- 1Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, and Center for Chronic Airway Diseases, Peking University Health Science Center, Peking University, Beijing, China
- 2Department of Hematology, Lymphoma Research Center, Peking University Third Hospital, Beijing, China
- 3Department of Respiratory and Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, Beijing, China
Background: Lianhuaqingwen (LHQW), a traditional Chinese medicine comprised of 13 herbal extracts renowned for their robust heat-clearing and detoxifying properties, has gained widespread utilization in China but has yet to garner similar recognition abroad. It is believed to exhibit efficacy in ameliorating symptoms in individuals afflicted with coronavirus disease 2019 (COVID-19). However, the precise impact of LHQW on viral shedding (VS), particularly in the context of mild or asymptomatic infections caused by the Omicron BF.4/5 or BF.7 variants of COVID-19, remained inadequately elucidated. Consequently, a real-world study was conducted, involving patients diagnosed with COVID-19, with the primary objective of ascertaining the effectiveness of LHQW in this specific clinical context.
Methods: We conducted an investigation on Omicron-infected patients through a single-center, propensity score-matched real-world study conducted at Xiaotangshan Fangcang Hospital from May to November 2022. A total of 3,368 COVID-19 patients were enrolled in the study, all of whom presented mild or asymptomatic infections caused by either BF.4/5 or BF.7 strains of the virus. Demographic and clinical data were systematically collected from medical records. Patients were allocated to receive treatment with LHQW (designated as the treatment group) or received no LHQW treatment (designated as the not-treated/no-treatment group). Viral load was quantified utilizing quantitative real-time PCR (qPCR), and the duration of VS was defined as the time interval between the initial negative test result and the date of COVID-19 diagnosis or symptom onset.
Results: The study encompassed a cohort of 3,368 patients, and following propensity score matching, a subset of 296 patients was meticulously chosen for subsequent analysis. Notably, baseline characteristics exhibited disparities between the treatment and not-treated/no-treatment groups. However, post-matching, these characteristics achieved a commendable level of comparability. Our findings unequivocally demonstrated that there existed no statistically significant disparity in VS. This holds true when comparing patients subjected to LHQW treatment against those not administered LHQW, as well as when contrasting individuals presenting asymptomatic and mild COVID-19 manifestations.
Conclusion: No statistically significant difference in VS was observed between patients who underwent LHQW treatment and those who did not. Additional investigations are imperative to provide a comprehensive assessment of LHQW’s efficacy, particularly in patients afflicted with severe COVID-19 or those infected with viral strains distinct from BF.4/5 or BF.7.
Introduction
COVID-19, precipitated by the SARS-CoV-2 virus, has disseminated on a global scale since late 2019, presenting formidable healthcare challenges. The disease manifests with symptoms such as fever, cough, respiratory distress, anosmia, and ageusia, and in severe instances, it can prove fatal (1, 2). The Omicron variant of the SARS-CoV-2 virus was first identified in November 2021 in Botswana and South Africa (3). Subsequently, Omicron variants BF.7 and BA.4/5 emerged as the predominant strains. These variants exhibit numerous mutations in their spike protein, which could potentially enhance transmissibility, confer resistance to antibodies, or lead to milder symptomatology when compared to earlier strains. Following infection, symptoms may endure until the virus is cleared from the body (4). Individuals with preexisting medical conditions are at an elevated risk of experiencing severe illness and life-threatening complications subsequent to contracting the Omicron variant of the virus (5).
Viral load and respiratory testing of SARS-CoV-2 are key to estimating infectivity, and shedding of infectious virus is necessary for continued transmission (6), therefore, VS is closely related to infectivity. SARS-CoV-2 can be transmitted in a variety of ways, including through the excrement of a person with COVID-19 into wastewater. Recent research has identified that SARS-CoV-2 virus strains in fecal water are constantly mutating and dominated by co-circulating variants (7). Many drugs can reduce VS, but there are not enough studies on them, so it is important to study the relationship between LHQW and VS.
Currently, supportive therapies stand as the cornerstone of COVID-19 management. Traditional Chinese medicine, owing to its extensive historical use and proven effectiveness in influenza patients (8), has been recently adapted for clinical COVID-19 management (9). Numerous potential antiviral agents have been subject to investigation (10). Among these, the widely employed Lianhuaqingwen (LHQW) capsule, developed by Shijiazhuang Yiling Pharmaceutical Co. Ltd. in Shijiazhuang, China, contains 13 herbal extracts with robust heat-clearing and detoxifying properties, thereby demonstrating effectiveness in mitigating lung inflammation. A prospective, multicenter, randomized controlled trial was conducted across 23 hospitals spanning 9 provinces in China to elucidate the potential of LHQW in ameliorating clinical symptoms such as fever, fatigue, and cough, while concurrently improving chest imaging and reducing symptom duration and expediting recovery time (11). In vitro experiments provided compelling evidence supporting LHQW’s anti-SARS-CoV-2 efficacy. LHQW was reported to demonstrate antiviral activity by inhibiting SARS-CoV-2 replication, suppressing the production of pro-inflammatory cytokines (TNF-α, IL-6, MCP-1, and IP-10), and influencing virion morphology (12). Previous research indicated that LHQW has shown effectiveness in treating infections caused by the Omicron strain of the novel coronavirus, with some studies suggesting its potential to facilitate viral clearance (11, 13–17). Nevertheless, there is a scarcity of relevant investigations regarding its influence on VS. The present study is constrained by an insufficient sample size and the absence of real-world studies in this context.
This study endeavors to assess and compare the therapeutic efficacy between the LHQW treatment group and the LHQW not-treated/no-treatment group in patients afflicted with the Omicron variant through real-world observations. Our findings offer a novel perspective on the utilization of LHQW in the context of COVID-19 treatment.
Methods and materials
Participant recruitment and data acquisition
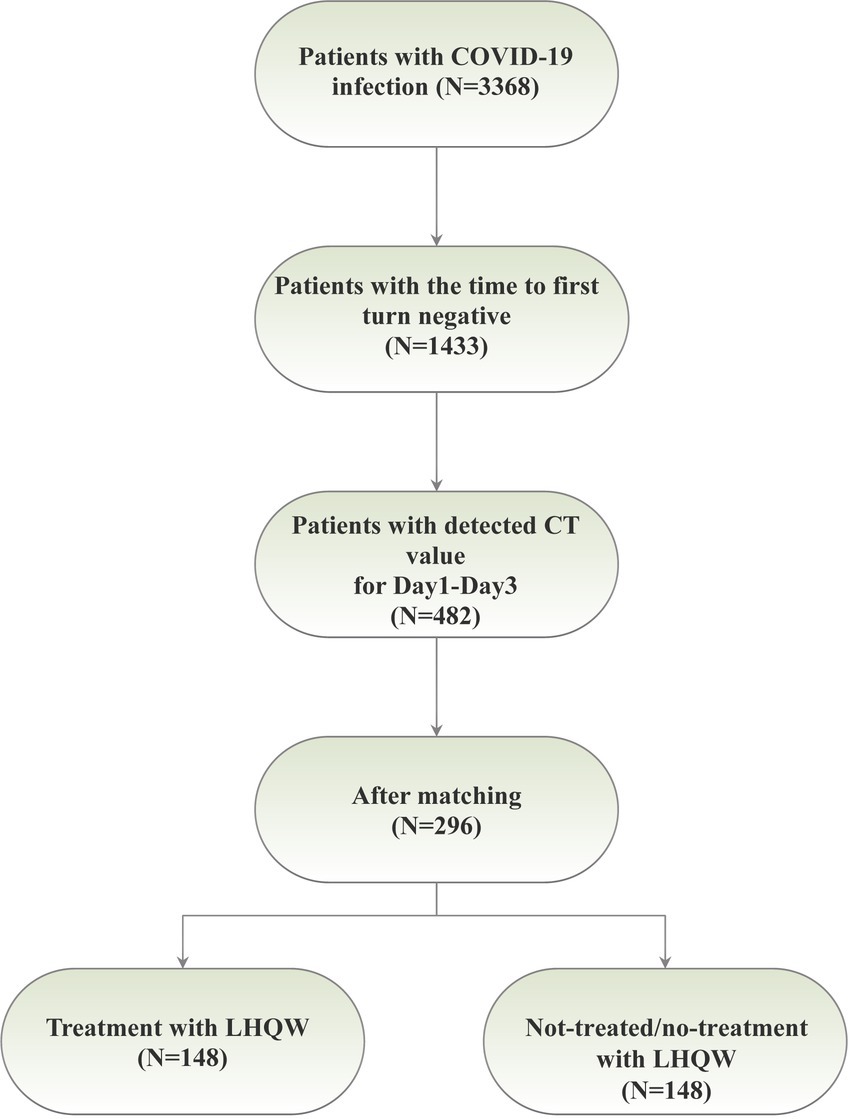
This research was conducted at Xiaotangshan Fangcang Hospital, located in Beijing, China, in strict accordance with the ethical principles delineated in the Declaration of Helsinki. The study protocol received approval from the Ethics Committee at Peking University Third Hospital. Participants were enrolled at Fangcang Hospital during two distinct time intervals: from May to June 2022 (for BA.4/5) and from October to November 2022 (for BF.7). In total, 3,368 patients were recruited for the study. The inclusion criteria encompassed individuals who tested positive for COVID-19 via nucleic acid testing, comprising asymptomatic infections (those diagnosed with COVID-19 but devoid of clinical symptoms) and mild patients (individuals exhibiting minor symptoms without pneumonia evident in medical imaging). Age was not a limiting factor. Exclusion criteria were defined to exclude patients in critical condition, those incapable of completing the nucleic acid test, or those who were transferred out of Fangcang Hospital during the study period. Population demographics and clinical characteristics were meticulously extracted from electronic health records. The severity of COVID-19 was categorized into two groups, namely asymptomatic infections and mild patients. We initially screened 482 patients with CT values recorded for Day 1 to Day 3, and subsequent to propensity score matching (PSM), a cohort of 296 patients was ultimately selected (Figure 1A).
Quantification of viral load through qPCR analysis
We diligently monitored VS at multiple, closely spaced time points. Nucleic acid testing was performed sequentially, with an average of 7 tests conducted per patient. The quantitative real-time PCR (qPCR) assay specifically targeted the open reading frame lab (ORFlab) and the nucleocapsid protein (N) as the designated target genes. Nasopharyngeal swab samples were processed following the instructions provided by the Marburg virus nucleic acid detection kit from Shanghai BioGerm Medical Technology Co., Ltd. (CHN). Viral RNA was efficiently extracted and detected from these samples. Viral load was quantified using qPCR and was denoted by the cycle threshold (CT) value. The CT cut-off threshold for determining the presence of VS was set at 35. If the CT values for both N and ORFlab were equal to or greater than 35, it was deemed a negative result; otherwise, it was classified as a positive result. In instances where a patient’s CT value exceeded 35, a follow-up nucleic acid test was scheduled to confirm a negative result on the subsequent day. Conversely, if the CT value remained below 35, daily nucleic acid testing was continued until a CT value exceeding 35 was obtained, subsequently leading to the confirmation of a negative result on the following day. The duration of viral shedding (VS) was calculated as the time interval between the date of the initial negative result and the date of COVID-19 diagnosis or symptom onset.
Results
The baseline demographic and clinical characteristics, both before and after propensity score matching (PSM), have been comprehensively summarized in Table 1. Following PSM, no statistically significant differences were observed between the LHQW-treated and LHQW-untreated groups. In both groups, a higher prevalence of male patients was noted compared to female patients. Furthermore, cases of asymptomatic infection exceeded those of mild infection in both cohorts. Additionally, a majority of patients in both groups did not report habits of smoking or alcohol consumption, and a higher proportion of patients were devoid of comorbidities such as hypertension, fever, and allergies. At the baseline, there were no statistically significant differences in CT values for the ORF1ab gene and N gene between the two groups. Moreover, the majority of patients did not receive alternative treatments such as Qinggan, Paxlovid, and Azvudine; however, it is worth noting that the distinction between the LHQW-treated and LHQW-not-treated/no-treatment groups in this regard was not statistically significant.
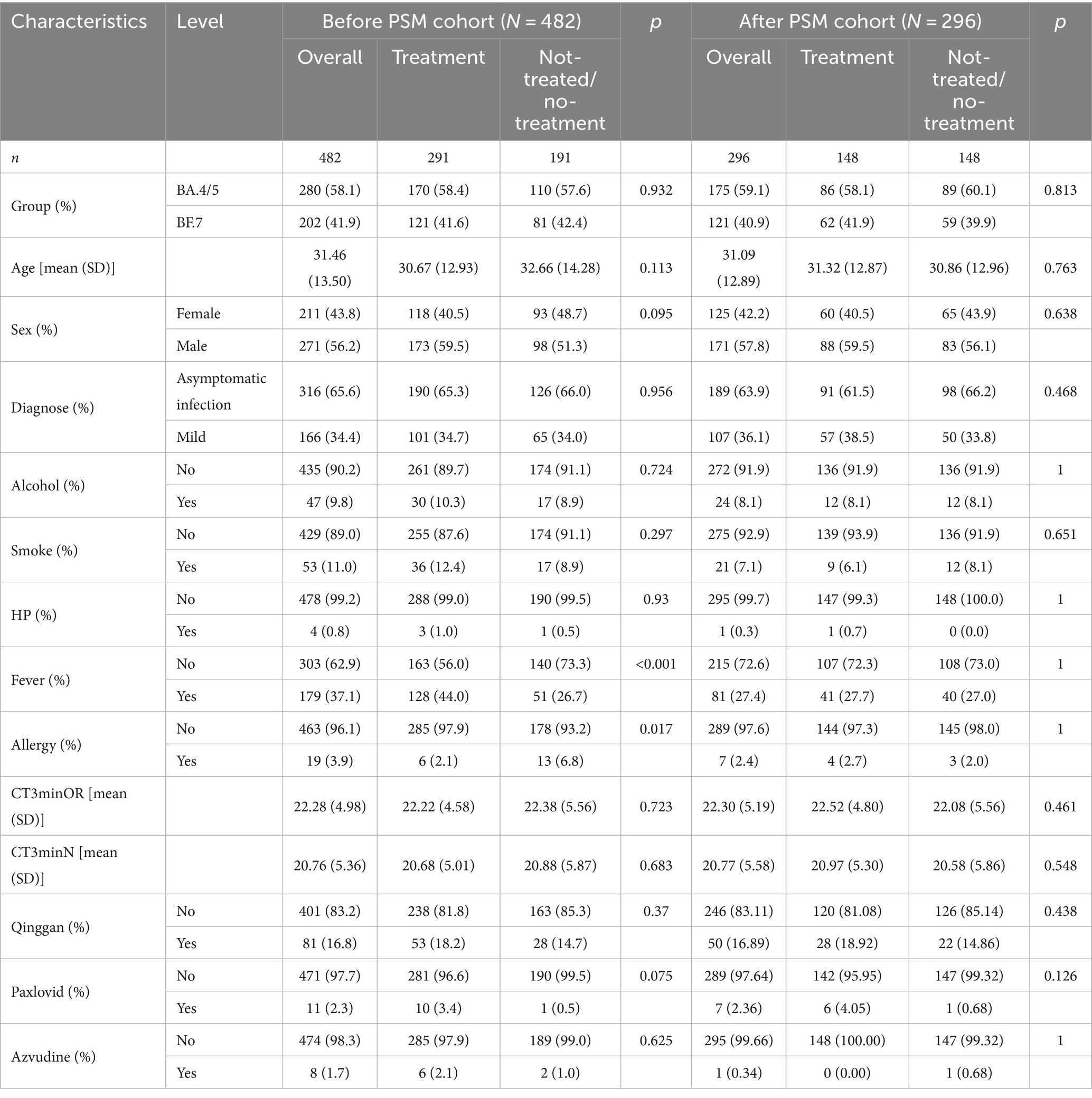
Table 1. Baseline characteristics of COVID19 patients treated with LHQW and untreated with LHQW before and after PSM.
Following the application of the PSM matching technique to balance the baseline characteristics of the two groups, we identified and selected 296 patients in a 1:1 ratio, comprising 148 patients in each of the LHQW-treated and LHQW-not-treated/no-treatment groups (Figure 1A). Upon scrutinizing the characteristics of both groups after matching, it was observed that the propensity score distribution of the two groups was concordant, and the matching score effect exhibited stability and reliability. This observation suggested that the data from both groups post-matching was more comparable (Figure 2A). In light of this, we conducted a thorough comparison of the disparities in baseline characteristics between the two patient groups following matching. The outcomes revealed no statistically significant differences in baseline characteristics between the two groups (Figure 2B). To further illustrate the effectiveness of the matching process, Figure 2C presents a visualization of the standardized mean difference (SMD) both before and after matching, underscoring the reduction in SMD achieved through the matching procedure. In the subsequent analysis, after matching, it was discerned that the LHQW-treated group and LHQW-not-treated/no-treatment group displayed analogous trends in terms of both N-CT and ORF-CT (Figures 3A–D). Furthermore, the investigation unveiled that there was no significant disparity in VS between the two groups, both among individuals with asymptomatic and mild infections (Figures 3E–H).
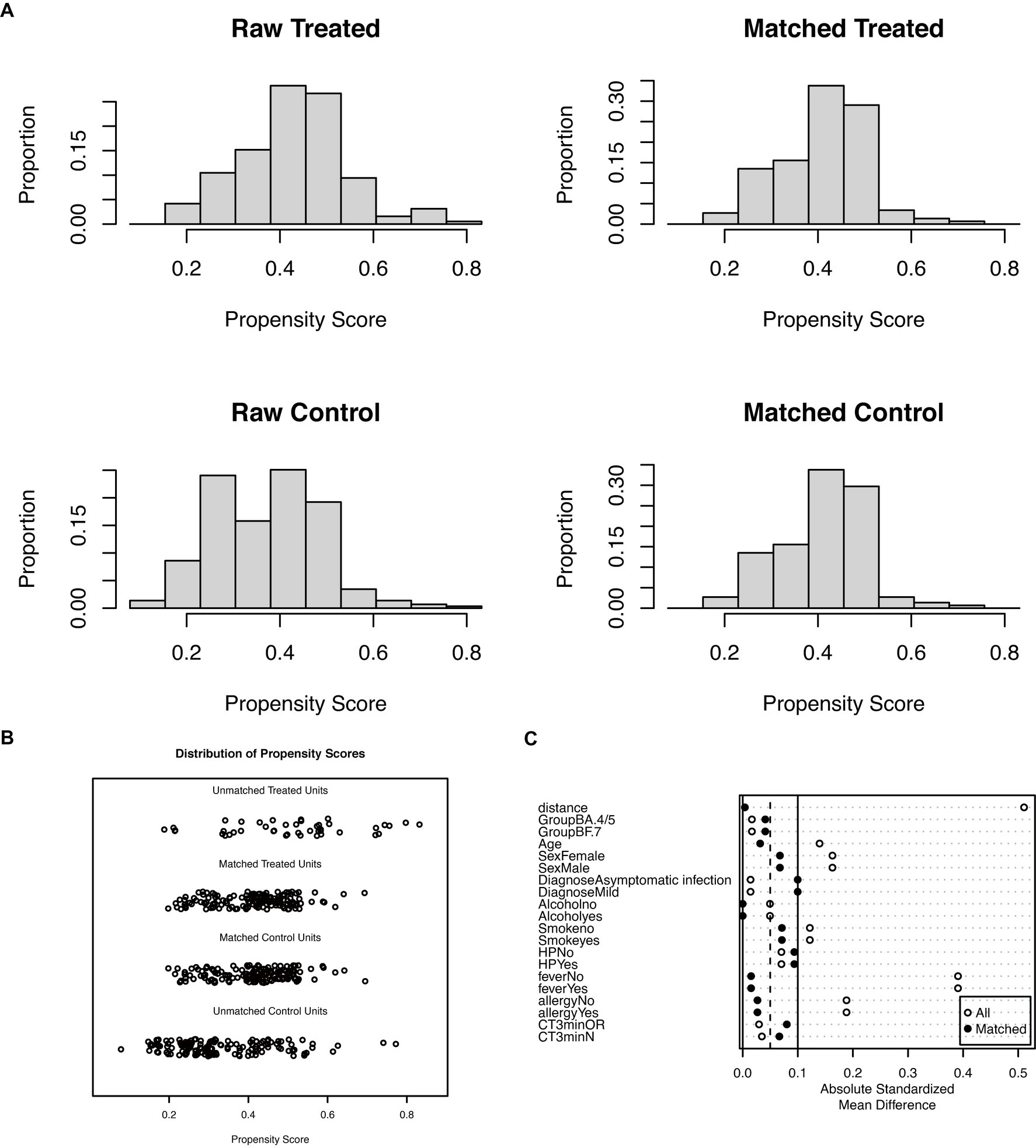
Figure 2. Propensity score before and after matching. (A) Histogram for propensity score of the raw and matched group. (B) Dotplot for propensity score of the raw and matched group. (C) Dotplot of the absolute standardized mean difference.
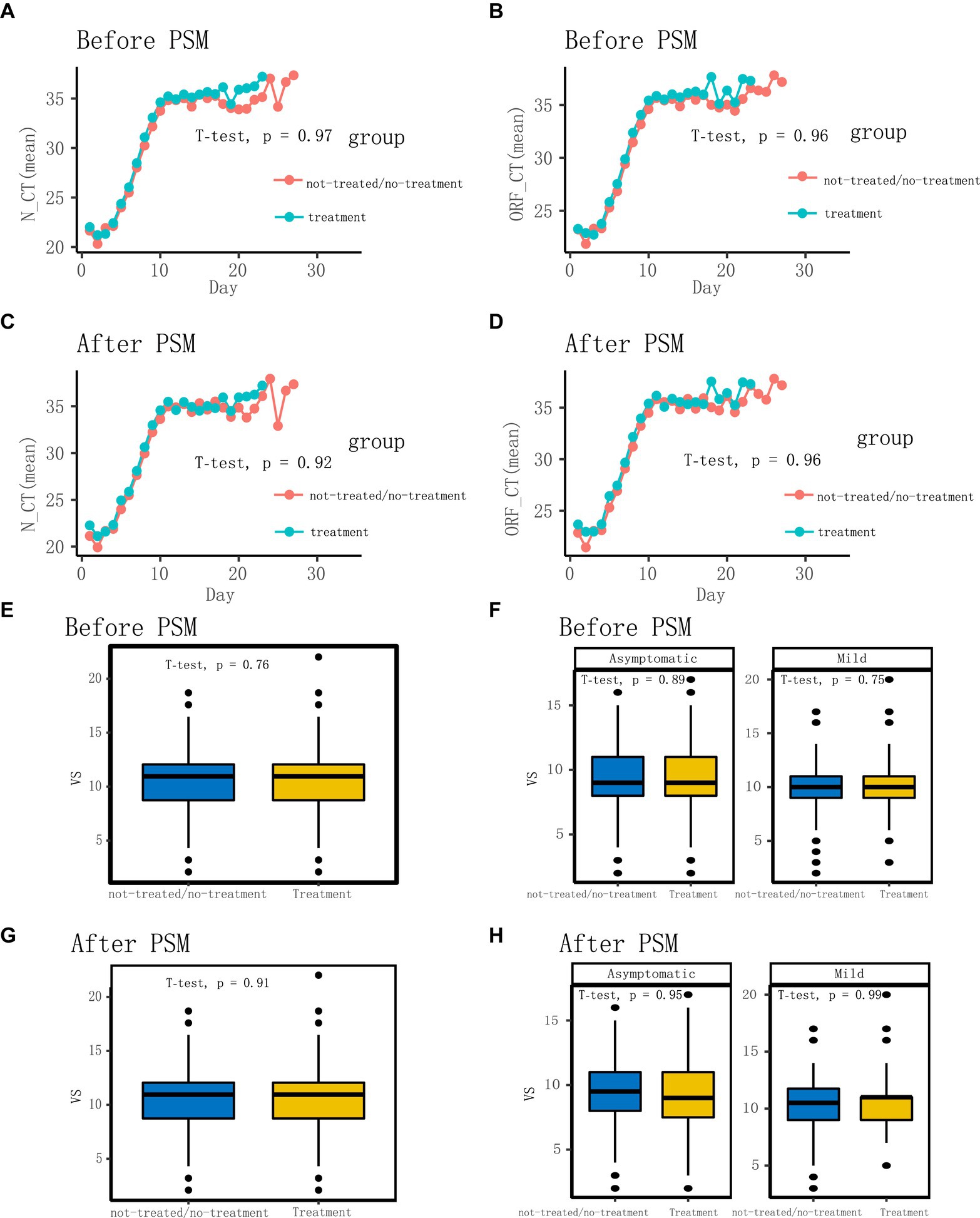
Figure 3. Comparison of characteristics before and after matching. (A) Comparison of N-CT (mean) between not-treated/no-treatment and treatment groups before matching. (B) Comparison of ORF-CT (mean) between not-treated/no-treatment and treatment groups before matching. (C) Comparison of N-CT (mean) between not-treated/no-treatment and treatment groups after matching. (D) Comparison of ORF-CT (mean) between not-treated/no-treatment and treatment groups after matching. (E,F) Comparison of VS between not-treated/no-treatment and treatment groups before matching. (G,H) Comparison of VS between not-treated/no-treatment and treatment groups after matching.
Discussion
The primary objective of this study was to conduct a comparative analysis of VS in COVID-19 patients who underwent LHQW treatment versus those who did not receive any treatment. Our study encompassed a substantial sample size comprising 3,368 patients afflicted with either the Omicron BF.7 or Omicron BA.4/5 variants, thereby ensuring the availability of robust and representative data for our analysis.
Prior research in the field has predominantly concentrated on assessing the effectiveness of LHQW in ameliorating clinical symptoms, with limited insights into its impact on VS (11–13, 15, 16, 18, 19). For instance, Academician Zhong’s recent publication reports findings from an international, multicenter, double-blind, randomized controlled study, primarily focusing on the alleviation of COVID-19 symptoms by LHQW, encompassing nasal congestion, sore throat, cough, fever, and other related symptoms (20). The results indicate that LHQW can significantly and expeditiously reduce the relief time and enhance the recovery rate of major COVID-19 symptoms. However, it is noteworthy that the mentioned study did not address the VS. Consequently, our research serves as a complementary study to Academician Zhong’s investigation into LHQW, addressing this specific aspect that was not covered in his study. A prospective, multicenter, randomized controlled trial spanning 23 hospitals across 9 provinces in China demonstrated the capacity of LHQW to alleviate clinical symptoms such as fever, malaise, and cough, while also leading to improvements in chest radiographic outcomes. However, it did not yield significant improvements in the conversion rate of viral assays (11), a result in line with our own findings. It’s noteworthy that their research pertained to the early SARS-CoV-2 strain, whereas our investigation centered on the Omicron variant, a subject they did not explore. Nevertheless, it’s worth noting that there are also conflicting studies in the literature that diverge from our results. A study involving children, with a total of 692 samples, revealed that the LHQW treatment group exhibited a reduction in the time required for VS (21). This suggested that LHQW might be efficacious in expediting reduction of VS in pediatric patients. We believed that our study came to the opposite conclusion for two main reasons, Firstly, the virus under investigation in the referenced study was the Omicron BF.2 variant, while our research focused on the Omicron BF.4/5 or BF.7 variants. Secondly, the demographic of the referenced study comprised children, in contrast to our study, which targeted the adult population. These distinctions in both the viral variants and the study populations were pivotal in understanding the differing outcomes observed. Conversely, another study encompassing a large adult population of 4,918 cases demonstrated that the LHQW treatment group exhibited improvements in the nucleic acid conversion rate (19). Numerous systematic reviews and meta-analyses have underscored the substantial efficacy of LHQW in the treatment of COVID-19 (14, 15, 22), reporting enhancements in clinical symptoms and a reduction in the progression to severe or critical conditions, but they did not specifically monitor VS. The most notable difference between our study and these prior investigations lies in the diverse endpoints used to evaluate LHQW efficacy, as well as the distinct populations of interest under study. For patients battling severe COVID-19, there is an unequivocal imperative to prioritize the amelioration of symptoms and prognosis. Conversely, in the case of patients experiencing mild or even asymptomatic COVID-19, the emphasis on symptom improvement becomes less pertinent. In such instances, directing our focus toward VS, as conducted in our study, assumes paramount significance. Furthermore, a study conducted by Fang et al. reported a synergistic effect between LHQW and dexamethasone in expediting the attainment of nucleic acid negativity in patients afflicted with severe COVID-19 (23). However, it’s crucial to acknowledge that the quantity and quality of the studies included in this line of research remain limited. Hence, the necessity for additional high-quality clinical trials incorporating additional observational indicators is evident to substantiate these findings (18, 23, 24).
This study is subject to several limitations. Firstly, it is important to acknowledge that this is a retrospective study rather than a prospective one, potentially affecting the robustness of our findings. Secondly, our study exclusively enrolled patients with mild and asymptomatic COVID-19 infections caused by the BA.4/5 or BF.7 strains. Consequently, the efficacy of LHQW in patients with severe COVID-19 or those infected with strains distinct from BA.4/5 or BF.7 remains uncharted territory and warrants further investigation.
In summary, our study has unveiled that there exists no substantial discrepancy in VS between the cohort of patients subjected to LHQW treatment and the not-treated/no-treatment group. This observation introduces a novel dimension to the discourse surrounding the utility of LHQW in the management of COVID-19. It underscores the necessity for additional research endeavors aimed at providing a comprehensive evaluation of its efficacy and feasibility in clinical practice. Furthermore, future cohorts should be expanded to encompass patients grappling with severe COVID-19 manifestations or those infected with viral strains divergent from BF.4/5 or BF.7. Such investigations will contribute to a more comprehensive understanding of the potential enhancements in VS attributed to LHQW in these distinct clinical contexts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Given the urgent demand for epidemiological and clinical data acquisition and the retrospective nature of our study, the Ethics Committees granted a waiver of prior informed consent. All data were meticulously analyzed in an anonymous fashion to safeguard the privacy of participants.
Author contributions
XG: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. CY: Writing – review & editing, Writing – original draft, Validation, Resources, Investigation, Data curation, Conceptualization. CW: Writing – review & editing, Writing – original draft, Validation, Resources, Investigation, Data curation, Conceptualization. ZD: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. JF: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. SY: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. PY: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. FB: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. HJ: Writing – review & editing, Writing – original draft, Validation, Investigation, Funding acquisition, Data curation. CC: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. YM: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. WZ: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. YS: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support for this research was provided through multiple sources, including the special fund of the National Clinical Key Specialty Construction Program, P. R. China (2023), the Capital’s Funds for Health Enhancement and Research (Grant No. 2022-2G-40910), National Natural Science Foundation of China (Grant No. 81800195), and Key Clinical Initiatives of Peking University Third Hospital (Grants BYSYZD2019026, BYSYDL2021006, BYSYZD2022014).
Acknowledgments
The authors extend their gratitude to the dedicated healthcare professionals who tirelessly served during the COVID-19 pandemic at the Beijing Xiaotangshan Fangcang shelter hospital, as well as to the patients under their care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Umakanthan, S, Sahu, P, Ranade, AV, Bukelo, MM, Rao, JS, Abrahao-Machado, LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. (2020) 96:753–8. doi: 10.1136/postgradmedj-2020-138234
2. Khan, M, Adil, SF, Alkhathlan, HZ, Tahir, MN, Saif, S, Khan, M, et al. COVID-19: a global challenge with old history, epidemiology and Progress so far. Molecules. (2020) 26:10039. doi: 10.3390/molecules26010039
3. Iuliano, AD, Brunkard, JM, Boehmer, TK, Peterson, E, Adjei, S, Binder, AM, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods – United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:146–52. doi: 10.15585/mmwr.mm7104e4
4. Andrews, N, Stowe, J, Kirsebom, F, Toffa, S, Sachdeva, R, Gower, C, et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. (2022) 28:831–7. doi: 10.1038/s41591-022-01699-1
5. Adjei, S, Hong, K, Molinari, N-AM, Bull-Otterson, L, Ajani, UA, Gundlapalli, AV, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods – United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1182–9. doi: 10.15585/mmwr.mm7137a4
6. Pilapil, JD, Notarte, KI, and Yeung, KL. The dominance of co-circulating SARS-CoV-2 variants in wastewater. Int J Hyg Environ Health. (2023) 253:114224. doi: 10.1016/j.ijheh.2023.114224
7. Puhach, O, Meyer, B, and Eckerle, I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. (2023) 21:147–61. doi: 10.1038/s41579-022-00822-w
8. Duan, Z-P, Jia, Z-H, Zhang, J, Liu, S, Chen, Y, Liang, LC, et al. Natural herbal medicine Lianhuaqingwen capsule anti-influenza a (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin Med J. (2011) 124:2925–33. doi: 10.3760/cma.j.issn.0366-6999.2011.18.024
9. Xia, JJCYD. Chinese medicine masters and academicians enter the national medical treatment expert group, and Chinese medicine deeply intervenes in the whole process of new coronary pneumonia diagnosis and treatment. National Administration of Traditional Chinese Medicine. (2020). Available at: http://www.natcm.gov.cn/xinxifabu/meitibaodao/2020-02-17/13175.html.
10. Wang, M, Cao, R, Zhang, L, Yang, X, Liu, J, Xu, M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
11. Hu, K, Guan, W-J, Bi, Y, Zhang, W, Li, L, Zhang, B, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. (2021) 85:153242. doi: 10.1016/j.phymed.2020.153242
12. Runfeng, L, Yunlong, H, Jicheng, H, Weiqi, P, Qinhai, M, Yongxia, S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. (2020) 156:104761. doi: 10.1016/j.phrs.2020.104761
13. Liu, M, Gao, Y, Yuan, Y, Yang, K, Shi, S, Zhang, J, et al. Efficacy and safety of integrated traditional Chinese and Western medicine for Corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res. (2020) 158:104896. doi: 10.1016/j.phrs.2020.104896
14. Zhuang, J, Dai, X, Wu, Q, Cai, H, Fu, X, Zhang, W, et al. A meta-analysis for Lianhua Qingwen on the treatment of coronavirus disease 2019 (COVID-19). Complement Ther Med. (2021) 60:102754. doi: 10.1016/j.ctim.2021.102754
15. Zeng, M, Li, L, and Wu, Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019 (COVID-19): Meta-analysis of randomized controlled trials. PLoS One. (2020) 15:e0238828. doi: 10.1371/journal.pone.0238828
16. Zhang, X, Cao, D, Liu, J, Zhang, Q, and Liu, M. Efficacy and safety of Lianhua Qingwen combined with conventional antiviral Western medicine in the treatment of coronavirus disease (covid-19) in 2019: protocol for a systematic review and meta-analysis. Medicine (Baltimore). (2020) 99:e21404. doi: 10.1097/MD.0000000000021404
17. Saravolatz, LD, Depcinski, S, and Sharma, M. Molnupiravir and Nirmatrelvir-ritonavir: Oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis. (2023) 76:165–71. doi: 10.1093/cid/ciac180
18. Ji, H. The Effects of Chinese Medicine Lianhua Qingwen on Inhibiting the Replication of Covid-19 Virus. Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare. (2022). doi: 10.5220/0011292900003438
19. Lu, Y, Zhang, M, Yang, Q-Q, Li, WJ, Yang, K, Hu, W, et al. Effectiveness and safety of Lianhua Qingwen capsules for COVID-19: a propensity-score matched cohort study. Evid Based Complement Alternat Med. (2023) 2023:1–7. doi: 10.1155/2023/6028554
20. Zheng, J-P, Ling, Y, Jiang, L-S, Mootsikapun, P, Lu, HZ, Chayakulkeeree, M, et al. Effects of Lianhuaqingwen capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial. Virol J. (2023) 20:277. doi: 10.1186/s12985-023-02144-6
21. Xu, X, Wu, H, Jin, G, Huang, J, Li, J, Zhou, J, et al. Efficacy of Lianhua Qingwen for children with SARS-CoV-2 omicron infection: a propensity score-matched retrospective cohort study. Phytomedicine. (2023) 111:154665. doi: 10.1016/j.phymed.2023.154665
22. Li, Y, Xiao, P, Liu, N, and Zhang, Z. Efficacy and safety of Chinese medicine Lianhua Qingwen for treating COVID-19: an updated meta-analysis. Front Pharmacol. (2022) 13:888820. doi: 10.3389/fphar.2022.888820
23. Fang, X, Me, Z, Zhang, K, Wang, Z, and He, P. Synergic role of dexamethasone and Lianhua Qingwen capsule accelerate nucleic acid negative conversion in severe COVID-19 patients. Research Square. (2021). doi: 10.21203/rs.3.rs-955194/v1
Keywords: Lianhua Qingwen, LHQW, SARS-CoV-2, COVID-19, omicron, real-world study
Citation: Gai X, Yan C, Wu C, Duan Z, Fan J, Yuan S, Yang P, Bao F, Jing H, Cai C, Ma Y, Zhang W and Sun Y (2024) Impact of Lianhua Qingwen on viral shedding in omicron mild/asymtomatic patients: a real-world study. Front. Med. 11:1357299. doi: 10.3389/fmed.2024.1357299
Edited by:
Yazine Mahjoub, Centre Hospitalier Universitaire (CHU) d'Amiens, FranceReviewed by:
Md. Maruf Ahmed Molla, Upstate Medical University, United StatesKin Israel Notarte, Johns Hopkins University, United States
Copyright © 2024 Gai, Yan, Wu, Duan, Fan, Yuan, Yang, Bao, Jing, Cai, Ma, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weilong Zhang, emhhbmd3bDIwMTJAMTI2LmNvbQ==; Yongchang Sun, c3VueUBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Xiaoyan Gai
Xiaoyan Gai Changjian Yan2†
Changjian Yan2† Shengren Yuan
Shengren Yuan Hongmei Jing
Hongmei Jing Yingmin Ma
Yingmin Ma Weilong Zhang
Weilong Zhang Yongchang Sun
Yongchang Sun