
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Med. , 20 May 2024
Sec. Ophthalmology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1359463
This article is part of the Research Topic Dry Eye Disease Syndrome - Volume II View all 8 articles
 Fatemeh Sanie-Jahromi1
Fatemeh Sanie-Jahromi1 Mehdi Khaki1
Mehdi Khaki1 Mojtaba Heydari1
Mojtaba Heydari1 Mohammad Hossein Nowroozzadeh1
Mohammad Hossein Nowroozzadeh1 Amin Reza Akbarizadeh2
Amin Reza Akbarizadeh2 Saeid Daneshamouz3
Saeid Daneshamouz3 Yaser NejatyJahromy1
Yaser NejatyJahromy1 Maryam Nejabat4
Maryam Nejabat4 Ahmad Mahmoudi1
Ahmad Mahmoudi1 Athar Zareei1
Athar Zareei1 Mahmood Nejabat1*
Mahmood Nejabat1*Background: The use of honey as an eye treatment encounters challenges due to its high osmolarity, low pH, and difficulties in sterilization. This study addresses these issues by employing a low concentration of honey, focusing on both in-vitro experiments and clinical trials for treating dry eye disease in corneal cells.
Methods: In the in-vitro experiment, we investigated the impact of a 1% honey-supplemented medium (HSM) on limbal stem cells (LSCs) and keratocytes using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay and real-time polymerase chain reaction (PCR) for BCL-2, BAX, and IL-1β gene expression. Simultaneously, in the clinical trial, 80 participants were divided into two groups, receiving either a 1% w/v honey ophthalmic formulation or a placebo for 3 months. Study outcomes included subjective improvement in dry eye symptoms, tear break-up time (TBUT), and Schirmer’s test results.
Results: MTT results indicated that 1% HSM did not compromise the survival of corneal cells and significantly reduced the expression of the IL-1β gene. Additionally, participants in the honey group demonstrated a higher rate of improvement in dry eye symptoms and a significant enhancement in TBUT values at the three-month follow-up. However, there was no significant difference between the study groups in terms of Schirmer’s test values. No adverse events were observed or reported.
Conclusion: In conclusion, 1% honey exhibits anti-inflammatory and anti-infective properties, proving effective in ameliorating dry eye symptoms and enhancing tear film stability in patients with dry eye disease.
Clinical Trial Registration: https://irct.behdasht.gov.ir/trial/63800.
Dry eye syndrome is a prevalent condition characterized by insufficient tear production or an imbalance in tear film composition, causing discomfort and visual disturbances (1). Its global prevalence ranges from 5 to 50%, varying based on geographical region and diagnostic criteria (2, 3). The condition significantly impacts the quality of life, leading to symptoms such as ocular discomfort, foreign material sensation, eye redness, blurred vision, and occasional pain (4). Existing treatments primarily focus on symptomatic relief and improving tear quality, including the use of artificial tear substitutes, lubricants, anti-inflammatory agents, and punctal occlusion (5). Despite these options, many patients continue to experience persistent symptoms, emphasizing the limitations of current treatments (6). Some individuals may also exhibit intolerance or inadequate response to conventional therapies, prompting the exploration of alternative approaches (7). Molecular and cellular events contribute to dry eye disease, with inflammation and corneal epithelial cell apoptosis identified as significant factors (8, 9). Anti-inflammatory treatments have shown utility in stimulating tear production and alleviating general dry eye symptoms (10).
Honey, a natural substance with recognized antimicrobial, anti-inflammatory, anti-cancer, and wound-healing properties, has been traditionally used for various therapeutic purposes, including eye-related conditions (11–17). While scientific evidence supporting honey’s ophthalmic use is limited, several studies have explored the efficacy of honey eye drops in managing dry eye, with promising results (18–21). However, varying honey concentrations (16.5 to 70%) in these studies raised concerns about increased osmolarity, acidity, and viscosity of eye drops, potentially causing irritation and discomfort (22). In this research, we addressed these challenges by using a diluted concentration of honey solution for both experimental (in-vitro) and clinical studies, focusing on corneal cells for managing dry eye disorder.
Our research aimed to evaluate the effects of a 1% w/v honey-supplemented medium (HSM) on the expression of apoptotic and inflammatory genes (BCL-2, BAX, and IL-1β) in corneal limbal stem cells (LSCs) and stromal keratocytes. Additionally, we assessed the anti-bacterial activity of HSM. Subsequently, we formulated an ophthalmic solution using 1% honey for the treatment of dry eye disease. Our focus on a lower dose aimed to mitigate potential osmolarity-related irritation and enhance overall treatment acceptability. Through our research, we aspire to contribute valuable insights into the therapeutic potential of a 1% honey ophthalmic formulation in managing dry eye syndrome, prioritizing patient comfort and tolerability.
Corneal tissue was obtained from a cadaver (a thirty-two years-old male) and used for corneal transplantation up to 48 h postmortem, the residual tissue after transplantation was transferred to the cell culture lab, under sterile conditions to be used for cell culture (23). Limbal biopsy (approximately 1 × 2 mm2) was extracted from the limbus and cultured in DMEM/F12 (Shell max, Iran) supplemented with 10% fetal bovine serum (FBS, Gibco, Germany) and 1% penicillin/streptomycin (Shell max, Iran). LSCs explants were cultured so that the epithelial layer was upward. After 15 min for the initial adhesion of tissue explants to the plate surface, the culture medium was gently added to the edge of the plate and gently tilted to spread throughout the culture dish. Approximately 7 days after the initial culture, LSCs showed growth from the bottom of the samples. Every 5 days, half of the culture medium was replaced with a fresh culture medium. When the cultures reached about 60–70% of confluence, the cells were characterized for the expression of LSCs markers including cytokeratin 19, vimentin (immunocytochemistry), CD44, P63, and ABCG2 (flow cytometry), and used for further analysis. In this study we intended to investigate the effect of 1% HSM on LSCs in their original cell architecture. As LSCs are susceptible to enzyme treatment and lose their cell–cell connections, all experiments were conducted using LSCs from primary cultures. This approach was specifically chosen to ensure the preservation of the cells’ intrinsic properties and to minimize any potential alterations in gene expression or functionality that might occur. Primary LSCs provide a more physiologically relevant model for assessing the effects of interventions.
Keratocyte cell culture was performed using the peripheral stroma of the same corneoscleral rime (a thirty-two years-old male) as described previously (24–26). Under sterile conditions, the corneal rim was relocated to the culture room. After rinsing the tissue with sterile phosphate buffer (PBS), the epithelial and endothelial layers were removed by knife scratching, and the rest of the tissue was cut into small segments, each roughly 1 mm in size. Stromal keratocyte explants were cultured in DMEM/F12 (Shell max, Iran) enriched with 10% FBS (Gibco, Germany) as well as 1% penicillin/streptomycin (Shell Max, Iran). Medium change and subculture were performed regularly every two to three days. Cells from passages 5–7 were checked for the expression of keratocytes markers (LUM and KERA) using polymerase chain reaction (PCR) and applied for further analysis.
Wax-free coriander honey from Koozeasal Co. (Product ID: hd-4, Esfahan, Iran), was analyzed and confirmed to be free from toxins or microorganisms.
Hydroxy methyl furfural (HMF) was measured using White’s spectrophotometric method. Honey samples were mixed with water and Carrez solutions, filtered, and analyzed using a UV–visible spectrophotometer at 284 and 336 nm. The HMF value was calculated using the provided equation.
The Lane-Eynon technique was used to quantify reducing sugars. Fehling’s solutions A and B were mixed with honey, and titration was performed with methylene blue as an indicator.
Fructose and glucose (F/G) were measured enzymatically. Fructose content was calculated by subtracting glucose content from total reducing sugars.
Sucrose content was determined by subtracting total reducing sugars from total sugar content, multiplied by a correction factor.
Proline content was measured by dissolving honey samples, adding formic acid and ninhydrin solution, heating, and reading the absorbance at 520 nm after the addition of propanol-water solution.
Honey (at concentration of 1% w/v) was dissolved in DMEM/F12 (Shell Max, Iran) at 37°C, then filtered through a 0.22 μm filter, and kept at 4°C until the time of the experiment. Before usage, the pH of HSM was determined and verified in terms of neutrality (pH ~7.2–7.4). Then, HSM-treated cells underwent relative gene expression analysis and cell viability assays, and their results were contrasted to those of controls (culture media without honey).
Using an MTT test, the impact of HSM on LSCs and keratocyte proliferation was assessed. Cells were cultured in a 96-well microplate at a concentration of 104 cells per well, and exposed to either 1% HSM or control media for 24 h at 37°C. Subsequently, 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (5 mg/mL PBS; GoldBio, United States) was added to each well and kept at 37°C for approximately 4 h. Crystals of formazan are created by live cells using MTT. After that, 100 μL acidic SDS [10% SDS (Parstous, Iran) in 0.01 M HCL] was added to each well and incubated at 37°C overnight. SDS helps dissolve cell membrane and formazan crystals and produces a purple solution, which optical density (OD) reflects the number of living cells. The OD of each well was then assessed at 545 nm wavelength using a microplate reader (BMG Labtech, Germany) and was used to evaluate keratocyte and LSCs survival in HSM vs. control groups.
LSCs and keratocytes (106 cells per 10 cm2 culture dish) were exposed to 1% HSM medium for 24 h at 37°C. We used the RNeasy kit (Parstous Co, Iran) for total RNA extraction and the Easy cDNA Synthesis Kit (Parstous, Iran) for cDNA synthesis. AlleleID software (v.7.5, Premier Biosoft International, Palo Alto, CA, United States) was utilized for primer design of BAX, BCL-2, IL-1β, and ACTB (house-keeping gene) (Table 1). The StepOneTM Real-Time PCR machine (Applied Biosystems) was used to run real-time PCR using RealQ Plus Master Mix Green (Ampliqon). PCR reactions were comprised of 7.5 μL master mix, 1.5 μL F and R primers, and 6 μL of DNA (total volume of 15 μL). Every PCR run had a single-step temperature profile with 40 cycles of 95°C hold phase for 15 min, 95°C denaturation phase for 10 s, and 61°C annealing and extension phase for 45 s. According to the 2−ΔΔCt formulation, relative RNA expression was quantified.
To assess the antimicrobial activity of 1% HSM on ocular pathogens, an antimicrobial susceptibility test was performed. Cultures of nine common human ocular pathogenic strains were obtained from the local hospital of the study. Species included Escherichia coli, Pseudomonas aeruginosa, Klebsiella, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, Staphylococcus haemolyticus, Enterococcus faecalis, and Acinetobacter. Every sample was cultured, and if microbial growth materialized, differential cultures and assays were run to distinguish between various bacterial strains. Disc diffusion method was used for antibacterial activity tests. Mueller–Hinton agar, placed into either 100 mm or 150 mm Petri dishes, was the medium utilized in this experiment. The agar had a pH in the range of 7.2 to 7.4. The Mueller–Hinton agar culture medium (Merck, Germany) was streaked to create a bacterial lawn after being inoculated with a straight saline suspension of isolated colonies that had been corrected to 0.5 McFarland turbidity standard. The plates were dried and the HSM stock solution was diluted to concentration of 1%. A volume of 25 μL of each dilution was loaded into sterile, blank discs 6 mm in diameter (Padtanteb, Iran), and the disks were fully dried before placing on the plates. Then the disk was gently placed on top of the agar and was lightly pressed down with the pancetta. After 16–18 h of incubation at 35°C, the area of inhibited bacterial growth was measured. Antibiotic disks as positive control used for the tests included: chloramphenicol, gentamicin, ciprofloxacin, and ofloxacin. Distilled water-loaded discs were used as negative controls, also a vehicle medium (culture medium without honey) was used as control. The concentration of the solution would be at its peak right adjacent to the disc and would gradually diminish as the distance from the disk increase. No colonies would grow in the zone of inhibition if the drug was effective against bacteria at doses higher than critical concentration, that is, the region of the agar where the concentration of the antibacterial agent was greater than or equal to this minimum effective concentration. This-together with the rate of antibiotic diffusion-was utilized to determine how susceptible the bacteria were to that specific antibiotic.
The study protocol was approved by the ethics committee of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1400.548) and registered with the Iranian Registry of Clinical Trials (IRCT20220117053744N1). Patients diagnosed with dry eye disease based on clinical signs and symptoms were recruited for the study. Inclusion criteria included a Schirmer test result of less than 5 mm in 5 min and tear break time of less than 10 s. Patients with blepharitis, meibomian gland disorder, a history of taking tetracycline or oral corticosteroids within the past 3 months, ocular surface disorders, previous eye surgery, or a history of allergies were excluded from the study.
To minimize bias and ensure the integrity of the study, blinding procedures were implemented. Both the participants and the researchers involved in data collection and analysis were blinded to the treatment assignment. The honey and placebo eye drops were visually indistinguishable, as they had the same appearance and packaging. Only the pharmacist responsible for preparing the eye drops had access to the treatment allocation information. Allocation concealment was maintained to prevent selection bias. The treatment assignments were concealed from the researchers enrolling participants and assigning them to their respective groups. The allocation sequence was generated by a randomization procedure using computer-generated random numbers. The treatment assignments were then placed in opaque, sealed envelopes sequentially numbered. Each envelope was opened only at the time of participant enrollment, ensuring that the treatment allocation remained concealed until the participants were assigned to their respective groups.
The treatment group received honey eye drops, administered three times a day for 3 weeks. Follow-up evaluations were conducted 6 weeks after completing the treatment. The control group received placebo formulation following the same time and duration protocol.
Honey eye drops were prepared in a clean room according to Good Manufacturing Practice (GMP) protocols. The concentration of honey used was 1% (w/v) in artificial tear eye drops. The honey solution underwent sterilization through 0.45 μm and 0.22 μm filtration, followed by packaging and labeling. Benzalkonium chloride (BAK) (5 μg/mL), hydroxypropyl methyl cellulose (0.001 g/mL) and dextran 70 (0.001 g/mL) was used in both the honey and placebo eye drops.
The subjective improvement in symptoms of dry eye, tear break-up time and Schirmer’s test results were used as study outcome. The ocular surface disease index (OSDI) questionnaire was employed to assess the subjective improvement of dry eye symptoms. All patients were asked regarding any adverse events experienced during the study period.
Three copies of each in-vitro experiment were performed. Data from in-vitro experiments were given as mean ± standard error of mean (SEM) and examined using GraphPad Prism software (version 6; San Diego, CA, United States) and an unpaired t-test. The significance threshold was established at p < 0.05. Descriptive statistics, such as mean and percentage, were used to analyze the clinical data. Statistical tests, including Mann–Whitney, Chi-square, and ANOVA tests, were performed using SPSS version 21 software to assess the significance of the clinical findings.
Our study acknowledged the complexity of limbal stem cell identification, adopting a multifaceted approach by incorporating both widely recognized (such as cytokeratin 19) and supplementary markers (such as vimentin). Cytokeratin 19 is a well-established marker of limbal epithelial cells. Vimentin is present in transitional limbal cells (27) and is a marker for EMT in limbal stem cells. Furthermore, by examining the cells’ biomolecular profiles in relation to their microenvironment, we utilized a panel of markers to identify and characterize LSCs. These included ABCG2, CD44, and P63, in accordance with the markers consensually associated with LSCs as highlighted in recent studies (28). Immunocytochemistry (ICC) results confirmed the identity of LSCs through the expression of cytokeratin 19 and vimentin markers in more than 90% of the cells (Figures 1A–F). The flow cytometry analysis revealed positive expression of CD44 (16.2%), P63 (75%), and ABCG2 (11.5%) as markers specific to LSCs. CD44 is a cell surface glycoprotein associated with cell adhesion, P63 is a transcription factor crucial for epithelial stem cell maintenance, and ABCG2 is an ATP-binding cassette transporter associated with stem cell properties. Together, these markers provided a comprehensive profile confirming the identity of limbal stem cells.
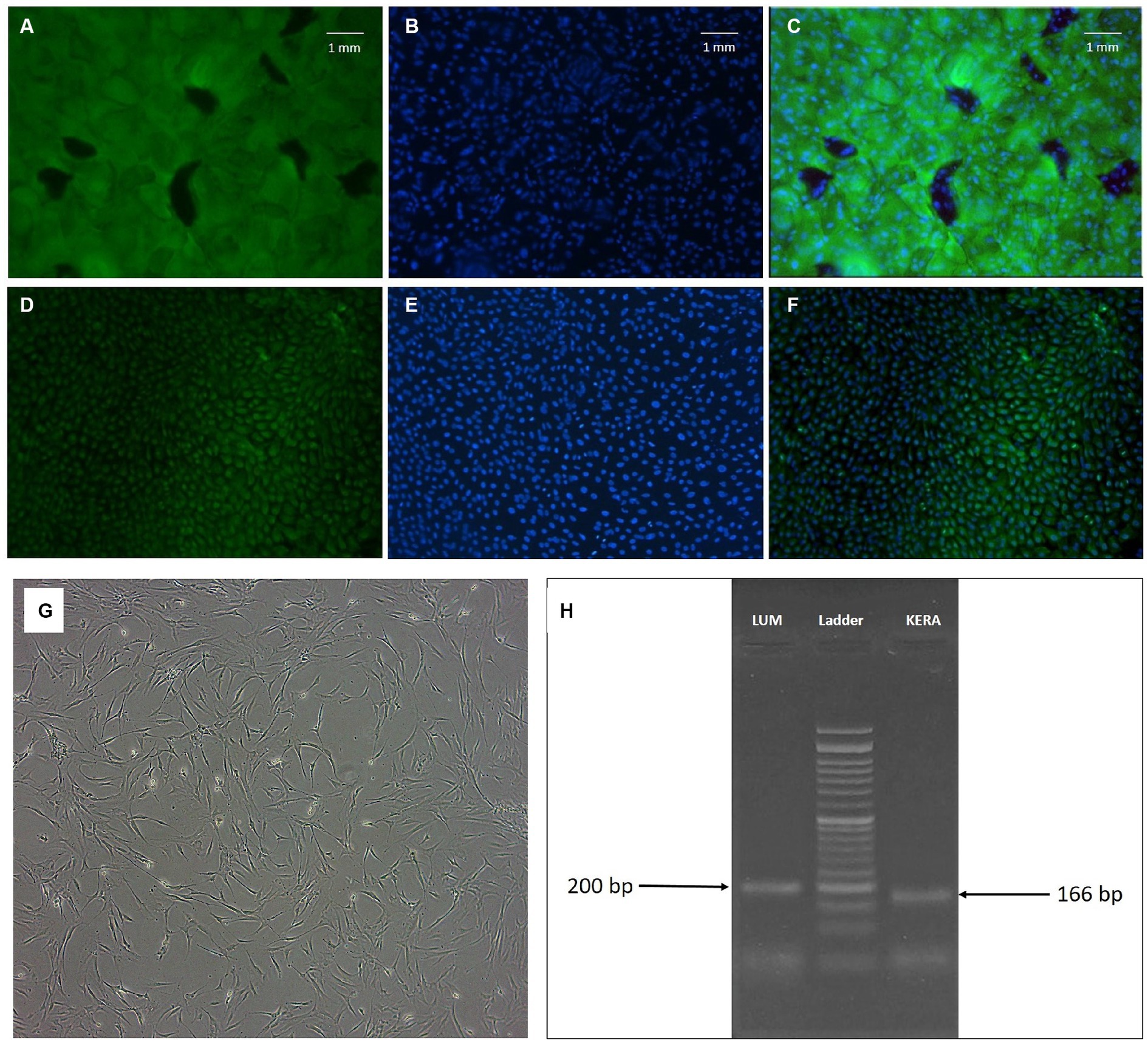
Figure 1. (A–F) Fluorescence microscopy of limbal epithelial stem cells. Positive cells for cytokeratin 19 (A) and vimentin (D) and their relevant DAPI field (B,E) are shown. (C,F) Fields represent the merged FITC and DAPI fields (magnification: ×100). (G,H) Keratocyte morphology and marker; (G): phase contrast microscopic image of human corneal keratocytes (passage 5, magnification ×40). Note the fibroblastic morphology of the cells; (H): agarose gel electrophoresis of LUM and KERA RT-PCR products of corneal keratocytes (24 and 26).
Keratocytes displayed the characteristic spindle-shaped morphology indicative of these cells (Figure 1G). To further confirm their identity, the expression of LUM and KERA genes, recognized markers for keratocytes, was examined by PCR. The agarose gel electrophoresis showed the presence of expected bands at 200 bp for LUM and 166 bp for KERA (Figure 1H). Lumican (LUM) and keratocan (KERA) are proteoglycans associated with the extracellular matrix of corneal stroma and are considered specific markers for keratocytes. The detection of these bands in the PCR analysis provided additional validation of the identity of keratocytes in the study.
In summary, the use of cytokeratin 19, vimentin, CD44, P63, ABCG2, LUM, and KERA as markers, combined with various analytical techniques, ensured a thorough and reliable characterization of limbal stem cells and keratocytes in this investigation.
Chemical analysis of coriander honey used in the ophthalmic formulation revealed notable characteristics, including a high percentage of reducing sugars (72.52% before and 73.56% after hydrolysis), low sucrose content (0.99 g/100 g), and balanced levels of fructose and glucose (36.3 g/100 g each, F/G ratio of 1.00). The presence of hydroxymethylfurfural (HMF) at 7.19 mg/kg, an indicator of honey quality, and a proline content of 734.7 mg/kg were also identified. These findings contribute valuable insights into the chemical composition of coriander honey, underscoring its significance as a key component in the ophthalmic formulation, potentially influencing the therapeutic efficacy and safety of the product. The summarized results are presented in Table 2.
The MTT assay results demonstrated that the 1% HSM did not adversely affect the survival of corneal LSCs and keratocytes, as evidenced by comparable viability to the control group. However, a non-significant reduction in cell proliferation was observed, with LSCs exhibiting 87.16 ± 10.03% viability compared to the control (100 ± 10.04%, p = 0.4167), and keratocytes showing 84.64 ± 1.37% viability compared to the control (100 ± 6.30%, p = 0.0758) (Figure 2).
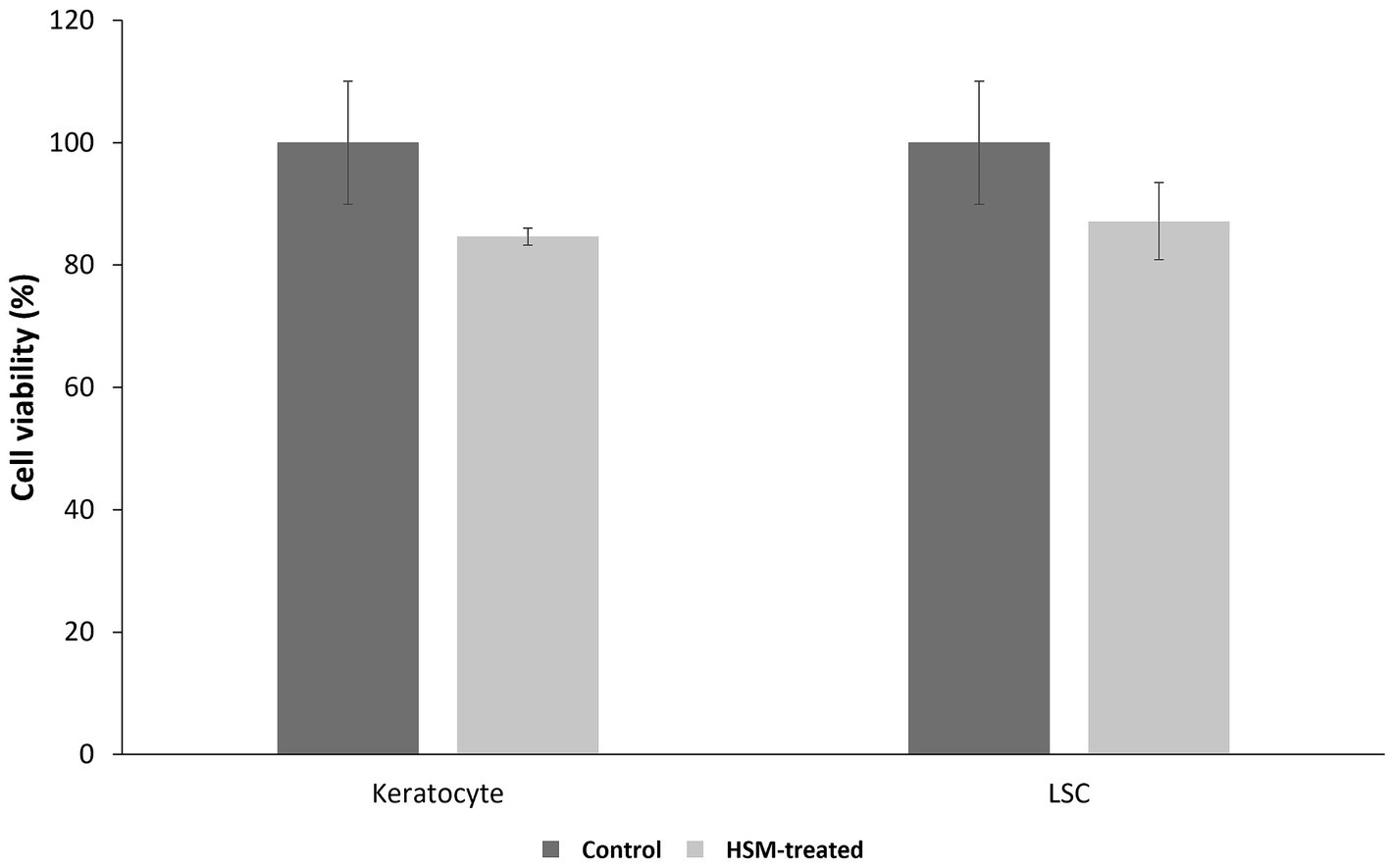
Figure 2. Cell viability of corneal LSCs and keratocytes after 24 h treatments with 1% HSM (treat, light bar) or the controls (dark bar) (n = 3, mean ± SEM). A t-test was used for statistical analysis.
The findings of our study indicate that the treatment of LSCs and keratocytes with 1% HSM did not result in a significant impact on the expression of BAX and BCL-2 genes, as illustrated in Figure 3. However, we observed a decreasing trend in the expression of the IL-1β gene in both LSCs and keratocytes treated with 1% HSM. Notably, this reduction reached statistical significance in keratocytes, indicating a potential anti-inflammatory effect of 1% HSM on these corneal cells. These results contribute to a better understanding of the molecular responses of LSCs and keratocytes to 1% HSM treatment, suggesting a specific influence on the expression of genes associated with inflammation in the keratocyte population.
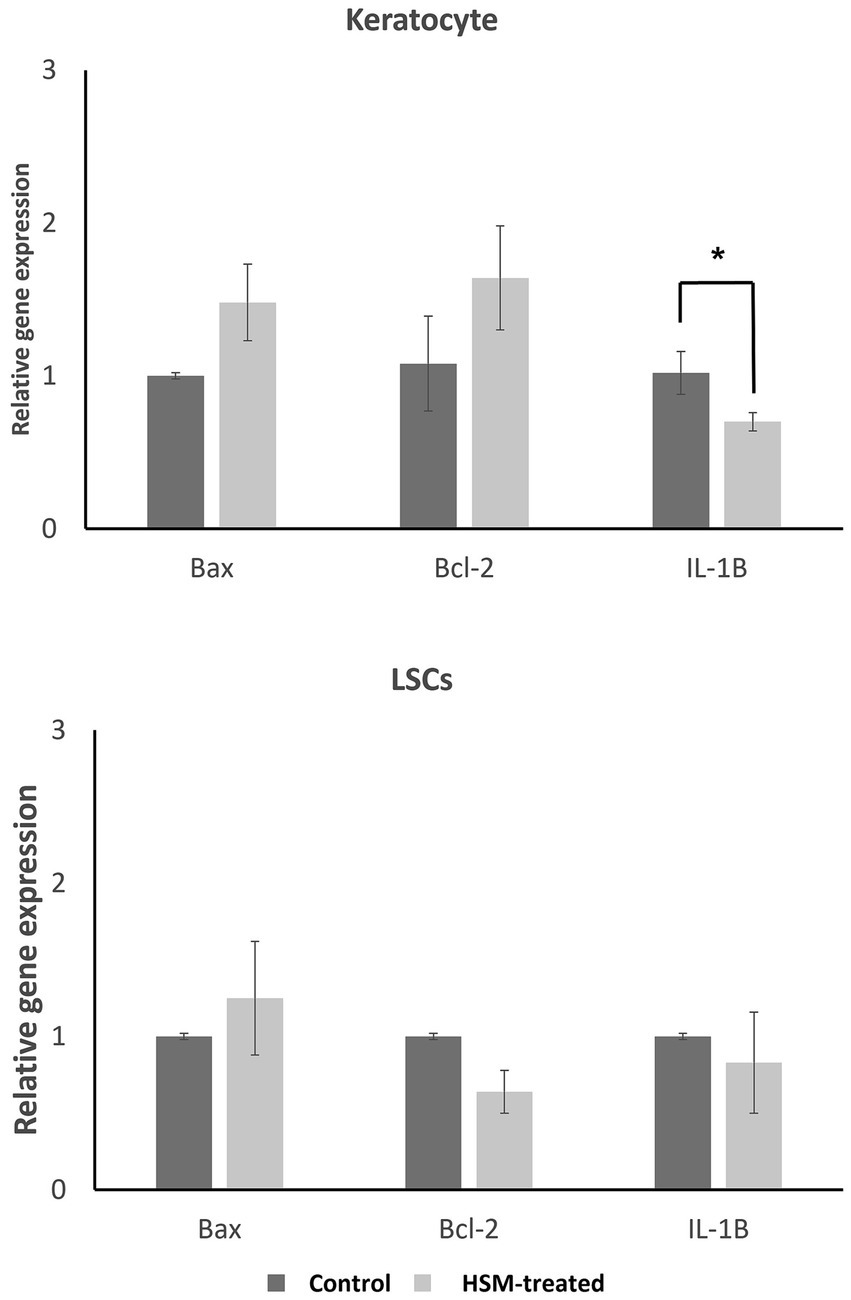
Figure 3. Relative gene expression of apoptotic genes (BCL-2, BAX) and inflammation marker (IL-1β) in LSCs and keratocytes treated by 1% HSM (treat, light bars) versus control (dark bars). The height of the bars indicates the mean value, and the error bars represent the SEM. Significant differences (p < 0.05) are highlighted with an asterisk (*). All pairs were compared using a student t-test.
The antibacterial activity of the 1% HSM solution was carefully evaluated by measuring the diameter of the zone of growth inhibition around the disks and comparing these measurements to those obtained with established antibiotics, including gentamicin, chloramphenicol, ciprofloxacin, and ofloxacin (Table 3). The length of the inhibitory zone is directly proportional to the inhibitory effect of the tested compound. Our analysis emphasizes that the notable antibacterial potential of 1% HSM was particularly evident against Staphylococcus aureus and Staphylococcus epidermidis, where it demonstrated comparable inhibitory effects in comparison to the reference antibiotics. Specifically, the zones of inhibition for these bacteria ranged from 10.00 mm to 15.44 mm, with Staphylococcus epidermidis showing the largest zone of inhibition at 15.44 mm. It is important to highlight that while 1% HSM showed promising results against these specific strains, it did not exhibit inhibitory effects against Acinetobacter and Pseudomonas aeruginosa. The vehicle medium (culture medium without honey) that was used as control showed growth for all the studied bacterial strains (see Table 3).
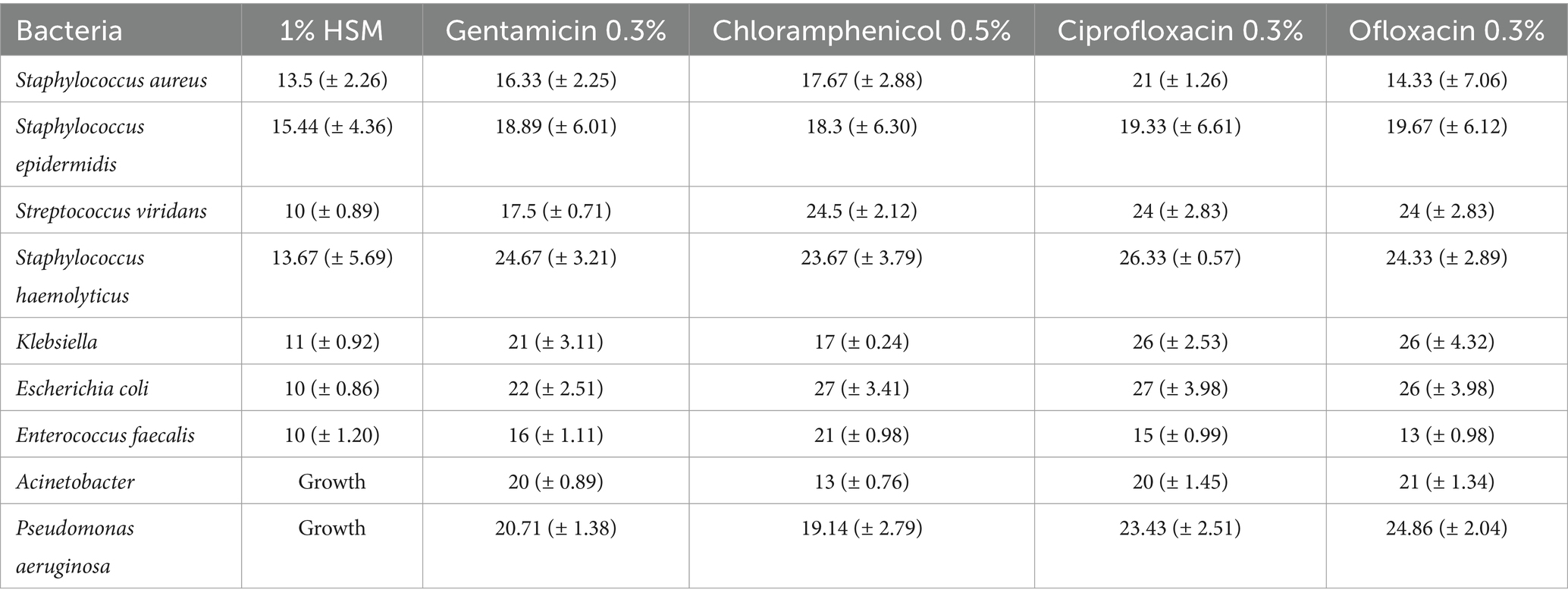
Table 3. The inhibition zone (mm) of 1% HSM in comparison to other antibiotics (in their clinically available concentration).
This outcome underscores the potential application of 1% HSM in targeting specific ocular pathogens and supports its consideration for inclusion in eye drop formulations, especially for infections known to be caused by susceptible bacterial strains.
In the present study, out of the initially assessed 112 participants, 80 were randomized into either the honey intervention group (40 participants) or the placebo intervention group (40 participants). Four participants in the honey group and 12 in the placebo group were lost to follow-up. Consequently, 36 participants in the honey group and 28 participants in the placebo group were included in the final analysis. The participants’ flow is summarized in the CONSORT flow diagram (Figure 4).
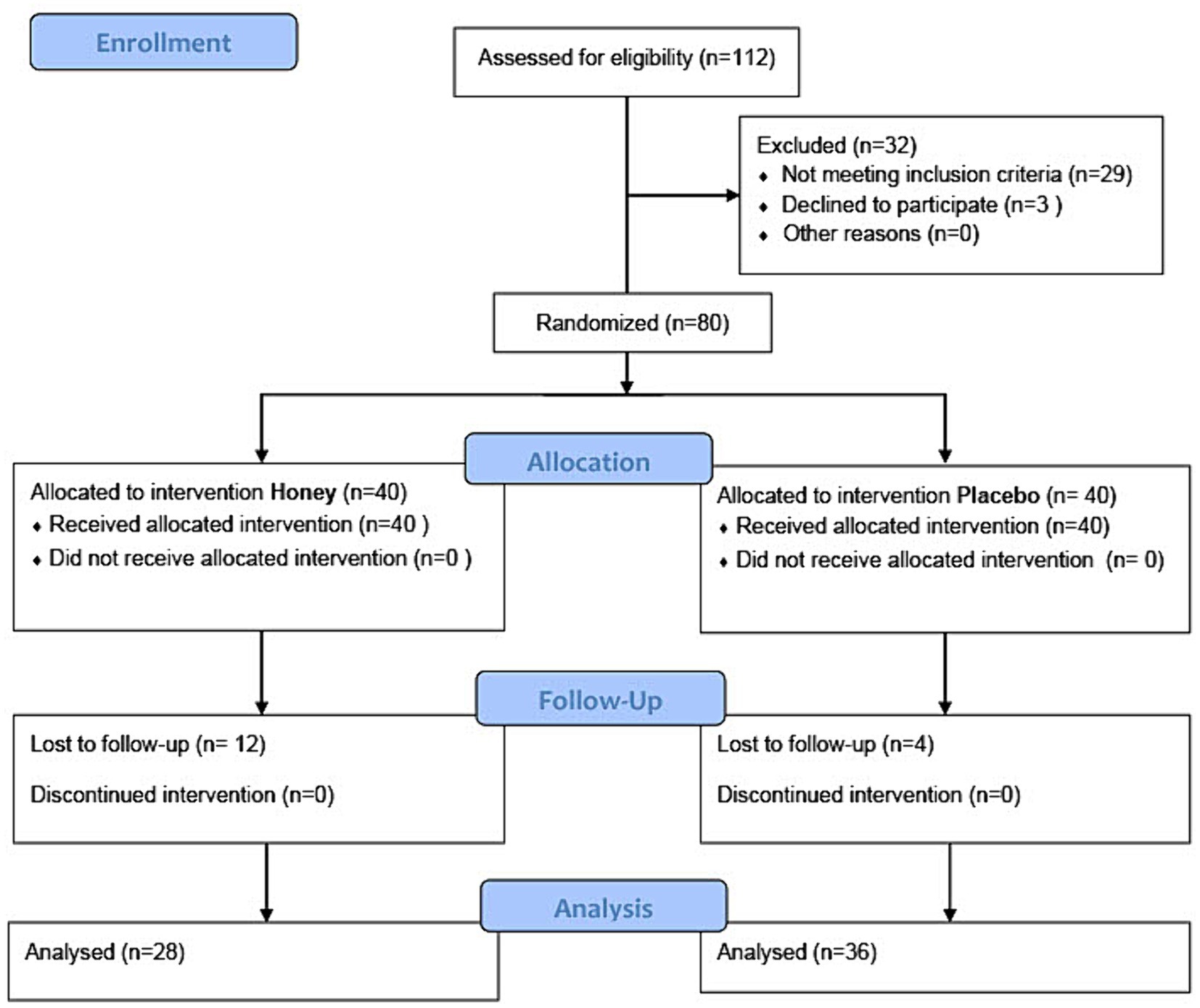
Figure 4. CONSORT flow diagram of the study showing the number of participants in each stage of study enrollment, allocation, follow-up, and analysis.
The baseline characteristics of participants in the honey and placebo groups were similar in terms of age and sex distribution. The mean age was 53.6 ± 11.8 years in the honey group and 53.1 ± 10.2 years in the placebo group. Sex distribution showed 47.5% females in the honey group and 43.3% females in the placebo group. Baseline measurements of Schirmer’s test and tear break-up time (TBUT) did not exhibit significant differences between the groups (Table 4).
The assessment of dry eye symptoms revealed a significantly higher percentage of participants experiencing improvement in the honey group (66.7%) compared to the placebo group (38.5%) (p = 0.029) (Table 5).
TBUT values at baseline were comparable between the honey and placebo groups. At the three-month follow-up, the honey group demonstrated a significant improvement in TBUT (9.7 ± 2.2 s) compared to the placebo group (8.9 ± 3.3 s) (p = 0.008) (Table 6).
Baseline Schirmer’s test values were similar between groups, and both groups showed a significant increase at the three-month follow-up (p < 0.001). However, no significant difference was observed between the honey and placebo groups regarding the improvement in Schirmer’s test results (p = 0.554) (Table 7).
No adverse events were observed or reported in patients treated with honey and placebo eye drops, indicating the safety of both interventions.
Tear film instability, eye irritation, and vision impairment collectively characterize dry eye, a multifactorial ocular surface condition (29). From a molecular perspective, inflammation and apoptosis emerge as key signaling pathways in the pathogenesis of dry eye disease (30, 31). The historical use of honey in eye care, attributed to its anti-inflammatory and anti-apoptotic properties (32–34), positions it as a promising therapeutic candidate for managing dry eye.
Our in-vitro investigations affirmed the potential of a 1% honey concentration in supporting the proliferation of corneal cells. Specifically, this concentration demonstrated no significant impact on the expression levels of crucial genes associated with cell survival (BCL-2) and apoptosis (BAX) (35). The consistent BCL-2/BAX ratio in 1% honey-treated cells compared to controls implies the safety of this concentration for cell survival without promoting apoptosis in corneal cells. Moreover, a significant decrease in the expression of IL-1β, a pro-inflammatory cytokine linked to dry eye disease (36–38), underscores the anti-inflammatory potential of 1% honey (39–43).
The choice of IL-1β was made based on its well-documented involvement in the pathogenesis of dry eye disease and its established role as a key mediator of ocular surface inflammation. IL-1β is known to play a crucial role in initiating and perpetuating inflammatory responses, including the release of other inflammatory mediators and the disruption of ocular surface homeostasis. However, there are other significant inflammatory markers for dry eye disease, including MMP-9. Of course, exploring the expression profiles of additional inflammatory markers in future studies could provide valuable insights into the heterogeneity of dry eye disease phenotypes and facilitate the development of targeted therapeutic interventions tailored to individual patients’ inflammatory profiles.
In addition to the promising outcomes observed in the anti-inflammatory and apoptosis gene expression studies, our bacterial test results further underscore the multifaceted therapeutic potential of 1% honey. The observed antimicrobial activity of 1% honey against common ocular pathogens, including Staphylococcus aureus and Staphylococcus epidermidis, aligns with the growing body of evidence supporting honey’s broad-spectrum antibacterial properties (44–46). Notably, the inhibition zones measured for these bacteria suggest a potent bacteriostatic, if not bactericidal, effect of low-concentration honey, which is particularly relevant given the rising concern over antibiotic resistance in ocular infections (47–49).
Moreover, the significant inhibitory effect of 1% honey against certain strains compared to reference antibiotics suggests an exciting avenue for future research, and could pave the way for novel, natural antimicrobial agents in the fight against drug-resistant bacterial infections.
Of course, in vivo studies are essential to confirm the safety and efficacy of honey-based ophthalmic formulations, considering the complex microenvironment of the eye and potential variability in honey’s composition.
Translating these in-vitro insights into clinical outcomes, participants in the honey group exhibited a higher rate of improvement in dry eye symptoms and a significant enhancement in TBUT compared to the placebo group. Notably, Schirmer’s test values showed no significant difference between the study groups (18–21). This suggests that the honey formulation may primarily address tear film stability and ocular surface comfort, distinct from directly stimulating tear production.
To address challenges associated with high osmolarity and sterilization difficulties, we deliberately opted for a low concentration (1%) of honey. This choice allowed for adjustable osmolarity and facilitated easy sterilization through multiple filtrations. The selection of coriander honey further contributed to the study, bringing forth a unique composition with therapeutic properties encompassing antibacterial, antioxidant, anti-cancer, and anti-inflammatory effects (50–55).
Our findings resonate with previous studies demonstrating the efficacy of honey in managing dry eye symptoms (21, 56, 57). The improvement in TBUT, coupled with a reduction in subjective dry eye symptoms, aligns with research employing honey eye drops in various concentrations. The observed positive effects on meibomian gland dysfunction (MGD), a significant contributor to dry eye (58, 59), support the notion that honey contributes to ocular surface stabilization by reducing dryness, inflammation, and bacterial overgrowth.
In conclusion, our study supports the therapeutic potential of a 1% honey eye drop formulation in managing dry eye symptoms and improving tear film stability (25). The molecular effects observed in vitro, combined with positive clinical outcomes, suggest that honey, even at a low concentration, may influence corneal cells at the molecular level, contributing to its observed efficacy. Future research should delve into the precise mechanisms and potential synergistic effects of honey in dry eye management, advancing our understanding and refining treatment strategies.
It should be noted that the seasonal batch variation of honey is an important consideration in studies investigating its therapeutic properties, including our research on its efficacy in treating dry eye disease. While we made efforts to minimize the impact of this variability by sourcing honey from a consistent supplier and adhering to standardized processing methods, the inherent differences in honey batches may still exist. These differences could potentially affect the concentration and composition of bioactive compounds in the honey, consequently influencing its biological effects on corneal cells and clinical outcomes in dry eye patients. There is a certain need for future studies to explore strategies for standardizing honey preparations or incorporating methods to account for batch variability, such as rigorous quality control measures and detailed characterization of honey constituents.
In conclusion, our study highlights the suitability of a 1% honey solution as an effective anti-inflammatory and anti-bacterial supplement for corneal cells. The use of a 1% honey eye drop formulation demonstrates promising outcomes in ameliorating dry eye symptoms and enhancing TBUT as illustrated in Figure 5. Nonetheless, to validate and further understand these findings, additional research is essential. Future studies should focus on optimizing treatment protocols and delving into the potential mechanisms that underlie the therapeutic effects of honey in the management of dry eye. Continued investigation into honey-based treatments may contribute a valuable addition to the array of therapies available for individuals grappling with dry eye syndrome.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Shiraz University of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
FS-J: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. MK: Data curation, Investigation, Methodology, Writing – original draft. MH: Formal analysis, Methodology, Writing – review & editing. MHN: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. AA: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. SD: Conceptualization, Investigation, Methodology, Writing – original draft. YN: Writing – review & editing. MarN: Data curation, Investigation, Methodology, Writing – original draft. AM: Writing – original draft. AZ: Writing – original draft. MahN: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Shiraz University of Medical Sciences (in-vitro study, Grant# 17857; clinical study, Grant# 20914).
The authors thank the Vice Chancellor of Shiraz University of Medical Sciences for supporting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BAX, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; HSM, Honey-supplemented-medium; IL, Interleukin; LSC, Limbal stem cells; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; PCR, Polymerase chain reaction; TBUT, Tear break-up time
1. Qian, L, and Wei, W. Identified risk factors for dry eye syndrome: a systematic review and meta-analysis. PLoS One. (2022) 17:e0271267. doi: 10.1371/journal.pone.0271267
2. McCann, P, Abraham, AG, Mukhopadhyay, A, Panagiotopoulou, K, Chen, H, Rittiphairoj, T, et al. Prevalence and incidence of dry eye and meibomian gland dysfunction in the United States: a systematic review and meta-analysis. JAMA Ophthalmol. (2022) 140:1181–92. doi: 10.1001/jamaophthalmol.2022.4394
3. Cai, Y, Wei, J, Zhou, J, and Zou, W. Prevalence and incidence of dry eye disease in Asia: a systematic review and meta-analysis. Ophthalmic Res. (2022) 65:647–58. doi: 10.1159/000525696
4. McGinnigle, S, Naroo, SA, and Eperjesi, F. Evaluation of dry eye. Surv Ophthalmol. (2012) 57:293–316. doi: 10.1016/j.survophthal.2011.11.003
5. O’Neil, EC, Henderson, M, Massaro-Giordano, M, and Bunya, VY. Advances in dry eye disease treatment. Curr Opin Ophthalmol. (2019) 30:166–78. doi: 10.1097/ICU.0000000000000569
6. Gomes, JA, and Santo, RM. The impact of dry eye disease treatment on patient satisfaction and quality of life: a review. Ocul Surf. (2019) 17:9–19. doi: 10.1016/j.jtos.2018.11.003
7. Heydari, M, Kalani, M, Ghasemi, Y, and Nejabat, M. The effect of ophthalmic and systemic formulations of Latilactobacillus sakei on clinical and immunological outcomes of patients with dry eye disease: a factorial, randomized, placebo-controlled, and triple-masking clinical trial. Probiotics Antimicrob Proteins. (2023) doi: 10.1007/s12602-023-10079-1 [Epub ahead of print].
8. Pflugfelder, SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. (2004) 137:337–42. doi: 10.1016/j.ajo.2003.10.036
9. Gao, J, Gelber-Schwalb, TA, Addeo, JV, and Stern, ME. Apoptosis in the lacrimal gland and conjunctiva of dry eye dogs In: DA Sullivan, DA Dartt, and MA Meneray, editors. Lacrimal gland, tear film, and dry eye syndromes 2: basic science and clinical relevance. Boston, MA: Springer (1998). 453–60.
10. Okanobo, A, Chauhan, SK, Dastjerdi, MH, Kodati, S, and Dana, R. Efficacy of topical blockade of interleukin-1 in experimental dry eye disease. Am J Ophthalmol. (2012) 154:63–71. doi: 10.1016/j.ajo.2012.01.034
11. Kuropatnicki, AK, Kłósek, M, and Kucharzewski, M. Honey as medicine: historical perspectives. J Apic Res. (2018) 57:113–8. doi: 10.1080/00218839.2017.1411182
12. Karegar-Borzi, H, Salehi, M, and Rahimi, R. Laūq: a sustained-release dosage form for respiratory disorders in traditional Persian medicine. J Evid Based Complementary Altern Med. (2016) 21:63–70. doi: 10.1177/2156587215587417
13. Nikhat, S, and Fazil, M. History, phytochemistry, experimental pharmacology and clinical uses of honey: a comprehensive review with special reference to Unani medicine. J Ethnopharmacol. (2022) 282:114614. doi: 10.1016/j.jep.2021.114614
14. Waheed, M, Hussain, MB, Javed, A, Mushtaq, Z, Hassan, S, Shariati, MA, et al. Honey and cancer: a mechanistic review. Clin Nutr. (2019) 38:2499–503. doi: 10.1016/j.clnu.2018.12.019
15. Zakerian, M, Roudi, F, Ramezani, M, Salari, R, Refahi, B, Ramezani, M, et al. Physicochemical properties, antioxidative and antibacterial activities of the Persian medicine-based maolasal honey: an experimental study. J Nutr Food Secur. (2022) 8. 94–101. doi: 10.18502/jnfs.v8i1.11773
16. Lawal, O, and Banjo, A. Survey for the usage of arthropods in traditional medicine in southwestern Nigeria. J Entomol. (2007) 4:104–12. doi: 10.3923/je.2007.104.112
17. Shenoy, R, Bialasiewicz, A, Khandekar, R, Al Barwani, B, and Al Belushi, H. Traditional medicine in Oman: its role in ophthalmology. Middle East Afr J Ophthalmol. (2009) 16:92–6. doi: 10.4103/0974-9233.53869
18. Li, AL, Li, SL, Kam, KW, and Young, AL. Randomised assessor-masked trial evaluating topical manuka honey (Optimel) in treatment of meibomian gland dysfunction. Br J Ophthalmol. (2022) 106:777–80. doi: 10.1136/bjophthalmol-2020-317506
19. Hu, J, Kong, L, Zhu, S, Ju, M, and Zhang, Q. Efficacy and safety of manuka honey for dry eye. Clin Exp Optom. (2023) 106:455–65. doi: 10.1080/08164622.2022.2106779
20. Craig, JP, Cruzat, A, Cheung, IMY, Watters, GA, and Wang, MTM. Randomized masked trial of the clinical efficacy of MGO manuka honey microemulsion eye cream for the treatment of blepharitis. Ocul Surf. (2020) 18:170–7. doi: 10.1016/j.jtos.2019.11.009
21. Albietz, JMS, and Katrina, L. Randomised controlled trial of topical antibacterial manuka (Leptospermum species) honey for evaporative dry eye due to meibomian gland dysfunction. Clin Exp Optom. (2017) 100:603–15. doi: 10.1111/cxo.12524
22. Dutescu, RM, Panfil, C, and Schrage, N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea. (2015) 34:560–6. doi: 10.1097/ICO.0000000000000368
23. Sanie-Jahromi, F, Eghtedari, M, Mirzaei, E, Jalalpour, MH, Asvar, Z, Nejabat, M, et al. Propagation of limbal stem cells on polycaprolactone and polycaprolactone/gelatin fibrous scaffolds and transplantation in animal model. Bioimpacts. (2020) 10:45. doi: 10.15171/bi.2020.06
24. Sanie-Jahromi, F, Mohsenzadeh, AH, Namjoyan, F, Gharegezloo, Z, and Nejabat, M. Effect of adlay seed extract on inflammation and fibrogenesis in human corneal activated keratocytes at transcriptional level. Exp Eye Res. (2023) 235:109641. doi: 10.1016/j.exer.2023.109641
25. Sanie-Jahromi, F, Nowroozzadeh, MH, Emadi, Z, Eghtedari, M, and Khajehahmadi, Z. Intra-stromal injection of honey-treated keratocytes as a cell-based therapy for experimental corneal laceration. J Complement Integr Med. (2023) 20:604–11. doi: 10.1515/jcim-2023-0076
26. Sanie-Jahromi, F, and Jahromi, MSS. In vitro effect of propofol on the expression of genes involved in inflammation and apoptosis in corneal activated keratocytes. Cornea. (2022) 43:105–10. doi: 10.1097/ICO.0000000000003378
27. Schlötzer-Schrehardt, U, Dietrich, T, Saito, K, Sorokin, L, Sasaki, T, Paulsson, M, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. (2007) 85:845–60. doi: 10.1016/j.exer.2007.08.020
28. Gouveia, RM, Lepert, G, Gupta, S, Mohan, RR, Paterson, C, and Connon, CJ. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat Commun. (2019) 10:1496. doi: 10.1038/s41467-019-09331-6
29. Janine, A. The epidemiology of dry eye disease: report of the epidemiological subcommittee of the international dry eye workshop. Ocul Surf. (2007) 5:93–107. doi: 10.1016/s1542-0124(12)70082-4
30. Yu, L, Yu, C, Dong, H, Mu, Y, Zhang, R, Zhang, Q, et al. Recent developments about the pathogenesis of dry eye disease: based on immune inflammatory mechanisms. Front Pharmacol. (2021) 12:732887. doi: 10.3389/fphar.2021.732887
31. Rao, SK, Mohan, R, Gokhale, N, Matalia, H, and Mehta, P. Inflammation and dry eye disease—where are we? Int J Ophthalmol. (2022) 15:820–7. doi: 10.18240/ijo.2022.05.20
32. Molan, PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World. (1999) 80:80–92. doi: 10.1080/0005772X.1999.11099430
33. Simon, A, Traynor, K, Santos, K, Blaser, G, Bode, U, and Molan, P. Medical honey for wound care—still the ‘latest resort’? Evid Based Complement Alternat Med. (2009) 6:165–73. doi: 10.1093/ecam/nem175
34. Mohamed, HK, Mobasher, MA, Ebiya, RA, Hassen, MT, Hagag, HM, El-Sayed, R, et al. Anti-inflammatory, anti-apoptotic, and antioxidant roles of honey, royal jelly, and propolis in suppressing nephrotoxicity induced by doxorubicin in male albino rats. Antioxidants. (2022) 11:1029. doi: 10.3390/antiox11051029
35. Antonsson, B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. (2001) 306:347–61. doi: 10.1007/s00441-001-0472-0
36. Chen, Y, Zhang, X, Yang, L, Li, M, Li, B, Wang, W, et al. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of dry eye mice. Mol Med Rep. (2014) 9:2015–23. doi: 10.3892/mmr.2014.2041
37. Solomon, A, Dursun, D, Liu, Z, Xie, Y, Macri, A, and Pflugfelder, SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. (2001) 42:2283–92.
38. Zhang, C, Xi, L, Zhao, S, Wei, R, Huang, Y, Yang, R, et al. Interleukin-1β and tumour necrosis factor-α levels in conjunctiva of diabetic patients with symptomatic moderate dry eye: case–control study. BMJ Open. (2016) 6:e010979. doi: 10.1136/bmjopen-2015-010979
39. Wu, D, Chen, L, Teh, J, Sim, E, Schlundt, J, and Conway, PL. Honeys with anti-inflammatory capacity can alter the elderly gut microbiota in an ex vivo gut model. Food Chem. (2022) 392:133229. doi: 10.1016/j.foodchem.2022.133229
40. Kim, HY, Jo, MJ, Nam, SY, Kim, KM, Choi, MB, and Lee, YH. Evaluating the effects of honey bee (Apis mellifera L.) venom on the expression of insulin sensitivity and inflammation-related genes in co-culture of adipocytes and macrophages. J Entomol Res. (2020) 50:236–44. doi: 10.1111/1748-5967.12431
41. Kocyigit, A, Guler, EM, and Kaleli, S. Anti-inflammatory and antioxidative properties of honey bee venom on Freund’s complete adjuvant-induced arthritis model in rats. Toxicon. (2019) 161:4–11. doi: 10.1016/j.toxicon.2019.02.016
42. Alfarisi, HAH, Ibrahim, M, Azahari, N, Mohamed, ZH, Hamdan, A, and Mohamad, C. Anti-inflammatory effects of trihoney in hypercholesterolemic atherosclerotic rabbits: a comparative study with atorvastatin. Malays J Med Health Sci. (2020) 16:230–6.
43. Gasparrini, M, Afrin, S, Forbes-Hernández, TY, Cianciosi, D, Reboredo-Rodriguez, P, Amici, A, et al. Protective effects of manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem Toxicol. (2018) 120:578–87. doi: 10.1016/j.fct.2018.08.001
44. Grecka, K, Kuś, PM, Worobo, RW, and Szweda, P. Study of the anti-staphylococcal potential of honeys produced in northern Poland. Molecules. (2018) 23:260. doi: 10.3390/molecules23020260
45. Attah, F. (2021). Antimicrobial effects of honey and its specific actions on cell walls, membranes and enzymes of some microbial pathogens. The Federal University of Technology Minna. Available at: http://repository.futminna.edu.ng:8080/jspui/handle/123456789/13219
46. Romário-Silva, D, Alencar, SM, Bueno-Silva, B, Sardi, JDCO, Franchin, M, Carvalho, RDPD, et al. Antimicrobial activity of honey against oral microorganisms: current reality, methodological challenges and solutions. Microorganisms. (2022) 10:2325. doi: 10.3390/microorganisms10122325
47. Asbell, PA, Sanfilippo, CM, Sahm, DF, and DeCory, HH. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. (2020) 138:439–50.
48. Bale, BI, Elebesunu, EE, Manikavasagar, P, Agwuna, FO, Ogunkola, IO, Sow, AU, et al. Antibiotic resistance in ocular bacterial infections: an integrative review of ophthalmic chloramphenicol. Trop Med Health. (2023) 51:15. doi: 10.1186/s41182-023-00496-x
49. Lee, AE, Niruttan, K, Rawson, TM, and Moore, LS. Antibacterial resistance in ophthalmic infections: a multi-centre analysis across UK care settings. BMC Infect Dis. (2019) 19:768. doi: 10.1186/s12879-019-4418-0
50. Akbari, E, Baigbabaei, A, and Shahidi, M. Determination of the floral origin of honey based on its phenolic profile and physicochemical properties coupled with chemometrics. Int J Food Prop. (2020) 23:506–19. doi: 10.1080/10942912.2020.1740249
51. Czipa, N, Borbély, M, and Győri, Z. Proline content of different honey types. Acta Aliment. (2012) 41:26–32. doi: 10.1556/AAlim.2011.0002
52. Czipa, N, Varga, MB, and Győri, Z. Antioxidant activity and total flavonoid content of honeys. Acta agrar Debr. (2010) 41:25–8. doi: 10.34101/actaagrar/41/2675
53. Hegazi, AG. Antimicrobial activity of different Egyptian honeys as comparison of Saudi Arabia honey. Res J Microbiol. (2011) 6:488–95. doi: 10.17311/jm.2011.488.495
54. Afrin, S, Haneefa, SM, Fernandez-Cabezudo, MJ, Giampieri, F, Al-Ramadi, BK, and Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: an evidence-based review. Nutr Res Rev. (2020) 33:50–76. doi: 10.1017/S0954422419000192
55. Chan-Zapata, I, and Segura-Campos, MR. Honey and its protein components: effects in the cancer immunology. J Food Biochem. (2021) 45:e13613. doi: 10.1111/jfbc.13613
56. Tan, J, Jia, T, Liao, R, and Stapleton, F. Effect of a formulated eye drop with Leptospermum spp honey on tear film properties. Br J Ophthalmol. (2020) 104:1373–7. doi: 10.1136/bjophthalmol-2019-315160
57. Wong, D, Albietz, JM, Tran, H, Du Toit, C, Li, AH, Yun, T, et al. Treatment of contact lens related dry eye with antibacterial honey. Cont Lens Anterior Eye. (2017) 40:389–93. doi: 10.1016/j.clae.2017.10.001
58. Nichols, KK, Foulks, GN, Bron, AJ, Glasgow, BJ, Dogru, M, Tsubota, K, et al. The international workshop on meibomian gland dysfunction: executive summary. Investig Ophthalmol Vis Sci. (2011) 52:1922–9. doi: 10.1167/iovs.10-6997a
Keywords: dry eye, honey, apoptosis, inflammation, corneal limbal stem cell, keratocytes
Citation: Sanie-Jahromi F, Khaki M, Heydari M, Nowroozzadeh MH, Akbarizadeh AR, Daneshamouz S, NejatyJahromy Y, Nejabat M, Mahmoudi A, Zareei A and Nejabat M (2024) Effect of low dose honey on the apoptosis and inflammation gene expression in corneal limbal stem cells and keratocytes and its efficacy as an ophthalmic formulation in the treatment of dry eye: in-vitro and clinical study. Front. Med. 11:1359463. doi: 10.3389/fmed.2024.1359463
Received: 21 December 2023; Accepted: 29 April 2024;
Published: 20 May 2024.
Edited by:
Alejandro Navas, Instituto de Oftalmología Fundación de Asistencia Privada Conde de Valenciana, I.A.P, MexicoReviewed by:
Alfredo Domínguez López, Instituto de Oftalmobiología Aplicada—Universidad de Valladolid, SpainCopyright © 2024 Sanie-Jahromi, Khaki, Heydari, Nowroozzadeh, Akbarizadeh, Daneshamouz, NejatyJahromy, Nejabat, Mahmoudi, Zareei and Nejabat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmood Nejabat, bmVqYWJhdG1Ac3Vtcy5hYy5pcg==, c2FuaWUuZmF0aUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.