- 1Zhuzhou Clinical College, Jishou University, Jishou, China
- 2Department of Anesthesiology, Zhuzhou Central Hospital, Zhuzhou, China
- 3Department of Radiology, Third Xiangya Hospital, Central South University, Changsha, China
Precision medicine, characterized by the personalized integration of a patient’s genetic blueprint and clinical history, represents a dynamic paradigm in healthcare evolution. The emerging field of personalized anesthesia is at the intersection of genetics and anesthesiology, where anesthetic care will be tailored to an individual’s genetic make-up, comorbidities and patient-specific factors. Genomics and biomarkers can provide more accurate anesthetic protocols, while artificial intelligence can simplify anesthetic procedures and reduce anesthetic risks, and real-time monitoring tools can improve perioperative safety and efficacy. The aim of this paper is to present and summarize the applications of these related fields in anesthesiology by reviewing them, exploring the potential of advanced technologies in the implementation and development of personalized anesthesia, realizing the future integration of new technologies into clinical practice, and promoting multidisciplinary collaboration between anesthesiology and disciplines such as genomics and artificial intelligence.
1 Introduction
Precision medicine, a paradigm that individualizes healthcare by integrating a patient’s genetic blueprint and clinical history (1), is a rapidly evolving field that has demonstrated its potential across a broad range of biomedical areas and addressed significant public health challenges (2). This approach is particularly paramount in the realm of anesthetic management for enhancing patient safety and optimizing therapeutic efficacy. Additionally, individualized medicine plays a crucial role within precision medicine (3). Traditional anesthetic protocols, while effective for the majority, often overlook the vast inter-individual variability in drug responses and procedural risks. These differences can lead to unpredictable responses or toxic effects in some individuals or subgroups, ultimately impacting patient outcomes (4).
Personalized medicine opens up new horizons in the field (5). The advent of personalized anesthesia heralds a shift toward a nuanced framework where anesthetic regimens are sculpted around the patient’s genetic predispositions, existing comorbidities, and specific physiological parameters. This granular customization aims to mitigate perioperative complications, fine-tune pain management, bolster enhanced recovery after surgery (ERAS) protocols, and enhance patient satisfaction.
Recent strides in genomic sequencing, biomarker identification, and innovations in monitoring modalities have been pivotal in catapulting personalized anesthesia from conceptualization to clinical practice. Pharmacogenomics, which focuses on identifying genetic variations that affect the pharmacodynamics and pharmacokinetics of drugs, has shed light on genetic polymorphisms that modulate anesthetic sensitivity and susceptibility to complications, playing an important role in personalized medicine (6). Concurrently, the emergence of novel biomarkers and cutting-edge monitoring technology has refined the predictive accuracy of anesthetic outcomes, facilitating more strategic intraoperative planning.
This study searched the PubMed database using “personalized anesthesia,” “pharmacogenomics,” “biomarkers,” “machine learning,” and “artificial intelligence” as search terms for articles published between 2000 and 2024. The articles retrieved included clinical trials, randomized controlled trials, and reviews. These articles were then categorized according to the content of their abstracts. This review elucidates the intricacies of personalized anesthesia within the framework of precision medicine, emphasizing the influence of genetic variables, comorbid conditions, and individual patient factors on anesthetic administration. It canvasses the burgeoning domain of pharmacogenomics, explores the trajectory of biomarker and monitoring technology development, and scrutinizes the impediments and prospective evolution of personalized anesthesia. Furthermore, it considers the growing impact of artificial intelligence (AI) and machine learning as pivotal tools in the evolution of anesthetic precision.
2 Genetic factors
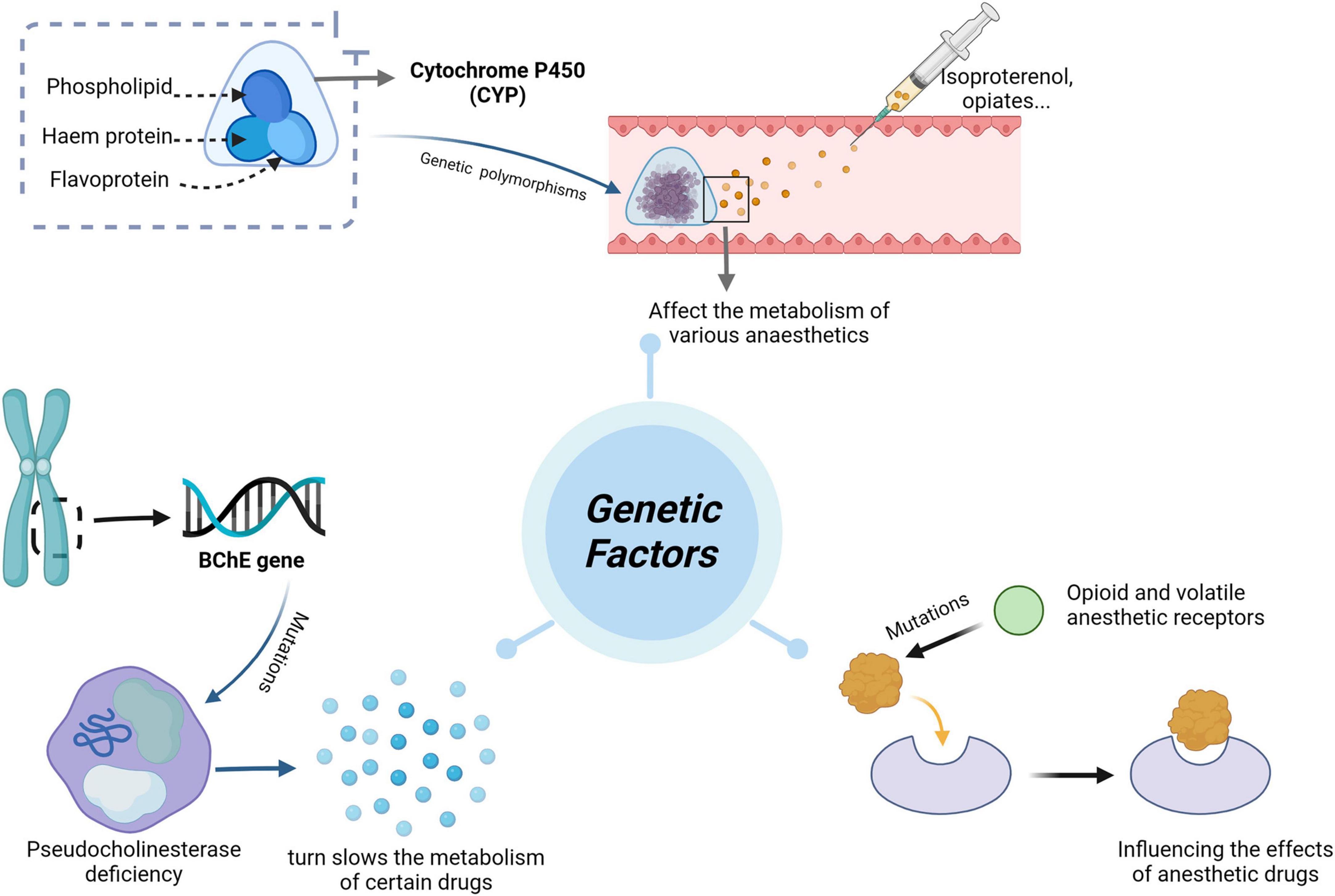
Pharmacogenomics (PGx) is the discipline of predicting drug efficacy and toxicity at the genetic level (7), dedicated to elucidating the genetic variations that underlie the pharmacodynamics and pharmacokinetics of legacy drugs. This knowledge can guide the clinical selection of optimal therapeutic agents at the most appropriate dosage, improving drug efficacy, reducing or avoiding adverse effects, enhancing prognosis, and saving healthcare costs (8). This emerging field has evolved from merely identifying gene-drug pairs to realizing their clinical applications (9). Recent studies support the premise that adverse drug reactions (ADRs) can be prevented through PGx testing, highlighting this approach’s potential to improve drug safety and optimize therapeutic efficacy (10, 11). Figure 1 illustrates the relationship between genetic factors and the effects of anesthesia.
2.1 CYP450
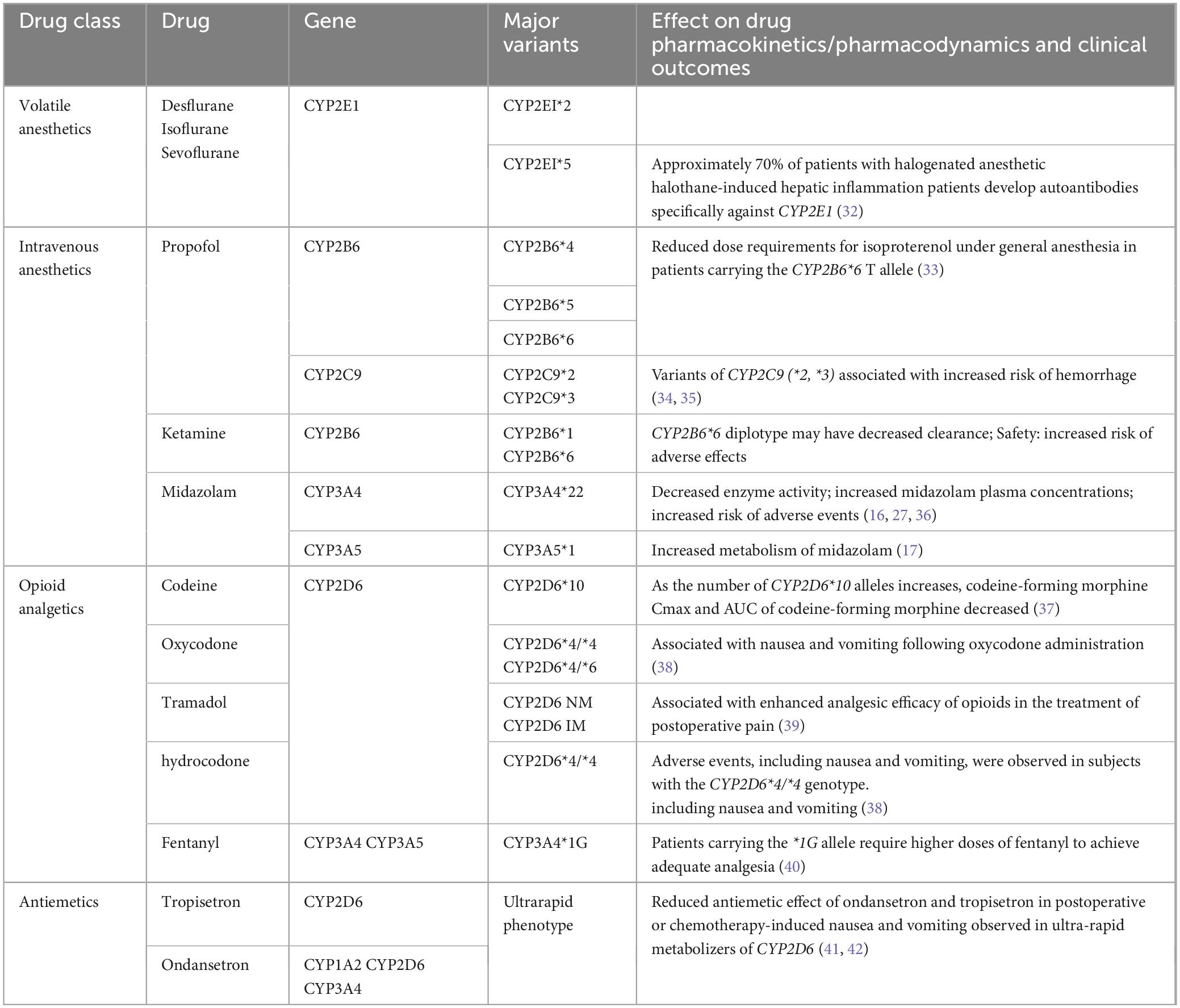
Cytochrome P450 (CYP) is a collection of structurally and functionally related isoenzymes belonging to the group of B cytochromes. This group includes flavoprotein (NADPH cytochrome C reductase), hemoglobin (P450), and phospholipids (phosphatidylcholine). These enzymes are found mainly in the liver (4), but also in the lungs, kidneys, brain, and, to a lesser extent, in the gastrointestinal tract, skin, and placental tissue. CYP proteins, particularly those of the CYP1, CYP2, and CYP3 families, are major contributors to drug metabolism in humans (12). Their function and expression are regulated by variables such as sex, age, and disease state (13). CYP450 is the first step in the metabolism of almost 80% of drugs (14). Table 1 summarizes the genotypes discussed in this chapter and their impact on drugs used in anesthetic practice.
Inhalational anesthetics are among the most commonly used general anesthetic agents in clinical anesthesia and have general anesthetic, analgesic, sedative, and amnestic effects. Between 20 and 50% of halothane, 2% of sevoflurane, less than 1% of isoflurane, and 0.1% of desflurane are biotransformed in the liver (15). Metabolism occurs in the liver and kidney via microsomal CYP2E1. Inhalational anesthetics enter the body and, due to their high lipophilicity, are rapidly absorbed into the circulation and distributed to the tissues; they are almost exclusively eliminated by the lungs. Therefore, their effects do not depend on common polymorphisms in genes encoding metabolic enzymes or drug transporter proteins (16, 17). Hepatotoxicity of halothane has been frequently reported (18). Hepatotoxicity of desflurane, sevoflurane, and isoflurane has also been occasionally reported (19, 20).
Propofol is the most commonly used parenteral anesthetic with sedative-hypnotic, anxiolytic, anticonvulsant, anti-inflammatory, antiemetic, antioxidant, and possibly neuroprotective effects. Differential responses to propofol may be due to polymorphisms in the gene encoding the metabolic enzyme CYP2B6 (7). Up to 70% of propofol binds to glucuronide via UGT1A9, while the remaining 30% of the drug is first hydroxylated via CYP2B6 (17). Iohom et al. (21) found that interpatient variability in response was associated with the presence of CYP2B6 variants (R487C, K262R, and Q172), but GABRE variants (mRNA358G/T, 20118C/T, 20326C/T, and 20502A/T) were not statistically significantly associated.
Ketamine, a potent analgesic, increases heart rate, blood pressure, and cardiac output. It acts mainly at NMDA receptors as a non-competitive blocker. Ketamine is metabolized primarily by two cytochrome P450 enzymes, CYP2B6 and CYP3A4, and is subsequently glucuronidated and excreted by the kidneys (22, 23). Li et al. (24) found that the CYP2B6*6 allele was associated with a significant reduction in steady-state ketamine plasma clearance in chronic pain patients.
Midazolam, a common benzodiazepine sedative-hypnotic, exhibits sedative-hypnotic, anxiolytic, anticonvulsant, myorelaxant, and amnesic properties. It is primarily metabolized by CYP3A4 and CYP3A5, and its metabolites bind to glucuronide (25). The CYP3A4*22 variant is associated with reduced enzyme function (26). POR is an important component of the CYP enzyme system, and POR28 is a common variant. One study found a 45% lower metabolism of midazolam in patients with the POR*28 variant compared to those with the POR*1/*1 genotype among CYP3A5 expressors (27).
Opioids, the most commonly used analgesics, are metabolized by CYP2B6, CYP2D6, CYP3A4, and CYP3A5. Tramadol is metabolized in the liver by CYP2D6 into its pharmacologically active metabolite, O-desmethyltramadol. Fentanyl is metabolized in the liver by CYP3A4 and CYP3A5 into desmethylfentanyl. CYP3A5*1 is the only functional allele known to enhance fentanyl metabolism (16). Codeine is metabolized in the liver into morphine by CYP2D6, and patients with poor CYP2D6 metabolism exhibit very low morphine plasma concentrations after codeine administration (28). The majority of oxycodone is metabolized by CYP3A4 into its inactive metabolite, noroxycodone, while a minority is metabolized by CYP2D6 into its active metabolite, oxymorphone (28, 29). Metabolism varies among CYP2D6 genotypes, and the analgesic effect of oxycodone is diminished in individuals with poor metabolism compared to those with extensive metabolism. Alternative medications should not be metabolized by CYP2D6 and therefore should not contain oxycodone, tramadol, or codeine, which are metabolized by CYP2D6 (30).
Ondansetron and tropisetron, commonly used to prevent post-operative nausea and vomiting (PONV), are 5-HT3 receptor antagonists. Ondansetron is metabolized in the liver by CYP1A2, CYP2D6, and CYP3A4, while tropisetron is primarily metabolized by CYP2D6. Ultra-fast CYP2D6 metabolizers of ondansetron experience a higher incidence of vomiting and reduced antiemetic efficacy. It is recommended that dosing be consistent for individuals with moderate and poor CYP2D6 metabolizing phenotypes. For ultra-fast metabolizers, antiemetics that do not rely on CYP2D6 substrates are advised (31).
2.2 Pseudocholinesterase
Pseudocholinesterase, or butyrylcholinesterase, is an esterase that is expressed throughout the body and encoded by the BChE gene on chromosome 3q26 (43). Mutations in this gene can lead to pseudocholinesterase deficiency, which in turn slows the metabolism of certain drugs, resulting in delayed metabolic conditions (44). Changes in pseudocholinesterase activity can cause prolonged apnea. Among these changes, the A variant (209A > G, Asp70Gly) and the K variant (1615G > A, Ala539Thr) are the most common (45). The elimination of ester-type anesthetics such as bupivacaine and procaine depends on plasma butyrylcholinesterase activity. Case studies have highlighted the long-term effects of epidural injections of chloroprocaine in patients with abnormal pseudocholinesterase activity (46, 47). Additionally, individuals with this deficiency may experience long-term paralysis after the administration of succinylcholine due to impaired drug metabolism (48, 49).
2.3 Receptor polymorphisms
With regard to receptor polymorphisms, opioid and volatile anesthetic receptors (e.g., mu opioid receptor (OPRM1) and gamma-aminobutyric acid type A (GABAA) receptors) have been shown to result in different patient responses to anesthetics. Opioid receptors are widely expressed in the central nervous system and peripheral tissues, and the μ-opioid receptor encoded by OPRM1 is a major binding site for opioids (50). More than 200 variant alleles of this gene have been identified. Genetic differences arising from variations in these genes are a major source of variability in opioid response (51). The OPRM1 118 A > G variant can alter μ-opioid receptor (MOR) signaling in the brain (52). Studies have shown that individuals with at least one OPRM1 118G allele have a blunted response to morphine compared to those with the 118 A/A genotype (53, 54). The OPRM1 A118G polymorphism has also been associated with post-operative side effects such as vomiting (55). Additionally, intrathecal fentanyl injections are significantly more analgesic in women carrying the OPRM1 304G allele (56).
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian brain and coordinates many physiological states, including sleep, anesthesia, and pain modulation (57). Barbiturates such as isoamylbarbital, pentobarbital, and secobarbital alter the activity of GABA-A and glycine receptors, inducing CNS depressant and sedative effects. Due to their tendency to cause respiratory depression in preoperative anesthesia, they have been replaced by benzodiazepines with safer pharmacological profiles (16, 17).
Midazolam and diazepam are orthosteric modulators of the GABA-A receptor. Choi et al. (58) found that patients with the AA genotype of GABRA1 (the α-1 subunit of the GABA receptor) rs4263535 have an increased risk of deep sedation. Malignant hyperthermia (MH) is a rare autosomal dominant disorder characterized by sudden onset of muscle spasms, rapid temperature increase, tachycardia, elevated heart rate, and an increased risk of heart failure, along with increased oxygen consumption, acidosis, and myoglobinuria. Mutations in the RYR1 gene, which encodes the ryanodine receptor on the sarcoplasmic reticulum, are one of the possible causes (59). Volatile anesthetics and succinylcholine are the most common triggers of MH in susceptible patients. Testing all patients suspected of having MH can reduce MH mortality (60). Anesthetics and their adjuvants are critical in surgery, and this pharmacogenomic evidence underscores the potential for genetic analysis to inform anesthetic selection and dosing, with the aim of minimizing adverse effects and maximizing therapeutic outcomes.
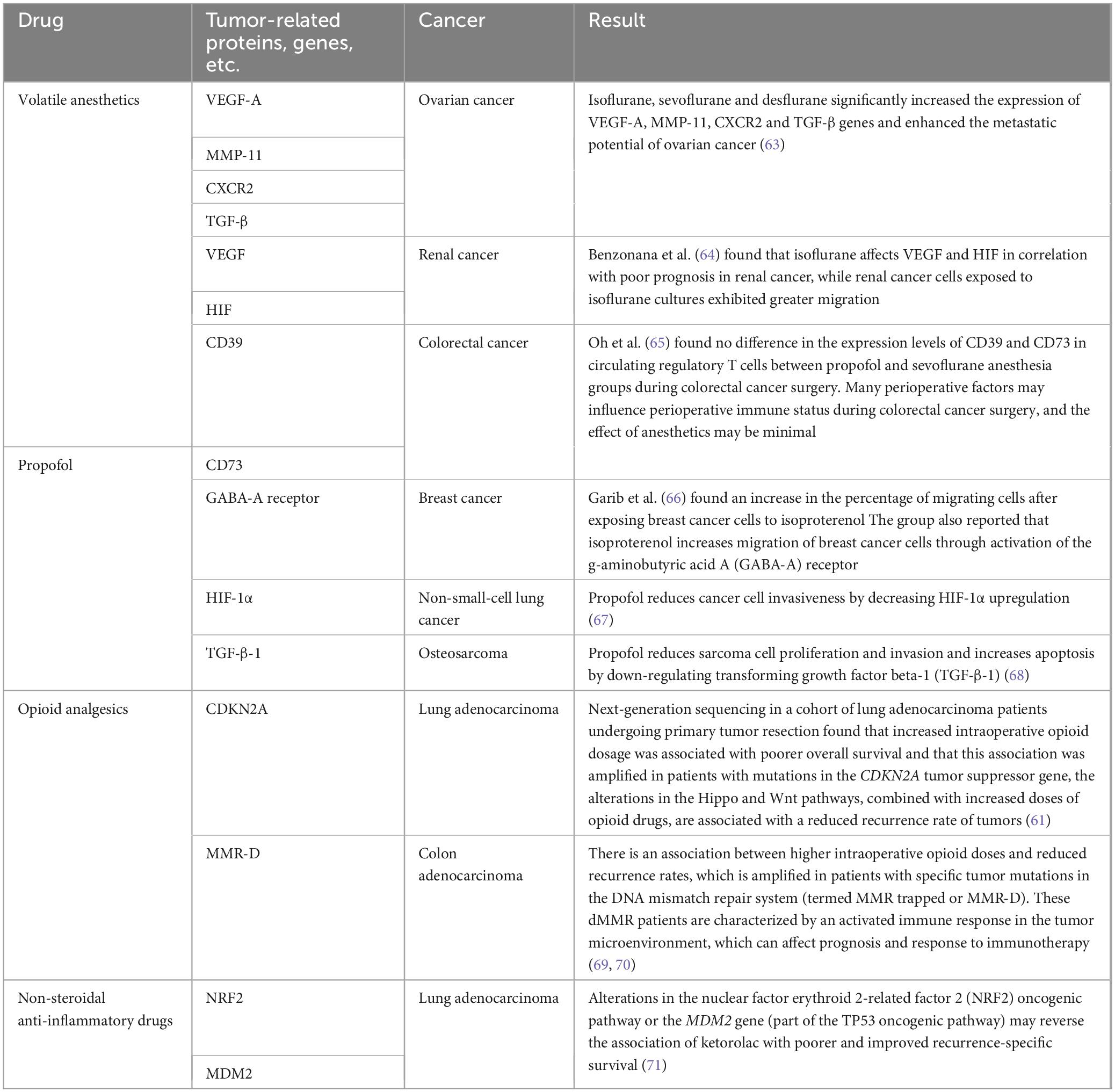
Anesthesiologists can use this knowledge to understand the effects of anesthetics on perioperative disease. Additionally, opioids, ketamine, and non-steroidal anti-inflammatory drugs are used in treating chronic cancer pain. Studies have shown that perioperative administration of opioids that interact with specific tumor genomes can alter survival outcomes (61). Table 2 lists some factors associated with anesthesia for certain cancers, although causality has not been established. These factors could ultimately influence anesthesia and analgesia in cancer patients by considering changes in perioperative and pain management interventions for specific cancer subtypes (62). Incorporating these genetic factors into clinical practice may help to tailor anesthesia care to match the genetic makeup of each patient.
3 Biomarkers
Biomarkers are not only key indicators of infection and host response dysregulation but also valuable tools for assessing treatment responses. They assist clinicians in predicting patient risks and serve as diagnostic and prognostic tools for clinical decision-making and risk stratification in clinical trials (72, 73). Beyond indicating systemic manifestations of infection and organ dysfunction, biomarkers offer insights into the biological basis of disease pathogenesis and treatment outcomes (73, 74). The detection of specific biomarkers during the perioperative period can enhance understanding of a patient’s condition, guide the development of tailored anesthetic regimens for various outcomes, and reduce patient risks while improving prognosis. For example, specific combinations of protein biomarkers can identify patients with adult respiratory distress syndrome (ARDS) who are most likely to benefit from interventions such as positive end-expiratory pressure (PEEP) or conservative fluid management strategies (75, 76).
Postoperative cognitive deficits are primarily categorized as postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) (77, 78). Postoperative delirium (POD) is an acute and transient dysfunction of the central nervous system (CNS) (79) occurring in 15–53% of elderly patients immediately after surgery (80). Unlike delirium, POCD is not a clinical diagnosis but rather a variable operational concept, defined by postoperative cognitive decline as measured by neuropsychological testing within the first three months after surgery (80). POD and POCD are closely associated with neuroinflammation (81) and several biomarkers can predict or diagnose the occurrence of both (see Table 3). An increasing number of preclinical studies have shown that general anesthetics cause long-term cognitive impairment (82). A recent meta-analysis revealed that the use of midazolam, propofol, desflurane, and sevoflurane was associated with a higher incidence of delirium compared to dexmedetomidine (83). The anti-inflammatory and immunomodulatory effects of dexmedetomidine have been shown to reduce acute POD (84). Moreover, the use of benzodiazepines, opioids, antihistamines, and dihydropyridines has been linked to an increased risk of delirium (85). Several studies have indicated that isoproterenol, dexmedetomidine, and fentanyl reduce the risk of cognitive impairment compared to agents like midazolam, lorazepam, pethidine, and morphine (86). However, O’Bryan et al. (87) found statistically that the choice of maintenance anesthetic had little effect on the perioperative inflammatory response. Instead, individual patient and surgical factors may have a greater influence on the inflammatory response. The impact of anesthetic agents on postoperative cognitive impairment warrants further investigation, and monitoring relevant biomarkers could aid in risk stratification and improving prognosis.
4 The role of artificial intelligence (AI) and machine learning in personalized anesthesia
Artificial Intelligence (AI), which analyzes and classifies complex patterns and large amounts of data, is increasingly being recognized in healthcare for its ability to analyze complex datasets, simulate human cognitive learning, and incrementally improve its performance. Its applications range from virtual patient assistance to medical imaging and diagnostic support. Machine learning, a subset of artificial intelligence, has demonstrated the capability to assimilate clinical data to guide decision-making (101). In other clinical settings, image analysis is an area where AI approaches hold great promise (102). The wider application of AI in endoscopy could improve benign adenoma detection rates and reduce both the costs and risks of unnecessary polypectomies (103). AI-assisted image analysis aimed at improving disease risk prediction and diagnosis could detect cancer metastases (104), diabetic retinopathy (105), and identify benign melanomas (106). AI-based image analysis has also become part of direct-to-consumer diagnostic tools for anemia (107). In an attempt to automate the classification of pediatric pneumonia based on lung ultrasound patterns, neural network algorithms were able to correctly identify pneumonic infiltrates in healthy lungs with over 90% sensitivity and 100% specificity (108).
The emergence of diagnostic decision support tools has brought about a paradigm shift in anesthesia practice, combining human expertise with the computational power of artificial intelligence (AI) and machine learning (ML) (109). Decision aids aim to prepare individuals for decision-making by providing accurate and balanced information about treatment options and outcomes, helping them make specific and considered choices about their treatment (110). These aids have shown effectiveness in helping patients recognize the value sensitivity of decisions, guiding them to consider benefits and harms, improving patient-provider communication, and providing guidance throughout the decision-making process (110). Additionally, decision aids assist patients in making informed healthcare decisions by offering detailed information about treatment options and outcomes (110, 111). AI is utilized for evidence-based clinical decision support (112), detecting adverse events, and using electronic health record (EHR) data to predict patients at risk of readmission (113). By accessing EHR data, AI has demonstrated potential to surpass physicians in diagnostic accuracy (114–117). Algorithms that combine imaging and EHR data with relevant medical records can predict malignancy on biopsy and differentiate between normal and abnormal screening results, significantly reducing missed breast cancer diagnoses (118). AI-enabled clinical decision support systems can reduce diagnostic errors, enhance decision support intelligence, and assist clinicians with EHR data extraction and documentation tasks. Moreover, Banegas et al. (119) found that the use of decision aids reduced decisional conflict and aided women at high risk for breast cancer in deciding whether to take prophylactic tamoxifen or raloxifene to reduce cancer risk.
The development of artificial intelligence in all aspects of anesthesia has brought significant benefits, including airway management, ultrasound-guided interventions, intelligent drug infusion systems, accurate intraoperative monitoring, and perioperative risk assessment (120). A randomized trial evaluating the performance of an automated inspired oxygen concentration (FiO2 closed-loop system) using a narrower SpO2 target range found that the time spent within the clinically determined alarm limit (86–94%) was as good as with two wider target ranges (121). In an attempt to automate the classification of pediatric pneumonia based on lung ultrasound patterns, a neural network algorithm was able to correctly identify pneumonic infiltrates in healthy lungs with over 90% sensitivity and 100% specificity (108).
In genetic diagnostics, particularly for rare genetic diseases, clinicians face the daunting task of distinguishing disease-causing variants from millions of benign variants (122). Advances in artificial intelligence are transforming healthcare (123). and are expected to address bottlenecks in diagnosing rare genetic diseases through electronic clinical decision support systems (eCDSS) (124–128). A well-integrated CDSS linked to an electronic health record (EHR) can simplify data analysis and eliminate the need for redundant data entry.
Clinical validation and implementation of enhanced decision support tools are still in their infancy compared to other functionalities, and there is ample room for research on artificial intelligence and automation in anesthesia. The introduction of artificial intelligence and machine learning in medicine has already helped healthcare professionals improve the quality of care they provide and is expected to continue to do so in the near future and beyond. As these technologies advance, they offer a pathway for a more predictive and personalized approach to anesthesia, highlighting the need for anesthetists to become proficient in these digital tools to enhance patient care.
5 Artificial intelligence and real-time tools
5.1 Monitoring technologies: enhancing perioperative safety and efficacy
Advancements in monitoring technologies have significantly augmented the anesthesiologist’s ability to individualize patient care and optimize perioperative outcomes. Techniques such as electroencephalography (EEG) for assessing the depth of anesthesia and near-infrared spectroscopy (NIRS) for cerebral oximetry are at the forefront of these advancements.
The cerebral oxygen index (COx), correlating local brain tissue oximetry (StO2) derived from NIRS with mean arterial pressure (MAP), has become a pivotal tool in monitoring cerebral oxygenation. Tissue ischemia, hypoxia, hyperoxia, and hyperoxic reperfusion enhance the production of reactive oxygen species, thereby inducing oxidative damage (129–131). Such intraoperative oxidative stress has been implicated in postoperative cerebral and renal injuries (132). Cerebral oximetry, a non-invasive and user-friendly technique, allows for the real-time estimation of cerebral oxygen saturation (133). Recent findings by Lopez et al. (134) suggest that traditional practices of over-oxygenation during surgery, commonly believed to be protective, may in fact be deleterious to cerebral tissues.
Near-infrared spectroscopy has proven to be a reliable surrogate for cerebral blood flow, offering earlier warnings of compromised perfusion compared to traditional indicators of cerebral ischemia (135). Painful stimuli received by the CNS produce nociception (136). Localized cortical activation in adults not only results in nociceptive sensations but also causes an increase in local blood flow to the activated area (137) which significantly exceeds the oxygen demand of the brain tissue, ultimately leading to an increase in the oxygen content of hemoglobin. Functional near-infrared spectroscopic imaging, equivalent to magnetic resonance in assessing brain function, utilizes the distinct optical properties of hemoglobin to non-invasively quantify changes in cortical hemodynamics (138). Thanaboriboon et al. (138) demonstrated an increased risk of cerebral de-oxygenation events (CDEs) during shoulder arthroscopy in the beach chair position. The risk of CDE is high, and factors that may affect cerebral perfusion and oxygenation should be carefully monitored. Additionally, a study using near-infrared spectroscopy during shoulder arthroscopy in the beach chair position found that CDEs were more likely to occur (139).
Electroencephalography, historically used for diagnosing neurological diseases, now plays a critical role in monitoring the depth of anesthesia (140, 141). Frontal cortex EEG signals exhibit characteristic responses to anesthetic agents, leading to the development of various devices since the 1990s that utilize these EEG frequency domain transformations (142). Monitoring the depth of anesthesia (DoA) via EEG remains a challenge for anesthesiologists, especially in the elderly, due to age-related decreases in brain activity (143, 144), complicating the distinction between awake and anesthetized states in individual patients.
5.2 Enhancing anesthetic precision with AI-integrated monitoring technologies
The integration of artificial intelligence (AI) with monitoring technologies marks a significant advancement in anesthesiology, leveraging AI’s formidable data processing and self-learning capabilities. By statistically analyzing the continuous data streams from anesthesia machines and monitors, AI can harmonize with technologies like electroencephalography (EEG) and near-infrared spectroscopy (NIRS), providing real-time feedback on anesthetic depth to optimize patient care.
Machine learning models, built upon AI foundations, have demonstrated their utility in perioperative anesthesia management. These models enhance the interpretation of EEG signals, facilitating nuanced analyzes of complex data streams for depth of anesthesia (DoA) monitoring. Studies have highlighted the efficacy of direct EEG signal analysis through AI and spectral analysis (120). Park et al. (145) developed a DoA system utilizing real-time EEG and deep neural network algorithms that surpass traditional bispectral index (BIS) systems in performance. Gu et al. (146) devised a monitoring system integrating multi-electroencephalographic frequencies and entropy features with neural networks to classify DoA stages with remarkable accuracy. Ramaswamy et al. (147) extracted EEG spectral features using clinical trial datasets, logistic regression, support vector machines, and random forest models, accurately predicting the depth of sedation in patients. Similarly, Mirsadeghi et al. (148) and Shalbaf et al. (149) have demonstrated the superior accuracy of machine learning algorithms over BIS in analyzing EEG features across various anesthesia depths. AI’s application extends to perioperative ultrasound, aiding anesthesiologists in swiftly and accurately interpreting images, enhancing the precision of perioperative assessments, and streamlining result analysis (148, 149).
The application of artificial intelligence (AI) extends beyond monitoring anesthesia depth, fundamentally enhancing perioperative ultrasound imaging. AI’s ability to swiftly and accurately process ultrasound images promises to revolutionize anesthesiologists’ workflows by improving the precision of perioperative diagnostics and reducing the time required for assessment analysis (120). Hayasaka et al. (150) successfully used AI to predict difficult intubations, while Hetherington et al. (151) designed a neural network model that identifies anatomical landmarks with up to 95% accuracy. AI also assists anesthetists in analyzing complex ultrasound data; this capability facilitates the performance of technically demanding procedures, such as epidural punctures and tube placements, by automatically locating vertebral bodies and intervertebral spaces. Furthermore, AI aids in analyzing complex ultrasound data, with machine learning algorithms now capable of autonomously measuring cardiac ejection fraction and assessing cardiac function—delivering results that rival the accuracy of cardiologists and offer greater consistency than traditional ultrasound evaluations (152).
Moreover, AI’s predictive capabilities extend beyond diagnostics to the logistical aspects of surgery, including predicting surgical duration, identifying cancelations in high-risk procedures, and estimating post-anesthesia care unit stays. These advancements pave the way for more tailored anesthesia management, catering to the unique needs of each surgical procedure and patient profile (153).
6 Comorbidities and patient factors
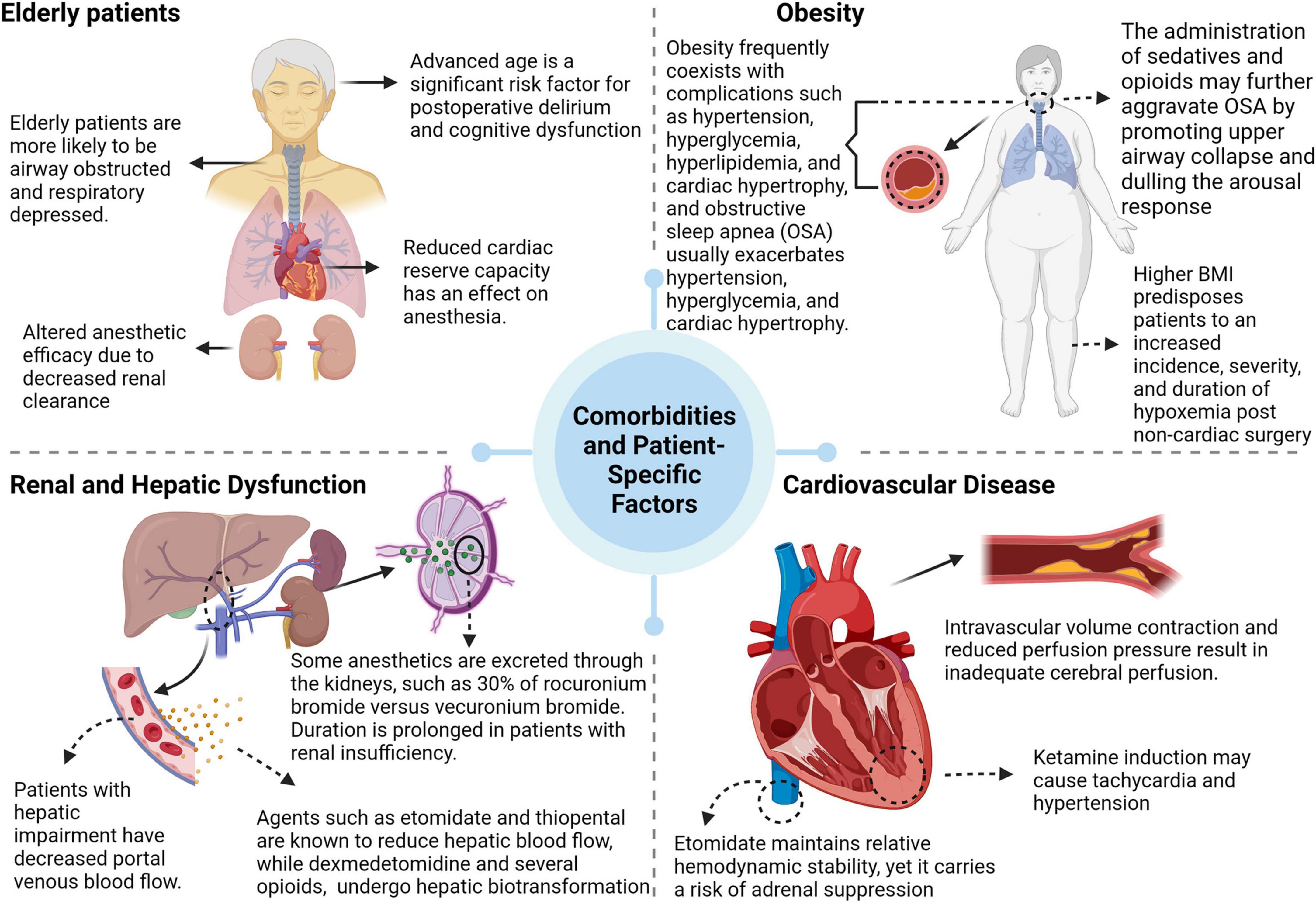
An individual’s response to anesthesia is significantly influenced by various comorbidities and patient-specific factors (see Figure 2).
Age, particularly in the elderly, is an important determinant. This population has reduced cardiac, pulmonary, and renal reserve capacities and often exhibits physical and cognitive impairments. Anesthesia induces a complex physiological response in this group, which is exacerbated by the age-related decline in renal clearance. This decline prolongs the elimination half-life of both hydrophilic and lipophilic drugs, causing pharmacokinetic and pharmacodynamic changes that ultimately increase susceptibility to sedation (154). The clearance of benzodiazepines decreases significantly with age, enhancing their effects and increasing the risk of sedation-related adverse events (155, 156). Additionally, interactions between sedatives and centrally acting drugs, including general anesthetics, often produce synergistic effects (157).
Obesity is a multifactorial state of physiological dysfunction resulting from a complex interaction of genetic, environmental, and endocrine factors. It is often associated with comorbidities such as hypertension, hyperglycemia, hyperlipidemia, cardiac hypertrophy, and obstructive sleep apnea (OSA). The perioperative period in obese patients is compromised by an increased risk of pulmonary complications, typically characterized by altered respiratory mechanics, including increased respiratory rate, decreased tidal volume, and increased airway resistance (158, 159). Notably, obesity is a predictor of difficult airway management and is significantly correlated with difficult intubation scenarios (160).
This reduction in patients with hepatic dysfunction (especially those with cirrhosis) may be exacerbated by a lack of compensatory increase in portal blood flow during anesthesia (161). Drugs such as etomidate and sodium thiopental are known to reduce hepatic blood flow, whereas dexmedetomidine and several opioids (except remifentanil) undergo hepatic biotransformation and therefore require dose adjustment in the presence of hepatic insufficiency (162, 163). At the same time, renal disease alters the pharmacokinetics and pharmacodynamics of anesthetic agents. Rocuronium bromide and vecuronium bromide depend on renal excretion for 30% of their elimination and may have a prolonged duration in the presence of renal insufficiency. In contrast, cis-atracurium and atracurium, which undergo Hoffman elimination, are unaffected by renal impairment (164).
Cardiovascular disease poses a major challenge in the perioperative period, where surgical trauma, anesthesia, and related factors can induce arrhythmias, myocardial ischemia, and hemodynamic changes that can seriously affect patient prognosis (165). Patients with chronic hypertension tend to be more sensitive to anesthetics and surgical procedures (166).
The choice of anesthetic modalities and anesthetic drugs should be assessed in light of the patient’s condition and individual differences, and a personalized anesthetic plan should be developed based on the patient’s specific physiological conditions.
7 Challenges and future directions
7.1 Challenges in the implementation of personalized anesthesia
Despite its promise, personalized anesthesia still faces a number of significant obstacles. The limited availability and high cost associated with pharmacogenomic testing pose significant barriers to its widespread use. In the United States, clinical pharmacogenomic testing laboratories must be accredited by organizations such as the College of American Pathologists, and false positives and false negatives can occur due to potential errors in the test design itself (167). In addition, results are not standardized and may vary from lab to lab (168, 169). Second, the translation of pharmacogenomics into clinical practice requires the availability of high-quality genotyping tests in a short period of time, and the correct interpretation of pharmacogenetic test results by clinicians requires an adequate clinical decision support infrastructure, so it is necessary to train healthcare professionals (170) and to attempt to apply artificial intelligence and machine learning to create drug response prediction models to analyze genomic and other “histologic” data (171) to allow patients to choose the right drug at the right dose. As technology advances and costs decrease, genotyping may become more accessible and practical in routine anesthesia practice.
7.2 Navigating the pharmacogenomic landscape in anesthesia
The application of pharmacogenomic testing in clinical practice is compounded by the complexity of interpreting genetic data, particularly in the context of polypharmacy. Drug-drug interactions must be meticulously considered alongside pharmacogenomic results to accurately predict phenotypic outcomes (172). For instance, a patient concurrently taking multiple medications that prolong the QT interval (QTc) may be predisposed to torsades de pointes, even if pharmacogenomic testing predicts a normal response. Similarly, pharmacological agents that act as inhibitors or inducers can significantly alter the functionality of drug-metabolizing enzymes, thus transforming the phenotype (172). The integration of pharmacogenomic results with other clinical factors—such as age, existing comorbidities, and current medications—is imperative to avoid suboptimal patient outcomes (173). Any variant of a gene can affect the efficacy and safety of a drug, and 95.12% of all genes have one or more variants. Therefore, the detection of variants within key genes is important. For instance, the presence of 64 variants within the rosuvastatin gene raises concerns about the impact of these variants, which could range from negligible to the induction of severe myopathy, affecting a significant portion of prescriptions (174).
Given the intricacies of pharmacogenomic data, the development of intuitive tools and comprehensive guidelines is essential to aid anesthesiologists in interpreting and applying genetic information to patient care, thereby facilitating the delivery of truly personalized anesthetic management.
7.3 Advancing education and training in anesthesiology
The evolving field of anesthesiology is increasingly incorporating the principles of genetics, pharmacogenomics and personalized medicine. To ensure that these advances are translated into improved patient care, anesthetists need to be educated and trained in these disciplines, either through books or courses. They should be able to detect genetic polymorphisms and biomarker changes and determine individual conditions, as well as be skilled in the use of EEG, ultrasound and other artificial intelligence decision support tools. This is not only an extension of existing knowledge, but also necessary for the modern anesthetist to deal with the complexity of an individual’s genetic profile when administering anesthesia.
7.4 Future research directions in personalized anesthesia
Future research efforts in personalized anesthesia are expected to refine and expand the scope of patient-specific anesthesia management. Areas of focus should include:
Genetic and biomarker discovery: The identification of novel genetic determinants and biomarkers that can reliably predict an individual’s response to anesthetics and analgesics is critical. As more and more large biobanks or sample libraries are linked to genomic data, this provides an opportunity for future pharmacogenomic studies to query genetic polymorphisms more easily. In addition, anesthesia-related biomarkers are under-researched, and a large number of clinical trials are needed to identify the appropriate markers that can predict the risk of perioperative complications and allow for pre-emptive intervention.
Health economics: It is imperative to evaluate the cost-effectiveness of personalized anesthesia strategies. Research should aim to delineate the economic benefits, such as reductions in healthcare costs and improvements in patient outcomes, attributable to the adoption of personalized approaches.
Technological innovation: The development and rigorous validation of advanced monitoring technologies are critical. These innovations should be capable of supporting the customization of anesthetic management, aligning with the nuances of individual physiological responses.
Clinical trials: There is a need for extensive, multicenter clinical trials to conclusively ascertain the efficacy and safety of personalized anesthesia modalities across diverse patient demographics and surgical disciplines. Such trials will be instrumental in establishing evidence-based guidelines and protocols.
As the field progresses, it is crucial that research in these areas is conducted with methodological rigor and a multidisciplinary approach, integrating insights from genomics, pharmacology, bioinformatics, and clinical anesthesiology.
8 Conclusion
Personalized anesthesia and precision medicine represent a paradigm shift in the field of anesthesiology, with rapid advancements heralding a new era of enhanced patient care. The integration of individual genetic profiles, specific comorbid conditions, and unique patient characteristics with the burgeoning fields of pharmacogenomics and biomarker discovery has the potential to significantly refine anesthetic management. When coupled with the latest in monitoring technologies, these insights empower anesthesiologists to customize treatment plans to the distinct requirements of each patient.
The path toward fully realizing the promise of personalized anesthesia is lined with challenges, including the need for widespread education and training in the relevant fields of genetics and pharmacogenomics, as well as the development of cost-effective and accessible technologies. Moreover, interdisciplinary collaboration is vital for advancing research and translating these innovations into routine clinical practice. As we navigate these challenges, the collective efforts of anesthesiologists, geneticists, and other healthcare professionals will be paramount in harnessing the full potential of personalized anesthesia to optimize patient outcomes and elevate the standard of care.
Author contributions
SZ: Conceptualization, Investigation, Writing – original draft. QQ: Conceptualization, Investigation, Writing – original draft. WX: Data curation, Methodology, Writing – original draft. SY: Methodology, Validation, Writing – original draft. MZ: Validation, Visualization, Writing – original draft. HT: Validation, Visualization, Writing – original draft. JP: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. JH: Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hunan Provincial Nature Fund (grant no. 2024JJ7664) of Hunan Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Orellana García L, Ehmann F, Hines P, Ritzhaupt A, Brand A. Biomarker and companion diagnostics-a review of medicinal products approved by the European medicines agency. Front Med. (2021) 8:753187. doi: 10.3389/fmed.2021.753187
2. Gu S, Luo Q, Wen C, Zhang Y, Liu L, Liu L, et al. Application of advanced technologies-nanotechnology, genomics technology, and 3D printing technology-in precision anesthesia: A comprehensive narrative review. Pharmaceutics. (2023) 15:2289. doi: 10.3390/pharmaceutics15092289
3. Zhang R, Wang Z, Li Y, Lu Y, Wang S, Yu W, et al. Usefulness of dynamic contrast-enhanced magnetic resonance imaging for predicting treatment response to vinorelbine-cisplatin with or without recombinant human endostatin in bone metastasis of non-small cell lung cancer. Am J Cancer Res. (2016) 6:2890–900.
4. Mohammadi-Yeganeh S, Bilanicz S, Dabbagh A. The Role of OMICS (genomics, epigenetics, transcriptomics, proteomics and metabolomics) in personalized anesthesia and perioperative medicine. In: A Dabbagh editor. Personalized Medicine in Anesthesia, Pain and Perioperative Medicine. Cham: Springer International Publishing (2021). p. 9–63.
5. Dabbagh A, Sabouri AS. The role of personalized medicine in current and future clinical practice of anesthesiology and perioperative medicine: Towards anesthesiomics. In: A Dabbagh editor. Personalized Medicine in Anesthesia, Pain and Perioperative Medicine. Cham: Springer International Publishing (2021). p. 1–8.
6. Hlavac V, Kovacova M, Elsnerova K, Brynychova V, Kozevnikovova R, Raus K, et al. Use of germline genetic variability for prediction of chemoresistance and prognosis of breast cancer patients. Cancers. (2018) 10:511. doi: 10.3390/cancers10120511
7. Bach-Rojecky L, Čutura T, Lozić M, Kliškinjić IH, Matišić V, Primorac D. Personalized anesthetic pharmacology. In: A Dabbagh editor. Personalized Medicine in Anesthesia, Pain and Perioperative Medicine. Cham: Springer International Publishing (2021). p. 65–92.
8. Manolio T, Chisholm R, Ozenberger B, Roden D, Williams M, Wilson R, et al. Implementing genomic medicine in the clinic: The future is here. Genet Med. (2013) 15:258–67. doi: 10.1038/gim.2012.157
9. Frick A, Benton C, Scolaro K, McLaughlin J, Bradley C, Suzuki O, et al. Transitioning pharmacogenomics into the clinical setting: Training future pharmacists. Front Pharmacol. (2016) 7:241. doi: 10.3389/fphar.2016.00241
10. Chan S, Ang X, Sani L, Ng H, Winther M, Liu J, et al. Prevalence and characteristics of adverse drug reactions at admission to hospital: A prospective observational study. Br J Clin Pharmacol. (2016) 82:1636–46. doi: 10.1111/bcp.13081
11. Dunnenberger H, Crews K, Hoffman J, Caudle K, Broeckel U, Howard S, et al. Preemptive clinical pharmacogenetics implementation: Current programs in five US medical centers. Annu Rev Pharmacol Toxicol. (2015) 55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835
12. van der Weide J, Hinrichs J. The influence of cytochrome P450 pharmacogenetics on disposition of common antidepressant and antipsychotic medications. Clin Biochem Rev. (2006) 27:17–25.
13. Zanger U, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. (2013) 138:103–41. doi: 10.1016/j.pharmthera.2012.12.007
14. Zhao M, Ma J, Li M, Zhang Y, Jiang B, Zhao X, et al. Cytochrome P450 enzymes and drug metabolism in humans. Int J Mol Sci. (2021) 22:12808. doi: 10.3390/ijms222312808
15. Kharasch ED, Hankins D, Cox K. Clinical isoflurane metabolism by cytochrome P450 2E1. Anesthesiology. (1999) 90:766–71. doi: 10.1097/00000542-199903000-00019
16. Xie S, Ma W, Guo Q, Liu J, Li W, McLeod H, et al. The pharmacogenetics of medications used in general anesthesia. Pharmacogenomics. (2018) 19:285–98. doi: 10.2217/pgs-2017-0168
17. Bach-Rojecky L, Vađunec D, Lozić M, Žunić K, Špoljar G, Čutura T, et al. Challenges in anesthesia personalization: Resolving the pharmacogenomic puzzle. Per Med. (2019) 16:511–25. doi: 10.2217/pme-2019-0056
18. Kharasch ED, Hankins D, Mautz D, Thummel K. Identification of the enzyme responsible for oxidative halothane metabolism: Implications for prevention of halothane hepatitis. Lancet. (1996) 347:1367–71. doi: 10.1016/s0140-6736(96)91011-9
19. Tung D, Yoshida E, Wang C, Steinbrecher U. Severe desflurane hepatotoxicity after colon surgery in an elderly patient. Can J Anaesth. (2005) 52:133–6. doi: 10.1007/BF03027717
20. Turillazzi E, D’Errico S, Neri M, Riezzo I, Fineschi V. A fatal case of fulminant hepatic necrosis following sevoflurane anesthesia. Toxicol Pathol. (2007) 35:840–5. doi: 10.1080/01926230701584148
21. Iohom G, Ni Chonghaile M, O’Brien J, Cunningham A, Fitzgerald D, Shields D. An investigation of potential genetic determinants of propofol requirements and recovery from anaesthesia. Eur J Anaesthesiol. (2007) 24:912–9. doi: 10.1017/S0265021507000476
22. Peltoniemi M, Hagelberg N, Olkkola K, Saari T. Ketamine: A review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin Pharmacokinet. (2016) 55:1059–77. doi: 10.1007/s40262-016-0383-6
23. Zanos P, Moaddel R, Morris P, Riggs L, Highland J, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol Rev. (2018) 70:621–60. doi: 10.1124/pr.117.015198
24. Li Y, Jackson K, Slon B, Hardy J, Franco M, William L, et al. CYP2B6*6 allele and age substantially reduce steady-state ketamine clearance in chronic pain patients: Impact on adverse effects. Br J Clin Pharmacol. (2015) 80:276–84. doi: 10.1111/bcp.12614
25. Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In: LL Brunton, R Hilal-Dandan, BC Knollmann editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Education (2017).
26. de Jonge H, Elens L, de Loor H, van Schaik R, Kuypers D. The CYP3A4*22 C>T single nucleotide polymorphism is associated with reduced midazolam and tacrolimus clearance in stable renal allograft recipients. Pharmacogenomics J. (2015) 15:144–52. doi: 10.1038/tpj.2014.49
27. Elens L, Nieuweboer A, Clarke S, Charles K, de Graan A, Haufroid V, et al. Impact of POR*28 on the clinical pharmacokinetics of CYP3A phenotyping probes midazolam and erythromycin. Pharmacogenet Genomics. (2013) 23:148–55. doi: 10.1097/FPC.0b013e32835dc113
28. Johnson J, Rolan P, Johnson M, Bobrovskaya L, Williams D, Johnson K, et al. Codeine-induced hyperalgesia and allodynia: Investigating the role of glial activation. Transl Psychiatry. (2014) 4:e482. doi: 10.1038/tp.2014.121
29. Balyan R, Mecoli M, Venkatasubramanian R, Chidambaran V, Kamos N, Clay S, et al. CYP2D6 pharmacogenetic and oxycodone pharmacokinetic association study in pediatric surgical patients. Pharmacogenomics. (2017) 18:337–48. doi: 10.2217/pgs-2016-0183
30. Gong L, Stamer U, Tzvetkov M, Altman R, Klein T. PharmGKB summary: Tramadol pathway. Pharmacogenet Genomics. (2014) 24:374–80. doi: 10.1097/FPC.0000000000000057
31. Bell G, Caudle K, Whirl-Carrillo M, Gordon R, Hikino K, Prows C, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin Pharmacol Ther. (2017) 102:213–8. doi: 10.1002/cpt.598
32. Eliasson E, Kenna J. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. (1996) 50:573–82.
33. Mourão A, de Abreu F, Fiegenbaum M. Impact of the Cytochrome P450 2B6 (CYP2B6) Gene Polymorphism c.516G>T (rs3745274) on Propofol Dose Variability. Eur J Drug Metab Pharmacokinet. (2016) 41:511–5. doi: 10.1007/s13318-015-0289-y
34. Kamali F, Wynne H. Pharmacogenetics of warfarin. Annu Rev Med. (2010) 61:63–75. doi: 10.1146/annurev.med.070808.170037
35. Lindh J, Lundgren S, Holm L, Alfredsson L, Rane A. Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin Pharmacol Ther. (2005) 78:540–50. doi: 10.1016/j.clpt.2005.08.006
36. Zhou S, Skaar D, Jacobson P, Huang R. Pharmacogenomics of medications commonly used in the intensive care unit. Front Pharmacol. (2018) 9:1436. doi: 10.3389/fphar.2018.01436
37. Wu X, Yuan L, Zuo J, Lv J, Guo T. The impact of CYP2D6 polymorphisms on the pharmacokinetics of codeine and its metabolites in Mongolian Chinese subjects. Eur J Clin Pharmacol. (2014) 70:57–63. doi: 10.1007/s00228-013-1573-x
38. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. (2007) 7:352–6. doi: 10.1111/j.1533-2500.2007.00153.x
39. Seripa D, Latina P, Fontana A, Gravina C, Lattanzi M, Savino M, et al. Role of CYP2D6 polymorphisms in the outcome of postoperative pain treatment. Pain Med. (2015) 16:2012–23. doi: 10.1111/pme.12778
40. Dong Z, Li H, Chen Q, Hu Y, Wu S, Tang L, et al. Effect of CYP3A4*1G on the fentanyl consumption for intravenous patient-controlled analgesia after total abdominal hysterectomy in Chinese Han population. J Clin Pharm Ther. (2012) 37:153–6. doi: 10.1111/j.1365-2710.2011.01268.x
41. Candiotti K, Birnbach D, Lubarsky D, Nhuch F, Kamat A, Koch W, et al. The impact of pharmacogenomics on postoperative nausea and vomiting: Do CYP2D6 allele copy number and polymorphisms affect the success or failure of ondansetron prophylaxis? Anesthesiology. (2005) 102:543–9. doi: 10.1097/00000542-200503000-00011
42. Kaiser R, Sezer O, Papies A, Bauer S, Schelenz C, Tremblay P, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol. (2002) 20:2805–11. doi: 10.1200/JCO.2002.09.064
43. Allderdice P, Gardner H, Galutira D, Lockridge O, LaDu B, McAlpine P. The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26. Genomics. (1991) 11:452–4. doi: 10.1016/0888-7543(91)90154-7
44. Masson P, Carletti E, Nachon F. Structure, activities and biomedical applications of human butyrylcholinesterase. Protein Pept Lett. (2009) 16:1215–24. doi: 10.2174/092986609789071207
45. Lando G, Mosca A, Bonora R, Azzario F, Penco S, Marocchi A, et al. Frequency of butyrylcholinesterase gene mutations in individuals with abnormal inhibition numbers: An Italian-population study. Pharmacogenetics. (2003) 13:265–70. doi: 10.1097/00008571-200305000-00005
46. Kuhnert B, Philipson E, Pimental R, Kuhnert PM. A prolonged chloroprocaine epidural block in a postpartum patient with abnormal pseudocholinesterase. Anesthesiology. (1982) 56:477–8. doi: 10.1097/00000542-198206000-00017
47. Monedero P, Hess P. High epidural block with chloroprocaine in a parturient with low pseudocholinesterase activity. Can J Anaesth. (2001) 48:318–9. doi: 10.1007/BF03019772
48. Acharya S, Bhattarai S, Shrimanker I, Gupta SS. A prolonged paralysis with succinylcholine in pseudocholinesterase deficiency: An undesired effect. QJM. (2022) 115:547–8. doi: 10.1093/qjmed/hcac103
49. Wecksell M, Koutsospyros D. Pseudocholinesterase deficiency in a octogenarian undergoing total intravenous anesthesia; implications F N. Middle East J Anaesthesiol. (2015) 23:157–62.
50. Bell G, Donovan K, McLeod H. Clinical implications of opioid pharmacogenomics in patients with cancer. Cancer Control. (2015) 22:426–32. doi: 10.1177/107327481502200408
51. Angst M, Phillips N, Drover D, Tingle M, Ray A, Swan G, et al. Pain sensitivity and opioid analgesia: A pharmacogenomic twin study. Pain. (2012) 153:1397–409. doi: 10.1016/j.pain.2012.02.022
52. Stamer U, Lehnen K, Höthker F, Bayerer B, Wolf S, Hoeft A, et al. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. (2003) 105:231–8. doi: 10.1016/s0304-3959(03)00212-4
53. Kelly L, Rieder M, van den Anker J, Malkin B, Ross C, Neely M, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. (2012) 129:e1343–7. doi: 10.1542/peds.2011-2538
54. Stamer U, Stüber F, Muders T, Musshoff F. Respiratory depression with tramadol in a patient with renal impairment and CYP2D6 gene duplication. Anesth Analg. (2008) 107:926–9. doi: 10.1213/ane.0b013e31817b796e
55. Kong Y, Yan T, Gong S, Deng H, Zhang G, Wang J. Opioid receptor mu 1 (OPRM1) A118G polymorphism (rs1799971) and postoperative nausea and vomiting. Am J Transl Res. (2018) 10:2764–80.
56. Landau R, Kern C, Columb M, Smiley R, Blouin J. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. (2008) 139:5–14. doi: 10.1016/j.pain.2008.02.023
57. Brown R, Basheer R, McKenna J, Strecker R, McCarley R. Control of sleep and wakefulness. Physiol Rev. (2012) 92:1087–1087. doi: 10.1152/physrev.00032.2011
58. Choi Y, Lee S, Yang K, Park J, Yoon S, Yoon S. Polymorphism rs4263535 in GABRA1 intron 4 was related to deeper sedation by intravenous midazolam. J Int Med Res. (2015) 43:686–98. doi: 10.1177/0300060515587580
59. Gonsalves S, Dirksen R, Sangkuhl K, Pulk R, Alvarellos M, Vo T, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for the use of potent volatile anesthetic agents and succinylcholine in the context of RYR1 or CACNA1S Genotypes. Clin Pharmacol Ther. (2019) 105:1338–44. doi: 10.1002/cpt.1319
60. Kim D. Malignant hyperthermia. Korean J Anesthesiol. (2012) 63:391–401. doi: 10.4097/kjae.2012.63.5.391
61. Connolly J, Tan K, Mastrogiacomo B, Dycoco J, Caso R, Jones G, et al. Intraoperative opioid exposure, tumour genomic alterations, and survival differences in people with lung adenocarcinoma. Br J Anaesth. (2021) 127:75–84. doi: 10.1016/j.bja.2021.03.030
62. Mincer J, Buggy D. Anaesthesia, analgesia, and cancer outcomes: Time to think like oncologists? Br J Anaesth. (2023) 131:193–6. doi: 10.1016/j.bja.2023.02.001
63. Iwasaki M, Zhao H, Jaffer T, Unwith S, Benzonana L, Lian Q, et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. (2016) 7:26042–56. doi: 10.18632/oncotarget.8304
64. Benzonana L, Perry N, Watts H, Yang B, Perry I, Coombes C, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. (2013) 119:593–605. doi: 10.1097/ALN.0b013e31829e47fd
65. Oh C, Park H, Piao L, Sohn K, Koh S, Hwang D, et al. Expression profiles of immune cells after propofol or sevoflurane anesthesia for colorectal cancer surgery: A prospective double-blind randomized trial. Anesthesiology. (2022) 136:448–58. doi: 10.1097/ALN.0000000000004119
66. Garib V, Lang K, Niggemann B, Zänker K, Brandt L, Dittmar T. Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells. Eur J Anaesthesiol. (2005) 22:609–15. doi: 10.1017/s026502150500102x
67. Xu Y, Du Q, Zhang M, Yun P, He C. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. (2013) 17:2486–94.
68. Yang W, Cai J, Zabkiewicz C, Zhang H, Ruge F, Jiang W. The effects of anesthetics on recurrence and metastasis of cancer, and clinical implications. World J Oncol. (2017) 8:63–70. doi: 10.14740/wjon1031e
69. Yuval J, Lee J, Wu F, Thompson H, Verheij F, Gupta H, et al. Intraoperative opioids are associated with decreased recurrence rates in colon adenocarcinoma: A retrospective observational cohort study. Br J Anaesth. (2022) 129:172–81. doi: 10.1016/j.bja.2022.04.024
70. Willis J, Reyes-Uribe L, Chang K, Lipkin S, Vilar E. Immune activation in mismatch repair-deficient carcinogenesis: More than just mutational rate. Clin Cancer Res. (2020) 26:11–7. doi: 10.1158/1078-0432.CCR-18-0856
71. Connolly J, Scarpa J, Gupta H, Tan K, Mastrogiacomo B, Dycoco J, et al. Intraoperative ketorolac may interact with patient-specific tumour genomics to modify recurrence risk in lung adenocarcinoma: An exploratory analysis. Br J Anaesth. (2021) 127:e82–5. doi: 10.1016/j.bja.2021.05.032
72. Dancey J, Dobbin K, Groshen S, Jessup J, Hruszkewycz A, Koehler M, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. (2010) 16:1745–55. doi: 10.1158/1078-0432.CCR-09-2167
73. De Gruttola V, Clax P, DeMets D, Downing G, Ellenberg S, Friedman L, et al. Considerations in the evaluation of surrogate endpoints in clinical trials. Summary of a National Institutes of Health workshop. Control Clin Trials. (2001) 22:485–502. doi: 10.1016/s0197-2456(01)00153-2
74. Póvoa P, Coelho L, Dal-Pizzol F, Ferrer R, Huttner A, Conway Morris A, et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. (2023) 49:142–53. doi: 10.1007/s00134-022-06956-y
75. Calfee C, Delucchi K, Parsons P, Thompson B, Ware L, Matthay M, et al. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir Med. (2014) 2:611–20. doi: 10.1016/S2213-2600(14)70097-9
76. Famous K, Delucchi K, Ware L, Kangelaris K, Liu K, Thompson B, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. (2017) 195:331–8. doi: 10.1164/rccm.201603-0645OC
77. Hartholt K, van der Cammen T, Klimek M. Postoperative cognitive dysfunction in geriatric patients. Z Gerontol Geriatr. (2012) 45:411–6. doi: 10.1007/s00391-012-0326-2
78. Reddy S, Irkal J, Srinivasamurthy A. Postoperative delirium in elderly citizens and current practice. J Anaesthesiol Clin Pharmacol. (2017) 33:291–9. doi: 10.4103/joacp.JOACP_180_16
79. Oh S, Park J. Postoperative delirium. Korean J Anesthesiol. (2019) 72:4–12. doi: 10.4097/kja.d.18.00073.1
80. Monk T, Weldon B, Garvan C, Dede D, van der Aa M, Heilman K, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. (2008) 108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e
81. Safavynia S, Goldstein P. The role of neuroinflammation in postoperative cognitive dysfunction: Moving from hypothesis to treatment. Front Psychiatry. (2019) 9:752. doi: 10.3389/fpsyt.2018.00752
82. Joris J, Kehlet H, Slim K. Postoperative cognitive dysfunction: Time for enhanced recovery after surgery programmes. Eur J Anaesthesiol. (2022) 39:733–4. doi: 10.1097/EJA.0000000000001684
83. Cui Y, Li G, Cao R, Luan L, Kla K. The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: A network meta-analysis. J Clin Anesth. (2020) 59:89–98. doi: 10.1016/j.jclinane.2019.06.028
84. Carr Z, Cios T, Potter K, Swick J. Does dexmedetomidine ameliorate postoperative cognitive dysfunction? A brief review of the recent literature. Curr Neurol Neurosci Rep. (2018) 18:64. doi: 10.1007/s11910-018-0873-z
85. Clegg A, Young J. Which medications to avoid in people at risk of delirium: A systematic review. Age Ageing. (2011) 40:23–9. doi: 10.1093/ageing/afq140
86. Hayhurst C, Pandharipande P, Hughes C. Intensive care unit delirium: A review of diagnosis, prevention, and treatment. Anesthesiology. (2016) 125:1229–41. doi: 10.1097/ALN.0000000000001378
87. O’Bryan L, Atkins K, Lipszyc A, Scott D, Silbert B, Evered L. Inflammatory biomarker levels after propofol or sevoflurane anesthesia: A meta-analysis. Anesth Analg. (2022) 134:69–81. doi: 10.1213/ANE.0000000000005671
88. Rump K, Adamzik M. Epigenetic mechanisms of postoperative cognitive impairment induced by anesthesia and neuroinflammation. Cells. (2022) 11:2954. doi: 10.3390/cells11192954
89. Mathew J, Grocott H, Phillips-Bute B, Stafford-Smith M, Laskowitz D, Rossignol D, et al. Lower endotoxin immunity predicts increased cognitive dysfunction in elderly patients after cardiac surgery. Stroke. (2003) 34:508–13. doi: 10.1161/01.str.0000053844.09493.58
90. Li X, Shao M, Wang J, Wang Y. Relationship between post-operative cognitive dysfunction and regional cerebral oxygen saturation and β-amyloid protein. J Zhejiang Univ Sci B. (2014) 15:870–8. doi: 10.1631/jzus.B1400130
91. Li X, Wen D, Zhao Y, Hang Y, Mandell M. Increase of beta-amyloid and C-reactive protein in liver transplant recipients with postoperative cognitive dysfunction. Hepatobiliary Pancreat Dis Int. (2013) 12:370–6. doi: 10.1016/s1499-3872(13)60058-2
92. Tian A, Ma H, Zhang R, Tan W, Wang X, Wu B, et al. Interleukin17A promotes postoperative cognitive dysfunction by triggering β-Amyloid accumulation via the transforming growth factor-β (TGFβ)/Smad signaling pathway. PLoS One. (2015) 10:e0141596. doi: 10.1371/journal.pone.0141596
93. Vacas S, Degos V, Tracey K, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. (2014) 120:1160–7. doi: 10.1097/ALN.0000000000000045
94. Lin F, Shan W, Zheng Y, Pan L, Zuo Z. Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem. (2021) 158:328–41. doi: 10.1111/jnc.15368
95. Lin G, Wang T, Chen M, Hu Z, Ouyang W. Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol Scand. (2014) 58:668–74. doi: 10.1111/aas.12320
96. Xie H, Huang D, Zhang S, Hu X, Guo J, Wang Z, et al. Relationships between adiponectin and matrix metalloproteinase-9 (MMP-9) serum levels and postoperative cognitive dysfunction in elderly patients after general anesthesia. Aging Clin Exp Res. (2016) 28:1075–9. doi: 10.1007/s40520-015-0519-9
97. Casey C, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. (2020) 143:47–54. doi: 10.1093/brain/awz354
98. Brown C, Lewis A, Probert J, Parish M, Tian J, Mandal K, et al. Perioperative neurofilament light plasma concentrations and cognition before and after cardiac surgery: A prospective nested cohort study. Anesthesiology. (2022) 137:303–14. doi: 10.1097/ALN.0000000000004327
99. Suárez-Calvet M, Capell A, Araque Caballero M, Morenas-Rodríguez E, Fellerer K, Franzmeier N, et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol Med. (2018) 10:e9712. doi: 10.15252/emmm.201809712
100. Evered L, Silbert B, Knopman D, Scott D, DeKosky S, Rasmussen L, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. (2018) 129:872–9. doi: 10.1097/ALN.0000000000002334
101. Diao J, Kohane I, Manrai A. Biomedical informatics and machine learning for clinical genomics. Hum Mol Genet. (2018) 27:R29–34. doi: 10.1093/hmg/ddy088
102. Matheny M, Whicher D, Thadaney Israni S. Artificial intelligence in health care: A report from the National academy of medicine. JAMA. (2020) 323:509–10. doi: 10.1001/jama.2019.21579
103. Mori Y, Kudo S, Misawa M, Mori K. Simultaneous detection and characterization of diminutive polyps with the use of artificial intelligence during colonoscopy. VideoGIE. (2019) 4:7–10. doi: 10.1016/j.vgie.2018.10.006
104. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. (2017) 318:2199–210. doi: 10.1001/jama.2017.14585
105. Ting D, Cheung C, Lim G, Tan G, Quang N, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. (2017) 318:2211–23. doi: 10.1001/jama.2017.18152
106. Han S, Kim M, Lim W, Park G, Park I, Chang S. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. (2018) 138:1529–38. doi: 10.1016/j.jid.2018.01.028
107. Mannino R, Myers D, Tyburski E, Caruso C, Boudreaux J, Leong T, et al. Smartphone app for non-invasive detection of anemia using only patient-sourced photos. Nat Commun. (2018) 9:4924. doi: 10.1038/s41467-018-07262-2
108. Correa M, Zimic M, Barrientos F, Barrientos R, Román-Gonzalez A, Pajuelo M, et al. Automatic classification of pediatric pneumonia based on lung ultrasound pattern recognition. PLoS One. (2018) 13:e0206410. doi: 10.1371/journal.pone.0206410
109. Sarwar S, Dent A, Faust K, Richer M, Djuric U, Van Ommeren R, et al. Physician perspectives on integration of artificial intelligence into diagnostic pathology. NPJ Digit Med. (2019) 2:28. doi: 10.1038/s41746-019-0106-0
110. Stacey D, Bennett C, Barry M, Col N, Eden K, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2011) 10:CD001431.
111. Waljee J, Rogers M, Alderman A. Decision aids and breast cancer: Do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. (2007) 25:1067–73. doi: 10.1200/JCO.2006.08.5472
112. Patel N, Michelini V, Snell J, Balu S, Hoyle A, Parker J, et al. Enhancing next-generation sequencing-guided cancer care through cognitive computing. Oncologist. (2018) 23:179–85. doi: 10.1634/theoncologist.2017-0170
113. Rajkomar A, Oren E, Chen K, Dai A, Hajaj N, Hardt M, et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. (2018) 1:18. doi: 10.1038/s41746-018-0029-1
114. Gulshan V, Peng L, Coram M, Stumpe M, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. (2016) 316:2402–10. doi: 10.1001/jama.2016.17216
115. Dande P, Samant P. Acquaintance to Artificial Neural Networks and use of artificial intelligence as a diagnostic tool for tuberculosis: A review. Tuberculosis. (2018) 108:1–9. doi: 10.1016/j.tube.2017.09.006
116. Mintz Y, Brodie R. Introduction to artificial intelligence in medicine. Minim Invasive Ther Allied Technol. (2019) 28:73–81. doi: 10.1080/13645706.2019.1575882
117. Qian H, Dong B, Yuan J, Yin F, Wang Z, Wang H, et al. Pre-consultation system based on the artificial intelligence has a better diagnostic performance than the physicians in the outpatient department of pediatrics. Front Med. (2021) 8:695185. doi: 10.3389/fmed.2021.695185
118. Akselrod-Ballin A, Chorev M, Shoshan Y, Spiro A, Hazan A, Melamed R, et al. Predicting breast cancer by applying deep learning to linked health records and mammograms. Radiology. (2019) 292:331–42. doi: 10.1148/radiol.2019182622
119. Banegas M, McClure J, Barlow W, Ubel P, Smith D, Zikmund-Fisher B, et al. Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer. Patient Educ Couns. (2013) 91:364–71. doi: 10.1016/j.pec.2012.12.014
120. Song B, Zhou M, Zhu J. Necessity and importance of developing ai in anesthesia from the perspective of clinical safety and information security. Med Sci Monit. (2023) 29:e938835. doi: 10.12659/MSM.938835
121. van den Heuvel M, van Zanten H, Bachman T, Te Pas A, van Kaam A, Onland W. Optimal target range of closed-loop inspired oxygen support in preterm infants: A randomized cross-over study. J Pediatr. (2018) 197:36–41. doi: 10.1016/j.jpeds.2018.01.077
122. Markello T, Adams D. Genome-scale sequencing to identify genes involved in Mendelian disorders. Curr Protoc Hum Genet. (2013) 79:6.13.1–6.13.19. doi: 10.1002/0471142905.hg0613s79.
123. Topol E. High-performance medicine: The convergence of human and artificial intelligence. Nat Med. (2019) 25:44–56. doi: 10.1038/s41591-018-0300-7
124. Birgmeier J, Haeussler M, Deisseroth C, Steinberg E, Jagadeesh K, Ratner A, et al. AMELIE speeds Mendelian diagnosis by matching patient phenotype and genotype to primary literature. Sci Transl Med. (2020) 12:eaau9113. doi: 10.1126/scitranslmed.aau9113
125. Birgmeier J, Deisseroth C, Hayward L, Galhardo L, Tierno A, Jagadeesh K, et al. AVADA: Toward automated pathogenic variant evidence retrieval directly from the full-text literature. Genet Med. (2020) 22:362–70. doi: 10.1038/s41436-019-0643-6
126. Clark M, Hildreth A, Batalov S, Ding Y, Chowdhury S, Watkins K, et al. Diagnosis of genetic diseases in seriously ill children by rapid whole-genome sequencing and automated phenotyping and interpretation. Sci Transl Med. (2019) 11:eaat6177. doi: 10.1126/scitranslmed.aat6177
127. James K, Clark M, Camp B, Kint C, Schols P, Batalov S, et al. Partially automated whole-genome sequencing reanalysis of previously undiagnosed pediatric patients can efficiently yield new diagnoses. NPJ Genom Med. (2020) 5:33. doi: 10.1038/s41525-020-00140-1
128. Shortliffe E, Sepúlveda M. Clinical decision support in the era of artificial intelligence. JAMA. (2018) 320:2199–200. doi: 10.1001/jama.2018.17163
129. Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. (2006) 34:453–63. doi: 10.1165/rcmb.2005-0223OC
130. Hazelton J, Balan I, Elmer G, Kristian T, Rosenthal R, Krause G, et al. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. (2010) 27:753–62. doi: 10.1089/neu.2009.1186
132. Billings F, Pretorius M, Schildcrout J, Mercaldo N, Byrne J, Ikizler T, et al. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. (2012) 23:1221–8. doi: 10.1681/ASN.2011090940
133. Lewis C, Parulkar S, Bebawy J, Sherwani S, Hogue C. Cerebral neuromonitoring during cardiac surgery: A critical appraisal with an emphasis on near-infrared spectroscopy. J Cardiothorac Vasc Anesth. (2018) 32:2313–22. doi: 10.1053/j.jvca.2018.03.032
134. Lopez M, Pandharipande P, Morse J, Shotwell M, Milne G, Pretorius M, et al. Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic Biol Med. (2017) 103:192–8. doi: 10.1016/j.freeradbiomed.2016.12.039
135. Ono M, Brady K, Easley R, Brown C, Kraut M, Gottesman R, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. (2014) 147:483–9. doi: 10.1016/j.jtcvs.2013.07.069
136. Peters A, McEwen B, Friston K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol. (2017) 156:164–88. doi: 10.1016/j.pneurobio.2017.05.004
137. Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. (2002) 25:621–5. doi: 10.1016/s0166-2236(02)02264-6
138. Thanaboriboon C, Vanichvithya P, Jinaworn P. What is the risk of intraoperative cerebral oxygen desaturation in patients undergoing shoulder surgery in the beach chair position? Clin Orthop Relat Res. (2021) 479:2677–87. doi: 10.1097/CORR.0000000000001864
139. Cox R, Jamgochian G, Nicholson K, Wong J, Namdari S, Abboud J. The effectiveness of cerebral oxygenation monitoring during arthroscopic shoulder surgery in the beach chair position: A randomized blinded study. J Shoulder Elbow Surg. (2018) 27:692–700. doi: 10.1016/j.jse.2017.11.004
140. Zhang X, Roy R, Jensen EW. EEG complexity as a measure of depth of anesthesia for patients. IEEE Trans Biomed Eng. (2001) 48:1424–33. doi: 10.1109/10.966601
141. Jameson L, Sloan T. Using EEG to monitor anesthesia drug effects during surgery. J Clin Monit Comput. (2006) 20:445–72. doi: 10.1007/s10877-006-9044-x
142. Fahy B, Chau D. The technology of processed electroencephalogram monitoring devices for assessment of depth of anesthesia. Anesth Analg. (2018) 126:111–7. doi: 10.1213/ANE.0000000000002331
143. Akeju O, Pavone K, Thum J, Firth P, Westover M, Puglia M, et al. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. Br J Anaesth (2015) 115(Suppl 1):i66–76. doi: 10.1093/bja/aev114
144. Purdon P, Sampson A, Pavone K, Brown E. Clinical electroencephalography for anesthesiologists: Part I: Background and basic signatures. Anesthesiology. (2015) 123:937–60. doi: 10.1097/ALN.0000000000000841
145. Park Y, Han S, Byun W, Kim J, Lee H, Kim SJA. Real-time depth of anesthesia monitoring system based on deep neural network with large EDO tolerant EEG analog front-end. IEEE Trans Biomed Circuits Syst. (2020) 14:825–37. doi: 10.1109/TBCAS.2020.2998172
146. Gu Y, Liang Z, Hagihira S. Use of multiple EEG features and artificial neural network to monitor the depth of anesthesia. Sensors. (2019) 19:2499. doi: 10.3390/s19112499
147. Ramaswamy S, Weerink M, Struys M, Nagaraj S. Dexmedetomidine-induced deep sedation mimics non-rapid eye movement stage 3 sleep: Large-scale validation using machine learning. Sleep. (2021) 44:zsaa167. doi: 10.1093/sleep/zsaa167
148. Mirsadeghi M, Behnam H, Shalbaf R, Jelveh Moghadam H. Characterizing awake and anesthetized states using a dimensionality reduction method. J Med Syst. (2016) 40:13. doi: 10.1007/s10916-015-0382-4
149. Shalbaf A, Saffar M, Sleigh J, Shalbaf R. Monitoring the depth of anesthesia using a new adaptive neurofuzzy system. IEEE J Biomed Health Inform. (2018) 22:671–7. doi: 10.1109/JBHI.2017.2709841
150. Hayasaka T, Kawano K, Kurihara K, Suzuki H, Nakane M, Kawamae K. Creation of an artificial intelligence model for intubation difficulty classification by deep learning (convolutional neural network) using face images: an observational study. J Intensive Care. (2021) 9:38. doi: 10.1186/s40560-021-00551-x
151. Hetherington J, Lessoway V, Gunka V, Abolmaesumi P, Rohling R. SLIDE: Automatic spine level identification system using a deep convolutional neural network. Int J Comput Assist Radiol Surg. (2017) 12:1189–98. doi: 10.1007/s11548-017-1575-8
152. Chen X, Owen C, Huang E, Maggard B, Latif R, Clifford S, et al. Artificial intelligence in echocardiography for anesthesiologists. J Cardiothorac Vasc Anesth. (2021) 35:251–61. doi: 10.1053/j.jvca.2020.08.048
153. Bellini V, Guzzon M, Bigliardi B, Mordonini M, Filippelli S, Bignami E. Artificial intelligence: A new tool in operating room management. Role of machine learning models in operating room optimization. J Med Syst. (2019) 44:20. doi: 10.1007/s10916-019-1512-1
154. Ekstein M, Gavish D, Ezri T, Weinbroum A. Monitored anaesthesia care in the elderly: Guidelines and recommendations. Drugs Aging. (2008) 25:477–500. doi: 10.2165/00002512-200825060-00003
155. Mehta P, Kochhar G, Kalra S, Maurer W, Tetzlaff J, Singh G, et al. Can a validated sleep apnea scoring system predict cardiopulmonary events using propofol sedation for routine EGD or colonoscopy? A prospective cohort study. Gastrointest Endosc. (2014) 79:436–44. doi: 10.1016/j.gie.2013.09.022
156. Jacobs J, Reves J, Marty J, White W, Bai S, Smith L. Aging increases pharmacodynamic sensitivity to the hypnotic effects of midazolam. Anesth Analg. (1995) 80:143–8. doi: 10.1097/00000539-199501000-00024
157. Eilers H, Niemann C. Clinically important drug interactions with intravenous anaesthetics in older patients. Drugs Aging. (2003) 20:969–80. doi: 10.2165/00002512-200320130-00002
158. Ball L, Hemmes S, Serpa Neto A, Bluth T, Canet J, Hiesmayr M, et al. Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br J Anaesth. (2018) 121:899–908. doi: 10.1016/j.bja.2018.04.021
159. Littleton S. Impact of obesity on respiratory function. Respirology. (2012) 17:43–9. doi: 10.1111/j.1440-1843.2011.02096.x
160. Langeron O, Masso E, Huraux C, Guggiari M, Bianchi A, Coriat P, et al. Prediction of difficult mask ventilation. Anesthesiology. (2000) 92:1229–36. doi: 10.1097/00000542-200005000-00009
161. Rahimzadeh P, Safari S, Faiz S, Alavian S. Anesthesia for patients with liver disease. Hepat Mon. (2014) 14:e19881. doi: 10.5812/hepatmon.19881
162. Thomson I, Fitch W, Hughes R, Campbell D, Watson R. Effects of certain i.v. Anaesthetics on liver blood flow and hepatic oxygen consumption in the greyhound. Br J Anaesth. (1986) 58:69–80. doi: 10.1093/bja/58.1.69
163. Mcclain R, Ramakrishna H, Aniskevich S, Cartwright J, Phar L, Pai S, et al. Anesthetic pharmacology and perioperative considerations for the end stage liver disease patient. Curr Clin Pharmacol. (2015) 10:35–46. doi: 10.2174/1574884709666140212110036
164. Brentjens T, Chadha R. Anesthesia for the patient with concomitant hepatic and renal impairment. Anesthesiol Clin. (2016) 34:645–58. doi: 10.1016/j.anclin.2016.06.002
165. Prys-Roberts C, Meloche R, Foëx P. Studies of anaesthesia in relation to hypertension. I. Cardiovascular responses of treated and untreated patients. Br J Anaesth. (1971) 43:122–37. doi: 10.1093/bja/43.2.122
166. Dabu-Bondoc S, Shelley KH. Management of comorbidities in ambulatory anesthesia: A review. Ambulatory Anesth. (2015) 2:39–51.
167. Tandy-Connor S, Guiltinan J, Krempely K, LaDuca H, Reineke P, Gutierrez S, et al. False-positive results released by direct-to-consumer genetic tests highlight the importance of clinical confirmation testing for appropriate patient care. Genet Med. (2018) 20:1515–21. doi: 10.1038/gim.2018.38
168. Bousman C, Dunlop B. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. Pharmacogenomics J. (2018) 18:613–22. doi: 10.1038/s41397-018-0027-3
169. Kalman L, Agúndez J, Appell M, Black J, Bell G, Boukouvala S, et al. Pharmacogenetic allele nomenclature: International workgroup recommendations for test result reporting. Clin Pharmacol Ther. (2016) 99:172–85. doi: 10.1002/cpt.280
170. Huang R, Ratain M. Pharmacogenetics and pharmacogenomics of anticancer agents. CA Cancer J Clin. (2009) 59:42–55. doi: 10.3322/caac.20002
171. Athreya A, Neavin D, Carrillo-Roa T, Skime M, Biernacka J, Frye M, et al. Pharmacogenomics-driven prediction of antidepressant treatment outcomes: A machine-learning approach with multi-trial replication. Clin Pharmacol Ther. (2019) 106:855–65. doi: 10.1002/cpt.1482
172. Shah R, Smith R. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br J Clin Pharmacol. (2015) 79:222–40. doi: 10.1111/bcp.12441
173. Nicholson W, Formea C, Matey E, Wright J, Giri J, Moyer A. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. (2021) 96:218–30. doi: 10.1016/j.mayocp.2020.03.011
Keywords: personalized anesthesia, precision medicine, pharmacogenomics, biomarkers, monitoring technologies, machine learning, artificial intelligence
Citation: Zeng S, Qing Q, Xu W, Yu S, Zheng M, Tan H, Peng J and Huang J (2024) Personalized anesthesia and precision medicine: a comprehensive review of genetic factors, artificial intelligence, and patient-specific factors. Front. Med. 11:1365524. doi: 10.3389/fmed.2024.1365524
Received: 04 January 2024; Accepted: 22 April 2024;
Published: 09 May 2024.
Edited by:
Renan Pedra de Souza, Universidade Federal de Minas Gerais, BrazilReviewed by:
Hui Yin Yow, University of Malaya, MalaysiaJoshua Samuel Mincer, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2024 Zeng, Qing, Xu, Yu, Zheng, Tan, Peng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmin Peng, NDQxODU3MzQ5QHFxLmNvbQ==; Jing Huang, aGoxMzg3MzM1MTMzOEAxNjMuY29t
†These authors have contributed equally to this work
 Shiyue Zeng1†
Shiyue Zeng1† Hongpei Tan
Hongpei Tan Jing Huang
Jing Huang