
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 14 February 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1330446
This article is part of the Research TopicInsights in Intensive Care Medicine and Anesthesiology: 2023View all 22 articles
 Jānis Verners Birnbaums1,2*
Jānis Verners Birnbaums1,2* Agnese Ozoliņa1,2*
Agnese Ozoliņa1,2* Leonids Solovjovs3
Leonids Solovjovs3 Zane Glāzniece-Kagane2
Zane Glāzniece-Kagane2 Jānis Nemme4
Jānis Nemme4 Ināra Logina1
Ināra Logina1Background: Erector spine plane block (ESPB) has been widely used in spinal surgery, although there are variable data about its efficacy.
Objectives: This study aimed to evaluate the efficacy of ESPB in elective lumbar spinal fusion surgery patients with two different surgical approaches.
Materials and methods: Retrospectively, 45 elective lumbar transpedicular fusion (TPF) surgery patients undergoing open surgery with different approaches [posterior transforaminal fusion approach (TLIF) or combined posterior and anterior approach (TLIF+ALIF)] were divided into 2 groups: general anesthesia (GA, n = 24) and general anesthesia combined with ESPB (GA + ESPB, n = 21). The primary outcome was to analyze the efficacy of ESPB in two different surgical approaches in terms of pain intensity in the first 48 h. Secondary: Fentanyl-free patients and opioid consumption in the first 24 h postoperatively. Comparative analysis was performed (SPSS® v. 28.0) (p < 0.05).
Results: Out of 45 patients (27 female), 21 received GA + ESPB and 24 received GA. The average age was 60.3 ± 14.3 years. Chronic back pain before the operation was registered in 56% of patients. ESPB was performed in 17 TLIF and in 4 TLIF+ALIF patients. ESPB significantly reduced pain intensity at rest in both surgical approaches 48 h after surgery (p < 0.05). The need for postoperative fentanyl infusion was significantly lower in the group treated with GA + ESPB in both surgical approaches than in those who only received GA (29% vs. 77% in TLIF and 0% vs. 80% in TLIF+ALIF); p = 0.01 and p = 0.004. Additionally, we observed that ESPB provides a good analgesic effect for up to 6.8 ± 3.2 h in the TLIF and 8.9 ± 7.6 h in the TLIF+ALIF approaches. Consequently, ESPB reduced the initiation of the fentanyl compared to GA alone, with a mean difference of 3.2 ± 4.2 h in the TLIF subgroup (p = 0.045) and 6.7 ± 5.3 h in TLIF +ALIF (p = 0.028). Only in the TLIF+ALIF approach, ESPB reduced the total fentanyl consumption compared to those with GA (1.43 ± 0.45 mg/24 h vs. 0.93 ± 0.68 mg/24 h; p = 0.015).
Conclusion: ESPB significantly reduced pain at rest after surgery, the number of patients requiring immediate postoperative fentanyl analgesia, and total fentanyl consumption in both surgical approaches, particularly in TLIF+ALIF. However, the application of ESPB does not always provide completely sufficient analgesia.
Since the human lifespan is rapidly increasing, there is also an increase in the number of patients with degenerative lumbar spondylosis, which is a cause of chronic back pain in up to 80% of cases (1). Nowadays, surgical interventions are gaining in popularity—spinal fusion operations in the US have increased by 77% in the period from 2002 to 2011 (2), and in the UK, the number of surgeries has increased by 63% from 2005 to 2015 (3).
The main indications for spinal fusion surgery are spinal stenosis, spondylolisthesis, and vertebral instability (4). After surgery, most severe pain is expected in the first 3–5 days postoperatively, with a tendency to progress into chronic pain (1, 5–7). Many spinal surgery patients suffer from chronic pain, depression, and restrictions on physical activities (8). Therefore, appropriate postoperative analgesia, with a reduction in opioid consumption, is of superior importance (9, 10).
Currently, the practice of anesthesiology is focused on opioid-sparing postoperative analgesia to avoid opioid-related side effects (2). Peripheral blocks, including erector spine plane block (ESPB), are essential components of multimodal analgesia, which helps to alleviate pain and increase the patient’s comfort (11). It has been widely used in spinal surgery, although there are variable data about its efficacy regarding different surgical approaches, duration of action, and impact on early rehabilitation (12). Recently, a large meta-analysis demonstrated that ESPB used in lumbar spinal surgery was effective in relieving postoperative pain and decreasing the perioperative consumption of opioids (13).
Still, it would be important to understand the impact of the ESPB on pain intensity and opioid consumption after spinal fusion surgeries using two surgical approaches: TLIF and TLIF+ALIF.
In our study, the aim was to look through our first clinical experience with and without ESPB for postoperative analgesia in TPF surgery patients. The primary outcome was to analyze the efficacy of ESPB on pain intensity in the first 48 h for lumbar spinal fusion surgeries with two different surgical approaches. The secondary outcomes were opioid consumption in the first 24 h postoperatively, and the number of fentanyl-free patients was evaluated.
This is a retrospective cohort study including 45 adult patients who underwent elective lumbar spinal fusion surgery in the Orto Clinic, Riga, Latvia, from 1 November 2019 to 30 April 2022. All spinal fusion surgeries were performed using two surgical approaches: either posterior transforaminal fusion (TLIF) surgery or combined surgery with posterior and anterior (TLIF+ALIF) approaches. The TLIF approach was performed on multiple surgery levels, but the ALIF approach was performed only on the L5-S1 level.
The inclusion criteria were 8 years of age or older, an ASA score of I–III, and elective lumbar spinal fusion surgery under general anesthesia. The exclusion criteria were known allergic reactions to local anesthetics, signs of local or general infection, pregnancy, history of mental disorders, and failed regional block (immediately reported pain intensity NRS > 6 after surgery).
All the ESPBs were performed by the same anesthesiologist for all included patients starting in September 2021, when ESPBs were introduced in the daily practice for TPF lumbar spinal surgeries. Until then, all patients underwent standardized general anesthesia (GA) without ESPB. Consequently, all patients retrospectively were allocated into two groups: the general anesthesia group (GA, N = 24) and GA combined with ESPB (GA + ESPB, N = 21). Of those who received GA, 13 underwent the TLIF approach, and 11 had the TLIF + ALIF approach. From those, who received GA + ESPB, 17 underwent TLIF, and only 4 underwent the TLIF + ALIF approach. The patient sample size was based on retrospectively available data, and the incidence of the TPF surgery approach was based on surgical indications; therefore, the sample size in the TLIF+ALIF approach receiving GA + ESPB was lower compared to other groups.
All patients, with or without ESPB block, received the same standardized GA. It included a premedication of 7.5 mg of oral Midazolam (Dormicum®, F. Hoffman-La Roche AG, Switzerland) for 30 min before transfer to the operating room. Induction of GA was provided with midazolam (Dormicum® 5 mg/mL, F. Hoffmann-La Roche Ltd., Switzerland) 2.5 mg, fentanyl (Fentanyl-Kalceks® 0.05 mg/mL, A/S Kalceks, Latvia) 1.5–2 μg/kg, propofol (Propofol® 10 mg/mL, Fresenius Kabi AG, Germany) 2 mg/kg, and cisatracurium (Nimbex®, 2 mg/mL, Aspen Pharma Ltd., Ireland) 0.2 μg/kg. Then the patient was intubated. Anesthesia was maintained with sevoflurane (Sevorane®, AbbVie S.r.l., Italy) MAC 0.8–1.2, intravenous fentanyl (Fentanyl-Kalceks® 0.05 mg/mL, A/S Kalceks, Latvia) infusion 0.5–1.5 μg/kg/h, and cisatracurium infusion 1–2 μg/kg/min.
For those who received ESPB, after the induction of GA, the patient was intubated and placed in the prone position. Bilateral ultrasound-guided ESPB at the lumbar (L2–L4) level was then performed depending on the spinal fusion level. A high-frequency linear ultrasound transducer was placed in a parasagittal orientation 3 cm laterally from the spinous process. At the spinal lumbar level, the only muscle identified superficial to the hyperdense transverse process is the erector spinae muscle. A 50 mm 22 G ultrasound needle (BRAUN®, Germany) was inserted in-plane in a cephalad-to-caudal direction until bone contact with the top of the transverse process. After slight retraction of the needle, 30 mL of 0.35% bupivacaine (Bupivacaine-Grindex, 5 mg/mL, Grindex, Latvia) with 200 mcg epinephrine (Adrenaline, 1 mg/mL, Sopharma Ad. Bulgaria) was injected between the transverse process and erector spinae, observing the cephalad to caudal spread of the local anesthetic. The same procedure was repeated on the contralateral side. During surgery, standard monitoring was performed according to the American Society of Anesthesiology standards.
Postoperatively, hemodynamic monitoring was followed regularly. Fluid management and oxygen supply were provided in the postoperative observational surgical unit for the first 24 h. The patient was assessed for pain control at 0, 1, 6, 12, 24, and 48 h after the surgery using the numeric pain rating scale (NRS). According to the local hospital guidelines, intravenous multimodal analgesia was provided with dexketoprofenum (Dolmen®, Berlin-Chemie/Menarini, Germany) 50 mg every 12 h, acetaminophen (Paracetamol, B. Braun Melsungen AG, Germany) 1 g every 6 h, and pregabalin orally (Lyrica®, Pfizer, United States) 150 mg every 24 h. For pain exacerbation, if NRS > 6, a fentanyl infusion of 2 mg/50 mL intravenously was started with a rate of 0.5–1 μg/kg/h depending on the response to analgesia. Afterward, total fentanyl consumption was calculated in the first 24 h after surgery. Thromboprophylaxis was provided with enoxaparin 40 mg (Clexane®, Sanofi-Aventis S.A. Spain) once daily from the first postoperative day.
Statistical analysis was performed using SPSS 26.0 (Statistical Package for Social Sciences). The Kolmogorov–Smirnov test was used to evaluate whether datasets conformed to a normal distribution. Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were presented as median ± IQR. Differences in data distribution between the groups were evaluated using a Mann–Whitney U-test for non-parametric datasets and a two-sample t-test or ANOVA for datasets conforming with normal distribution. A chi-square test was used for sets of nominal variables. Statistical significance was assumed if the two-tailed p < 0.05.
In total, 45 patients 18 (40%) men and 27 (60%) women were included. The mean age was 60.3 ± 14.3 years. All patients were scheduled for elective lumbar spinal fusion surgery. Of those, 30 patients underwent TLIF, of whom 17 received GA + ESPB and 13 received GA. TLIF+ALIF was performed in 15 patients, of whom 4 received GA + ESPB and 11 received GA. In total, 21 (47%) patients received GA + ESPB, and 24 (53%) were included in the GA group. As shown in Table 1, patients undergoing TLIF+ALIF with GA had a higher body mass index (BMI) compared to those receiving GA + ESPB; p = 0.04. Analyzed comorbidities and ASA class were similarly distributed between patients with the two lumbar spinal fusion surgical approaches. Chronic pain (> 3 months) was identified before surgery in 56% of all analyzed cases. All patients with the TLIF+ALIF approach in the GA + ESPB group had a history of chronic pain in contrast to patients with the TLIF approach in the GA group (p = 0.01). Lumbar spinal fusion surgery is most often performed at one (47%) or two (33%) vertebral levels. Less often, spinal fusion surgery was performed at four or five levels (4.4%).

Table 1. Distribution of patients in two lumbar spinal fusion surgery approaches according to the type of anesthesia.
As shown in Figures 1, 2, the pain was assessed at 0, 1, 6, 12, 24, and 48 h after the surgery. We found significantly lower pain scores in GA + ESPB vs. GA patients at several time points: 6 h after the surgery, pain at rest was NRS 0 vs. 2.18 ± 1.2; p < 0.001 in TLIF patients. Similarly, in TLIF+ALIF surgery patients, the pain score at rest was lower already 1 h after the surgery, NRS 0.94 ± 1.3 vs. 2.5 ± 2.3; p = 0.04 in the GA + ESPB group compared to the GA group.
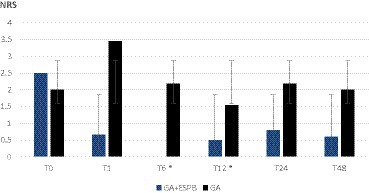
Figure 1. Pain score at rest between patients with general anesthesia with and without erector spinae plane block undergoing lumbar spinal fusion surgery with posterior transforaminal fusion approach. GA: general anesthesia; ESPB: erector spinae plane block; NRS: numeric rating scale; T0: before the surgery; T1: 1 h after the surgery; T6: 6 h after the surgery; T12: 12 h after the surgery; T24: 24 h after the surgery; T48: 48 h after the surgery; * – statistically significant difference.
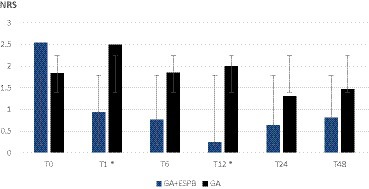
Figure 2. Pain score at rest between patients with general anesthesia with and without erector spinae plane block undergoing lumbar spinal fusion surgery with a combined posterior transforaminal and anterior surgical approach. GA: general anesthesia; ESPB: erector spinae plane block; NRS: numeric rating scale; T0: before the surgery; T1: 1 h after the surgery; T6: 6 h after the surgery; T12: 12 h after the surgery; T24: 24 h after the surgery; T48: 48 h after the surgery; * – statistically significant difference.
Finally, 12 h after surgery, the mean pain score at rest in the GA + ESPB vs. GA group was lower in both surgery approaches: TLIF approach was 1.54 ± 1.2 vs. 0.5 ± 066; p = 0.004 and in the TLIF+ALIF approach was 2 ± 1.2 vs. 0.25 ± 0.5; p = 0.015, respectively.
In total, 73%, or 33 patients out of 45, required additional fentanyl analgesia after surgery without differences according to the type of anesthesia, as reflected in Table 2. Fentanyl immediately after surgery was less often started in those receiving GA + ESPB vs. GA alone, respectively, in 29% vs. 77% (TLIF) and in 0% vs. 82% (TLIF+ALIF); p = 0.01 and p = 0.004. Additionally, we observed that ESPB provides a good analgesic effect for up to 6.8 ± 3.2 h in the TLIF and 8.9 ± 7.6 h in the TLIF+ALIF approaches. Therefore, patients with ESPB had lower total fentanyl consumption, particularly those undergoing the TLIF+ALIF approach, 1.4 ± 0.45 mg/24 h vs. 0.9 ± 0.7 mg/24 h; p = 0.01, as depicted in Table 2.
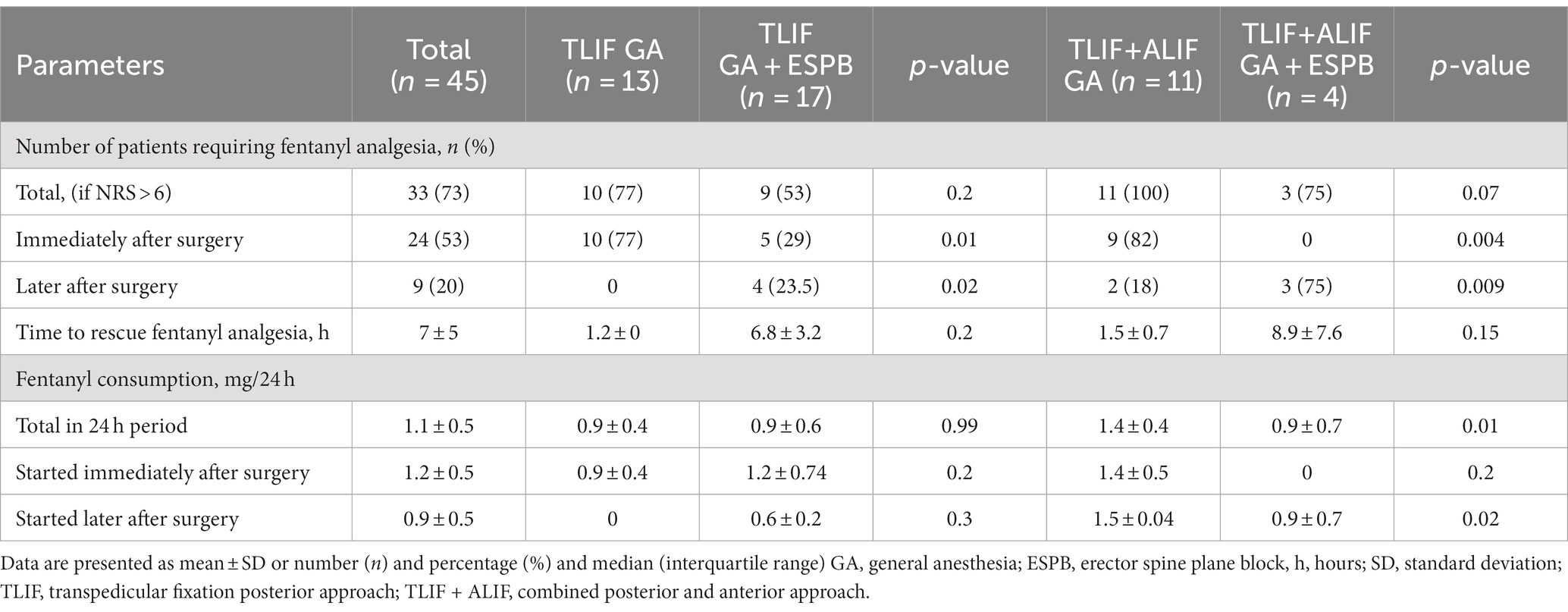
Table 2. Distribution of opioid consumption in two lumbar spinal fusion surgery approaches according to the type of anesthesia.
In this retrospective pilot study, we demonstrated that ESPB might be suitable for lumbar spinal fusion surgery patients with two different surgical approaches: posterior (TLIF) or combined posterior and anterior (TLIF+ALIF). We observed that ESPB reduces pain at rest in the 48 h postoperative period, providing a good analgesic effect for up to 6.8 ± 3.2 h in the TLIF and 8.9 ± 7.6 h in the TLIF+ALIF approaches. Additionally, it reduces the number of patients requiring immediate postoperative fentanyl analgesia, the initiation of the fentanyl after surgery, and the total fentanyl consumption compared to GA alone in both surgical approaches, particularly in the TLIF+ALIF approach.
Opioid requirement reduction is essential, particularly in spinal surgery patients who might be at high risk of developing chronic back pain syndrome without showing enough satisfaction after surgery. Moreover, the application of opioids is associated with major side effects such as nausea and vomiting, sedation, urinary retention, ileus, and respiratory depression. These side effects promote a longer hospital stay and a longer recovery time. In our study, 56% of patients suffered from chronic back pain (> 3 months) already before surgery (60% with TLIF and 46% with the TLIF+ALIF approach). According to other studies, for every 10% of the patients suffering from severe pain in the postoperative period, there is a 30% risk of the development of chronic pain (9, 14–22). As a result, multimodal analgesia with the application of regional blocks is gaining in popularity (23–26).
We must say that the applications of ESPB are not always allowed to fully avoid fentanyl administration for postoperative pain control. In total, 33 patients out of 45 required additional fentanyl analgesia after surgery, without differences according to the type of anesthesia. Still, ESPB allowed to reduce the total fentanyl consumption in the 24 h postoperative period with the greatest analgesic effect produced in patients with the TLIF+ALIF approach, where 81% of patients did not require fentanyl in the early postoperative period and it was started on average 8.9 ± 7.6 h after the surgery. Thereby, the total fentanyl consumption in 24 h was considerably lower in the GA + ESPB group compared to GA for those with the TLIF+ALIF approach, with MD 0.5 ± 0.23 mg/24 h; p = 0.015.
Interestingly, 29.4% of TLIF patients in the GA+ ESPB group required fentanyl analgesia early after the operation, but after the TLIF +ALIF approach, none of the patients required immediate fentanyl infusion. That might be explained by the small group of patients who received ESPB for TLIF+ALIF surgery.
ESPB might be useful as a part of multimodal analgesia because it also considerably reduces the initiation of fentanyl analgesia immediately after surgery. Similar results were reported by Liang et al. in a 2021 meta-analysis. They demonstrated that patients receiving ESPB in spinal fusion surgeries in the lumbar region less often required rescue analgesia (RR = 0.39, from 0.19 to 0.80, p = 0.01). Moreover, rescue analgesia was asked for later compared to patients without ESPB. The application of ESPB prolonged the time until rescue analgesia was started on average for 6.15 h (from 2.19 to 10.12; p = 0.002) (27). Our study confirmed this data, where fentanyl analgesia immediately after the surgery was more often started in the GA group vs. GA + ESPB group in both the TLIF (77% vs. 29%) and TLIF+ALIF (82% vs. 0%) approaches.
We found that the ESPB reduces total 24 h fentanyl consumption, particularly in TLIF+ALIF approach patients (MD 0.5 mg/24 h). Other studies had shown marked opioid reduction in the ESPB group patients (MD, −18.69; 95% CI, −27.95 to −9.42; p < 0.0001) in various spinal surgeries (20, 26–37). However, still great non-homogeneity in results regarding opioid reduction is shown. Wu et al. in 2019 found a small difference in opioid consumption in the first 24 h (MD, −2.6; 95% CI, −4.82 to −0.38; p < 0.0001) (27, 38). Our study showed a mean difference in fentanyl consumption of 0.26 mg. When recalculated to intravenous morphine equivalents, it is 1.3 mg. We might conclude that the different types of surgery, such as laminectomy or decompression, require a greater dose of opioids for postoperative analgesia compared with lumbar discectomy (39–44).
We also evaluated pain intensity at rest after surgery in the first 48 h postoperative period. High pain levels are usually expected after spinal fusion surgeries (4). It is reported that the peak in pain intensity after spinal surgeries in the lumbar region is usually felt 4 h after the surgery, and it gradually decreases during the next 72 h (28, 45–48). That is why we aimed to evaluate the pain level in the first 48 h after the surgery. Furthermore, there is no common opinion about when to measure pain—at rest or movement (4, 14, 49–54). We found statistically significant differences in the pain intensity between two applied types of anesthesia: 1, 6, and 12 h after surgery. In all included patients, the mean pain intensity at rest in the GA group was NRS 3, but it is important to specify that this pain level in most of the patients (87.5%) was observed when fentanyl analgesia was used. The authors describe ESPB in a wide range of spinal surgeries—decompressions, discectomies, and fusion surgeries—and in some cases, the type of surgery is not always specified (4, 11, 27, 28, 39, 55). Still, there is no clear understanding of the mechanism of action and distribution of local anesthetics after ESPB. Most likely, the analgesic effect is provided by the distribution of the local anesthetic in the dorsal and ventral nerve roots from the interfacial space between the processus transversus and the erector spine muscle group; however, systemic absorption of the local anesthetic cannot be excluded (6, 23, 35, 56–64).
The postoperative pain intensity can be affected not only by surgical trauma but also by risk factors for chronic pain (65–68). Other publications demonstrate that chronic pain before surgery increases the risk of postoperative chronic pain by 2.6 times (9, 24, 69–71). In our study, half of the patients (56%) suffered from chronic back pain (>3 months) already before surgery (60% with the TLIF approach and 46% with the TLIF+ALIF approach), but we did not evaluate the incidence of postoperative chronic pain 3 months after the surgery since the study was retrospectively designed.
The duration of the ESPB depends on the volume and concentration of the local anesthetic, as well as on the application of adjuvants (23, 54, 72–75). Rizkkalla et al., in the meta-analysis (2021) of 15 studies, showed that the duration of ESPB varied from 4 to 72 h and this was influenced by the type of local anesthetic, its volume (20–40 mL), and if the block was unilateral or bilateral (55). In our study, we unified the dose of the local anesthetic and used 30 mL of bupivacaine 0.35% bilaterally, knowing that 20 mL in the lumbar region distributes up to 2–3 levels (76) and the expected duration of the ESPB is 6–8 h after bilateral block with the 20 mL of bupivacaine 0.25% reported by Singh et al. (77). We added 200 μg epinephrin, decreasing the systemic absorption of the local anesthetic (78–81). We observed that our regimen had not affected the prolongation of the block when compared to other studies (55, 77, 82). There is no certainty about the optimal dose of the local anesthetic or volume. Studies show variable doses of the local anesthetic for spinal surgeries: 10–40 mL of ropivacaine, levobupivacaine, bupivacaine (in concentrations 0.5, 0.25%, or 0.375%), and lidocaine (in concentrations 1% or 2%), not exceeding the maximal dose (21, 67, 75).
Since the anatomical structures are being impacted during the spinal surgery, there might be different analgesic effects of ESPB, which can be affected by the changes in the anatomical structures of the spine (3, 83–85). That emphasizes the importance of the evaluation of the sensor block before and after surgery. It might be the restriction of our study that routinely ESPB was performed after induction of anesthesia without the evaluation of a sensor block. Still, we speculate that it might be hard to distinguish if the analgesic effect is always achieved by ESPB or by the systemic absorption of the local anesthetic after surgery (11, 86–88).
We admit as a major limitation of this study that it was a retrospectively designed pilot study to evaluate our first experience with ESPB. Therefore, we were not able to reach equal distributions of different TPF surgery approaches between the GA and GA + ESPB groups. The patient group in the TLIF+ALIF approach receiving GA + ESPB was too small (four patients), which may lead to a type 2 error in statistical analysis. Although we reached a statistically significant difference in 24 h fentanyl consumption in the TLIF+ALIF approach, it is still too early to draw any scientific or clinically relevant conclusions.
In contrast, we did not reach a statistically significant difference in the 24 h fentanyl consumption in the TLIF group, also indicating a too low analyzed patient sample size. Nevertheless, the data were precisely manually collected by going through each medical history, surgery, and anesthesia performed by the same surgeon and the same anesthesiologist, and postoperative care was strongly standardized for all analyzed patients. According to ASA classes and co-morbidities most patients were homogenic, although some heterogenicity was noticed in those with TLIF+ALIF approach and GA + ESPB, these patients more often presented chronic pain, anxiety and depression.
Assuming a medium effect size of 0.5, a significance level of 0.05, a power of 0.80, and a potential attrition rate of 10%, the minimum sample size required for each group would be approximately 64 participants.
Retrospective data from our first clinical experience with ESPB in TPF surgery patients indicate that ESPB might be an effective component of multimodal analgesia in lumbar spinal fusion surgery patients with TLIF or TLIF+ALIF surgical approaches. ESPB significantly reduced pain at rest after surgery, the number of patients requiring immediate postoperative fentanyl analgesia, and total fentanyl consumption in both surgical approaches, particularly in TLIF+ALIF. However, the application of ESPB does not always provide sufficient analgesia to completely avoid fentanyl administration after the surgery in the 24 h postoperative period. Further prospective analysis, including more patients, is necessary to confirm the effectiveness of both TPF approaches.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Prof. Olafs Brūvers Theology Asoc. Prof. Santa Purviņa Pharmacology Asoc. Prof. Voldemārs Arnis Rehabilitology Prof. Regīna Kleina Pathology Prof. Guntars Pupelis Surgery Asoc. Prof. Viesturs Liguts Toxicology Doc. Iveta Jankovska orthodontology Doc. Kristaps Circenis Lecturer Ilvija Razgale. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JB: Methodology, Writing – original draft. AO: Supervision, Conceptualization, Data curation, Methodology, Writing – review & editing. LS: Conceptualization, Formal analysis, Writing – review & editing. ZG-K: Data curation, Writing – original draft. JN: Formal analysis, Writing – original draft. IL: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication has been supported by funding Nr.3-FD.1-1/99/IE24 from Rīgas Stradiņš University.
Many thanks to Spinal Surgeon Dr. Gulbis, who performed surgeries for all the patients analyzed in the study. And thanks to the Ortho Clinic for excellent collaboration in conducting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ramdas, J, and Jella, V. Prevalence and risk factors of low back pain. Int J Adv Med. (2018) 5:1120. doi: 10.18203/2349-3933.ijam20183413
2. Weiss, AJ, and Elixhauser, A. “Trends in Operating Room Procedures in U.S. Hospitals, 2001-2011” Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Agency for Healthcare Research and Quality (US). (2014).
3. Jin, F, Li, Z, Tan, W, Ma, H, Li, X, and Lu, H. Preoperative versus postoperative ultrasound-guided rectus sheath block for improving pain, sleep quality and cytokine levels in patients with open midline incisions undergoing transabdominal gynecological surgery: a randomized-controlled trial. BMC Anesthesiol. (2018) 18:19. doi: 10.1186/s12871-018-0485-9
4. Sayers, A, Wylde, V, Lenguerrand, E, Beswick, AD, Gooberman-Hill, R, Pyke, M, et al. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res. (2016) 68:237–45. doi: 10.1002/acr.22656
5. Soffin, EM, Vaishnav, AS, Wetmore, DS, Barber, L, Hill, P, Gang, CH, et al. Design and implementation of an enhanced recovery after surgery (ERAS) program for minimally invasive lumbar decompression spine surgery. Spine. (2019) 44:E561–70. doi: 10.1097/BRS.0000000000002905
6. Patil, H, Garg, N, Navakar, D, and Banabokade, L. Lumbar spine surgeries under spinal anesthesia in high-risk patients: a retrospective analysis. World Neurosurg. (2019) 124:779–82. doi: 10.1016/j.wneu.2019.01.023
7. Papadopoulos, EC, Girardi, FP, Sama, A, Pappou, IP, Urban, MK, and Cammisa, FP. Lumbar microdiscectomy under epidural anesthesia: a comparison study. Spine J. (2006) 6:561–4. doi: 10.1016/j.spinee.2005.12.002
8. Fletcher, D, Stamer, UM, Pogatzki-Zahn, E, Zaslansky, R, Tanase, NV, Perruchoud, C, et al. Chronic postsurgical pain in Europe. Eur J Anaesthesiol. (2015) 32:725–34. doi: 10.1097/EJA.0000000000000319
9. Forero, M, Adhikary, SD, Lopez, H, Tsui, C, and Chin, KJ. The erector spinae plane block. Reg Anesth Pain Med. (2016) 41:621–7. doi: 10.1097/AAP.0000000000000451
10. Hospital Admitted Patient Care Activity, 2015–16. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2015-16. (Accessed July 10, 2019).
11. Saadawi, M, Layera, S, Aliste, J, Bravo, D, Leurcharusmee, P, and Tran, DQ. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. (2021) 68:110063. doi: 10.1016/j.jclinane.2020.110063
12. Ueshima, H, Ozawa, T, Toyone, T, and Otake, H. Efficacy of the thoracolumbar Interfascial plane block for lumbar Laminoplasty: a retrospective study. Asian Spine J. (2017) 11:722–5. doi: 10.4184/asj.2017.11.5.722
13. Fu, MY, Hao, J, Ye, LH, Jiang, W, Lv, YW, Shen, JL, et al. Efficacy and safety of erector spinae plane block for perioperative pain Management in Lumbar Spinal Surgery: a systematic review and Meta-analysis of randomized controlled trials. J Pain Res. (2023) 16:1453–75. doi: 10.2147/JPR.S402931
14. Liang, X, Zhou, W, and Fan, Y. Erector spinae plane block for spinal surgery: a systematic review and meta-analysis. Korean J Pain. (2021) 34:487–500. doi: 10.3344/kjp.2021.34.4.487
15. Qiu, Y, Zhang, T-J, and Hua, Z. Erector spinae plane block for lumbar spinal surgery: a systematic review. J Pain Res. (2020) 13:1611–9. doi: 10.2147/JPR.S256205
16. Oh, SK, Lim, BG, Won, YJ, Lee, DK, and Kim, SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. (2022) 78:110647. doi: 10.1016/j.jclinane.2022.110647
17. Rizkalla, JM, Holderread, B, Awad, M, Botros, A, and Syed, IY. The erector spinae plane block for analgesia after lumbar spine surgery: a systematic review. J Orthop. (2021) 24:145–50. doi: 10.1016/j.jor.2021.02.006
18. De Cassai, A, and Tonetti, T. Local anesthetic spread during erector spinae plane block. J Clin Anesth. (2018) 48:60–1. doi: 10.1016/j.jclinane.2018.05.003
19. Adhikary, SD, Bernard, S, Lopez, H, and Chin, KJ. Erector spinae plane block versus retrolaminar block. Reg Anesth Pain Med. (2018) 1:1. doi: 10.1097/AAP.0000000000000798
20. Vidal, E, Giménez, H, Forero, M, and Fajardo, M. Bloqueo Del Plano Del Músculo Erector Espinal: Estudio Anatómico-Cadavérico Para Determinar Su Mecanismo de Acción. Rev Esp Anestesiol Reanim. (2018) 65:514–9. doi: 10.1016/j.redar.2018.07.004
21. Schwartzmann, A, Peng, P, Maciel, MA, and Forero, M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anesth/J Can Anesth. (2018) 65:1165–6. doi: 10.1007/s12630-018-1187-y
22. Chin, KJ, Lirk, P, Hollmann, MW, and Schwarz, SKW. Mechanisms of action of fascial plane blocks: a narrative review. Reg Anesth Pain Med. (2021) 46:618–28. doi: 10.1136/rapm-2020-102305
23. Mistry, T, and Vadera, H. Erector spinae plane block: anatomical landmark-guided technique. Saudi J Anaesth. (2019) 13:268–9. doi: 10.4103/sja.SJA_780_18
24. Celik, M, Tulgar, S, Ahiskalioglu, A, and Alper, F. Is high volume lumbar erector spinae plane block an alternative to Transforaminal epidural injection? Evaluation with MRI. Reg Anesth Pain Med. (2019) 44:906–7. doi: 10.1136/rapm-2019-100514
25. Soliman, MAR, Khan, A, Aguirre, AO, Ruggiero, N, Levy, BR, Mariotti, BL, et al. Effectiveness and safety of continuous infusion regional anesthesia pumps for pain after Thoracopelvic fusion surgery for persistent spinal pain syndrome. World Neurosurg. (2021) 154:815–21. doi: 10.1016/j.wneu.2021.08.013
26. Attari, MA, Mirhosseini, SA, Honarmand, A, and Safavi, MR. Spinal anesthesia versus general anesthesia for elective lumbar spine surgery: a randomized clinical trial. J Res Med Sci. (2011) 16:524–9.
27. Garg, B, Ahuja, K, Khanna, P, and Sharan, AD. Regional anesthesia for spine surgery. Clin Spine Surg. (2020) 34:163–70. doi: 10.1097/BSD.0000000000001096
28. West, JL, De Biase, G, Bydon, M, Bojaxhi, E, Mendhi, M, Quiñones-Hinojosa, A, et al. What is the learning curve for lumbar spine surgery under spinal anesthesia? World Neurosurg. (2022) 158:310–6. doi: 10.1016/j.wneu.2021.10.172
29. Ivanusic, J, Konishi, Y, and Barrington, MJ. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. (2018) 43:567–71. doi: 10.1097/AAP.0000000000000789
30. Forero, M, Rajarathinam, M, Adhikary, SD, and Chin, KJ. Erector spinae plane block for the Management of Chronic Shoulder Pain: a case report. Can J Anesth/J Can Anesth. (2017) 65:288–93. doi: 10.1007/s12630-017-1010-1
31. Bang, S, Chung, J, Kwon, W, Yoo, S, Soh, H, and Lee, SM. Erector spinae plane block for multimodal analgesia after wide midline laparotomy. Medicine. (2019) 98:e15654. doi: 10.1097/MD.0000000000015654
32. Yao, Y, Fu, S, Dai, S, Yun, J, Zeng, M, Li, H, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth. (2020) 63:109783. doi: 10.1016/j.jclinane.2020.109783
33. Schwenk, ES, and Mariano, ER. Designing the ideal perioperative pain management plan starts with multimodal analgesia. Korean J Anesthesiol. (2018) 71:345–52. doi: 10.4097/kja.d.18.00217
34. Waldmann, D, Hartung, H, and Roth, HJ. Examinations of Upper Gastrointestinal Bleeding-Value of emergency endoscopy (Author’s Transl). Zentralbl Chir. (1977) 102:262–9.
35. Tulgar, S, Selvi, O, Senturk, O, Ermis, MN, Cubuk, R, and Ozer, Z. Clinical experiences of ultrasound-guided lumbar erector spinae plane block for hip joint and proximal femur surgeries. J Clin Anesth. (2018) 47:5–6. doi: 10.1016/j.jclinane.2018.02.014
36. Chung, K, and Kim, ED. Continuous erector spinae plane block at the lower lumbar level in a lower extremity complex regional pain syndrome patient. J Clin Anesth. (2018) 48:30–1. doi: 10.1016/j.jclinane.2018.04.012
37. Chin, KJ, Forero, M, and Adhikary, SD. Reply to Dr Ueshima and Dr Murouchi. Reg Anesth Pain Med. (2017) 42:124–5. doi: 10.1097/AAP.0000000000000531
38. Munshey, F, Caruso, TJ, Wang, EY, and Tsui, BCH. Programmed intermittent bolus regimen for erector spinae plane blocks in children. Anesth Analg. (2018) 1:e63–e66.
39. Balaban, O, and Aydın, T. Lumbar erector spinae plane catheterization for continuous postoperative analgesia in Total knee arthroplasty: a case report. J Clin Anesth. (2019) 55:138–9. doi: 10.1016/j.jclinane.2018.12.017
40. López, MB, Cadórniga, ÁG, González, JML, Suárez, ED, Carballo, CL, and Sobrino, FP. Erector spinae block. A narrative review. Central Eur J Clin Res. (2018) 1:28–39. doi: 10.2478/cejcr-2018-0005
41. Finneran, JJ, Gabriel, RA, and Khatibi, B. Erector spinae plane blocks provide analgesia for breast and axillary surgery. Reg Anesth Pain Med. (2018) 43:101–2. doi: 10.1097/AAP.0000000000000695
42. Macaire, P, Ho, N, Nguyen, T, Nguyen, B, Vu, V, Quach, C, et al. Ultrasound-guided continuous thoracic erector spinae plane block within an enhanced recovery program is associated with decreased opioid consumption and improved patient postoperative rehabilitation after open cardiac surgery—a patient-matched, controlled before-and-after study. J Cardiothorac Vasc Anesth. (2019) 33:1659–67. doi: 10.1053/j.jvca.2018.11.021
43. Goel, VK, Chandramohan, M, Murugan, C, Shetty, AP, Subramanian, B, Kanna, RM, et al. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: a prospective, randomized, case-control study. Spine J. (2021) 21:1873–80. doi: 10.1016/j.spinee.2021.06.015
44. Finnerty, D, Ní Eochagáin, A, Ahmed, M, Poynton, A, Butler, JS, and Buggy, DJ. A randomised trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia. (2021) 76:1499–503. doi: 10.1111/anae.15488
45. Yörükoğlu, HU, İçli, D, Aksu, C, Cesur, S, Kuş, A, and Gürkan, Y. Erector spinae block for postoperative pain Management in Lumbar Disc Hernia Repair. J Anesth. (2021) 35:420–5. doi: 10.1007/s00540-021-02920-0
46. Zhu, L, Wang, M, Wang, X, Wang, Y, Chen, L, and Li, J. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: a randomized controlled trial. Pain Physician. (2021) 24:E161–8. doi: 10.36076/ppj.2021.24.E161-E168
47. Yeşiltaş, S, Abdallah, A, Uysal, Ö, Yilmaz, S, Çinar, İ, and Karaaslan, K. The efficacy of intraoperative freehand erector spinae plane block in lumbar spondylolisthesis: a randomized controlled study. Spine. (2021) 46:E902–10. doi: 10.1097/BRS.0000000000003966
48. Yu, Y, Wang, M, Ying, H, Ding, J, Wang, H, and Wang, Y. The analgesic efficacy of erector spinae plane blocks in patients undergoing posterior lumbar spinal surgery for lumbar fracture. World Neurosurg. (2021) 147:1–7. doi: 10.1016/j.wneu.2020.10.175
49. Zhang, Q, Wu, Y, Ren, F, Zhang, X, and Feng, Y. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. (2021) 68:110090. doi: 10.1016/j.jclinane.2020.110090
50. Eskin, MB, Ceylan, A, Özhan, MÖ, and Atik, B. Ultrasound-guided erector spinae block versus mid-transverse process to pleura block for postoperative analgesia in lumbar spinal surgery. Anaesthesist. (2020) 69:742–50. doi: 10.1007/s00101-020-00848-w
51. Aksu, C, and Gürkan, Y. Aksu approach for lumbar erector spinae plane block for pediatric surgeries. J Clin Anesth. (2019) 54:74–5. doi: 10.1016/j.jclinane.2018.10.043
52. De Cassai, A, Bonvicini, D, Correale, C, Sandei, L, Tulgar, S, and Tonetti, T. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. (2019) 85:85 (3). doi: 10.23736/S0375-9393.18.13341-4
53. Melvin, JP, Schrot, RJ, Chu, GM, and Chin, KJ. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anesth/J Can Anesth. (2018) 65:1057–65. doi: 10.1007/s12630-018-1145-8
54. Tulgar, S, Aydin, ME, Ahiskalioglu, A, De Cassai, A, and Gurkan, Y. Anesthetic techniques: focus on lumbar erector spinae plane block. Local Reg Anesth. (2020) 13:121–33. doi: 10.2147/LRA.S233274
55. Calandese, F, and Adduci, A. Erector spinae plane block for acute postoperative pain management after anterior thoracolumbar spine surgery. J Clin Anesth. (2019) 52:55–6. doi: 10.1016/j.jclinane.2018.08.014
56. Tulgar, S, and Senturk, O. Ultrasound guided erector spinae plane block at L-4 transverse process level provides effective postoperative analgesia for Total hip arthroplasty. J Clin Anesth. (2018) 44:68. doi: 10.1016/j.jclinane.2017.11.006
57. Tulgar, S, Selvi, O, Senturk, O, Serifsoy, TE, and Thomas, DT. Ultrasound-guided erector spinae plane block: indications, complications, and effects on acute and chronic pain based on a single-center experience. Cureus. (2019) 11:e3815. doi: 10.7759/cureus.3815
58. Kim, E, and Alshoubi, A. Fluoroscopic-guided erector spinae plane block for spine surgery. Saudi J Anaesth. (2022) 16:229–31. doi: 10.4103/sja.sja_694_21
59. Ahiskalioglu, A, Alici, HA, and Ari, MA. Ultrasound guided low thoracic erector spinae plane block for Management of Acute Herpes Zoster. J Clin Anesth. (2018) 45:60–1. doi: 10.1016/j.jclinane.2017.12.018
60. Elkoundi, A, Chouikh, C, Baite, A, Bensghir, M, Bakkali, H, and Lalaoui, SJ. Successful erector spinae plane block without ultrasound guidance in a severely cardiovascular compromised patient. J Clin Anesth. (2019) 53:50. doi: 10.1016/j.jclinane.2018.10.002
61. De Cassai, A, Sgarabotto, C, and Dal Cin, S. Old approach for a new indication: shamrock sign for ESP block. Reg Anesth Pain Med. (2019) 44:256. doi: 10.1136/rapm-2018-100170
62. Tulgar, S, Unal, OK, Thomas, DT, and Ozer, Z. A novel modification to ultrasound guided lumbar erector spinae plane block: Tulgar approach. J Clin Anesth. (2019) 56:30–1. doi: 10.1016/j.jclinane.2019.01.016
63. Singh, N, Nagaraja, P, Ragavendran, S, Asai, O, Bhavya, G, Manjunath, N, et al. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain Management in Cardiac Surgery. Ann Card Anaesth. (2018) 21:323–7. doi: 10.4103/aca.ACA_16_18
64. Gaio-Lima, C, Costa, CC, Moreira, JB, Lemos, TS, and Trindade, HL. Bloqueo Continuo En El Plano Del Músculo Erector Del Espinal Para Analgesia En Cirugía Torácica Pediátrica: Informe de Un Caso. Rev Esp Anestesiol Reanim. (2018) 65:287–90. doi: 10.1016/j.redar.2017.11.010
65. Ueshima, H, and Otake, H. RETRACTED: clinical experiences of erector spinae plane block for children. J Clin Anesth. (2018) 44:41. doi: 10.1016/j.jclinane.2017.10.021
66. De la Cuadra-Fontaine, JC, Concha, M, Vuletin, F, and Arancibia, H. Continuous erector spinae plane block for thoracic surgery in a pediatric patient. Pediatr Anesth. (2017) 28:74–5. doi: 10.1111/pan.13277
67. Muñoz-Leyva, F, Mendiola, WE, Bonilla, AJ, Cubillos, J, Moreno, DA, and Chin, KJ. In reply to “continuous erector spinae plane (ESP) block: optimizing the analgesia technique”. J Cardiothorac Vasc Anesth. (2018) 32:e3–4. doi: 10.1053/j.jvca.2018.03.033
68. Singh, S, Pandey, R, and Chowdhary, N. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in Choledochal cyst resection surgery. Saudi J Anaesth. (2018) 12:499–500. doi: 10.4103/sja.SJA_188_18
69. Jaiswal, V, Jain, K, and Puri, A. Erector spinae plane block: relatively new block on horizon with a wide Spectrum of application – a case series. Indian J Anaesth. (2018) 62:809–13. doi: 10.4103/ija.IJA_263_18
70. De Cassai, A, Tonetti, T, Galligioni, H, and Ori, C. Bloqueio Do Plano Do Eretor Da Espinha Com Técnica de Múltiplos Cateteres Para Esofagectomia Aberta: Relato de Caso. Braz J Anesthesiol. (2019) 69:95–8. doi: 10.1016/j.bjan.2018.06.001
71. G, N.; Tariq1, Z.; Niraj; G2*; Internationals, O. Continuous erector spinae plane (ESP) analgesia in different open abdominal surgical procedures: a case series. J Anesth Surg. (2018) 5:57–60. doi: 10.15436/2377-1364.18.1853
72. Yang, H-M, Choi, YJ, Kwon, HJ, O, J, Cho, TH, and Kim, SH. Comparison of Injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. (2018) 73:1244–50. doi: 10.1111/anae.14408
73. Harbell, MW, Seamans, DP, Koyyalamudi, V, Kraus, MB, Craner, RC, and Langley, NR. Evaluating the extent of lumbar erector spinae plane block: an anatomical study. Reg Anesth Pain Med. (2020) 45:640–4. doi: 10.1136/rapm-2020-101523
74. Karaca, O, and Pinar, HU. Is high dose lumbar erector spinae plane block safe? J Clin Anesth. (2020) 62:109721. doi: 10.1016/j.jclinane.2020.109721
75. Tulgar, S, Ahiskalioglu, A, De Cassai, A, and Gurkan, Y. Efficacy of bilateral erector spinae plane block in the Management of Pain: current insights. J Pain Res. (2019) 12:2597–613. doi: 10.2147/JPR.S182128
76. De Cassai, A, Andreatta, G, Bonvicini, D, Boscolo, A, Munari, M, and Navalesi, P. Injectate spread in ESP block: a review of anatomical investigations. J Clin Anesth. (2020) 61:109669. doi: 10.1016/j.jclinane.2019.109669
77. Singh, S, Choudhary, NK, Lalin, D, and Verma, VK. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol. (2019) 32:330–4. doi: 10.1097/ANA.0000000000000603
78. Bhoi, D, Acharya, P, Talawar, P, and Malviya, A. Continuous erector spinae plane local anesthetic infusion for perioperative analgesia in pediatric thoracic surgery. Saudi J Anaesth. (2018) 12:502–3. doi: 10.4103/sja.SJA_243_18
79. Josh Luftig, PA, Mantuani, D, Herring, AA, Dixon, B, Clattenburg, E, and Nagdev, A. The authors reply to the optimal dose and volume of local anesthetic for erector spinae plane blockade for posterior rib fractures. Am J Emerg Med. (2018) 36:1103–4. doi: 10.1016/j.ajem.2018.03.051
80. Neal, JM, Barrington, MJ, Fettiplace, MR, Gitman, M, Memtsoudis, SG, Mörwald, EE, et al. The third American Society of Regional Anesthesia and Pain Medicine practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. (2018) 43:113–23. doi: 10.1097/AAP.0000000000000720
81. El-Boghdadly, K, Pawa, A, and Chin, KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. (2018) 11:35–44. doi: 10.2147/LRA.S154512
82. Elder, JB, Hoh, DJ, and Wang, MY. Postoperative continuous paravertebral anesthetic infusion for pain control in lumbar spinal fusion surgery. Spine. (2008) 33:210–8. doi: 10.1097/BRS.0b013e318160447a
83. Restrepo-Garces, CE, Chin, KJ, Suarez, P, and Diaz, A. Bilateral continuous erector spinae plane block contributes to effective postoperative analgesia after major open abdominal surgery. A A Case Rep. (2017) 9:319–21. doi: 10.1213/XAA.0000000000000605
84. Ezhevskaya, AA, Mlyavykh, SG, and Anderson, DG. Effects of continuous epidural anesthesia and postoperative epidural analgesia on pain management and stress response in patients undergoing major spinal surgery. Spine. (2013) 38:1324–30. doi: 10.1097/BRS.0b013e318290ff26
85. Kurnutala, LN, Dibble, JE, Kinthala, S, and Tucci, MA. Enhanced recovery after surgery protocol for lumbar spinal surgery with regional anesthesia: a retrospective review. Cureus. (2021) 13:e18016. doi: 10.7759/cureus.18016
86. Jadon, A, Swarupa, C, and Amir, M. Fluoroscopic-guided erector spinae plane block: a feasible option. Indian J Anaesth. (2018) 62:806–8. doi: 10.4103/ija.IJA_411_18
87. Chin, KJ, Malhas, L, and Perlas, A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery. Reg Anesth Pain Med. (2017) 42:372–6. doi: 10.1097/AAP.0000000000000581
Keywords: ESPB, erector spine plane block, regional anesthesia, postoperative pain, lumbar spinal fusion surgery, pain, ultrasound
Citation: Birnbaums JV, Ozoliņa A, Solovjovs L, Glāzniece-Kagane Z, Nemme J and Logina I (2024) Efficacy of erector spine plane block in two different approaches to lumbar spinal fusion surgery: a retrospective pilot study. Front. Med. 11:1330446. doi: 10.3389/fmed.2024.1330446
Received: 30 October 2023; Accepted: 15 January 2024;
Published: 14 February 2024.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Marco Echeverria-Villalobos, Oho State University, United StatesCopyright © 2024 Birnbaums, Ozoliņa, Solovjovs, Glāzniece-Kagane, Nemme and Logina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jānis Verners Birnbaums, anZiaXJuYmF1bXNAZ21haWwuY29t; Agnese Ozoliņa, YWduZXNlLm96b2xpbmFAaWNsb3VkLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.