
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 20 June 2024
Sec. Obstetrics and Gynecology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1348884
Objective: This study aims to assess the comprehensive and integrated modulatory effects of acupuncture and electroacupuncture on various ovarian dysfunctions.
Methods: We systematically searched for articles on animal experiments related to polycystic ovary syndrome (PCOS), premature ovarian failure (POF), premature ovarian insufficiency (POI), and perimenopausal syndrome (PMS) across multiple databases, including PubMed, Web of Science, Cochrane Library, Embase, and four Chinese language databases. The search covered the period from inception to November 2023. We conducted a comparative analysis between the acupuncture group and the model group (untreated) based on eligible literature. Our primary outcomes encompassed serum sex hormones (Luteinizing hormone, Follicle-stimulating hormone, Testosterone, Estradiol, Progesterone, and Anti-Müllerian hormone) and ovarian weight. Dichotomous data were synthesized to establish the relative risk (RR) of notable post-treatment improvement, while continuous data were pooled to determine the standardized mean difference (SMD) in post-treatment scores between the groups. Statistical analyses, including sensitivity analysis, Egger's test, and the trim-and-fill method, were executed using Stata 15.0 software.
Results: The meta-analysis encompassed 29 articles involving a total of 623 rats. In comparison to rat models of PCOS, the experimental group exhibited a reduction in serum levels of LH, T and LH/FSH ratio. However, no statistically significant differences were observed in AMH, FSH, E2 levels, and ovarian weight between the two groups. In the ovarian hypoplasia model rats, both acupuncture and electroacupuncture interventions were associated with an increase in E2 levels. However, the levels of LH and FSH did not exhibit a significant difference between the two groups.
Conclusions: Acupuncture or electroacupuncture facilitates the restoration of ovarian function primarily through the modulation of serum sex hormones, exerting regulatory effects across various types of ovarian dysfunction disorders.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022316279
Ovarian dysfunction, primarily attributed to endocrine disorders, manifests as menstrual irregularities and, in severe cases, infertility (1–5). In the last few decades, hormone replacement therapy (HRT) has been employed as an intervention for individuals grappling with ovarian dysfunction. Nevertheless, prolonged usage of HRT is associated with heightened risks of breast cancer, coronary heart disease, and venous thromboembolism. Furthermore, its efficacy in enhancing clinical pregnancy rates remains insubstantial (6, 7). Women of reproductive age frequently encounter the development of polycystic ovaries attributed to hormonal imbalances, resulting in delayed follicular development (8, 9). Additionally, stress and various factors affecting ovarian function can contribute to conditions such as diminished ovarian reserve (DOR), premature ovarian insufficiency (POI), and even premature ovarian failure (POF) (10). As women age, ovarian function naturally diminishes, leading to the onset of perimenopausal syndrome (PMS) and significantly impacting their daily lives (11, 12). The array of ovarian dysfunctions mentioned earlier constitutes factors associated with endocrine disruptions in women, resulting in compromised oocyte quality, diminished fertility, and premature aging.
Presently, acupuncture stands as a widely utilized intervention for addressing abnormal ovarian function, attributed to its well-tolerated side effects (13, 14). Acupuncture has been found to improve ovarian function, promote ovulation, relieve pain, ease emotional anxiety, and even delay diminished ovarian function (15–20). Extensive research has indicated that acupuncture not only enhances ovarian function and fosters ovulation but also provides relief from pain, mitigates emotional anxiety, and has the potential to postpone diminished ovarian function (21). Animal experiments and the subsequent integrated data analysis derived from these experiments can, to a certain extent, yield valuable a priori information. This information proves instrumental in guiding experimental or trial design and, in some cases, even informs clinical decision-making. The meta-analysis of preclinical animal studies plays a pivotal role in translational medicine, contributing to the development of more precise medical treatments (22, 23). While animal experiments offer valuable insights, their limitations include a low repetition rate of results. In an effort to delve further into the efficacy of acupuncture and minimize unnecessary attrition of animals in experiments, this study strategically categorized the PCOS animal model as a high-response group for ovarian function, while DOR, POI, POF, and PMS were collectively considered a low-response group for ovarian function. The aim was to investigate the effectiveness of acupuncture as a standalone intervention across different abnormal ovarian functions, providing a precise theoretical foundation for the acupuncture treatment of ovarian dysfunction diseases. Notably, this study protocol has been prospectively registered with Prospero (CRD42022316279).
The search spanned from the inception of databases until November 2023, covering the Chinese National Knowledge Infrastructure (CNKI), Wanfang Data (WFDB), VIP Information Database, China Biology Medicine (CBM Disc), as well as international databases including PubMed, Embase, Cochrane Library, and Web of Science. Employing a combination of subject terms and free words, the search aimed to comprehensively capture relevant literature. Additionally, a manual search for references in pertinent literature and reviews was conducted (refer to Appendix 1) to ensure a thorough examination and avoidance of any potential omissions.
The research was considered eligible if it met the following criteria:
1. Female Sprague Dawley (SD) rats were utilized as animal models for PCOS, POF, POI, DOR, and PMS.
2. Implementation of either simple acupuncture or electroacupuncture as the intervention.
3. Formation of experimental groups comprising both the model and acupuncture cohorts, with the control group consisting solely of the model group. In instances where included articles featured more than two groups, only the acupuncture and model groups were retained.
4. Assessment encompassing at least one of the following outcome indicators: luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), estradiol (E2), anti-Müllerian hormone (AMH), LH/FSH ratio, ovarian weight (OW), ovarian volume (OV), and follicle number (FN). Notably, all hormone tests were conducted using serum samples.
The research that satisfied any of the following conditions are excluded:
1. Disruption of ovarian tissue morphology during the modeling process.
2. Utilization of acupuncture or electroacupuncture as a pre-treatment intervention before or during the construction of animal models.
3. In cases where data were duplicated across multiple instances, only the complete study was considered.
4. Studies lacking sufficient data were also excluded.
Two reviewers (W.H.J. and L.L.Y.) independently performed literature screening, data extraction, and quality assessment for the trials. In cases of disagreement, a third party (Z.Y.M.) was consulted, and a consensus was reached. Titles and abstracts were meticulously reviewed, with articles not meeting the eligibility criteria being excluded. In instances of missing data, attempts were made to acquire the necessary information by reaching out to the corresponding authors via email. For articles presenting data solely in image format, two independent authors (L.Y.) utilized the webplot digitizer (https://apps.automeris.io/wpd/) to extract mean difference (MD) and standard deviation (SD) values from the images. The extracted data were cross-checked, and any disagreements were resolved through consultation with a third party (J.L.X. and L.Y.).
The characteristics and general information were systematically extracted and organized in tabular form. The extracted data encompassed details such as authors, publication date, animal species, intervention methods, modeling techniques, and study outcomes.
The chosen studies underwent evaluation based on the guidelines outlined in the Animal Research: Reporting of In Vivo Experiments (ARRIVE) protocol (24, 25). To gauge the quality of each study, a ratio of the obtained score to the maximum possible score was calculated. This process resulted in three distinct quality intervals: studies scoring between 0.8–1 were categorized as “excellent,” those with scores between 0.5–0.8 were deemed “average,” while scores below 0.5 were categorized as “poor” (21, 26, 27).
The risk of bias in the selected studies was assessed using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) tool (28). Each item within the tool was categorized as “yes,” “no,” or “unclear” to assign scores to the respective articles. Studies with at least one item identified as having a high risk of bias were classified as having an overall high risk of bias. Those with unclear risk of bias for at least one item were considered to be at an unclear risk of bias, while studies demonstrating low risk of bias across all items were rated as having a low risk of bias.
Two authors (Z.Y.M. and H.J.H.) independently conducted the quality and risk of bias assessments. Any discrepancies were resolved through discussion or, when necessary, by seeking the judgment of a third expert (L.Y.) until a consensus was reached.
In this study, serum sex hormone levels, ovarian weight, ovarian volume, and the number of follicles in both the disease and intervention groups were treated as continuous data in comparison to the control group. Standardized mean difference (SMD) was employed to evaluate the outcome indicators. The meta-analysis for each serum sex hormone level and ovarian morphology was conducted using Stata 15.0, with a confidence interval (CI) set at 95%.
If the P > 0.10 and I2 < 50%, indicating minimal heterogeneity among studies, the fixed-effects model is selected. Conversely, if these conditions are not met, the random-effects model is employed. Due to insufficient data on the main factors, we refrained from conducting subgroup analyses to investigate heterogeneity at this stage (29). However, we intend to perform future subgroup analyses, exploring variables such as different acupuncture protocols, shorter (≤2 weeks) or longer (>2 weeks) durations, and variations in animal model species.
For outcome indices with 10 or more included studies, we will conduct sensitivity analyses to explore the causes of heterogeneity. Additionally, Egger's method will be employed to test for publication bias. If the P < 0.05, indicating the presence of publication bias, appropriate measures, such as trimming or adjustments, will be considered to address the identified bias.
A total of 1,093 publications were initially identified. Following rigorous selection criteria, 29 articles, encompassing a total of 623 rats, were ultimately included in the present meta-analysis. The distribution of these studies comprised 18 articles focused on PCOS, five on POF, three on PMS, zero on DOR and an additional three on PMS (Figure 1).
Twelve studies employed manual acupuncture, while 17 studies utilized electroacupuncture as the intervention method. Ovarian function was assessed through the collection of specimens, encompassing both serum samples and ovarian tissue. Detailed baseline characteristics of the included studies are provided in Table 1.
A comprehensive evaluation of 21 items was conducted in accordance with the ARRIVE guidelines. Based on the quality score-to-maximum score ratio, items 18, 19, and 20 were deemed suboptimal. Meanwhile, items 2, 3, 4, 6, 7, 9, 10, 12, 14, 16, 17, and 21 were classified as having an average rating, while items 1, 5, 8, 9, 11, 13, and 15 demonstrated an excellent level of adherence. The final aggregate score, calculated as 0.65, was considered to be of an average quality. A detailed breakdown of the quality assessment for the identified studies is presented in Table 2.
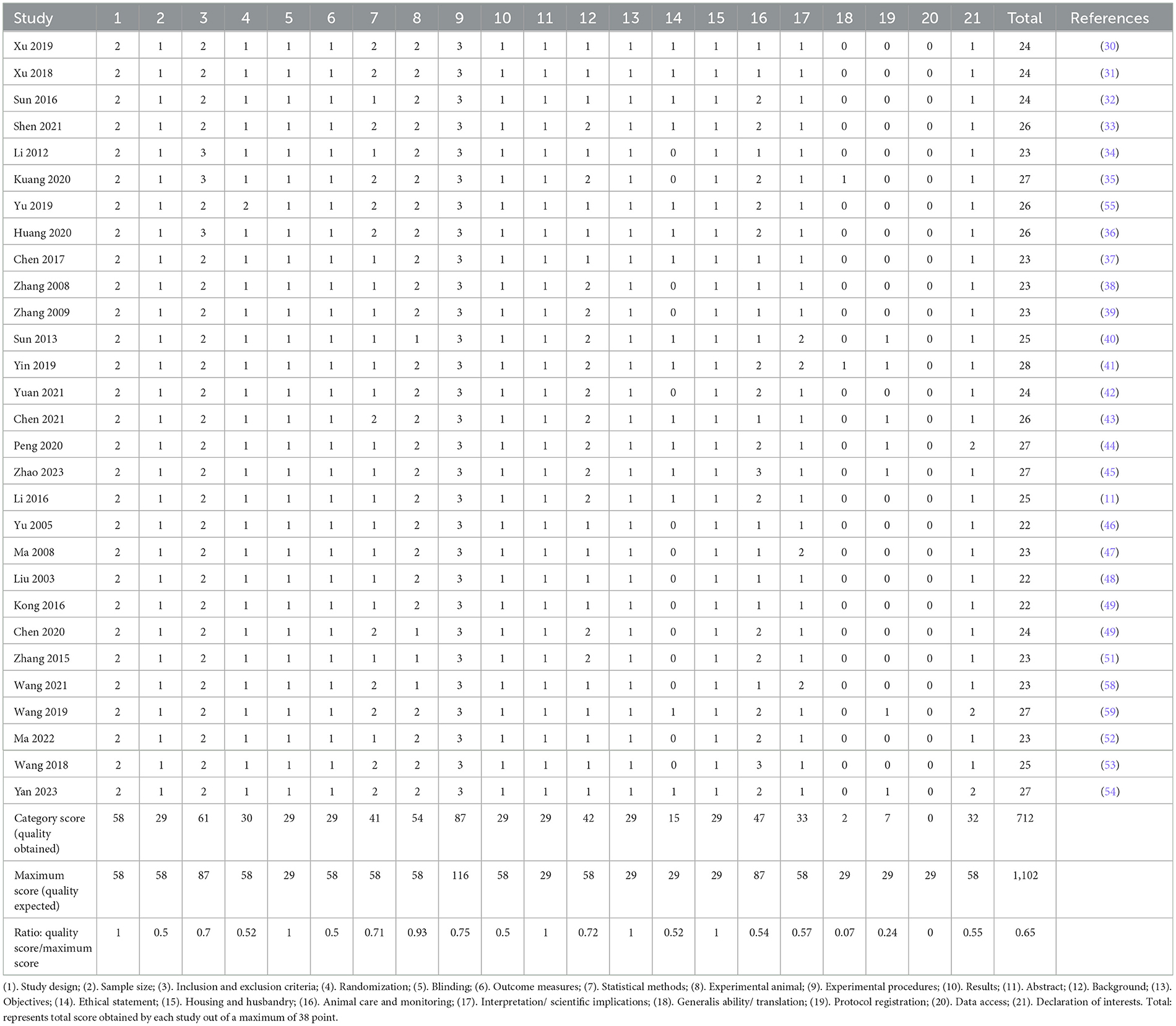
Table 2. Quality assessment according to the Animal Research Reporting In Vivo Experiment (ARRIVE) guidelines.
The quality assessment was conducted using the SYRCLE risk assessment tool for animal experiments. The selected studies comprised seven with a low risk of sequence generation (30, 31, 42, 47, 50, 53, 55), eight with a low risk in baseline characteristics (35, 36, 43, 44, 52–55), and three with a low risk of random results (34, 37, 39). Additionally, all 29 studies exhibited a low risk in terms of incomplete result data, selective result reporting, and bias from other sources. A comprehensive overview of the risk of bias is provided in Table 3.
The serum levels of LH, FSH, T, E2, LH/FSH ratio and AMH were subjected to analysis across the selected studies (30–40, 42–45, 55–57). The findings indicated the following: in comparison to the model group, the LH level demonstrated a significant decrease in the acupuncture group [acupuncture group n = 191, model group n = 190, I2 = 84.40%, SMD = −1.90 (95% CI −2.60 to −1.19); Z = 10.76, P = 0.000]. Similarly, the T level exhibited a significant reduction in the acupuncture group [acupuncture group n = 154, model group n = 153, I2 = 86.94%, SMD = −3.14 (95% CI −4.10 to −2.18); Z = 14.77, P = 0.023], and the ratio also showed a significant decrease in the acupuncture group [acupuncture group n = 49, model group n = 49, I2 = 86.94%, SMD = −3.13 (95% CI −4.93 to −1.33); Z = 3.41, P = 0.001]. Conversely, the E2 level [acupuncture group n = 153, model group n = 152, I2 = 94.47%, SMD = 0.85 (95% CI −0.64 to 2.33); Z = 0.88, P = 0.40], the FSH level [acupuncture group n = 149, model group n = 148, I2 = 91.59%, SMD = −0.67 (95% CI −1.60 to 0.27); Z = −1.05, P = 0.31], and the AMH level [acupuncture group n = 40, model group n = 40, I2 = 92.06%, SMD = 0.32 (95% CI −1.57 to 2.21); Z = 0.28, P = 0.80] did not exhibit a significant difference in the acupuncture group. The corresponding forest plots for each outcome measurement are illustrated in Figures 2A–F.
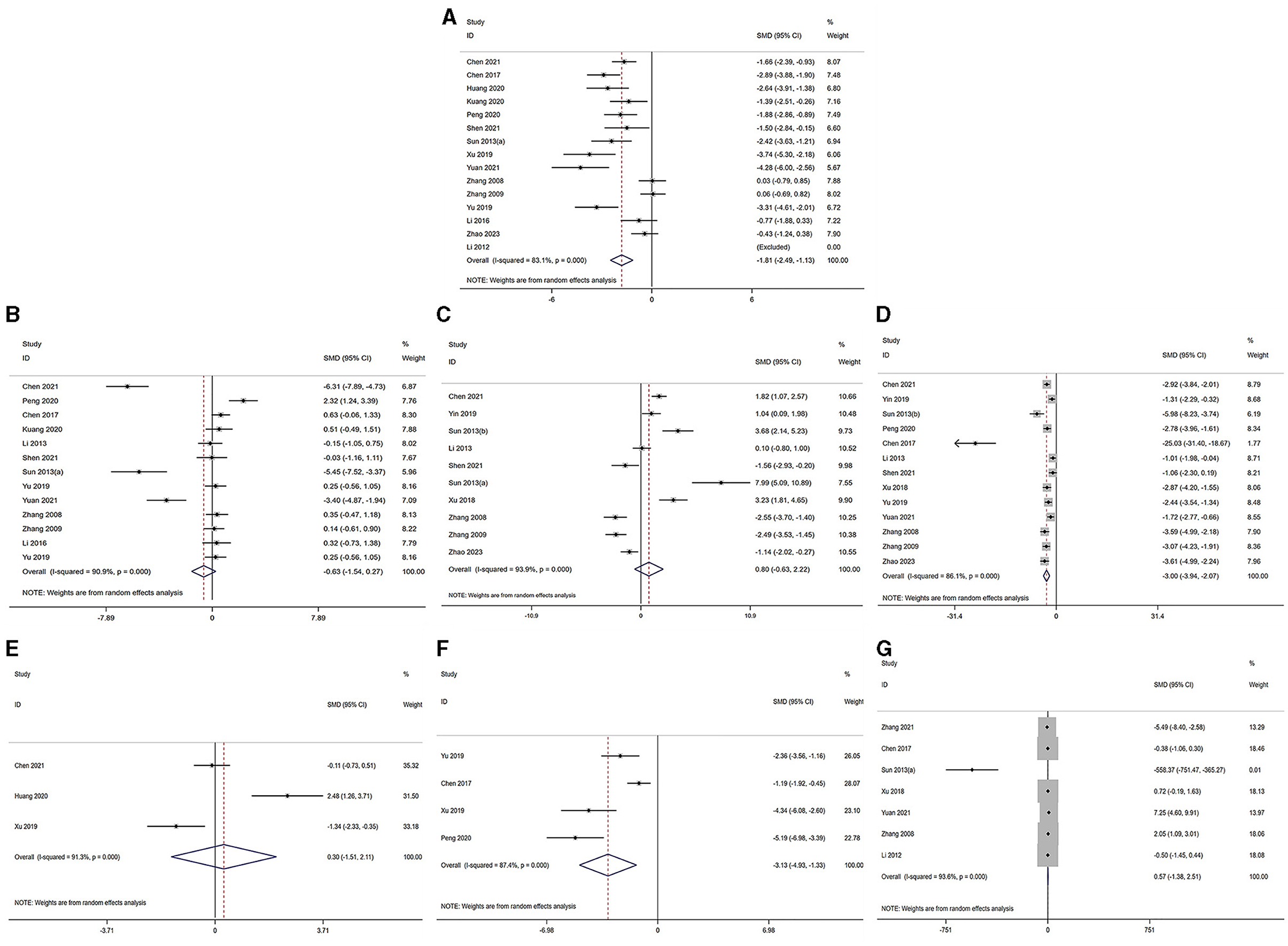
Figure 2. Forest plot of comparison acupuncture vs. control in model of PCOS. The statistical method is random effect. (A) Forest map of LH; (B) Forest map of FSH; (C) Forest map of E2; (D) Forest map of T; (E) Forest map of AMH; (F) Forest map of LH/FSH; (G) Forest map of OW. LH, luteinizing hormone; FSH, follicle-stimulating hormone; T, testosterone; E2, estradiol; AMH, Anti-Müllerian hormone; OW, Ovarian weight.
The analysis of ovarian weight, as reported in studies (31, 32, 34, 37, 38, 42, 45), revealed that in the acupuncture group, there was no significant difference in ovarian weight [acupuncture group n = 82, model group n = 81, I2 = 91.6%, SMD = 0.76 (95% CI −1.25 to 2.77); Z = 0.21, P = 0.838] when compared to the model group. Further details pertaining to the ovarian morphology in PCOS are presented in Figure 2G.
Based on the disease characteristics, we classified POI, POF, and PMS into the category of decreased ovarian function. The serum levels of LH, FSH, and E2 were analyzed across studies (46, 47, 49–54, 58–60). The outcomes are as follows: Serum E2 level [acupuncture group n = 113, model group n = 113, I2 = 78.00%, SMD = 1.87 (95% CI 1.16–2.58); Z = 3.33, P = 0.008] demonstrated a significant increase compared to the model group. LH level [acupuncture group n = 82, model group n = 82, I2 = 94.14%, SMD = −1.81 (95% CI −3.65 to 0.02); Z = −1.75, P = 0.124] and FSH (acupuncture group n = 96, model group n = 96, I2 = 92.61%, SMD = −2.30 [95% CI −3.78 to 0.81]; Z = −2.19, P = 0.06) in the acupuncture group were not significantly different from the model group. These findings are visually represented in Figure 3.
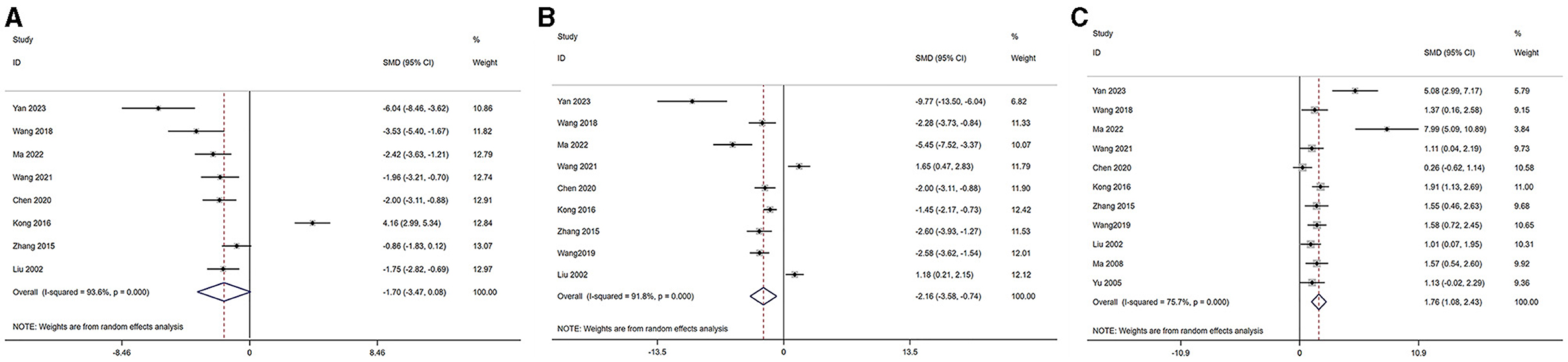
Figure 3. Forest plot of comparison: acupuncture vs. control in model of decreased ovarian function. The statistical method is random effect. (A) Forest map of LH; (B) Forest map of FSH; (C) Forest map of E2. LH, luteinizing hormone; FSH, follicle-stimulating hormone; T, testosterone; E2, estradiol.
In this study, a sensitivity analysis was conducted for each outcome index that included more than 10 pieces of literature. The analysis encompassed 15 articles on LH analysis, 13 articles on FSH analysis, 10 articles on E2 analysis, 13 articles on T analysis in PCOS, and 11 articles on E2 analysis in decreased ovarian function (Figure 4). Remarkably, the omission of Chen's study in 2021 (43) significantly influenced the stability of results in the E2 analysis within the context of PCOS. This impact could potentially be attributed to variations in the modeling drugs employed in this particular study, introducing heterogeneity in the outcomes. Furthermore, the heterogeneity of other results persisted, as evidenced by the continued detection using Egger's test.
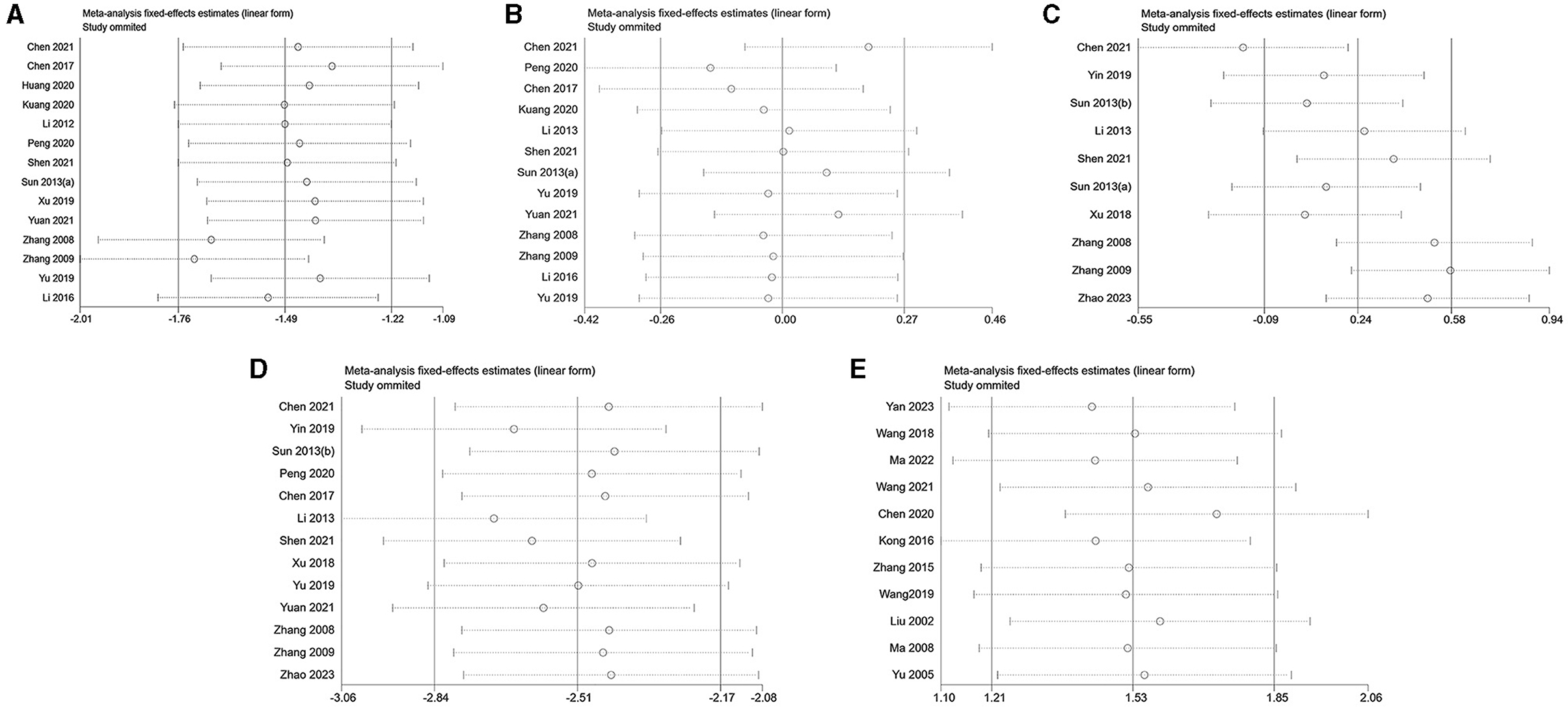
Figure 4. Sensitivity analysis. (A) LH of PCOS; (B) FSH of PCOS; (C) E2 of PCOS; (D) T of PCOS; (E) E2 of decreased ovarian function.
Egger's test was employed to examine the bias within the included literature, encompassing 15 types of literature in LH analysis, 13 types of literature in FSH analysis, 13 types of literature in T analysis in PCOS, and 11 articles on E2 analysis in decreased ovarian function. The findings reveal that a P < 0.05 for LH and E2 indicates the presence of publication bias, necessitating supplementary correction methods such as the trim-and-fill technique. Conversely, the analysis of FSH and T indicated a relatively low likelihood of publication bias, as summarized in Table 4.
In Figure 5, no additional data for LH, FSH, T in PCOS, and E2 in decreased ovarian function are apparent. The analysis indicates that the data for LH in PCOS and E2 in decreased ovarian function have undergone no filling study, suggesting the stability of the results.
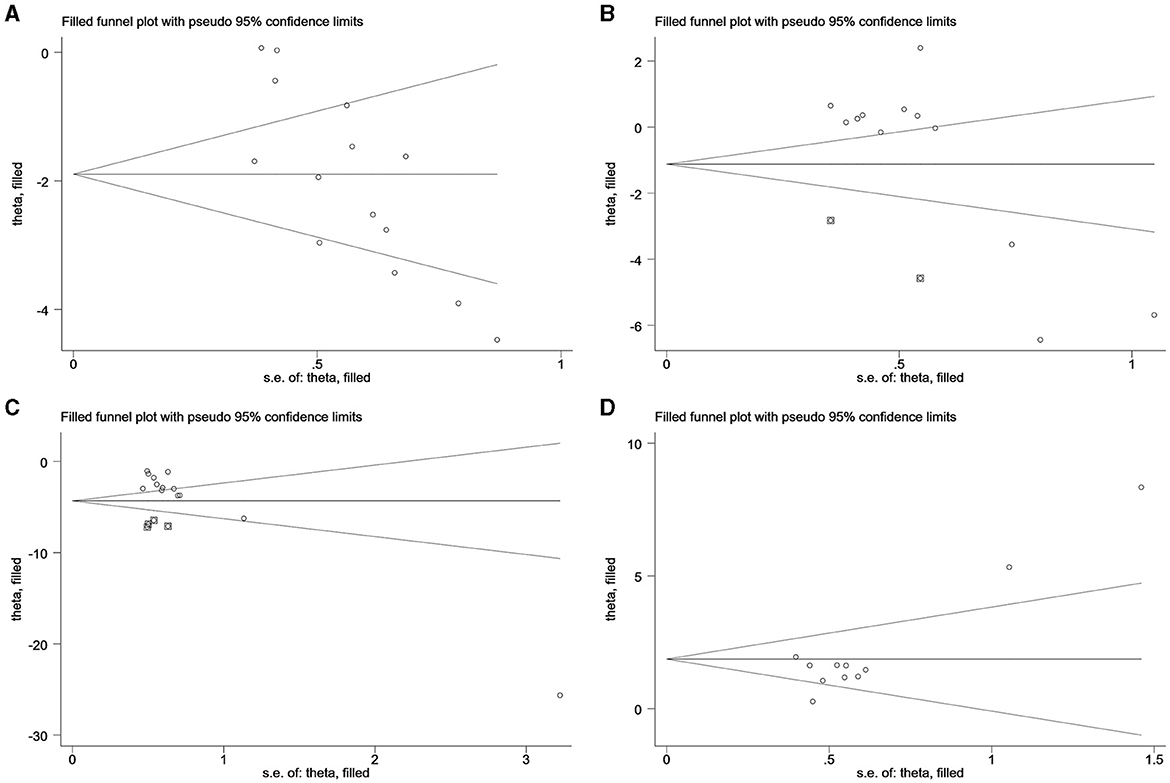
Figure 5. Trim and filling method. (A) LH of PCOS; (B) FSH of PCOS; (C) T of PCOS; (D) E2 of decreased ovarian function.
For LH analysis, two rounds of LINEAR iterations were performed with no additional articles identified, resulting in Q = 83.59, P = 0.00 < 0.05. This signifies the stability of the original results. Subsequently, a random-effects model was employed, yielding a combined result of IOR = 0.15 for the effect indicators, and a 95% CI of (0.07–0.30).
Similarly, the analysis of E2 underwent two rounds of LINEAR iterations with no additional articles added, resulting in Q = 45.56, P = 0.00 < 0.05, indicating the stability of the original results. The subsequent use of a random-effects model produced a combined effect indicator of IOR = 6.47, with a 95% CI of (3.18–13.16).
Notably, two points in the FSH plot and four points in the T plot, indicated as squares, are suggested as potential effect sizes for future inclusion. Combining the above forest plots ensures the symmetry of the funnel plots, eliminating publication bias by consistently incorporating articles with similar results from corresponding studies expressed in the plots.
In this comprehensive meta-analysis, our objective was to address the effectiveness of acupuncture in ameliorating ovarian dysfunction, drawing insights from the collective data of 623 rats across 29 articles. The study outcomes revealed that acupuncture led to a noteworthy reduction in serum LH, T and LF/FSH ratio levels in rats modeled with PCOS. Additionally, acupuncture exhibited a significant increase in serum E2 levels in rats modeled with decreased ovarian function. Intriguingly, despite these observed effects, acupuncture did not yield a significant alteration in ovarian volume in the context of PCOS.
Absolutely, our statement provides a concise and accurate summary of the crucial roles played by LH and FSH in ovarian function. These hormones, secreted by the pituitary gland, are instrumental in orchestrating the complex processes involved in the development of follicles and oocytes within the ovaries. FSH is essential for promoting the maturation of follicles, working in tandem with LH to facilitate ovulation. LH, in turn, acts synergistically with FSH, particularly during the late follicular stage, to promote the development and optimal functioning of follicles. The regulation of the hypothalamus-pituitary-ovary axis (HPOA) by LH and FSH is a key aspect of maintaining ovarian function. This regulatory mechanism plays a central role in promoting follicular development and finely tuning the secretion of estrogen, thereby contributing significantly to the overall regulation of the female reproductive system (61). Our statement aptly captures the interconnected nature of sex hormone regulation and how acupuncture can impact these dynamics. T serves as a crucial precursor in the biosynthesis of E2 and exerts a negative feedback regulatory effect on the hypothalamus-pituitary axis (62, 63). LH plays a significant role in the regulation of T synthesis, and therefore, the effects of acupuncture on T align with its impact on LH. For PCOS, characterized by increased LH secretion, elevated LH/FSH ratio, and elevated T levels in some patients, research findings indicate that both acupuncture and electroacupuncture can significantly reduce serum LH, T, and LH/FSH levels. This suggests that acupuncture holds promise in effectively addressing sex hormone level disorders in PCOS and potentially restoring abnormal ovarian function. E2 is a key component in follicular development and maturation, exerts both positive and negative feedback effects on the hypothalamus (64, 65). Additionally, E2 plays a decisive role in maintaining ovarian function and mitigating ovarian aging (66). This highlights the potential of acupuncture to influence a range of sex hormones, contributing to the restoration of hormonal balance and, consequently, improved ovarian function. The study outcomes reveal that both acupuncture and electroacupuncture interventions have the potential to enhance serum E2 levels across various degrees of ovarian hypoplasia, thereby contributing to the alleviation of the ovarian hypoplasia process. However, the observation of no significant difference in ovarian weight between the acupuncture group and the model group in PCOS suggests the need for cautious interpretation. This lack of significance may be associated with the relatively short duration of acupuncture treatment in animal experiments and the limited number of articles included in this study (67). It underscores the importance of considering these factors and highlights the necessity of supplementing relevant outcome indicators in future studies to provide a more comprehensive understanding of the impact of acupuncture on ovarian weight in PCOS models.
In the current study, it was observed that only six of the included articles proposed the collection of serum samples during the inter-estrous period in model animals (30, 31, 35, 42, 45, 53). However, a sensitivity analysis was conducted to assess the impact of excluding any of the articles that sampled during the interestrus period from LH, FSH, and T analyses. Remarkably, the results indicated that such exclusions had no significant effect on the overall meta-analysis results. Given the similarity between the animal estrous cycle and the human menstrual period, it is noteworthy that there are substantial changes in serum sex hormones across different estrous cycles (68). This factor may exert an influence on the outcomes of our study (69). Follicular development exhibits a close association with the level of E2, especially in patients with PCOS during the anogenital follicular phase. Here, an increase in E2 may play a role in suppressing oocyte meiosis, potentially resulting in abnormal oocyte maturation (70). In the context of the early stages of PMS, a reduction in LH and T levels indicates a decline in ovarian function. It is crucial to recognize that hormonal variations at different points in time can have diverse effects on ovarian function and follicular development. Therefore, when investigating alterations in sex hormones, the changes in the motility cycle emerge as a significant factor that cannot be overlooked.
This meta-analysis puts forth the proposition that acupuncture exhibits the capacity to reduce elevated hormones, elevate lowered hormones, and normalize disordered hormone levels in the treatment of dysfunctional ovarian diseases. Hence, our inference is that the regulation of ovarian dysfunction through acupuncture or electroacupuncture primarily occurs via the modulation of serum sex hormones.
Additionally, previous studies have elucidated the multifaceted impact of acupuncture or electroacupuncture on ovarian function. These effects encompass diverse mechanisms, such as modulating protein content within ovarian tissue, altering sex hormone receptor activity, modifying gene expression associated with reproductive factors, influencing metabolic factors, and modulating various signaling pathways (41, 48, 71–78), and alteration of the expression of various proteins in the hypothalamus (79, 80). For example, most PCOS patients are usually accompanied by insulin resistance (IR), and lipocalin can increase insulin sensitivity and promote glucose metabolism, which is a key signaling factor in the regulation of glucose-lipid metabolism, and plays an important role in regulating the modulation of reproductive and metabolic disorders (81–85). Studies have demonstrated that acupuncture can ameliorate glycolipid metabolism in PCOS by downregulating lipocalin receptor expression, upregulating lipocalin protein expression, and activating the AMP-activated protein kinase (AMPK) pathway (86–88). POF, POI, and PMS are characterized by reductions in ovarian volume and cell numbers. However, acupuncture has been shown to increase granulosa cell and follicle numbers by activating the Phosphoinositide 3-kinase/Protein Kinase B (PI3K/AKT) signaling pathway, thereby improving ovarian function and fertility (59, 89). In conclusion, the confirmed regulatory effect of acupuncture or electroacupuncture on ovarian function provides a novel therapeutic perspective for the treatment of diseases associated with ovarian function (Figure 6).
The study did not undertake a comprehensive analysis of all indices. Given the inclusion of diverse diseases and the inconsistent changes observed in serum sex hormones and ovarian morphology across these conditions, the effects of each hormone were individually accounted for. This approach allowed for a nuanced explanation of hormonal changes across different diseases.
A notable portion of the articles did not account for the impact of different estrous cycles on outcomes during serum sample extraction. Considering the relevance of estrous cycle recovery in animal models as an important indicator of acupuncture response in diseases related to ovarian dysfunction, future studies should meticulously consider the fluctuation of sex hormone levels across different estrous cycles. Researchers are advised to choose outcome indicators judiciously to more accurately reflect acupuncture efficacy.
The reliance on specific outcome indicators to gauge the integrative modulatory effects of acupuncture on disorders of abnormal ovarian function may potentially constrain the comprehensive interpretation of acupuncture efficacy. And since changes in serum sex hormones are a common indicator for assessing the efficacy of acupuncture in clinical trials, we likewise chose serum sex hormones as an observational indicator of efficacy in our study. However, in future research endeavors, we should take full advantage of the opportunity provided by animal experiments to incorporate additional outcome indicators. This approach would better summarize the multifaceted effects of acupuncture on ovarian function. For example, parameters such as protein content, gene expression and signaling pathway alterations must be studied in depth.
The predominance of studies focused on PCOS in this analysis may restrict the generalizability of the findings to other ovarian dysfunction diseases. Nevertheless, it is essential to highlight that this study marks the first endeavor to evaluate the effects of acupuncture or electroacupuncture on improving animal models of ovarian dysfunction across various diseases, providing insights into the underlying mechanisms of acupuncture or electroacupuncture. The aim of this study was to comprehensively observe the efficacy of acupuncture therapy on ovarian dysfunction diseases, and the previous analysis of acupuncture and electroacupuncture by subgroups did not appear to have a significant impact on the analytical results, so this study did not describe the subgroup analysis of acupuncture and electroacupuncture. And due to the limited number of articles we screened, especially POF, PMS and POI, subgroup analysis of acupuncture and electroacupuncture was difficult to achieve, so future studies should further include more articles to further analyze and analyze the efficacy of acupuncture and electroacupuncture.
Finally, this study observed the effects of acupuncture on various ovarian dysfunctions through animal experiments. In future research, we will focus on observing whether acupuncture has similar therapeutic effects in clinical studies, which will require further in-depth analysis and exploration.
This meta-analysis underscores that acupuncture treatment has the potential to enhance ovarian function in PCOS, POF, POI, and PMS model rats by effectively regulating serum sex hormones. The findings of this review provide a valuable foundation for future research into the therapeutic effects of acupuncture or electroacupuncture. It is anticipated that this comprehensive review will serve as a noteworthy reference in this evolving field of research, encouraging further exploration and insights into the application of acupuncture in the context of ovarian dysfunction.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YZ: Writing – review & editing, Writing – original draft. YL: Writing – review & editing. LL: Writing – review & editing. JH: Writing – review & editing. HW: Writing – review & editing. LJ: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received financial support from several sources, including grants from the National Natural Science Foundation of China (Nos. 82105028 and 82074549), the Xinglin Scholar Research Promotion Project of Chengdu University of Traditional Chinese Medicine (No. OJRC2022009), the Hundred Talents Program of the Hospital of Chengdu University of Traditional Chinese Medicine (No. 22-Q29), and the Young Elite Scientists Sponsorship Program by CACM (No. CACM-2023-QNRC2-B03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1348884/full#supplementary-material
1. Leong I. Reproductive endocrinology: restoring ovarian function. Nat Rev Endocrinol. (2018) 14:66. doi: 10.1038/nrendo.2017.171
2. Perheentupa A, Huhtaniemi I. Aging of the human ovary and testis. Mol Cell Endocrinol. (2009) 299:2–13. doi: 10.1016/j.mce.2008.11.004
3. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
4. Rebar RW, Keator CS. Expanding our knowledge of premature ovarian insufficiency. Fertil Steril. (2021) 115:328–9. doi: 10.1016/j.fertnstert.2020.09.145
5. Blumenfeld Z. The ovarian hyperstimulation syndrome. Vitam Horm. (2018) 107:423–51. doi: 10.1016/bs.vh.2018.01.018
6. Wang Y, Huang R, Li X, Zhu Q, Liao Y, Tao T, et al. High concentration of chemerin caused by ovarian hyperandrogenism may lead to poor IvF outcome in polycystic ovary syndrome: a pilot study. Gynecol Endocrinol. (2019) 35:1072–7. doi: 10.1080/09513590.2019.1622087
7. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women principal results from the women's health initiative randomized controlled trial. JAMA. (2002) 288:321–33. doi: 10.1001/jama.288.3.321
8. Li Y, Chen C, Ma Y, Xiao J, Luo G, Li Y, et al. Multi-system reproductive metabolic disorder: significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. (2019) 228:167–75. doi: 10.1016/j.lfs.2019.04.046
9. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr Rev. (2016) 37:467–520. doi: 10.1210/er.2015-1104
10. Liu H, Jiang C, La B, Cao M, Ning S, Zhou J, et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through Ampk/Foxo3a signaling pathway. Stem Cell Res Ther. (2021) 12:317. doi: 10.1186/s13287-021-02382-x
11. Li RX, Ma M, Xiao XR, Xu Y, Chen XY Li B. Perimenopausal syndrome and mood disorders in perimenopause: prevalence, severity, relationships, and risk factors. Medicine. (2016) 95:e4466. doi: 10.1097/MD.0000000000004466
12. Lan J, Wu C, Liang W, Shen J, Zhuo Z, Hu L, et al. An effective treatment of perimenopausal syndrome by combining two traditional prescriptions of chinese botanical drugs. Front Pharmacol. (2021) 12:744409. doi: 10.3389/fphar.2021.744409
13. Chang R, Chung PH, Rosenwaks Z. Role of acupuncture in the treatment of female infertility. Fertil. (2002) 78:1149–53. doi: 10.1016/S0015-0282(02)04348-0
14. Wang RR, Su MH, Liu LY, Lai YY, Guo XL, Gan D, et al. Systematic review of acupuncture to improve ovarian function in women with poor ovarian response. Front Endocrinol. (2023) 14:1028853. doi: 10.3389/fendo.2023.1028853
15. Liu Y, Wu L, Yao S, Wu C, Lu L, Yi W. Acupuncture for infertile women without undergoing assisted reproductive techniques (ART) a systematic review and meta-analysis. Medicine. (2019) 98:e16463. doi: 10.1097/MD.0000000000016463
16. Ye Y, Zhou CC, Hu HQ, Fukuzawa I, Zhang HL. Underlying mechanisms of acupuncture therapy on polycystic ovary syndrome: evidences from animal and clinical studies. Front Endocrinol. (2022) 13:1035929. doi: 10.3389/fendo.2022.1035929
17. Xi D, Chen B, Tao H, Xu Y, Chen G. The risk of depressive and anxiety symptoms in women with premature ovarian insufficiency: a systematic review and meta-analysis. Arch Womens Ment Health. (2023) 26:1–10. doi: 10.1007/s00737-022-01289-7
18. Smith CA, de Lacey S, Chapman M, Ratcliffe J, Norman RJ, Johnson NP, et al. The effects of acupuncture on the secondary outcomes of anxiety and quality of life for women undergoing IVF: a randomized controlled trial. Acta Obstet Gynecol Scand. (2019) 98:460–9. doi: 10.1111/aogs.13528
19. Xia J-f, Inagaki Y, Zhang J-f, Wang L, Song P-p. Chinese medicine as complementary therapy for female infertility. Chin J Integr Med. (2017) 23:245–52. doi: 10.1007/s11655-016-2510-5
20. Robinson N. Integrating acupuncture: are there positive health outcomes for women? J Zhejiang Univ Sci B. (2017) 18:233–8. doi: 10.1631/jzus.B1600260
21. Xiao LY, Li Z, Du YZ, Shi HY, Yang SQ, Zhang YX, et al. Acupuncture for hypertension in animal models: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:8171636. doi: 10.1155/2021/8171636
22. Ritskes-Hoitinga M, Leenaars M, Avey M, Rovers M, Scholten R. Systematic reviews of preclinical animal studies can make significant contributions to health care and more transparent translational medicine. Cochrane Database Syst Rev. (2014) 2014:ED000078. doi: 10.1002/14651858.ED000078
23. Sandercock P, Roberts I. Systematic reviews of animal experiments. Lancet. (2002) 360:586. doi: 10.1016/S0140-6736(02)09812-4
24. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. (2020) 18:e3000410. doi: 10.1371/journal.pbio.3000410
25. Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: explanation and elaboration for the arrive guidelines 2.0. PLoS Biol. (2020) 18:e3000411. doi: 10.1371/journal.pbio.3000411
26. Javed F, Kellesarian SV, Abduljabbar T, Abduljabbar AT, Akram Z, Vohra F, et al. Influence of involuntary cigarette smoke inhalation on osseointegration: a systematic review and meta-analysis of preclinical studies. Int J Oral Maxillofac Surg. (2018) 47:764–72. doi: 10.1016/j.ijom.2017.11.009
27. Andrade EF, Orlando DR, Araujo AMS, de Andrade J, Azzi DV, de Lima RR, et al. Can resveratrol treatment control the progression of induced periodontal disease? A systematic review and meta-analysis of preclinical studies. Nutrients. (2019) 11:953. doi: 10.3390/nu11050953
28. Hooijmans CR, Rovers MM, Vries RBd, Leenaars M, Ritskes-Hoitinga M, Langendam MW. Syrcle's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
29. Mu J, Furlan AD, Lam WY, Hsu MY, Ning Z, Lao L. Acupuncture for chronic nonspecific low back pain. Cochrane Database Syst Rev. (2020) 12:CD013814. doi: 10.1002/14651858.CD013814
30. Xu G, Zhang AD, Wang X, Liu JD, Feng JW, Chen YL. Effect of electroacupuncture at different acupoints on follicle development and related factors in serum and ovary tissues of PCOS rats. Acupunct Res. (2019) 44:740–6. doi: 10.13702/j.1000-0607.190041
31. Xu G, Wang Q, Zhang AD, Liu JD, Feng GW, Chen YL. Effects of electroacupuncture at different acupoints on hyperandrogenemia and androgen receptor expression in the ovaries of polycystic ovary syndrome rats. Acupunct Res. (2018) 43:543–9. doi: 10.13702/j.1000-0607.170688
32. Sun J, Zhao JM Ji R, Liu HR, Shi Y, Jin CL. Effects of electroacupuncture on ovarian hyperandrogenism in polycystic ovary syndrome rats. Acupunct Res. (2013) 38:465–72. doi: 10.13702/j.1000-0607.2013.06.008
33. Shen LY, Chang FX, Pan L, Liu XJ, Yang ZF, Hu H. Effects of electroacupuncture on hypothalamic kisspeptin protein expression in polycystic ovary syndrome rats. Acupunct Res. (2021) 46:106–10. doi: 10.13702/j.1000-0607.200497
34. Li J, Cui W, Sun W, Wen J. Regulatory effects of electroacupuncture on cytokines and tumor necrosis factor-A expression in polycystic ovary syndrome rats. Chin J Trad Chin Med Inform. (2012) 19:26–8.
35. Kuang HY, Zhang L, Li W, Zhang YS, Feng WT. Study on the expression of metabolic factors in adipose tissue of polycystic ovary syndrome rats treated with electroacupuncture. Shanghai Acupunct J. (2020) 39:914–21. doi: 10.13460/j.issn.1005-0957.2020.13.0003
36. Huang J, Tang CL, Liao DM. Effects of electroacupuncture on autophagy-related factors in polycystic ovary syndrome rats. Acupunct Res. (2020) 45:640–4, 51. doi: 10.13702/j.1000-0607.190744
37. Chen PD, Yang ZX, Liu F, Qiu TT, Ling WL, Qiu XL. Effects of electroacupuncture at ren mai meridian acupoints on hormones in polycystic ovary syndrome rats. J Qiqihar Med Coll. (2017) 38:252–4.
38. Zhang WY, Huang GY, Liu J, Zhang MM, Wang W. Effects of acupuncture on the expression of Tgf-A and Egfr in the ovaries of polycystic ovary syndrome rats. Journal of Microcirc. (2008) 18:4–7.
39. Zhang WY, Huang GY, Liu J. Influences of acupuncture on infertility of rats with polycystic ovarian syndrome. Chin J Integr Tradit West Med. (2009) 29:997–1000.
40. Sun J, Jin C, Wu H, Zhao J, Cui Y, Liu H, et al. Effects of electro-acupuncture on ovarian P450arom, P450c17α and mRNA expression induced by letrozole in PCOS rats. PLoS ONE. (2013) 8:e79382. doi: 10.1371/journal.pone.0079382
41. Yin Y. Effects of Acupuncture on Hpo Axis and Fsh Camp Signaling Pathway in Rats with Ovarian Reserve Dysfunction [Master]. Beijing: China Academy of Chinese Medical Sciences (2020).
42. Yuan CP, Peng LM, Wang S, Wang MY. Effects of acupuncture treatment on overall efficacy, hormone levels, and inflammatory markers in rats with polycystic ovary syndrome. J Tradit Chin Med. (2021) 49:60–4. doi: 10.19664/j.cnki.1002-2392.210139
43. Chen XH, Tang HL, Liang YY, Wu PT, Xie LH, Ding Y, et al. Acupuncture regulates the autophagy of ovarian granulosa cells in polycystic ovarian syndrome ovulation disorder by inhibiting the Pi3k/Akt/Mtor pathway through Lncmeg3. Biomed Pharmacother. (2021) 144:112288. doi: 10.1016/j.biopha.2021.112288
44. Peng Y, Guo L, Gu A, Shi B, Ren Y, Cong J, et al. Electroacupuncture alleviates polycystic ovary syndrome-like symptoms through improving insulin resistance, mitochondrial dysfunction, and endoplasmic reticulum stress via enhancing autophagy in rats. Mol Med. (2020) 26:73. doi: 10.1186/s10020-020-00198-8
45. Zhao XD, Li ZH, Hu JW, Chen YL, Xu G. Effects of electroacupuncture on the secretion function of ovarian cells and kisspeptin/Kiss1r system in rats with polycystic ovarian syndrome. Acupunct Res. (2023) 48:804–11. doi: 10.13702/j.1000-0607.20220481
46. Yu XX, Wu QY, Huang J, Cai J, Cui DP, Wang WB, et al. Effects of electroacupuncture on serum testosterone, estradiol, and progesterone levels in elderly female rats. Shanghai Acupunct J. (2005) 24:37–9. doi: 10.13460/j.issn.1005-0957.2005.09.025
47. Ma XP, Dai M, Shi Z, Zhao TP, Tan LY, Jiang B, et al. Effects of acupuncture on ovarian morphology and serum E2 levels in perimenopausal rats. Jiangsu Tradit Chin Med. (2008) 40:80–2.
48. Liu H, Wang B, Wu G. Effect of Electroacupuncture on neuroendocrine immune network in climacteric rats. JiangSu Tradit Chin Med. (2003) 24:49–51.
49. Kong SP, Wang YG, Li MY, Zhou SH. Effects of kidney-tonifying and liver-regulating acupuncture method on hormones in rats with premature ovarian insufficiency. World J Integr Tradit West Med. (2016) 6:114–6, 35. doi: 10.13935/j.cnki.sjzx.160134
50. Chen YR, Dong F, Li CH, Chen H, Xiao T. Effects of acupuncture at different time points on central and peripheral hormone levels in rats with premature ovarian insufficiency. Shanghai Acupunct J. (2020) 39:220–5. doi: 10.13460/j.issn.1005-0957.2020.02.0220
51. Zhang YM, Yu B, Chen J, Zhao ZS, Wang JL, Huang FS, et al. Effects of acupuncture treatment on the Pi3k/Akt/Mtor signaling pathway in rats with premature ovarian insufficiency. Chin Acupunct. (2015) 35:53–8. doi: 10.13703/j.0255-2930.2015.01.016
52. Ma MN Si JH, Jin XF Li JR, Yan J. Effects of ‘Zhibian' to ‘Shuidao' acupuncture technique on the Hpo axis and Bcl-2, Bax proteins in rats with premature ovarian insufficiency. Acupunct Clin J. (2022) 38:60–5. doi: 10.19917/j.cnki.1005-0779.022175
53. Wang WM, Wang Y, Wu JN, Yang LK, Liu ZS. Experimental study on the improvement of ovarian function in rats with premature ovarian insufficiency model by electroacupuncture at acupoints ‘Zhongliao' and ‘Tianshu'. Chin Acupunct. (2018) 38:519–26. doi: 10.13703/j.0255-2930.2018.05.018
54. Yan J, Ma MN, Zhao JY, Wang HY, Yin LY, Jin XF. Effects of “Zhibian” to “Shuidao” acupuncture on the expression of the death receptor pathway Fas/Fadd/Caspase-8 in rats with premature ovarian insufficiency. Chin Acupunct. (2023) 43:537–44. doi: 10.13703/j.0255-2930.20220301-k0002
55. Yu XQ, Li YJ. Effects of electroacupuncture on hormones and Tsp-1 expression in ovarian tissues of polycystic ovary syndrome model rats. J Hunan Univ Chin Med. (2019) 39:511–5.
56. Li W, Zhang YS, Kuang HY, Feng WT. Effects of Electroacupuncture on glucose and lipid metabolism disorders in polycystic ovary syndrome model rats. Acupunct Clin J. (2016) 32:70–3.
57. Shi Y, Li L, Zhou J, Sun J, Chen L, Zhao J, et al. Efficacy of electroacupuncture in regulating the imbalance of Amh and Fsh to improve follicle development and hyperandrogenism in PCOS rats. Biomed Pharmacother. (2019) 113:108687. doi: 10.1016/j.biopha.2019.108687
58. Wang S-C, Jiang Y-M, Qiu LR, Su M. Efficacy of needling acupoints of Guanyuan (Cv4), Sanyinjiao (Sp6), Zusanli (St36), Pishu (Bl20), Shenshu (Bl23), Zigong (Ex-Ca1) on expression of P38 mitogen-activated protein kinase in ovarian tissue in rats with premature ovarian failure induced by cyclophospha- mide. J Tradit Chin Med. (2021) 41:953–8. doi: 10.19852/j.cnki.jtcm.2021.06.012
59. Wang SQ, Lin SJ, Zhu MM, Li CL, Chen SL, Pu L, et al. Acupuncture reduces apoptosis of granulosa cells in rats with premature ovarian failure via restoring the Pi3k/Akt signaling pathway. Int J Mol Sci. (2019) 20:6311. doi: 10.3390/ijms20246311
60. Liu HY, Wu GY, Wang B, Wang H. Experimental study on acupuncture treatment for menopausal syndrome. J Tianjin Univ Tradit Chin Med. (2002) 21:45–6.
61. Palermo R. Differential actions of Fsh and Lh during folliculogenesis. Reprod Biomed Online. (2007) 15:326–37. doi: 10.1016/S1472-6483(10)60347-1
62. Adashi EY. Endocrinology of the ovary. Human Reprod. (1994) 9:815–27. doi: 10.1093/oxfordjournals.humrep.a138602
63. Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. (2015) 3:980–92. doi: 10.1016/S2213-8587(15)00284-3
64. Salehpour S, Hosseini S, Nazari L, Saharkhiz N, Zademodarres S. Effects of orlistat on serum androgen levels among Iranian obese women with polycystic ovarian syndrome. JBRA Assist Reprod. (2018) 22:180–4. doi: 10.5935/1518-0557.20180033
65. Chauvin S, Cohen-Tannoudji J, Guigon CJ. Estradiol signaling at the heart of folliculogenesis: its potential deregulation in human ovarian pathologies. Int J Mol Sci. (2022) 23:512. doi: 10.3390/ijms23010512
66. Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. (2011) 46:685–93. doi: 10.1016/j.exger.2011.04.006
67. Xuemin S. Study of the relationship between acupuncture dose and effect. Acupunct Herbal Med. (2021) 1:3–9. doi: 10.1097/HM9.0000000000000009
68. Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reductions in plasma estradiol and progesterone during the estrous cycle of C57bl/6j mice. Endocrinology. (1992) 130:805–10. doi: 10.1210/endo.130.2.1733727
69. Zang L, Zhang Q, Zhou Y, Zhao Y, Lu L, Jiang Z, et al. Expression pattern of G protein-coupled estrogen receptor 1 (GPER) in human cumulus granulosa cells (CGCs) of patients with PCOS. Syst Biol Reprod Med. (2016) 62:184–91. doi: 10.3109/19396368.2016.1164260
70. Xu XL, Huang ZY, Yu K, Li J, Fu XW, Deng SL. Estrogen biosynthesis and signal transduction in ovarian disease. Front Endocrinol. (2022) 13:827032. doi: 10.3389/fendo.2022.827032
71. Yu X, Li Y. Effect of Electroacupuncture on sex hormone and Tsp-1 Expression in ovary of PCOS model rats. J Hunan Univ Tradit Chin Med. (2019) 39:511–5.
72. Zhong YZ, Lin J, Xie PP, Chen JJ, et al. Mechanism of action of acupuncture in the treatment of polycystic ovary syndrome in rats by regulating the Akt/Mtor autophagy pathway through LNCRNA Meg3. J. Shanxi Med. Univ. (2022) 53:44854. doi: 10.13753/j.issn.1007-6611.2022.04.011
73. Lin J. To Explore the Mechanism of Acupuncture Effect on Ovulation Disorder in Pcos from Lncmeg3 Mediated Pi3k/Akt/Mtor Regulation of Autophagy in Ovarian Granulosa Cells [Doctor]. Guangzhou: Guangzhou University of Traditional Chinese Medicine (2020).
74. Geng ZX, Nie XL, Liu P, et al. Effect of electroacupuncture on ferroptosis in mice with premature ovarian failure. Shanghai J Acupunct Moxibust. (2023) 42:175–81. doi: 10.13460/j.issn.1005-0957.2022.13.2020
75. Guo M, Yang YH, Lu ZL, et al. Electroacupuncture improves ovulatory dysfunction and sex hormone levels in rats with polycystic ovary syndrome model by inhibiting leptin/protocin axis. J. Guangzhou Univ. Chinese Med. (2022) 39:2102–8. doi: 10.13359/j.cnki.gzxbtcm.2022.09.023
76. He Z, Hu L, Shen X, Zhu Q. Regulatory effect of electroacupuncture at different combinations of acupoints on reproductive endocrine immune network function in perimenopausal rats. J Anhui Coll Tradit Chin Med. (2010) 29:40–4.
77. Si J. Study on the Effect of Zhibian Channel Penetrating Acupuncture on Sex Hormone and Expression of Bcl-2 and Bax in Rats with Early-Onset Ovarian Insufficiency [Master]. Taiyuan: Shanxi University of Traditional Chinese Medicine (2020).
78. Bai YY, Kang ZY. Progress in the study of the mechanism of acupuncture in treating ovarian reserve hypoplasia. J. Liaoning Univ. Tradit. Chinese Med. (2023) 25:161–5. doi: 10.13194/j.issn.1673-842x.2023.05.032
79. Shen L, Chang X, Pan L, Liu X, Yang Z, Hu H. Effect of electroacupuncture on expression of kisspeptin protein in hypothalamus of rats with polycystic ovary syndrome. Acupunct Res. (2021) 46:106–10. doi: 10.13702/j.1000-0607.200497
80. Fu L, Zhao J, Liu Y. Regulating effect of acupuncture ‘Zusanli', ‘Shenshu', ‘Guanyuan' and ‘Sanyinjiao' on gonadal axis in perimenopausal rats. Med Innov China. (2011) 8:12–3.
81. Singh A, Choubey M, Bora P, Krishna A. Adiponectin and chemerin: contrary adipokines in regulating reproduction and metabolic disorders. Reprod Sci. (2018) 25:1462–73. doi: 10.1177/1933719118770547
82. Begum M, Choubey M, Tirumalasetty MB, Arbee S, Mohib MM, Wahiduzzaman M, et al. Adiponectin: a promising target for the treatment of diabetes and its complications. Life. (2023) 13:2213. doi: 10.20944/preprints202310.0782.v1
83. Groth SW. Adiponectin and polycystic ovary syndrome. Biol Res Nurs. (2010) 12:62–72. doi: 10.1177/1099800410371824
84. Shirazi FKH, Khodamoradi Z, Jeddi M. Insulin resistance and high molecular weight adiponectin in obese and non-obese patients with polycystic ovarian syndrome (PCOS). BMC Endocr Disord. (2021) 21:45. doi: 10.1186/s12902-021-00710-z
85. Bril F, Ezeh U, Amiri M, Hatoum S, Pace L, Chen YH, et al. Adipose tissue dysfunction in polycystic ovary syndrome. J Clin Endocrinol Metab. (2023) 109:10–24. doi: 10.1210/clinem/dgad356
86. Gonzalez Garcia B, Garcia Vicente AM, Palomar Munoz A, Poblete Garcia VM, Jimenez Londono GA, Soriano Castrejon AM. Incidental pathologic extracardiac uptake of (99m)Tc-tetrofosmin in myocardial perfusion imaging: importance of patient background evaluation. Rev Esp Med Nucl Imagen Mol. (2015) 34:383–6. doi: 10.1016/j.remn.2015.03.005
87. Jing Z, Ping Y, Qingyi Z, Zhihai H, Yi W, Guizhi M, et al. Electroacupuncture improves follicular development and metabolism and regulates the expression of adiponectin, Ampk and Acc in an Obese Rat Model of Polycystic Ovary Syndrome. Acupunct Med. (2022) 41:151–62. doi: 10.1177/09645284221107690
88. Dou Z, Ma S-H, Song J-Y, Xia T. Retrospective analysis on pregnancy outcomes and fat-related factors of treatment of endomorph PCOS Infertility patients by acupuncture of 8 acupoints around umbilicus. Zhen Ci Yan Jiu. (2021) 46:158–63. doi: 10.13702/j.1000-0607.200119
Keywords: polycystic ovary syndrome, premature ovarian failure, perimenopausal syndrome, premature ovarian insufficiency, acupuncture, electroacupuncture, ovarian function, animal experiments
Citation: Zhao Y, Lan Y, Liu L, Hao J, Wang H and Ji L (2024) Efficacy of acupuncture in animal models of various ovarian dysfunctions: a systematic review and meta-analysis. Front. Med. 11:1348884. doi: 10.3389/fmed.2024.1348884
Received: 03 December 2023; Accepted: 03 June 2024;
Published: 20 June 2024.
Edited by:
Mayank Choubey, NYU Grossman Long Island School of Medicine, United StatesReviewed by:
Atul Kumar, Columbia University, United StatesCopyright © 2024 Zhao, Lan, Liu, Hao, Wang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Lan, bGFueWluZ3RjbUAxNjMuY29t; Laixi Ji, amlsYWl4aXRjbUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.