
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 19 March 2024
Sec. Nuclear Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1373260
This article is part of the Research Topic Advances in PET-CT Imaging View all 14 articles
The integration of diagnostic CT scans into PET/CT facilitates a comprehensive single examination, presenting potential advantages for patients seeking a thorough one-shot check-up. The introduction of iodinated contrast media during PET scanning raises theoretical concerns about potential interference with uptake quantification, due to the modification of tissue density on CT. Nevertheless, this impact appears generally insignificant for clinical use, compared to the intrinsic variability of standardized uptake values. On the other hand, with the growing indications of PET, especially 18F-FDG PET, contrast enhancement increases the diagnostic performances of the exam, and provides additional information. This improvement in performance achieved through contrast-enhanced PET/CT must be carefully evaluated considering the associated risks and side-effects stemming from the administration of iodinated contrast media. Within this article, we present a comprehensive literature review of contrast enhanced PET/CT, examining the potential impact of iodinated contrast media on quantification, additional side-effects and the pivotal clinically demonstrated benefits of an all-encompassing examination for patients. In conclusion, the clinical benefits of iodinated contrast media are mainly validated by the large diffusion in PET protocols. Contrary to positive oral contrast, which does not appear to offer any major advantage in patient management, intravenous iodine contrast media provides clinical benefits without significant artifact on images or quantification. However, studies on the benefit–risk balance for patients are still lacking.
A recent editorial has brought forth the question: “PET/contrast-enhanced CT in oncology: to do, or not to do, that is the question” (1).
Positron Emission Tomography (PET) is a molecular imaging technique that enhances diagnostic performance (2), therapeutic response monitoring (3), prognosis evaluation (4, 5) and modifies the management of patients with hematological (6) or solid malignancy (7). However, it is sensitive to attenuation. Unlike Single Photon Emission Tomography (SPECT), PET reconstruction needs the detection of two simultaneous 511 keV photons produced by β + annihilation. The increased interaction probability of at least one of two photons versus only one results in a decreased sensitivity with patient’s depth.
This attenuation has been historically corrected with a 511 keV attenuation map generated using a 68Germanium transmission source. The primary drawback of this technique was its inherent slowness, significantly elongating the examination duration. Subsequent PET generation introduced a shift where attenuation correction map was substituted with a simulated high-energy attenuation map derived from Computed Tomography (CT). The first hybrid PET/CT systems offered enhanced accuracy in pinpointing the anatomical uptake location.
Gradually, PET manufacturers integrated the same diagnostic CT used as in radiology. With the potentially comparable performances capabilities, the redundancy between radiological CT and PET/CT prompted physicians to enhance the CT parameters of PET for minimizing overall patient’s exposure, especially in oncology, and the economic impact (8).
The quest to the one-stop-shop anatomic and metabolic exam potentially required contrast-enhanced CT but the attenuation map modification by contrast medium could potentially lead to PET artefacts (9, 10). In this article, we propose a comprehensive literature review on the physical and clinical impacts of intravenous and positive oral contrast-enhanced CT in PET (cePET/CT).
The standardized uptake value (SUV) is the main quantitative parameter in PET to assess the radiopharmaceutical concentrations in tissues, while accounting for radioactive decay.
SUV body weighted (SUVbw or SUW) is determined by the ratio of the activity concentration in the tissue under examination to the activity concentration in the entire body.
However, SUVbw assumed a uniform distribution of the radiopharmaceutical throughout the body which was not the case. Particularly for the mainly used radiopharmaceuticals, the activity level in white adipose tissue was considerably low and led to an SUVbw overestimation in patients with obesity (11). To rectify this, a more accurate approach involved scaling the SUV according to the lean body mass (SUVlbm or SUL) (12) for adults or to the body surface area (SUA) in pediatric patients (13).
Furthermore, various other factors could potentially interfere with radiopharmaceutical distribution, such as injected activity, post-injection uptake time, blood glucose level, attenuation correction (14).
There was also multiple methods for quantifying SUV in a region of interest (ROI). SUVmean represented the average activity in the ROI while SUVmax captured only the maximum pixel value. However, SUVmax was more sensitive to noise (15) and SUVmean tended to lower the quantitative values with a better repeatability (16). An alternative approach could involve SUVpeak which computed the mean pixel value in the vicinity of the pixel with the maximum value and is less sensitive to changes in reconstruction parameters (17).
On anthropomorphic phantom with and without Iodine Contrast Media (ICM), the study conducted by Razac et al. revealed a marginal absolute difference ΔSUVmax and ΔSUVmean, of 0.2 and 0.4, respectively (18). This disparity was more pronounced in the most metabolically active simulated lesion (ΔSUV = 1.5 for a SUVmax of 22). Nevertheless, these discrepancies were not clinically or statistically significant. These findings corroborated those of Bunyaviroch et al. which indicated a maximum SUV relative difference of 7% on phantom studies and a lower variance with 5.9% in clinical application (19). A variability of up to 8% was also found in conventional and digital systems complying with EARL accreditation but a more than 30% SUV difference could be observed on a limited number of lesions (17).
In clinical practice, the SUV fluctuation was heightened in highly contrast-enhanced regions, although these differences were not deemed significant (20, 21). Even when the variability was statistically significant, the authors did not observe any discernible clinical impact (22). This ICM SUV induced difference remained relatively negligible compared to the 20–30% global SUV variability in tumors (16). For this reason, it was recommended to use a 25% SUV decreased threshold for tumor reduction and a 33% SUV increased threshold for tumor progression in follow-up (23).
To mitigate the effects of ICM on attenuation correction, numerous research groups have explored how to refine the injection protocol, such as adjusting the ICM dose, concentration, or flow rate.
Regarding dose adjustment, an adaptation to the body surface area demonstrated a decrease in SUV variability, compared to a fixed-dose approach, and an improved interpatient homogeneity of contrast enhancement (24).
Similarly, the same researchers compared the effects of ICM dosage, finding no significant difference between 300 and 370 mgI/ml (25). When using an even higher iodine concentration of 400 mgI/ml, in a multiphase contrast enhanced CT protocol, Aschoff et al. noted only a minimal to negligible influence on 2-[18F]FDG (18F-FDG) quantification (26). It was advisable to opt for a single-phase CT rather than a multiphasic protocol to minimize coregistration errors (27).
Similarly to ICM, positive oral contrast agents could influence SUV values. This effect was demonstrated on phantom with SUV overestimation for high-density oral contrast agent (28) and the absence of significant artifact for low-density barium oral contrast agents (29). However, in a small patient cohort, Otsuka et al. did not find a significant correlation between SUV and Hounsfield density (30).
On simulated PET reconstructions, Dizendorf et al. demonstrated that oral contrast agents overestimated PET attenuation coefficients by 26.2% with only a small effect on SUV PET. The error was measured at 4.4% and did not appear to be clinically significant (31).
In clinical use and because of their route of administration, positive oral contrast media were remarkably safe and side effects were exceedingly rare (32). Most complications were observed with intravenous contrast agents.
ICM allowed the enhancement of vascular structures and tissue contrast. They were classified into two major categories: highly hyperosmolar ICM and hypo- or iso-osmolar non-ionic ICM. Both types could induce side effects, with a higher prevalence seen in ionic ICM. This discrepancy in side effects prevalence was the reason why non-ionic ICM were preferred (33).
Warming iodine contrast media at 37°C could also reduce the risk of allergic or physiologic reactions (34).
While ionic ICM previously resulted in side effects for 12% of patients, the use of non-ionic ICM had significantly decreased this occurrence to 0.7–3.1% and the most severe reactions have been drastically reduced from 0.22 to 0.02–0.04%.
ICM reactions were mostly non-fatal and manifested in 70% of patients within the first 5 min following ICM injection (35).
The majority of ICM reactions were non-allergic. Hyperosmolality induced fast vascular volume changes or direct chemotoxicity which could lead to physiological responses such as flushing, nausea and altered taste. Another reaction came from a non-allergic hypersensitivity caused by the direct release of histamine from mast cells and basophils. This mechanism could result in allergic-like symptoms like urticaria. For mild reactions, simple monitoring or H1-antihistamines treatment was generally sufficient.
IgE-mediated allergy was uncommon. In more severe cases involving laryngeal edema, corticosteroid therapy was often initiated while resuscitation measures were implemented during anaphylactic shocks (36).
For at-risk patients, a prophylactic treatment could be implemented (H1-antihistamines, corticosteroids) (37).
ICM increased the risk of acute kidney injury within 48 h following injection. This risk, previously overestimated, could now be prevented by hydration when clearance was below 30 mL/min/1.73 m2 or for high-risk patients without contra-indication (38). As contrast enhanced CT was optional in PET, it might be advisable to refrain from administering ICM to these patients. For the specific case of myeloma, a meta-analysis suggested that no special precaution was needed if the calcium level was within the normal range (39).
Metformin is an oral antihyperglycemic medication commonly prescribed for diabetes. In the context of ICM injection, patients might potentially develop lactic acidosis coupled with renal failure (35). The European Society of Urogenital Radiology (ESUR) guidelines and American College of Radiology manual recommended discontinuing the treatment for 48 h and monitoring renal function when the baseline clearance was below 30 mL/min/1.73 m2 or if there were signs of acute renal failure.
Compared to the low injected volume of radiopharmaceuticals, ICM injection is carried out at higher pressure and for a larger volume. The risk of extravasation reported in the literature ranged from 0.1 to 0.9% and was increased when using an automatic injector (40, 41) or in cancer patients (42).
The risks associated with extravasation increased with osmolality but also depended on its direct toxicity. This toxicity was notably more pronounced with ionic ICM, as well as the anatomical location or volume. While most cases were relatively benign resulting in minor issues like skin erythema, there was potential for more severe side effects such as compartment syndrome or necrosis (40).
The widespread use of non-ionic ICM usually did not expose patients to the risk of severe complications. Therefore, a surgical consultation might be advised only for volumes exceeding 150 mL or in case of compressive signs (impaired perfusion or altered sensibility) (40).
A typical radiological dose of ICM contains a substantial amount of free iodine, equivalent to the iodine needs for several months. When the body encountered excess iodine, the Wolff-Chaikoff effect was triggered, causing a fast downregulation in thyroid hormone synthesis. Prolonged exposure to high iodine levels could disrupt this regulatory mechanism, potentially resulting in either hyperthyroidism or hypothyroidism.
Moreover, this excess iodine load had the potential to exacerbate existing thyroid pathology or even directly cause thyroid toxicity (43).
While ICM themselves were not inherently toxic, their presence in source waters raised concerns due to the formation of potentially toxic transformation products detected in drinking water (44). Specifically, ICM could react with commonly used disinfectants like chlorine, leading to formation of iodinated disinfection byproducts (iodo-DBP). Studies indicated that these iodo-DBP were highly genotoxic or cytotoxic, surpassing the conventional DBPs in toxicity. This situation highlighted concerns regarding the effectiveness of current treatment technologies and raised serious questions about disinfecting water containing ICM (45). Recent proposals suggested measures aimed at reducing and collecting ICM residues (46).
These potential side effects needed to be balanced against the clinical benefits of an enhanced CT for the patients. Table 1 summarizes the main advantages and disadvantages of ICM injection in PET/CT.
Squamous cell carcinoma (SCC) represents the most common head and neck tumor type (95%). 18F-FDG cePET/CT with dual phase has been proved superior to conventional imaging by MRI or CT for diagnosis and staging of patients with laryngeal carcinoma, with an higher rate of regional nodal, distant metastasis, and synchronous tumors (5, 47). More globally, 18F-FDG PET/CT is a recognized modality for the staging and follow-up of head and neck SCC (48, 49). In cases with cervical lymph node metastasis from an unknown primary tumor, 18F-FDG PET/CT revealed primary tumors that went undetected by CT or MRI in about 25% of cases (50). However, in those studies the difference between PET/CT and cePET/CT was not evaluated.
Prognosis for head and neck SCC is partly influenced by Human Papilloma Virus (HPV) status, with evidence that virally induced tumors responded far better to radiotherapy (51, 52). Using the hypoxia-specific tracer 18F-fluoroazomycin arabinoside (FAZA), Saksø et al. demonstrated that the risk of locoregional recurrence was higher among patients with more hypoxic, non-HPV tumors (57% [21–94%]), when comparing to less hypoxic, non-HPV tumors, with a risk difference of 45% [4–86%] (53).
Integrated 18F-FDG PET/perfusion CT showed that tumoral perfusion was significantly increased compared to surrounding soft tissue, especially for advanced tumors, and that meant blood flow was decreased in HPV-negative tumors (54).
Suenaga et al. showed that cePET/CT and PET/CT statistically showed larger AUC than contrast enhanced CT (ceCT) for recurrent head and neck squamous cell carcinoma (55). Even though minimal, the difference between cePET/CT and PET/CT for local recurrence reached a significant level (p = 0.039).
These works highlighted the distinctions between HPV-positive and negative tumors and emphasized the utility of analyzing the microvasculature features of tumoral head and neck SCC to predict their aggressiveness. This illustrated the necessity of integrating non-morphologic parameters, and also looking beyond the SUV uptake.
18F-FDG ceCT had a higher predictive positive value for any PET pathologic findings than CT in the whole gastrointestinal tract, as well as in the separate evaluation of the upper and lower gastrointestinal tract (56). The sensitivity for the detection of a malignant lesion was 100% for ceCT and 29.4% for CT (p = 0.0001). The false negative rate for any pathology was 31.1% for ceCT and 68.9% for CT; this rate was however lower in the lower gastro-intestinal tract for CT (12.5% vs. 37.5% for ceCT).
18F-FDG PET and ceCT seemed to have similar value in the detection of unsuspected recurrence of high-risk colorectal cancer in a patient-based analysis: sensitivity and specificity of 86 and 88%, 86 and 92%, 86 and 85%, respectively for PET, ceCT and cePET/CT (57). However, the combined assessment of cePET/CT improved the accuracy in the lesion-based analysis: sensitivity of 56, 71 and 97%, respectively for PET, ceCT and cePET/CT.
Regarding rectal tumors, cePET/CT was superior to non-enhanced PET/CT for precise definition of regional nodal status in rectal cancer with a better characterization of pararectal, internal iliac and obturator lymph nodes (58).
A retrospective study explored the diagnostic value of the cross-modality fusion images provided by 18F-FDG PET/CT and ceCT in differentiating malignant from benign pancreatic lesions and staging pancreatic cancer (59). The authors found higher sensitivity and accuracy of cePET/CT compared to PET/CT and ceCT conducted individually both for diagnosing pancreatic malignant tumor and peripancreatic vessel invasion. Regarding regional lymph node metastasis, there was no significant differences between the three methods: however, regarding distant metastasis, cePET/CT improved sensitivity and negative predictive value in comparison to ceCT alone. cePET/CT had also higher sensitivity and accuracy than PET/CT, but the difference was not statistically significant.
Considering neuroendocrine tumors, a recent study emphasized that ceCT in 68Ga-DOTATATE PET should be included for staging. The overall lesion-based sensitivity, specificity, negative predictive value and positive predictive value were 97% versus 85, 86% versus 73, 93% versus 72, 93% versus 85%, respectively, for full-dose cePET/CT and low dose PET/CT (60).
In the case of positive oral contrast media, several studies have demonstrated an improvement in digestive distension which was a potential help for diagnosis (61–63), but few have been able to demonstrate a clinical benefit. Chen et al. reported a more accurate delayed PET/CT with laxative-augmented contrast medium than conventional PET/CT for the evaluation of colorectal foci (64) and Guo et al. reported a case of enterovesical fistula revealed with oral contrast (65). The main challenge in assessing the impact of oral contrast agents was the hyperfixation of digestive structures (66), particularly due to distension, increased motility, and irritative phenomena (30). Regarding these digestive fixations, they were less pronounced with negative oral contrast agents (67–69) or those with low iodine density (62, 70).
Considering malignant ovarian tumors, 18F-FDG cePET/CT outperformed ceCT with sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of 96 versus 84%, 92 versus 59%, 90 versus 59%, 97 versus 84%, and 95 versus 76%, respectively for cePET/CT and PET/CT alone (71). cePET/CT represented an accurate imaging modality for staging ovarian cancer (72).
Regarding ovarian cancer recurrence, cePET/CT seemed to be more accurate. Some data suggested higher sensitivity, specificity, and accuracy of cePET/CT: 86.9, 95.9, and 92.5%, respectively, (compared to 78.3, 95.0, and 88.3%, respectively for PET/CT) (p = 0.023 at least) (73). Another study found a better identification of smaller peritoneal/lymph node lesions close to physiological FDG uptake sources with cePET/CT (74). With a better accuracy compared to non-contrast PET/CT and enhanced CT, cePET/CT could lead to changes in patient management for 39% of them (75).
Similarly, for uterine cancer, cePET/CT seemed to perform slightly better (sensitivity and accuracy) for nodal staging (p = 0.046 and 0.047) (76). cePET/CT was accurate for recurrence, reducing the frequency of equivocal interpretations (77) and leading to more appropriate subsequent clinical management than that resulting from PET alone or ceCT alone (78).
A previous study recommended to perform 18F-FDG PET/CT instead of cePET/CT for staging of malignant melanoma patients (79). Comparison between CT and ceCT alone clearly revealed higher sensitivity and specificity for ceCT. However, when directly comparing lesion-based evaluation of combined PET/CT and cePET/CT, there was a difference in sensitivity of 3% only and no difference in specificity. As a limit, this study was conducted on a non-time-of-flight PET/CT system.
Integrated cePET/CT could improve the evaluation of pelvic lymphatic pathways nodal status in patients with malignant lymphoma (external and internal iliac, common iliac lymph nodes); diagnostic accuracies of retroperitoneal lymph nodes seemed to be similar between PET/CT and cePET/CT (80). However, the contribution of ceCT in nodal staging (Ann Arbor) seemed to remain limited (81). Similarly, the response evaluation applying the Deauville score and Lugano classification criteria remained unchanged with cePET/CT (82).
Thus, cePET/CT could be performed in the management of lymphoma patients, especially for a precise target delineation before radiotherapy (83).
CePET/CT approach should also be considered in pediatric exams. It could offer dose savings at similar image quality for children and young adults with lymphoma who had indications for both PET and diagnostic CT examinations (84).
18F-FDG cePET/CT showed similar results compared with CT/MRI in the detection of primary renal tumors, but it was superior to conventional methods in the detection of metastasis and staging (85). Once again, in this study, the authors did not compare directly PET/CT and cePET/CT.
Similarly, the same authors also showed higher diagnostic accuracy of 18F-FDG cePET/CT for staging bladder cancer (89% vs. 57% for conventional imaging: CT and MRI), with upstaging in 37% of patients, resulting in changes of patient management.
The use of contrast has also been described as useful in 18F-FDG cePET/CT as an initial imaging modality in patients presenting with metastatic malignancy of undefined primary origin (86).
In the specific case of lung and breast cancers, although these cancers were frequent, the clinical contribution of iodinated contrast injection has not been studied. It had only been demonstrated for lung cancers that non-ionic contrast injection did not cause significant artifact (21).
Figure 1 illustrates the improved visualization of low-contrast lesions, especially in difficult areas for diagnosis.
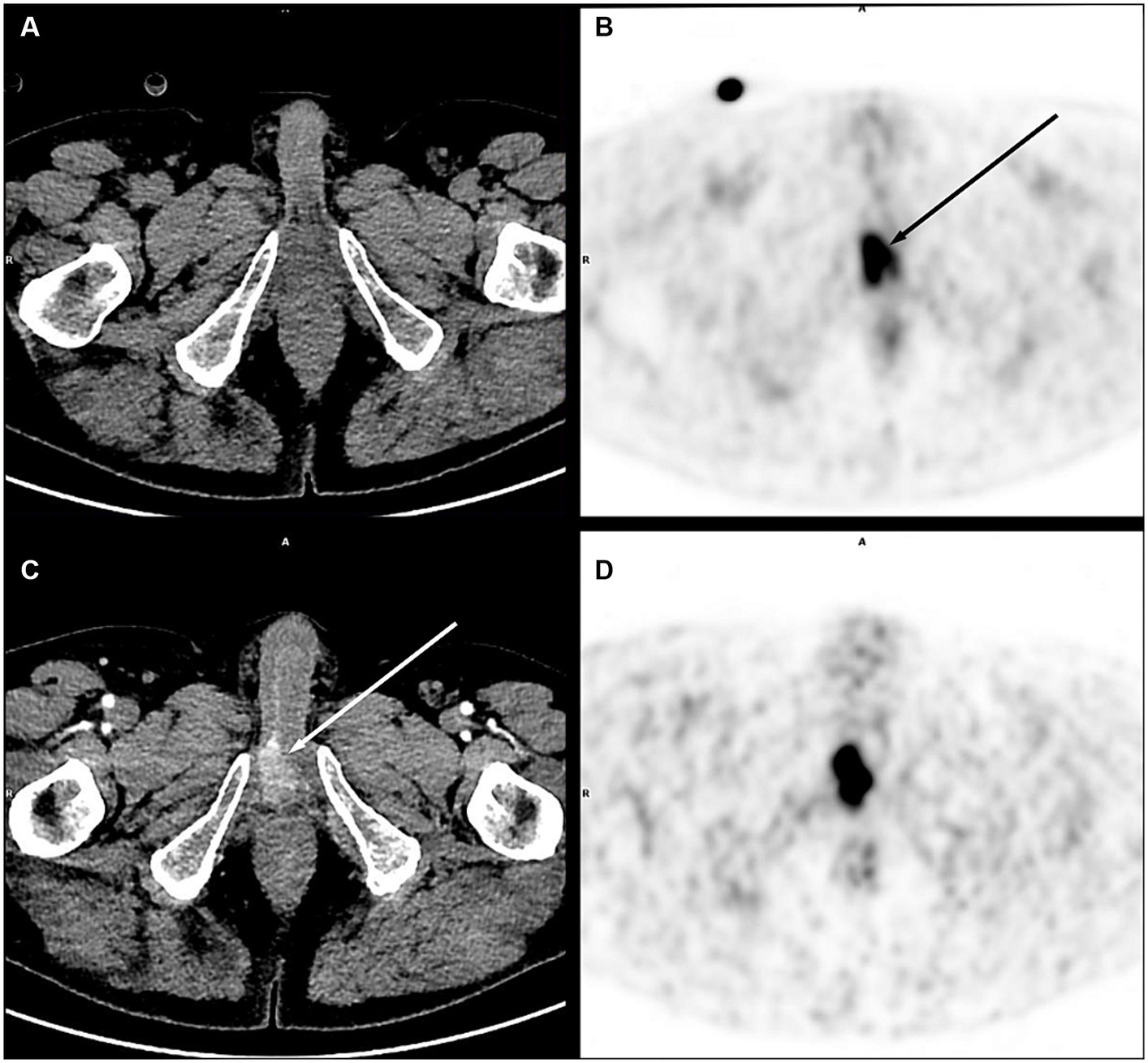
Figure 1. Right corpora cavernosa metastasis from penile carcinoma. on PET images (early acquisition B, late acquisition D), there was a focal uptake [(B) black arrow], without significant lesion on unenhanced CT (A), in an area close to the physiological urinary activity. With contrast medium injection on a dedicated acquisition (C), this uptake corresponded to a metastatic lesion clearly visible on CT (white arrow).
A recent study focused on the diagnostic challenge in suspected infected aortic aneurysms, showing the high diagnostic accuracy of PET/CT for the detection of infection (sensitivity between 85 and 100% vs. between 63 and 88% for ceCT) (87). However, the authors raised the question of specificity because of false positive findings. The combined acquisition and analysis of PET and ceCT could help to improve this specificity.
In vasculitis, cePET/CT could be useful for identifying stenotic lesions in Takayasu arteritis, but data are insufficient to support its routine use for giant cell arteritis large vessel vasculitis. Guidelines recommended a low-dose CT prior to ceCT for attenuation correction and subsequent SUV calculations (88).
Recent guidelines in the management of infectious endocarditis recommended cePET/CT, as it allowed the detection of metabolic findings (FDG uptake distribution and intensity) and anatomical findings (endocarditis-related lesions like abscess) within a single imaging procedure, resulting in the clinical clarification of indeterminate findings and change in the management of the patients. This might be particularly helpful in complex settings like aortic grafts (89).
Lastly, contrast enhancement with an ICM enabled the detection of others pathologies, mostly not visible in PET/CT, such as lesions below the system resolution or pulmonary embolism, which were common in oncology (90).
Despite being widely used for prostate cancer in many countries, only a few studies on parathyroid glands have been published. In a meta-analysis, Piccardo et al. found a better pooled sensitivity for 4D cePET/CT compared to PET/CT in primary hyperparathyroidism (Sensitivity of 0.93 and 0.89, respectively) with an identical detection rate (0.86) (91). In a cohort comprising 44 primary hyperparathyroidism patients, the same researchers confirmed the higher sensitivity of cePET/CT over PET/CT (with sensitivity of 1.0 and 0.8, respectively) and a better detection rate of 0.72 compared to 0.56, respectively (92).
For neuroendocrine tumors, 68Ga-DOTATOC cePET/CT demonstrated a minimal increase in sensitivity and specificity compared to unenhanced exam (93). Ruf et al. recommended the same multiphase protocol for cePET/CT as for CT scan (94, 95).
Although the injection of contrast media did not yield a significant difference in diagnostic performance between PET/CT and cePET/CT, contrast enhancement seemed to improve the delineation of genitourinary system and increased the diagnostic certainty and interobserver agreement (96, 97).
However, CT acquisition during the contrast urinary excretion allowed for a better identification of the urinary tract. In a large retrospective study of 247 patients, Rosar et al. demonstrated that CT urography increased diagnostic confidence (in 48.6% of patients) while providing substantial support for interpretation (24.3%). In 12.1% of patients, urography changed the disease staging with a potential impact on patient management (98).
Tulipan et al. also showed that iodinated contrast agent sedimentation in the bladder created an activity gradient that improved visualization of the prostatic bed and the posterior bladder (99).
The one-stop-shop PET exam with ICM blurs the boundary between nuclear medicine and radiology. Depending on the country, this may rise issues of legislation and reimbursement. In addition, the choice of cePET/CT injection protocol is not standardized and differs from one nuclear medicine department to another (100).
The use of iodinated contrast media (ICM) in PET/CT scans enhanced the overall examination performance by combining the PET sensitivity and specificity with those of diagnostic enhanced CT. This synergistic performance enhancement was achievable through an all-in-one examination, improving patient dosimetry, facilitating pathology management, and decreasing the administered volumes of ICM especially in the field of oncology. In contrast, although positive oral contrast media enhanced distension and contrast of digestive structures, their clinical utility in PET imaging appeared more modest.
However, the ICM injection was not exempt from side effects, most of which were moderate. For the most severe forms, the additional risk remained low, as most patients would have undergone an ICM enhanced CT as part of their assessment. Apart from contraindications, injecting less than 150 mL of non-ionic ICM into patients with a renal clearance greater than 30 mL/min/1.75 m2 could maximize the safety of ICM use in PET as long as no benefit–risk studies have been carried out.
GM: Writing – original draft, Writing – review & editing. CC: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Scialpi, M, Moschini, TO, and De Filippis, G. PET/contrast-enhanced CT in oncology: "to do, or not to do, that is the question". Radiol Med. (2022) 127:925–7. doi: 10.1007/s11547-022-01496-3
2. Kim, K, Ha, M, and Kim, SJ. Comparative study of different imaging modalities for diagnosis of bone metastases of prostate cancer: a Bayesian network meta-analysis. Clin Nucl Med. (2024) 49:312–8. doi: 10.1097/RLU.0000000000005078
3. Wahl, RL, Jacene, H, Kasamon, Y, and Lodge, MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. (2009) 50:122S–50S. doi: 10.2967/jnumed.108.057307
4. Xia, Q, Liu, J, Wu, C, Song, S, Tong, L, Huang, G, et al. Prognostic significance of (18)FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging. (2015) 15:19. doi: 10.1186/s40644-015-0055-z
5. Al-Ibraheem, A, Abdlkadir, AS, Al-Adhami, D, Hejleh, TA, Mansour, A, Mohamad, I, et al. The prognostic and diagnostic value of [(18)F]FDG PET/CT in untreated laryngeal carcinoma. J Clin Med. (2023) 12:3514. doi: 10.3390/jcm12103514
6. Valls, L, Badve, C, Avril, S, Herrmann, K, Faulhaber, P, O'Donnell, J, et al. FDG-PET imaging in hematological malignancies. Blood Rev. (2016) 30:317–31. doi: 10.1016/j.blre.2016.02.003
7. Al-Ibraheem, A, AlSharif, A, Abu-Hijlih, R, Jaradat, I, and Mansour, A. Clinical impact of (18)F-FDG PET/CT on the Management of Gynecologic Cancers: one center experience. Asia Ocean J Nucl Med Biol. (2019) 7:7–12. doi: 10.22038/AOJNMB.2018.11208
8. Picchio, M, Mansueto, M, Crivellaro, C, Guerra, L, Marcelli, S, Arosio, M, et al. PET/CT and contrast enhanced CT in single vs. two separate sessions: a cost analysis study. Q J Nucl Med Mol Imaging. (2012) 56:309–16.
9. Annunziata, S, Testart, N, Auf der Springe, K, Cuzzocrea, M, Nicod Lalonde, M, Schaefer, N, et al. Contrast enhanced CT on PET/CT imaging in clinical routine: an international survey. Front Med (Lausanne). (2023) 10:1290956. doi: 10.3389/fmed.2023.1290956
10. Strobel, K, Heinrich, S, Bhure, U, Soyka, J, Veit-Haibach, P, Pestalozzi, BC, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med. (2008) 49:1408–13. doi: 10.2967/jnumed.108.051466
11. Sarikaya, I, Albatineh, AN, and Sarikaya, A. Revisiting weight-normalized SUV and lean-body-mass-normalized SUV in PET studies. J Nucl Med Technol. (2020) 48:163–7. doi: 10.2967/jnmt.119.233353
12. Keramida, G, and Peters, AM. The appropriate whole body metric for calculating standardised uptake value and the influence of sex. Nucl Med Commun. (2019) 40:3–7. doi: 10.1097/MNM.0000000000000935
13. Sarikaya, I, and Sarikaya, A. Assessing PET parameters in oncologic (18)F-FDG studies. J Nucl Med Technol. (2020) 48:278–82. doi: 10.2967/jnmt.119.236109
14. Adams, MC, Turkington, TG, Wilson, JM, and Wong, TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. (2010) 195:310–20. doi: 10.2214/AJR.10.4923
15. Boellaard, R, Krak, NC, Hoekstra, OS, and Lammertsma, AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. (2004) 45:1519–27.
16. de Langen, AJ, Vincent, A, Velasquez, LM, van Tinteren, H, Boellaard, R, Shankar, LK, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. (2012) 53:701–8. doi: 10.2967/jnumed.111.095299
17. Koopman, D, Jager, PL, Slump, CH, Knollema, S, and van Dalen, JA. SUV variability in EARL-accredited conventional and digital PET. EJNMMI Res. (2019) 9:106. doi: 10.1186/s13550-019-0569-7
18. Abdul Razak, HR, Nordin, AJ, Ackerly, T, Van Every, B, Martin, R, and Geso, M. Quantifying the effects of iodine contrast media on standardised uptake values of FDG PET/CT images: an anthropomorphic phantom study. Australas Phys Eng Sci Med. (2011) 34:367–74. doi: 10.1007/s13246-011-0088-y
19. Bunyaviroch, T, Turkington, TG, Wong, TZ, Wilson, JW, Colsher, JG, and Coleman, RE. Quantitative effects of contrast enhanced CT attenuation correction on PET SUV measurements. Mol Imaging Biol. (2008) 10:107–13. doi: 10.1007/s11307-007-0126-z
20. Mawlawi, O, Erasmus, JJ, Munden, RF, Pan, T, Knight, AE, Macapinlac, HA, et al. Quantifying the effect of IV contrast media on integrated PET/CT: clinical evaluation. AJR Am J Roentgenol. (2006) 186:308–19. doi: 10.2214/AJR.04.1740
21. An, YS, Sheen, SS, Oh, YJ, Hwang, SC, and Yoon, JK. Nonionic intravenous contrast agent does not cause clinically significant artifacts to 18F-FDG PET/CT in patients with lung cancer. Ann Nucl Med. (2007) 21:585–92. doi: 10.1007/s12149-007-0066-3
22. Behrendt, FF, Temur, Y, Verburg, FA, Palmowski, M, Krohn, T, Pietsch, H, et al. PET/CT in lung cancer: influence of contrast medium on quantitative and clinical assessment. Eur Radiol. (2012) 22:2458–64. doi: 10.1007/s00330-012-2515-1
23. Lodge, MA. Repeatability of SUV in oncologic (18)F-FDG PET. J Nucl Med. (2017) 58:523–32. doi: 10.2967/jnumed.116.186353
24. Behrendt, FF, Rebière, M, Goedicke, A, Pietsch, H, Palmowski, K, Kuhl, CK, et al. Contrast medium injection protocol adjusted for body surface area in combined PET/CT. Eur Radiol. (2013) 23:1970–7. doi: 10.1007/s00330-013-2781-6
25. Verburg, FA, Apitzsch, J, Lensing, C, Kuhl, CK, Pietsch, H, Mottaghy, FM, et al. Body surface area adapted iopromide 300 mg/ml versus 370 mg/ml contrast medium injection protocol: influence on quantitative and clinical assessment in combined PET/CT. Eur J Radiol. (2013) 82:2348–52. doi: 10.1016/j.ejrad.2013.09.013
26. Aschoff, P, Plathow, C, Beyer, T, Lichy, MP, Erb, G, Öksüz, M, et al. Multiphase contrast-enhanced CT with highly concentrated contrast agent can be used for PET attenuation correction in integrated PET/CT imaging. Eur J Nucl Med Mol Imaging. (2012) 39:316–25. doi: 10.1007/s00259-011-1919-5
27. Brechtel, K, Klein, M, Vogel, M, Mueller, M, Aschoff, P, Beyer, T, et al. Optimized contrast-enhanced CT protocols for diagnostic whole-body 18F-FDG PET/CT: technical aspects of single-phase versus multiphase CT imaging. J Nucl Med. (2006) 47:470–6.
28. Antoch, G, Jentzen, W, Freudenberg, LS, Stattaus, J, Mueller, SP, Debatin, JF, et al. Effect of oral contrast agents on computed tomography-based positron emission tomography attenuation correction in dual-modality positron emission tomography/computed tomography imaging. Investig Radiol. (2003) 38:784–9.
29. Cohade, C, Osman, M, Nakamoto, Y, Marshall, LT, Links, JM, Fishman, EK, et al. Initial experience with oral contrast in PET/CT: phantom and clinical studies. J Nucl Med. (2003) 44:412–6.
30. Otsuka, H, Kubo, A, Graham, M, and Nishitani, H. The relationship between standard uptake value (SUV) and Hounsfield unit (HU) of oral contrast agent for FDG-PET/CT study. J Med Investig. (2004) 51:226–9. doi: 10.2152/jmi.51.226
31. Dizendorf, E, Hany, TF, Buck, A, von Schulthess, GK, and Burger, C. Cause and magnitude of the error induced by oral CT contrast agent in CT-based attenuation correction of PET emission studies. J Nucl Med. (2003) 44:732–8.
32. Pickhardt, PJ. Positive Oral contrast material for abdominal CT: current clinical indications and areas of controversy. AJR Am J Roentgenol. (2020) 215:69–78. doi: 10.2214/AJR.19.21989
33. Urrutia, M, Macharaviaya, A, and Rodriguez, R. Adverse reactions to contrast media for intravenous use. A comparison between ionic and nonionic media. Rev Med Panama. (1995) 20:20–4.
34. Nawras, M, Alyousif, Z, Beran, A, and Elsamaloty, H. The relationship between iodinated contrast material temperature and adverse reactions: a meta-analysis of 307,329 injections. Clin Imaging. (2023) 100:54–9. doi: 10.1016/j.clinimag.2023.05.006
35. Rose, TA Jr, and Choi, JW. Intravenous imaging contrast media complications: the basics that every clinician needs to know. Am J Med. (2015) 128:943–9. doi: 10.1016/j.amjmed.2015.02.018
37. Hsieh, C, Wu, SC, Kosik, RO, Huang, YC, and Chan, WP. Pharmacological prevention of hypersensitivity reactions caused by iodinated contrast media: a systematic review and meta-analysis. Diagnostics (Basel). (2022) 12:1673. doi: 10.3390/diagnostics12071673
38. Davenport, MS, Perazella, MA, Yee, J, Dillman, JR, Fine, D, McDonald, RJ, et al. Use of intravenous iodinated contrast Media in Patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. (2020) 294:660–8. doi: 10.1148/radiol.2019192094
39. Stacul, F, Bertolotto, M, Thomsen, HS, Pozzato, G, Ugolini, D, Bellin, MF, et al. Iodine-based contrast media, multiple myeloma and monoclonal gammopathies: literature review and ESUR contrast media safety committee guidelines. Eur Radiol. (2018) 28:683–91. doi: 10.1007/s00330-017-5023-5
40. Mandlik, V, Prantl, L, and Schreyer, AG. Contrast media extravasation in CT and MRI - a literature review and strategies for therapy. Rofo. (2019) 191:25–32. doi: 10.1055/a-0628-7095
41. Heshmatzadeh Behzadi, A, Farooq, Z, Newhouse, JH, and Prince, MR. MRI and CT contrast media extravasation: a systematic review. Medicine (Baltimore). (2018) 97:e0055. doi: 10.1097/MD.0000000000010055
42. Silva, HCS, Bitencourt, AGV, and Chojniak, R. Extravasation of iodinated contrast medium in cancer patients undergoing computed tomography. Radiol Bras. (2018) 51:236–41. doi: 10.1590/0100-3984.2017.0064
43. Andreucci, M, Solomon, R, and Tasanarong, A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int. (2014) 2014:741018:1–20. doi: 10.1155/2014/741018
44. Duirk, SE, Lindell, C, Cornelison, CC, Kormos, J, Ternes, TA, Attene-Ramos, M, et al. Formation of toxic iodinated disinfection by-products from compounds used in medical imaging. Environ Sci Technol. (2011) 45:6845–54. doi: 10.1021/es200983f
45. Sengar, A, and Vijayanandan, A. Comprehensive review on iodinated X-ray contrast media: complete fate, occurrence, and formation of disinfection byproducts. Sci Total Environ. (2021) 769:144846. doi: 10.1016/j.scitotenv.2020.144846
46. Dekker, HM, Stroomberg, GJ, and Prokop, M. Tackling the increasing contamination of the water supply by iodinated contrast media. Insights Imaging. (2022) 13:30. doi: 10.1186/s13244-022-01175-x
47. Tatar, G, Cermik, TF, Karagoz, Y, Gundogan, C, Karacetin, D, Yildiz, E, et al. The value of whole-body contrast-enhanced 18F-FDG PET/CT imaging in the diagnosis and staging of patients with laryngeal carcinoma. Nucl Med Commun. (2018) 39:334–42. doi: 10.1097/MNM.0000000000000809
48. Sheikhbahaei, S, Taghipour, M, Ahmad, R, Fakhry, C, Kiess, AP, Chung, CH, et al. Diagnostic accuracy of follow-up FDG PET or PET/CT in patients with head and neck Cancer after definitive treatment: a systematic review and Meta-analysis. AJR Am J Roentgenol. (2015) 205:629–39. doi: 10.2214/AJR.14.14166
49. Kyzas, PA, Evangelou, E, Denaxa-Kyza, D, and Ioannidis, JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. (2008) 100:712–20. doi: 10.1093/jnci/djn125
50. Rusthoven, KE, Koshy, M, and Paulino, AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. (2004) 101:2641–9. doi: 10.1002/cncr.20687
51. Lassen, P, Primdahl, H, Johansen, J, Kristensen, CA, Andersen, E, Andersen, LJ, et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. (2014) 113:310–6. doi: 10.1016/j.radonc.2014.11.032
52. Economopoulou, P, de Bree, R, Kotsantis, I, and Psyrri, A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front Oncol. (2019) 9:827. doi: 10.3389/fonc.2019.00827
53. Sakso, M, Mortensen, LS, Primdahl, H, Johansen, J, Kallehauge, J, Hansen, CR, et al. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: long-term results from the DAHANCA 24 trial (NCT01017224). Radiother Oncol. (2020) 151:126–33. doi: 10.1016/j.radonc.2020.08.006
54. Nesteruk, M, Lang, S, Veit-Haibach, P, Studer, G, Stieb, S, Glatz, S, et al. Tumor stage, tumor site and HPV dependent correlation of perfusion CT parameters and [18F]-FDG uptake in head and neck squamous cell carcinoma. Radiother Oncol. (2015) 117:125–31. doi: 10.1016/j.radonc.2015.09.026
55. Suenaga, Y, Kitajima, K, Ishihara, T, Sasaki, R, Otsuki, N, Nibu, K, et al. FDG-PET/contrast-enhanced CT as a post-treatment tool in head and neck squamous cell carcinoma: comparison with FDG-PET/non-contrast-enhanced CT and contrast-enhanced CT. Eur Radiol. (2016) 26:1018–30. doi: 10.1007/s00330-015-3902-1
56. Brendle, CB, Aschoff, P, Kratt, T, Schraml, C, Reimold, M, Claussen, CD, et al. Is there any additional benefit of contrast-enhanced CT as part of routine PET/CT protocols for the differentiation of suspicious incidental gastrointestinal 2-deoxy-(18)F-FDG uptake? Korean J Radiol. (2013) 14:951–9. doi: 10.3348/kjr.2013.14.6.951
57. Jimenez Londono, GA, Garcia Vicente, AM, Sanchez Perez, V, Jimenez Aragon, F, Leon Martin, A, Cano Cano, JM, et al. (1)(8)F-FDG PET/contrast enhanced CT in the standard surveillance of high risk colorectal cancer patients. Eur J Radiol. (2014) 83:2224–30. doi: 10.1016/j.ejrad.2014.08.016
58. Tateishi, U, Maeda, T, Morimoto, T, Miyake, M, Arai, Y, and Kim, EE. Non-enhanced CT versus contrast-enhanced CT in integrated PET/CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging. (2007) 34:1627–34. doi: 10.1007/s00259-007-0455-9
59. Zhang, J, Zuo, CJ, Jia, NY, Wang, JH, Hu, SP, Yu, ZF, et al. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J Gastroenterol. (2015) 21:2988–96. doi: 10.3748/wjg.v21.i10.2988
60. Apitzsch, J, Verburg, FA, Mottaghy, F, and Heinzel, A. Use of full-dose contrast-enhanced CT for extrahepatic staging using Gallium-68-DOTATATE PET/CT in patients with neuroendocrine tumors. Diagn Interv Radiol. (2021) 27:573–9. doi: 10.5152/dir.2021.19424
61. Cronin, CG, Prakash, P, and Blake, MA. Oral and IV contrast agents for the CT portion of PET/CT. AJR Am J Roentgenol. (2010) 195:W5–w13. doi: 10.2214/AJR.09.3844
62. Blake, MA, Setty, BN, Cronin, CG, Kalra, M, Holalkere, NS, Fischman, AJ, et al. Evaluation of the effects of oral water and low-density barium sulphate suspension on bowel appearance on FDG-PET/CT. Eur Radiol. (2010) 20:157–64. doi: 10.1007/s00330-009-1527-y
63. Groves, AM, Kayani, I, Dickson, JC, Townsend, C, Croasdale, I, Syed, R, et al. Oral contrast medium in PET/CT: should you or shouldn't you? Eur J Nucl Med Mol Imaging. (2005) 32:1160–6. doi: 10.1007/s00259-005-1833-9
64. Chen, YK, Chen, JH, Tsui, CC, Chou, HH, Cheng, RH, and Chiu, JS. Use of laxative-augmented contrast medium in the evaluation of colorectal foci at FDG PET. Radiology. (2011) 259:525–33. doi: 10.1148/radiol.11101193
65. Guo, L, and Shen, G. Enterovesical fistula in a lymphoma patient revealed by FDG PET/CT with an Oral contrast agents. Clin Nucl Med. (2024) 49:e38–9. doi: 10.1097/RLU.0000000000004954
66. Otsuka, H, Graham, MM, Kubo, A, and Nishitani, H. The effect of oral contrast on large bowel activity in FDG-PET/CT. Ann Nucl Med. (2005) 19:101–8. doi: 10.1007/BF03027388
67. Meyer, SA, and Gawde, S. Utility of negative oral contrast (milk with 4% fat) in PET-CT studies. Indian J Nucl Med. (2012) 27:151–5. doi: 10.4103/0972-3919.112719
68. Antoch, G, Kuehl, H, Kanja, J, Lauenstein, TC, Schneemann, H, Hauth, E, et al. Dual-modality PET/CT scanning with negative oral contrast agent to avoid artifacts: introduction and evaluation. Radiology. (2004) 230:879–85. doi: 10.1148/radiol.2303021287
69. Sun, XG, Huang, G, Liu, JJ, and Wan, LR. Comparison of the effect of positive and negative oral contrast agents on (18)F-FDG PET/CT scan. Hell J Nucl Med. (2009) 12:115–8.
70. Otero, HJ, Yap, JT, Patak, MA, Erturk, SM, Israel, DA, Johnston, CJ, et al. Evaluation of low-density neutral oral contrast material in PET/CT for tumor imaging: results of a randomized clinical trial. AJR Am J Roentgenol. (2009) 193:326–32. doi: 10.2214/AJR.08.1565
71. Tawakol, A, Abdelhafez, YG, Osama, A, Hamada, E, and El Refaei, S. Diagnostic performance of 18F-FDG PET/contrast-enhanced CT versus contrast-enhanced CT alone for post-treatment detection of ovarian malignancy. Nucl Med Commun. (2016) 37:453–60. doi: 10.1097/MNM.0000000000000477
72. Kitajima, K, Murakami, K, Yamasaki, E, Kaji, Y, Fukasawa, I, Inaba, N, et al. Diagnostic accuracy of integrated FDG-PET/contrast-enhanced CT in staging ovarian cancer: comparison with enhanced CT. Eur J Nucl Med Mol Imaging. (2008) 35:1912–20. doi: 10.1007/s00259-008-0890-2
73. Kitajima, K, Ueno, Y, Suzuki, K, Kita, M, Ebina, Y, Yamada, H, et al. Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT scans for diagnosing ovarian cancer recurrence. Eur J Radiol. (2012) 81:3557–62. doi: 10.1016/j.ejrad.2012.03.020
74. Massollo, M, Fiz, F, Bottoni, G, Ugolini, M, Paparo, F, Puppo, C, et al. To enhance or not to enhance? The role of contrast medium (18)F-FDG PET/CT in recurrent ovarian carcinomas. Medicina (Kaunas). (2021) 57:561. doi: 10.3390/medicina57060561
75. Kitajima, K, Murakami, K, Yamasaki, E, Domeki, Y, Kaji, Y, Fukasawa, I, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent ovarian cancer: comparison with integrated FDG-PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. (2008) 35:1439–48. doi: 10.1007/s00259-008-0776-3
76. Kitajima, K, Suzuki, K, Senda, M, Kita, M, Nakamoto, Y, Sakamoto, S, et al. Preoperative nodal staging of uterine cancer: is contrast-enhanced PET/CT more accurate than non-enhanced PET/CT or enhanced CT alone? Ann Nucl Med. (2011) 25:511–9. doi: 10.1007/s12149-011-0496-9
77. Kitajima, K, Suzuki, K, Nakamoto, Y, Onishi, Y, Sakamoto, S, Senda, M, et al. Low-dose non-enhanced CT versus full-dose contrast-enhanced CT in integrated PET/CT studies for the diagnosis of uterine cancer recurrence. Eur J Nucl Med Mol Imaging. (2010) 37:1490–8. doi: 10.1007/s00259-010-1440-2
78. Kitajima, K, Murakami, K, Yamasaki, E, Domeki, Y, Kaji, Y, Morita, S, et al. Performance of integrated FDG-PET/contrast-enhanced CT in the diagnosis of recurrent uterine cancer: comparison with PET and enhanced CT. Eur J Nucl Med Mol Imaging. (2009) 36:362–72. doi: 10.1007/s00259-008-0956-1
79. Pfluger, T, Melzer, HI, Schneider, V, La Fougere, C, Coppenrath, E, Berking, C, et al. PET/CT in malignant melanoma: contrast-enhanced CT versus plain low-dose CT. Eur J Nucl Med Mol Imaging. (2011) 38:822–31. doi: 10.1007/s00259-010-1702-z
80. Morimoto, T, Tateishi, U, Maeda, T, Arai, Y, Nakajima, Y, and Edmund, KE. Nodal status of malignant lymphoma in pelvic and retroperitoneal lymphatic pathways: comparison of integrated PET/CT with or without contrast enhancement. Eur J Radiol. (2008) 67:508–13. doi: 10.1016/j.ejrad.2007.08.017
81. Chiaravalloti, A, Danieli, R, Caracciolo, CR, Travascio, L, Cantonetti, M, Gallamini, A, et al. Initial staging of Hodgkin's disease: role of contrast-enhanced 18F FDG PET/CT. Medicine (Baltimore). (2014) 93:e50. doi: 10.1097/MD.0000000000000050
82. Paone, G, Raditchkova-Sarnelli, M, Ruberto-Macchi, T, Cuzzocrea, M, Zucca, E, Ceriani, L, et al. Limited benefit of additional contrast-enhanced CT to end-of-treatment PET/CT evaluation in patients with follicular lymphoma. Sci Rep. (2021) 11:18496. doi: 10.1038/s41598-021-98081-x
83. Milgrom, SA, Rechner, L, and Berthelsen, A. The optimal use of PET/CT in the management of lymphoma patients. Br J Radiol. (2021) 94:20210470. doi: 10.1259/bjr.20210470
84. Qi, Z, Gates, EL, O'Brien, MM, and Trout, AT. Radiation dose reduction through combining positron emission tomography/computed tomography (PET/CT) and diagnostic CT in children and young adults with lymphoma. Pediatr Radiol. (2018) 48:196–203. doi: 10.1007/s00247-017-4019-2
85. Gundogan, C, Cermik, TF, Erkan, E, Yardimci, AH, Behzatoglu, K, Tatar, G, et al. Role of contrast-enhanced 18F-FDG PET/CT imaging in the diagnosis and staging of renal tumors. Nucl Med Commun. (2018) 39:1174–82. doi: 10.1097/MNM.0000000000000915
86. Jain, A, Srivastava, MK, Pawaskar, AS, Shelley, S, Elangovan, I, Jain, H, et al. Contrast-enhanced [18F] fluorodeoxyglucose-positron emission tomography-computed tomography as an initial imaging modality in patients presenting with metastatic malignancy of undefined primary origin. Indian J Nucl Med. (2015) 30:213–20. doi: 10.4103/0972-3919.158529
87. Husmann, L, Huellner, MW, Ledergerber, B, Eberhard, N, Kaelin, MB, Anagnostopoulos, A, et al. Diagnostic accuracy of PET/CT and contrast enhanced CT in patients with suspected infected aortic aneurysms. Eur J Vasc Endovasc Surg. (2020) 59:972–81. doi: 10.1016/j.ejvs.2020.01.032
88. Writing Group, Reviewer Group, Members of EANM Cardiovascular, Members of EANM Infection & Inflammation, Members of Committees, SNMMI Cardiovascular, Members of Council, PET Interest Group, Members of ASNC, EANM Committee CoordinatorSlart, RHJA. FDG-PET/CT(a) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET interest group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. (2018) 45:1250–69. doi: 10.1007/s00259-018-3973-8
89. Delgado, V, Ajmone Marsan, N, de Waha, S, Bonaros, N, Brida, M, Burri, H, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. (2023) 44:3948–4042. doi: 10.1093/eurheartj/ehad193
90. Lubetsky, A. Pulmonary embolism in Cancer patients: a review. Isr Med Assoc J. (2022) 24:179–82.
91. Piccardo, A, Bottoni, G, Boccalatte, LA, Camponovo, C, Musumeci, M, Bacigalupo, L, et al. Head-to-head comparison among (18)F-choline PET/CT, 4D contrast-enhanced CT, and (18)F-choline PET/4D contrast-enhanced CT in the detection of hyperfunctioning parathyroid glands: a systematic review and meta-analysis. Endocrine. (2021) 74:404–12. doi: 10.1007/s12020-021-02798-8
92. Piccardo, A, Trimboli, P, Rutigliani, M, Puntoni, M, Foppiani, L, Bacigalupo, L, et al. Additional value of integrated (18)F-choline PET/4D contrast-enhanced CT in the localization of hyperfunctioning parathyroid glands and correlation with molecular profile. Eur J Nucl Med Mol Imaging. (2019) 46:766–75. doi: 10.1007/s00259-018-4147-4
93. Mayerhoefer, ME, Schuetz, M, Magnaldi, S, Weber, M, Trattnig, S, and Karanikas, G. Are contrast media required for (68)Ga-DOTATOC PET/CT in patients with neuroendocrine tumours of the abdomen? Eur Radiol. (2012) 22:938–46. doi: 10.1007/s00330-011-2328-7
94. Ruf, J, Heuck, F, Schiefer, J, Denecke, T, Elgeti, F, Pascher, A, et al. Impact of multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology. (2010) 91:101–9. doi: 10.1159/000265561
95. Ruf, J, Schiefer, J, Furth, C, Kosiek, O, Kropf, S, Heuck, F, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: spotlight on the CT phases of a triple-phase protocol. J Nucl Med. (2011) 52:697–704. doi: 10.2967/jnumed.110.083741
96. Winiger, A, Pérez Lago, MDS, Lehnick, D, Roos, JE, and Strobel, K. The value of intravenous contrast medium in PSMA PET/CT imaging in patients with biochemical recurrence of prostate cancer. Nucl Med Commun. (2021) 42:1239–46. doi: 10.1097/MNM.0000000000001453
97. Iravani, A, Hofman, MS, Mulcahy, T, Williams, S, Murphy, D, Parameswaran, BK, et al. (68)Ga PSMA-11 PET with CT urography protocol in the initial staging and biochemical relapse of prostate cancer. Cancer Imaging. (2017) 17:31. doi: 10.1186/s40644-017-0133-5
98. Rosar, F, Hügle, MJ, Ries, M, Bartholomä, M, Maus, S, Fries, P, et al. Benefit of including CT urography in [68Ga]PSMA-11 PET/CT with low-dose CT: first results from a larger prostate cancer cohort analysis. Q J Nucl Med Mol Imaging. (2022) 66:280–9. doi: 10.23736/S1824-4785.20.03224-0
99. Tulipan, AJ, Guzman, AJ, Haslerud, TM, Foldnes, K, Kvernenes, OH, Honoré, A, et al. Enhancing PSMA-PET/CT with intravenous contrast: improved tracer clearance in the prostate bed. Nuklearmedizin. (2022) 61:394–401. doi: 10.1055/a-1821-8112
Keywords: PET/CT, iodine, contrast enhanced, one-shot, examination
Citation: Metrard G, Cohen C and Bailly M (2024) Comprehensive literature review of oral and intravenous contrast-enhanced PET/CT: a step forward? Front. Med. 11:1373260. doi: 10.3389/fmed.2024.1373260
Received: 19 January 2024; Accepted: 06 March 2024;
Published: 19 March 2024.
Edited by:
Nataliya Lutay, Skåne University Hospital, SwedenReviewed by:
Natale Quartuccio, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyCopyright © 2024 Metrard, Cohen and Bailly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilles Metrard, Z2lsbGVzLm1ldHJhcmRAY2h1LW9ybGVhbnMuZnI=
†These authors have contributed equally to this work
‡ORCID: Gilles Metrard, https://orcid.org/0000-0002-6479-4271
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.