
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 23 February 2024
Sec. Nuclear Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1370762
Primary pancreatic lymphoma (PPL) is a rare malignancy, which is defined as a mass centered in pancreas with involvement of contiguous lymph nodes and distant spread may exist. Accurate diagnosis of PPL prior to pathological confirmation remains challenging, underscoring the critical significance of preoperative imaging assessments. This case report collected two instances of PPL that underwent initial evaluation via 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) between August 2021 and July 2022. Correspondingly, pertinent literature encompassing 18F-FDG PET/CT data related to PPL was meticulously reviewed. Including our aforementioned pair of cases, a cumulative total of 25 instances of PPL were assembled. The distinctive profile of 18F-FDG PET/CT images of PPL predominantly manifests as hypermetabolic lesions with diminished density. Primarily characterized by singular lesions and comparatively substantial volumetric dimensions, a total of eleven cases revealed contiguous lymph node engagement, with five instances displaying distant dissemination encompassing lymph nodes in multiple locations. Amongst these, ten patients underwent sequential 18F-FDG PET/CT follow-up post-intervention. In comparison to pancreatic carcinoma, PPL lesions exhibited heightened hypermetabolism, augmented volumetric proportions, and distinct patterns of distant metastasis. This study indicates that the pivotal role of 18F-FDG PET/CT in the diagnosis and assessment of therapeutic efficacy in PPL is unequivocal. Combined with the clinical attributes of patients, the integration of 18F-FDG PET/CT augments the differential diagnostic capacity differentiating PPL from pancreatic carcinoma.
Primary pancreatic lymphoma (PPL) constitutes a rare malignancy, with an incidence rate of less than 2% among extranodal non-Hodgkin lymphomas in populations with good immunocompetent (1). The World Health Organization (WHO) has defined PPL as predominantly lymphomatous proliferation centered within the confines of the pancreas. However, involvement of contiguous lymph nodes within the pancreatic region and the possibility of distant dissemination remain plausible (2, 3), with potential associations to immunodeficiency as an underlying etiological factor. Owing to its rarity and a lack of distinct clinical manifestations, PPL poses a considerable diagnostic challenge prior to histopathological assessment, frequently leading to misdiagnoses akin to other pancreatic space-occupying lesions, such as pancreatic carcinoma (4).
Functional imaging stands as a pivotal achievement in the realm of medical imaging, enabling the simultaneous provision of metabolic and anatomical insights. Foremost among these modalities, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has emerged as a widely embraced functional imaging technique. It assumes a pivotal role in discerning differential diagnoses, facilitating staging procedures, and monitoring the trajectory of lymphomas (5, 6). However, owing to the infrequent occurrence of PPL, there exists a dearth of literature detailing the distinctive 18F-FDG PET/CT features pertinent to PPL. Notably, no comprehensive systematic review has hitherto encapsulated the value of 18F-FDG PET/CT in the context of PPL.
We report two PPL cases that underwent assessment via 18F-FDG PET/CT and discuss the potential utility of 18F-FDG PET/CT in the realm of PPL through a literature review. It is pertinent to underscore that this retrospective study has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study has been approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
A 70-year-old female presented with a one-month history of unexplained pain below the xiphoid process. She had undergone a myomectomy three decades earlier. Abdominal contrast-enhanced computed tomography (CT) disclosed a slightly hypodense space-occupying lesion within the pancreatic head, accompanied by mild dilatation of the pancreatic duct but without bile duct dilatation. The enhanced scan exhibited relatively modest enhancement. No irregularities emerged during tumor marker assessments, including CA-199, CEA, and CA-125. Laboratory analyses indicated hepatic function impairment (ALT: 539°U/L, AST: 472°U/L, GLD: 140.5°U/L, γ-GT: 615°U/L, ALP: 821°U/L, TBIL: 178.9°μmol/L, DB: 131.5°μmol/L, IBIL: 47.4°μmol/L). In order to pursue further assessment, the patient underwent an 18F-FDG PET/CT examination. The maximum intensity projection (MIP) of the 18F-FDG PET/CT divulged a hypermetabolic lesion within the upper abdomen (Figure 1A). A 9.5 × 8.3 × 7.1 cm irregular lesion situated at the pancreatic head exhibited noteworthy FDG uptake, characterized by a maximal standardized uptake value (SUVmax) of 22.4 (Figures 1B–E). Mild to moderately FDG-avid peripancreatic lymph nodes, displaying indistinct boundaries with the pancreatic mass (Figures 1B–E). Additionally, mildly hypermetabolic retroperitoneal lymph nodes were detected (Figures 1F, G). Subsequent to these findings, the patient underwent a pancreatic tumor biopsy and gastrojejunostomy under general anesthesia. The conclusive histopathological diagnosis unveiled diffuse large B-cell lymphoma of the pancreatic head, identified as the non-germinal center type. Subsequently, this patient commenced a low-dose CHOP chemotherapy regimen combined with rituximab (R-miniCHOP). Encouragingly, the patient displayed commendable tolerance and remained devoid of pronounced adverse reactions. At present, the patient is engaged in the sixth cycle of chemotherapy.
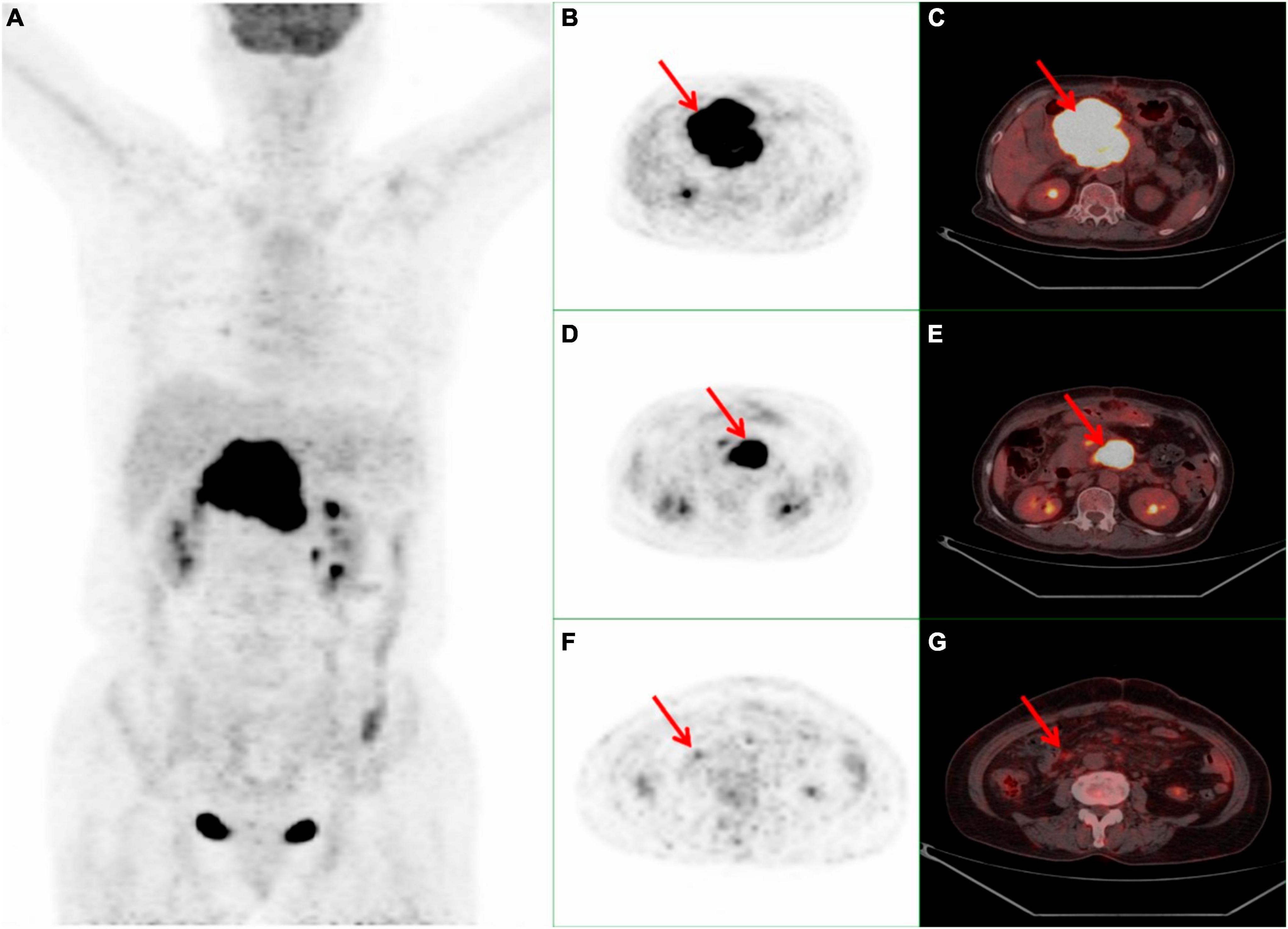
Figure 1. Whole body 18F-FDG PET/CT scan of a 70-year-old female with PPL. (A) MIP demonstrated a hypermetabolic lesion in the superior abdominal. (B,C) A 9.5 × 8.3 × 7.1 cm high FDG-avid irregular occupying at the head of the pancreas with a SUVmax 22.4 (red arrow). (D, E) Peripancreatic hypermetabolic enlarged lymph nodes (red arrow). (F, G) Suspicious mild hypermetabolic retroperitoneal lymph node with (red arrow). PPL, primary pancreatic lymphoma; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; SUVmax, maximal standardized uptake value.
A 31-year-old male presented with a persistent post-prandial stomach flatulence for a duration of 2°months, accompanied by abdominal pain lasting for 1°month. The abdominal pain exhibited mild tenderness and was not concomitant with any additional discomfort. The patient bore a medical history marked by ankylosing spondylitis and hemorrhoids. The scrutiny of tumor markers rendered abnormal outcomes: sSCC-Ag at 1.800°ng/ml, ferritin at 459.10°ng/ml, CA125 at 171.00°U/ml, and CA19-9 at 12.00°U/ml. Abdominal ultrasonography revealed no aberrations, while electronic gastroscopy indicated chronic non-atrophic gastritis. The abdominal contrast-enhanced CT unveiled occupying lesions within the pancreas, coupled with lymph node enlargement within the retroperitoneal space. Subsequent 18F-FDG PET/CT illustrated a hypermetabolic lesion situated in the upper abdomen, with peripheral subsidiary lesions evident in the maximum intensity projection (MIP) (Figure 2A). Notably, the thoracic spine also exhibited mild hypermetabolism (Figure 2A). 18F-FDG PET/CT delineated a conspicuously intense hypermetabolic lesion (SUVmax 22.0) situated at the head and neck of the pancreas. Concurrently, hypermetabolic lymph nodes in the posterior pancreatic region were observed (Figures 2B–E). Additionally, mild hypermetabolic (SUVmax 2.3) lymph nodes were found in left clavicular region and left internal mammary (Figures 2F–I). Subsequent CT-guided puncture biopsy of the pancreas yielded a diagnosis of non-Hodgkin T-cell lymphoma. Bone marrow puncture results were normal. Upon diagnosis, the patient initiated chemotherapy, commencing with one cycle of CHOP-E, followed by six cycles of CHOPE combined with chidamide, and culminating in one cycle of cyclophosphamide and etoposide. Following the administration of five chemotherapy cycles, the patient experienced neutropenia and fever, with a neutrophil count of 1.24 × 109/L and a body temperature of 37.8°C. The condition improved in response to anti-infection treatment. At present, the patient is undergoing the eighth cycle of chemotherapy.

Figure 2. Whole body 18F-FDG PET/CT scan of a 31-year-old male with PPL. (A) MIP showed a hypermetabolic mass in the superior abdominal and peripheral lesions. And mild hypermetabolic of FDG were observed in the thoracic spine. (B, C) A 5.5 × 5.4 cm high FDG-avid mass at the head and neck of the pancreas with SUVmax 22.0 (red arrow). (D,E) Hypermetabolic lymph nodes in the posterior of pancreatic region (red arrow). (F,G) Left clavicular region lymph node with mild FDG hypermetabolism (red arrow). (H, I) Suspicious mild hypermetabolism within the left internal mammary lymph nodes (red arrow). PPL, primary pancreatic lymphoma; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; MIP, maximum intensity projection; SUVmax, maximal standardized uptake value; CT, computed tomography.
We present two cases of primary pancreatic lymphoma diagnosed with 18F-FDG PET/CT assistance in this article. For further investigation, we conducted an exhaustive literature review encompassing publications until July 2023, sourced from PubMed, Embase, and Web of Science databases. The predetermined inclusion criteria comprised the following aspects: Publications authored in the English language. Works involving human subjects. Cases of PPL that were accompanied by 18F-FDG PET/CT examination data.
The systematic search of the literature, a cumulative total of 19 articles (including 23 instances of PPL) (7–25) met the stipulated inclusion criteria. Incorporating our own two cases, the collective review encapsulated a total of 25 instances of PPL (including 31 lesions). The data assimilation process encompassed a spectrum of aspects, including age, gender, presenting symptoms, pathological findings, treatment modalities, prognosis, laboratory examination results, as well as morphological and PET imaging attributes of the respective cases. We conducted a comprehensive analysis of the data in the literatures and a descriptive statistical analysis of the counting data recorded in the literature. Data sets exhibiting a normal distribution were represented statistically in the format of mean ± standard deviation, whereas those deviating from the norm were expressed in the format of M (P25, P75).
The clinical manifestations of primary pancreatic lymphoma lack specificity, the clinical datas of our PPL cases are summarized in Table 1. Among the cases, a predominant male preponderance was evident, exhibiting a male-to-female ratio of 2.125:1. A total of 24 cases were documented with clinical symptoms, with gastrointestinal symptoms, particularly abdominal pain, prevailing as the most commonly reported clinical manifestation (15 out of 24 cases, 62.5%). All cases in our study were subjected to pathological confirmation, and DLBCL emerged as the principal subtype (40%). Our results are consistent with previous studies (26, 27).
Past investigations revealed laboratory evaluations often disclose abnormal liver function and escalated pancreatic enzyme levels, whereas elevated tumor markers assume less prevalence. Remarkably, certain patients showcase an increase in CA199 (4, 26, 28, 29). Aberrant liver function might be attributed to a sizable mass situated at the pancreatic head, causing obstructions within the common bile duct. In Our cases, sixteen cases possessed recorded laboratory test results, as detailed in Table 2, 80% (4 out of 5) of our cases characterized by irregular liver function had lesions located within the pancreatic head. And three cases evidenced elevated tumor markers (including CEA, CA199, and CA125), two cases demonstrated heightened levels of pancreatic enzymes (lipase and amylase).
In the realm of treatment, chemotherapy represents the primary modality for PPL (30). Solely relying on surgical intervention typically yields inferior prognoses compared to cases treated exclusively with chemotherapy. Consequently, surgery is better positioned as a diagnostic or palliative strategy (26, 31, 32). The post-chemotherapy prognosis for PPL is generally promising (26, 33), while advanced age (≥60 years), female gender, unmarried status, higher staging, and absence of chemotherapy emerge as adverse prognostic indicators for PPL (31, 32). Relapses within PPL predominantly manifest at distant sites, with pancreatic relapses being comparatively infrequent. Remarkably, a risk of central nervous system (CNS) relapse is also underscored (26, 32). In our cases, a Eighteen cases underwent chemotherapy, with the rituximab-combined CHOP regimen (R-CHOP) standing out as the prevailing therapeutic approach. Only one patient succumbed to septic shock 5°weeks post-diagnosis, while the remaining cases continue to exhibit vitality.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) offers the dual advantage of functional and morphological imaging. On CT scans, discerning a relatively sizeable mass in the pancreatic head devoid of pancreatic duct dilation reinforces the suspicion of PPL. Notable features include a pancreatic duct that generally remains either normal or exhibits mild displacement and constriction and invasive pancreatic masses infiltrating the pancreatic borders, along with enlarged lymph nodes below the renal vein level. Furthermore, untreated PPL seldom demonstrates calcification or necrosis. Bile duct dilation attributed to obstruction predominantly characterizes PPL situated at the pancreatic head (34, 35). The prevailing CT characteristics gleaned from the PPL cases incorporated within this study correspond with localized, substantial, low-density masses, with only three instances featuring mild pancreatic duct dilation. A substantial proportion (19 out of 25 cases, 76%) exhibited solitary lesions, while the remaining six cases demonstrated the presence of dual lesions. Notably, no preferential localization for lesions emerged, with 17 lesions situated in the pancreatic head and neck region, and 14 lesions located within the body and tail of the pancreas. The lesion dimensions were relatively expansive, with the median of maximum diameter recorded in 21 cases is 4.30 (3.15, 7.80) cm. Moreover, two cases of pancreatic head lesions engendered intrahepatic bile duct dilation, aligning seamlessly with prior investigations. The morphological imaging findings for the array of PPL cases are meticulously compiled within Table 3.
The most distinctive PET hallmark of PPL resides in the conspicuous hypermetabolic lesion discernible within the pancreas. Among the accumulated cases, a total of 16 lesions featured the documentation of the maximal standardized uptake value (SUVmax), yielding an average SUVmax of 14.37 ± 7.95, with a range spanning from 4.10 to 28.00. Furthermore, eleven instances evidenced the involvement of contiguous lymph nodes, of which five cases exhibited evidence of distant dissemination, encompassing lymph nodes in various locations including the splenic hilum, left kidney hilum, lower esophageal region, left supraclavicular region, and left internal mammary region. Notably, one case disclosed FDG uptake within the left adrenal gland, attributed to tumor invasion. The PET imaging findings for the array of PPL cases are meticulously compiled within Table 4.
The versatility of 18F-FDG PET/CT extends to its ability to discern glycometabolic changes within lesions prior to observable anatomical alterations, rendering it an invaluable asset for baseline assessment and the assessment of lymphoma treatment efficacy (5, 6, 36). In our study, two instances (9, 20) unveiled smaller pancreatic lesions that evaded detection via CT and MRI, accompanied by comparatively lower FDG uptake than their larger counterparts. Notably, among our cases, ten subjects underwent post-treatment 18F-FDG PET/CT evaluations, uncovering two cases of recurrence within the pancreas and peripancreatic lymph nodes. Following a change in chemotherapy regimen, subsequent scans signaled remission. The remaining cases indicated a state of remission on 18F-FDG PET/CT scans post-treatment.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT)’s significance in staging diverse lymphoma categories, particularly DLBCL, is profound. Moreover, for indolent lymphomas, 18F-FDG PET/CT emerges as a pivotal tool for identifying histological transformations from low-grade indolent lymphomas to more aggressive high-grade forms (36). One of the rationale behind 18F-FDG PET/CT’s superior staging potential compared to contrast-enhanced CT lies in its heightened sensitivity in detecting bone marrow involvement. Nevertheless, consensus remains elusive regarding whether diffuse FDG uptake in bone marrow should be construed as a definitive marker of bone marrow involvement (37). Prior investigations have suggested that diffuse bone marrow uptake in DLBCL signifies an elevated likelihood of bone marrow invasion. Conversely, diffuse bone marrow uptake in Hodgkin lymphoma (HL) is more likely indicative of bone marrow inflammatory changes (38). Nonetheless, as these studies feature relatively modest sample sizes, further research is warranted to elucidate this matter. An illustrative case within our study exhibited mild diffuse hypermetabolism in the thoracic vertebrae, despite normal findings from a bone marrow biopsy.
According to the WHO’s classification, PPL can indeed entail contiguous region lymph node involvement and distant spread, while the core of the lesion resides within the pancreas. Our study underscores that PPL commonly involves contiguous region lymph nodes, but distant spread is less prevalent. The distinction between PPL and secondary pancreatic lymphoma hinges on the status of lesions beyond the pancreas, with 18F-FDG PET/CT emerging as pivotal in this regard. 18F-FDG PET/CT effectively detects the primary lesion and distant spread of PPL, proficiently demarcating it from secondary pancreatic lymphoma. Compared with the 18F-FDG PET/CT findings of PPL, there are more lesions in other parts of secondary pancreatic lymphoma and higher uptake of FDG. Intriguingly, the pancreatic lesions in secondary pancreatic lymphoma are similar to those of PPL, characterized predominantly by sizeable hypermetabolic masses (39).
The precise differentiation between PPL and pancreatic carcinoma assumes paramount importance. Given the relatively favorable prognosis associated with PPL following chemotherapy (26, 33), accurate distinction from pancreatic carcinoma holds potential to avert unnecessary surgical interventions. At times, PPL proves elusive to traditional diagnostic techniques like computed tomography (CT) or magnetic resonance imaging (MRI). Of the cases assimilated within this study, 14 instances garnered a preliminary diagnostic conclusion via traditional imaging (CT or MRI) prior to pathological confirmation. Regrettably, a mere three cases received a lymphoma diagnosis, yielding a diagnostic accuracy of 21.4%, and predominantly manifesting as misdiagnoses spanning pancreatic cancer or malignant pancreatic masses. Pancreatic carcinoma exhibits a higher prevalence among males, with its zenith incidence occurring beyond the age of 70 years (40). In accordance with extant literature reports (27), PPL also displays a male predilection, yet its onset is characteristically earlier than that of pancreatic carcinoma, with a median age of 67. Additionally, a study (41) uncovered elevated CA199 levels in instances of pancreatic carcinoma (406.81 ± 352.09), while liver function (ALT, AST, ALP, etc.) and pancreatic enzymes were generally in a normal range. This stands in contrast to the laboratory findings in cases of PPL. Through an exhaustive scrutiny of antecedent literature (42, 43) including 230 instances of pathologically confirmed pancreatic carcinoma, it is established that pancreatic carcinoma predominantly manifests as hypoattenuated lesions during CT enhancement scans, occasionally displaying isodensity. The typical maximum diameter of isodensity pancreatic carcinoma lesions seldom exceeds 2 cm, while their hypoattenuated counterparts demonstrate an average maximum diameter of approximately 3 cm. Moreover, characteristic imaging attributes of pancreatic carcinoma encompass pancreatic ductal truncation, dilation of pancreatic ducts, and cholangiectasis (42, 44). Contrasting with the PPL cases expounded within this exposition, it becomes evident that PPL lesions generally exhibit relatively greater dimensions. Notably, the dilation of the bile duct and pancreatic duct is a rarity in PPL cases; however, akin to pancreatic carcinoma, both maladies predominantly showcase hypoattenuated lesions upon contrast-enhanced CT imaging.
As per antecedent investigations, both pancreatic carcinoma and PPL predominantly exhibit hypermetabolic lesions on 18F-FDG PET/CT scans. In the study by Moon (45), the SUVmax of 21 pancreatic carcinoma lesions registered at 6.8 ± 3.0, with a range spanning 2−12. An examination by Zhang et al. (41) on 40 patients with pancreatic carcinoma unveiled an average SUVmax of 7.30 ± 3.21 for 40 pancreatic carcinoma lesions. In a study conducted by Lee et al. (46) encompassing 87 cases of pancreatic carcinoma, the median SUVmax for pancreatic carcinoma lesions was 4.4, oscillating between 2.1 and 17.3. Drawing from the aforementioned investigations, when juxtaposed against the SUVmax value of PPL documented within our discourse (14.37 ± 7.95), it becomes apparent that the SUVmax of pancreatic carcinoma cases tends to be lower in comparison to those of PPL cases. Furthermore, the metastatic dissemination patterns for these two conditions deviate. Predominantly, pancreatic adenocarcinoma metastasizes to the liver, subsequently to the peritoneum, abdominal lymph nodes, and harbors a notable likelihood for lung metastasis. As a rule, pancreatic adenocarcinoma engages in multi-organ metastasis (47). In our analysis, PPL demonstrates a scarcity of distant dissemination, with sporadic instances of distant lymph node metastasis, thereby markedly diverging from the distant metastatic mode typified by pancreatic adenocarcinoma.
In summation, 18F-FDG PET/CT, functioning as a non-invasive functional imaging modality, assumes a pivotal role in diagnosing, initially assessing, and gauging the efficacy of treatment for PPL. Concurrently, the amalgamation of 18F-FDG PET/CT image attributes with clinical profiles and laboratory analyses possesses the potential to furnish more insightful information, facilitating the discrimination between PPL and pancreatic carcinoma.
Primary pancreatic lymphoma (PPL) constitutes a rare malignancy, with an incidence rate of less than 2% among extranodal non-Hodgkin lymphomas in populations with good immunocompetent. Owing to its rarity and a lack of distinct clinical manifestations, PPL poses a considerable diagnostic challenge prior to histopathological assessment, frequently leading to misdiagnoses akin to other pancreatic space-occupying lesions, such as pancreatic carcinoma.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has emerged as a widely embraced functional imaging technique. It assumes a pivotal role in discerning differential diagnoses, facilitating staging procedures, and monitoring the trajectory of lymphomas. However, owing to the infrequent occurrence of PPL, there exists a dearth of literature detailing the distinctive 18F-FDG PET/CT features pertinent to PPL.
The purpose of this article is to explore the potential value of 18F-FDG PET/CT in the diagnosis and treatment framework of PPL. By reporting two cases and literature review, a total of 25 cases (including 31 lesions) assessed by 18F-FDG PET/CT before treatment were included. The results of this study reveal that 18F-FDG PET/CT has important value in the diagnosis of PPL and the differential diagnosis between PPL and pancreatic carcinoma, and can effectively evaluate the therapeutic effect of PPL.
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Medical Ethics Committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JW: Conceptualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. HL: Formal Analysis, Investigation, Methodology, Writing – review & editing. JZ: Formal Analysis, Investigation, Methodology, Writing – review & editing. XL: Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hedgire SS, Kudrimoti S, Oliveira IS, Nadkarni N, McDermott S, Hahn PF, et al. Extranodal lymphomas of abdomen and pelvis: imaging findings and differential diagnosis. Abdomin Radiol. (2017) 42:1096–112. doi: 10.1007/s00261-016-0964-8
2. Hamilton SR, Aaltonen LA editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press (2000).
3. Facchinelli D, Boninsegna E, Visco C, Tecchio C. Primary pancreatic lymphoma: recommendations for diagnosis and management. J Blood Med. (2021) 12:257–67. doi: 10.2147/jbm.S273095
4. Rad N, Khafaf A, Mohammad Alizadeh AH. Primary pancreatic lymphoma: what we need to know. J Gastrointest Oncol. (2017) 8:749–57. doi: 10.21037/jgo.2017.06.03
5. Even-Sapir E, Lievshitz G, Perry C, Herishanu Y, Lerman H, Metser U. Fluorine-18 fluorodeoxyglucose Pet/Ct patterns of extranodal involvement in patients with non-hodgkin lymphoma and Hodgkin’s disease. PET Clin. (2006) 1:251–63. doi: 10.1016/j.cpet.2006.04.007
6. Paes FM, Kalkanis DG, Sideras PA, Serafini AN. Fdg Pet/Ct of extranodal involvement in non-hodgkin lymphoma and hodgkin disease. Radiographics. (2010) 30:269–91. doi: 10.1148/rg.301095088
7. Chun K, Ahn B, Song I. A case of primary pancreatic lymphoma mimicking pancreatic adenocaecinoma. HPB. (2011) 13 (Suppl. 3):174–244.
8. Yoon SN, Lee MH, Yoon JK. F-18 Fdg positron emission tomography findings in primary pancreatic lymphoma. Clin Nucl Med. (2004) 29:574–5. doi: 10.1097/01.rlu.0000135269.00531.f8
9. Abe Y, Tamura K, Sakata I, Ishida J, Mukai M, Ohtaki M, et al. Unique intense uptake demonstrated by (18)F-Fdg positron emission tomography/computed tomography in primary pancreatic lymphoma: a case report. Oncol Lett. (2010) 1:605–7. doi: 10.3892/ol_00000107
10. Savari O, Al-Duwal Z, Wang Z, Ganesan S, Danan-Rayes R, Ayub S. Pancreatic lymphoma: a cytologic diagnosis challenge. Diagn Cytopathol. (2020) 48:350–5. doi: 10.1002/dc.24349
11. Yadav R, Agarwal GR, Gupta Y, Sharma V, Gupta A. Primary pancreatic lymphoma-diagnosed on computed tomography: a rare case report. Radiol Case Rep. (2022) 17:1831–5. doi: 10.1016/j.radcr.2022.02.058
12. Nakamura T, Ito T, Abe Y, Izutsu K, Gibo J, Itaba S, et al. Primary pancreatic low-grade mucosa-associated lymphoid tissue lymphoma presenting with multiple masses. Clin J Gastroenterol. (2008) 1:168–73. doi: 10.1007/s12328-008-0028-x
13. León-Asuero-Moreno I, Martín-Marcuartu JJ, de-Bonilla-Damiá Á, Jiménez-Hoyuela-García JM. Primary pancreatic lymphoma in a (18)F-Fdg Pet/Ct study. Rev Esp Med Nucl Imagen Mol. (2021) 40:249–50. doi: 10.1016/j.remnie.2020.09.005
14. Cagle BA, Holbert BL, Wolanin S, Tappouni R, Lalwani N. Knife wielding radiologist: a case report of primary pancreatic lymphoma. Eur J Radiol Open. (2018) 5:141–6. doi: 10.1016/j.ejro.2018.08.007
15. Jonnalagadda PB, Sankar S, Suresh KP, Chinni VA. Primary mantle cell lymphoma of pancreas-rare case. Sch J Med Case Rep. (2022) 1:12–6.
16. Yamai T, Ikezawa K, Daiku K, Maeda S, Abe Y, Kai Y, et al. Primary pancreatic mantle cell lymphoma diagnosed via endoscopic ultrasound-guided fine-needle aspiration. Case Rep Gastroenterol. (2021) 15:482–7. doi: 10.1159/000515570
17. Shapira G, Fisher Y, Ilivitzki A. Bifocal primary pancreatic Burkitt’s lymphoma in a 4-year-old child. J Clin Ultras. (2017) 45:171–4. doi: 10.1002/jcu.22372
18. Liu CH, Deng MW, Ji R, Wang YQ, Ng KK, Qiu S, et al. Pylorus-Preserving pancreaticoduodenectomy with reconstruction of portal vein for primary pancreatic lymphoma: one case report. Sci Educ Publish. (2019) 7:52–5.
19. Okamoto T, Sasaki T, Nishimura N, Takamatsu M, Mori C, Mie T, et al. Pancreatic follicular lymphoma: a report of two cases and literature review. Clin J Gastroenterol. (2021) 14:1756–65. doi: 10.1007/s12328-021-01507-2
20. Bozzoli V, Tisi MC, Pianese L, Tumini S, Rufini V, Calcagni ML, et al. Primary pancreatic lymphoma in a patient with maturity onset diabetes of the young type 3. Mediterranean J Hematol Infect Dis. (2012) 4:e2012005. doi: 10.4084/mjhid.2012.005
21. Zafar Y, Kaur A, Banno F, Anuj S. Primary pancreatic lymphoma: an uncommon presentation in the pancreatic tail. Cureus. (2019) 11:e5479. doi: 10.7759/cureus.5479
22. Boninsegna E, Zamboni GA, Facchinelli D, Triantopoulou C, Gourtsoyianni S, Ambrosetti MC, et al. Ct imaging of primary pancreatic lymphoma: experience from three referral centres for pancreatic diseases. Insights Imaging. (2018) 9:17–24. doi: 10.1007/s13244-017-0585-y
23. Anand D, Lall C, Bhosale P, Ganeshan D, Qayyum A. Current update on primary pancreatic lymphoma. Abdomin Radiol. (2016) 41:347–55. doi: 10.1007/s00261-015-0620-8
24. Wang P, Cheng X, Huo L, Li F. Primary pancreatic lymphoma on Fdg Pet/Ct. Clin Nucl Med. (2020) 45:830–2. doi: 10.1097/rlu.0000000000003205
25. Nakaji S, Hirata N, Shiratori T, Kobayashi M, Fujii H, Ishii E, et al. A case of primary pancreatic lymphoblastic lymphoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Clin J Gastroenterol. (2014) 7:180–4. doi: 10.1007/s12328-014-0462-x
26. Sadot E, Yahalom J, Do RK, Teruya-Feldstein J, Allen PJ, Gönen M, et al. Clinical features and outcome of primary pancreatic lymphoma. Ann Surg Oncol. (2015) 22:1176–84. doi: 10.1245/s10434-014-4176-6
27. Mukhija D, Nagpal SJ, Sohal DP. Epidemiology, tumor characteristics, and survival in patients with primary pancreatic lymphoma: a large population-based study using the seer database. Am J Clin Oncol. (2019) 42:454–8. doi: 10.1097/coc.0000000000000544
28. Segaran N, Sandrasegaran K, Devine C, Wang MX, Shah C, Ganeshan D. Features of primary pancreatic lymphoma: a bi-institutional review with an emphasis on typical and atypical imaging features. World J Clin Oncol. (2021) 12:823–32. doi: 10.5306/wjco.v12.i9.823
29. Prayer L, Schurawitzki H, Mallek R, Mostbeck G. Ct in pancreatic involvement of non-hodgkin lymphoma. Acta Radiol. (1992) 33:123–7.
30. Battula N, Srinivasan P, Prachalias A, Rela M, Heaton N. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. (2006) 33:192–4. doi: 10.1097/01.mpa.0000227910.63579.15
31. Mishra MV, Keith SW, Shen X, Bar Ad V, Champ CE, Biswas T. Primary pancreatic lymphoma: a population-based analysis using the seer program. Am J Clin Oncol. (2013) 36:38–43. doi: 10.1097/COC.0b013e3182354bbb
32. Facchinelli D, Sina S, Boninsegna E, Borin A, Tisi MC, Piazza F, et al. Primary pancreatic lymphoma: clinical presentation, diagnosis, treatment, and outcome. Eur J Haematol. (2020) 105:468–75. doi: 10.1111/ejh.13468
33. Ramesh J, Hebert-Magee S, Kim H, Trevino J, Varadarajulu S. Frequency of occurrence and characteristics of primary pancreatic lymphoma during endoscopic ultrasound guided fine needle aspiration: a retrospective study. Digest Liver Dis. (2014) 46:470–3. doi: 10.1016/j.dld.2013.12.016
34. Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. Am J Roentgenol. (2000) 174:671–5. doi: 10.2214/ajr.174.3.1740671
36. Zanoni L, Mattana F, Calabrò D, Paccagnella A, Broccoli A, Nanni C, et al. Overview and recent advances in Pet/Ct imaging in lymphoma and multiple myeloma. Eur J Radiol. (2021) 141:109793. doi: 10.1016/j.ejrad.2021.109793
37. Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA. Fdg Pet/Ct for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. (2014) 41:565–74. doi: 10.1007/s00259-013-2623-4
38. Salaun PY, Gastinne T, Bodet-Milin C, Campion L, Cambefort P, Moreau A, et al. Analysis of 18f-Fdg pet diffuse bone marrow uptake and splenic uptake in staging of Hodgkin’s lymphoma: a reflection of disease infiltration or just inflammation? Eur J Med Mol Imaging. (2009) 36:1813–21. doi: 10.1007/s00259-009-1183-0
39. Dong A, Cui Y, Gao L, Wang Y, Zuo C, Yang J. Patterns of Fdg uptake in pancreatic Non-Hodgkin’s lymphoma lesions. Abdomin Imaging. (2014) 39:175–86. doi: 10.1007/s00261-013-0041-5
40. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. (2019) 10:10–27. doi: 10.14740/wjon1166
41. Zhang J, Jia G, Zuo C, Jia N, Wang H. (18)F- Fdg Pet/Ct helps differentiate autoimmune pancreatitis from pancreatic cancer. BMC Cancer. (2017) 17:695.
42. Ishigami K, Yoshimitsu K, Irie H, Tajima T, Asayama Y, Nishie A, et al. Diagnostic value of the delayed phase image for iso-attenuating pancreatic carcinomas in the pancreatic parenchymal phase on multidetector computed tomography. Eur J Radiol. (2009) 69:139–46. doi: 10.1016/j.ejrad.2007.09.012
43. Psar R, Urban O, Cerna M, Rohan T, Hill M. Improvement of the diagnosis of isoattenuating pancreatic carcinomas by defining their characteristics on contrast enhanced computed tomography and endosonography with fine-needle aspiration (Eus-Fna). Diagnostics. (2021) 11:776. doi: 10.3390/diagnostics11050776
44. Kim JH, Park SH, Yu ES, Kim MH, Kim J, Byun JH, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced Ct: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. (2010) 257:87–96. doi: 10.1148/radiol.10100015
45. Moon SY, Joo KR, So YR, Lim JU, Cha JM, Shin HP, et al. Predictive value of maximum standardized uptake value (Suvmax) on 18f-Fdg Pet/Ct in patients with locally advanced or metastatic pancreatic cancer. Clin Nucl Med. (2013) 38:778–83. doi: 10.1097/RLU.0b013e31829f8c90
46. Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-Fdg Pet/Ct in patients with pancreatic cancer. J Nucl Med. (2014) 55:898–904. doi: 10.2967/jnumed.113.131847
Keywords: primary pancreatic lymphoma, 18F-fluorodeoxyglucose positron emission tomography/computed tomography, case report, pancreatic carcinoma, therapeutic efficacy
Citation: Wang J, Zhou Y, Liu H, Zhou J and Li X (2024) 18F-FDG PET/CT assists the diagnosis of primary pancreatic lymphoma: Two case reports and literature review. Front. Med. 11:1370762. doi: 10.3389/fmed.2024.1370762
Received: 15 January 2024; Accepted: 09 February 2024;
Published: 23 February 2024.
Edited by:
Domenico Albano, University of Brescia, ItalyReviewed by:
Rexhep Durmo, IRCCS Local Health Authority of Reggio Emilia, ItalyCopyright © 2024 Wang, Zhou, Liu, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, OTMyNzU2MDI0QHFxLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.