
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 12 June 2024
Sec. Pulmonary Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1380236
This article is part of the Research Topic Case Reports in Pulmonary Medicine 2023 View all 15 articles
Blau syndrome (BS), is an autoinflammatory granulomatosis disease characterized by a distinct triad of skin, joint, and eye disorders similar to those of sarcoidosis, but the lung involvement frequently observed in sarcoidosis are rare. Granulomas from patients with BS displayed a distinct morphology indicating an exuberant chronic inflammatory response. Patients with BS may have granulomatous lung lesions, which require early diagnosis. To determine whether therapeutic intervention is needed for lung lesions, examining transbronchial lung cryobiopsy specimens and accumulating cases of BS with lung involvement could be contributed to improving BS management in the future.
Juvenile sarcoidosis, known as Blau syndrome (BS), is an autoinflammatory granulomatosis disease characterized by a distinct triad of skin, joint, and eye disorders (1). Nucleotide-binding oligomerization domain 2 (NOD2) has been identified as the gene responsible for this disease, and the autosomal dominant or sporadic gene mutations induce autoactivation of nuclear factor kappa B (2).
The symptoms of BS are similar to those of sarcoidosis, but the lymphadenopathy and lung involvement frequently observed in sarcoidosis are rare, and the first case of interstitial lung disease in BS was reported in 2007 (3). In the present case, the patient had early-onset BS at the age of 2 years, and the M513T mutation in the NOD2 was identified and previously reported (2). Transbronchial lung cryobiopsy (TBLC) was performed on the tiny but diffuse granular shadows in the lungs, and pathological examination of the specimens confirmed the granulomas. There have been very few reports of lung lesions in BS, and to date, no report has pathologically proven granulomas in the lung and adult-onset lung granulomas in BS.
Herein, a 22-years-old man was referred to our department for the examination of extensive bilateral lung granular shadows. On admission to receive TBLC, the vital signs of the patient were body temperature of 36.3°C, regular pulse of 75 bpm, SpO2 of 99% in room air, and auscultation for the heart and lungs was normal. He had been having dry cough for 3 months before bronchoscopic examination (the day X-3M) (Figure 1), and painful swelling of the right knee and right wrist, and diffuse tiny scaly erythematous in abdomen, back and extremities of cutaneous with mild pigmentation, were accompanied with emergence of cough.
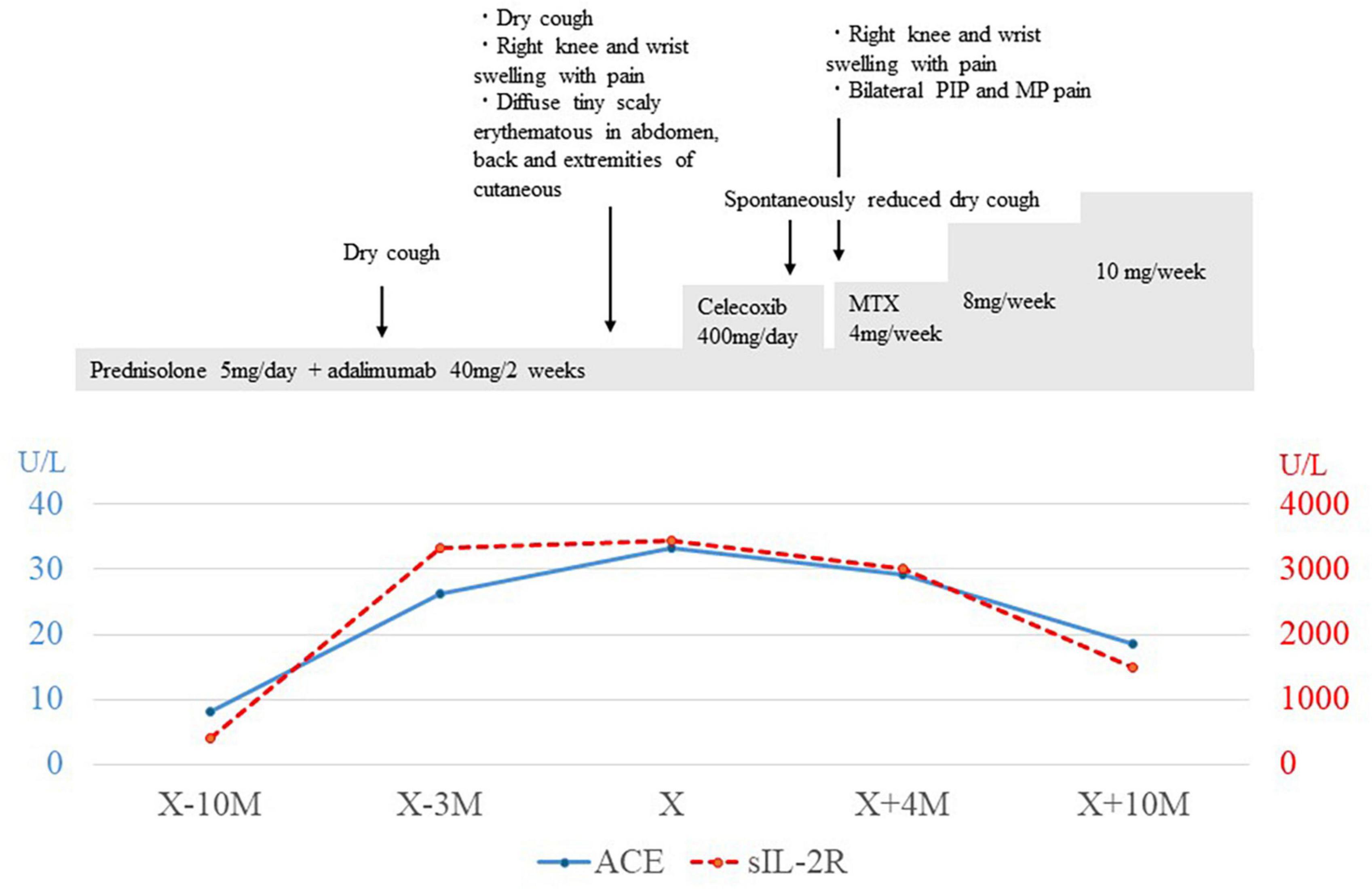
Figure 1. Clinical and treatment course. X indicated the day of bronchoscopic examination was performed, and X-10M indicated the day of 10 month (M) before the day of bronchoscopic examination was performed. Changes in serum levels of angiotensin converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R) were in blue line and red-dot line, each. PIP, proximal interphalangeal; MP, metacarpophalangeal; MTX, Methotrexate.
Laboratory examination revealed a normal range of leukocytes (4800 cells/μL) and fraction of neutrophils (56%), eosinophils (2.9%), basophils (1.0%), monocytes (16.9%), and lymphocytes (23.2%), but elevated angiotensin-converting enzyme (33.4 U/L), soluble interleukin 2 receptor (3440 U/ml), and matrix metalloproteinase-3 (233 ng/ml). The electrocardiogram showed incomplete right bundle branch block, spirometry showed a normal range of forced vital capacity (83.7%), forced expiratory volume in 1 second (83.1%), and DLCO (84.6%). Chest X-rays and CT scans showed diffuse fine granular shadows extending into the bilateral lungs (Figures 2A–C) and minor swelling of the mediastinal lymph nodes (Figure 2D).
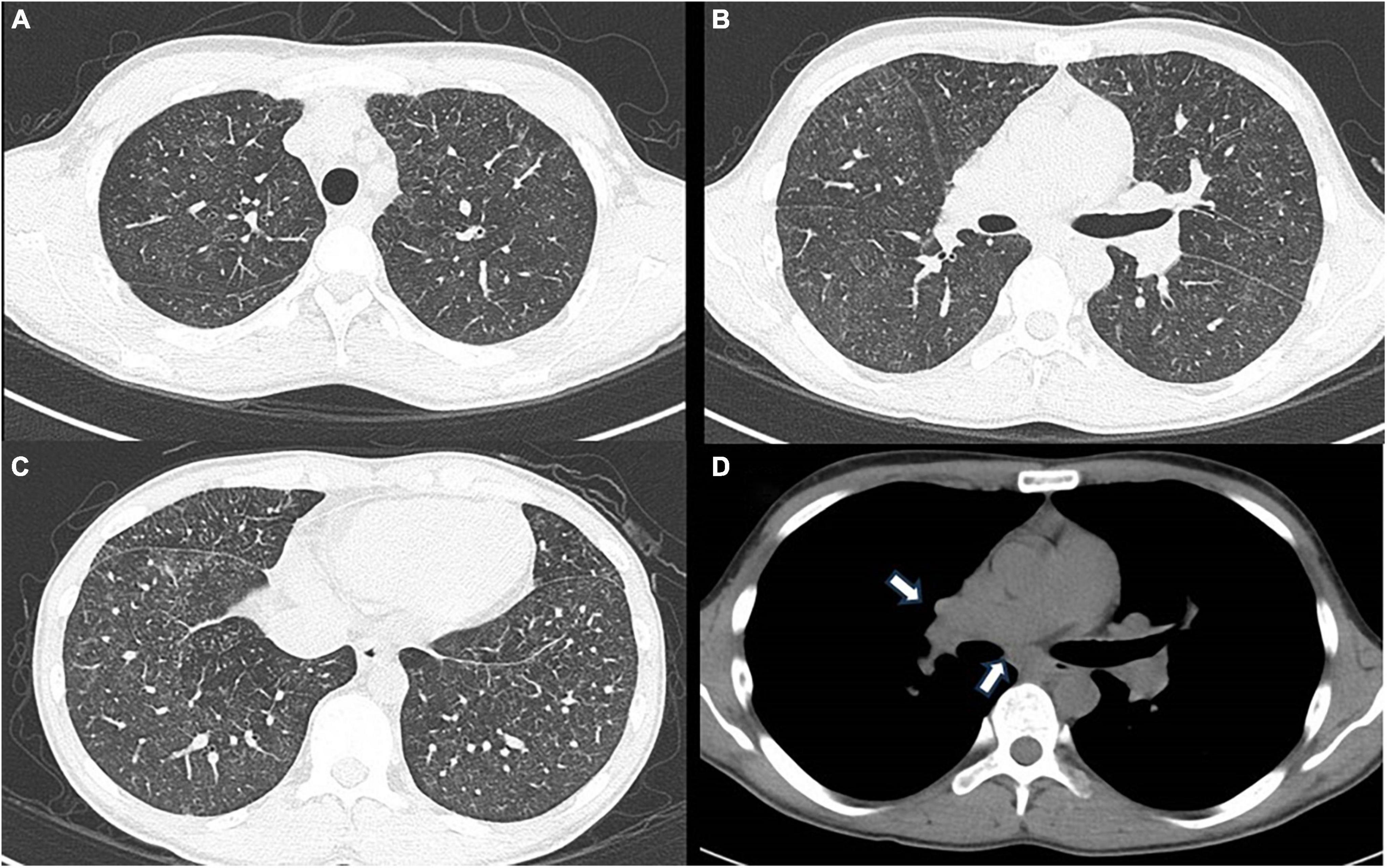
Figure 2. (A) Chest computed tomography using the lung window setting shows diffuse ground-glass shadows presumed to be fine nodules in the bilateral upper lobes, (B) middle and lingular lobes, (C) and bilateral lower lobes. (D) Chest computed tomography using the mediastinum window setting shows minor swelling of mediastinal lymph nodes, indicated by white arrows.
The patient was treated with prednisolone (5–10 mg/day) and human IgG1 monoclonal antibody specific for tumor necrosis factor-α (TNF-α, adalimumab) at a dose of 40 mg once every two weeks, which was introduced 2 years ago to treat arthritis and reduce the adverse effects of steroids. Differential diagnosis for granular shadows in the bilateral lungs suggested fungal and acid-fast bacillus infections, drug-induced granulomatosis, lymphoma, vasculitis, and granulomas due to BS.
Bronchoscopy was performed on the lesions of the lung field and mediastinal lymph nodes on day X (Figure 1). The lumen of the main bronchus showed reticulated dilation of the capillaries, appearing like sarcoidosis. Bronchoalveolar lavage fluid (BALF) was collected from the left B5a, and 101/150 mL of lavage was recovered. The cell fraction of BALF was predominantly lymphocytes (87%), and the CD4/8 ratio was not elevated (0.97). Transbronchial needle biopsy (TBNA) was performed on longitudinal lymph nodes #7 and #2R, where access was feasible. TBLC was performed on right B8a, right B4a, and right B3a. In a previous report, granulomas from patients with BS displayed a distinct morphology characterized by large polycyclic granulomas with dense lymphocytic coronas, forming a large granulomatous complex without inter granulomatous sclerosis, indicating an exuberant chronic inflammatory response (4). In the present case, specimens obtained by TBLC were sufficiently large to observe complex of granulomas and inter granulomas, and those specimens showed non-caseating epithelioid granulomas scattered around the alveolar septum, bronchi, and vessels forming a large granulomatous complex without inter granulomatous sclerosis at low magnification (Figure 3A). At high magnification, prominent epithelioid cells and multinucleated giant cells around bronchioles were observed, similar to previous reports on polycyclic granuloma observed in lymph nodes or skin biopsy specimens from BS patients (4), but in the present case, they were not seen in granulomas with dense lymphocytic coronas (Figure 3B). TBNA specimens contained epithelioid cells. The cultures of cells obtained from saline for washing needle of TBNA and for probe of TBLC, BALF and an aspirated sputum sample through bronchoscopy did not reveal bacterial or fungus infections and mycobacterial infection by Ziehl-Neelsen stain and culture using mycobacterium growth indicator tube (MGIT) system. Based on clinical, hematological, and pathological findings, the lung lesions were diagnosed as pulmonary granulomas caused by BS. While an additional dose of prednisolone was considered for the therapy of cough and lung lesions, taking into account the lack of SpO2 decrease, the patient’s young age, the increased risk of adverse effects from additional prednisolone, the absence of infection and also the possibility of spontaneous resolution of granulomatous lesions, the patient was followed up for 2 months without escalating dose of prednisolone or additional immunosuppressant. Celecoxib, a cox-2 inhibitor, was additionally administered for arthralgia of the knees and wrists, and arthralgia was alleviated. Cough gradually decreased spontaneously subsequent two months (X+2M), and chest X-ray also showed a slight improving. However, after four months form bronchoscopic examination (X+4M), right knee and right wrist, proximal interphalangeal (PIP) joint pain and metacarpophalangeal (MP) joint pain were aggravated again. Since chief complaint of the patient was joint pain and prednisolone and methotrexate (MTX) and/or azathioprine were recommended for pulmonary sarcoidosis (5), MTX 4mg/2weeks was started for the arthralgia. The dose was subsequently increased, and pain was under control. No adverse effects due to MTX have appeared. Despite worsening arthralgia on the day X+4, cough and the lung lesions have remained without recurrence, and those were no subsequent deterioration. Changes in serum angiotensin converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R) levels appeared to reflect well the manifestation of cough and lung lesions and their subsequent resolution (Figure 1).
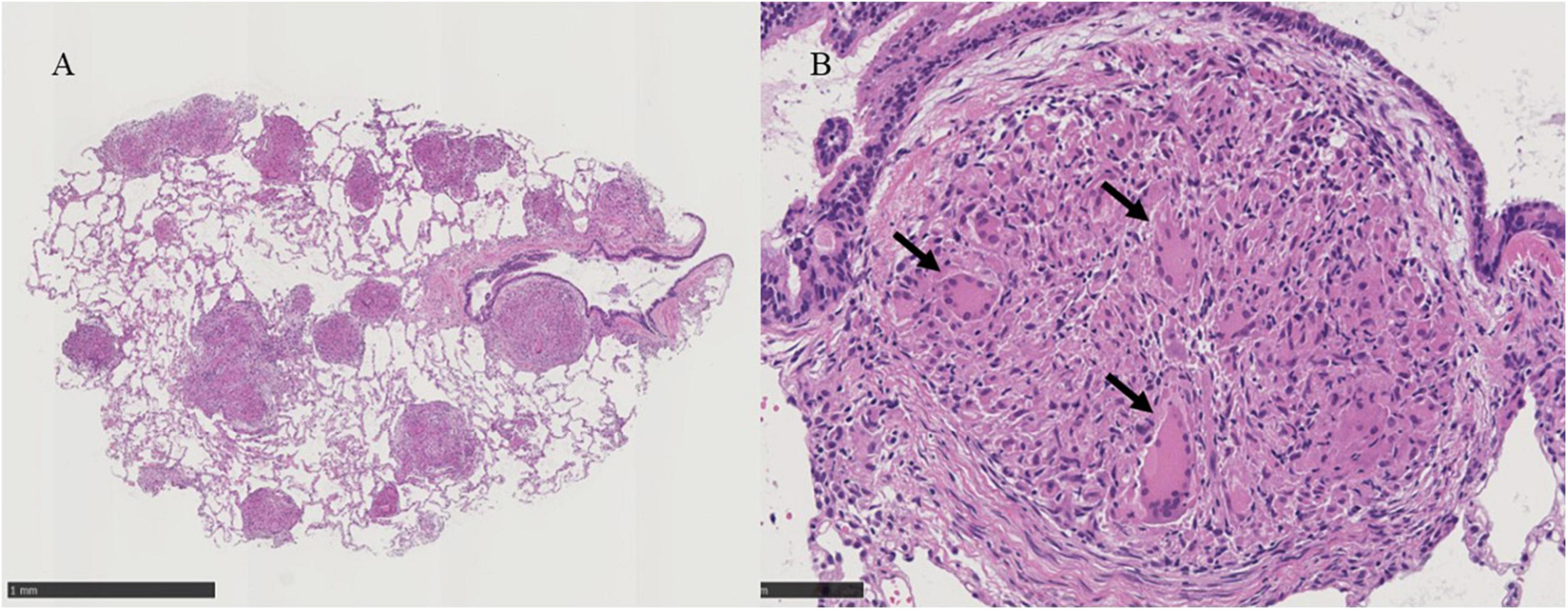
Figure 3. (A) Histological examinations of the lung tissue obtained by transbronchial lung cryobiopsy show non-caseating epithelioid granulomas scattered around the alveolar septum, bronchi, vessels and large complex of granulomas without inter granulomatous sclerosis at X30 magnification, black scale bar = 1mm. (B) Prominent epithelioid cells and multinucleated giant cells around bronchioles are indicated by black arrows in the panel at X200 magnification, black scale bar = 100 μm.
Blau syndrome is characterized by dermatitis, arthritis, and uveitis related to NOD2 mutation, but in recent years, granulomatous lesions other than the triad of BS have also been considered as a phenotype of BS (3). The background of the manifestation of lung lesions in this patient was speculated from three aspects. The first was whether the frequency of pulmonary lesions differed depending on the type of NOD2 mutations, the second was whether there was a trigger for enhancing NOD2 pathway activation, and the third was whether there were triggers for pulmonary lesion formation observed in adult sarcoidosis. First, BS patients who have been reported to having pulmonary lesions with NOD2 mutation were a 23-years-old male with amino acid substitution Glu498Gly (not R334Q, R334W and L489F) (6), a six-teen-years-old male with R334 Q (3), a two-years-old and 7 months boy with pulmonary hemorrhage due to bronchial granuloma with the R334Q, a two-years-old boy with R334W and a nineteen-year-old male of this presenting with M513T. R334W is most common in BS and R334Q is also frequent but M513T is less frequent in BS (7). The activation levels of NF-κb by NOD2 mutations are similar among R334Q, R334W and M513T (7). In terms of these, it is not possible to determine the mutation that predispose to pulmonary lesions in BS. Second, NOD2 mutation leads to autoactivation of NF-κb, but external factors might further amplify the activation of the pathway from NOD2 to NF-κb. NOD2 is activated by muramyl-dipeptide MurNAc-L-Ala-D-isoGln (MDP), a conserved proteoglycan (PGN) found in almost all bacteria and also mycobacterium tuberculosis activates via MDP. Single strand RNA virus including influenza A or RS virus induce NOD2 expression (8). In the presenting patient, there was no tuberculosis or influenza infection at the emergence of the pulmonary lesions, but RS virus infection was not examined, so the involvement of RS virus infection could not be ruled out as a trigger for developing lung lesions in present case. BCG vaccination also activates NOD2 (7), but there was no BCG vaccination in this patient. There is a report that propionibacterium acnes (P. acnes) had been detected on the skin specimen of a patient with BS (9), but whether P. acnes was involved in pulmonary lesion was unknown because staining using anti-propionibacterium acnes monoclonal antibody (PAB) was not performed on specimens of present patient. Third, Mycobacterium tuberculosis, cutibacterium acnes, metals, carbon, silicon inorganic antigens could be antigen for sarcoidosis (10), but there was no history of inhalation of these antigens in present case. Examination with polarized light to rule out foreign body was not done on specimens.
In an ex vivo study using pluripotent stem cells from BS patients with the NOD2 R334W mutation, abnormal cytokine expressions in monocytes were induced by interferon-γ stimulation, and those expressions were inhibited by anti-TNF-α antibody or pan Janus kinase inhibitor, tofacitinib (11, 12). In the present case, despite the therapy with prednisolone and adalimumab, lung lesions appeared accompanied with worsening arthralgia and skin rash, but at the time of recurrence of worsening arthralgia, cough and lung lesion did not appear. MTX administration was started at the time of recurrence of arthralgia and MTX has been continued since then, and there has been no recurrence of lung lesions. It was unclear whether MTX contributed to the suppression of recurrence of pulmonary symptoms and lesions. In two previous case reports of pulmonary involvement in adolescent (16-years-old) and adult (23-years-old) BS, one case report of adult BS recommended the use of prednisolone and immunosuppressive agents (6). In another 16-years-old BS case, disease control was achieved with prednisolone 10 mg/day combined with anti-TNF-α (infiliximab) 10 mg/kg every 8 weeks (3). In that case report, biopsy of the lung lesion was not performed, but a biopsy was performed on the posterior auricular lymph node. The pathological findings demonstrated discrete non-necrotizing granulomas with a multinucleated cell, but large granulomatous complexes without inter-granulomatous sclerosis, which could be found in present case by TBLC were difficult to determine from the literature (3). In presented case, when pulmonary symptoms and granulomas appear again, it may be useful to monitor the changes in ACE and sIL-2R levels and consider the use of immunosuppressive agents if the pulmonary lesions worsen.
Unlike sarcoidosis, the spontaneous remission of BS is rare, and the syndrome eventually leads to sequelae such as blindness and joint contractures. Thus, early diagnosis are considered necessary, even in the lungs. The diagnostic rate was reported to be 37–90% for transbronchial lung biopsy (TBLB) and 80–92% for TBLC in sarcoidosis, a major disease presenting with granulomatous lesions of the lungs (13, 14). The similarities and differences between pulmonary granulomatous lesions in BS and sarcoidosis remain unresolved.
Accordingly, accumulating knowledge from pathological examination on TBLC specimens would be useful in determining the timing of therapeutic intervention for lung lesion and that can contribute to improving management for BS in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
YS: Writing−review and editing, Writing−original draft, Visualization, Project administration, Investigation, Formal analysis, Data curation, Conceptualization. YK: Writing−review and editing, Writing−original draft, Investigation, Data curation. AyT: Writing−review and editing, Writing−original draft, Data curation. AkT: Writing−review and editing, Writing−original draft, Methodology. KI: Investigation, Writing−review and editing, Writing−original draft, Visualization, Validation. SN: Writing−review and editing, Writing−original draft, Supervision.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blau E. Familial granulomatous arthritis, iritis, and rash. J Pediatr. (1985) 107:689–93. doi: 10.1016/s0022-3476(85)80394-2
2. Matsuda T, Kambe N, Ueki Y, Kanazawa N, Izawa K, Honda Y, et al. Clinical characteristics and treatment of 50 cases of Blau syndrome in Japan confirmed by genetic analysis of the NOD2 mutation. Ann Rheum Dis. (2020) 79:1492–9. doi: 10.1136/annrheumdis-2020-217320
3. Becker M, Martin T, Doyle T, Rosé C. Interstitial pneumonitis in Blau syndrome with documented mutation in CARD15. Arthritis Rheum. (2007) 56:1292–4. doi: 10.1002/art.22509
4. Janssen C, Rose C, De Hertogh G, Martin T, Bader Meunier B, Cimaz R, et al. Morphologic and immunohistochemical characterization of granulomas in the nucleotide oligomerization domain 2-related disorders Blau syndrome and Crohn disease. J Allergy Clin Immunol. (2012) 129:1076–84. doi: 10.1016/j.jaci.2012.02.004
5. Japan Society of Sarcoidosis and other Granulomatous Disorders. Guide to Sarcoidosis Clinical Practice. (2020). Available online at. https://www.jssog.com/wp/wp-content/themes/jssog/images/system/guidance/1-3-1.pdf
6. Chauhan K, Michet C. A case of blau syndrome. Case Rep Rheumatol. (2014) 2014:216056. doi: 10.1155/2014/216056
7. Okafuji I, Nishikomori R, Kanazawa N, Kambe N, Fujisawa A, Yamazaki S, et al. Role of the NOD2 genotype in the clinical phenotype of Blau syndrome and early-onset sarcoidosis. Arthritis Rheum. (2009) 60:242–50. doi: 10.1002/art.24134
8. Wiese K, Coates B, Ridge K. The role of nucleotide-binding oligomerization domain-like receptors in pulmonary infection. Am J Respir Cell Mol Biol. (2017) 57(2):151–61. doi: 10.1165/rcmb.2016-0375TR
9. Okazaki F, Wakiguchi H, Korenaga Y, Nakamura T, Yasudo H, Uchi S, et al. A novel mutation in early-onset sarcoidosis/Blau syndrome: an association with Propionibacterium acnes. Pediatr Rheumatol Online J. (2021) 19:18. doi: 10.1186/s12969-021-00505-5
10. Chen C, Luo N, Dai F, Zhou W, Wu X, Zhang J. Advance in pathogenesis of sarcoidosis: triggers and progression. Heliyon. (2024) 10:e27612. doi: 10.1016/j.heliyon.2024.e27612
11. Matsuda T, Kambe N, Takimoto-Ito R, Ueki Y, Nakamizo S, Saito M, et al. Potential Benefits of TNF targeting therapy in Blau syndrome, a NOD2-associated systemic autoinflammatory granulomatosis. Front Immunol. (2022) 13:895765. doi: 10.3389/fimmu.2022.895765
12. Ueki Y, Takimoto-Ito R, Saito M, Tanizaki H, Kambe N. Tofacitinib, a suppressor of NOD2 expression, is a potential treatment for Blau syndrome. Front Immunol. (2023) 14:1211240. doi: 10.3389/fimmu.2023.1211240
13. Pedro C, Melo N, Bastos H, Magalhães A, Fernandes G, Martins N. Role of bronchoscopic techniques in the diagnosis of thoracic sarcoidosis. J Clin Med. (2019) 8:1327. doi: 10.3390/jcm8091327
Keywords: NOD2, cryobiopsy, lung, sarcoidosis, granulomas, Blau syndrome, bronchoscopy, interstitial lung disease
Citation: Shimizu Y, Kushima Y, Tanaka A, Takemasa A, Ishida K and Niho S (2024) Pulmonary granulomas confirmed in Blau syndrome using TBLC specimens: Case report. Front. Med. 11:1380236. doi: 10.3389/fmed.2024.1380236
Received: 01 February 2024; Accepted: 20 May 2024;
Published: 12 June 2024.
Edited by:
Daniel Prantner, University of Maryland, United StatesReviewed by:
Jeffrey Don McBride, University of Oklahoma Health Sciences Center, United StatesCopyright © 2024 Shimizu, Kushima, Tanaka, Takemasa, Ishida and Niho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuo Shimizu, eWFzdW8tc0Bkb2treW9tZWQuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.