- 1Neuroscience Center, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2Hematology, Stem Cell Transplant and Cellular Therapy Department, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 3Radiology Department, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 4Department of Biostatistics, Epidemiology, and Scientific Computing, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 5Department of Medicine, AlFaisal University, Riyadh, Saudi Arabia
- 6Department of Medicine University of Alberta, Edmonton, AB, Canada
Introduction: Posterior reversible encephalopathy syndrome (PRES) is a serious neurological syndrome that may develop following immunosuppressive therapy for stem cell transplantation (SCT). We report 8 patients with sickle cell disease (SCD) who developed PRES, which is likely to be related to immunosuppression.
Methods: This is retrospective cohort analysis of the SCD registry at the King Faisal Specialist Hospital and Research Center (KFSHRC) in Riyadh, Saudi Arabia. Inclusion criteria included all adults SCD patients who underwent SCT from 2011 until 2022. We explored all cases of PRES in patients with SCT. PRES was diagnosed with MRI imaging showing reversible vasogenic cerebral edema associated with neurological symptoms including severe headache, seizures, encephalopathy, delirium, and visual disturbances.
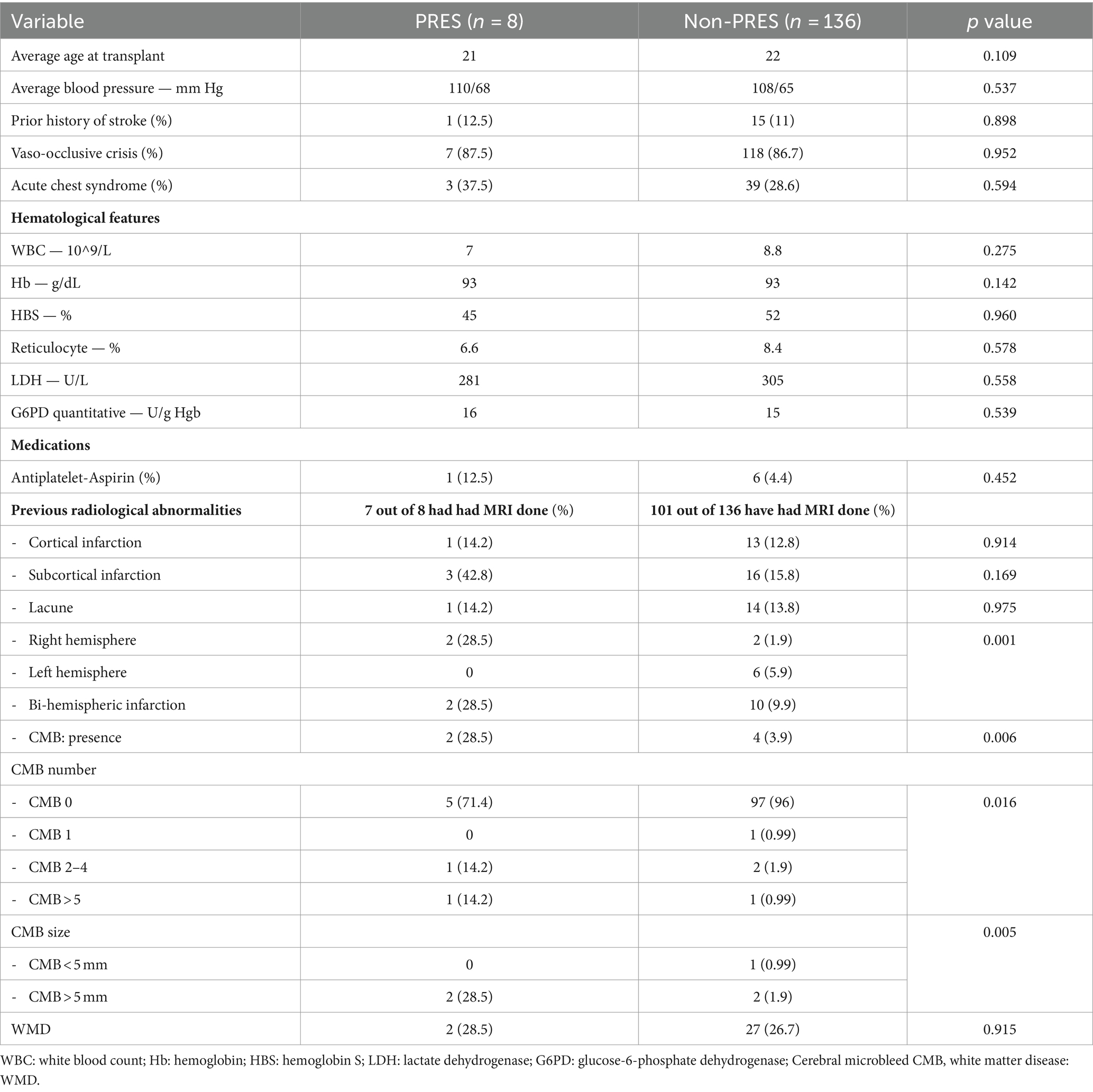
Results: During ten years follow-up (2011–2022) we found 8 patients with PRES (age range between 14 to 37 years at diagnosis) PRES occurred 8 to 124 days following SCT in 7 cases and one patient developed PRES 8 months prior to SCT. All patients were on immunosuppressive medications, including tacrolimus, cyclosporine, sirolimus and or mycophenolate mofetil. Headache, seizures, visual hallucinations, confusion, and drowsiness were the most common presenting symptoms. MRI showed abnormalities in the occipital, parietal and frontal lobes in most cases. Recovery was complete in all patients and no recurrences were noted. Two patients had graft versus host disease (GVHD). We compared risk factors for PRES among the 8 cases and 136 SCT in SCD patients who did not develop PRES. There was a significant association between PRES and imaging abnormalities, including previous bi-hemispheric infarctions (p = 0.001), and cerebral microbleeds (CBMs). PRES was strongly associated with presence (p = 0.006), size (p = 0.016) and number (p = 0.005) of CMBs.
Conclusion: PRES can develop days to weeks following SCT in patients with SCD, and is associated with immunosuppressive therapy, previous bi-hemispheric infarctions and CMB. Prompt recognition and intervention leads to good recovery.
Introduction
Posterior reversible encephalopathy syndrome (PRES) refers to the development of reversible vasogenic cerebral edema leading to neurological symptoms including severe headache, seizures, encephalopathy, delirium, and visual disturbances (1). Common underlying etiologies that may precipitate PRES include sudden increase in blood pressure, autoimmune disorders and medications, especially cytotoxic drugs and eclampsia (2). Diagnosis is usually confirmed with brain imaging, particularly MRI which shows characteristic changes suggestive of vasogenic edema, especially in the occipital, temporal and parietal regions (2). While the underlying mechanisms are not fully understood, inflammation and endothelial cell dysfunction resulting in increased leakage of the blood brain barrier (BBB) are important contributing factors (3).
PRES has been reported with several hematological disorders including thalassemia and sickle cell disease (SCD) (4–6). In SCD, most cases have been reported in children in close proximity to stem cell transplantation (SCT). PRES in adult SCD patients is rare (7–11). To our knowledge, there are only two reports of PRES in SCD in proximity to SCT (7, 8). We report 8 SCD cases from our institution who underwent SCT and developed PRES.
Methods
We reviewed the Stem Cell Transplantation database at the King Faisal Specialist Hospital and Research Center (KFSHRC) in Riyadh, Kingdom of Saudi Arabia (KSA) for all patients who underwent stem cell transplantation for SCD between 2011 and 2022. This database includes all SCD patients indicated for SCT, indications of SCT include: SCD with CNS events (stroke, TIA, CNS vasculopathy), SCD with more than two acute chest syndromes in last 2 years, more than two joints with avascular necrosis, more than 2 vaso-occlusive crisis per year for past 2 years, sickle cell nephropathy, recurrent priapism.
We studied the SCD transplant database for potential risk factors for PRES in SCT treated patients. These variables include demographic data, clinical features, laboratory investigation, previous radiological abnormalities and medications. We also evaluated the presence of any acute illness, hypertensive crises, medications changes and the proximity to the timing of conditioning and the development of PRES. The diagnosis of PRES is made with brain MRI showing reversible vasogenic edema in bilateral cortical or/and subcortical areas. We used chi-square and Fisher’s exact tests for association between demographics, clinical, laboratory and radiological variables and PRES.
Results and summary
There were 144 patients who underwent SCT for SCD. PRES developed in 8 patients. The details of their clinical presentations and laboratory features are shown in Tables 1, 2. There was no difference in the age at the time of SCT, hematological or clinical parameters between the two groups. While there was no significant difference in the long-term blood pressures between the two groups, 7 of 8 patients were hypertensive during the acute PRES illness with systolic blood pressure between 140 and 170 mmHg. The time between the SCT and development of PRES varied from 8 days to 4 months after the transplant. The time interval between SCT and PRES was less than 40 days in 5 of 7 cases.
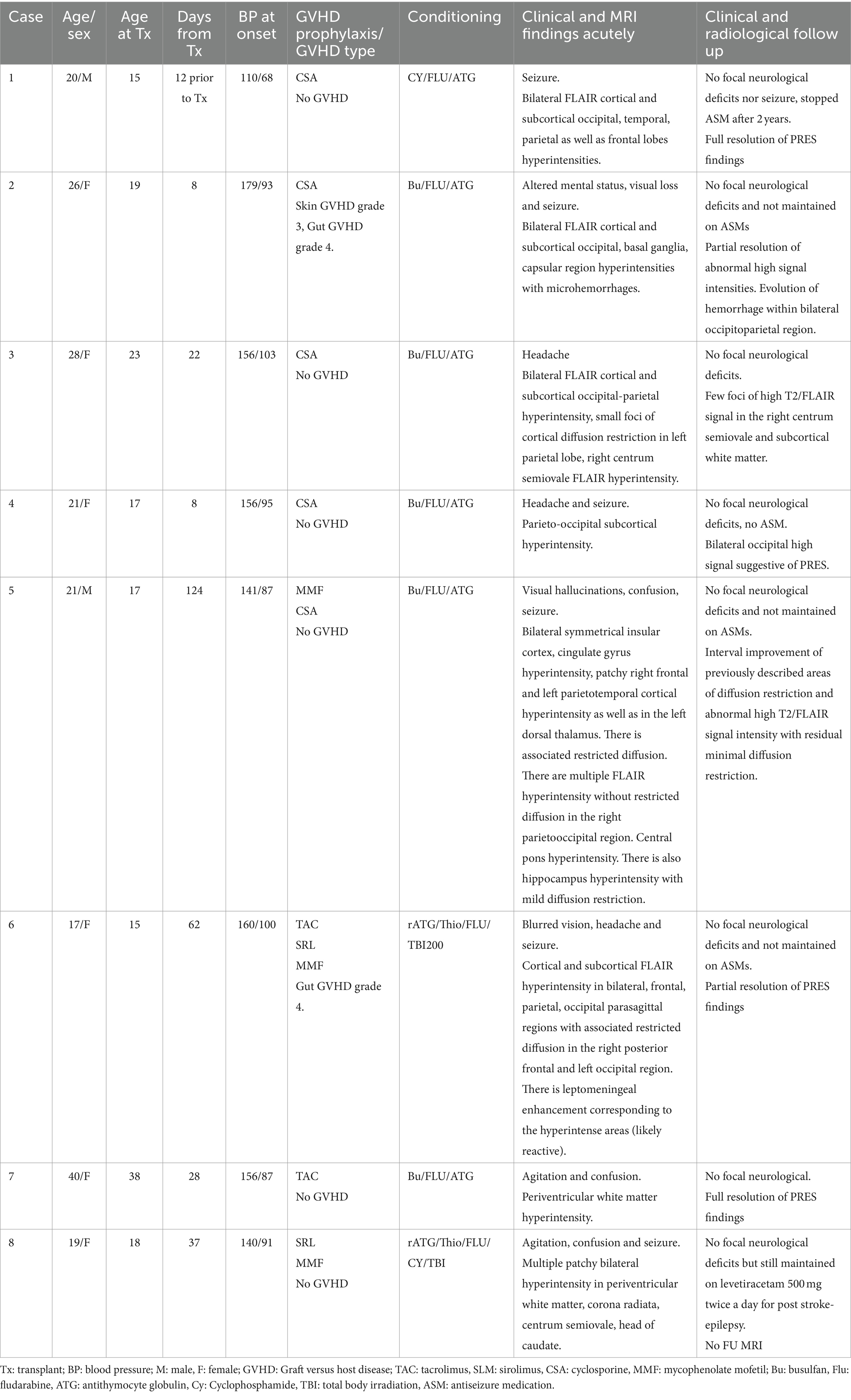
Table 2. Clinical details, medications and radiological features of the patients presenting with PRES.
The most common symptoms associated with PRES at the time of admission included sudden confusion, headache, visual symptoms, seizures, agitation and drowsiness. As shown in Table 2, most patients had mild disease, defined as cortical and/or subcortical edema without hemorrhage, mass effect or herniation (12), and all made full recovery. No patients developed recurrent PRES in follow-up ranging from 1 to 7 years.
Details of GVHD prophylaxis and conditioning regimen are shown in Table 2. Tacrolimus, sirolimus, mycophenolate and cyclosporine were the most used medications for GVHD prophylaxis. The conditioning medications are also shown in Table 2 and included busulfan (Bu)/fludarabine (Flu), antithymocyte globulin (ATG) or Flu/Cyclophosphamide (Cy)/ATG in most patients.
The imaging findings are shown in Figure 1. The diagnosis of PRES was confirmed on MRI imaging in most patients. The hyperintensities on FLAIR images were most frequently seen bilaterally cortically and sub-cortically in the occipital, parietal and temporal lobes. No patients developed ICH or SAH.
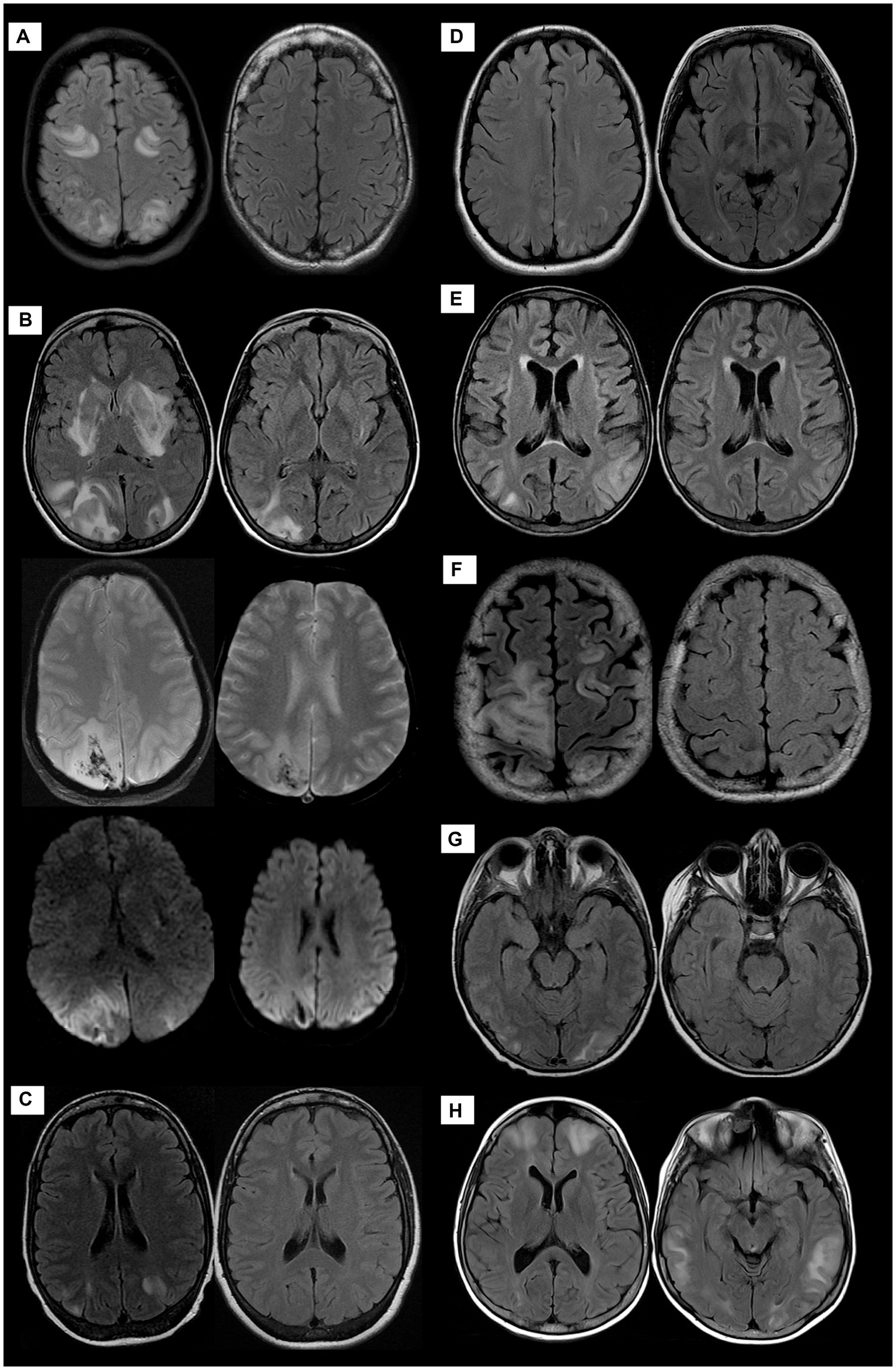
Figure 1. MRI of the brain of all patients demonstrating PRES manifestations at variable degrees; patient 1 (A): the initial exam of AXIAL FLAIR weighted images-(WI)-left side- shows predominantly sub-cortical signal alteration involving bilateral fronto-parietal and occipital lobes with complete resolution on the follow-up study right side. Patient 2 (B) Cortical and sub-cortical diffuse signal alteration at the bilateral parieto-occipital lobes with diffusion restriction and hemorrhage evident by blooming artifacts on SWI as well as unusual signal alteration at bilateral basal ganglia. Follow-up images at right side exhibit significant improvement with residual signal alteration of hemorrhagic PRES. Patient 3 (C), Patient 4 (D), and Patient 5 (E): AXIAL FLAIR WI demonstrate predominantly sub-cortical signal alteration involving bilateral parieto-occipital lobes with complete resolution on follow-up images right side except for patient 4, no follow-up images. Patient 6 (F), Patient 7 (G), and Patient 8 (H) AXIAL FLAIR WIs demonstrate predominantly sub-cortical white matter signal alteration at different distributions, mainly parieto-occipital to a lesser extent involving fronto-temporal lobes with complete resolution on follow-up images right side except for patient 8, no follow-up images.
A significant association was observed between imaging abnormalities and PRES. The presence of bi-hemispheric infarctions (p = 0.001), presence (p = 0.006), size (p = 0.016) and number (p = 0.005) of cerebral-microbleed (CMB) were higher risk of PRES. There was no significant association between the presence or severity of white matter hyperintensities [as measured by Fazekas score (13)] and the risk of PRES.
Discussion
We report eight patients with SCD who developed PRES after SCT, four of whom are adults. The time between the SCT and development of PRES varied from 8 days to 4 months after transplant. The time to PRES was less than 40 days from SCT in 5 of 7 cases and 8 months prior to SCT in one patient. We did not identify any preceding precipitating factors, and the clinical course was benign with no recurrences in our patients. We noted that the presence of previous bi-hemispheric cerebral infarction and CMBs increased the risk of PRES. The presence of white matter hyperintensities did not appear to be associated with increased risk.
Although rare in adults, PRES has been frequently reported in children with SCD. Chronic hemolysis can lead to the release of erythrocyte damage-associated molecular protein molecules (cDAMPs). In addition, Toll-like receptor 4 promotes the release of oxygen reactive species. These together with neutrophil extracellular traps, can result in endothelial cell injury and injury to the blood–brain barrier (8). Conditioning chemotherapy can also result in endothelial injury and increase the risk of PRES in children (8, 14). Endothelial cell dysfunction is also noted after HSCT, similarly, is driven by the predominant presence of pro-inflammatory factors such as complement activation, cDAMPs, and Toll-like receptors (15). Complement activation is not known if it has a role in the pathophysiology of the endothelial dysfunction in PRES, compared to post-HSCT. Genetic abnormalities in the complement pathway have been associated with some thrombotic microangiopathy syndromes, for instance transplant-associated thrombotic microangiopathy (16). Furthermore, thrombotic microangiopathy can develop with or without SCT in patients with SCD and has been successfully treated with eculizumab to improve symptoms of PRES (8).
SCT and preconditioning are frequently associated with neurotoxicity including PRES (14). The combination of preexisting endothelial injury from chronic on-going hemolysis and the additional injury from cytotoxic/immunosuppressive medications can place patients with SCD at a greater risk of thrombotic thrombocytopenia, veno-occlusive disease and PRES (17, 18). In children with SCD with SCT, PRES has been reported in up to 22% of patients in one large study from Rome (19). The incidence of PRES seems to be significantly less frequent in adult SCD patients and SCT, with only two previous cases prior to our report (7, 8). The reasons for such age-related discrepancy are not yet clear. We cannot exclude the possibility that this may be related to under-reporting of cases in adults.
In children with hemoglobinopathies, PRES has been reported in patients treated with calcineurin inhibitors (cyclosporin, sirolimus and tacrolimus) (4, 6, 19, 20). In one series of children with SCD and thalassemia who underwent SCT from Rome (age range 2–17 years old), PRES was reported in 31 of 222 cases. All patients had been treated with cyclosporine or tacrolimus and PRES developed in most patients within 100 days from SCT (19). In another series of 313 consecutive SCT in children from Bologna, PRES was reported in 26 (8.3%) patients and occurred in higher frequency in patients with hemoglobinopathies (20). The time from SCT to PRES ranged from 5 to 352 days. The authors noted an association between fludarabine-based conditioning and the risk of PRES in these children.
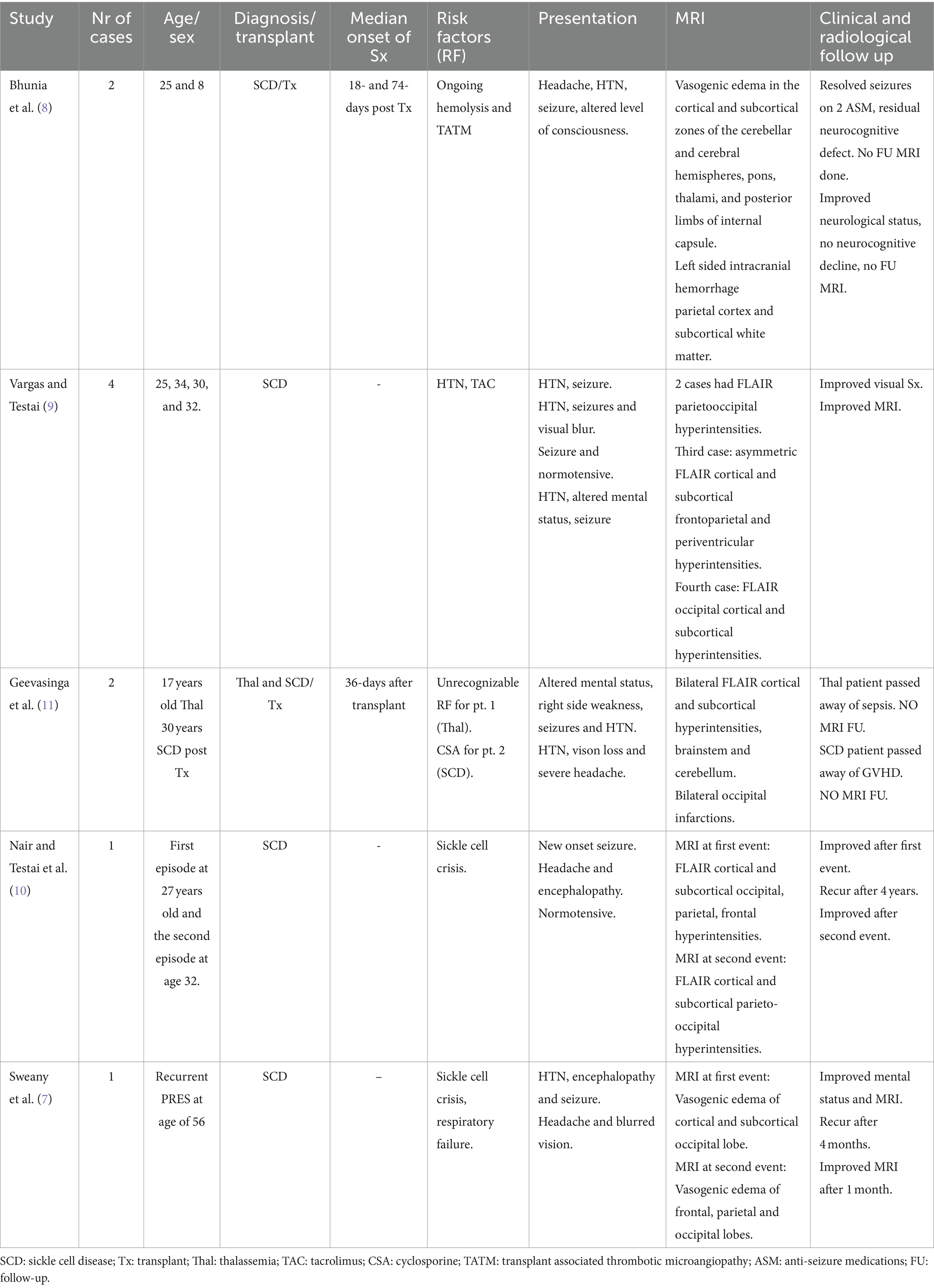
As shown in Table 3, there are rare reports of PRES in adult patients with SCD (7–11). To our knowledge, there are only two previous cases of SCD in which SCT was complicated by PRES. Sweany et al. reported 3 patients with recurrent PRES in patients with SCD, systemic lupus erythematosus and acute myeloid leukemia. All patients had evidence of endothelial cell injury (schistocyte formation and increased LDH). PRES was associated with SCT in one of the three cases. The time interval between SCT and PRES was not mentioned (7). Bhunia et al. reported one pediatric and one adult patient with SCD who developed PRES following SCT (8). Interestingly, the authors considered transplant-associated thrombotic microangiopathy as predisposing to PRES and treatment with eculizumab seems to result in quick recovery (8). Recurrent episodes of PRES in SCD have also been reported by Nair and Testai. The patient did not undergo SCT (10). Throughout eight years of follow-up, we did not observe any recurrent episodes of PRES in our patients.
Differentiation of PRES from ischemic or hemorrhagic stroke is important and can sometimes be challenging. Sickle cell disease patients are at an increased risk of stroke and moyamoya disease (21). The clinical presentation is mostly with focal neurological symptoms; and the imaging, especially MRI-DWI shows characteristic findings that are helpful in making the correct diagnosis (1, 2). Early diagnosis, especially in patients with ischemic stroke, is important as the patients can be candidates for reperfusion treatment with intravenous alteplase or endovascular clot removal. Patients with PRES may require rapid blood pressure reduction, which can sometimes be harmful in acute ischemic stroke.
There are strengths to our study. This is a comprehensive review from an academic center with experience and a large volume of stem cell transplantation for SCD. The patients were evaluated by hematologists and neurologists and had extensive investigations to determine the etiology. The follow-up is comprehensive and clinical course was compared to a large number of SCD transplanted patients who did not develop PRES.
However, there are limitations to the study. On the one hand, this is a single center study which reflects the experience from the Kingdom of Saudi Arabia and therefore the results cannot be generalized. On the other hand, the data in the transplant registry was collected prospectively, our study is a retrospective analysis and carries the risk of inadequate data collection and evaluation. Future research is warranted to include more patients with collaboration with other transplant institutes to test the clinical implications of findings noted on MRI as predictor of PRES in SCD patients and for which any intervention for endothelial dysfunction could play a role.
In summary, we present four adult SCD patients post SCT in addition to two prior reported cases. While most patients were hypertensive, we did not identify an acute hypertensive or sickle cell crisis in any patient preceding PRES. Immunosuppressive treatment or conditioning may have been contributing factors in some patients. MRI can be used as a probable predictive tool of PRES in patients with SCD undergoing SCT. The clinical course was mild in most patients and none experienced recurrence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee (REC) of King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AAlA: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. AAlQ: Conceptualization, Data curation, Methodology, Visualization, Writing – review & editing. GM: Data curation, Formal analysis, Writing – review & editing. FAlo: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MAlS: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AAlS: Conceptualization, Data curation, Formal analysis, Writing – review & editing. SA: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AH: Conceptualization, Data curation, Formal analysis, Writing – review & editing. AAlr: Conceptualization, Data curation, Writing – review & editing. AAlK: Conceptualization, Data curation, Writing – review & editing. AAlH: Conceptualization, Data curation, Writing – review & editing. MAlZ: Conceptualization, Data curation, Writing – review & editing. AS: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. FA-A: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing. FAlM: Conceptualization, Data curation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Geocadin, RG . Posterior reversible encephalopathy syndrome. N Engl J Med. (2023) 388:2171–8. doi: 10.1056/NEJMra2114482
2. Fugate, JE, and Rabinstein, AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. (2015) 14:914–25. doi: 10.1016/S1474-4422(15)00111-8
3. Gewirtz, AN, Gao, V, Parauda, SC, and Robbins, MS. Posterior reversible encephalopathy syndrome. Curr Pain Headache Rep (2021); 25,:19. doi: 10.1007/s11916-020-00932-1
4. Shash, H, Aldaama, S, Omer, H, and Alafghani, S Different clinicoradiological characteristics of posterior reversible encephalopathy syndrome in pediatric oncology and post-bone marrow transplantation cases: a retrospective study. Front Neurol (2022), 13::13. doi: 10.3389/fneur.2022.836033
5. Krishnamurti, L, Neuberg, DS, Sullivan, KM, Kamani, NR, Abraham, A, Campigotto, F, et al. Bone marrow transplantation for adolescents and young adults with sickle cell disease: results of a prospective multicenter pilot study. Am J Hematol (2019). 94: 446–454. doi: 10.1002/ajh.25401
6. Thompson, D, Harrington, Y, and De, FJ. The incidence of posterior reversible encephalopathy syndrome is increased in BMT for Haemoglobinopathies. Blood. (2013) 122:4579–9. doi: 10.1182/blood.V122.21.4579.4579
7. Sweany, JM, Bartynski, WS, and Boardman, JF. “Recurrent” posterior reversible encephalopathy syndrome. J Comput Assist Tomogr. (2007) 31:148–56. doi: 10.1097/01.rct.0000233127.21303.b9
8. Bhunia, N, Abu-Arja, R, Bajwa, RPS, Auletta, JJ, and Rangarajan, HG. Successful treatment with eculizumab for posterior reversible encephalopathy syndrome due to underlying transplant-associated thrombotic microangiopathy in patients transplanted for sickle cell disease. Pediatric Blood Cancer. (2019) 66:e27912. doi: 10.1002/pbc.27912
9. Vargas, A, and Testai, FD. Posterior reversible encephalopathy syndrome in adult sickle-cell patients: case series and literature review. J Clin Neurosci. (2019) 70:249–50. doi: 10.1016/j.jocn.2019.08.070
10. Nair, A, and Testai, FD. Recurrent posterior reversible encephalopathy syndrome in a sickle cell patient. J Natl Med Assoc. (2011) 103:170–2. doi: 10.1016/s0027-9684(15)30267-4
11. Geevasinga, N, Cole, C, Herkes, GK, Barnett, Y, Lin, J, and Needham, M. Sickle cell disease and posterior reversible leukoencephalopathy. J Clin Neurosci. (2014) 21:1329–32. doi: 10.1016/j.jocn.2013.10.028
12. Hinduja, A . Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol. (2020) 11:14. doi: 10.3389/fneur.2020.00071
13. Andere, A, Jindal, G, Molino, J, Collins, S, Merck, D, Burton, T, et al. Volumetric white matter Hyperintensity ranges correspond to Fazekas scores on brain MRI. J Stroke Cerebrovasc Dis. (2022) 31:106333. doi: 10.1016/j.jstrokecerebrovasdis.2022.106333
14. Bartynski, WS, Zeigler, ZR, Shadduck, RK, and Lister, J. Pretransplantation conditioning influence on the occurrence of cyclosporine or FK-506 neurotoxicity in allogeneic bone marrow transplantation. AJNR Am J Neuroradiol. (2004) 25:261–9.
15. Neubauer, K, and Zieger, B. Endothelial cells and coagulation. Cell Tissue Res (2022);387:391–398. doi: 10.1007/s00441-021-03471-2
16. Gavriilaki, E, Sakellari, I, Chatzikonstantinou, T, Mallouri, D., Batsis, I., Vardi, A., et al. Endothelial and complement activation as predictors of survival in adult allogeneic hematopoietic cell transplantation. Hema (2020);5:e487. doi: 10.1097/HS9.0000000000000487
17. Abusin, GA, Abu-Arja, R, Bajwa, RPS, Horwitz, EM, Auletta, JJ, and Rangarajan, HG. Severe transplant-associated thrombotic microangiopathy in patients with hemoglobinopathies. Pediatr Blood Cancer. (2017) 64:e26503. doi: 10.1002/pbc.26503
18. Jodele, S . Double trouble: complement-mediated thrombotic microangiopathy in patients with hemoglobinopathies after stem cell transplantation. Pediatr Blood Cancer. (2017) 64:e26566. doi: 10.1002/pbc.26566
19. Gaziev, J, Marziali, S, Paciaroni, K, Isgrò, A, di Giuliano, F, Rossi, G, et al. Posterior reversible encephalopathy syndrome after hematopoietic cell transplantation in children with Hemoglobinopathies. Biol Blood Marrow Transplant. (2017) 23:1531–40. doi: 10.1016/j.bbmt.2017.05.033
20. Zama, D, Masetti, R, Cordelli, DM, Vendemini, F, Giordano, L, Milito, G, et al. Risk factor analysis of posterior reversible encephalopathy syndrome after allogeneic hematopoietic SCT in children. Bone Marrow Transplant. (2014) 49:1538–40. doi: 10.1038/bmt.2014.182
Keywords: posterior reversible encephalopathy syndrome, stem cell transplantation, sickle cell disease, bi-hemispheric infarctions, cerebral microbleeds
Citation: BinAmir HA, AlAhmari A, AlQahtani A, Mohamed G, Alotaibi F, AlShamrani M, AlSaeed A, AlGhanmi S, Heji A, Alreshaid A, AlKawi A, AlHazzani A, AlZawahmah M, Shuaib A, Al-Ajlan F and AlMohareb F (2024) Posterior reversible encephalopathy syndrome post stem cell transplantation in sickle cell disease: case series and literature review. Front. Med. 11:1330688. doi: 10.3389/fmed.2024.1330688
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Christos Demosthenous, G. Papanikolaou General Hospital, GreeceChristos Varelas, G. Papanikolaou General Hospital, Greece
Copyright © 2024 BinAmir, AlAhmari, AlQahtani, Mohamed, Alotaibi, AlShamrani, AlSaeed, AlGhanmi, Heji, Alreshaid, AlKawi, AlHazzani, AlZawahmah, Shuaib, Al-Ajlan and AlMohareb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahad Al-Ajlan, ZmFsYWpsYW45N0BrZnNocmMuZWR1LnNh
 Hussain A. BinAmir
Hussain A. BinAmir Ali AlAhmari
Ali AlAhmari AlWaleed AlQahtani3
AlWaleed AlQahtani3 Ammar AlKawi
Ammar AlKawi Ashfaq Shuaib
Ashfaq Shuaib