- 1Transplant Center, University Hospital Munich, Ludwig-Maximilians-University (LMU), Munich, Germany
- 2Division of Nephrology, Department of Medicine IV, University Hospital Munich, Ludwig-Maximilians-University (LMU), Munich, Germany
- 3Department of Nephrology and Rheumatology, Augustinum Klinik München, Munich, Germany
- 4Institute of Laboratory Medicine, University Hospital Munich, Ludwig-Maximilians-University (LMU), Munich, Germany
- 5Department of General, Visceral and Transplantation Surgery, University Hospital Munich, Ludwig-Maximilians-University (LMU), Munich, Germany
The calcineurin inhibitor tacrolimus, which is available as an immediate- or extended-release formulation, is the standard-of-care immunosuppression after kidney transplantation with low rejection rates, especially in the first year after transplantation. However, its highly variable metabolism rate, narrow therapeutic window, and nephrotoxic side effects require close drug monitoring and individual dosing. Here, we describe first the application of extended-release tacrolimus (ER-Tac) twice daily with beneficial effects in a kidney transplant recipient under extensive therapeutic drug monitoring. A 47-year-old female kidney transplant recipient, who was identified as a fast metabolizer for tacrolimus, presented with declining allograft function and low tacrolimus through levels over time and 8 years after a second kidney transplantation despite the administration of high doses of ER-Tac once daily. Therefore, the area under the concentration–time curve (AUC) showed exceedingly high blood levels of ER-Tac. The latest biopsy of the kidney transplant showed arteriolar hyalinosis with pole vessel stenosis as a sign of chronic transplant vasculopathy and transplant glomerulopathy as a sign of chronic humoral rejection. After the exclusion of other options for immunosuppressive therapy due to the patient’s high immunological risk, the patient was switched from ER-Tac once daily to ER-Tac twice daily. After switching to ER-Tac twice daily, the AUC for oral tacrolimus decreased and the transplant function improved despite higher tacrolimus trough levels and a lower total dose administered. This case highlights the importance of careful therapeutic drug monitoring with the performance of an AUC in the follow-up management of kidney transplant recipients.
1 Introduction
The calcineurin inhibitor (CNI) tacrolimus either as prolonged-, extended-, or immediate-release administration is part of the standard-of-care immunosuppressive therapy after kidney transplantation (1). Therefore, the acute rejection rates in tacrolimus-based immunosuppressive therapy, especially in the first year after kidney transplantation, are lower in contrast to other immunosuppressive regimes (2). However, a typical side effect of CNIs like tacrolimus is nephrotoxicity due to vasoconstriction. Histological lesions such as arteriolar hyalinosis can be associated with chronic CNI nephrotoxicity (3). Furthermore, the highly variable metabolism rate of tacrolimus and its narrow therapeutic window require close drug monitoring and individual dosing (4). Fast tacrolimus metabolism is associated with reduced kidney transplant function and survival, the tacrolimus metabolism rate being defined as the drug trough concentration (C) normalized by the corresponding daily tacrolimus dose (D) (5, 6). Therefore, a C/D ratio of <1.05 ng/mL*1/mg indicates fast tacrolimus metabolism (6).
Tacrolimus is available as an immediate-release formulation, which must be given twice a day, or as an extended- or prolonged-release formulation, which should normally be given once daily (1). The application of extended-release tacrolimus (ER-Tac) twice a day with a possible positive effect, especially in “fast tacrolimus metabolism,” has not been described so far (7).
2 Case description
We report the case of a 47-year-old female kidney transplant recipient who presented with declining allograft function 8 years after a second kidney transplantation and the resulting unusual application of extended-release tacrolimus twice daily (BID).
Kidney transplantation had previously been performed after HLA-incompatible living donation with a donor-specific antibody (DSA), specifically anti-HLA DQ7, HLA-mismatch of 1-1-2, and 82% panel-reactive antibodies. The initial immunosuppression included rituximab, plasmapheresis, and anti-thymocyte globulin (ATG) due to high immunological risk, as well as immediate-release tacrolimus (IR-Tac), mycophenolate mofetil, and prednisolone in the follow-up. Over time, the pre-known DSAs were detectable with high signal intensity (MFI approximately 20.000) for years despite the administration of tacrolimus, high-dose antimetabolite [mycophenolate mofetil (CellCept) 1 g twice daily], and persistent steroid administration (prednisolone 5 mg once daily) in this patient.
The dosage of mycophenolate mofetil and prednisolone was continued at this dose due to the immunological risk and was not changed over time.
To reduce peak levels of calcineurin inhibitors, IR-Tac twice a day was switched to extended-release tacrolimus (ER-Tac) once daily during long-term follow-up (8). After that, ER-Tac trough levels were in the lower range despite the administration of high doses (12 mg daily), a normal BMI (20 kg/m2) with a body weight of 60 kg, and correct administration, which was critically assessed by anamnesis regarding medication intake and eating behavior as well as review of concomitant medication. A C/D ratio of <1.05 ng/mL*1/mg revealed fast tacrolimus metabolism in this patient. According to the relatively high dose, the area under the concentration–time curve (AUC) showed exceedingly high blood levels of ER-Tac (Figure 1A).

Figure 1. (A) Serum levels of tacrolimus [ng/ml] over 24 h. Blue line: Serum levels of extended-release tacrolimus (ER-Tac) once daily (SID, 12 mg); red line: serum levels of extended-release tacrolimus (ER-Tac) twice daily (BID, 2 × 5 mg). The AUC (area under the concentration–time curve) was calculated using PRISM by GraphPad. The time points used to calculate the AUC are marked on the x-axis. (B) Estimated glomerular filtration rate (eGFR) [ml/min/1.73m2] according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (blue line) and extended-release tacrolimus (ER-Tac) trough levels over time (red line). The black dashed line marks the switch from ER-Tac once daily (SID) to ER-Tac twice daily (BID).
There was a history of a previous graft biopsy 7 years after transplantation in this patient which revealed a mild cellular reaction (BANFF borderline), discrete signs of glomerulitis (without C4d positivity in immunohistochemistry), and transplant glomerulopathy (verified by electron microscopy) according to the BANFF lesion scores (t1, i2, v0, g1, ptc0, ci1, ct1, cv2, cg1b, mm1, ah1, aah0, ti2, and i-IFTA1). Based on these findings, the patient was treated with high-dose steroids and immunoglobulins.
Over time, the patient presented with declining eGFR and increased proteinuria. An indication biopsy performed again showed arteriolar hyalinosis with pole vessel stenosis as a sign of chronic transplant vasculopathy, which is likely aggravated by calcineurin inhibitor toxicity, as well as further evidence of transplant glomerulopathy, which is likely associated with chronic (non-active) antibody-mediated rejection (ABMR) in the absence of histological signs of acute rejection (9).
Switching to a selective co-stimulation blockade of T-cell activation with belatacept or to a mammalian target of rapamycin (mTOR) inhibitor-based regimen was not considered a suitable option because of the patient’s high immunological risk (2, 10).
To reduce the progress of worsening allograft function due to calcineurin inhibitor toxicity and to achieve an optimized therapeutic drug level, the administration of ER-Tac twice daily in adjusted dosage was prescribed after discussion of all possible options of immunosuppressive therapy and after informing the patient in detail about the unusual use. After switching to ER-Tac twice daily (BID), the AUC for oral tacrolimus (Figure 1A) and the transplant function fortunately improved despite higher tacrolimus trough levels and a lower total dose administered (Figure 1B). The patient tolerated the non-standard application of ER-Tac (BID) with no side effects, no negative effects on glucose hemostasis monitored by fasting glucose, and no doubts about her adherence. Rather, her general condition improved against the background of having at least temporarily decelerated the rapid function decline of the kidney transplant. The transplant function is still stable 3 years after switching to ER-Tac twice daily (as of March 2024).
3 Discussion
To our knowledge, this is the first described case in the application of ER-Tac twice a day with beneficial effects in a kidney transplant recipient under extensive therapeutic drug monitoring.
The use of prolonged- or extended-release formulations of tacrolimus, usually taken once a day, is a part of the clinical standard of care for kidney transplant recipients (1). Recently, a meta-analysis indicated that the conversion from IR-Tac twice daily to ER-Tac once daily may decrease serum creatinine in kidney transplant recipients with a follow-up duration of more than 48 weeks, but at the same time, eGFR remained unchanged (11). At least many randomized controlled studies could show that the administration of ER-Tac once daily with less peak levels (and therefore in total reduced maximum plasma concentrations) does not appear to have an impact on either the efficacy or safety of this formulation and is an effective immunosuppressant treatment in kidney transplant recipients (7, 8, 12, 13). However, the nephrotoxicity of tacrolimus according to its peak levels and frequently unfavorable AUC with a risk of developing transplant glomerulopathy remains a limiting factor for graft survival, particularly in fast metabolizers (14, 15). This can be observed in immediate and extended-release formulations of tacrolimus (5, 6, 15).
Concerning therapeutic drug monitoring, the AUC is considered a better surrogate marker of systemic tacrolimus exposure than the maximum plasma concentration and is strongly associated with clinical outcomes (7). Furthermore, it is essential to identify the high peak levels of tacrolimus, which could cause renal toxicity. However, the determination of the AUC (including maximum concentration) for oral tacrolimus remains challenging and time-consuming in the clinical routine. Therefore, we performed only one AUC under each formulation for ER-Tac. Approaches of finger prick measurements might be a future option to easily include AUC in routine follow-up management of kidney transplant recipients (16). Other considerations in therapeutic drug monitoring include measuring tacrolimus concentrations in intracellular rather than whole blood. This is because most of the tacrolimus measured in whole blood is bound to erythrocytes and plasma proteins and thus represents the pharmacologically inactive fraction (17).
However, the off-label use of ER-Tac twice a day suggests critical discussion. First, in this case, after conversion to ER-Tac twice daily, trough levels increased (with two peaks), but the 24 h total AUC decreased (as seen in Figure 1A). This could be related to the improved transplant function with better eGFR. These potential beneficial effects must be weighed against the risk of long-term under immunosuppression and our patient’s history with histologically detected transplant glomerulopathy and preexisting DSAs (18). According to general data, chronic rejection is the major cause of death-censored transplant failure after kidney transplantation in the long-term follow-up (19). Second, several investigations revealed significantly higher peak levels and AUC of IR-Tac after the morning dose compared to after the evening dose, suggesting a circadian dependence in the clinical pharmacokinetics of tacrolimus (20). In the present case, the peak level in the evening was higher during the administration of ER-Tac twice daily, which could be due to the retard formulation. However, this requires further observation. Third, the application of ER-Tac once daily may improve patient adherence, which is an independent risk factor for the development of de novo DSAs (21). This advantage could be lost when taking ER-Tac twice a day. Therefore, the possibility of switching to the more frequent ER-Tac twice a day should be weighed carefully against possible patient’s non-adherence. Fourth, the absolute effect of the intervention seems quite modest. The reduction in AUC was in line with the dose reduction, although the switch to ER-Tac twice daily was accompanied by higher trough levels of tacrolimus. The improvement in renal function is probably due to the lower AUC, but other factors such as adherence, favorable hydration state or daily blood pressure, physical activity of the patient, or hyperfiltration of the kidney transplant might be involved. Finally, based on our observation, we hypothesize a particular advantage for high metabolizers taking ER-Tac twice daily. Thus, a further limitation of our case report is the absence of data on our patient’s specific CYP genotyping. However, the C/D ratio for IR-and ER-Tac strongly suggests fast tacrolimus metabolism in our patient (6). Our case report should focus on transplant recipients requiring high doses of extended- or prolonged-release tacrolimus, which are likely to result in high peak levels when taken as a single dose. Distributing the administration into two doses can result in lower peak and higher trough levels, thereby avoiding unnecessary dose increase and toxicity.
In conclusion, the administration of ER-Tac twice daily (BID) might be beneficial for selected patients with fast tacrolimus metabolism. However, in this special application, careful therapeutic drug monitoring including AUC is necessary and the transplant community should critically discuss this off-label drug use. As of today, the patient was converted 3 years ago and the kidney transplant function is stable, but the follow-up course of the transplant under ER-Tac twice a day needs to be further monitored closely.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LF: Formal analysis, Writing – original draft. LK: Data curation, Writing – review & editing. KN: Formal analysis, Methodology, Writing – review & editing. TS: Visualization, Writing – review & editing. MJS: Funding acquisition, Supervision, Writing – review & editing. DK: Supervision, Writing – review & editing, Funding acquisition. BM: Writing – review & editing, Supervision. MS: Writing – review & editing, Formal analysis, Methodology, Software. MF: Conceptualization, Supervision, Writing – review & editing. SK: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ong, SC, and Gaston, RS. Thirty years of tacrolimus in clinical practice. Transplantation. (2021) 105:484–95. doi: 10.1097/TP.0000000000003350
2. Ekberg, H, Tedesco-Silva, H, Demirbas, A, Vítko, Š, Nashan, B, Gürkan, A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. (2007) 357:2562–75. doi: 10.1056/NEJMoa067411
3. Naesens, M, Kuypers, DR, and Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. (2009) 4:481–508. doi: 10.2215/CJN.04800908
4. Schutte-Nutgen, K, Tholking, G, Suwelack, B, and Reuter, S. Tacrolimus - pharmacokinetic considerations for clinicians. Curr Drug Metab. (2018) 19:342–50. doi: 10.2174/1389200219666180101104159
5. Thölking, G, Filensky, B, Jehn, U, Schütte-Nütgen, K, Koch, R, Kurschat, C, et al. Increased renal function decline in fast metabolizers using extended-release tacrolimus after kidney transplantation. Sci Rep. (2021) 11:15606. doi: 10.1038/s41598-021-95201-5
6. Thölking, G, Fortmann, C, Koch, R, Gerth, HU, Pabst, D, Pavenstädt, H, et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One. (2014) 9:e111128. doi: 10.1371/journal.pone.0111128
7. Banas, B, Kramer, BK, Kruger, B, Kamar, N, and Undre, N. Long-term kidney transplant outcomes: role of prolonged-release tacrolimus. Transplant Proc. (2020) 52:102–10. doi: 10.1016/j.transproceed.2019.11.003
8. Tanzi, MG, Undre, N, Keirns, J, Fitzsimmons, WE, Brown, M, and First, MR. Pharmacokinetics of prolonged-release tacrolimus and implications for use in solid organ transplant recipients. Clin Transpl. (2016) 30:901–11. doi: 10.1111/ctr.12763
9. el-Zoghby, ZM, Stegall, MD, Lager, DJ, Kremers, WK, Amer, H, Gloor, JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. (2009) 9:527–35. doi: 10.1111/j.1600-6143.2008.02519.x
10. Huber, M, Kemmner, S, Renders, L, and Heemann, U. Should belatacept be the centrepiece of renal transplantation? Nephrol Dial Transplant. (2016) 31:1995–2002. doi: 10.1093/ndt/gfw226
11. Chao, S, Jia, L, Zhu, K, Chen, L, and Niu, Y. The effect of tacrolimus conversion from immediate-to extended-release formulation on renal function in renal transplant patients: a meta-analysis. Front Pharmacol. (2023) 14:1226647. doi: 10.3389/fphar.2023.1226647
12. Albano, L, Banas, B, Klempnauer, JL, Glyda, M, Viklicky, O, Kamar, N, et al. OSAKA trial: a randomized, controlled trial comparing tacrolimus QD and BD in kidney transplantation. Transplantation. (2013) 96:897–903. doi: 10.1097/TP.0b013e3182a203bd
13. Krämer, BK, Charpentier, B, Bäckman, L, Silva, HT, Mondragon-Ramirez, G, Cassuto-Viguier, E, et al. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. (2010) 10:2632–43. doi: 10.1111/j.1600-6143.2010.03256.x
14. Egeland, EJ, Reisaeter, AV, Robertsen, I, Midtvedt, K, Strøm, EH, Holdaas, H, et al. High tacrolimus clearance - a risk factor for development of interstitial fibrosis and tubular atrophy in the transplanted kidney: a retrospective single-center cohort study. Transpl Int. (2019) 32:257–69. doi: 10.1111/tri.13356
15. Thölking, G, Schütte-Nütgen, K, Schmitz, J, Rovas, A, Dahmen, M, Bautz, J, et al. A low tacrolimus concentration/dose ratio increases the risk for the development of acute Calcineurin inhibitor-induced nephrotoxicity. J Clin Med. (2019) 8:1586. doi: 10.3390/jcm8101586
16. Nierychlweski, K, Kemmner, S, Stangl, M, Schwarz, M, and Fischereder, M. Poster presentations (therapeutic drug monitoring (TDM) of tacrolimus from capillary blood microsamples (CMS)). Transpl Int. (2020) 33:16–36.
17. Udomkarnjananun, S, Eiamsitrakoon, T, de Winter, BCM, van Gelder, T, and Hesselink, DA. Should we abandon therapeutic drug monitoring of tacrolimus in whole blood and move to intracellular concentration measurements? Br J Clin Pharmacol. (2023). doi: 10.1111/bcp.15946
18. Aubert, O, Loupy, A, Hidalgo, L, Duong van Huyen, JP, Higgins, S, Viglietti, D, et al. Antibody-mediated rejection due to preexisting versus De novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. (2017) 28:1912–23. doi: 10.1681/ASN.2016070797
19. Sellarés, J, de Freitas, DG, Mengel, M, Reeve, J, Einecke, G, Sis, B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. (2012) 12:388–99. doi: 10.1111/j.1600-6143.2011.03840.x
20. Baraldo, M, and Furlanut, M. Chronopharmacokinetics of ciclosporin and tacrolimus. Clin Pharmacokinet. (2006) 45:775–88. doi: 10.2165/00003088-200645080-00002
21. Wiebe, C, Gibson, IW, Blydt-Hansen, TD, Karpinski, M, Ho, J, Storsley, LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. (2012) 12:1157–67. doi: 10.1111/j.1600-6143.2012.04013.x
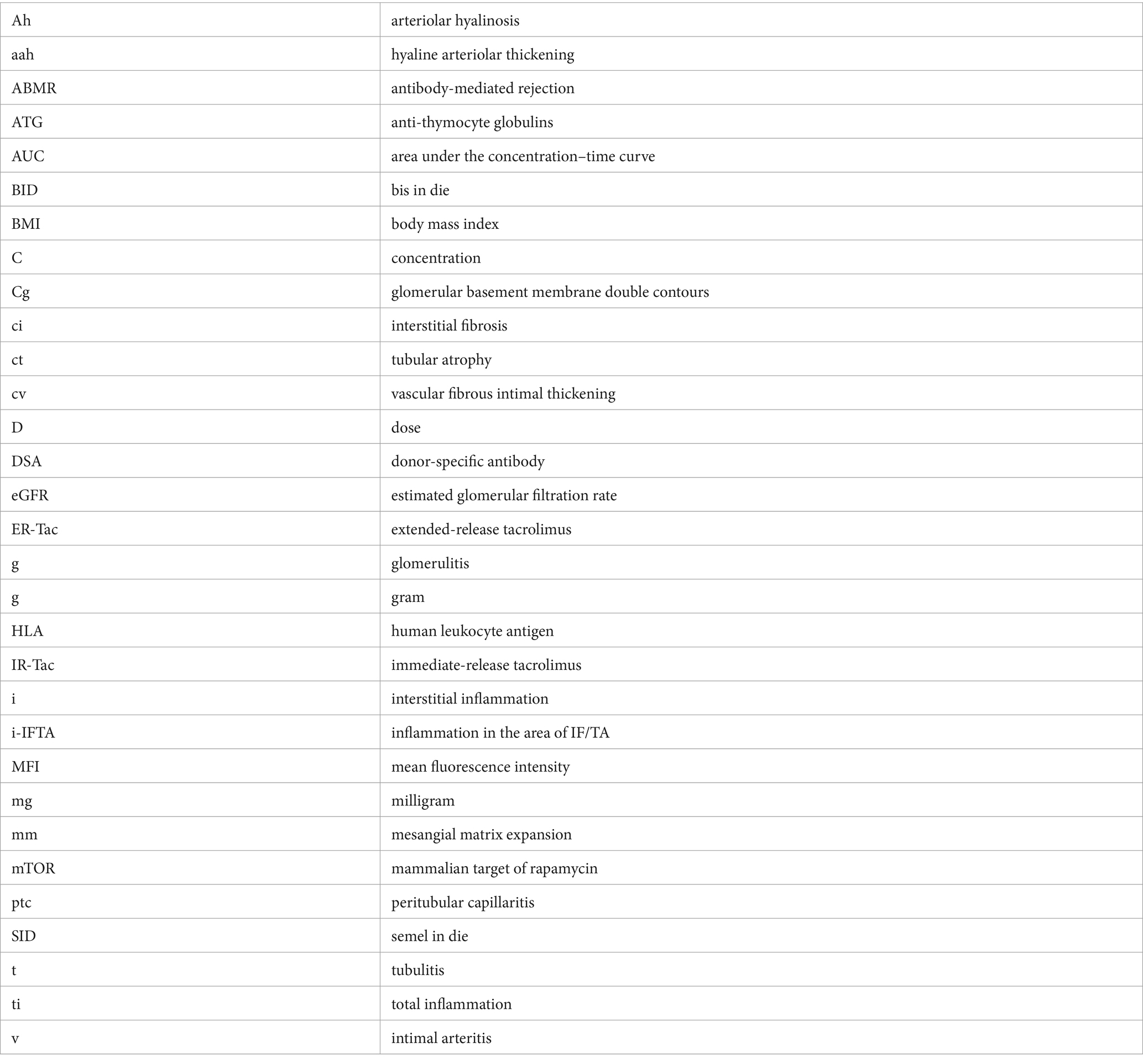
Glossary
Keywords: tacrolimus, kidney transplantation, calcineurin inhibitor toxicity, extended-release tacrolimus, prolonged-release tacrolimus
Citation: Füessl L, Kreuzer L, Nierychlewski K, Seibt T, Stangl MJ, Koliogiannis D, Meiser B, Schwarz M, Fischereder M and Kemmner S (2024) The use of extended-release tacrolimus twice a day might be beneficial for selected kidney transplant recipients: a case report. Front. Med. 11:1336035. doi: 10.3389/fmed.2024.1336035
Edited by:
Laila-Yasmin Mani, University Hospital of Bern, SwitzerlandReviewed by:
Orsolya Horváth, Semmelweis University, HungaryKarl Martin Wissing, University Hospital Brussels, Belgium
Copyright © 2024 Füessl, Kreuzer, Nierychlewski, Seibt, Stangl, Koliogiannis, Meiser, Schwarz, Fischereder and Kemmner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan Kemmner, stephan.kemmner@tum.de
 Louise Füessl1,2
Louise Füessl1,2