
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 12 April 2024
Sec. Geriatric Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1350064
This article is part of the Research Topic Risk Factors and Prevention Strategy in Intervertebral Disc Herniation View all 7 articles
Objective: This study aimed to compare the clinical efficacy and safety of reduction vs. arthrodesis in situ with transforaminal lumbar interbody fusion (TLIF) for low-grade lumbar spondylolisthesis.
Study design: Systematic review and meta-analysis.
Methods: A comprehensive literature search was implemented in PubMed, Embase, and Cochrane Library databases. Randomized or non-randomized controlled trials that were published until July 2023 that compared reduction vs. arthrodesis in situ techniques with minimally invasive or open-TLIF for low-grade spondylolisthesis were selected. The quality of the included studies was evaluated by the Newcastle–Ottawa Scale (NOS). Data were extracted according to the predefined outcome measures, including operation time and intraoperative blood loss; short- and long-time follow-up of visual analog scale (VAS) back pain (VAS-BP) and Oswestry Disability Index (ODI); slippage and segmental lordosis; and the complication and fusion rate.
Results: Five studies (n = 495 patients) were finally included. All of them were retrospective cohort studies with Evidence Level II. The pooled data revealed that both techniques had similar patient-reported outcomes (VAS, ODI, and good and excellent rate) during short- and long-term follow-up. In addition, no significant differences were observed in the fusion and complication rates. However, although the reduction group did achieve better slippage correction, it was associated with increased operation time and intraoperative blood loss compared with the in situ arthrodesis group.
Conclusions: Based on the available evidence, intraoperative reduction does not result in better clinical outcomes in low-grade spondylolisthesis after minimally invasive or open-TLIF, and the in situ arthrodesis technique could be an alternative.
Lumbar spondylolisthesis is defined as the forward slippage of the superior vertebrae relative to the inferior vertebrae (1). In addition, according to the degree of slippage assessed by radiology, Meyerding proposed that 1%−25% slippage represents Grade 1, 26%−50% represents Grade 2, 51%−75% represents Grade 3, and 76%−100% slippage represents Grade 4; low-grade spondylolisthesis represents from 1% up to 50% slippage (2). In addition, based on the etiology of spondylolisthesis, Wiltse classified it into five types, namely, congenital, isthmic, degenerative, traumatic, and pathologic (3). For patients with symptoms of degenerative or isthmic spondylolisthesis, such as persistent mechanical pain or low back pain and/or overt neurological deficits, surgical management has been confirmed to have a greater advantage than conservative treatment strategies (4–6). To date, various surgical techniques have been performed to deal with symptoms of spondylolisthesis. Furthermore, the main aim is to stabilize the spinal segment and decompress the neural elements (7–9). In 1982, Harms first described the transforaminal lumbar interbody fusion (TLIF) technique (10). Since then, it has gained popularity as an effective management strategy for lumbar spondylolisthesis (11). TLIF reduces the retraction of nerve roots and the thecal sac and preserves the structural integrity of the posterior column (12, 13). The clinical indications for TLIF surgery are as follows: (1) Grade 1 or 2 degenerative or isthmus lumbar spondylolisthesis without accompanying neurological symptoms or with only unilateral neurological symptoms; (2) discogenic lower back pain with ineffective conservative treatment; (3) intervertebral disc herniation accompanied by lumbar instability (including extreme lateral disc herniation); (4) patients with single-segment lumbar disc herniation who require revision after surgery; and (5) formation of false joints between vertebrae. Moreover, with the development of operative instruments, the TLIF technique has developed into a minimally invasive procedure, which was first described by Foley in 2002 (14). With the potential advantages of smaller incisions, less soft tissue trauma, and faster recovery, the minimally invasive TLIF (MIS-TLIF) technique has become more and more popular (15, 16). In addition, the indications for MIS-TLIF are also constantly expanding, while the contraindications are comparatively shrinking. However, no consensus has been reached on the correlation between reduction and clinical outcomes in patients with low-grade spondylolisthesis. Does intraoperative reduction result in better outcomes after TLIF? This study was performed to estimate the clinical efficacy and safety between reduction and arthrodesis in situ with MIS/open-TLIF for low-grade spondylolisthesis.
The systematic review and meta-analysis was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). A comprehensive literature retrieval was implemented in PubMed, Embase, and Cochrane Library databases. Randomized or non-randomized controlled trials published up to July 2023, which compared reduction vs. arthrodesis in situ with MIS/open-TLIF for low-grade spondylolisthesis were achieved. For maximum sensitivity of the search strategy, the following terms were used: (1) transforaminal lumbar interbody fusion OR TLIF OR minimally invasive transforaminal lumbar interbody fusion OR MIS-TLIF; (2) reduction OR without reduction OR in situ; and (3) spondylolisthesis; and (4) (1), (2), AND (3). The retrieval was limited to studies published in English. Two reviewers screened the titles and abstracts of all identified studies independently, and full-text copies of relevant studies were obtained. Reference lists of the retrieved studies and previous reviews were manually checked to identify additional potentially relevant research studies. Differences between them were resolved by discussion with a third reviewer.
The inclusion criteria were as follows:
(1) Study design: randomized or non-randomized cohort trial; (2) patients: low-grade spondylolisthesis with mechanical low back pain and/or radiculopathy; (3) intervention measures: reduction vs. arthrodesis in situ with TLIF/MIS-TLIF and pedicular screw fixation; and (4) predefined outcome measures: operation time and intraoperative blood loss; short- and long-time follow-up of VAS back pain (VAS-BP) and Oswestry Disability Index (ODI); slippage and segmental lordosis; and the complication and fusion rate.
The exclusion criteria were as follows:
(1) patients suffering from spinal trauma, infectious diseases, or tumors; (2) those who opted for/were eligible for other operative procedures such as posterolateral fusion (PLF) and posterior lumbar intervertebral fusion (PLIF); (3) those who have experienced previous lumbar surgery; (4) follow-up time of < 1 year; and (5) repeated studies, animal research studies, non-comparative studies, biomechanical or cadaveric studies, case reports, and reviews.
We extracted data from each study based on the following terms: (1) first author and year of publication; (2) study design; (3) country; (4) patient demographics; (5) surgical procedure; (6) sample size; (7) type and grade of spondylolisthesis; (10) follow-up time; (11) predefined outcome measures: operation time and intraoperative blood loss; short- and long-term follow-up of VAS-BP and ODI; slippage and segmental lordosis; good and excellent rate; and the complication and fusion rate. When the same participants were included in several publications, we retained only the study with the maximum sample size to avoid duplication of dates. In addition, we defined a follow-up that ranged from 3 to 6 months after surgery as a short-term follow-up and a follow-up of at least 2 years as a long-term follow-up.
All statistical analyses were performed using Review Manager Version 5.3 (Cochrane Collaboration), and heterogeneity among studies was calculated using the chi-square test and quantified via calculating I2 statistic. For the pooled effects, weighted mean difference (WMD) was calculated for continuous variables according to the consistency of measurement units, while relative risk (RR) was used for dichotomous variables. In the present study, the continuous variables were summarized by WMD and 95% confidence intervals (95% CIs), while the dichotomous variables were represented using RR and 95% CIs. For I2 < 50%, we preferred the fixed-effects model; otherwise, the random-effects model was used. All P-values were two-sided, and a P-value < 0.05 was considered statistically significant.
The flow diagram Figure 1 shows the filtering process for relative studies. A total of 347 studies were initially obtained by electronic and manual searching. According to the inclusion and exclusion criteria that were set previously, five articles (18–22) were finally included for quantitative analysis.
Five retrospective cohort studies (18–22) included 495 patients (278 in the reduction group and 217 in the arthrodesis in situ group) with low-grade lumbar spondylolisthesis. The baseline characteristics of patients in each study are shown in Table 1. The summary of outcome measures for the two interventions is presented in Table 2. Their quality was assessed according to the Newcastle–Ottawa Scale (NOS) (23). In this scale, scores range from 0 (lowest quality) to 9 (highest quality), and the range 7–9 represents good or high quality, while the range 0–3 represents poor or low quality. Two investigators graded the risk of bias of included studies independently. In addition, all studies were rated with a total score of more than 5 (Table 3), signifying relatively moderate to high quality.
Five studies (n = 495 patients; 278 patients underwent the reduction procedure and 217 were included in the arthrodesis in situ group) reported the operation time in the form of mean ± standard deviation. The pooled data showed that the reduction group had a longer operation time than the arthrodesis in situ group [P = 0.02 < 0.05, WMD 10.41 (1.45, 19.37); Figure 2]. χ2 tests showed no statistical evidence of heterogeneity (P = 0.55 > 0.05, I2 = 0%).
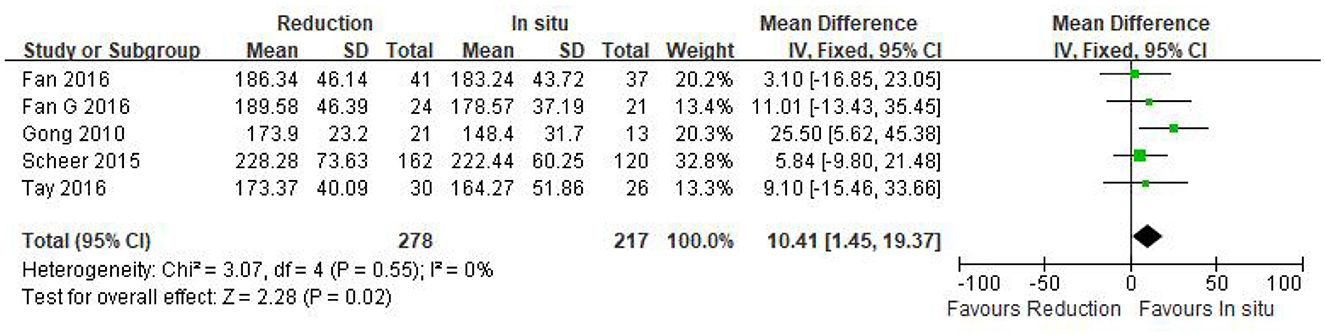
Figure 2. Weighted mean difference of the operation time between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV inverse variance.
Intraoperative blood loss was reported in five eligible studies (n = 495 patients; 278 in the reduction group and 217 in the arthrodesis in situ group). A forest plot of the pooled results indicated more intraoperative blood loss in the reduction group than in the arthrodesis in situ group [P = 0.03 < 0.05, WMD 44.17 (3.86, 84.48); Figure 3]. However, significant heterogeneity was detected by the chi-squared test (P < 0.05, I2 = 78%).
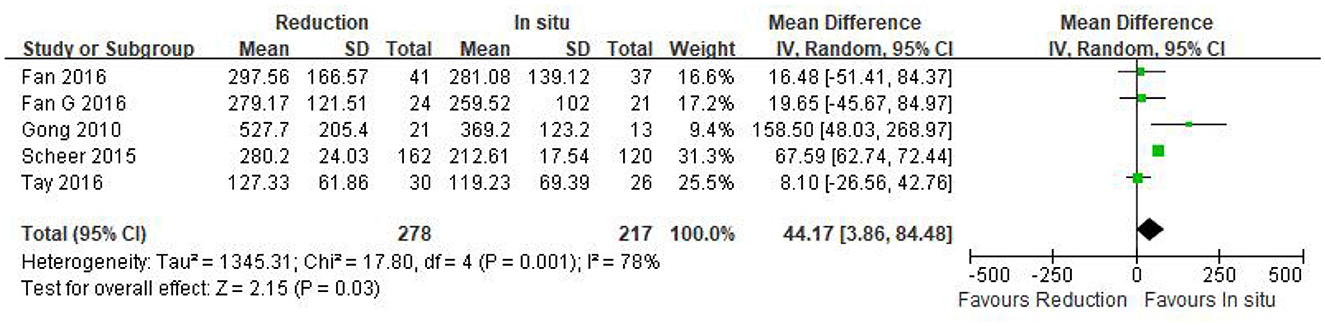
Figure 3. Weighted mean difference of intraoperative blood loss between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV, inverse variance.
Three eligible studies (n = 157 patients; 86 in the reduction group and 71 in the arthrodesis in situ group) estimated the slippage degree. In addition, no statistically significant difference was observed in preoperative slippage between both groups [P = 0.86 > 0.05, WMD −0.28 (−3.39, 2.83), heterogeneity: Tau2 = 2.38, Chi2 = 2.91, df = 2, P = 0.23, I2 = 31%; Figure 4].
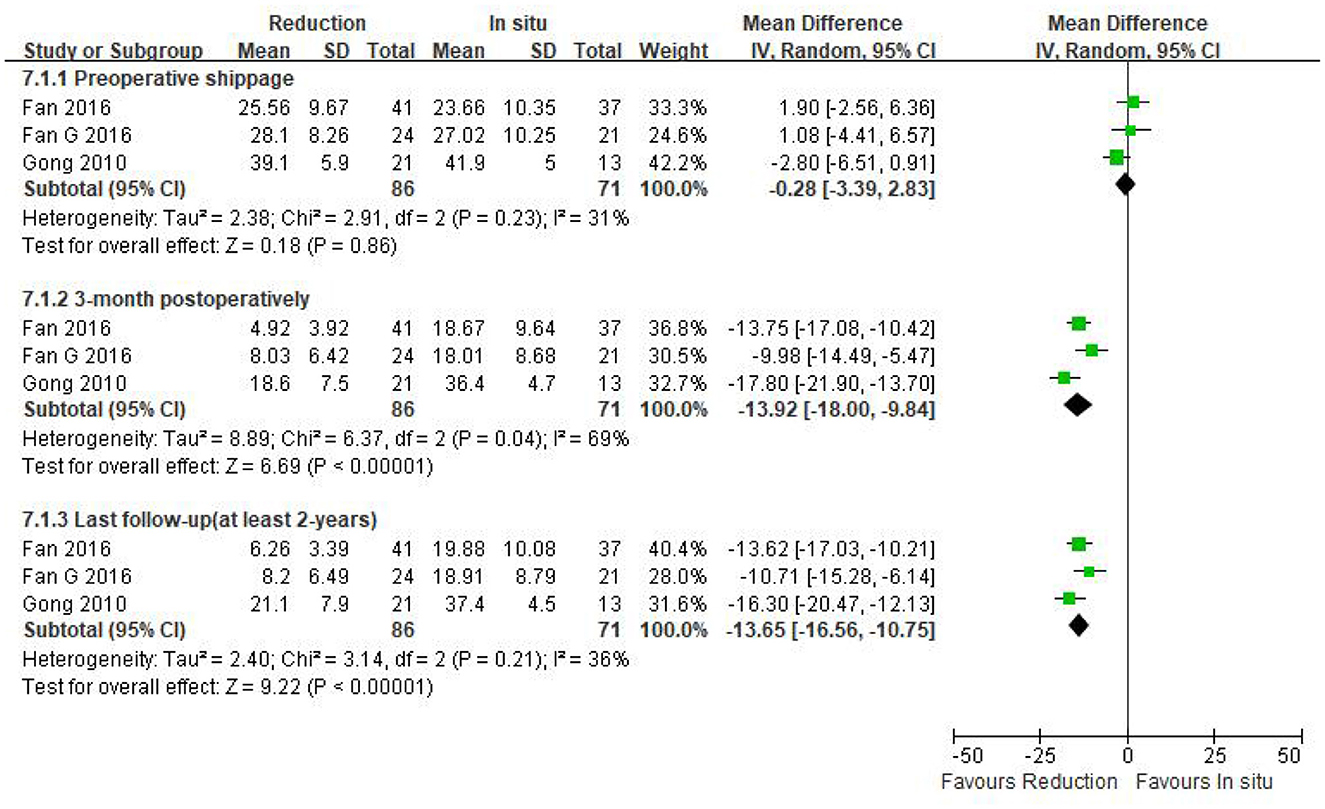
Figure 4. Weighted mean difference of preoperative slippage and postoperative slippage at short- and long-term follow-up between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV, inverse variance.
The same three studies as above (n = 157 patients; 86 patients in the reduction group and 71 in the arthrodesis in situ group) reported postoperative slippage at short- and long-term follow-up. It is clear that the reduction group was associated with better slippage correction than the arthrodesis in situ group during short- and long-term follow-up [P < 0.05, WMD −13.92 (−18.00, −9.84), heterogeneity: Tau2 = 8.89, Chi2 = 6.37, df = 2, P = 0.04, I2 = 69%; P < 0.05, WMD −13.65 (−16.56, −10.75), heterogeneity: Tau2 = 2.40, Chi2 = 3.14, df = 2, P = 0.21, I2 = 36%; respectively; Figure 4].
Data regarding preoperative VAS-BP were available in four studies (n = 213 patients; 116 patients underwent intraoperative reduction and 97 underwent arthrodesis in situ). No statistically significant difference was observed in the preoperative VAS-BP score between both groups [P = 0.78 > 0.05, WMD 0.07 (−0.39, 0.52); heterogeneity: Tau2 = 0.10, Chi2 = 7.44, df = 3, P = 0.06, I2 = 60%; Figure 5].
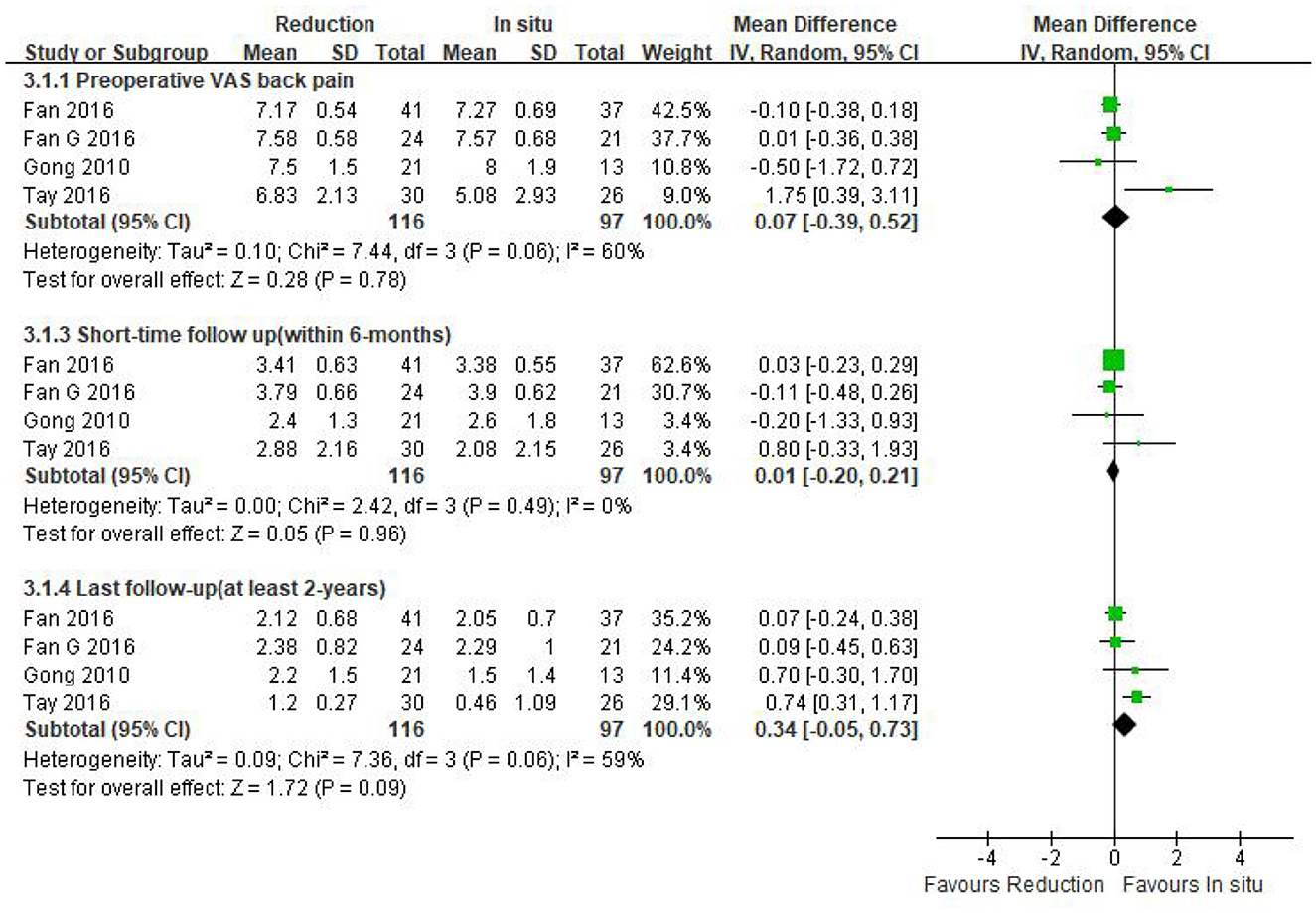
Figure 5. Weighted mean difference of preoperative VAS-BP and postoperative VAS-BP during short- and long-term follow-up between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV, inverse variance.
Four studies mentioned above (n = 213 patients; 116 in the reduction group and 97 in the arthrodesis in situ group) provided the postoperative VAS-BP score during short- and long-term follow-up. The pooled result indicated that no statistical difference was detected in either short-term or long-term follow-up of VAS-BP between the two groups [P = 0.96 > 0.05, WMD 0.01 (−0.20, 0.21), heterogeneity: Tau2 = 0.00, Chi2 = 2.42, df = 3, P = 0.49, I2 = 0%; P = 0.09 > 0.05, WMD 0.34 (−0.05, 0.73), heterogeneity: Tau2 = 0.09, Chi2 = 7.36, df = 3, P = 0.06, I2 = 59%; respectively; Figure 5].
The above information indicated that the patient-reported outcomes of preoperative and postoperative back pain were similar in both groups.
Preoperative ODI was reported in four eligible studies (n = 213 patients; 116 patients underwent intraoperative reduction and 97 underwent arthrodesis in situ). No significant difference was observed in the preoperative ODI between the two groups [P = 0.60 > 0.05, WMD −0.96 (−4.57, 2.64); Figure 6]. Moreover, no statistical heterogeneity was detected by the chi-square test (P = 0.82 > 0.05, I2 = 0%).
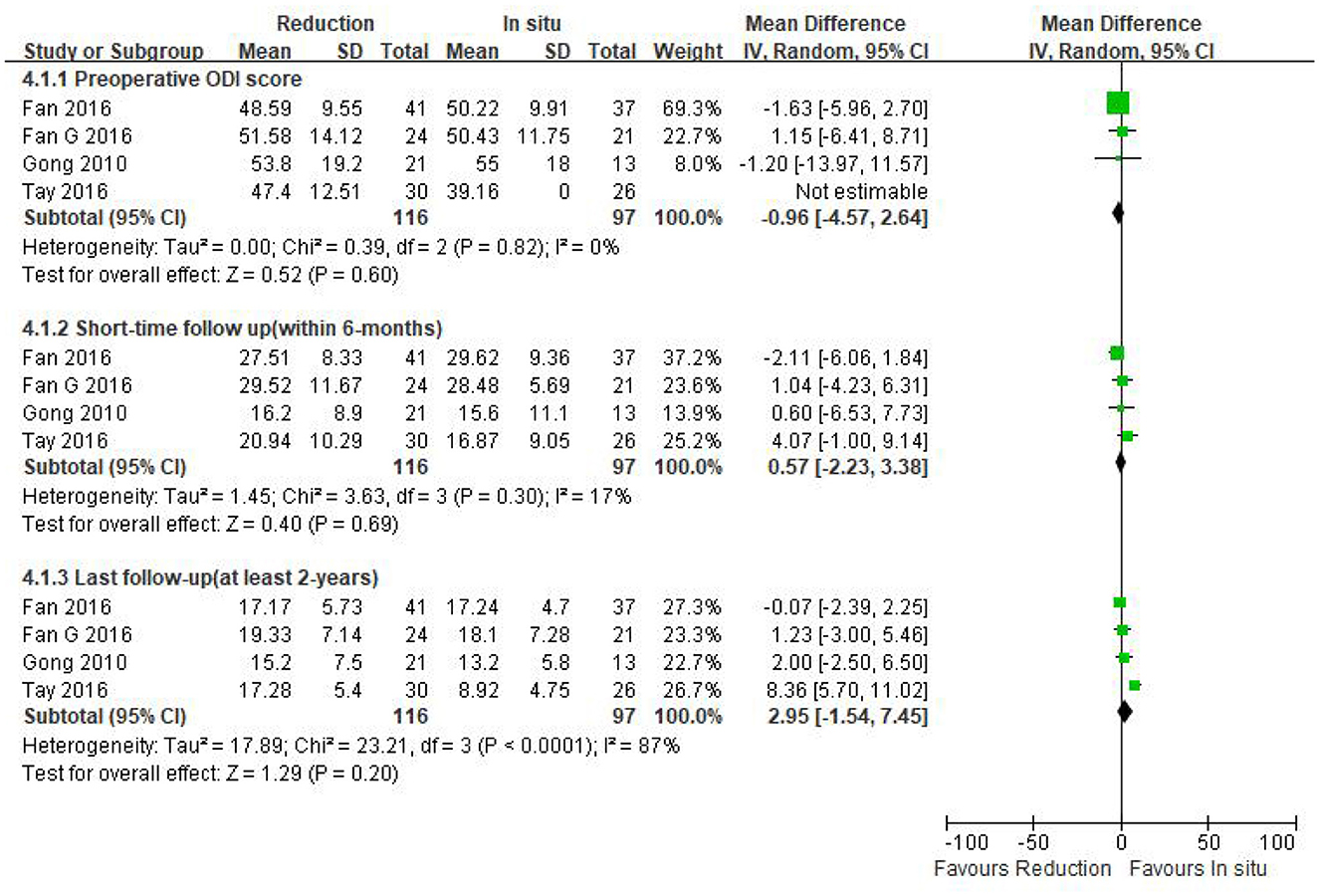
Figure 6. Weighted mean difference of preoperative ODI and postoperative ODI during short- and long-term follow-up between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV, inverse variance.
The same four studies mentioned above (n = 213 patients; 116 in the reduction group and 97 in the arthrodesis in situ group) estimated the short- and long-term follow-up of the ODI. In addition, no statistical difference was detected in the postoperative ODI during short- and long-term follow-up between the two groups [P = 0.69 > 0.05, WMD 0.57 (−2.23, 3.38), heterogeneity: Tau2 = 1.45, Chi2 = 3.63, df = 3, P = 0.30, I2 = 17%; P = 0.20 > 0.05, WMD 2.95 (−1.54, 7.45), heterogeneity: Tau2 = 17.89, Chi2 = 23.21, df = 3, P < 0.05, I2 = 87%; respectively; Figure 6].
In summary, the above result indicated that the patient-reported outcomes of both preoperative and postoperative function were similar in both groups.
Four eligible studies (n = 213 patients; 116 in the reduction group and 97 in the arthrodesis in situ group) reported good and excellent rates based on the MacNab criteria (24). The pooled plot shows that no statistical difference was observed in the good and excellent rate between the two groups [P = 0.72 > 0.05, RR 0.98 (0.89, 1.08); Figure 7]. χ2 tests showed no statistical evidence of heterogeneity (P = 0.89 > 0.05, I2 = 0%).
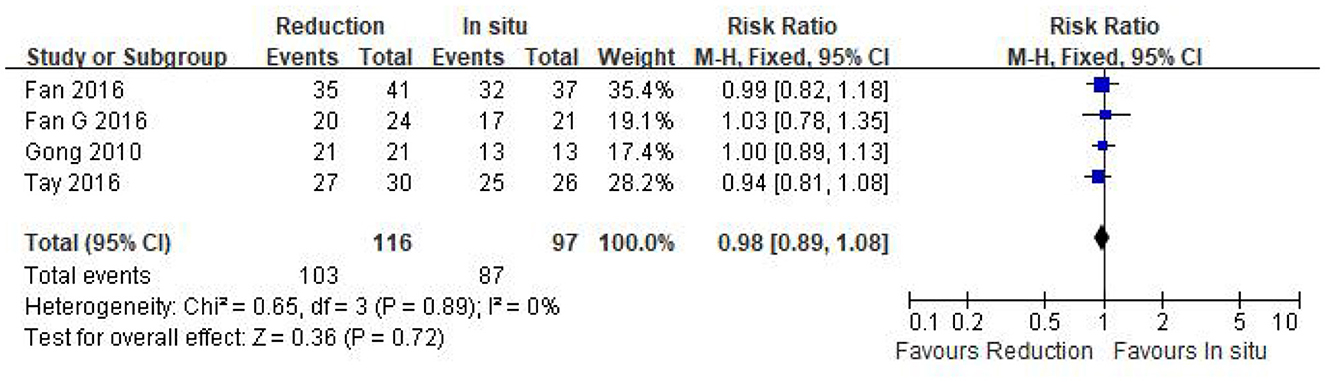
Figure 7. Relative ratio of the good and excellent rate between the reduction group and the arthrodesis in situ group. CI, confidence interval; M-H Mantel–Haenszel.
Only two studies (n = 90 patients; 51 in the reduction group and 39 in the arthrodesis in situ group) estimated segmental lordosis before surgery and at the last follow-up. No statistically significant difference was observed in preoperative and the last follow-up of segmental lordosis between the two groups [P = 0.11 > 0.05, WMD 2.87 (−0.63, 6.36); P = 0.51 > 0.05, WMD 0.99 (−1.92, 3.89); respectively; Figure 8]. In addition, no statistical heterogeneity was detected by the chi-squared test (P = 0.99 > 0.05, I2 = 0%; P = 0.66 > 0.05, I2 = 0%; respectively).
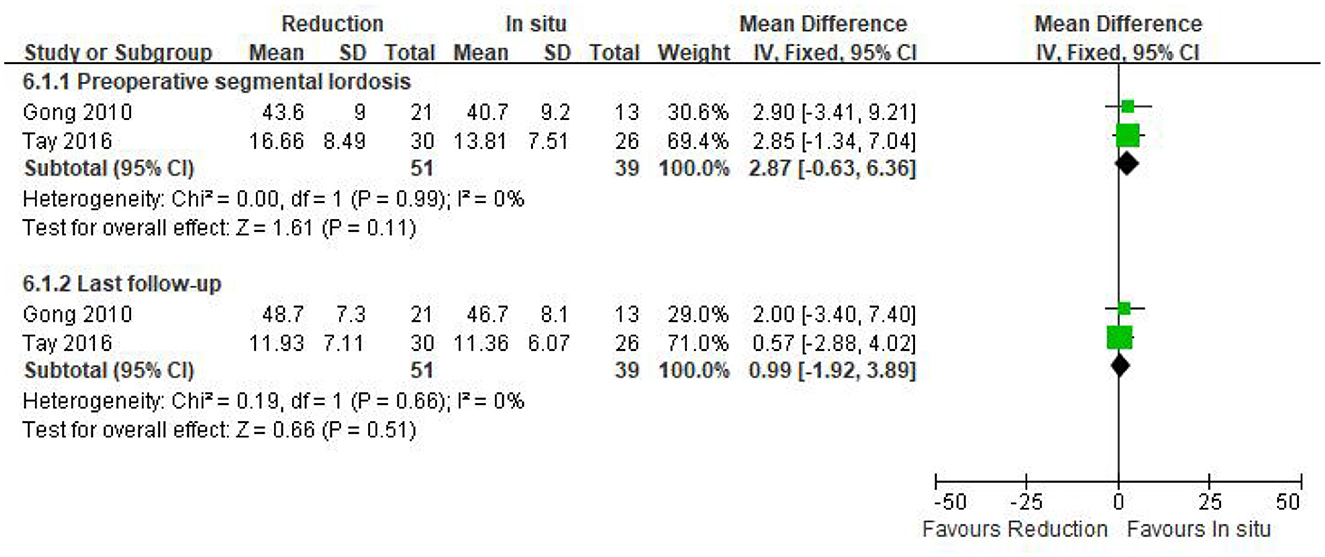
Figure 8. Weighted mean difference of preoperative and last follow-up segmental lordosis between the reduction group and the arthrodesis in situ group. SD, standard deviation; CI, confidence interval; IV, inverse variance.
Data regarding the fusion rate were available in all of the five studies (n = 495 patients; 278 patients underwent intraoperative reduction and 217 underwent arthrodesis in situ). No statistical difference was detected in the fusion rate between the two groups [P = 0.20 > 0.05, WMD 1.08 (0.96, 1.22); heterogeneity: Tau2 = 0.01, Chi2 = 14.38, df = 4, P < 0.05, I2 = 72%; Figure 9].
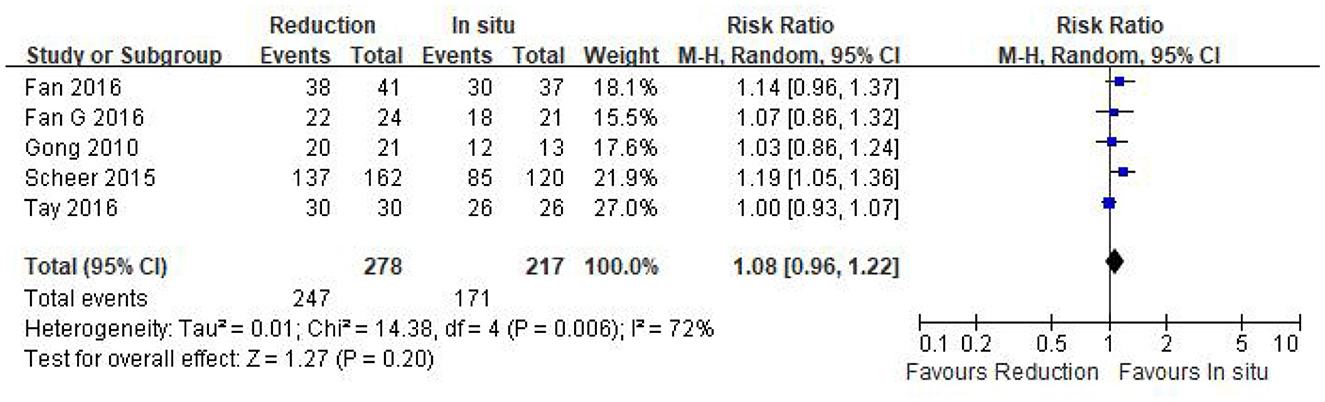
Figure 9. Relative ratio of the fusion rate between the reduction group and the arthrodesis in situ group. CI, confidence interval; M-H, Mantel–Haenszel.
The surgery-related complication rate was assessed in five eligible studies (n = 495 patients; 278 in the reduction group and 217 in the arthrodesis in situ group). The pooled result indicated that no statistical difference was observed in the surgery-related complication rate between the two groups [P = 0.84 > 0.05, RR 1.05 (0.66, 1.69); heterogeneity: Chi2 = 5.05, df = 4, P = 0.28, I2 = 21%; Figure 10].
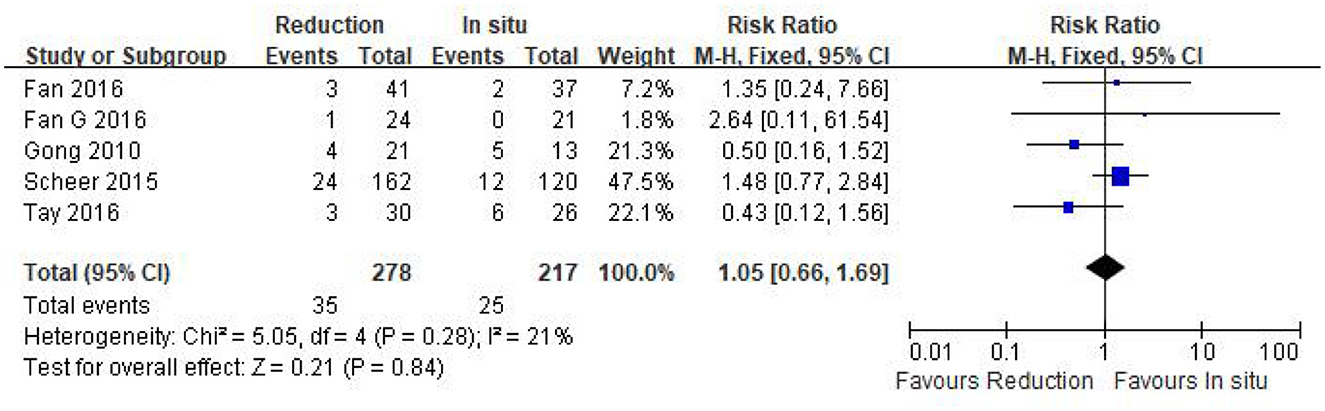
Figure 10. Relative ratio of the surgery-related complication rate between the reduction group and the arthrodesis in situ group. CI, confidence interval; M-H, Mantel–Haenszel.
The initial surgical strategy for lumbar spondylolisthesis was laminectomy, followed by lumbar interbody fusion. The implementation of fusion surgery can effectively improve the clinical prognosis in patients with symptomatic lumbar spondylolisthesis. Compared with PLIF, the TLIF technique reduces the retraction of the thecal sac and nerve roots and preserves the structural integrity of the posterior column, which reduces the risk of various complications, including neurological tissue damage. In addition, interbody fusion can maintain the height of the intervertebral space and restore the height of the intervertebral foramen, thereby improving or relieving the narrowing of the intervertebral foramen, and the normal anatomical curvature of the corresponding segment can be reconstructed to restore or maintain the overall physiological curvature of the spine. However, whether intentional reduction with TLIF is necessary for the surgical treatment of low-grade lumbar spondylolisthesis remains controversial. Only one meta-analysis study (25) has been performed on this subject, which concluded that no statistical difference was observed in terms of operation time, estimated blood loss, patient-reported outcomes, and fusion rate and complication rate. However, in the aforementioned study, patients in one included study underwent posterolateral fusion (PLF) (26), patients in three studies underwent PLIF (27–29), and patients in the remaining three studies underwent MIS/open-TLIF (18, 21, 22). The uncontrolled confounding factors for surgical operations might exert an effect on their results. To our knowledge, several surgical techniques have been performed to treat symptomatic spondylolisthesis, which could not be treated by conservative treatment strategies. Moreover, the advent of minimally invasive technologies for spine surgery led to the logical progression of open-TLIF to MIS-TLIF (30). The TLIF technique has been proven to be an effective management strategy for spondylolisthesis as it preserves the structural integrity of the posterior column and reduces the retraction of thecal sac and nerve roots (12, 13). In addition, our study aims to compare the clinical efficacy and safety of reduction vs. arthrodesis in situ with only the MIS/open-TLIF technique for low-grade spondylolisthesis, as, currently, there is still a lack of powerful clinical evidence. The pooled results in our study revealed that both techniques had similar patient-reported outcomes (VAS, ODI, and good and excellent rate) during short- and long-term follow-up, and no significant difference was observed in the fusion and complication rate, as well as the segmental lordosis. Although the reduction group did achieve better slippage correction, it was associated with increased operation time and intraoperative blood loss compared with the arthrodesis in situ group.
Intentional reduction of the slipped vertebrae may be a useful procedure, and the idea remains attractive in theory. It restores the spinal column into a more anatomic alignment. Fan reported that significant advantages in slippage decrease were observed in the reduction group (20). And, as shown in our study, the reduction group did achieve better slippage correction. In addition, some studies considered that intraoperative reduction might relieve early muscular fatigue and hypolordosis-induced back pain and prevent disc degeneration of the adjacent segment (31, 32). However, no evidence-based conclusion was reached to support the above viewpoint (29, 33). The pooled plot in our study indicated that the reduction group had increased operation time and blood loss, which might seem reasonable, especially in MIS-TLIF technology.
Patient-reported outcomes such as VAS-BP and ODI are of paramount importance when estimating the clinical effect of a certain technique (34). We predefined a follow-up that ranged from 3 to 6 months after surgery as a short-term follow-up and a follow-up of at least 2 years as a long-term follow-up. In our study, both techniques had similar VAS-BP and ODI during short- and long-term follow-up. The primary goal of intervention for spinal diseases is to relieve the pain and restore function (35, 36), and the excessive pursuit of anatomic restoration in MIS/open-TLIF procedures may not help in improving pain and function in patients (20). In addition, for the complication and fusion rate, Bai et al.'s (25) paper of meta-analysis demonstrated no statistical difference, which was again confirmed by our findings. In a word, intraoperative reduction in MIS/open-TLIF procedures, while safe, did not result in better clinical outcomes in patients with low-grade lumbar spondylolisthesis.
Moreover, bone quality is an important factor in the success of the reduction of slippage in the vertebrae. It is not easy to obtain the reduction, especially in patients with osteoporosis. Surgeons may fail in intraoperative reduction due to screw loosening in low-bone mass patients. However, the index of bone density was not mentioned in all five included studies in our meta-analysis. For intraoperative screw loosening after reduction, bone cement could be used to reinforce the pedicle screws.
We recognize the limitations of our study. First, all studies included in this meta-analysis were retrospective cohort studies and were inherently prone to methodology defects. Although all studies were rated with a total score of more than 5 according to the NOS (23), which represented relatively moderate to high quality, the validity of the data available might be weakened due to selection bias and other biases. In addition, the quality of evidence based on GRADE (37) was very low (Table 4), and the strength of the recommendations was relatively weak. Second, a relatively small number of participants and various definitions of complications among the studies might exert an effect on the results because of the limited statistical power and homogeneity. Third, the decision for intraoperative reduction was made primarily based on surgeon preference, and diverse MIS/open-TLIF technical specifications and postoperative management applied by diverse surgeons in different treatment centers might also have influenced the results. Moreover, data about health-related quality of life (HRQOL) were not available, which would be necessary to know if patients undergoing intraoperative reduction had an improvement in the HRQOL score over the patients who underwent arthrodesis in situ.
Based on the available evidence, intraoperative reduction does not result in better clinical outcomes in low-grade spondylolisthesis after MIS/open-TLIF, and the arthrodesis in situ technique could be an alternative. In view of the limitations of this study, a multicenter, large sample, well-designed randomized controlled study is essential to draw a more convincing conclusion.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
RQ: Validation, Writing – original draft, Writing – review & editing. MZ: Data curation, Investigation, Methodology, Software, Writing – review & editing. PZ: Software, Supervision, Validation, Writing – review & editing. AG: Software, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Qin R, Liu B, Zhou P, Yao Y, Hao J, Yang K, et al. Minimally invasive versus traditional open transforaminal lumbar interbody fusion for the treatment of single-level spondylolisthesis grades 1 and 2: a systematic review and meta-analysis. World Neurosurg. (2019) 122:180–9. doi: 10.1016/j.wneu.2018.10.202
2. Meyerding HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coil Surg. (1956) 26(5 Part 1):566–91.
3. Majid K, Fischgrund JS. Degenerative lumbar spondylolisthesis: trends in management. J Am Acad Orthop Surg. (2008) 16:208–15. doi: 10.5435/00124635-200804000-00004
4. Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. (2007) 356:2257–70. doi: 10.1056/NEJMoa070302
5. Ekman P, Moller H, Hedlund R. The long-term effect of posterolateral fusion in adult isthmic spondylolisthesis: a randomized controlled study. Spine J. (2005) 5:36–44. doi: 10.1016/j.spinee.2004.05.249
6. Moller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis–a prospective randomized study: part 1. Spine. (2000) 25:1711–5. doi: 10.1097/00007632-200007010-00016
7. Uysal M, Circi E, Ozalay M, Derincek A, Cinar M. The surgical treatment for a rare case of double-level isthmic spondylolisthesis in L4 and L5 lumbar spine: decompression, reduction and fusion. Eur J Orthop Surg Traumatol. (2012) 22:21–4. doi: 10.1007/s00590-012-0993-0
8. Etemadifar MR, Hadi A, Masouleh MF. Posterolateral instrumented fusion with and without transforaminal lumbar interbody fusion for the treatment of adult isthmic spondylolisthesis: a randomized clinical trial with 2-year follow-up. J Craniovertebr Junction Spine. (2016) 7:43–9. doi: 10.4103/0974-8237.176623
9. Schulte TL, Ringel F, Quante M, Eicker SO, Muche-Borowski C, Kothe R. Surgery for adult spondylolisthesis: a systematic review of the evidence. Eur Spine J. (2016) 25:2359–67. doi: 10.1007/s00586-015-4177-6
10. Harms J, Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl). Z Orthop Ihre Grenzgeb. (1982) 120:343–7. doi: 10.1055/s-2008-1051624
11. Lee N, Kim KN, Yi S, Ha Y, Shin DA, Yoon DH, et al. Comparison of outcomes of anterior-, posterior- and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg. (2017) 101:216–26. doi: 10.1016/j.wneu.2017.01.114
12. Karikari IO, Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine. (2010) 35:S294–301. doi: 10.1097/BRS.0b013e3182022ddc
13. Tsahtsarlis A, Wood M. Minimally invasive transforaminal lumber interbody fusion and degenerative lumbar spine disease. Eur Spine J. (2012) 21:2300–5. doi: 10.1007/s00586-012-2376-y
14. Foley KT, Lefkowitz MA. Advances in minimally invasive spine surgery. Clin Neurosurg. (2002) 49:499−517.
15. Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. (2012) 19:829–35. doi: 10.1016/j.jocn.2011.10.004
16. Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop. (2009) 33:1683–8. doi: 10.1007/s00264-008-0687-8
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
18. Gong K, Wang Z, Luo Z. Reduction and transforaminal lumbar interbody fusion with posterior fixation versus transsacral cage fusion in situ with posterior fixation in the treatment of Grade 2 adult isthmic spondylolisthesis in the lumbosacral spine. J Neurosurg Spine. (2010) 13:394–400. doi: 10.3171/2010.3.SPINE09560
19. Scheer JK, Auffinger B, Wong RH, Lam SK, Lawton CD, Nixon AT, et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis in 282 patients: in situ arthrodesis versus reduction. World Neurosurg. (2015) 84:108–13. doi: 10.1016/j.wneu.2015.02.037
20. Fan G, Zhang H, Guan X, Gu G, Wu X, Hu A, et al. Patient-reported and radiographic outcomes of minimally invasive transforaminal lumbar interbody fusion for degenerative spondylolisthesis with or without reduction: a comparative study. J Clin Neurosci. (2016) 33:111–8. doi: 10.1016/j.jocn.2016.02.037
21. Fan G, Gu G, Zhu Y, Guan X, Hu A, Wu X, et al. Minimally invasive transforaminal lumbar interbody fusion for isthmic spondylolisthesis: in situ versus reduction. World Neurosurg. (2016) 90:580–7. doi: 10.1016/j.wneu.2016.02.033
22. Tay KS, Bassi A, Yeo W, Yue WM. Intraoperative reduction does not result in better outcomes in low-grade lumbar spondylolisthesis with neurogenic symptoms after minimally invasive transforaminal lumbar interbody fusion—a 5-year follow-up study. Spine J. (2016) 16:182–90. doi: 10.1016/j.spinee.2015.10.026
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
24. Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. (1971) 53:891–903. doi: 10.2106/00004623-197153050-00004
25. Bai X, Chen J, Liu L, Li X, Wu Y, Wang D, et al. Is reduction better than arthrodesis in situ in surgical management of low-grade spondylolisthesis? A system review and meta analysis. Eur Spine J. (2016) 26:606–18. doi: 10.1007/s00586-016-4810-z
26. Benli IT, Cicek H, Kaya A. Comparison of sagittal plane realignment and reduction with posterior instrumentation in developmental low or high dysplastic spondylolisthesis. Kobe J Med Sci. (2006) 52:151–69.
27. Audat ZM, Darwish FT, Al BM, Obaidat MM, Haddad WH, Bashaireh KM, et al. Surgical management of low grade isthmic spondylolisthesis; a randomized controlled study of the surgical fixation with and without reduction. Scoliosis. (2011) 6:14. doi: 10.1186/1748-7161-6-14
28. Lian X-F, Hou T-S, Xu J-G, Zeng B-F, Zhao J, Liu X-K, et al. Posterior lumbar interbody fusion for aged patients with degenerative spondylolisthesis: is intentional surgical reduction essential? Spine J. (2013) 13:1183–9. doi: 10.1016/j.spinee.2013.07.481
29. Lian X, Hou T, Xu J, Zeng B, Zhao J, Liu XK, et al. Single segment of posterior lumbar interbody fusion for adult isthmic spondylolisthesis: reduction or fusion in situ. Eur Spine J. (2014) 23:172–9. doi: 10.1007/s00586-013-2858-6
30. Phan K, Rao PJ, Kam AC, Mobbs RJ. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J. (2015) 24:1017–30. doi: 10.1007/s00586-015-3903-4
31. Kida K, Tadokoro N, Kumon M, Ikeuchi M, Kawazoe T, Tani T. Can cantilever transforaminal lumbar interbody fusion (C-TLIF) maintain segmental lordosis for degenerative spondylolisthesis on a long-term basis? Arch Orthop Trauma Surg. (2014) 134:311–5. doi: 10.1007/s00402-014-1925-8
32. Floman Y, Millgram MA, Ashkenazi E, Smorgick Y, Rand N. Instrumented slip reduction and fusion for painful unstable isthmic spondylolisthesis in adults. J Spinal Disord Tech. (2008) 21:477–83. doi: 10.1097/BSD.0b013e31815b1abf
33. Wang W, Aubin CE, Cahill P, Baran G, Arnoux PJ, Parent S, et al. Biomechanics of high-grade spondylolisthesis with and without reduction. Med Biol Eng Comput. (2015) 54:619–28. doi: 10.1007/s11517-015-1353-0
34. Lee MJ, Mok J, Patel P. Transforaminal lumbar interbody fusion: traditional open versus minimally invasive techniques. J Am Acad Orthop Surg. (2018) 26:124–31. doi: 10.5435/JAAOS-D-15-00756
35. Mccormick JD, Werner BC, Shimer AL. Patient-reported outcome measures in spine surgery. J Am Acad Orthop Surg. (2013) 21:99–107. doi: 10.5435/JAAOS-21-02-99
36. Shi L, Chen Y, Miao J, Shi J, Chen D. Reduction of slippage influences surgical outcomes of grade II and III lumbar isthmic spondylolisthesis. World Neurosurg. (2018) 120:e1017–23. doi: 10.1016/j.wneu.2018.08.217
Keywords: transforaminal lumbar interbody fusion, reduction, arthrodesis in situ, spondylolisthesis, meta-analysis
Citation: Qin R, Zhu M, Zhou P and Guan A (2024) Does intraoperative reduction result in better outcomes in low-grade lumbar spondylolisthesis after transforaminal lumbar interbody fusion? A systematic review and meta-analysis. Front. Med. 11:1350064. doi: 10.3389/fmed.2024.1350064
Received: 05 December 2023; Accepted: 20 March 2024;
Published: 12 April 2024.
Edited by:
Xinhua Li, Shanghai General Hospital, ChinaCopyright © 2024 Qin, Zhu, Zhou and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongqing Qin, cWlucm9uZ3FpbmcxMjNAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.