecomastia. Following chemotherapy, a substantial decrease in β-hCG levels was observed, aligning with existing literature. In addition to the abnormal laboratory results, elevated white blood cell count may indicate an underlying infection or inflammation. Elevated levels of D-dimer, C-reactive protein, and fibrinogen may suggest a hypercoagulable state, which could be related to the patient’s malignancy. Elevated tumor markers and neuron-specific enolase levels may indicate an underlying malignancy. Elevated prolactin and testosterone levels and a low estradiol level may suggest an endocrine disorder, potentially related to the patient’s breast development and gynecomastia. Furthermore, positive EBV antibody IgG suggests a previous Epstein–Barr virus infection, which may have contributed to the patient’s condition.
The pathogenesis of extragonadal choriocarcinoma has been a subject of prolonged debate, currently revolving around three hypotheses (73). First, the tumor is postulated to arise from retained primordial germ cells that undergo abnormal migration during embryogenesis (74). Second, it is proposed that the lesion originates from the transformation of a nontrophoblastic neoplasm (75). The third hypothesis suggests that the tumor results from the metastasis of choriocarcinoma from the gonads, accompanied by the spontaneous regression of the primary choriocarcinoma in the gonads.
Histologically, choriocarcinoma is distinguished by a biphasic pattern featuring mononucleated cytotrophoblast cells (including intermediate trophoblast cells) alongside multinucleated syncytiotrophoblasts, with an absence of chorionic villi. The former cells organize lamellarily to form a villous structure, while the latter secrete β-hCG and human placental lactogen (HPL), observable at the tumor progression margin (76–78). Immunohistochemistry plays a crucial role in the differential diagnosis of relevant diseases (79). GATA binding protein 3 (GATA-3), Spalt-like transcription factor 4 (SALL-4), Cytokeratin (CK) AE1/AE3, and 3-beta-hydroxysteroid dehydrogenase type 1 (HSD3B1) have been identified as potential immunohistochemical markers for gestational choriocarcinoma (80, 81). A high Ki-67 proliferation index is noted in over 90% of choriocarcinoma cases (1). Immunohistochemistry holds significance in the differential diagnosis of relevant diseases.
The imaging characteristics of choriocarcinoma lack distinctive features that differentiate it from other types of germ cell tumors, making its initial diagnosis challenging. However, a comprehensive pre-operative workup, including clinical imaging assessments, remains crucial. There is a scarcity of studies reporting imaging features of retroperitoneal choriocarcinoma in men (82). On CT scans, the lesion typically exhibits a large central necrosis with common occurrences of bleeding and ring enhancement of solid components at the tumor margins. MRI findings include a mixed high signal on T1WI and T2WI associated with combined hemorrhage. Striped hypointensity on both T1WI and T2WI at the tumor margin suggests the possibility of old hemorrhage. In our presented case, CT revealed a cystic solid tumor with eggshell-like calcification at the margin, and an enhancement scan displayed circumferential enhancement, with patchy non-enhanced necrotic areas within the lesion. Due to the rapid growth of choriocarcinoma, histologically, the lesion lacks interstitial blood vessels, relying on the invasion of blood vessels for nutrition, leading to frequent internal necrosis. While CT served as the initial diagnostic modality in our case study, it proved insufficient in revealing metastatic details.
18F-FDG PET/CT emerges as a noninvasive tool, showcasing its exceptional utility in discerning the metabolic activity of tumors. It proves particularly advantageous in delineating the staging of choriocarcinoma, tracking relapse, and assessing therapeutic response (18, 24, 33, 47). PET/CT effectively identifies occult choriocarcinoma lesions that may elude detection through conventional imaging methods such as MRI or CT (30). In the case under scrutiny, 18F-FDG PET/CT provided comprehensive insights into the extent of systemic involvement of the tumor. Another pivotal role of 18F-FDG PET/CT in choriocarcinoma management lies in its ability to monitor treatment response. Radiological assessment using 18F-FDG PET/CT should be incorporated at the end of treatment and annually during follow-up. The existing literature on choriocarcinoma is predominantly comprised of case reports, highlighting its heightened metabolic state with significant 18F-FDG uptake, indicative of robust trophoblastic proliferation and the tumor’s highly aggressive nature (13). However, a subset of cases has demonstrated low 18F-FDG accumulation in metastatic nodules from choriocarcinoma, potentially influenced by hemorrhagic and/or necrotic components affecting 18F-FDG avidity (83). The reported SUVmax range for choriocarcinoma spans from 2.0 to 27.5, encompassing various studies (13, 16, 20, 21, 23, 26, 28, 30, 31, 33, 36, 45–47, 49, 51, 53, 54, 60–62, 64, 83).
The treatment approach for choriocarcinoma is contingent upon the disease’s stage (66). Non-gestational choriocarcinomas often receive a diagnosis in advanced or metastatic stages. Inaba et al. propose neoadjuvant chemotherapy to reduce tumor volume or high-dose chemotherapy before cytoreductive surgery (84). Currently, there is no standardized chemotherapy regimen for primary choriocarcinoma in males, with high-intensity chemotherapy regimens commonly employed, similar to those used for female choriocarcinoma. Frequently utilized chemotherapy protocols include EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine) and TP (paclitaxel and cisplatin). It is acknowledged that male patients often develop resistance to cytotoxic chemotherapy, leading to a grim prognosis. Factors such as poor response to chemotherapy, high disease burden, brain metastasis, and hemoptysis at the time of diagnosis correlate with shorter survival times in male primary choriocarcinoma patients, with a median overall survival of approximately 6 months and a 1-month mortality rate of 23.8% (85–90). In this study, the patient received treatment with etoposide, cisplatin, and bleomycin; however, metastases remained uncontrolled, resulting in the patient’s demise due to increased intracranial pressure and secondary epilepsy exacerbated by enlarged brain metastases. The overall survival was only 5 months. A consistent phenomenon observed in patients with poor prognoses was a rapid decrease in β-hCG to a lower level during treatment, followed by a sharp rise during disease relapse (91). Liu et al. suggest that, for advanced patients, a combination of adjuvant chemoradiotherapy with palliative surgery is recommended. If serum β-hCG drops to a normal level and residual lesions persist, salvage surgery to achieve an R0 status is considered worthwhile (76).
As the medical field advances, ongoing exploration and application of new treatment modalities persist. In a pre-clinical model, PD-L1 overexpression was identified in gestational trophoblastic specimens, suggesting the potential role of this ligand in tumor-immune evasion (92). Veras et al. (93) reported PD-L1 expression in human placentas and gestational trophoblastic diseases, including choriocarcinoma. Ghorani et al. (92) documented four cases of drug-resistant gestational trophoblastic neoplasia treated with pembrolizumab. Among these cases, all displayed PD-L1 overexpression, and three out of four patients achieved remission. The lack of response in one patient was attributed to the absence of tumor-infiltrating lymphocytes. In a recent phase II, single-arm, prospective trial (CAP 01), the efficacy and safety of camrelizumab (PD-1 inhibitor) combined with apatinib (vascular endothelial growth factor (VEGF) receptor inhibitor) were evaluated in patients with high-risk chemo-refractory or relapsed gestational trophoblastic neoplasia. The study included 20 patients (19 with gestational choriocarcinoma and one with placental site trophoblastic tumor). Notably, 50% of enrolled patients achieved a complete response with the combination of the two drugs, and none of the patients with a complete response experienced disease recurrence after discontinuation of the treatment (94). Contrastingly, in a study by Adra et al. (95), only one of three male patients with choriocarcinoma exhibited PD-L1 overexpression, and none of the three patients achieved an objective response to pembrolizumab treatment. These findings suggest that PD-1/PD-L1 blockade therapy may not be universally effective in all male patients. It is posited that the therapeutic efficacy of PD-1/PDL1 blockade varies based on clinicopathological features such as PD-L1 overexpression and the presence of tumor-infiltrating lymphocytes.
In conclusion, non-gestational choriocarcinoma represents a rare entity in clinical practice and should be considered in young men presenting with gynaecomastia and elevated β-hCG levels alongside normal gonads. Thus, we advocate for a more comprehensive inquiry into medical history and a systematic examination. Male primary choriocarcinoma is often associated with widespread metastatic disease, rapid disease progression, and a poor prognosis. Early diagnosis is paramount for formulating an optimal management strategy and minimizing widespread metastasis to achieve the best clinical outcome. The case elucidated in this report contributes to a deeper understanding of the disease for clinicians. The 18F-FDG PET/CT examination not only visually delineates the lesion’s location and extent but also serves as a cornerstone for clinical tumor staging, providing valuable support for treatment monitoring and subsequent follow-up.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WH: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Writing – review & editing. ZB: Formal analysis, Supervision, Writing – review & editing. XX: Conceptualization, Data curation, Writing – review & editing. LL: Data curation, Formal analysis, Writing – review & editing. ZS: Conceptualization, Data curation, Writing – review & editing. LK: Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bogani, G, Ray-Coquard, I, Mutch, D, Vergote, I, Ramirez, PT, Prat, J, et al. Gestational choriocarcinoma. Int J Gynecol Cancer. (2023) 33:1504–14. doi: 10.1136/ijgc-2023-004704
2. Han, C, Zhou, Y, Ma, J-A, Liu, J, Jiang, Y-N, and Zhang, H-X. A promising treatment option for refractory male primary choriocarcinoma: report of two cases. Transl Cancer Res. (2020) 9:3054–60. doi: 10.21037/tcr.2020.02.05
3. Wang, Y, Wang, Z, Zhu, X, Wan, Q, Han, P, Ying, J, et al. Intestinal metastasis from choriocarcinoma: a case series and literature review. World J Surg Oncol. (2022) 20:173. doi: 10.1186/s12957-022-02623-0
4. Rejlekova, K, Cursano, MC, De Giorgi, U, and Mego, M. Severe complications in testicular germ cell Tumors: the Choriocarcinoma syndrome. Front Endocrinol (Lausanne). (2019) 10:218. doi: 10.3389/fendo.2019.00218
5. Xu, S, Song, X, Jin, C, and Li, Y. Tubal choriocarcinoma presented as ruptured ectopic pregnancy: a case report and review of the literature. World J Surg Oncol. (2020) 18:245. doi: 10.1186/s12957-020-02021-4
6. Murshed, KA, Kanbour, A, Akhtar, M, and Al, HS. Primary mediastinal choriocarcinoma presenting as cutaneous metastasis with resistance to chemotherapy: case report and literature review. J Cutan Pathol. (2021) 48:81–5. doi: 10.1111/cup.13777
7. Vegh, GL, Szigetvári, I, Soltesz, I, Major, K, Batorfi, J, Dancso, J, et al. Primary pulmonary choriocarcinoma: a case report. J Reprod Med. (2008) 53:369–72.
8. Jiang, F, Xiang, Y, Feng, F-Z, Ren, T, Cui, Z-M, and Wan, X-R. Clinical analysis of 13 males with primary choriocarcinoma and review of the literature. Onco Targets Ther. (2014) 7:1135–41. doi: 10.2147/OTT.S62561
9. Seckl, MJ, Fisher, RA, Salerno, G, Rees, H, Paradinas, FJ, Foskett, M, et al. Choriocarcinoma and partial hydatidiform moles. Lancet. (2000) 356:36–9. doi: 10.1016/S0140-6736(00)02432-6
10. Massie, RJ, Shaw, PJ, and Burgess, M. Intracranial choriocarcinoma causing precocious puberty and cured with combined modality therapy. J Paediatr Child Health. (1993) 29:464–7. doi: 10.1111/j.1440-1754.1993.tb03022.x
11. Reilley, MJ, and Pagliaro, LC. Testicular choriocarcinoma: a rare variant that requires a unique treatment approach. Curr Oncol Rep. (2015) 17:2. doi: 10.1007/s11912-014-0430-0
12. Lowe, K, Paterson, J, Armstrong, S, Walsh, S, Groome, M, and Mowat, C. Metastatic testicular Choriocarcinoma: a rare cause of upper GI bleeding. ACG Case Rep J. (2016) 3:36–8. doi: 10.14309/crj.2015.94
13. Maruoka, Y, Abe, K, Baba, S, Isoda, T, Matsuo, Y, Kubo, Y, et al. A case of pulmonary choriocarcinoma metastasis with unusual FDG-PET and CT findings: correlation with pathology. Ann Nucl Med. (2012) 26:835–9. doi: 10.1007/s12149-012-0644-x
14. Dose, J, Bohuslavizki, K, Hüneke, B, Lindner, C, and Jänicke, F. Detection of intramural Choriocarcinoma of the uterus with 18F-FDG-PET. Mol Imaging Biol. (2000) 3:37–40. doi: 10.1016/s1095-0397(00)00036-4
15. Gazzilli, M, Albano, D, Ardighieri, L, Bertagna, F, and Giubbini, R. Primary nasal-ethmoid choriocarcinoma detected by 18F-FDG PET/CT: a rare tumor with complete remission. Nucl Med Rev Cent East Eur. (2020) 23:105–7. doi: 10.5603/NMR.a2020.0012
16. Numnum, TM, Leath, CA, Straughn, JM, Conner, MG, and Barnes, MN. Occult choriocarcinoma discovered by positron emission tomography/computed tomography imaging following a successful pregnancy. Gynecol Oncol. (2005) 97:713–5. doi: 10.1016/j.ygyno.2005.01.049
17. Hebart, H, Erley, C, Kaskas, B, Mayer, R, König, M, Einsele, H, et al. Positron emission tomography helps to diagnose tumor emboli and residual disease in choriocarcinoma. Ann Oncol. (1996) 7:416–8. doi: 10.1093/oxfordjournals.annonc.a010611
18. Huang, C-Y, Chen, C-A, Hsieh, C-Y, and Cheng, W-F. Intracerebral hemorrhage as initial presentation of gestational choriocarcinoma: a case report and literature review. Int J Gynecol Cancer. (2007) 17:1166–71. doi: 10.1111/j.1525-1438.2007.00934.x
19. Tripathi, M, D’Souza, MM, Jain, J, Srivastava, M, Jaimini, A, Jain, N, et al. Metastatic choriocarcinoma with tumor thrombus in the right atrium and pulmonary vessels: diagnosis and therapy monitoring with F-18 flurodeoxyglucose PET/CT. Clin Nucl Med. (2009) 34:381–5. doi: 10.1097/RLU.0b013e3181a3461f
20. Rao, M, Chen, Y, Zhu, Y, Huang, Z, and Zhang, L. Primary pancreatic choriocarcinoma revealed on FDG PET/CT. Clin Nucl Med. (2015) 40:76–8. doi: 10.1097/RLU.0000000000000584
21. Huang, W, Qiu, Y, Zhou, Y, Yang, Q, and Kang, L. Primary pancreatic Choriocarcinoma with hepatic metastases on 18 F-FDG PET/CT. Clin Nucl Med. (2023) 48:553–6. doi: 10.1097/RLU.0000000000004666
22. Trübenbach, J, Pereira, PL, Huppert, PE, Farnsworth, C, Mayer, R, Feine, U, et al. Primary choriocarcinoma of the pulmonary artery mimicking pulmonary embolism. Br J Radiol. (1997) 70:843–5. doi: 10.1259/bjr.70.836.9486052
23. Zhuang, H, Yamamoto, AJ, Ghesani, N, and Alavi, A. Detection of choriocarcinoma in the lung by FDG positron emission tomography. Clin Nucl Med. (2001) 26:723. doi: 10.1097/00003072-200108000-00020
24. Cimarelli, S, Deshayes, E, Mognetti, T, Biron, P, Desuzinges, C, Rivoire, M, et al. F-18 FDG PET/CT imaging in a case of primary choriocarcinoma in the retroperitoneum. Clin Nucl Med. (2009) 34:449–51. doi: 10.1097/RLU.0b013e3181a7d0cd
25. Joshua, AM, Carter, JR, and Beale, P. The use of taxanes in choriocarcinoma; a case report and review of the literature. Gynecol Oncol. (2004) 94:581–3. doi: 10.1016/j.ygyno.2004.05.036
26. Sone, T, Yoshikawa, K, and Fukunaga, M. Pulmonary tumor embolism from choriocarcinoma: detection with F-18 FDG positron emission tomography. Clin Nucl Med. (2008) 33:773–4. doi: 10.1097/RLU.0b013e318187f0d7
27. Kidd, D, Plant, GT, Scaravilli, F, McCartney, AC, Stanford, M, and Graham, EM. Metastatic choriocarcinoma presenting as multiple intracerebral haemorrhages: the role of imaging in the elucidation of the pathology. J Neurol Neurosurg Psychiatry. (1998) 65:939–41. doi: 10.1136/jnnp.65.6.939
28. Aleem, J, Qureshi, PAAA, Babar, N, Sultan, A, and Rehman, AU. Metastatic Choriocarcinoma of the breast: a rare entity. Cureus. (2022) 14:e22417. doi: 10.7759/cureus.22417
29. Goldfarb, JA, Dinoi, G, Mariani, A, and Langstraat, CL. A case of multi-agent drug resistant choriocarcinoma treated with Pembrolizumab. Gynecol Oncol Rep. (2020) 32:100574. doi: 10.1016/j.gore.2020.100574
30. Du, Y, Zhang, X, Sun, S, Sun, M, Yang, D, Yu, X, et al. Case report: 18F-FDG PET/CT and laparoscopic nephron sparing surgery in the Management of Bleeding from Renal Metastases of Choriocarcinoma. Front Oncol. (2022) 12:829190. doi: 10.3389/fonc.2022.829190
31. Wang, P, Ren, D, Guo, C, Ding, X, Cao, Y, Zhao, P, et al. A rare case of pulmonary artery embolism with choriocarcinoma: a case report and literature review. Oncol Lett. (2023) 26:490. doi: 10.3892/ol.2023.14077
32. Sekine, R, Hyodo, M, Kojima, M, Meguro, Y, Suzuki, A, Yokoyama, T, et al. Primary hepatic choriocarcinoma in a 49-year-old man: report of a case. World J Gastroenterol. (2013) 19:9485–9. doi: 10.3748/wjg.v19.i48.9485
33. Sironi, S, Picchio, M, Mangili, G, Garavaglia, E, Zangheri, B, Messa, C, et al. [18F] fluorodeoxyglucose positron emission tomography as a useful indicator of metastatic gestational trophoblastic tumor: preliminary results in three patients. Gynecol Oncol. (2003) 91:226–30. doi: 10.1016/s0090-8258(03)00437-2
34. Gasparri, R, Sedda, G, Brambilla, D, Girelli, L, Diotti, C, and Spaggiari, L. When a differential diagnosis is fundamental: Choriocarcinoma mimicking lung carcinoma. J Clin Med. (2019) 8:2018. doi: 10.3390/jcm8112018
35. Gvinianidze, L, Panagiotopoulos, N, Woo, WL, Borg, E, and Lawrence, D. The challenging management of lung choriocarcinoma. J Thorac Dis. (2014) 6:E220–2. doi: 10.3978/j.issn.2072-1439.2014.09.18
36. Snoj, Z, Kocijancic, I, and Skof, E. Primary pulmonary choriocarcinoma. Radiol Iugoslavica. (2017) 51:1–7. doi: 10.1515/raon-2016-0038
37. Göksel, S, Akın, S, Akdoğan, RA, Rakıcı, S, Abdioğlu, GY, and Ayvaz, MA. 18F-FDG PET/CT imaging of metastatic testicular Choriocarcinoma mimicking gastric cancer which initial symptom is melena. Mol Imaging Radionucl Ther. (2021) 30:47–9. doi: 10.4274/mirt.galenos.2020.65668
38. Francischetti, IMB, Cajigas, A, Suhrland, M, Farinhas, JM, and Khader, S. Incidental primary mediastinal choriocarcinoma diagnosed by endobronchial ultrasound-guided fine needle aspiration in a patient presenting with transient ischemic attack and stroke. Diagn Cytopathol. (2017) 45:738–43. doi: 10.1002/dc.23719
39. Pakkala, AK, Nekarakanti, PK, Nagari, B, Bansal, AK, Shroff, G, and Uppin, MS. Primary hepatic Choriocarcinoma with pregnancy: a diagnostic and therapeutic challenge. Korean J Gastroenterol. (2023) 81:91–4. doi: 10.4166/kjg.2022.116
40. Su, H-M, Hu, C, Wu, C-S, Du, W-N, Tsay, D-G, and Peng, N-J. Poor FDG avidity in a case of metastatic pulmonary choriocarcinoma. Clin Nucl Med. (2011) 36:826–7. doi: 10.1097/RLU.0b013e31821a289e
41. Lee, AJ, Im, YJ, Shim, S-H, Lee, SJ, Kim, TJ, and So, KA. Successful treatment of nongestational Choriocarcinoma in a 15-year-old girl: a case report. J Pediatr Adolesc Gynecol. (2021) 34:231–3. doi: 10.1016/j.jpag.2020.11.004
42. Lehmann, M, Hosa, H, Bartl, T, Tsibulak, I, Polterauer, S, Pötsch, N, et al. Combined chemotherapy and pembrolizumab salvages multi-chemotherapy agent and avelumab resistant choriocarcinoma: a case report. Gynecol Oncol Rep. (2023) 49:101259. doi: 10.1016/j.gore.2023.101259
43. Usta, TA, Karacan, T, Ozyurek, E, Naki, MM, Omeroglu, SN, and Demirkiran, F. Primary renal artery choriocarcinoma causing secondary renovascular hypertension. Int J Surg Case Rep. (2014) 5:1197–9. doi: 10.1016/j.ijscr.2014.11.056
44. Clair, KH, Gallegos, N, and Bristow, RE. Successful treatment of metastatic refractory gestational choriocarcinoma with pembrolizumab: a case for immune checkpoint salvage therapy in trophoblastic tumors. Gynecol Oncol Rep. (2020) 34:100625. doi: 10.1016/j.gore.2020.100625
45. Logan, D, Apurvi, P, Mittun, P, Alexander, JT, and Richard, T. Infantile choriocarcinoma with cutaneous, liver, and lung metastases. Available at: https://appliedradiology.com/articles/infantile-choriocarcinoma-with-cutaneous-liver-and-lung-metastases (Accessed December 4, 2023).
46. Shaw, S-W, Wang, C-W, Ma, S-Y, Ng, K-K, and Chang, T-C. Exclusion of lung metastases in placental site trophoblastic tumor using [18F] fluorodeoxyglucose positron emission tomography: a case report. Gynecol Oncol. (2005) 99:239–42. doi: 10.1016/j.ygyno.2005.06.037
47. Hyun, K, Jeon, HW, Kim, KS, Choi, KB, Park, JK, Park, HJ, et al. Bullae-forming pulmonary metastasis from Choriocarcinoma presenting as pneumothorax. Korean J Thorac Cardiovasc Surg. (2015) 48:435–8. doi: 10.5090/kjtcs.2015.48.6.435
48. Pessanha, I, Heitor, F, Furtado, E, Campos, AP, and Gonçalves, I. Long-term survival after choriocarcinoma transmitted by liver graft: a successful report in pediatric transplantation. Pediatr Transplant. (2022) 26:e14135. doi: 10.1111/petr.14135
49. Chen, Y, Liu, L, Zheng, W, and Zhang, X. Successful treatment of choriocarcinoma with multiple organ metastases after term delivery: a case report. Int J Clin Exp. (2017) 10:5468–74.
50. Kohler, A, Welsch, T, Sturm, A-K, Baretton, GB, Reissfelder, C, Weitz, J, et al. Primary choriocarcinoma of the liver: a rare, but important differential diagnosis of liver lesions. J Surg Case Rep. (2018) 2018, 2018:rjy 068. doi: 10.1093/jscr/rjy068
51. Dlewati, MM, Gonzalez, T, Razi, SS, Hussain, SF, and Bennett, J. Primary pulmonary Choriocarcinoma treated with neoadjuvant chemotherapy and lobectomy: a case report. Cureus. (2022) 14:e21931. doi: 10.7759/cureus.21931
52. Dasgupta, S, and Mohapatra, D. Histological conversion of seminoma of testis to metastatic choriocarcinoma in left cervical lymph node: an unusual phenomenon. J Dr. NTR Univ Health Sciences. (2021) 10:200–4. doi: 10.4103/jdrntruhs.jdrntruhs_18_21
53. Huang, K-G, Abdullah, NA, Adlan, A-S, Ueng, S-H, Ho, T-Y, and Lee, C-L. Successful surgical treatment of recurrent choriocarcinoma with laparoscopic resection of intraperitoneal pelvic tumor. Taiwan J Obstet Gynecol. (2013) 52:290–3. doi: 10.1016/j.tjog.2013.04.027
54. Chatterjee, T, Martial, A, Settypalli, S, and Lindahl, L. A rare case of combined Choriocarcinoma and placental site trophoblastic tumor presenting as skin lesion: a case report. Am J Case Rep. (2022) 23:e936451. doi: 10.12659/AJCR.936451
55. Zhou, Q, Zhou, Y, Ouyang, Y, Chen, W, and Zhou, X. Hepatoid adenocarcinoma of the stomach with metastatic choriocarcinoma of the liver: a case report of a rare subtype of gastric cancer with a complex treatment course. Front Surg. (2022) 9:968891. doi: 10.3389/fsurg.2022.968891
56. Matsuo, S, Tomita, E, Fukuhara, K, Kasuda, S, Suzuki, K, and Tsukamoto, Y. Metastatic gestational choriocarcinoma in lung incidentally found by hemoptysis and confirmed by DNA genotyping, highly suggesting the index antecedent pregnancy of a girl. Human Pathology: Case Reports. (2019) 18:200345. doi: 10.1016/j.ehpc.2019.200345
57. Røge, R, Simonsen, C, and Petersen, AC. Primary mediastinal Choriocarcinoma in an elderly patient with concurrent Goserelin-treated prostate adenocarcinoma. Case Rep Pathol. (2019) 2019:1–3. doi: 10.1155/2019/2734815
58. Kazemi, NY, Langstraat, C, and John, WS. Non-gestational choriocarcinoma with hyperprogression on pembrolizumab: a case report and review of the literature. Gynecol Oncol Rep. (2022) 39:100923. doi: 10.1016/j.gore.2022.100923
59. Pan, W, and Hou, J. Pembrolizumab for treatment of a male with primary mediastinal choriocarcinoma: a case report. Transl Cancer Res. (2022) 11:3416–20. doi: 10.21037/tcr-22-766
60. Kamata, S, Sakurada, A, Sato, N, Noda, M, and Okada, Y. A case of primary pulmonary choriocarcinoma successfully treated by surgery. Gen Thorac Cardiovasc Surg. (2017) 65:361–4. doi: 10.1007/s11748-016-0666-8
61. Iijima, Y, Akiyama, H, Nakajima, Y, Kinoshita, H, Mikami, I, Uramoto, H, et al. Solitary lung metastasis from gestational choriocarcinoma resected six years after hydatidiform mole: a case report. Int J Surg Case Rep. (2016) 28:231–3. doi: 10.1016/j.ijscr.2016.09.048
62. Hirotsu, A, Hiramatsu, Y, Kawata, S, Matsumoto, T, Ozaki, Y, Kikuchi, H, et al. Rapid recurrence of primary gastric choriocarcinoma after complete resection. Int J Surg Case Rep. (2019) 57:138–41. doi: 10.1016/j.ijscr.2019.03.045
63. Rehman, T, Hameed, A, Beharry, N, Du Parcq, J, and Bano, G. An unusual cause of gynaecomastia in a male. Endocrinol Diabetes Metab Case Rep. (2019) 2019:19–0060. doi: 10.1530/EDM-19-0060
64. Guo, N, Yin, R, Li, Q, Song, L, and Wang, D. Postmenopausal choriocarcinoma: a rare case report and review of the literature. Menopause. (2018) 25:239–41. doi: 10.1097/GME.0000000000000968
65. Richie, JP. Re: a meta-analysis of the risk of boys with isolated cryptorchidism developing testicular cancer in later life. J Urol. (2013) 190:1045. doi: 10.1016/j.juro.2013.05.078
66. Zhang, P, Wang, Y, and Xiong, L. Gastrointestinal bleeding caused by metastatic testicular choriocarcinoma: a case report and literature review. World J Surg Oncol. (2022) 20:205. doi: 10.1186/s12957-022-02670-7
67. Smith, ZL, Werntz, RP, and Eggener, SE. Testicular cancer: epidemiology, diagnosis, and management. Med Clin North Am. (2018) 102:251–64. doi: 10.1016/j.mcna.2017.10.003
68. Ma, Y, Wang, C, Sun, P-L, Zhu, Y, Huang, Z-K, and Jin, S-X. A case of male primary pulmonary Choriocarcinoma. Chin Med J. (2018) 131:3001–3. doi: 10.4103/0366-6999.247205
69. Marka, A, Hoyt, BS, and LeBlanc, RE. Cutaneous metastasis of Choriocarcinoma in 2 male patients: a rare presentation of an aggressive malignancy that Dermatopathologists must recognize. Am J Dermatopathol. (2019) 41:50–4. doi: 10.1097/DAD.0000000000001209
70. Berkowitz, RS, and Goldstein, DP. Current management of gestational trophoblastic diseases. Gynecol Oncol. (2009) 112:654–62. doi: 10.1016/j.ygyno.2008.09.005
71. Shabani, S, Pritchard, N, Padhya, TA, and Mifsud, M. Head and neck cutaneous metastasis of testicular choriocarcinoma. BMJ Case Rep. (2020) 13:e233337. doi: 10.1136/bcr-2019-233337
72. Chen, X, Xu, L, Chen, X, Teng, X, and Zheng, S. Testicular choriocarcinoma metastatic to skin and multiple organs. Two case reports and review of literature. J Cutan Pathol. (2010) 37:486–90. doi: 10.1111/j.1600-0560.2009.01296.x
73. Zhang, J, Wang, Z-J, Yang, B, Wei, Y-Y, Yang, L, Hu, Y, et al. Biochemical remission by chemoradiotherapy in male mediastinal choriocarcinoma with diffuse lung metastasis: a case report. Oncol Lett. (2016) 11:2615–8. doi: 10.3892/ol.2016.4248
74. Fine, G, Smith, RW, and Pachter, MR. Primary extragenital choriocarcinoma in the male subject. Case report and review of the literature. Am J Med. (1962) 32:776–94. doi: 10.1016/0002-9343(62)90167-5
75. Deshpande, JR, and Kinare, SG. Choriocarcinomatous transformation in metastases of an anaplastic lung carcinoma--a case report. Indian J Cancer. (1987) 24:161–6.
76. Liu, X, Zhang, X, Pang, Y, Ma, Y, Zhang, X, and Liu, P. Clinicopathological factors and prognosis analysis of 39 cases of non-gestational ovarian choriocarcinoma. Arch Gynecol Obstet. (2020) 301:901–12. doi: 10.1007/s00404-020-05502-9
77. Nishino, K, Yamamoto, E, Ikeda, Y, Niimi, K, Yamamoto, T, and Kajiyama, H. A poor prognostic metastatic nongestational choriocarcinoma of the ovary: a case report and the literature review. J Ovarian Res. (2021) 14:56. doi: 10.1186/s13048-021-00810-3
78. Wang, Q, Guo, C, Zou, L, Wang, Y, Song, X, Ma, Y, et al. Clinicopathological analysis of non-gestational ovarian choriocarcinoma: report of two cases and review of the literature. Oncol Lett. (2016) 11:2599–604. doi: 10.3892/ol.2016.4257
79. Han, SN, Amant, F, Leunen, K, Devi, UK, Neven, P, and Vergote, I. EP-EMA regimen (etoposide and cisplatin with etoposide, methotrexate, and dactinomycin) in a series of 18 women with gestational trophoblastic neoplasia. Int J Gynecol Cancer. (2012) 22:875–80. doi: 10.1097/IGC.0b013e31824d834d
80. Stichelbout, M, Devisme, L, Franquet-Ansart, H, Massardier, J, Vinatier, D, Renaud, F, et al. SALL4 expression in gestational trophoblastic tumors: a useful tool to distinguish choriocarcinoma from placental site trophoblastic tumor and epithelioid trophoblastic tumor. Hum Pathol. (2016) 54:121–6. doi: 10.1016/j.humpath.2016.03.012
81. Hui, P. Gestational trophoblastic Tumors: a timely review of diagnostic pathology. Arch Pathol Lab Med. (2019) 143:65–74. doi: 10.5858/arpa.2018-0234-RA
82. Sha, L, Ma, Y, Zheng, Y, and Zhao, X. Imaging manifestations of primary retroperitoneal choriocarcinoma in a male. Asian J Surg. (2023) 46:1500–1. doi: 10.1016/j.asjsur.2022.09.071
83. Chang, TC, Yen, TC, Li, YT, Wu, YC, Chang, YC, Ng, KK, et al. The role of 18F-fluorodeoxyglucose positron emission tomography in gestational trophoblastic tumours: a pilot study. Eur J Nucl Med Mol Imaging. (2006) 33:156–63. doi: 10.1007/s00259-005-1873-1
84. Inaba, H, Kawasaki, H, Hamazaki, M, Okugawa, T, Uchida, K, Honzumi, M, et al. A case of metastatic ovarian non-gestational choriocarcinoma: successful treatment with conservative type surgery and myeloablative chemotherapy. Pediatr Int. (2000) 42:383–5. doi: 10.1046/j.1442-200x.2000.01236.x
85. Winter, CC, and Trepashko, DW. Rare solitary metastasis to subcutaneous tissue from choriocarcinoma of testis. Urology. (1989) 33:320–1. doi: 10.1016/0090-4295(89)90276-8
86. Yokoi, K, Tanaka, N, Furukawa, K, Ishikawa, N, Seya, T, Horiba, K, et al. Male choriocarcinoma with metastasis to the jejunum: a case report and review of the literature. J Nippon Med Sch. (2008) 75:116–21. doi: 10.1272/jnms.75.116
87. Ji, YS, and Park, SH. Clinical experience of male primary Choriocarcinoma at the Samsung medical Center. Cancer Res Treat. (2021) 53:874–80. doi: 10.4143/crt.2020.1066
88. Lu, W-G, Ye, F, Shen, Y-M, Fu, Y-F, Chen, H-Z, Wan, X-Y, et al. EMA-CO chemotherapy for high-risk gestational trophoblastic neoplasia: a clinical analysis of 54 patients. Int J Gynecol Cancer. (2008) 18:357–62. doi: 10.1111/j.1525-1438.2007.00999.x
89. Amikura, T, Aoki, Y, Banzai, C, Yokoo, T, Nishikawa, N, Sekine, M, et al. Metastatic choriocarcinoma successfully treated with paclitaxel and carboplatin after interstitial lung disease induced by EMA-CO. Gynecol Oncol. (2006) 102:573–5. doi: 10.1016/j.ygyno.2006.02.031
90. Zhao, J, Lv, WG, Feng, FZ, Wan, XR, Liu, JH, Yi, XF, et al. Placental site trophoblastic tumor: a review of 108 cases and their implications for prognosis and treatment. Gynecol Oncol. (2016) 142:102–8. doi: 10.1016/j.ygyno.2016.05.006
91. Belliveau, RE, Wiernik, PH, and Sickles, EA. Blood carcinoembryonic antigen, Regan isoenzyme, and human chorionic gonadotrophin in a man with primary mediastinal choriocarcinoma. Lancet. (1973) 1:22–4. doi: 10.1016/s0140-6736(73)91227-0
92. Ghorani, E, Kaur, B, Fisher, RA, Short, D, Joneborg, U, Carlson, JW, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. (2017) 390:2343–5. doi: 10.1016/S0140-6736(17)32894-5
93. Veras, E, Kurman, RJ, Wang, T-L, and Shih, I-M. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. (2017) 36:146–53. doi: 10.1097/PGP.0000000000000305
94. Huang, M, Pinto, A, Castillo, RP, and Slomovitz, BM. Complete serologic response to Pembrolizumab in a woman with Chemoresistant metastatic Choriocarcinoma. J Clin Oncol. (2017) 35:3172–4. doi: 10.1200/JCO.2017.74.4052
Keywords: male, primary choriocarcinoma, prognosis, computed tomography, 18F-FDG, PET/CT
Citation: Huang W, Zheng Z, Bao Z, Xiao X, Li L, Sun Z and Kang L (2024) A poor prognostic male choriocarcinoma with multiple systemic metastases: a case report and the literature review. Front. Med. 11:1382672. doi: 10.3389/fmed.2024.1382672
Received: 06 February 2024; Accepted: 08 March 2024;
Published: 20 March 2024.
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, ItalyReviewed by:
Mahboubeh Nabavinia, The Research Institute at Nationwide Children’s Hospital, United StatesCopyright © 2024 Huang, Zheng, Bao, Xiao, Li, Sun and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Kang, a2FuZ2xlaUBiam11LmVkdS5jbg==; Zhaonan Sun, emhhb25hbl9zdW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.