
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 01 May 2024
Sec. Nephrology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1366010
 Yibeltal Yismaw Gela1*
Yibeltal Yismaw Gela1* Liknaw Workie Limenh2
Liknaw Workie Limenh2 Wudneh Simegn3
Wudneh Simegn3 Wondim Ayenew3
Wondim Ayenew3 Gashaw Sisay Chanie4
Gashaw Sisay Chanie4 Abdulwase Mohammed Seid4
Abdulwase Mohammed Seid4 Alemante Tafese Beyna5
Alemante Tafese Beyna5 Dereje Esubalew6
Dereje Esubalew6 Melese Legesse Mitku7
Melese Legesse Mitku7 Assefa Kebad Mengesha5
Assefa Kebad Mengesha5 Mihret Melese1
Mihret Melese1Background: Poor sleep quality is a common concern in chronic kidney disease (CKD) patients, which can accelerate the progression of chronic renal disease and negatively impact their health-related quality of life, potentially leading to greater morbidity and mortality rates. It can also have an effect on the immune system, cognitive function, and emotional well-being of CKD patients. Furthermore, poor sleep quality may contribute to drug noncompliance and decreased participation in the entire treatment plan. Nonetheless, no research has been undertaken in Ethiopia on the prevalence of poor sleep quality and its associated factors among CKD patients.
Objective: This study aimed to assess the prevalence of poor quality of sleep and associated factors among chronic kidney disease patients at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals in 2020.
Methods: A cross-sectional study design was implemented at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals between February and April 2020. The study participants were chosen through systematic random sampling techniques. The Pittsburgh Sleep Quality Index (PSQI), a validated assessment tool, was utilized to measure sleep quality. A PSQI total score > 5 was used as an indicator of poor sleep quality. Subsequently, the data obtained were entered into Epi Data version 3.0 and then transferred to STATA 14 for analysis. Both bivariable and multivariable binary logistic regression analyses were performed to recognize factors associated with poor sleep quality. In the multivariable logistic regression analysis, variables demonstrating a p-value of ≤0.05 were considered statistically associated to poor sleep quality.
Results: In this study, 424 CKD patients were included. Among screened CKD patients, 42.9% tested positive for poor sleep quality with a 95% CI (38 to 47%). Independent predictors of poor sleep quality among CKD patients were common mental disorder [AOR = 1.8, 95% CI (1.19–2.89)], anemia [AOR = 2.7, 95% CI (1.71–4.36)], declined eGFR between 60 and 89.9 [AOR = 1.6; 95% CI (2.28–5.54)], 30–59.9 [AOR = 2.6, 95% CI (1.53–4.43)], and ≤ 30 [AOR = 3.8, 95% CI (1.17–12.61)], age > 50 years [AOR = 1.7(1.11–2.69)] and duration of disease 2.9 [AOR = 2.9, 95% CI (1.77–4.90)].
Conclusion: In our study, almost 1 out of 2 CKD patients assessed for poor sleep quality tested positive. It was noted that poor sleep quality was more frequent among CKD patients with common mental disorders, anemia, decreased eGFR levels, individuals aged over 50 years, and those with a longer duration of the disease. Consequently, it’s advised to regularly screen these CKD patients for poor sleep quality.
Chronic kidney disease (CKD) is defined by chronic and permanent kidney deterioration, resulting in the kidney’s incapacity to function (1). The prevalence of CKD has recently been quickly growing as a result of higher rates of hypertension, diabetes, and obesity (2). According to institutional research study done in Ethiopia, the prevalence of CKD ranges from 22.1 to 38.6% (3–5).
Poor quality of sleep is difficulty initiating or maintaining sleep, waking up too early or unrefreshing sleep, despite adequate opportunity and circumstances for sleep, leading to impairment of daytime function (6). Sleep disorders present frequently with CKD with substantial impact on both the patient and the health system (7).
The prevalence of poor sleep quality in patients with CKD ranges from 37 to 97%, with the highest prevalence observed among hemodialysis patients in Turkey (8–13). Low-quality sleep can hasten the progression of CKD (14, 15) as well as have a negative influence on health-related quality of life (16), resulting in increased morbidity and death (17–20). It also has an impact on CKD patients’ immune systems, cognitive capabilities, and emotional well-being (21). Furthermore, poor sleep quality might lead to drug noncompliance and decreased engagement in the overall treatment plan (22, 23).
Healthcare providers often overlook sleep disorders (24), which are common but frequently unnoticed in chronic kidney disease patients (6). There have been no studies conducted in Ethiopia to determine the prevalence of poor sleep quality and its contributing factors among CKD patients. Therefore, this study aims to assess the prevalence of poor sleep quality and associated factors among CKD patients.
An institution-based cross-sectional study was conducted from February to April, 2020 at the University of Gondar Comprehensive Specialized Hospital and Felege Hiwot Referral Hospital.
During the data collecting period, all adult CKD patients encountered at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals were included in the study. However, CKD patients on dialysis were excluded from the study.
The sample size was calculated using the following single proportion formula, N= [(Zα/2)2×p(1−p)d2] = [(1.96)2×0.5(1−0.5)(0.05)2] = 385, where N: sample size, p: estimated prevalence value (50%), d: margin of sampling error tolerated (5%), Zα/2 (1.96): critical value at 95% confidence interval of certainty.
After adding 10% of the non-response rate, a total of 424 chronic kidney disease patients were selected.
During the data collection period, 350 patients were seen at the follow-up clinic of the University of Gondar Comprehensive Specialized Hospital, while 450 patients were encountered at the Felege Hiwot Referral Hospital. Employing the proportionate random sampling technique, 186 CKD patients were chosen from the University of Gondar Comprehensive Specialized Hospital, and 238 from the Felege Hiwot Referral Hospital. A total of 424 CKD patients were recruited using systematic random sampling, employing a K value of 2. The initial participant was selected using a lottery method, followed by every second patient for the interviews (Figure 1).
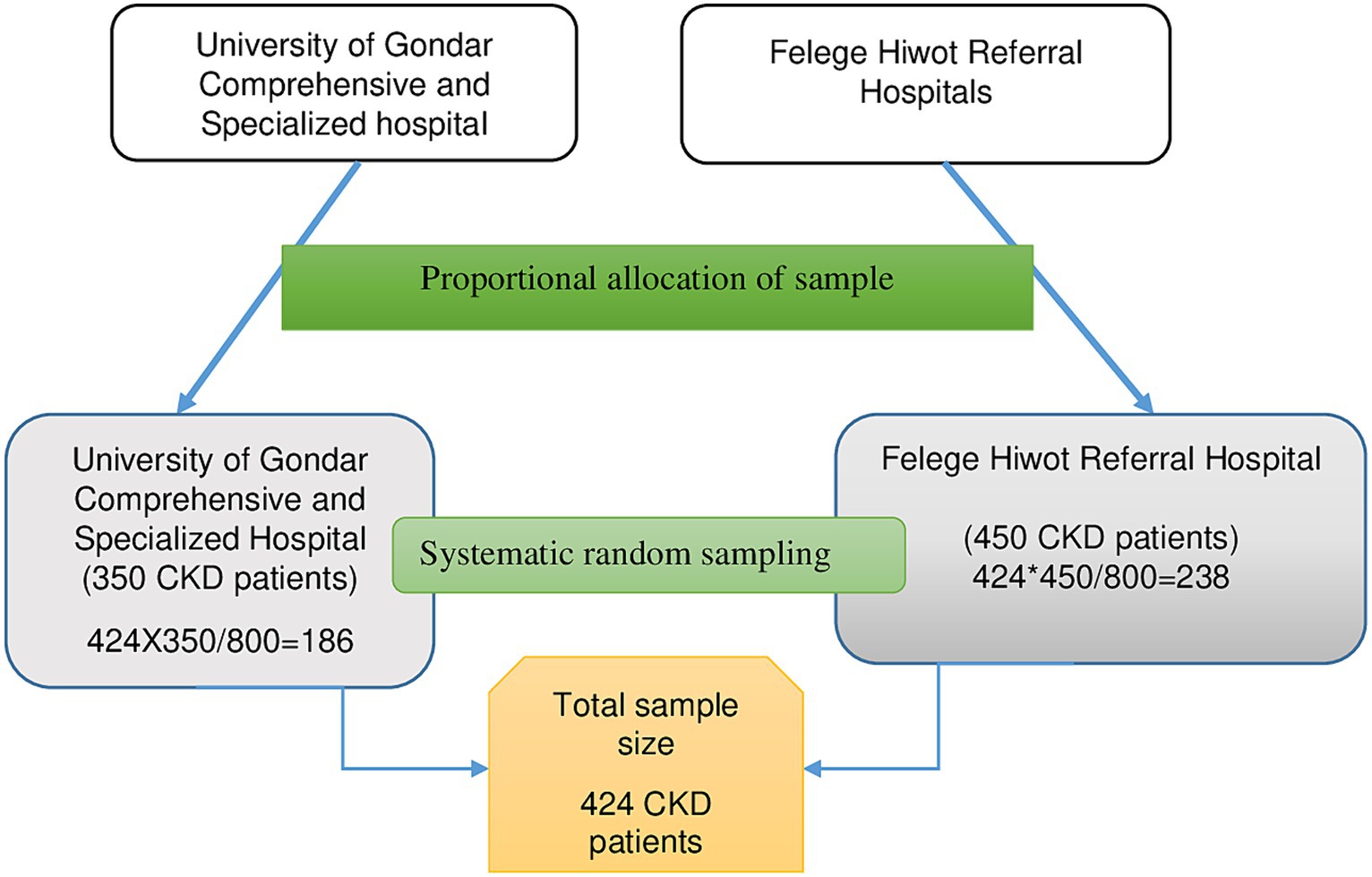
Figure 1. Illustrates the sampling process used to select chronic kidney disease patients at the University of Gondar Comprehensive Specialized Hospital and Felege Hiwot Referral Hospital, 2020.
Chronic kidney disease (CKD) is characterized by a sustained abnormality in kidney structure or function lasting for at least 3 months. This may manifest as a glomerular filtration rate (GFR) below 60 mL/min/1.73 m2, albuminuria (urine albumin ≥30 mg per 24 h or urine albumin-to-creatinine ratio [ACR] ≥30 mg/g), abnormalities in urine sediment, histology, or imaging indicating kidney damage, renal tubular disorders, or a history of kidney transplantation (1, 25).
The estimated glomerular filtration rate (eGFR) was calculated using epidemiology of collaboration (EPI) equations (26, 27).
Quality of sleep was screened using Pittsburgh Sleep Quality Index with optimal cut-off scores above 5 (28).
Physical activity: according to World Health Organization (WHO) is adults and older engage in 150–300 min of moderate-intensity aerobic physical activity per week, or 75–150 min of vigorous-intensity aerobic physical activity throughout the week (29).
Common mental disorders (CMDs) was screened using SRQ-F (Self-Reporting Questionnaire-Falk Institute) which contains 29 items. Those patients who scored ≥8 out of 29 scores in the last 1 month were screened as positive for CMD (30).
Social support is assistance provided when financial, social, or psychological problems arise. The Oslo Social Support Scale tool was used to screen social support status, which includes three items scored out of 14 and is defined as weak support (3–8), moderate support (9–11), and strong support (12–14) depending on the results (31).
Anemia is defined as having a hemoglobin concentration below 13 g/dL in men and below 12 g/dL in women (32).
Comorbidity: the presence of chronic kidney disease and one or more of the following diseases: HIV/AIDS, hypertension, cardiovascular diseases, and diabetic mellitus.
Participants were categorized based on their body mass index (BMI) into different groups: those with a BMI of ≤18.5 kg/m2 were classified as underweight, individuals with a BMI between 18.5 kg/m2 and 24.9 kg/m2 were categorized as having a normal weight, and those with a BMI between 25 kg/m2 and 29.9 kg/m2 were classified as overweight. A BMI exceeding 30 kg/m2 was considered indicative of obesity (33, 34).
Data were collected using interviewer-administered structured questionnaire which consists of sociodemographic characteristics, Medical Record Review, physical measurements (weight and height), Pittsburgh Sleep Quality Index and Oslo Social Support Scale.
The Pittsburgh Sleep Quality Index (PSQI) is a validated assessment tool used to evaluate sleep quality in Ethiopia (30). It consists of 19 items organized into seven components, assessing sleep duration, efficiency, latency, disturbances, daytime dysfunction, frequency of sleep medication use, and subjective sleep quality. Each item is rated on a scale of 0 to 3. The scores from these components are combined to generate a total score ranging from 0 to 21. A higher total score, known as the global score, indicates lower sleep quality. A PSQI global score of >5 is used as a cutoff to identify poor sleep quality, with a sensitivity of 89.6% and specificity of 86.5% (35).
The data undertook cleaning, coding, and entry into Epi-Data 3. Subsequently, it was exported to STATA 14 for analysis. Continuous variables were represented using the mean and standard deviation, while categorical variables were described through frequency distribution and pie charts.
Both bi-variable and multi-variable logistic regression analyses were conducted. In the bi-variable logistic regression, variables displaying an association with poor sleep quality at p ≤ 0.25 were included in the multi-variable regression model. Within the multi-variable logistic regression, variables demonstrating a p-value of ≤0.05 with a 95% confidence interval were considered significantly associated with poor sleep quality. The model’s fitness was assessed using the Hosmer and Lemeshow test; the model was considered fitted as the value obtained (0.12) exceeded the threshold of 0.05.
Furthermore, to ascertain the reliability of the Pittsburgh Sleep Quality Index, the Cronbach’s alpha test was conducted, yielding a reliability coefficient of 0.79. This value indicates good reliability of the tool in measuring sleep quality.
The questionnaire was translated into the Amharic language by a language expert, followed by retranslation into English by another expert to ensure consistency. The principal investigator provided training to the data collectors regarding the Pittsburgh Sleep Quality Index, Oslo Social Support Scale, Medical Record Review, as well as the procedure for measuring height and weight.
This research involved 424 patients diagnosed with chronic kidney disease. The average age of the patients was 53.8 years, with a standard deviation of 16.8 years. Among the CKD patients, the majority, comprising 256 individuals (60.4%), were male. Additionally, 177 participants (41.6%) had attended primary school, 222 (52.5%) were employed, 321 (75.7%) practiced Orthodox Christianity, and 333 (78.5%), were married (Table 1).
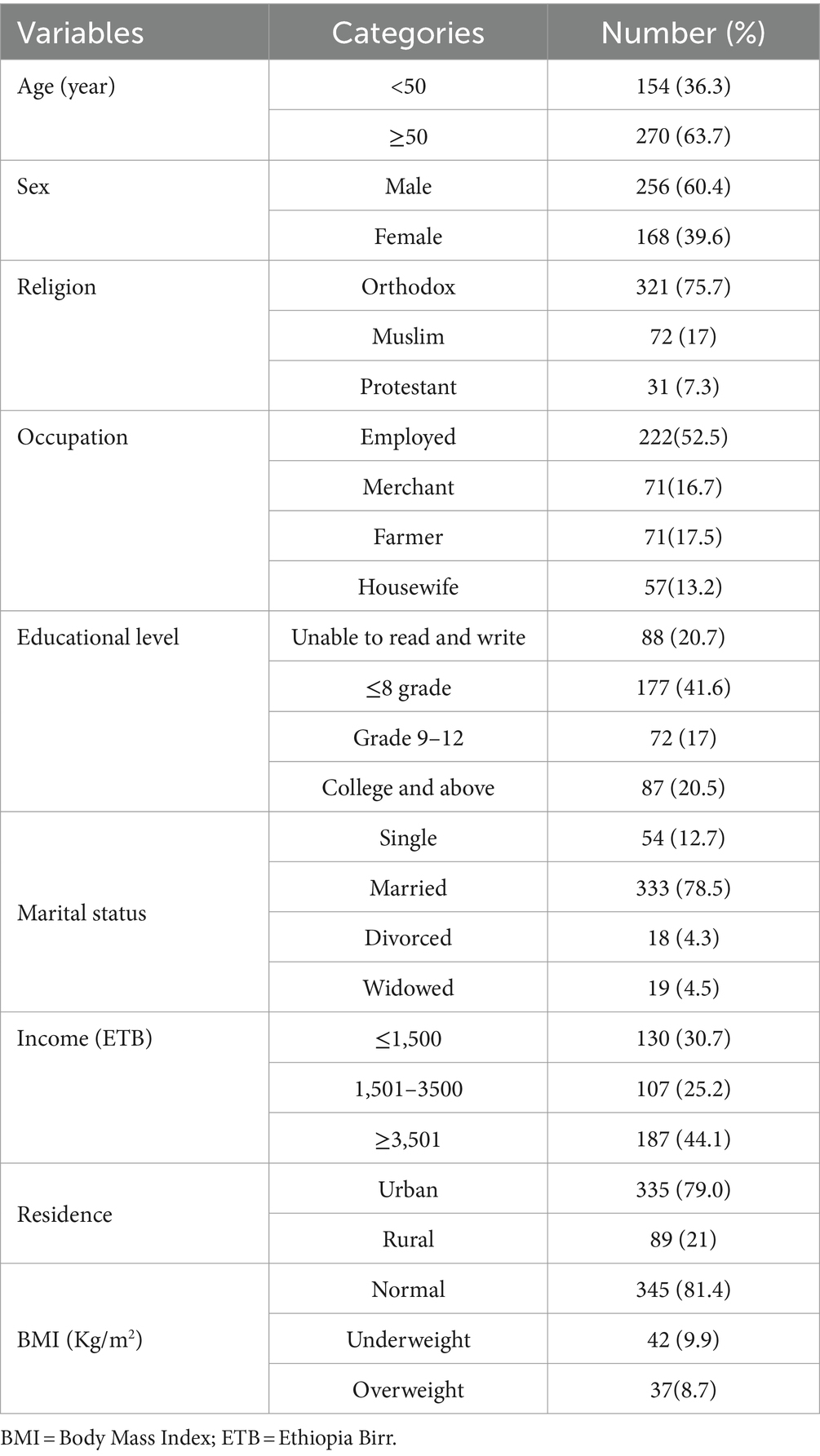
Table 1. Socio-demographic characteristics of chronic kidney disease patients at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals, 2020.
The mean creatinine level among CKD patients were 1.8 mg/dL (SD ± 1.4). One hundred nighty three (45%) patients had eGFR ≥90 mL/min/m2 and 119 (28.1%) of them were anemic. At the time of data collection, 54% of CKD patients’ duration since diagnosis was 5 years and less. Two hundred ninety-one (68.6%) CKD patients had comorbidities (Table 2).
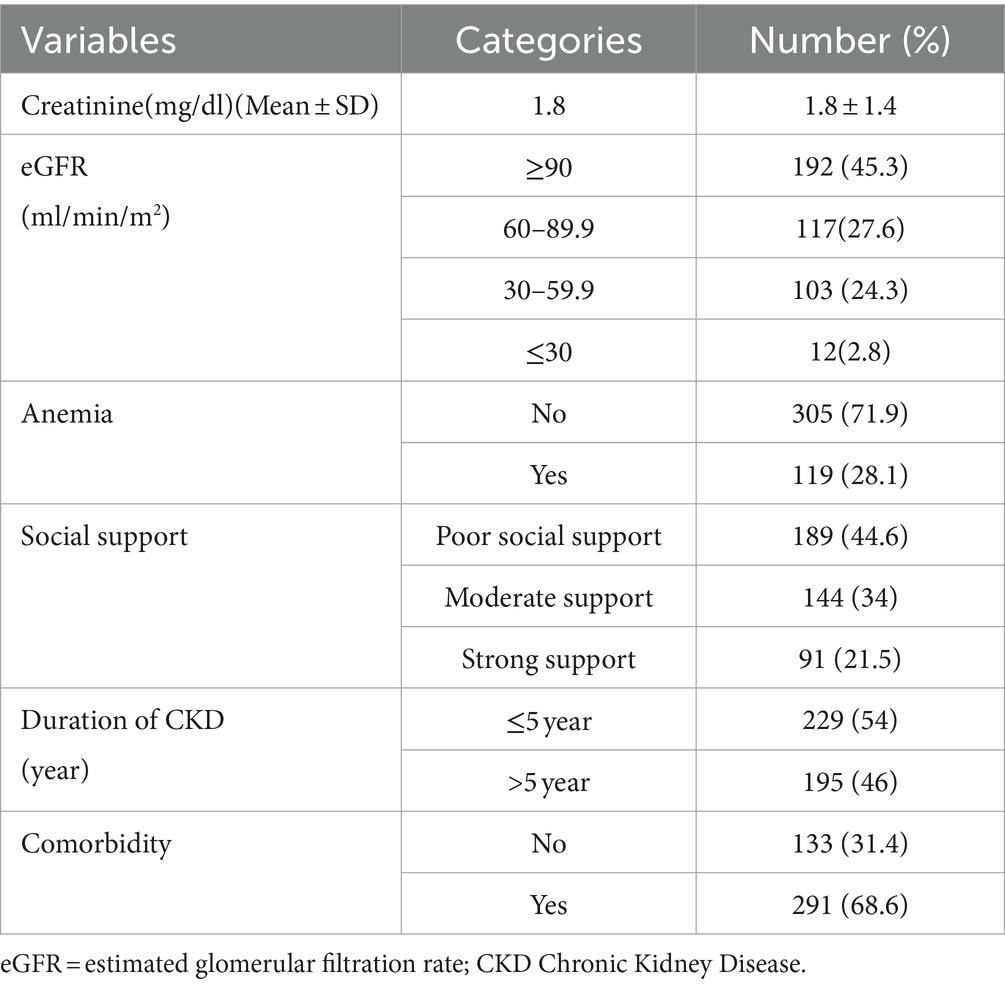
Table 2. Clinical characteristics of chronic kidney disease patients at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals, 2020.
Among screened CKD patients for poor sleep quality, 182 (42.9%) was positive for poor quality of sleep with a 95% CI (38 to 47%). (Figure 2). The value of Pittsburgh Sleep Quality Index (PSQI), Mean ± SD was 4.8 ± 6.11.
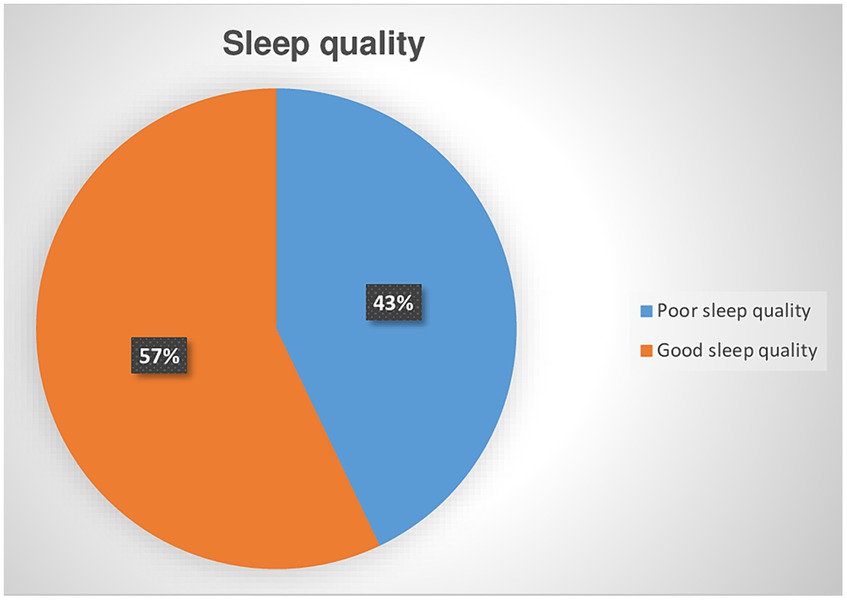
Figure 2. Prevalence of poor sleep quality among chronic kidney disease patients at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals in 2020.
Among variables entered into bivariable logistic regression, common mental disorder, duration of CKD, anemia, physical activity, estimated glomerular filtration rate (eGFR), and age were associated with poor quality of sleep at p-value of ≤0.25. However, in multi-variable logistic regression analysis anemia, common mental disorders, disease duration, eGFR, and age were variables significantly associated with poor quality of sleep at p-value of ≤0.05.
The odds of poor sleep quality among CKD patients with common mental disorders were 1.8 times higher compared to CKD patients without common mental disorders (AOR = 1.8, 95% CI 1.19–2.89). Among anemic CKD patients, the odds of poor sleep quality were 2.7 times higher compared to non-anemic CKD patients (AOR = 2.7, 95% CI 1.71–4.36).
The odds of poor sleep quality among CKD patients with eGFR between 60 and 89.9 mL/min/m2, 30–59.9 mL/min/m2, and ≤ 30 mL/min/m2 were 2.9 (AOR = 2.9, 95% CI 1.77–4.90), 2.6 (AOR = 2.6, 95% CI 1.53–4.43), and 3.8 (AOR = 3.8, 95% CI 1.17–12.61) times higher, respectively, compared to CKD patients with eGFR ≥90 mL/min/m2.
Among CKD patients, the odds of poor sleep quality were 1.7 times higher for those above 50 years old compared to those ≤50 years old (AOR = 1.7, 95% CI 1.11–2.69). Additionally, the odds of poor sleep quality were 1.6 times higher for CKD patients with a duration of over 5 years compared to those with a duration ≤5 years (AOR = 1.6, 95% CI 1.03–2.46) (Table 3).
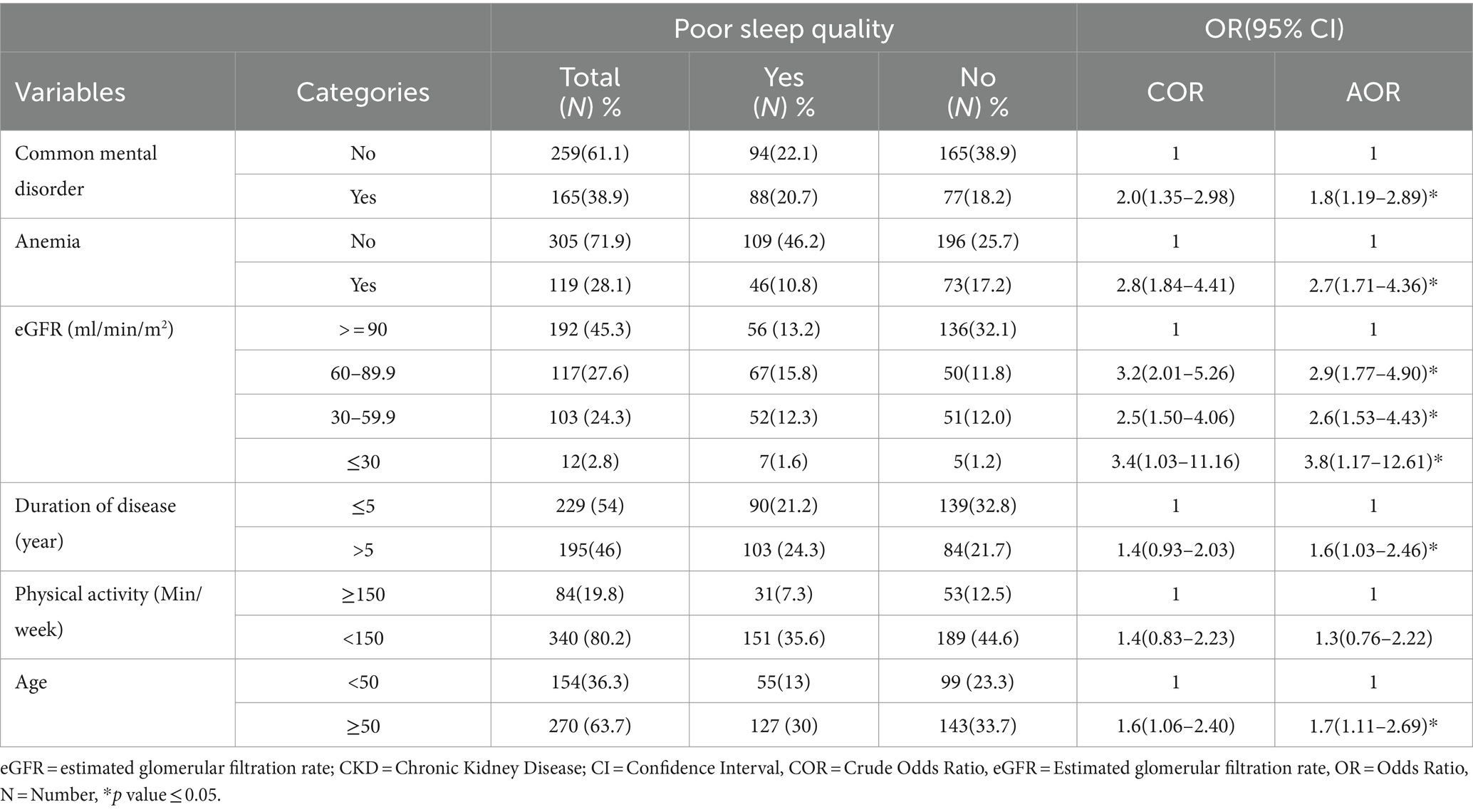
Table 3. Factors associated with poor quality of sleep among CKD patients at the University of Gondar Comprehensive Specialized and Felege Hiwot Referral Hospitals, 2020.
The purpose of this study was to determine the prevalence of poor sleep quality and contributing factors among CKD patients in Ethiopia. Nearly half of the CKD patients had poor sleep quality. Age, anemia, disease duration, and common mental disorder all had significant associations with poor sleep quality in CKD patients.
In our study, the prevalence of poor quality of sleep was found to be 42.9% with 95% CI (38 to 47%), which was similar to the reported prevalence from Turkey (42.2%) (18), but higher than the prevalence observed in two other studies from Saudi Arabia (36.4%) (22), and Japan (37%) (36). Possible reasons for these variations could be attributed to differences in sample sizes, with Saudi Arabia having a smaller sample size, and variations in the assessment tool’s cutoff value, such as Japan using a cutoff of ≥6 to evaluate sleep quality.
The observed prevalence in this study was less compared to Nigeria (50.2%) (8) and China (66.4%) (37), both of which involved solely stage 3–5 CKD patients, as well as Pakistan (65.8%) (38) and India (86.5%) (39). In the Pakistan and India studies, patients undergoing dialysis were also included, and they utilized a PSQI cutoff value of ≥5 to define poor sleep quality, potentially contributing to the higher prevalence of poor sleep quality in those studies.
A lower estimated glomerular filtration rate (eGFR) has been established as a predictor of poor sleep quality in people with CKD, correlating with prior research findings (8, 37). The factors that contribute to sleep problems in CKD patients are numerous and complex. The impairment of baroreceptor reflex function is a common problem in CKD. This defect causes an imbalance in sympathetic and vagal activity, resulting in increased sympathetic nervous system activity and decreased vagal tone (40). Normally, there is a natural decrease in sympathetic activity and a rise in vagal tone during sleep, which is interrupted in CKD patients (41). Chronic inflammation and stimulation of the hypothalamic–pituitary–adrenal axis and sympathetic nervous system are frequent in CKD patients (42). These factors have been linked to sleep disruptions. Furthermore, uremic toxin buildup in the cerebrovascular circulation in CKD may disturb the normal functioning of brain cells involved in sleep induction (43). The hypothalamus and basal forebrain play a crucial role in regulating sleep–wake cycles and are sensitive to changes in metabolic and hormonal status, often disrupted in chronic kidney disease (CKD). The activity of the c-fos protein (FOS) in ventrolateral preoptic neurons (VLPO) of the hypothalamus is associated with the sleep pattern. The expression of FOS in VLPO neurons of the hypothalamus is linked to the activation of neurons that initiate sleep, and this expression is affected by uremia in CKD patients, resulting in sleep disturbances. FOS protein, an immediate-early gene product, is found in a group of ventrolateral preoptic neurons specifically activated during sleep (44, 45).
Patients with end-stage kidney disease (ESKD) have higher amounts of orexin, a neuropeptide that increases alertness, which may contribute to poor sleep quality (46). Furthermore, CKD patients have lower amounts of melatonin, a hormone important in regulating sleep–wake cycles (41, 47).
In summary, the numerous causes of poor sleep quality in CKD patients include uremic toxins, disruption in circadian rhythms, fluid imbalance, electrolyte imbalance, changes in hormone balance, activation of sympathetic nervous system and inflammation. As the disease duration increases patients with CKD are more likely to develop poor sleep quality.
The presence of anemia in CKD patients was significantly associated with a poor quality of sleep similar with others studies (38, 48). The exact underlying mechanisms for the association of anemia and poor sleep quality remain unclear. One potential explanation is that anemia could increase insomnia risk through a shared gene (48), fatigue as a symptom of anemia may induce sleep problems (49). Another potential mechanism of this phenomenon anemia causes a decrease in the blood’s oxygen-carrying capacity (50). Hypoxia can alter the brain’s regulatory centers involved in sleep–wake cycles, thereby causing sleep disorders (50). Again anemic people may suffer increased respiratory effort during sleep, a condition known as sleep-disordered breathing. This greater effort may be caused by the body’s attempt to compensate for low oxygen (51).
Another factor associated with poor sleep quality was older age, which was supported by other studies (9, 18, 22, 37, 52). Aging is associated with decreased ability to maintain sleep, reduced nocturnal sleep duration, and decreased deep sleep (53).
Older adults often experience an increased frequency of awakenings during the night. These awakenings may occur due to a variety of reasons, including changes in bladder function leading to more frequent trips to the bathroom, discomfort from various health conditions, or a decrease in the ability to maintain deep sleep (9). Older individuals may find it challenging to return to sleep after waking during the night. Factors such as pain, discomfort, medication side effects, or underlying health conditions can contribute to prolonged periods of wakefulness during the night. While the need for sleep remains relatively constant throughout adulthood, older adults often experience a reduction in the total duration of sleep at night. This reduction can be influenced by changes in sleep architecture, more frequent awakenings, or a shift in the circadian rhythm (53).
Common mental disorder was significantly associated with poor quality of sleep which is supported by other studies (37, 38). Common mental disorders, which often coincide with poor sleep quality, frequently exhibit changes in neurotransmitter levels specifically serotonin, dopamine, and norepinephrine as well as disruptions in the hypothalamic–pituitary–adrenal (HPA) axis and heightened reactivity to stressors. These factors contribute to the sleep disturbances commonly observed in individuals with these mental health conditions.
In individuals experiencing depression, disruptions in the circadian rhythm are often linked to abnormalities in the circadian locomotor output cycles kaput gene. These abnormalities can affect the proper functioning of the suprachiasmatic nuclei (SCN), which serve as the body’s internal pacemakers. Consequently, these disturbances in the SCN can contribute to poor sleep quality among individuals with depression (54).
Moreover, heightened sleep reactivity due to stress is a common occurrence in various mental health disorders. This heightened reactivity refers to an increased sensitivity to environmental or internal stimuli that can disrupt sleep. Individuals with mental health conditions, including depression, often experience difficulties falling asleep or staying asleep due to heightened reactivity to stressors, further impacting their sleep quality (54).
The Pittsburgh Sleep Quality Index (PSQI) is primarily used to screen and identify poor sleep quality rather than diagnose specific sleep disorders. It assesses various sleep aspects over a defined period, offering insights into sleep habits. However, it does not diagnose specific sleep disorders or underlying causes. Its single-time assessment provides a snapshot of sleep quality, offering a general overview rather than tracking changes over time.
Almost 1 out of 2 CKD patients assessed for poor sleep quality tested positive. It was noted that poor sleep quality was more frequent among CKD patients with common mental disorders, anemia, decreased eGFR levels, individuals aged over 50 years, and those with a longer duration of the disease. Consequently, it’s advised to regularly screen these CKD patients for sleep quality.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by University of Gondar Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. LL: Writing – original draft, Writing – review & editing. WS: Writing – original draft, Writing – review & editing. WA: Writing – original draft, Writing – review & editing. GC: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. DE: Writing – original draft, Writing – review & editing. MMi: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. MMe: Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We extend our sincere gratitude to the University of Gondar for sponsoring this study. We also acknowledge and appreciate the study participants for their willingness, cooperation, and valuable contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, Body Mass Index; CKD, Chronic Kidney Disease; CMD, Common Mental Disorder; eGFR, Estimated Glomerular Filtration Rate; PSQI, Pittsburgh Sleep Quality Index.
1. Drueke, TB, Wong, G, Ronco, P, Rovin, B, Agarwal, A, Hans Anders, B, et al. KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney. Kidney Int Suppl. (2018) 8:91–165. doi: 10.1016/j.kisu.2018.06.001
2. Chen, T, and Harris, DC. Challenges of chronic kidney disease prevention. MJA. (2015) 203:209–10. doi: 10.5694/mja15.00241
3. Kore, C, and Yohannes, H. Prevalence of chronic kidney disease and associated factors among patients with kidney problems public hospitals in Addis Ababa, Ethiopia. J Kidney. (2018) 04:1–5. doi: 10.4172/2472-1220.1000162
4. Bahrey, D, Gebremedhn, G, Mariye, T, Girmay, A, Aberhe, W, Hika, A, et al. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes. (2019) 12:562. doi: 10.1186/s13104-019-4610-8
5. Damtie, S, Biadgo, B, Baynes, HW, Ambachew, S, Melak, T, Asmelash, D, et al. Chronic kidney disease and associated risk factors assessment among diabetes mellitus patients at A tertiary hospital, Northwest Ethiopia. Ethiop J Health Sci. (2018) 28:691–700. doi: 10.4314/ejhs.v28i6.3
6. Pierratos, A, and Hanly, J. Sleep disorders over the full range of chronic kidney disease. Blood Purif. (2011) 31:146–50. doi: 10.1159/000321859
7. Birhanu, TE, Getachew, B, Gerbi, A, and Dereje, D. Prevalence of poor sleep quality and its associated factors among hypertensive patients on follow up at Jimma University medical. J Hum Hypertens. (2021) 35:94–100. Available from:. doi: 10.1038/s41371-020-0320-x
8. Adejumo, OA, Edeki, IR, Mamven, M, Oguntola, OS, Okoye, OC, Akinbodewa, AA, et al. Sleep quality and associated factors among patients with chronic kidney disease. BMJ Open. (2023) 13:e074025–8. doi: 10.1136/bmjopen-2023-074025
9. L Hong, T, P shan, C, H yin, C, King, E, H chieh, Y, Y Luan, H, et al. Insomnia and poor sleep in CKD: a systematic review and Meta-analysis. Kidney Med. (2022) 4:100458. doi: 10.1016/j.xkme.2022.100458
10. Iliescu, EA, Yeates, KE, and Holland, DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. (2004) 19:95–9. doi: 10.1093/ndt/gfg423
11. Kamal, M, Zaki, NFW, Yousef, EA, Eltoraby, E, and Dilshad, M. An Egyptian study of sleep disorders and its correlates in end - stage renal disease patients receiving hemodialysis. Sleep Vigil. (2020) 4:213–20. doi: 10.1007/s41782-020-00095-4
12. Zhang, J, Wang, C, Gong, W, Peng, H, Tang, Y, Li, CC, et al. Association between sleep quality and cardiovascular damage in pre-dialysis patients with chronic kidney disease. BMC Nephrol. (2014) 15:1–9. doi: 10.1186/1471-2369-15-131
13. Şahin, AZ, Özdemir, N, Şahin, ŞK, and Bahadir, D. The effect of sleep quality of patients under hemodialysis on death anxiety, depression and pain. Genel Tıp Derg. (2023) 33:481–4. doi: 10.54005/geneltip.1225349
14. Australian Institute of Health and Welfare. Sleep problems as a risk factor for chronic conditions, AIHW, Australian Government, AIHW (2021). doi: 10.25816/d2d7-p797 (Accessed April 16, 2024)
15. Parvan, K, Lakdizaji, S, Roshangar, F, and Mostofi, M. Quality of sleep and its relationship to quality of life in hemodialysis patients. J caring Sci. (2013) 2:295–304. doi: 10.5681/jcs.2013.035
16. Lindner, AV, Novak, M, Bohra, M, and Mucsi, I. Insomnia in patients with chronic kidney disease. Semin Nephrol. (2015) 35:359–72. doi: 10.1016/j.semnephrol.2015.06.007
17. Mujahid, M, Nasir, K, Qureshi, R, Dhrolia, M, and Ahmad, A. Comparison of the quality of sleep in patients with chronic kidney disease and end-stage renal disease. Cureus. (2022) 14:4. doi: 10.7759/cureus.23862
18. Böbrek, K, Olan, H, Kötü, H, and Kalitesiyle, U. The effect of factors related to poor sleep quality on renal failure progression in patients with chronic kidney disease. J Contemp Med. (2020) 10:499–504. doi: 10.16899/jcm.788100
19. Lu, JL, Freire, AX, Molnar, MZ, Kalantar-zadeh, K, and Kovesdy, CP. Association of Chronic Insomnia with Mortality and Adverse Renal outcomes. Mayo Clin Proc. (2018) 93:1563–70. doi: 10.1016/j.mayocp.2018.05.032
20. Brekke, FB, Waldum, B, Amro, A, Østhus, TBH, Dammen, T, Gudmundsdottir, H, et al. Self-perceived quality of sleep and mortality in Norwegian dialysis patients. Hemodial Int. (2014) 18:87–94. doi: 10.1111/hdi.12066
21. Matharaarachchi, S, Domaratzki, M, Marasinghe, C, and Muthukumarana, S. Modeling and feature assessment of the sleep quality among chronic kidney disease patients. Sleep Epidemiol. (2021) 2:100041. doi: 10.1016/j.sleepe.2022.100041
22. Alshammari, B, Alkubati, SA, Pasay-an, E, and Alrasheeday, A. Sleep quality and its affecting factors among hemodialysis patients. Healthcare. (2023) 11:2536. doi: 10.3390/healthcare11182536
23. Kaneez, M, Muhammad, S, Zaidi, J, A Bin, Z, Rehan, M, Sarwar, Z, et al. Sleep quality and compliance to medical therapy among hemodialysis patients with moderate-to- severe depression: A cross-sectional study. Cureus. (2021) 13:5–11. doi: 10.7759/cureus.13477
24. Weisbord, SD, Fried, LF, Mor, MK, Resnick, AL, Unruh, ML, Palevsky, PM, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. (2006) 2:960–7. doi: 10.2215/CJN.00990207
25. Milik, A, and Hrynkiewicz, E. KDIGO 2012 clinical practice guideline for the Evaluationand Managementof chronic kidney disease. Off J Int Soc Nephrol. (2014) 19:4477–83.
26. Levey, AS, Inker, LA, and Coresh, J. Narrative review GFR estimation: from physiology to public health. Am J Kidney Dis. (2014) 63:820–34. doi: 10.1053/j.ajkd.2013.12.006
27. Hogg, RJ, Furth, S, Lemley, KV, Portman, R, Schwartz, GJ, Coresh, J, et al. National Kidney Foundation’s kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. (2003) 111:1416–21. doi: 10.1542/peds.111.6.1416
28. Salahuddin, M, Maru, TT, Kumalo, A, Pandi-perumal, SR, Bahammam, AS, and Manzar, D. Validation of the Pittsburgh sleep quality index in community dwelling Ethiopian adults. Health Qual Life Outcomes. (2017) 15:58–7. Available from:. doi: 10.1186/s12955-017-0637-5
29. Bull, FC. World Health Organization WHO guidelines on physical activity and sedentary behaviour for children and adolescents, adults and older adults for consultation only. British journal of sports medicine. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
30. Youngmann, R, Zilber, N, Workneh, F, and Giel, R. Adapting the SRQ for Ethiopian populations: A culturally-sensitive psychiatric screening instrument. Transcult Psychiatry. (2008) 45:566–89. doi: 10.1177/1363461508100783
31. Kocalevent, RD, Berg, L, Beutel, ME, Hinz, A, Zenger, M, Härter, M, et al. Social support in the general population: standardization of the Oslo social support scale (OSSS-3). BMC Psychol. (2018) 6:4–11. doi: 10.1186/s40359-018-0249-9
32. McLean, E, Cogswell, M, Egli, I, Wojdyla, D, and De Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. (2009) 12:444–54. doi: 10.1017/S1368980008002401
33. Anderson, AK. Prevalence of Anemia, overweight/obesity, and undiagnosed hypertension and diabetes among residents of selected communities in Ghana. Int J Chronic Dis. (2017) 2017:1–7. doi: 10.1155/2017/7836019
34. Tianyi, FL, Agbor, VN, Njamnshi, AK, and Atashili, J. Factors associated with the prevalence of cognitive impairment in a rural elderly Cameroonian population: A community-based study in sub-Saharan Africa. Dement Geriatr Cogn. (2019) 47:104–13. doi: 10.1159/000496825
35. Buysse, DJ, Reynolds, CF, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: A new instrument psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
36. Yamamoto, R, Shinzawa, M, Isaka, Y, Yamakoshi, E, Imai, E, Ohashi, Y, et al. Sleep quality and sleep duration with CKD are associated with progression to ESKD (2018) 13:1825–32. doi: 10.2215/CJN.01340118,
37. Han, Y, Song, X, Liu, Y, Zhang, W, Li, J, Tu, Y, et al. The effects of depression and age on sleep disturbances in patients with non - dialysis stage 3 – 5 chronic kidney disease: a single - center study. Int Urol Nephrol. (2020) 52:739–48. doi: 10.1007/s11255-020-02416-y
38. Shafi, ST, and Shafi, T. A comparison of quality of sleep between patients with chronic kidney disease not on hemodialysis and end-stage renal disease on hemodialysis in a developing country. Ren Fail. (2017) 39:623–8. doi: 10.1080/0886022X.2017.1361836
39. Aggarwal, HK, Jain, D, Dabas, G, and Yadav, RK. Prevalence of depression, anxiety and insomnia in chronic kidney disease patients and their co-relation with the demographic variables (2017) 38:35–44. doi: 10.1515/prilozi-2017-0020,
40. Neumann, J, Ligtenberg, G, Klein, II, and Koomans, HABP. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. (2004) 65:1568–76. doi: 10.1111/j.1523-1755.2004.00552.x
41. Maung, SC, El, SA, Chapman, C, Cohen, D, Cukor, D, Maung, SC, et al. Sleep disorders and chronic kidney disease. World J Nephrol. (2016) 5:224–32. doi: 10.5527/wjn.v5.i3.224
42. Luo, Q, Li, N, Zhu, Q, Yao, X, Hu, J, Abulimiti, A, et al. Non-dipping blood pressure pattern is associated with higher risk of new-onset diabetes in hypertensive patients with obstructive sleep apnea: UROSAH data. Front Endocrinol. (2023) 14:1083179. doi: 10.3389/fendo.2023.1083179
43. Hirotsu, C, Tufik, S, Bergamaschi, CT, Tenorio, NM, Araujo, P, and Andersen, ML. Sleep pattern in an experimental model of chronic kidney disease. Am J Physiol Renal Physiol. (2010) 299:F1379–88. doi: 10.1152/ajprenal.00118.2010
44. Kim, YO, Choi, EJ, Ahn, HJ, Park, CW, Yang, CW, Jin, DC, et al. The possible role of c-fos protein in hypothalamus in sleep disturbance in chronic uremic rats. Nephron. (1999) 83:139–45. doi: 10.1159/000045491
45. Stefani, E, Toro, L, Perozo, E, Bezanilla, F, Sigg, D, Lecar, H, et al. Activation of ventrolateral preoptic neurons during sleep. Science. (1994) 66:238
46. Y ling, C, Y fang, C, K chi, F, S kai, L, H yuen, C, J yeh, Y, et al. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. (2009):247–51. doi: 10.1093/ndt/gfn439
47. Koch, BCP, Putten, KVan Der, Someren, EJWVan, and Wielders, JPM. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol Dial Transplant. (2009), 25, 513–519, doi: 10.1093/ndt/gfp493;
48. Neumann, SN, J juan, L, X dong, Y, Shua, C, Cran, M, Murray-kolb, LE, et al. Anemia and insomnia: a cross-sectional study and meta-analysis. Chin Med J. (2021) 134:675–81. doi: 10.1097/CM9.0000000000001306
49. Jackowska, M, Kumari, M, and Steptoe, A. Sleep and biomarkers in the English longitudinal study of ageing: associations with C-reactive protein, fibrinogen, dehydroepiandrosterone sulfate and hemoglobin. Psychoneuroendocrinology. (2013) 38:1484–93. doi: 10.1016/j.psyneuen.2012.12.015
50. Murray-kolb, LE, Simonsick, EM, Ferrucci, L, Allen, R, Payne, ME, and Adam, P. Association between non-Iron-deficient Anemia and insomnia symptoms in community-dwelling older adults: the Baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. (2018) 73:380–5. doi: 10.1093/gerona/glw332
51. Gerontol, J, Sci, AB, Sci, M, Gottesman, RF, Sojkova, J, Beason-held, LL, et al. Patterns of regional cerebral blood flow associated with low hemoglobin in the Baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. (2012) 67:963–9. doi: 10.1093/gerona/gls121
52. Firoz, MN, Shafipour, V, Jafari, H, Hosseini, SH, and Charati, JY. Sleep quality and depression and their association with other factors in hemodialysis patients. Glob J Health Sci. (2016) 8:121–7. doi: 10.5539/gjhs.v8n8p121
53. Gorgoni, M, and De Gennaro, L. Sleep in the aging brain. Brain Sci. (2021) 11:1–7. doi: 10.3390/brainsci11020229
Keywords: poor sleep quality, chronic kidney disease, PSQI, Ethiopia, cross-sectional study
Citation: Gela YY, Limenh LW, Simegn W, Ayenew W, Chanie GS, Seid AM, Beyna AT, Esubalew D, Mitku ML, Mengesha AK and Melese M (2024) Poor sleep quality and associated factors among adult chronic kidney disease patients. Front. Med. 11:1366010. doi: 10.3389/fmed.2024.1366010
Received: 05 January 2024; Accepted: 11 April 2024;
Published: 01 May 2024.
Edited by:
Dalinda Isabel Sánchez-Vidaña, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Maria-Eleni Roumelioti, University of New Mexico, United StatesCopyright © 2024 Gela, Limenh, Simegn, Ayenew, Chanie, Seid, Beyna, Esubalew, Mitku, Mengesha and Melese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yibeltal Yismaw Gela, yibeltalyismaw7@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.