
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 04 July 2024
Sec. Ophthalmology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1351013
This article is part of the Research Topic Dry Eye Disease Syndrome - Volume II View all 8 articles
Purpose: The purpose of this study is to characterize and discuss the difference between software-detected non-invasive tear break-up time (NIBUT) and the traditional clinical method of fluorescein break-up time (FBUT).
Methods: Tear interferometry with the KOWA DR-1α (Kowa, Japan) and a standardized comprehensive ocular surface/tear evaluation were performed in 307 eyes. Software-detected NIBUT in the KOWA DR-1α images and the investigator-detected FBUT were compared.
Results: Software-detected NIBUT was significantly shorter than investigator-measured FBUT. NIBUT was 3.1 ± 2.5 s (mean ± SD), whereas FBUT was 4.8 ± 3.0 s. This difference was due to three different patterns or conditions: a spot break immediately after eyelid opening, moderate to severe keratitis sicca, and epithelial basement membrane corneal dystrophy (EBMD). In these cases, rapid tear film disruption was not captured by FBUT. A spot break immediately after eye opening that rapidly disappears was observed with conjunctivochalasis. This type of break-up may be difficult to detect using fluorescein because the human eye cannot catch such rapid blinks or post-blink events. In the second group with severe corneal epithelial disease, break-up may occur over the entire corneal surface upon eye opening, and distinct fluorescein tear break-up may not be identified because of poor dye dilution or spread over the corneal surface, whereas the non-invasive break-up is not solution-dependent, and the software can detect a distinct appearance. In the third group with EBMD, it is possible that focal break-up in the fluorescein pattern over the epithelial elevations, which might be missed visually, can be detected by software in video images.
Conclusion: We found that software-detected NIBUT is more sensitive in detecting tear break-up, can identify certain tear film disruptions that are missed by traditional FBUT, and may be more useful in distinguishing certain tear disorders.
Tear instability is the defining feature of dry eye disease (1). Traditionally, this has been visually detected as discontinuities in the fluorescein-stained tear film, termed fluorescein break-up time (FBUT). Non-invasive methods have been developed to detect break-up in an image (2). The KOWA DR-1α uses interferometry to image the tear lipid layer. Break-up in these interferometric images can be detected visually, and software has been developed and fine-tuned to detect and measure break-up (3). Dry eye is a heterogeneous disease that includes conditions with reduced tear volume and conditions with adequate tear volume that have alterations of the corneal or conjunctival surfaces, such as corneal epithelial basement membrane disease (EBMD) or conjunctivochalasis, which can mechanically disrupt tear distribution and stability (4). In eyes with diffuse EBMD, the initial site of fluorescein tear break-up may be missed, or tear break-up may occur simultaneously in several areas. Furthermore, in eyes with severe aqueous deficiency, instilled fluorescein dye may not adequately spread or mix with the tear film to allow proper visualization. Additionally, fluorescein may self-quench due to poor dilution (5). Consequently, FBUT may be difficult to evaluate or may not be accurately detected in these conditions. The purpose of this study is to compare the utility of software-detected non-invasive tear break-up in KOWA DR-1α interferometry images to FBUT measurements for a variety of tear disorders, with particular attention to these conditions.
This study was approved by the Baylor College of Medicine Institutional Review Board (IRB; Protocol Number H-51925), and all research adhered to the tenets of the Declaration of Helsinki. A retrospective chart review was conducted on all patients who received a comprehensive ocular surface examination for dry eye at the Alkek Eye Center from 2019 to 2022. Patients who had keratoneuralgia or non-tear film-related eye discomfort were excluded from the study.
All patients underwent a standard panel of tear film and ocular surface tests in the following order: a Symptom Assessment in Dry Eye (SANDE) symptom questionnaire, interferometric analysis of tear stability with the KOWA DR-1α, optical coherence tomography measurement of tear meniscus height (Avanti, Optovue, CA), biomicroscopic examination, fluorescein tear break-up time (FBUT), cornea fluorescein staining, and conjunctival lissamine green staining. These tests were performed according to previously reported methods (6). The severity of the ocular surface disease was graded 0–3 using previously reported severity criteria (7, 8). Dry eye was classified as aqueous deficiency, meibomian gland disease, conjunctivochalasis, or other categories based on previously published criteria (6, 9).
FBUT was measured by applying a drop of fluorescein dye to the lower tarsal conjunctiva using a fluorescein strip (BioGlo, HUB, Rancho Cucamonga, CA) wetted with a drop of preservative-free saline (Addipak, Teleflex, Research Triangle Park, NC). The patient was instructed to blink twice to distribute the fluorescein, then blink and keep the eye open while the time elapsed from the last blink to the appearance of the first break in the continuous layer of fluorescein, observed using a slit-lamp (Haag-Streit, Haag Streit, Koeniz, Switzerland) under cobalt blue light, was measured as the FBUT using a stopwatch.
The KOWA DR-1α instrument was calibrated daily using a standardized procedure. The light intensity control knob was set to the 10 o’clock position and the observation area switching knob was set to wide. The examination was performed for 30 s in each eye, and the video data were saved.
For a software analysis of NIBUT, an image classification model was developed to detect tear break-up non-invasively (3) by modifying the ResNet50 model. It was pre-trained on the ImageNet dataset to build a convolutional neural network model for detecting the characteristics of tear interferometric images by performing transfer learning on images extracted from videos recorded by the KOWA DR-1α. The model can detect three classes of tear break-up based on their shape: area break, spot break, and line break.
The software used in the analysis of the KOWA DR-1α was also used to detect inter-blink intervals and tear break-up. NIBUT using the software was measured as the time elapsed between the last blink and the detection of the first break-up. In our previous study, it was verified that there was a good correlation between investigator-visually detected and software-detected tear break-up time in the KOWA DR-1α interferometric fringe images (10). The investigator observed and checked the video frame-by-frame to identify the initial break in order to avoid overlooking and detecting errors.
Statistical tests were carried out using a statistical programming language R (version 3.6.1, The R Foundation for Statistical Computing, Vienna, Austria). Software-detected NIBUT and visually measured FBUT were compared using a linear mixed model where the random effect was patients.
A total of 307 examinations were performed on 204 patients over a 3-year period. Tear and ocular surface clinical data are shown in Table 1.
Software-detected NIBUT was significantly shorter than investigator-measured FBUT. NIBUT was 3.1 ± 2.5 s (mean ± SD), whereas FBUT was 4.8 ± 3.0 s (p < 0.001). This difference was due to three different patterns or conditions: spot break immediately after eyelid opening, moderate to severe keratitis sicca, and epithelial basement membrane corneal dystrophy (EBMD).
This type of break appeared as a bubble in the precorneal tear film. Spot break was detected by software in 55 eyes, and among these, 10 eyes with this type of break-up pattern had conjunctivochalasis (CCh; Table 2). Of these 10 eyes, we found that five eyes had a larger difference between FBUT and NIBUT (Table 3; Figure 1). In fluorescein break-up, a similar ratio of spot breaks to total breaks was noted in eyes with CCh (28%) compared to the ratio in all cases (31%). In contrast, software-detected NIBUT revealed that the ratio of spot breaks to total breaks was higher in CCh than in all cases (28 vs. 18%, respectively, Table 4). These data indicate that spot breaks are more likely to be detected by the software in eyes with CCh than other tear dysfunction conditions compared to FBUT.
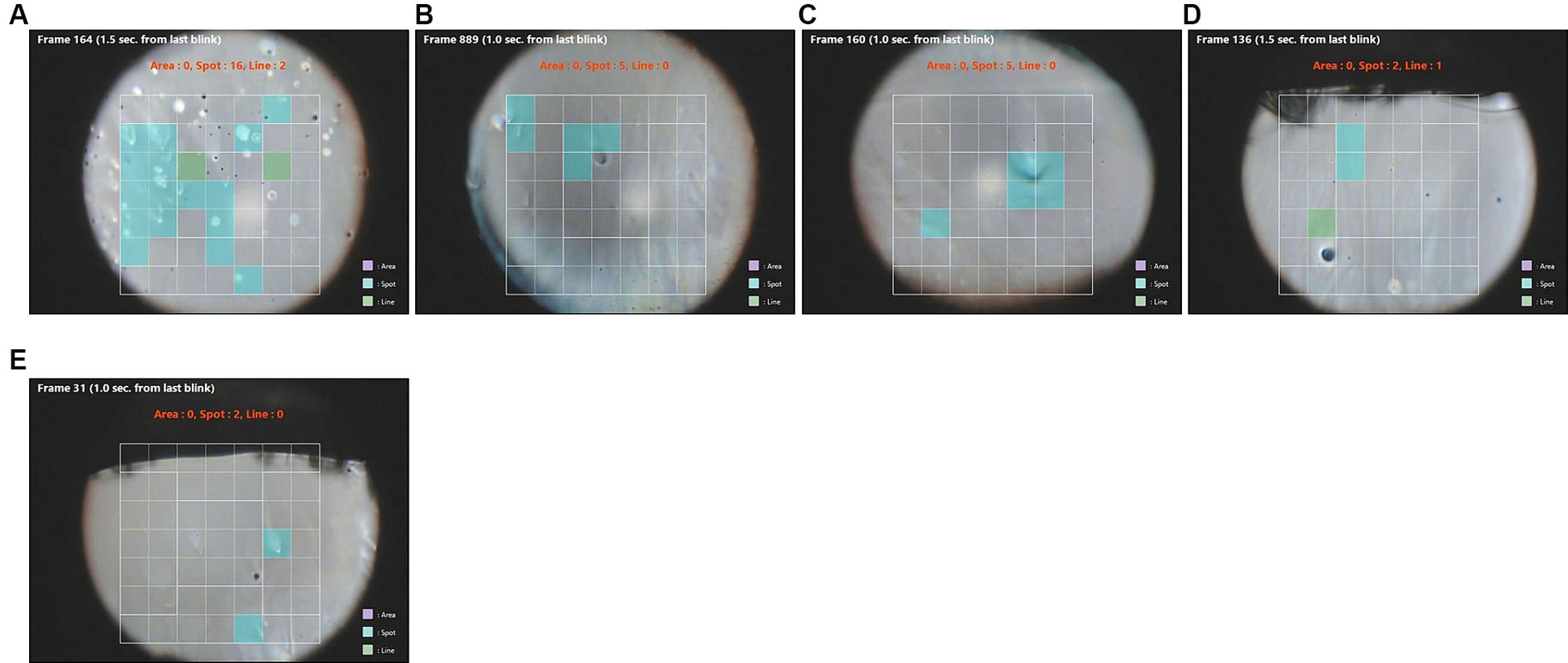
Figure 1. (A–E) Images of spot break-up after a blink (blue squares) in five different patients. NIBUT was more rapid than FBUT in these eyes (Table 2). Green squares are areas of line break-up.
In eyes with severe corneal fluorescein staining (scores >7), diffuse area break-up occurring over the entire corneal surface was detected in the interferometric images by software earlier than fluorescein break-up in seven eyes (Table 5; Figure 2). This group included eyes with low (<273 um) or elevated (>345 um) tear meniscus heights (Table 5). A higher ratio of area break was noted in eyes with corneal fluorescein staining scores of >7 overall compared to all cases (Table 6). Area breaks were detected in 77% (24/31) of eyes with corneal fluorescein staining scores >7, whereas they were detected in only 22% (68/307) of break-ups in all eyes.
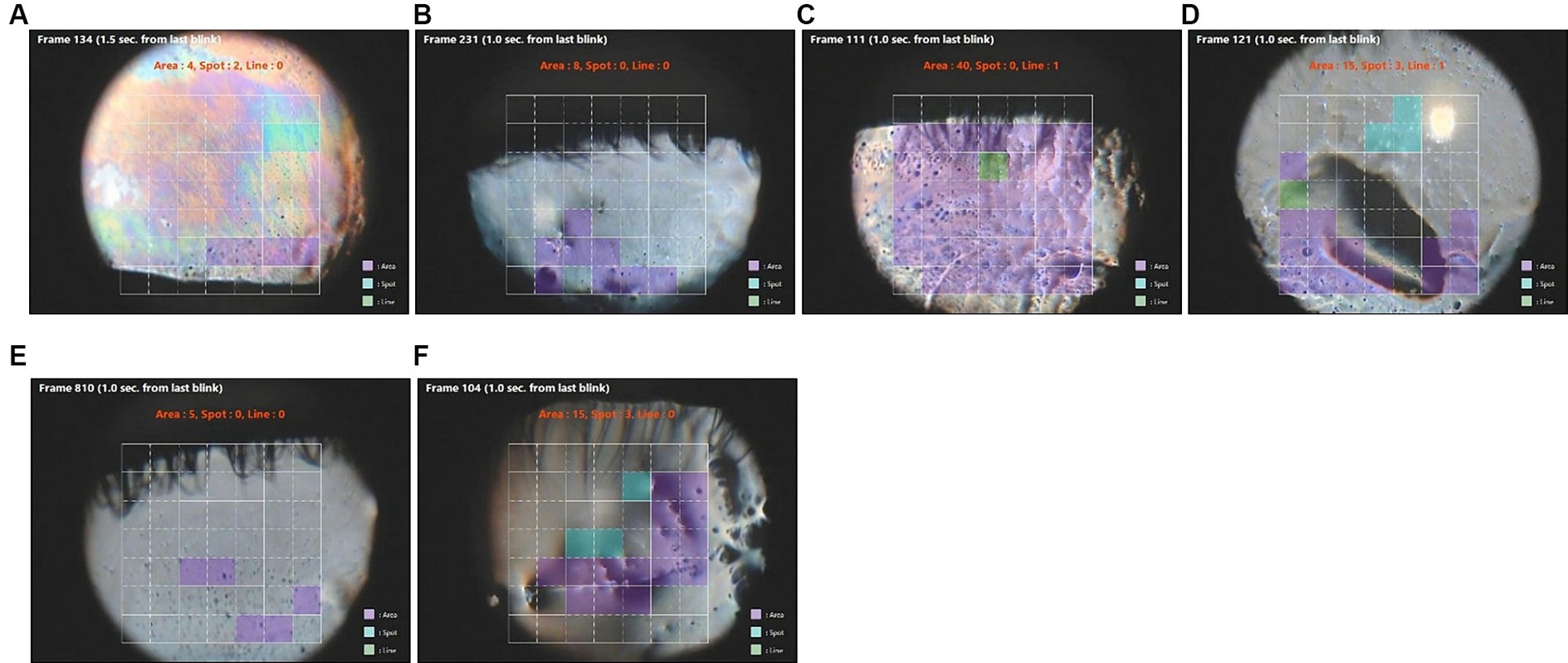
Figure 2. Images of rapid area tear break-up (purple squares) detected in video images in eyes with severe keratitis sicca (fluorescein staining scores >7). (A–F) correspond to the ID numbers in Table 5 (A = 8, B = 29, C = 69, D = 190, E = 231. F = 278). Green squares are areas of line break-up and blue squares are areas of spot break-up.
NIBUT in the KOWA DR-1α image was more rapid than FBUT in four eyes with epithelial basement membrane disease (EBMD) associated with either ATD or CCh (Table 7). This suggests that there is a tear break-up over these basement membrane deposits that can be software-detected but not visually with fluorescein. The basement membrane deposits were visible prior to the blink in three of these eyes (Table 7 and Figures 3A,C,E).

Figure 3. Images of tear break-up in eyes with epithelial basement membrane disease (EBMD). Green squares are areas of line break-up, blue squares are areas of spot break-up, and purple squares are areas of area break-up. (A) ID 100 R eye before blinking, (B) ID 100 R eye break-up detected in colored squares, (C) ID 100 L eye before blinking, (D) ID 100 L eye break-up detected in colored squares, (E) ID 114 R eye before blinking, (F) ID 114 R eye break-up detected in colored squares, (G) ID 114 L eye break-up detected in colored squares, (H) ID 212 R eye break-up detected in colored squares, and (I) ID 212 L break-up detected in colored squares.
This study evaluated the utility of measuring NIBUT using the KOWA DR-1α. Software-measured NIBUT was significantly faster than investigator-measured FBUT. Upon deeper analysis, the difference was found to be largely attributed to three conditions: CCh, severe keratitis sicca defined by high corneal fluorescein staining scores, and certain eyes with EBMD. These findings suggest that software may be able to detect discontinuities in interferometric images of the tear film earlier than they can be detected visually in the fluorescein-stained tear film. This suggests that software-detected NIBUT may be more sensitive in identifying an unstable tear film.
Different patterns of fluorescein tear break-up have been identified, and certain patterns have a reported association with specific disorders (e.g., area break-up with severe aqueous deficiency) (11). Our findings with the KOWA DR-1α software detection would support this concept. Rapid area break-up was found with increased frequency in eyes with severe keratitis sicca, and spot break-up immediately after eye opening was associated with CCh.
A previous study using the KOWA DR-1α has reported that NIBUT was longer than FBUT (12). In this study, the reasons why NIBUT is more sensitive than the fluorescein method in detecting tear break in these conditions remain to be determined, but it could be related to factors that have been modeled to affect fluorescein tear break-up, including tear volume, thickness, osmolarity, and tear spread (13). Quenching of fluorescence has been observed in eyes with low tear volume (5). Changes in the corneal epithelium in severe KCS, such as reduced production of membrane mucins and increased expression of cornified envelope precursors, may affect tear diffusion and adherence of tear fluid to the corneal epithelium (14). Lid parallel folds in conjunctivochalasis mechanically disrupt the tear meniscus, which can sequester tears and impede the mixing of fluorescein. These factors may interfere with the detection of fluorescein break-up (9, 15). Additionally, the rapid spot break in CCh occurring as the eyelid rises during a blink may have been overlooked in the fluorescein method. This is because spot breaks might be small (less than a few percent of the observed area) or are likely to disappear and/or move to various portions of the cornea during the upward movement of aqueous tear fluid after the eye opening, which can be overlooked. Evaluation of the tear lipid layer in interferometric images may be more sensitive in detecting tear break-up than fluorescein in some eyes with EBMD. It is possible that fluorescein does not break-up over these deposits, but software can detect a distinct interferometric appearance. It is interesting that all of the eyes with EBMD that had more rapid NIBUT had associated CCh or ATD.
In summary, software-detected non-invasive tear break-up appears to be more sensitive in detecting tear break-up in certain conditions. Software-detected NIBUT may prove to be valuable in the diagnostic classification of tear disorders, and the ability to detect break-up time and patterns may improve when the software is trained with a larger library of images containing a variety of tear dysfunction conditions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Baylor College of Medicine Institutional Review Board (IRB; Protocol number H-51925). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective data review.
SP: Formal analysis, Investigation, Writing – original draft. YK: Formal analysis, Software, Writing – original draft. ST: Formal analysis, Software, Writing – review & editing. TK: Formal analysis, Software, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported through an unrestricted grant from Research to Prevent Blindness, New York, NY (SP), The Hamill Foundation, Houston, TX (SP), and the Sid W. Richardson Foundation, Fort Worth, TX (SP).
SP is a paid consultant for Kowa. YK, ST, and TK are employees of Kowa.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tsubota, K, Pflugfelder, SC, Liu, Z, Baudouin, C, Kim, HM, Messmer, EM, et al. Defining dry eye from a clinical perspective. Int J Mol Sci. (2020) 21:9271. doi: 10.3390/ijms21239271
2. Wolffsohn, JS, Arita, R, Chalmers, R, Djalilian, A, Dogru, M, Dumbleton, K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. (2017) 15:539–74. doi: 10.1016/j.jtos.2017.05.001
3. Kikukawa, Y, Tanaka, S, Kosugi, T, and Pflugfelder, SC. Non-invasive and objective tear film breakup detection on interference color images using convolutional neural networks. PLoS One. (2023) 18:e0282973. doi: 10.1371/journal.pone.0282973
4. Balci, O . Clinical characteristics of patients with conjunctivochalasis. Clinic ophthalmol. (2014) 8:1655–60. doi: 10.2147/opth.S61851
5. Paugh, JR, Tse, J, Nguyen, T, Sasai, A, Chen, E, de Jesus, MT, et al. Efficacy of the fluorescein tear breakup time test in dry eye. Cornea. (2020) 39:92–8. doi: 10.1097/ico.0000000000002148
6. Tung, CI, Perin, AF, Gumus, K, and Pflugfelder, SC. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am J Ophthalmol. (2014) 157:301–310.e1. doi: 10.1016/j.ajo.2013.09.024
7. Pflugfelder, SC, Geerling, G, Kinoshita, S, Lemp, MA, McCulley, JP, Nelson, D, et al. Management and therapy of dry eye disease: report of the management and therapy Subcommittee of the International dry eye WorkShop (2007). Ocul Surf. (2007) 5:163–78. doi: 10.1016/s1542-0124(12)70085-x
8. Rao, K, Farley, WJ, and Pflugfelder, SC. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Invest Ophthalmol Vis Sci. (2010) 51:844–9. doi: 10.1167/iovs.09-3875
9. Meller, D, and Tseng, SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. (1998) 43:225–32. doi: 10.1016/S0039-6257(98)00037-X
10. Pflugfelder, S, Nakhleh, L, Kikukawa, Y, Tanaka, S, and Kosugi, T. Non-invasive tear break-up detection with the Kowa DR-1α and its relationship to dry eye clinical severity. Int J Mol Sci. (2022) 23:14774. doi: 10.3390/ijms232314774
11. Yokoi, N, and Georgiev, GA. Tear film-oriented diagnosis and tear film-oriented therapy for dry eye based on tear film dynamics. Invest Ophthalmol Vis Sci. (2018) 59:DES13–22. doi: 10.1167/iovs.17-23700
12. Itokawa, T, Suzuki, T, Koh, S, and Hori, Y. Evaluating the differences between fluorescein tear break-up time and noninvasive measurement techniques. Eye Contact Lens. (2023) 49:104–9. doi: 10.1097/icl.0000000000000966
13. Braun, RJ, King-Smith, PE, Begley, CG, Li, L, and Gewecke, NR. Dynamics and function of the tear film in relation to the blink cycle. Prog Retin Eye Res. (2015) 45:132–64. doi: 10.1016/j.preteyeres.2014.11.001
14. Pflugfelder, SC, and Stern, ME. The cornea in keratoconjunctivitis sicca. Exp Eye Res. (2020) 201:108295. doi: 10.1016/j.exer.2020.108295
Keywords: dry eye, tear stability, tear break-up time, interferometry, artificial intelligence
Citation: Pflugfelder SC, Kikukawa Y, Tanaka S and Kosugi T (2024) The utility of software-detected non-invasive tear break-up in comparison to fluorescein tear break-up measurements. Front. Med. 11:1351013. doi: 10.3389/fmed.2024.1351013
Received: 06 December 2023; Accepted: 18 June 2024;
Published: 04 July 2024.
Edited by:
Yonathan Garfias, National Autonomous University of Mexico, MexicoReviewed by:
Takashi Itokawa, Toho University Omori Medical Center, JapanCopyright © 2024 Pflugfelder, Kikukawa, Tanaka and Kosugi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen C. Pflugfelder, c3RldmVucEBiY20uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.