- 1Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Pediatrics, Peking University Third Hospital, Beijing, China
Purpose: Prostate-specific membrane antigen (PSMA)-targeted imaging has gained increasing interest in its application in prostate cancer lesion detection. Compared with 68Galium (68Ga), 18Fluoride (18F)-labeled imaging agent has easier syntheses, lower price, and a longer half-time. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid positron emission tomography (18F-DCFPyL PET) has been recently approved by the U.S. Food and Drug Administration. Several studies have proven its superiority to conventional imaging techniques in detecting prostate cancer lesions. However, the impact of 18F-DCFPyL PET on the management of patients with prostate cancer is not well established. Thus, we performed a systematic review and meta-analysis of available data to evaluate the impact of 18F-DCFPyL PET on the management of patients with prostate cancer.
Methods: The PubMed, Embase, Scopus, and Cochrane databases were searched up to April 2024. Studies that reported the proportion of changes in management after 18F-DCFPyL PET was performed in patients with prostate cancer were included. The Grading of Recommendations Assessment, Development, and Evaluation system was used for the quality evaluation of the included studies. The proportion of changes in management was pooled using a random effects model. Meta-regression analyses were performed to assess the potential correlation between the PET positivity and management changes.
Results: Fourteen studies (3,078 patients with prostate cancer) were included in our review and analysis. The pooled percentage of management changes was 43.5% (95% confidence interval [CI]: 33–54%). In patients with biochemical recurrent and for primary staging, the pooled percentage was 50% (95% CI: 39–60%) and 22% (95% CI: 15–29%), respectively. In the meta-regression analyses, PET positivity was detected as a significant predictor of management change (p = 0.0023).
Conclusion: 18F-DCFPyL PET significantly affects the management of patients with prostate cancer. Higher PET positivity rate significantly correlated with a higher proportion of management changes in patients with prostate cancer. However, more studies are still needed to confirm the important role of 18F-DCFPyL PET in the management of prostate cancer.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#myprospero, CRD42022339178.
Introduction
Prostate cancer is one of the most commonly diagnosed malignant diseases in men. It is projected that there will be approximately 2.3 million new cases and 0.7 million deaths by 2040 worldwide (1). Thus, it is important to accurately classify patients in the primary stage of the disease and closely monitor disease prognosis resulting from therapy decisions. Treatment decision-making process is usually significantly affect by many factors, such as extent and extraprostatic metastasis, clinicopathological status, and patient preferences (2). Besides, in patients with biochemical recurrence (BCR), it is appropriate to locate the lesions because its role as an indicator of disease progress is important for further disease management.
The most recent European Association of Urology (EAU) guidelines recommend at least one cross-sectional abdominopelvic imaging [computed tomography (CT) or magnetic resonance imaging (MRI)] and a bone scan for evaluating the extent of extra-prostatic disease in intermediate or high-risk prostate cancer (3). However, conventional imaging modalities detect malignancies mainly from morphological or osteoblastic activity data, and this becomes a major challenge when lesions develop in atypical locations, are small, or are from other benign pathologic processes such as fractures or infection. Once the BCR was detected following radical prostatectomy, only 11–14% of patients had a positive CT result for possible recurrent lesions (4). The pooled specificity of choline-based positron emission tomography (PET) imaging was proven higher than that of bone scan with fewer false-positive lesions (0.99 [95% CI: 0.93–1.00] vs. 0.82 [95% CI: 0.78–0.85]) (5). However, prostate-specific antigen (PSA) levels and kinetics dramatically affect the sensitivity of choline-based PET (6–8). Therefore, more accurate and advanced imaging modalities that can guide the management of prostate cancer patients are still needed in clinical practice.
Recently, prostate-specific membrane antigen (PSMA)-targeted PET tracers have gained increasing interest for imaging and selection of therapy in patients with prostate cancer. PSMA is a type II integral membrane glycoprotein produced by the prostatic epithelium, and is strongly overexpressed in prostate cancer cells (9, 10). Moreover, PSMA expression increases progressively in high-grade prostate tumor cells and metastatic lesions (11, 12). The most widely used PSMA ligand is 68Ga-PSMA-11 and rising clinical research is focused on its prostate cancer indications (13–17). However, its application has some disadvantages. 68Ga has a short half-life of 68 min and synthesis decreases as generators decay. In contrast, 18F has longer half-life, less positron energy, and possibly lower costs. Thus, 18F-labeled PSMA ligand is a promising alternative (18).
2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) is a widely used agent in clinical practice, and was recently approved by the U.S. Food and Drug Administration (FDA). It is a second-generation fluorinated PSMA-targeted PET radiotracer, and several studies have demonstrated that it holds promise in clinical application (19). Results from a study including 248 patients who were administered 18F-DCFPyL showed a rather high detection rate (17/29 scans, 59%) even with serum PSA value <0.5 ng/mL, and an overall detection rate of 86.3% (20). A recent meta-analysis by Pan et al. (21) showed that the pooled detection rates of 18F-DCFPyL-labeled PSMA PET/CT in prostate cancer was 92%. The pooled detection rate was 89% for PSA ≥ 0.5 ng/mL and 49% for PSA < 0.5 ng/mL.
Although the diagnostic performance of 18F-DCFPyL PET has been evaluated, its impact on changes in the treatment management of patients with prostate cancer has not been systematically reviewed. Therefore, in this systematic review and meta-analysis, we investigated the impact of 18F-DCFPyL PET on changes in the management of patients with prostate cancer.
Materials and methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered in the International Prospective Register of Systematic Reviews database (registration no. CRD 42022339178).
Literature search
This work was performed according to the PRISMA statement. A systematic literature search was conducted through PubMed, Embase, Scopus, and Cochrane databases until April 2024. The search query was performed based on the following string of terms: (prostate OR prostatic) AND (18F-DCFPyL) AND (positron emission tomography OR PET) AND (impact OR change OR alter OR modification OR influence). All relevant studies were assessed, and bibliographies of the retrieved articles were evaluated for any relevant papers. Two reviewers independently performed the assessment and evaluation. Disagreements were reviewed and resolved by a third reviewer.
Study selection
Two investigators (HW and HMZ) independently screened and assessed the titles, abstracts, and full texts of the eligible studies. Inclusion criteria were: (1) patients with primary staging (PS) or BCR prostate cancer (2) 18F-DCFPyL PET as “intervention,” (3) conventional imaging methods including CT, MRI, bone scan (BS) and other imaging modalities as “comparator,” (4) proportion of patients who have changed the disease management method as “outcome,” and (5) prospective or retrospective studies as “study design.” The exclusion criteria were as follows: (1) conference abstracts, review articles, editorials, and comments, and (2) overlapping cohort. Only the most recently published or largest study was included when there were multiple articles based on a similar population. Discrepancies were resolved by consulting a third investigator (QJ).
Data extraction
Two investigators (HW and GNL) independently extracted data using a pre-specified method. Baseline characteristics, including authors, publication years, countries and institutions, study design, management plan, PET device, injected dose, uptake time, PET positive rate, and percentage of therapy alteration, were extracted from individual studies. Discrepancies were resolved by consulting a third investigator (QJ).
Assessment of study quality
The quality of studies included in our study was evaluated by two independent reviewers according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (22). They were assessed in four areas: study design, patient selection, publication, and indirectness. The studies included in our meta-analysis were not randomized trials (comparing management before and after 18F-DCFPyL PET) and were rated down because blinding was impossible. Quality assessment were performed by two independent reviewers (HXH and JND), and all discrepancies were generally resolved by consensus or decided by the senior reviewer (QJ).
Data synthesis and analysis
In our meta-analysis, the impact of 18F-DCFPyL PET on the management of prostate cancer patients was the primary outcome, which represents the proportion of patients who underwent a change in therapy methods based on 18F-DCFPyL PET imaging findings. The secondary outcomes were the subgroup analyses for studies in patients with BCR and for PS.
After PET examination, 23.6% of cases underwent additional imaging, which cause overall 87.3% of management plan (23). In Metser et al. (24) and Liu et al.’s (25) studies, they have reported intend/recorded or actual/proposed treatment management, the recorded and actual numbers were analyzed.
Meta-analyses were conducted using R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria, Package: meta). Heterogeneity was evaluated using Cochran’s Q test and Higgins and Thompson’s I2 test. Publication bias was assessed using funnel plots and Egger’s test, random effect was used for pooling, and meta-regression analyses were performed to investigate if PET positivity rate is the possible predictor of management change.
One study (26) reported the PET positivity rate from three readers, we used the mean of these values. One study (27) reported the PET positivity rate of local region, lymph node and metastasis, the number of metastatic detection rate was used. One study (28) included patients for PS and with BCR, the PET positivity rate was reported separately, and we used the mean value.
Results
Literature search
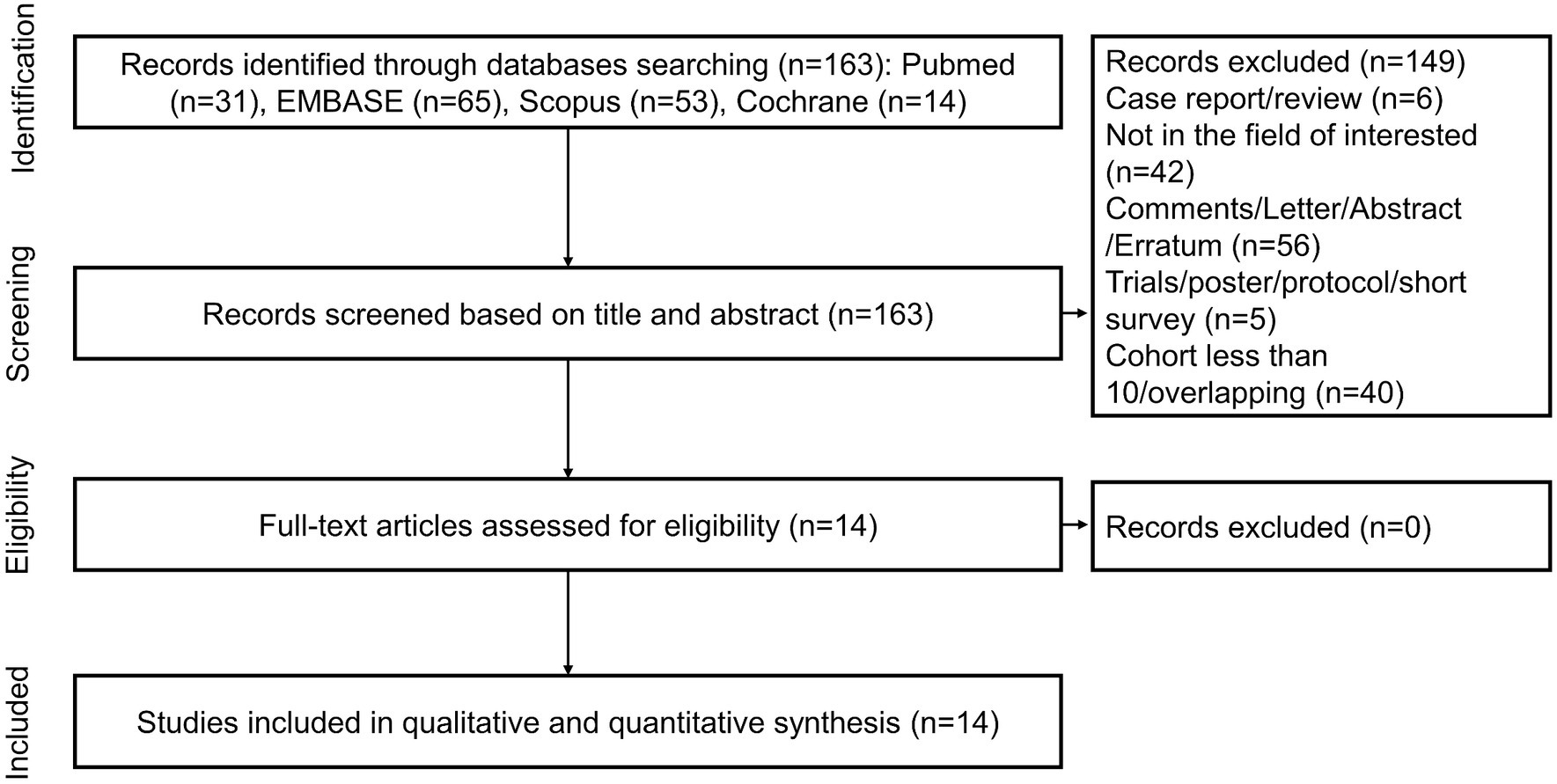
A total of 163 unique records were identified from the literature search and screened for titles, abstracts, and full texts. With the removal of 149 papers after screening the titles and abstracts, 14 articles were included in full-text reviews, and all studies were ultimately selected. Figure 1 shows the study selection process in detail.
Study characteristics
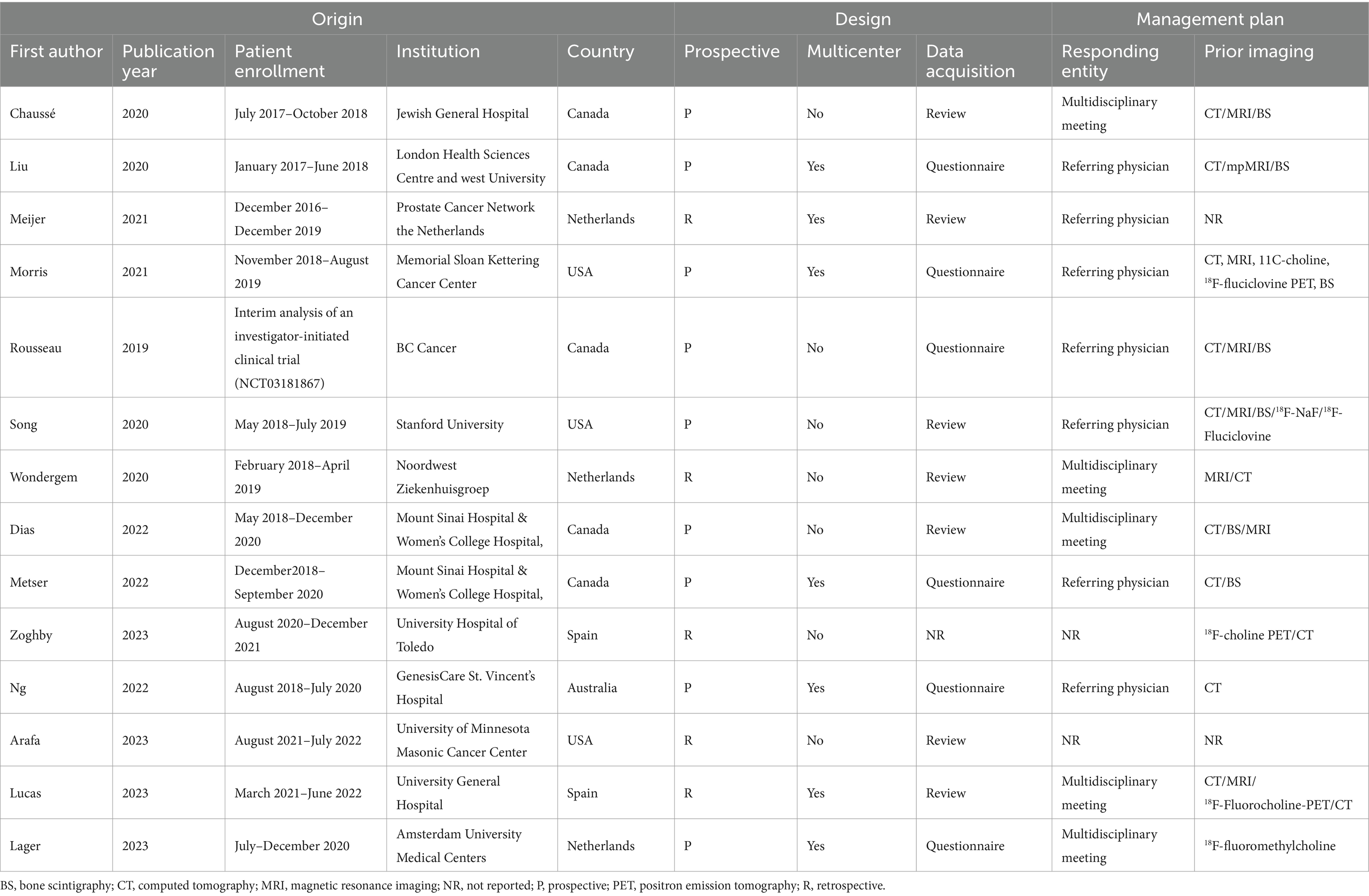
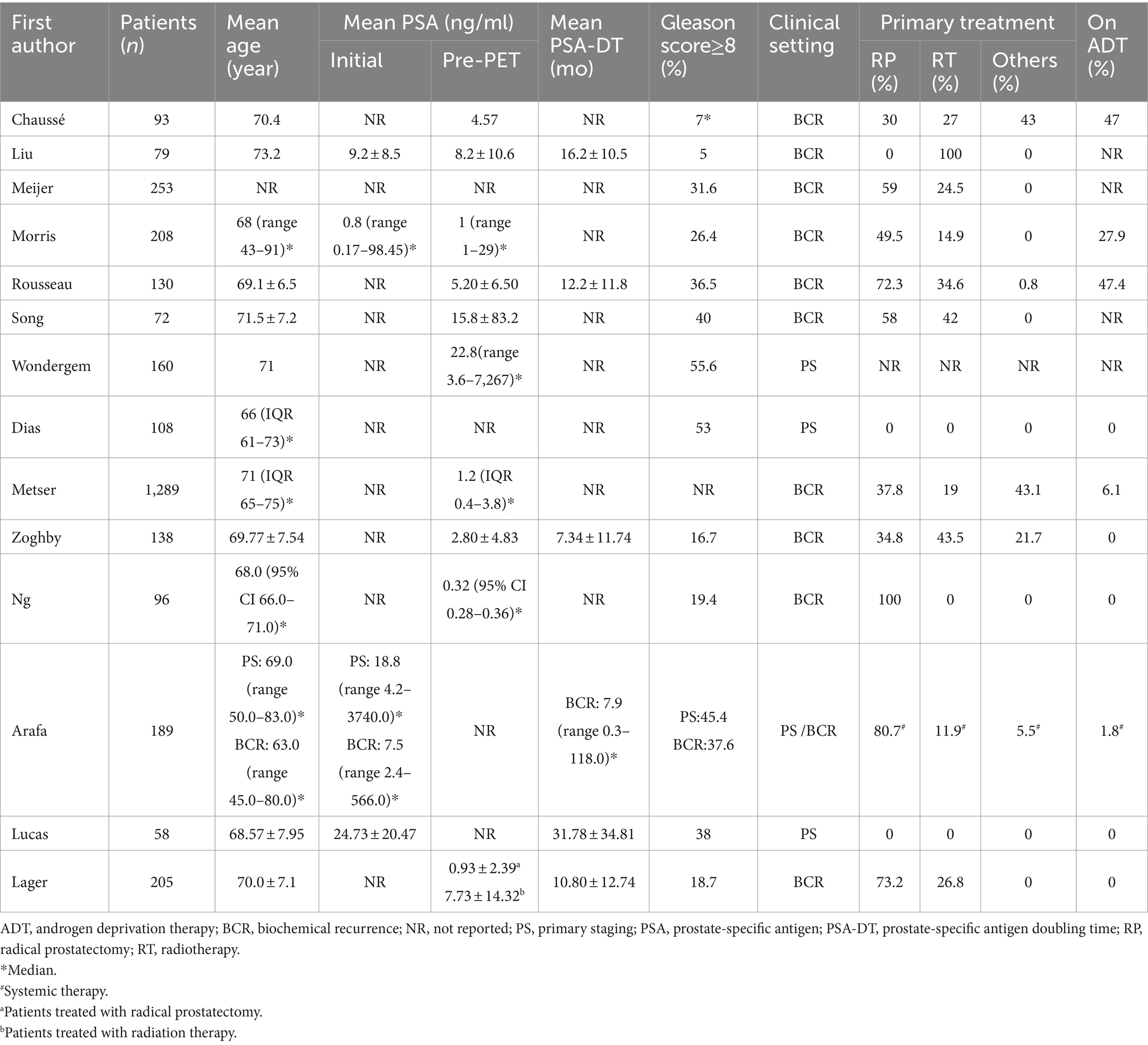
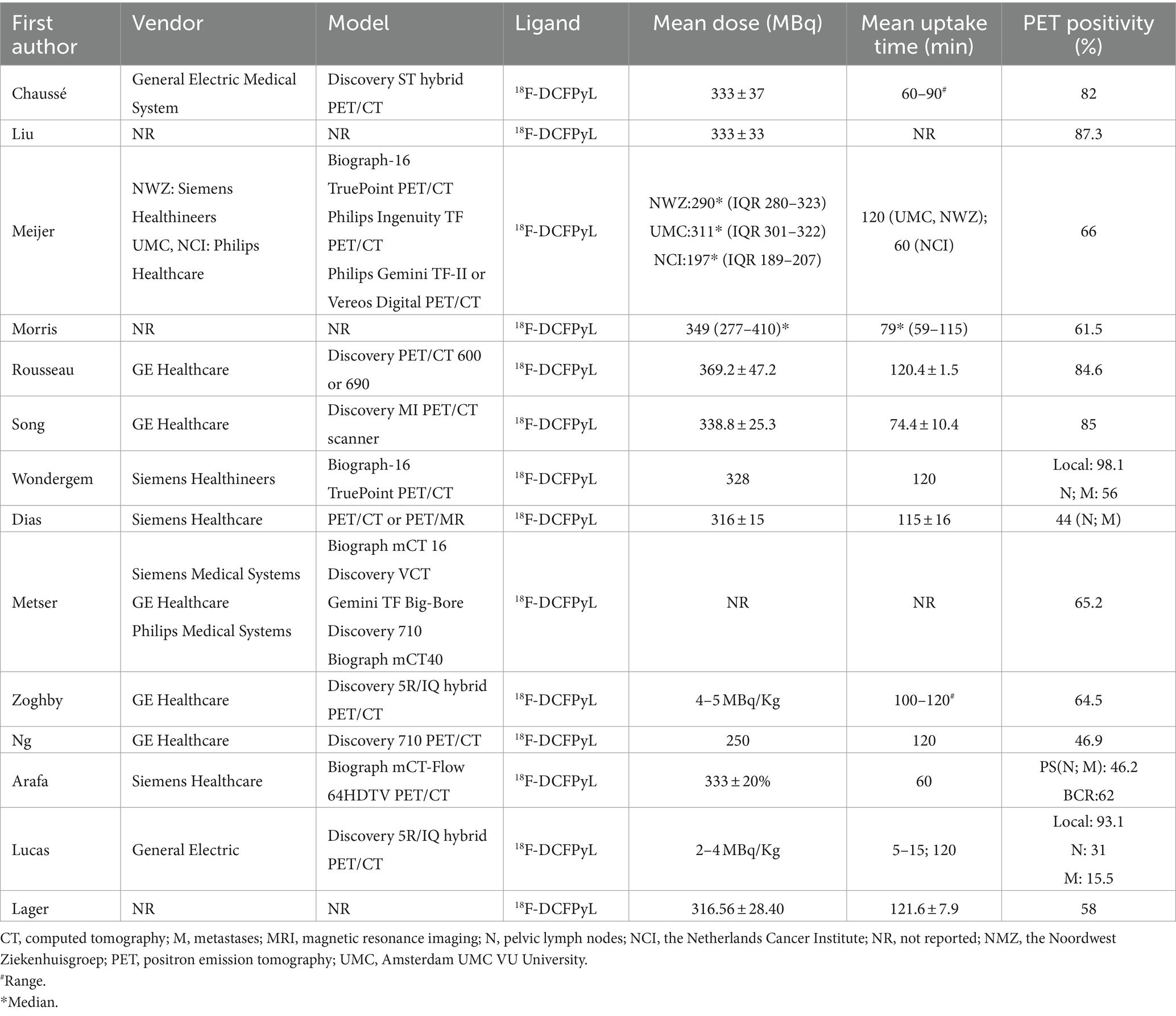
Study, patient, and PET characteristics are described in Tables 1–3 and Supplementary Table 1. The studies were conducted in Canada, the Netherlands, the United States, Spain, and Australia. There are 9 studies were prospective, and 5 were retrospective. The mean age range of the patients was 69–73 year. Serum mean PSA levels before 18F-DCFPyL PET were 0.32–15.8 ng/mL, respectively. The mean injected 18F-DCFPyL dose was 250–369 MBq, with mean uptake times ranging from 60 to 120 min. Reported patient management changes were BCR detection in 11 studies, PS in 4 studies, and both outcomes were reported in one study. PET positivity was reported in all studies, with values ranging from 15.5 to 87% (15.5% was reported as the detection rate of metastatic lesion, and for lymph node and local detection, the rate was 31, 93.1% respectively).
Quality assessment
In terms of bias, all included studies were rated down because blinding was impossible for therapeutic decisions based on conventional and 18F-DCFPyL PET imaging techniques. In terms of publication, two studies were rated down because of potential industry influence. Morris et al. (26) reported 18F-DCFPyL patent ownership and authors were employed in Progenics Pharmaceuticals. Liu et al. (25) reported personal fees from the industry and employment of a close family member by Roche Canada. All included studies have reported management changes and 5 studies were rated up due to a large effect size (> 50%) (23–26, 29). There was no other rating up or down in any of the studies selected for our review and analysis. Ultimately, the quality of evidence was high in 3 studies (23, 24, 29) and moderate in 11 studies (25–28, 30–36). Figure 2 shows the funnel plot and Egger’s test (p = 0.2239); there was no significant publication bias.
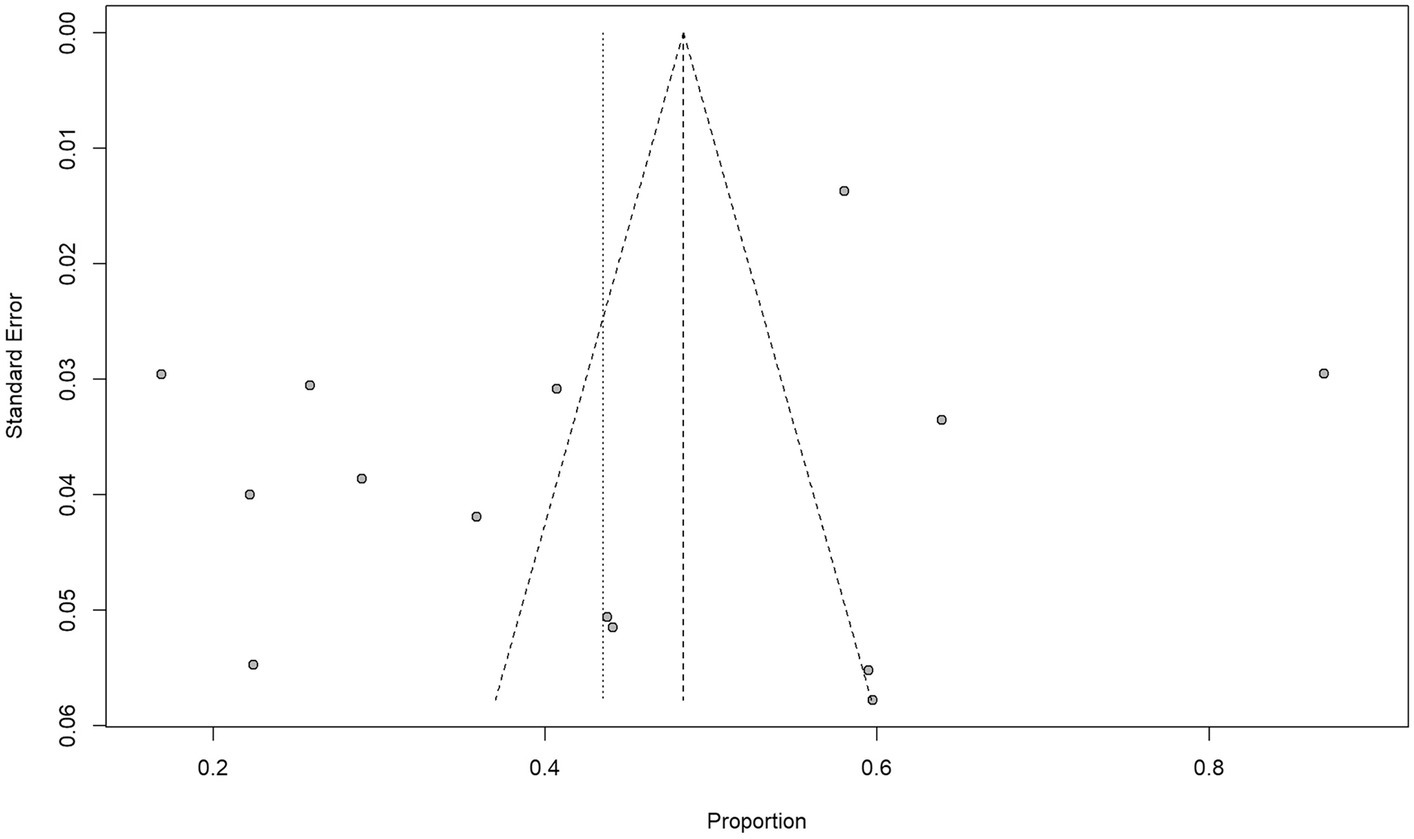
Figure 2. Funnel plot and Egger’s test suggest that the possibility of significant publication bias is low (p = 0. 2239).
Efficacy evaluation
The impact of 18F-DCFPyL PET on patient management in all included studies, divided into BCR intent and PS intent, is shown in Figure 3. The proportion of management changes in individual studies varied from 17 to 87%. The pooled percentage of management changes was 50% (95% confidence interval [CI]: 39–60%) in patients with BCR (11 studies). Substantial heterogeneity was observed based on the Q test (p < 0.01) and Higgins and Thompson’s I2 statistic (I2 = 97%). For the four studies reporting patients for PS, the pooled proportion was 22% (95% CI: 15–29%), and heterogeneity was observed (Q test: p = 0.06; Higgins and Thompson’s I2 statistics: I2 = 59%). The overall pooled percentage of management changes 43.5% (95% CI: 33–54%, Q test: p < 0.01; Higgins and Thompson’s I2 statistics: I2 = 98%) (Supplementary Figure 1).
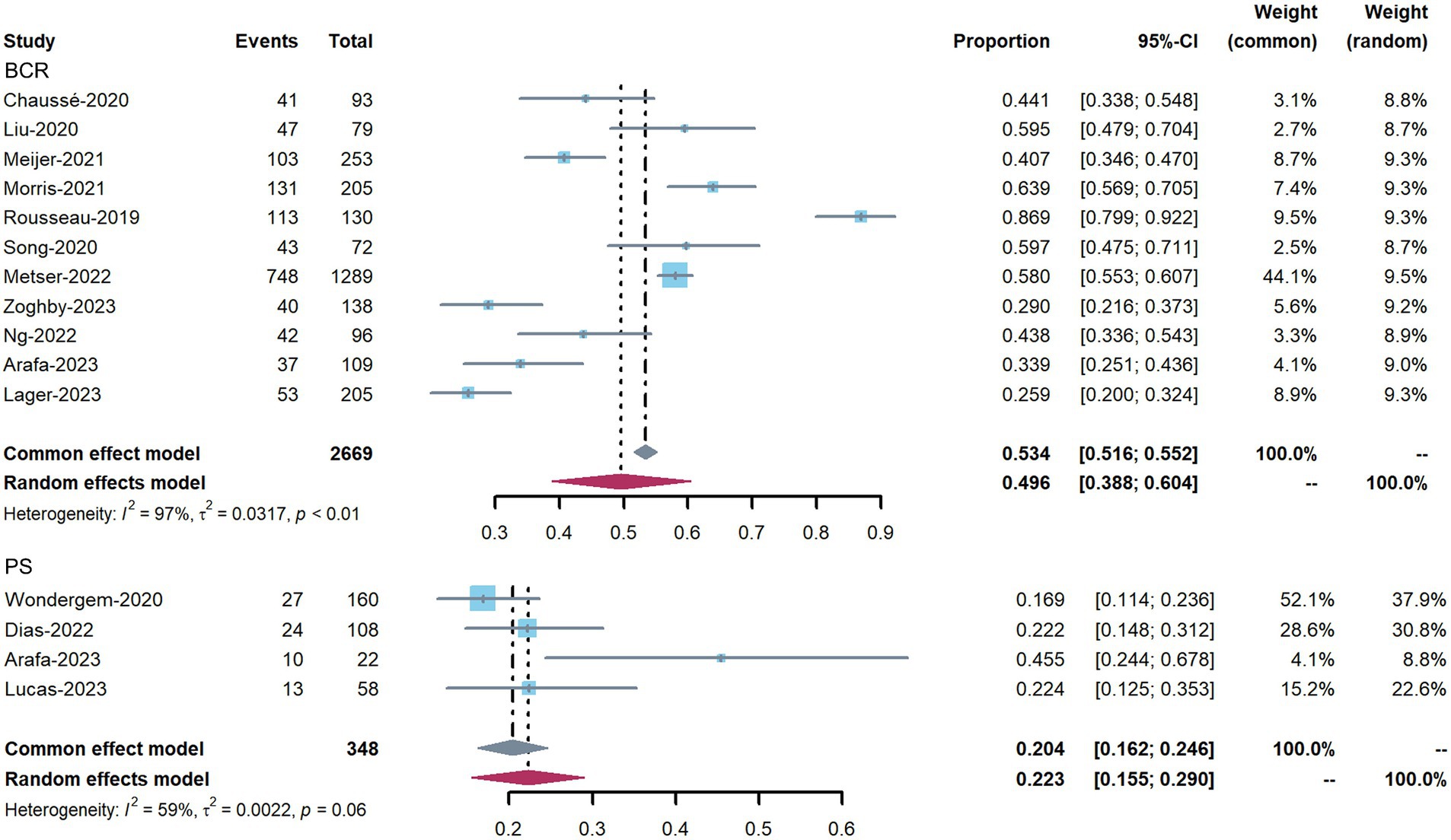
Figure 3. Forest plots showing the pooled proportion of management changes before and after 18F-DCFPyL PET, and classified into PS and BCR changes.
Correlation between 18F-DCFPyL PET positivity and management change
Figure 4 shows a clear tendency for greater 18F-DCFPyL PET positivity rate with a higher proportion of patient management changes (p = 0.0023). In addition, meta-regression analysis demonstrated that every 1% increase in PET positivity correlated with a 0.7% increase in management change.

Figure 4. Bubble plot of the correlation between 18F-DCFPyL PET positivity and rate of management change using meta-regression analysis (p = 0.0023).
Discussion
In the current meta-analysis, we assessed the impact of 18F-DCFPyL PET imaging on the change in treatment methods of patients with prostate cancer. 18F-DCFPyL PET led to a pooled proportion of 43.5%, indicating that the performance of 18F-DCFPyL PET could affect the therapy decisions. In addition, we evaluated studies in which management change in patients with BCR detection or PS, and the pooled proportions were 50 and 22%, respectively.
A previous meta-analysis of the use of 68Ga-PSMA PET reported a similar pooled proportion (54%) of management changes, and the implemented and intended changes were 54 and 51%, respectively (37). These similar results were expected, which have confirmed the fact that PSMA-targeted molecular imaging has superior detection rates compared with conventional imaging modalities. Other novel PSMA ligands, such PSMA-1007 (38) and rhPSMA-7 (39), have been developed. However, due to limited research, the overall comparison among these tracers is lacking. Moreover, a positive correlation was observed between 18F-DCFPyL PET positivity and the proportion of management changes. Notably, Han et al. has reported that there was a 0.55% increase in management change for every 1% increase in 68Ga-PSMA PET positivity (37). In our study, a significant correlation between 18F-DCFPyL PET positivity and management change was confirmed, and the number of increase in management change for every 1% increase in 18F-DCFPyL PET was 0.7.
Several meta-analyses have reported that 18F-DCFPyL PET has relatively good sensitivity and specificity for the detection of both primary and metastatic prostate cancer lesions. The pooled detection rate of 18F-DCFPyL PET in prostate cancer patients was 92%. The pooled detection rate was 89% for PSA ≥ 0.5 ng/mL and 49% for PSA < 0.5 ng/mL (21). Another meta-analysis revealed that the pooled detection rate of 18F-DCFPyL PET in biochemically recurrent prostate cancer was 81% (95% CI: 76.9–85.1%). The pooled detection rate was 88.8% for PSA ≥ 0.5 ng/mL (95% CI: 86.2–91.3%) and 47.2% for PSA < 0.5 ng/mL (95% CI: 32.6–61.8%) (40). Moreover, in the included studies, 18F-DCFPyL PET was able to detect more lesions compared with conventional imaging, which is important to the patient because metastatic lesions could alter therapy methods. 18F-DCFPyL PET provides extra information in molecular level, which enables the detection of tiny metastatic lesions even in atypical location. There is increasing evidence that 18F-DCFPyL PET outperforms conventional imaging modalities in both PS (41) and BCR (20) of prostate cancer patients, and our results could potentially support the findings. Both 68Ga-PSMA-11 and 18F-DCFPyL were approved by the FDA, it is necessary to compare them comprehensively to provide evidence for selection of hospitals or institutions. Previous and the current studies have demonstrated a comparable detection rate of these two ligands. Thus, some institutions could consider the costs without harming patients’ management options. Moreover, new generations of 18F-labled PSMA-ligand have been proved with faster clearance and better tumor-to-background ratio, thus, 18F-labeled PSMA-ligand is a promising alternative option for prostate cancer patients.
This systematic review had several limitations. First, substantial heterogeneity was observed (I2 = 97, 98%). To our knowledge, baseline characteristics of patients (serum PSA level, Gleason score, risk stratification), clinical settings (PS, BCR), and types of primary treatment (radical prostatectomy, radiation therapy, systematic treatment) might be attributed to the results. It is already well known that different treatment patterns exist in the same region, likely because of differences in country, institution, specialty, and patient preference (37, 42–44). Besides, the conventional imaging modalities of the selected study are inconsistent, which could cause inequality among disease staging results. The lack of a reference standard for the interpretation of PSMA imaging could potentially cause bias, and more studies with histopathological confirmation are needed. Moreover, some reasons for the heterogeneity remain unexplained. Therefore, caution should be exercised when comparing and applying our pooled proportions. Although 18F-DCFPyL has been approved by the FDA, the number of related studies is limited. Thus, we could only include 14 studies, and none of them could be a blinded trial. Besides, 5 studies were retrospectively designed, and the risk of overestimating the pooled estimates and selection bias will increase. In addition, management changes between retrospective and prospective studies were not assessed because of the small sample size. Moreover, although it has proven that 18F-DCFPyL PET led to a change in management in approximately half of patients with prostate cancer, the correlation between alteration and outcomes or prognoses is still unknown. Finally, some studies lack post-operative histological validation and follow-up imaging; therefore, the interpretation of these results should be considered with caution. Thus, further clinical studies with standardized follow-up protocols and histological validation are needed to compare the outcomes of these tracers. The current study is based on 18F-DCFPyL PET; therefore, pooled evidence for PSMA-targeted imaging, including 68Ga-labeled PSMA is earnestly needed. Further studies are required to clarify this issue.
Conclusion
The pooled proportion of prostate cancer patients experienced management changes was 43.5%, and 18F-DCFPyL PET has a significant impact on treatment options. Higher PET positivity rate is significantly associated with a higher proportion of management changes. Prospective studies with larger sample sizes and better follow-up are, therefore, urgently needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HW: Software, Writing – original draft, Conceptualization, Data curation, Methodology. HZ: Conceptualization, Data curation, Methodology, Writing – review & editing. GL: Conceptualization, Data curation, Methodology, Writing – review & editing. JD: Data curation, Methodology, Writing – review & editing. HH: Data curation, Investigation, Methodology, Writing – review & editing. QJ: Formal analysis, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1355236/full#supplementary-material
References
1. Ferlay, J, Laversanne, M, Ervik, M, Lam, F, Colombet, M, Mery, L, et al. Global Cancer observatory: Cancer tomorrow. Lyon, France: International Agency for Research on Cancer (2020).
2. Litwin, MS, and Tan, HJ. The diagnosis and treatment of prostate Cancer: a review. JAMA. (2017) 317:2532–42. doi: 10.1001/jama.2017.7248
3. EAU. EAU Guidelines presented at the EAU annual congress Amsterdam. Arnhem The Netherlands: EAU Guidelines Office (2022). 2022 p.
4. Beresford, MJ, Gillatt, D, Benson, RJ, and Ajithkumar, T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol. (2010) 22:46–55. doi: 10.1016/j.clon.2009.10.015
5. Shen, G, Deng, H, Hu, S, and Jia, Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. (2014) 43:1503–13. doi: 10.1007/s00256-014-1903-9
6. Brogsitter, C, Zöphel, K, and Kotzerke, J. 18F-choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur J Nucl Med Mol Imaging. (2013) 40:18–27. doi: 10.1007/s00259-013-2358-2
7. Ceci, F, Herrmann, K, Castellucci, P, Graziani, T, Bluemel, C, Schiavina, R, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-Centre trial. Eur J Nucl Med Mol Imaging. (2014) 41:2222–31. doi: 10.1007/s00259-014-2872-x
8. Treglia, G, Ceriani, L, Sadeghi, R, Giovacchini, G, and Giovanella, L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med. (2014) 52:725–33. doi: 10.1515/cclm-2013-0675
9. Cimadamore, A, Cheng, M, Santoni, M, Lopez-Beltran, A, Battelli, N, Massari, F, et al. New prostate Cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol. (2018) 8:653. doi: 10.3389/fonc.2018.00653
10. Silver, DA, Pellicer, I, Fair, WR, Heston, WD, and Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. (1997) 3:81–5.
11. Mannweiler, S, Amersdorfer, P, Trajanoski, S, Terrett, JA, King, D, and Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. (2009) 15:167–72. doi: 10.1007/s12253-008-9104-2
12. Minner, S, Wittmer, C, Graefen, M, Salomon, G, Steuber, T, Haese, A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. (2011) 71:281–8. doi: 10.1002/pros.21241
13. Schwarzenboeck, SM, Rauscher, I, Bluemel, C, Fendler, WP, Rowe, SP, Pomper, MG, et al. PSMA ligands for PET imaging of prostate Cancer. J Nucl Med. (2017) 58:1545–52. doi: 10.2967/jnumed.117.191031
14. Afshar-Oromieh, A, Malcher, A, Eder, M, Eisenhut, M, Linhart, H, Hadaschik, B, et al. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. (2013) 40:486–95. doi: 10.1007/s00259-012-2298-2
15. Perera, M, Papa, N, Christidis, D, Wetherell, D, Hofman, MS, Murphy, DG, et al. Sensitivity, specificity, and predictors of positive 68Ga–prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. (2016) 70:926–37. doi: 10.1016/j.eururo.2016.06.021
16. Einspieler, I, Rauscher, I, Düwel, C, Krönke, M, Rischpler, C, Habl, G, et al. Detection efficacy of hybrid 68Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by Phoenix criteria. J Nucl Med. (2017) 58:1081–7. doi: 10.2967/jnumed.116.184457
17. Hofman, MS, Hicks, RJ, Maurer, T, and Eiber, M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. (2018) 38:200–17. doi: 10.1148/rg.2018170108
18. Ferreira, G, Iravani, A, Hofman, MS, and Hicks, RJ. Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution. Cancer Imaging. (2019) 19:23. doi: 10.1186/s40644-019-0211-y
19. Chen, Y, Pullambhatla, M, Foss, CA, Byun, Y, Nimmagadda, S, Senthamizhchelvan, S, et al. 2-(3-{1-Carboxy-5-[(6-[18F] Fluoro-Pyridine-3-carbonyl)-amino]-Pentyl}-Ureido)-Pentanedioic acid, [18F] DCFPyL, a PSMA-based PET imaging agent for prostate Cancer [18F] DCFPyL synthesis and in vivo evaluation. Clin Cancer Res. (2011) 17:7645–53. doi: 10.1158/1078-0432.CCR-11-1357
20. Wondergem, M, Jansen, BHE, van der Zant, FM, van der Sluis, TM, Knol, RJJ, van Kalmthout, LWM, et al. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. (2019) 46:1911–8. doi: 10.1007/s00259-019-04385-6
21. Pan, KH, Wang, JF, Wang, CY, Nikzad, AA, Kong, FQ, Jian, L, et al. Evaluation of 18F-DCFPyL PSMA PET/CT for prostate Cancer: a meta-analysis. Front Oncol. (2020) 10:597422. doi: 10.3389/fonc.2020.597422
22. Balshem, H, Helfand, M, Schünemann, HJ, Oxman, AD, Kunz, R, Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
23. Rousseau, E, Wilson, D, Lacroix-Poisson, F, Krauze, A, Chi, K, Gleave, M, et al. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate Cancer. J Nucl Med. (2019) 60:1587–93. doi: 10.2967/jnumed.119.226381.
24. Metser, U, Zukotynski, K, Mak, V, Langer, D, Mac Crostie, P, Finelli, A, et al. Effect of 18F-DCFPyL PET/CT on the Management of Patients with recurrent prostate Cancer: results of a prospective multicenter registry trial. Radiology. (2022) 303:414–22. doi: 10.1148/radiol.211824
25. Liu, W, Zukotynski, K, Emmett, L, Chung, HT, Chung, P, Wolfson, R, et al. Utilization of salvage and systemic therapies for recurrent prostate Cancer as a result of 18F-DCFPyL PET/CT restaging. Adv Radiat Oncol. (2021) 6:100553. doi: 10.1016/j.adro.2020.08.010
26. Morris, MJ, Rowe, SP, Gorin, MA, Saperstein, L, Pouliot, F, Josephson, D, et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate Cancer: results from the CONDOR phase III Multicenter Study. Clin Cancer Res. (2021) 27:3674–82. doi: 10.1158/1078-0432.ccr-20-4573
27. Lucas Lucas, C, García Zoghby, L, Amo-Salas, M, Soriano Castrejón, ÁM, and García Vicente, AM. Diagnostic and therapeutic impact of PET/CT with 18F-DCFPyL versus 18F-Fluorocholine in initial staging of intermediate−/high-risk prostate cancer: a pilot study. Ann Nucl Med. (2023) 37:551–60. doi: 10.1007/s12149-023-01859-4
28. Arafa, AT, Jain, A, Skrobanek, P, Humphrey, B, Froelich, JW, and Antonarakis, ES. Impact of piflufolastat F-18 PSMA PET imaging on clinical decision-making in prostate cancer across disease states: a retrospective review. Prostate. (2023) 83:863–70. doi: 10.1002/pros.24527
29. Song, H, Harrison, C, Duan, H, Guja, K, Hatami, N, Franc, BL, et al. Prospective evaluation of 18F-DCFPyL PET/CT in biochemically recurrent prostate Cancer in an academic center: a focus on disease localization and changes in management. J Nucl Med. (2020) 61:546–51. doi: 10.2967/jnumed.119.231654
30. Chaussé, G, Ben-Ezra, N, Stoopler, M, Levett, JY, Niazi, T, Anidjar, M, et al. Diagnostic performance of 18F-DCFPyL positron emission tomography/computed tomography for biochemically recurrent prostate cancer and change-of-management analysis. Can Urol Assoc J. (2021) 15:173–8. doi: 10.5489/cuaj.6817
31. Meijer, D, van Leeuwen, PJ, Oosterholt, PMJ, Bodar, YJL, van der Poel, HG, Hendrikse, NH, et al. Management impact of 18F-DCFPyL PET/CT in hormone-sensitive prostate cancer patients with biochemical recurrence after definitive treatment: a multicenter retrospective study. Eur J Nucl Med Mol Imaging. (2021) 48:2960–9. doi: 10.1007/s00259-021-05222-5
32. Wondergem, M, van der Zant, FM, Broos, WAM, Roeleveld, TA, Donker, R, Ten Oever, D, et al. 18F-DCFPyL PET/CT for primary staging in 160 high-risk prostate cancer patients; metastasis detection rate, influence on clinical management and preliminary results of treatment efficacy. Eur J Nucl Med Mol Imaging. (2021) 48:521–31. doi: 10.1007/s00259-020-04782-2
33. Basso Dias, A, Finelli, A, Bauman, G, Veit-Haibach, P, Berlin, A, Ortega, C, et al. Impact of 18F-DCFPyL PET on staging and treatment of unfavorable intermediate or high-risk prostate Cancer. Radiology. (2022) 304:600–8. doi: 10.1148/radiol.211836
34. García-Zoghby, L, Lucas-Lucas, C, Amo-Salas, M, Soriano-Castrejón, ÁM, and García-Vicente, AM. Head-to-head comparison of [18F]F-choline and imaging of prostate-specific membrane antigen, using [18F] DCFPyL PET/CT, in patients with biochemical recurrence of prostate Cancer. Curr Oncol. (2023) 30:6271–88. doi: 10.3390/curroncol30070464
35. Ng, M, Guerrieri, M, Wong, LM, Taubman, K, Sutherland, T, Benson, A, et al. Changes in management after 18F-DCFPyL PSMA PET in patients undergoing Postprostatectomy radiotherapy, with early biochemical response outcomes. J Nucl Med. (2022) 63:1343–8. doi: 10.2967/jnumed.121.263521.
36. Oprea-Lager, DE, Gontier, E, García-Cañamaque, L, Gauthé, M, Olivier, P, Mitjavila, M, et al. [18F] DCFPyL PET/CT versus [18F] fluoromethylcholine PET/CT in biochemical recurrence of prostate Cancer (PYTHON): a prospective, open label, cross-over, comparative study. Eur J Nucl Med Mol Imaging. (2023) 50:3439–51. doi: 10.1007/s00259-023-06301-5
37. Han, S, Woo, S, Kim, YJ, and Suh, CH. Impact of 68Ga-PSMA PET on the Management of Patients with prostate Cancer: a systematic review and meta-analysis. Eur Urol. (2018) 74:179–90. doi: 10.1016/j.eururo.2018.03.030
38. Rahbar, K, Afshar-Oromieh, A, Bögemann, M, Wagner, S, Schäfers, M, Stegger, L, et al. 18F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: biodistribution, tumour detection and activity kinetics. Eur J Nucl Med Mol Imaging. (2018) 45:1329–34. doi: 10.1007/s00259-018-3989-0
39. Oh, SW, Wurzer, A, Teoh, EJ, Oh, S, Langbein, T, Kronke, M, et al. Quantitative and qualitative analyses of biodistribution and PET image quality of novel Radiohybrid PSMA, 18F-rhPSMA-7, in patients with prostate Cancer. J Nucl Med. (2019) 61:702–9. doi: 10.2967/jnumed.119.234609
40. Sun, J, Lin, Y, Wei, X, Ouyang, J, Huang, Y, and Ling, Z. Performance of 18F-DCFPyL PET/CT imaging in early detection of biochemically recurrent prostate Cancer: a systematic review and meta-analysis. Front Oncol. (2021) 11:649171. doi: 10.3389/fonc.2021.649171
41. Jansen, BHE, Bodar, YJL, Zwezerijnen, GJC, Meijer, D, van der Voorn, JP, Nieuwenhuijzen, JA, et al. Pelvic lymph-node staging with 18F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer-the SALT trial. Eur J Nucl Med Mol Imaging. (2021) 48:509–20. doi: 10.1007/s00259-020-04974-w
42. Kim, SP, Tilburt, JC, Karnes, RJ, Ziegenfuss, JY, Han, LC, Shah, ND, et al. Variation in treatment recommendations of adjuvant radiation therapy for high-risk prostate cancer by physician specialty. Urology. (2013) 82:807–13. doi: 10.1016/j.urology.2013.04.060
43. Touijer, KA, Ahallal, Y, and Guillonneau, BD. Indications for and anatomical extent of pelvic lymph node dissection for prostate cancer: practice patterns of uro-oncologists in North America. Urol Oncol. (2013) 31:1517–21.e1-2. doi: 10.1016/j.urolonc.2012.04.021
Keywords: 18F-DCFPyL, PET imaging, PET positivity, prostate cancer, management change
Citation: Wang H, Zhu H, Li G, Dai J, Huang H and Jia Q (2024) Effect of 18F-DCFPyL PET on changes in management of patients with prostate cancer: a systematic review and meta-analysis. Front. Med. 11:1355236. doi: 10.3389/fmed.2024.1355236
Edited by:
Anne Roivainen, University of Turku, FinlandReviewed by:
Sazan Rasul, Medical University of Vienna, AustriaDamodara Naidu Kommi, University of Virginia, United States
Copyright © 2024 Wang, Zhu, Li, Dai, Huang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Jia, amlhcWJ5c3lAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hui Wang
Hui Wang HongMei Zhu1†
HongMei Zhu1† GuanNan Li
GuanNan Li JiaoNa Dai
JiaoNa Dai Qiong Jia
Qiong Jia