
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 12 April 2024
Sec. Family Medicine and Primary Care
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1342792
This article is part of the Research TopicExploring the Interaction between Health-promoting and Health Risk Behaviours in HealthView all 16 articles
Background: This research aims to investigate the relationship between Life’s Essentials 8 (LE8), the American Heart Association’s latest indicator, and periodontitis. The purpose is to provide guidance on preventative measures.
Methods: Data for our investigation were obtained from the National Health and Nutrition Examination Survey (NHANES) 2009–2014, with a total of 8,784 participants eligible. LE8 scores were compiled from 8 index scores (the score for each component of diet, physical activity, nicotine exposure, sleep duration, body mass index, blood lipids, blood glucose, and blood pressure). Periodontitis was classified by the Centers for Disease Control and Prevention and American Academy of Periodontology (CDC/AAP). The study utilized multivariable logistic analyses to investigate the potential correlation.
Results: After controlling for all covariates, LE8 was discovered to have a significant negative correlation with periodontitis prevalence [0.91 (0.88, 0.94)]. This trend continued to hold statistical significance even after converting LE8 into a categorical variable. Furthermore, a noteworthy adverse correlation was discovered across both genders, specifically males [0.35 (0.22, 0.55)] and females [0.39 (0.25, 0.60)], as well as for the majority of categorical classifications, namely ethnicity, age, education level, and marital status. However, only the age subgroups displayed some degree of significant difference from each other.
Conclusion: Life’s essential 8 was negatively associated with periodontitis, but more prospective trails are needed to confirm our findings.
Periodontitis is a chronic inflammatory disease that damages the supporting tissue of the teeth, leading to the pathological resorption of the alveolar bone around the teeth, recession of the gingival tissues, and even loss of the teeth (1, 2). This condition poses a public health concern, impacting the oral and overall health of individuals across the world (3).
Matlila ‘s report represented the first reference to the relationship between oral infection and acute myocardial infarction (4). Subsequent research has shown that periodontitis not only affects systemic diseases but can also be influenced by them (5). Diseases such as cardiovascular disease trigger an immune response in the host, and the resulting metabolic dysfunction can cause chronic metabolic inflammatory disease. This, as one of the risk factors for periodontitis, can increase its morbidity (6, 7). Both periodontitis and cardiovascular disease are multifactorial conditions triggered by genetic, environmental, and lifestyle habits. Common risk factors for both diseases include increasing age, smoking, alcohol misuse, ethnicity, education and socioeconomic status, male gender, diabetes, and obesity. Several cross-sectional studies, case analyses, and epidemiological investigations indicate a significant correlation between chronic periodontitis and cardiovascular disease (8–11).
In 2010, the American Heart Association (AHA) defined “ideal cardiovascular health” as the presence of seven factors and behaviors that increase the chances of living a life free of cardiovascular disease and stroke (12). These seven factors and behaviors, including diet, physical activity, smoking, body mass index, total cholesterol, blood pressure, and blood glucose are known as “life’s simple 7 (LS7),” and are considered to be the core elements of building a healthier life (12). Studies have consistently demonstrated that higher LS7 scores are associated with greater cardiovascular health and reduced all-cause mortality in various populations (13–15). In 2022, the American Heart Association (AHA) established the Life’s Essential 8 (LE8) score, building upon the LS7 framework by introducing sleep as a novel cardiovascular health (CVH) determinant. According to the AHA, Life’s Essential 8 serves as a pivotal indicator for enhancing and preserving CVH, which can lower the incidence of heart disease, stroke, and other significant medical conditions (16–18). However, studies have not yet existed that have explored the relationship between LE8 and the incidence of periodontitis.
Hence, this study’s primary goal was to investigate this relationship, using nationally representative data from the National Health and Nutrition Examination Survey (NHANES).
The research data for this study were obtained from the NHANES survey from 2009–2014. NHANES is a population-based survey designed to collect health and nutritional information on the household population in the U.S.A (19). All participants provided written informed consent to conduct all survey procedures by relevant guidelines and standards.1 Over the 6 years of data from 2009–2014, as shown in Figure 1, a total of 8,784 respondents were finally included in the analysis of the study, with the following inclusion criteria: 18 years of age or older; having complete baseline information on the population; having received the NHANES “Oral Health-Periodontal Examination” and having recorded all measurements as required by the periodontal classification algorithm; and having the eight indicators as are necessary for a complete LE8.
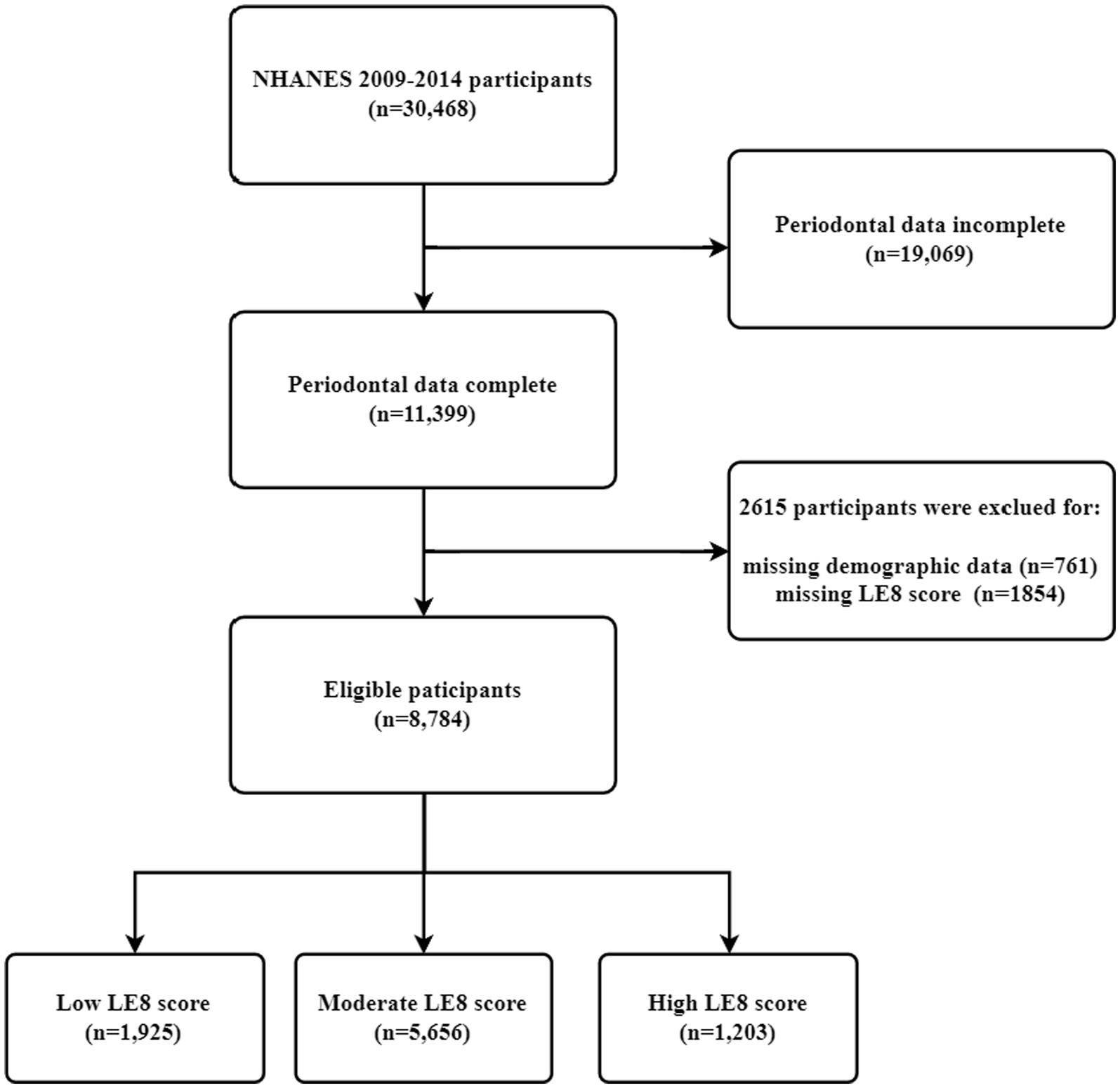
Figure 1. Study population selection (N = 8,784). NHANES, National Health and Nutrition Examination Survey; LE8, Life’s Essential 8.
The LE8 metrics comprised four health behaviors and four health factors, namely diet, physical activity, nicotine exposure, sleep health, body mass index (BMI), non-high-density lipoprotein (HDL) cholesterol, blood glucose, and blood pressure (BP). These factors were evaluated as shown in Supplementary Table S1, where the HEI-2015 (The Healthy Eating Index-2015) was used to assess dietary levels on a scale from 0–100. Higher HEI scores indicated better diet quality (20). Day 1 total nutrient intake (DR1TOT) from NHANES was employed to compute the 13 elements of the HEI-2015 (21). Supplementary Table S2 provides specific guidance on how to calculate HEI-2015. The remaining 7 components can be derived directly from NHANES. LE8 scores were classified as low (0–49), moderate (50–79), or high (≥80) (15, 22).
The program titled “Oral Health - Periodontal Screening” under the NHANES 2009–2014 performs measurements in six regions of every tooth with a maximum of 28 teeth. It contains two sets of clinical periodontal measurements - clinical attachment loss (CAL) and probing depth (PD). The periodontitis classification system was established based on case definitions from the Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC/AAP). Severe periodontitis was determined by the presence of ≥2 interproximal areas with a CAL of ≥6 mm that were not on the same tooth and the presence of ≥1 interproximal area with a PD of ≥5 mm. Moderate periodontitis was defined as the presence of two or more interproximal sites with a probing pocket depth exceeding or equal to 5 mm, not located on the same tooth, or two or more interproximal sites with clinical attachment level exceeding or equal to 4 mm, not found on the same tooth, as previously established (23, 24). Moderate/severe periodontitis cases were identified as patients with periodontitis, while all other cases (no/mild periodontitis) were classified as the reference group (24, 25).
Covariates included age (<40, 40–60, >60), gender (male, female), race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, different race/including multiracial), marital status (divorced/separated/married, married/cohabiting with a partner, never married), poverty-to-income ratio (categorized as low-income <1. 3, moderate-income 1.3–3.5, high income ≥3.5) (26), and educational attainment (less than high school, high school, some college or above).
All analyses were performed using R (version 4.2) and Empowerstats (version 5.0) (27, 28), and all statistical analyses were weighted according to the NHANES guidelines. Participants with high LE8 scores were considered to have LE8 scores of 80–100, moderate LE8 scores of 50 ~ 79, and low LE8 scores of 0 ~ 49 (16). We used chi-square tests and t-tests for LE8 trichotomous tests to assess demographic characteristics. To investigate the correlation between LE8 and periodontitis, we conducted a series of multiple linear regression analyses, examining the relationship between LE8 scores (per 10 points) and the prevalence of periodontitis. Finally, we conducted subgroup analyses to determine any differences in the above correlations based on gender, age, race, income, education, and marital status.
A study comprising 8,784 participants with an average age of 51.65 years was conducted, with 50.59% of the sample being male, as displayed in Table 1. The population prevalence of periodontitis was 49.44%. The LE8 scores were classified into low, medium, and high groups (0–49, 50–79, and 80–100) in agreement with the requirements prescribed by AHA. Overall, the prevalence of moderate to severe periodontitis was 49.44%. This decreased consistently with increasing LE8 group scores: 58.56% for the low group, 49.68% for the medium, and 33.25% for the high (p < 0.001). Moreover, higher LE8 scores were associated with a higher likelihood of being female, having higher education and income levels, and displaying significant differences in race and marital status.
The relationship between LE8 and periodontitis is presented in Table 2. It was found that LE8 scores had a negative association with the prevalence of periodontitis in all models. In the unadjusted model 1, the prevalence of periodontitis in the high LE8 group was 0.35 (95% confidence interval, 0.3, 0.4). In Model 3, after controlling for all covariates, including age, gender, race, income, marital status, and education level, the study revealed that the risk of periodontitis was reduced by 0.09% for every increase of 10 points in the LE8 score. In the high LE8 group, the risk decreases by 0.3%, and the results are statistically significant. Figure 2 illustrates the non-linear correlation between LE8 and periodontitis.
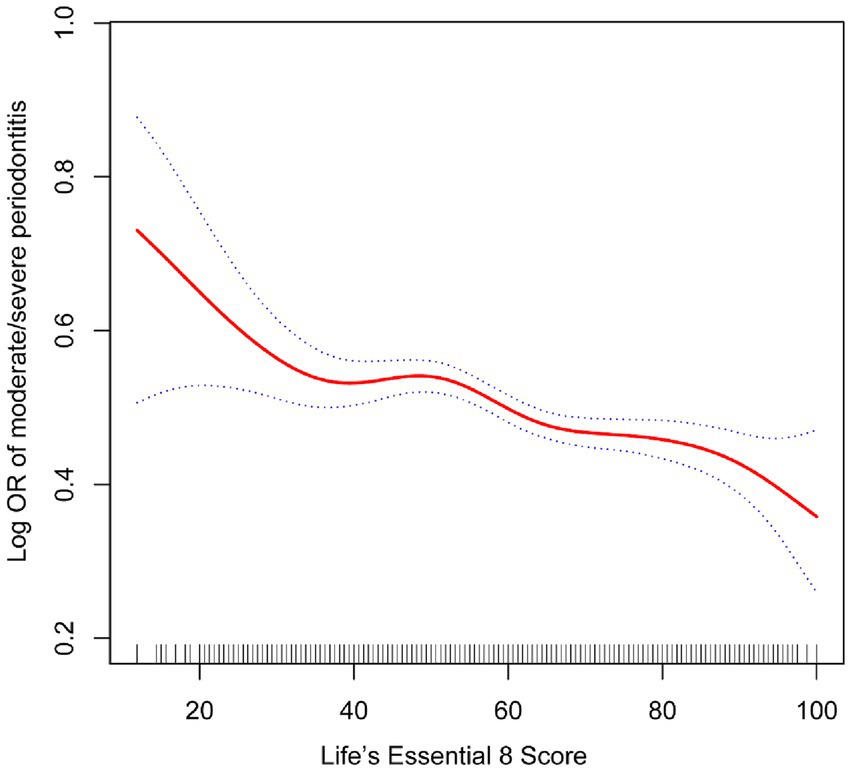
Figure 2. The association between life’s essential 8 and periodontitis. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit.
We conducted subgroup analyses of each covariate to determine potential effect factors. To illustrate the correlation between the two variables, we analyzed the association between LE8 score(per 10 points)and periodontitis. Table 3 presents results indicating age altering the association between LE8 scores and periodontitis. While several factors, including gender, race, income, education level, and marital status, had statistically significant effects within subgroups, they did not modify the correlation between LE8 and periodontitis between subgroups.
In this cross-sectional study of large-scale, population-based survey data, we identified a negative association between LE8 scores and periodontitis. The negative association between LE8 scores and periodontitis was more significant in participants aged 40–60, according to subgroup analyses. As LE8 is a recent improvement for assessing cardiovascular health, the current report enhances the considerable evidence of an association between cardiovascular health and periodontitis (16). Improving LE8 scores may offer clinical benefits as a viable and effective means to promote periodontal well-being.
Cardiovascular health is a broader, more positive concept than simply the lack of illness. To measure cardiovascular health, the American Heart Association established LS7 and LE8 in 2010 and 2022 as tools for defining and quantifying cardiovascular health (16). The most recent LE8 employs a combination of two domains and eight metrics to determine one’s cardiovascular health. These metrics cover various health behaviors, including physical activity, diet, sleep health, and nicotine exposure, alongside health factors like blood pressure, blood glucose, blood lipids, and BMI.
A large number of studies have reported significant associations between most of the health factors and health behavior indicators in LS7 or LE8 and periodontitis individually, such as blood glucose (29), blood pressure (30), lipids (31), BMI (32), nicotine exposure (32, 33), and diet (34). However, to our knowledge, no study has evaluated the association between LS7 or LE8 as independent factors and periodontitis.
Healthy behaviors, such as a balanced diet and reduced nicotine exposure, can potentially prevent periodontitis. A cross-sectional study examining data from the US NHANES between 2009 and 2014 found that most identified dietary patterns were not correlated with periodontitis severity. However, a diet comprising mainly salads, fruits and vegetables, and plain water or tea was associated with reduced levels of CAL (34). Further data from the Hamburg City Health Study (HCHS), which examined 6,209 participants, indicated a noteworthy correlation between increased adherence to both the DASH diet and the Mediterranean diet and reduced likelihood of periodontal disease. It is essential to note that the Mediterranean Eating Pattern for Americans (MEPA) tool and the Healthy Eating Index scores-2015 (HEI-2015) in the LE8 scores can determine dietary healthiness. However, it is imperative to understand that these metrics do not assess the healthiness of all dietary patterns of individuals or populations (16). Nicotine exposure is described as using combustible tobacco, inhaling nicotine-delivery systems (NDS), or being exposed to secondhand smoke (16). A 2018 systematic review and meta-regression analysis reported an 85% increase in the risk of periodontitis due to cigarette smoking (35). Another study based on Mendelian randomization found a robust and reliable association between genetic predisposition to smoking and periodontitis (32). Exposure to environmental tobacco smoke has been positively linked to periodontitis endpoints, as demonstrated by prior research (33).
Interestingly, the risk of developing or progressing periodontitis in ex-smokers was not significantly different from that in never-smokers. The precise molecular mechanisms responsible for the negative correlation between smoking and periodontal tissue health are not fully understood (36). Still, current research suggests that it may be linked to smoking or nicotine exposure disrupting inflammation and the host’s response to periodontal pathogens, alterations to the subgingival microbial community, and hindered tissue healing potential (37).
Health factors such as good body mass index, lipids, blood glucose, and blood pressure contribute to maintaining periodontal health. In 2021, the results of a systematic total and meta-analysis based on a cohort study by Stöhr et al. (38) showed that there is a bi-directional positive correlation between periodontal disease and diabetes mellitus, i.e., diabetes mellitus increases the risk of periodontitis, and periodontal inflammation negatively affects blood glucose control. The association between diabetes and periodontal disease is underpinned by various factors, including hyperglycemia, genetic, microbial, and lifestyle co-predisposing factors, which culminate in advanced glycosylation end products (39). Additionally, poorly controlled diabetes can lead to elevated levels of IL1-β, TNF-α, IL-6, RANKL/OPG, and oxygen metabolites in the gingiva, which can contribute to the destruction of periodontal tissues (40). In addition, associations between obesity, hypertension, and dyslipidemia have also been reported with periodontal diseases (31, 41–44). On the other hand, improvement in systemic blood pressure and lipid levels is expected with periodontal treatment (30, 31).
The study discovered a link between LE8 (a marker of cardiovascular health) and periodontal health outcomes. This confirms previous research linking periodontitis and cardiovascular disease (45). Patients with periodontitis have an elevated risk of developing coronary heart disease and atherosclerosis (46–48). Effective periodontal care can significantly decrease the likelihood of experiencing cardiovascular events, including cardiac death and myocardial infarction, according to a report (49). The correlation between periodontitis and cardiovascular disease may be linked to bacteremia caused by the entry of periodontal pathogenic bacteria into the vascular system, an increase in the systemic inflammatory response due to periodontitis, genetic factors, and environmental risk factors (47).
Our research expands on the LE8, a clinical cardiovascular health assessment tool that is comprehensive and easy to use to indicate periodontal health outcomes. Adherence to the ideal Lifestyle Index 8 could be suitable for preventing and managing periodontal disease and cardiovascular health.
Our study has several strengths. First, NHANES utilizes a complex multistage probability sampling design that draws a representative noninstitutionalized resident population to ensure higher data quality. As a result, extrapolating the results to the entire U.S. civilian noninstitutional population is highly reliable. Second, we investigated for the first time the association between a new indicator, LE8, and periodontitis, which increases the feasibility of self-determination of periodontitis risk by people in their daily lives, increasing periodontitis prevention awareness and decreasing morbidity.
However, there are several limitations to the present study. Firstly, it is limited due to the cross-sectional nature of NHANES, which precludes the inference of causality and necessitates support from numerous future prospective studies. Secondly, data on health behaviors such as diet, exercise, sleep, and smoking are self-reported and may be prone to recall bias. Lastly, there are some unmeasured influences, and the experimental data may have errors.
In summary, the proposed LE8 index by the American Heart Association revealed a negative correlation with the risk of periodontitis. Furthermore, the association was stronger in participants aged between 40 and 60 years, and LE8 exhibited a nonlinear correlation with the incidence of periodontitis. These findings imply that improving the LE8 index could be an effective measure for the tertiary prevention of periodontitis.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
KH: Writing – original draft. HZ: Data curation, Writing – review & editing. WS: Software, Writing – original draft. SL: Investigation, Writing – review & editing. JL: Methodology, Writing – original draft. ZM: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Capital Health Development Research Program, Award number: SF-2022-4-7101 and Beijing Shunyi District Research and Development Program, Award Number: Shunyi2023Q06.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1342792/full#supplementary-material
1. Slots, J . Periodontitis: facts, fallacies and the future. Periodontol 2000. (2017) 75:7–23. doi: 10.1111/prd.12221
2. Kwon, T, Lamster, IB, and Levin, L. Current concepts in the Management of Periodontitis. Int Dent J. (2021) 71:462–76. doi: 10.1111/idj.12630
3. Sanz, M, Herrera, D, Kebschull, M, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47 Suppl 22:4–60. doi: 10.1111/jcpe.13290
4. Mattila, KJ . Dental infections as a risk factor for acute myocardial infarction. Eur Heart J. (1993) 14 Suppl K:51–3. doi: 10.1002/ccd.1810300422
5. Akinkugbe, AA, and Papapanou, PN. The "sufficient cause" model framework applied to the periodontitis-systemic diseases link. J Periodontol. (2021) 92:343–7. doi: 10.1002/JPER.20-0148
6. Teles, F, Wang, Y, Hajishengallis, G, et al. Impact of systemic factors in shaping the periodontal microbiome. Periodontol 2000. (2021) 85:126–60. doi: 10.1111/prd.12356
7. Hajishengallis, G, and Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. (2021) 21:426–40. doi: 10.1038/s41577-020-00488-6
8. Ionel, A, Lucaciu, O, Bondor, C, et al. Assessment of the relationship between periodontal disease and cardiovascular disorders: a questionnaire-based study. Clujul Med. (2016) 89:534–41. doi: 10.15386/cjmed-639
9. Tonetti, MS, and Van Dyke, TE. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases [J]. J Clin Periodontol. (2013) 40:S24–9. doi: 10.1111/jcpe.12089
10. Herrera, D, Molina, A, Buhlin, K, et al. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000. (2020) 83:66–89. doi: 10.1111/prd.12302
11. Vázquez-Reza, M, López-Dequidt, I, Ouro, A, et al. Periodontitis is associated with subclinical cerebral and carotid atherosclerosis in hypertensive patients: a cross-sectional study [J]. Clin Oral Investig. (2023) 27:3489–98. doi: 10.1007/s00784-023-04958-8
12. Sacco, RL . The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). (2011) 12:255–7. doi: 10.2459/JCM.0b013e328343e986
13. Nève, G, Wagner, J, Knaier, R, et al. Ideal Life's simple 7 score relates to macrovascular structure and function in the healthy population. Nutrients. (2022) 14. doi: 10.3390/nu14173616
14. Del Brutto, OH, Mera, RM, Recalde, BY, et al. Life's simple 7 and all-cause mortality. A population-based prospective cohort study in middle-aged and older adults of Amerindian ancestry living in rural Ecuador. Prev Med Rep. (2022) 25:101668. doi: 10.1016/j.pmedr.2021.101668
15. Shetty, NS, Parcha, V, Patel, N, et al. AHA Life's essential 8 and ideal cardiovascular health among young adults. Am J Prev Cardiol. (2023) 13:100452. doi: 10.1016/j.ajpc.2022.100452
16. Lloyd-Jones, DM, Allen, NB, Anderson, CAM, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
17. Li, C, Li, Y, Zhao, M, et al. Using the new "Life's essential 8" metrics to evaluate trends in cardiovascular health among US adults from 2005 to 2018: analysis of serial cross-sectional studies. JMIR Public Health Surveill. (2023) 9:e45521. doi: 10.2196/45521
18. Ueno, K, Kaneko, H, Okada, A, et al. Association of four health behaviors in Life's essential 8 with the incidence of hypertension and diabetes mellitus. Prev Med. (2023) 175:107685. doi: 10.1016/j.ypmed.2023.107685
20. Kaschalk-Woods, E, Fly, AD, Foland, EB, et al. Forecasting your future: nutrition matters curriculum with teacher training promotes students to try new fruits and vegetables. Curr Dev Nutr. (2020) 4:nzaa101. doi: 10.1093/cdn/nzaa101
21. Krebs-Smith, SM, Pannucci, TE, Subar, AF, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
22. Lloyd-Jones, DM, Ning, H, Labarthe, D, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new "Life's essential 8" metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. (2022) 146:822–35. doi: 10.1161/CIRCULATIONAHA.122.060911
23. Eke, PI, Page, RC, Wei, L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. (2012) 83:1449–54. doi: 10.1902/jop.2012.110664
24. Eke, PI, Dye, BA, Wei, L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. (2015) 86:611–22. doi: 10.1902/jop.2015.140520
25. SSY, AL, Natto, ZS, Midle, JB, et al. Association between time since quitting smoking and periodontitis in former smokers in the National Health and nutrition examination surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. doi: 10.1002/JPER.18-0183
26. Ogden, CL, Fakhouri, TH, Carroll, MD, et al. Prevalence of obesity among adults, by household income and education - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. (2017) 66:1369–73. doi: 10.15585/mmwr.mm6650a1
27. Xie, R, and Liu, M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol. (2022) 13:857110. doi: 10.3389/fendo.2022.857110
28. Lu, J, Li, H, and Wang, S. The kidney reabsorption-related magnesium depletion score is associated with increased likelihood of abdominal aortic calcification among US adults. Nephrol Dial Transplant. (2023) 38:1421–9. doi: 10.1093/ndt/gfac218
29. Kocher, T, König, J, Borgnakke, WS, et al. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000. (2018) 78:59–97.
30. Czesnikiewicz-Guzik, M, Osmenda, G, Siedlinski, M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. (2019) 40:3459–70. doi: 10.1093/eurheartj/ehz646
31. Mirzaei, A, Shahrestanaki, E, Malmir, H, et al. Association of periodontitis with lipid profile: an updated systematic review and meta-analysis. J Diabetes Metab Disord. (2022) 21:1377–93. doi: 10.1007/s40200-022-01071-7
32. Larsson, SC, and Burgess, S. Appraising the causal role of smoking in multiple diseases: a systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine. (2022) 82:104154. doi: 10.1016/j.ebiom.2022.104154
33. Akinkugbe, AA, Slade, GD, Divaris, K, et al. Systematic review and meta-analysis of the association between exposure to environmental tobacco smoke and periodontitis endpoints among nonsmokers. Nicotine Tob Res. (2016) 18:2047–56. doi: 10.1093/ntr/ntw105
34. Wright, DM, McKenna, G, Nugent, A, et al. Association between diet and periodontitis: a cross-sectional study of 10,000 NHANES participants. Am J Clin Nutr. (2020) 112:1485–91. doi: 10.1093/ajcn/nqaa266
35. Leite, FRM, Nascimento, GG, Scheutz, F, et al. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med. (2018) 54:831–41. doi: 10.1016/j.amepre.2018.02.014
36. Caggiano, M, Gasparro, R, D'Ambrosio, F, et al. Smoking cessation on periodontal and Peri-implant health status: a systematic review. Dent J. (2022) 10. doi: 10.3390/dj10090162
37. Apatzidou, DA . The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol 2000. (2022) 90:45–61. doi: 10.1111/prd.12449
38. Stöhr, J, Barbaresko, J, Neuenschwander, M, et al. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. (2021) 11:13686. doi: 10.1038/s41598-021-93062-6
39. Nibali, L, Gkranias, N, Mainas, G, et al. Periodontitis and implant complications in diabetes [J]. Periodontol 2000. (2022) 90:88–105. doi: 10.1111/prd.12451
40. Polak, D, and Shapira, L. An update on the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. (2018) 45:150–66. doi: 10.1111/jcpe.12803
41. Martin-Cabezas, R, Seelam, N, Petit, C, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J. (2016) 180:98–112. doi: 10.1016/j.ahj.2016.07.018
42. Pamuk, F, and Kantarci, A. Inflammation as a link between periodontal disease and obesity. Periodontol 2000. (2022) 90:186–96. doi: 10.1111/prd.12457
43. Moura-Grec, PG, Marsicano, JA, Carvalho, CA, et al. Obesity and periodontitis: systematic review and meta-analysis. Ciênc Saúde Colet. (2014) 19:1763–72. doi: 10.1590/1413-81232014196.13482013
44. Issrani, R, Reddy, J, Bader, AK, et al. Exploring an association between body mass index and Oral health-a scoping review. Diagnostics. (2023) 13. doi: 10.3390/diagnostics13050902
45. Sanz, M, Marco Del Castillo, A, Jepsen, S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47:268–88. doi: 10.1111/jcpe.13189
46. Larvin, H, Kang, J, Aggarwal, VR, et al. Risk of incident cardiovascular disease in people with periodontal disease: a systematic review and meta-analysis. Clin Exp Dent Res. (2021) 7:109–22. doi: 10.1002/cre2.336
47. Herrera, D, Sanz, M, Shapira, L, et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: consensus report of the joint workshop by the European Federation of Periodontology (EFP) and the European arm of the world Organization of Family Doctors (WONCA Europe). J Clin Periodontol. (2023) 50:819–41. doi: 10.1111/jcpe.13807
48. de Oliveira, C, Watt, R, and Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish health survey. BMJ. (2010) 340:c2451. doi: 10.1136/bmj.c2451
Keywords: periodontitis, life’s essential 8, NHANES, epidemiology, risk factor(s)
Citation: Hou K, Zhang H, Song W, Li S, Liu J and Ma Z (2024) Association between life’s essential 8 and periodontitis: a study based on NHANES 2009–2014. Front. Med. 11:1342792. doi: 10.3389/fmed.2024.1342792
Received: 16 December 2023; Accepted: 25 March 2024;
Published: 12 April 2024.
Edited by:
Huixuan Zhou, Beijing Sport University, ChinaReviewed by:
Florence Carrouel, Université Claude Bernard Lyon 1, FranceCopyright © 2024 Hou, Zhang, Song, Li, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: KeGui Hou, S291a2FraUBxcS5jb20=;Zhaofeng Ma, bWF6aGFvZmVuZzIwMjJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.