- 1Department of Respiratory Medicine, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 2Department of Endocrinology, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
- 3Department of General and Practice, The Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
Purpose: The safety and efficacy of vaccination in people with hypertension (HTN) is important. There are currently a few data on the immunogenicity and safety of inactivated SARS-CoV-2 vaccinations in hypertension patients.
Methods: After receiving a two-dose immunization, 94 hypertension adult patients and 74 healthy controls (HCs) in this study, the evaluation included looking at antibodies (Abs) against receptor binding domain (RBD) IgG, SARS-CoV-2 neutralizing antibodies (NAbs), RBD-specific B cells, and memory B cells (MBCs).
Results: There was no discernible difference in the overall adverse events (AEs) over the course of 7 or 30 days between HTN patients and HCs. HTN patients had lower frequencies of RBD-specific memory B cells and the seropositivity rates and titers of Abs compared with HCs (all, p < 0.05). HTN patients with cardiovascular and cerebrovascular conditions (CCVD) have lower titers of CoV-2 NAb than in HCs. The titers of both Abs in HTN declined gradually over time.
Conclusion: Inactivated COVID-19 vaccinations were safe in hypertension patients; however humoral immune was limited, especially merged CCVD and declined gradually over time.
Introduction
Since its discovery in 2019 (1), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to devastate public health across the world. Even though several antiviral drug and specific monoclonal antibodies are currently available for the therapy of COVID-19, immunization is still essential for preventing COVID-19. HTN has been shown to have a higher risk of acquiring severe COVID-19 (2–4), therefore, the SARS-CoV-2 vaccines may be advantageous for this particular population.
Currently, hypertension still poses a threat to global public health. Numerous epidemiological studies (5, 6) have shown that the prevalence of hypertension in China ranges from 23.2% to 44.7%. Most individuals with hypertension, in particular, have co-occurring cardiovascular and cerebrovascular conditions (CCVD).
Recent studies have shown that HTN patients are protected after receiving a full vaccination, but the immune response may be lower than in HCs (3, 7–9). However, according to a study, there is no correlation between Ab response and hypertension (10). Because of this, it is currently unclear whether HTN patients in China may get the inactivated COVID-19 vaccination without harm and elicit a powerful immune response.
Following vaccination, antibody-secreting cells (ASCs), which are found in bone marrow and MBCs, mediate chronic humoral immunity. When an infection recurs, MBCs multiply quickly differentiate into ASCs, which aids in the defense of the body (11, 12). However, there has been little information available about how RBD+-specific MBCs response in HTN.
Previous studies have shown that COVID-19 vaccines are safe and effective (13–15). Due to the lack of information from clinical research, doctors might not be able to address concerns of patients about the safety and efficacy of COVID-19 vaccines in the HTN populations. Therefore, we evaluate the safety and immunogenicity of inactivated vaccinations, and this trial enrolled 94 adult HTN patients and 74 adult HCs.
Materials and methods
Study design
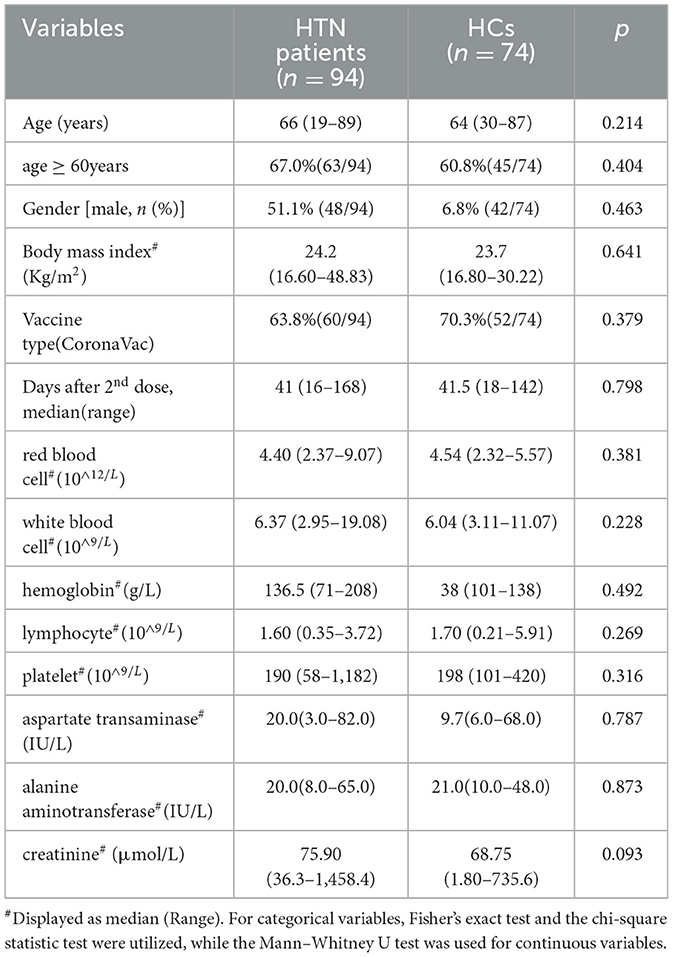
Participants in this study were systematically recruited at the Second Affiliated Hospital of Chongqing Medical University between 6 May 2021 and 3 December 2021. The diagnosis of hypertension was made based on the guidelines as follows: adults with an average SBP ≥140 mmHg or DBP ≥90 mmHg, and all patients who had the condition stayed stable and had good blood pressure management due to oral antihypertensive medications.
The key inclusion criteria for all participants were: (i) after two-dose vaccination (BBIBP-CorV or CoronaVac), (ii) age ≥ 18 years, key exclusion criteria were: (i) history of COVID-19 infection, (ii) autoimmune disease or use of immunosuppressants, and (iii) pregnancy. It is sure that all participants did not experience “white coat hypertension”.
All HTN patients were split into four groups for further analysis: secondary hypertension group (SH) (n = 16). Furthermore, essential hypertension will be classified into grades 1 to 3 depending on the grade in which patients received their hypertension diagnosis [essential grades 1 (EHPG1), 140 mmHg ≤ SBP < 159 mmHg or 90 mmHg ≤ DBP < 99 mmHg, n = 11, essential grades 2(EHPG2], 160 mmHg ≤ SBP < 179 mmHg or 100 mmHg ≤ DBP < 109 mmHg, n = 24, essential grades 3(EHPG3), SBP ≥ 180 mmHg or DBP ≥ 110 mmHg, n = 43).
All HTN patients were split into two subgroups according to with or without CCVD (CCVD: mainly include coronary atherosclerotic heart disease, hypertensive heart disease, cerebral infarction, and atherosclerosis): HTN with CCVD group (n=56) or without CCVD group (n=38).
To standardize the timing of vaccination (“1 month” was defined as 14–45 days, “2 months” as 46–75 days, “3 months” as 76–105 days, and over 3 months).
Record of adverse events
A questionnaire was used to evaluate AEs 7 and 30 days after the 2nd dose of vaccine. The Chinese Medical and Drug Administration scale was then used to classify all AEs (2019 version).
Antibody testing
S-RBD IgG and SARS-CoV-2 NAbs of blood samples were detected by MAGLUMI 2000 (Snibe, shenzhen, China). Based on the kit instructions, the threshold of anti-RBD IgG Abs which is >1.00 AU/ml is seropositive, and the threshold of CoV-2 NAbs that is >0.15 μg/ml is seropositive; on the contrary, less than or equal to the critical value is seronegative.
Detection of SARS-CoV-2-specific B cells
The stained peripheral blood mononuclear cells were tested by Beckman flow cytometry (Beckman Coulter, Inc., California, USA). The specific steps were as follows: first, we mixed the biotinylated SARS-CoV-2 spike RBD protein (40592-V08H2-B, Sino biological, Beijing, China) with streptavidin-BV421 (405225, Biolegend, California, USA) in a 4:1 mole ratio and leave for 1 h to get the antigen probe; second, peripheral blood mononuclear cells were obtained from whole heparinized blood; the density gradient centrifugation was performed by Histopaque (10771, Sigma–Aldrich, St Louis, Missouri, USA) and then cleaned by cytometric staining buffer (FACS, 2%FBS) cells, besides we added antigen probe (1:33.3), fluorescence-coupled antibody [anti-human IgG Fc (410722, Biolegend), anti-human IgM (314524, Anti-human) CD3 (300430, Biolegend), anti-human CD19 (302212, Biolegend), anti-human CD21 (354918, Biolegend), and anti-human CD27 (356406, Biolegend)] into the cells and dyed at 4°C for 30 min under dark condition. After being re-suspended with FACS buffer, the samples were tested on the machine. The data were analyzed by Flow Jo software (V10.0.7).
Statistical analysis
The median (range) test was used for ordinal variable analysis, and the chi-square test or Fisher's exact test was used for categorical variable analysis. Mann–Whitney U test and Kruskal–Wallis test were used for continuous variables. All results of multiple comparisons were corrected using Dunn's multiple comparisons test and Bonferroni. To examine the relationship between Abs and RBD-specific B cells, Spearman's rank correlation was used. The factors that significantly impacted Ab titers were identified using univariate and multivariate ordinal linear regression analyses. For statistical analysis, GraphPad Prism version 9.2.0 and SPSS 26 (IBM Corp., Armonk, NY, USA) were used. *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Participants' characteristics
The study cohort included 168 participants. As shown in Table 1, comparisons between the HTN patients and HCs found no significant differences in median age, percentage of men, mean body mass index, vaccine type, median number of days after 2nd vaccination, results of routine blood tests (white blood cells, hemoglobin, lymphocytes, and platelets), liver function markers (aspartate transaminase and alanine aminotransferase), and creatinine (Table 1).
Vaccine safety
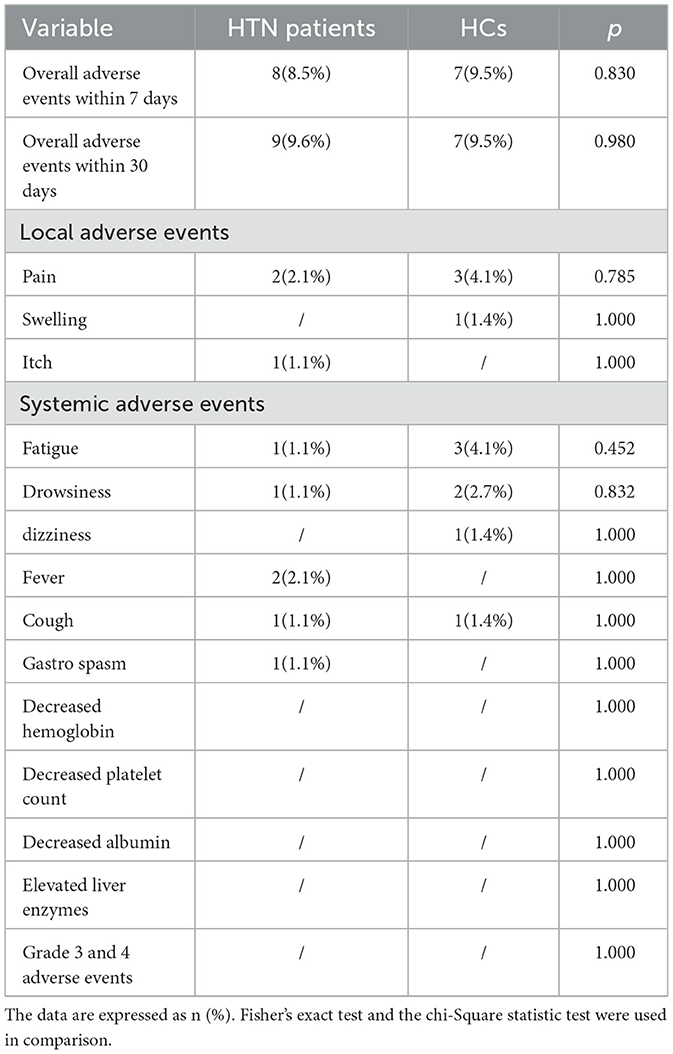
As shown in Table 2, after immunization, the incidence of negative outcomes after 7 days was comparable in HTN (8.5% vs. 9.5%, p = 0.830) compared with controls. The most frequent local adverse event was pain at the injection site, which occurred in 2.1% (2/94) of HTN patients and 4.1% (3/74) of HCs. The most frequent systemic adverse event in HTN patients was fever (2.1%), while the most frequent adverse event in healthy controls was weariness (4.1%). Self-reports of AEs of all participants indicated that they were all minor (grades 1 and 2). After extending the observation period to 30 days, only one additional occurrence of mild AEs—including one instance of pain at the injection site—was observed in HTN patients, and there were no new adverse events in the healthy controls.
Humoral immune response to inactivated SARS-CoV-2 vaccines in HTN
The seropositivity rates of both Abs were lower in HTN patients than HCs in Table 3. The titers of both Abs in HTN patients were significantly lower than in HCs (median [IQR], 2.80 [0.86–5.46] vs. 3.73 [1.78–8.04], p = 0.020, 0.21 [0.12–0.34] vs. 0.26 [0.18–0.44], p = 0.009, respectively) (Figures 1A,B). HTN patients were lower the frequencies of RBD-specific B cells, RBD+ resting MBCs, and RBD-specific MBCs compared with HCs, but higher frequencies of RBD+ atypical MBCs compared with HCs(median [IQR], 17.05 [13.58–22.20] vs. 21.05 [17.60–24.53], p = 0.001, 10.20 [1.52–19.63] vs. 17.10 [11.88–24.28], p = 0.001, and 37.10 [25.35–51.20] vs. 40.25 [31.70–50.05], p = 0.048, 33.70 [25.95–50.43] vs. 25.65 [18.48–35.93], p = 0.001, respectively) (Figure 1C).
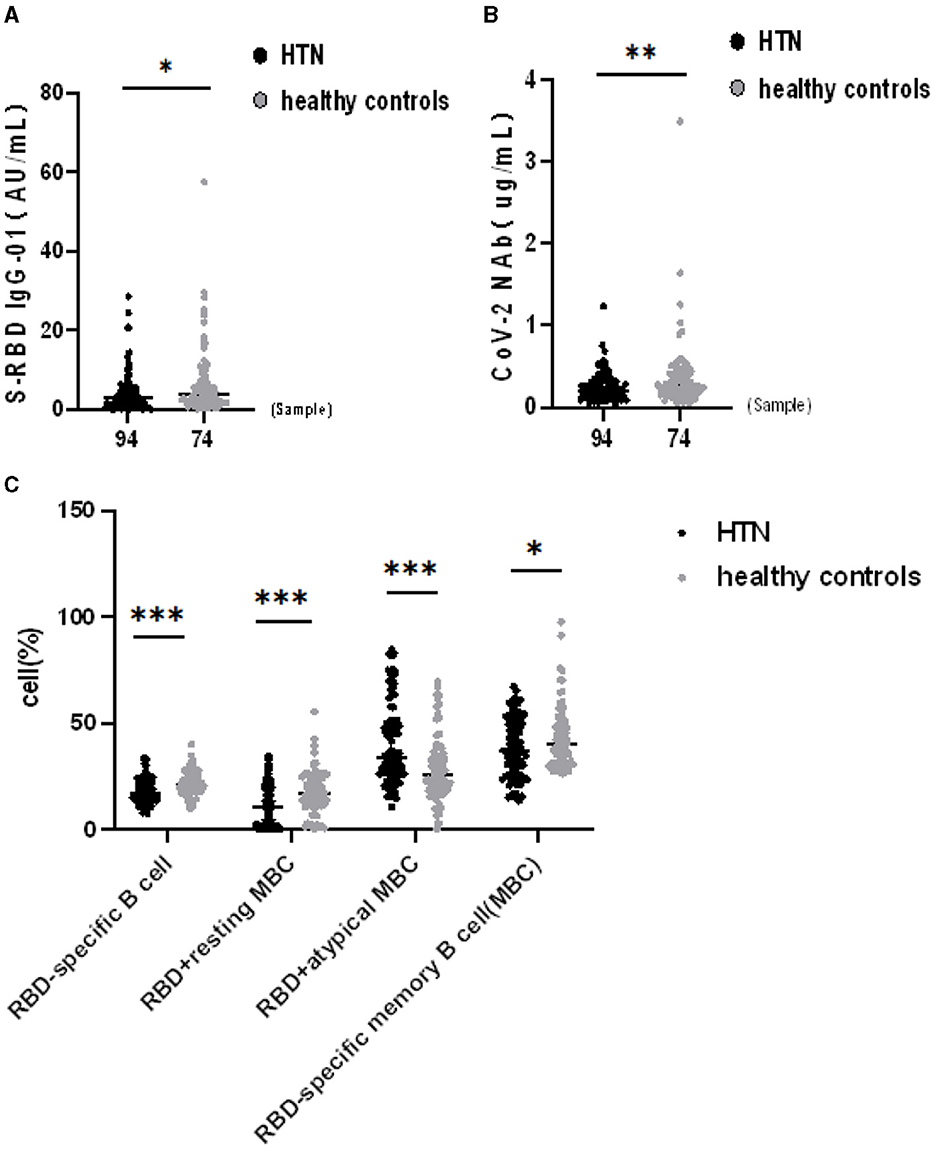
Figure 1. (A–C) Humoral immune responses to inactivated SARS-CoV-2 vaccines in HTN. *p <0.05, **p <0.01, ***p < 0.001.
Humoral immune response to inactivated SARS-CoV-2 vaccines in HTN subgroups
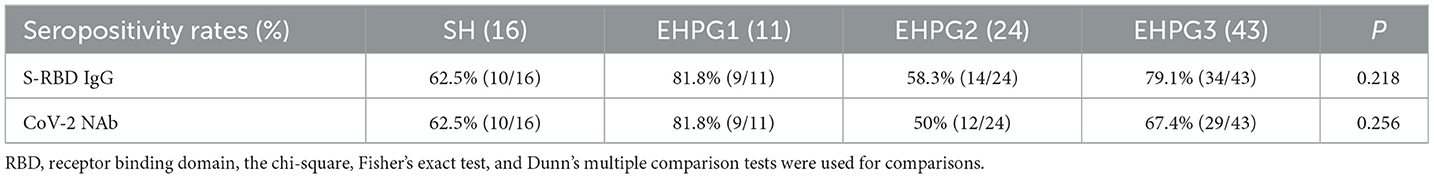
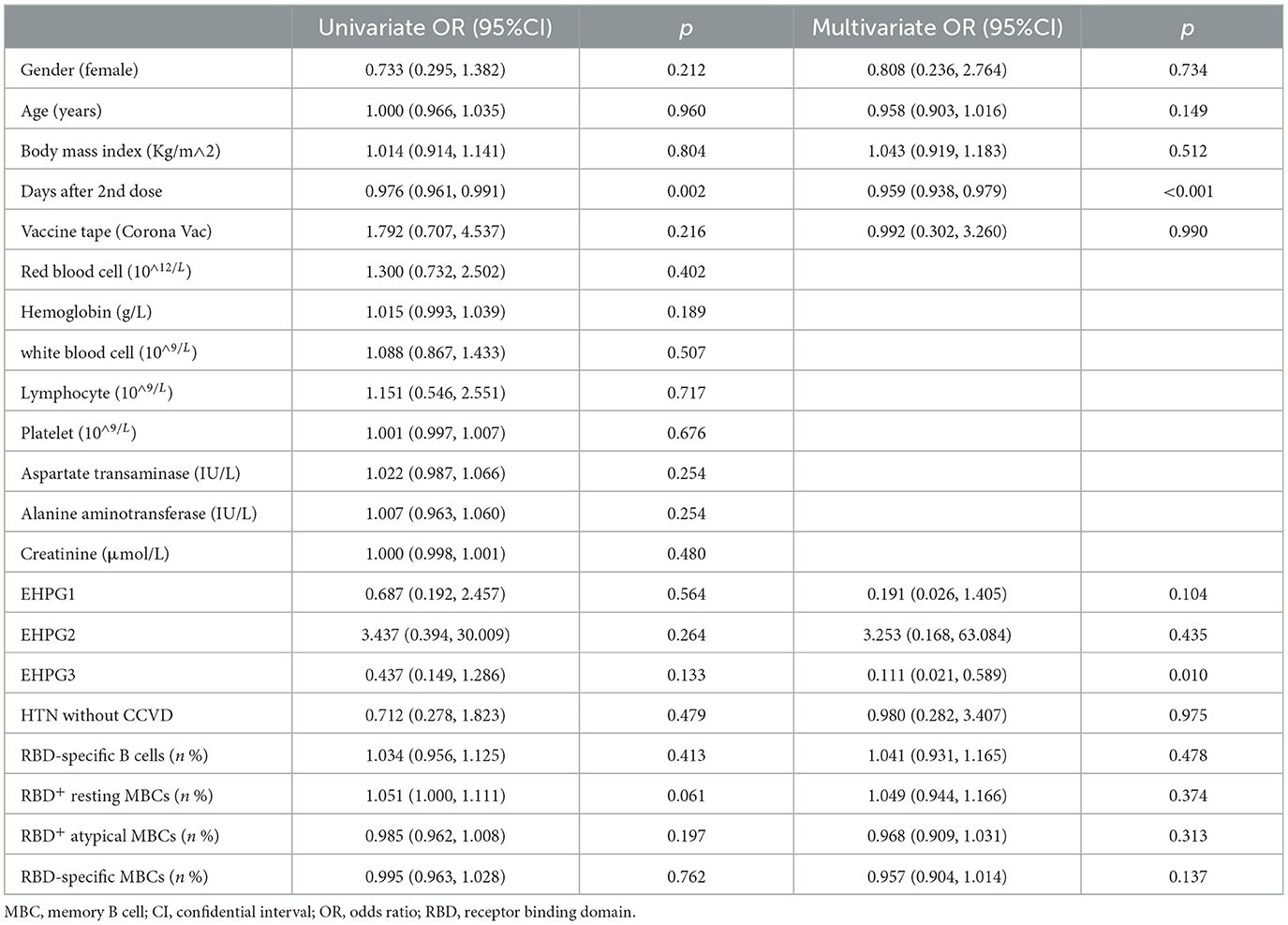
Following that, we discovered that the seropositivity rates of both Abs were similar in SH, EHPG1, 2, and 3 (Table 4).
In a subsequent research, the seropositivity rates of anti-RBD IgG and CoV-2 NAbs in SH, EHPG1, 2, and 3 showed no difference after analysis (68.8% vs. 81.8% vs. 58.3% vs. 79.1%, p = 0.280 and 62.5% vs. 81.8% vs. 50.0% vs. 67.4%, p = 0.274, respectively). The titers of anti-RBD IgG and CoV-2 NAbs were similar to SH, EHPG1, 2, and 3 (3.06 [0.88–5.48] vs. 2.17 [1.21–4.63] vs. 2.12 [0.56–4.61] vs. 3.07 [1.40–8.43], p = 0.284 and 0.21 [0.13–0.34] vs. 0.21 [0.17–0.34] vs. 0.18 [0.10–0.25] vs. 0.22 [0.14–0.39], p = 0.258, respectively) (Figures 2A,B).
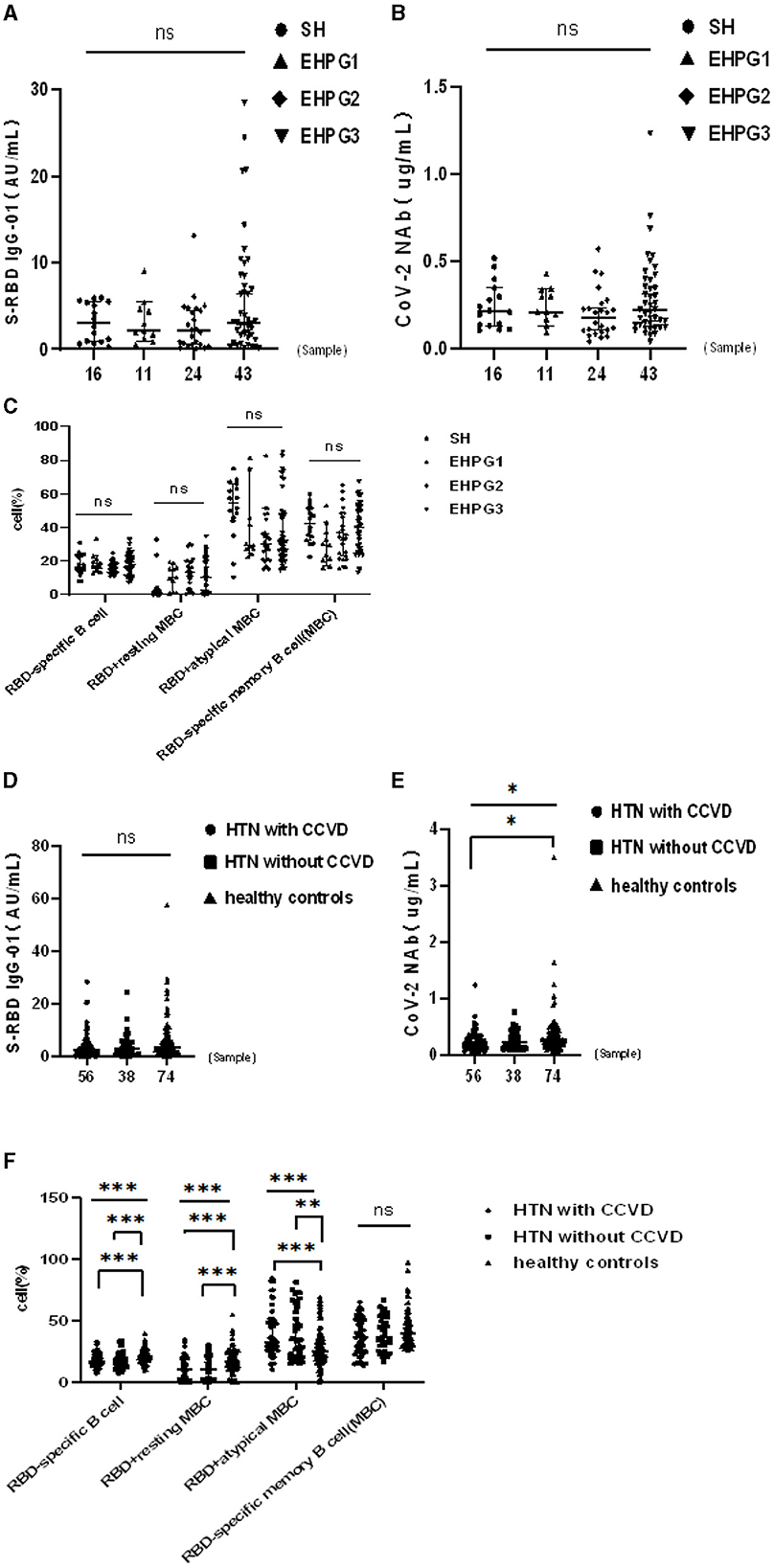
Figure 2. (A–F) Humoral immune responses to inactivated SARS-CoV-2 vaccines in HTN subgroups. *p <0.05, **p < 0.01, ***p < 0.001.
However, there was no difference in the frequencies of RBD-specific B cells, RBD+ resting MBCs, RBD+ atypical MBCs, and RBD-specific memory B cells (MBCs) of SH or EHPG 1, 2, or 3 (Figure 2C).
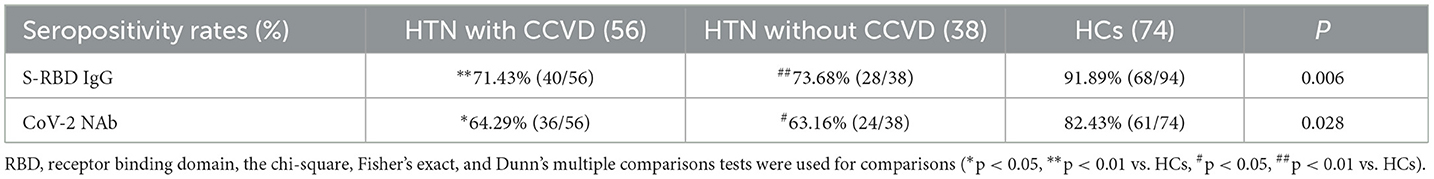
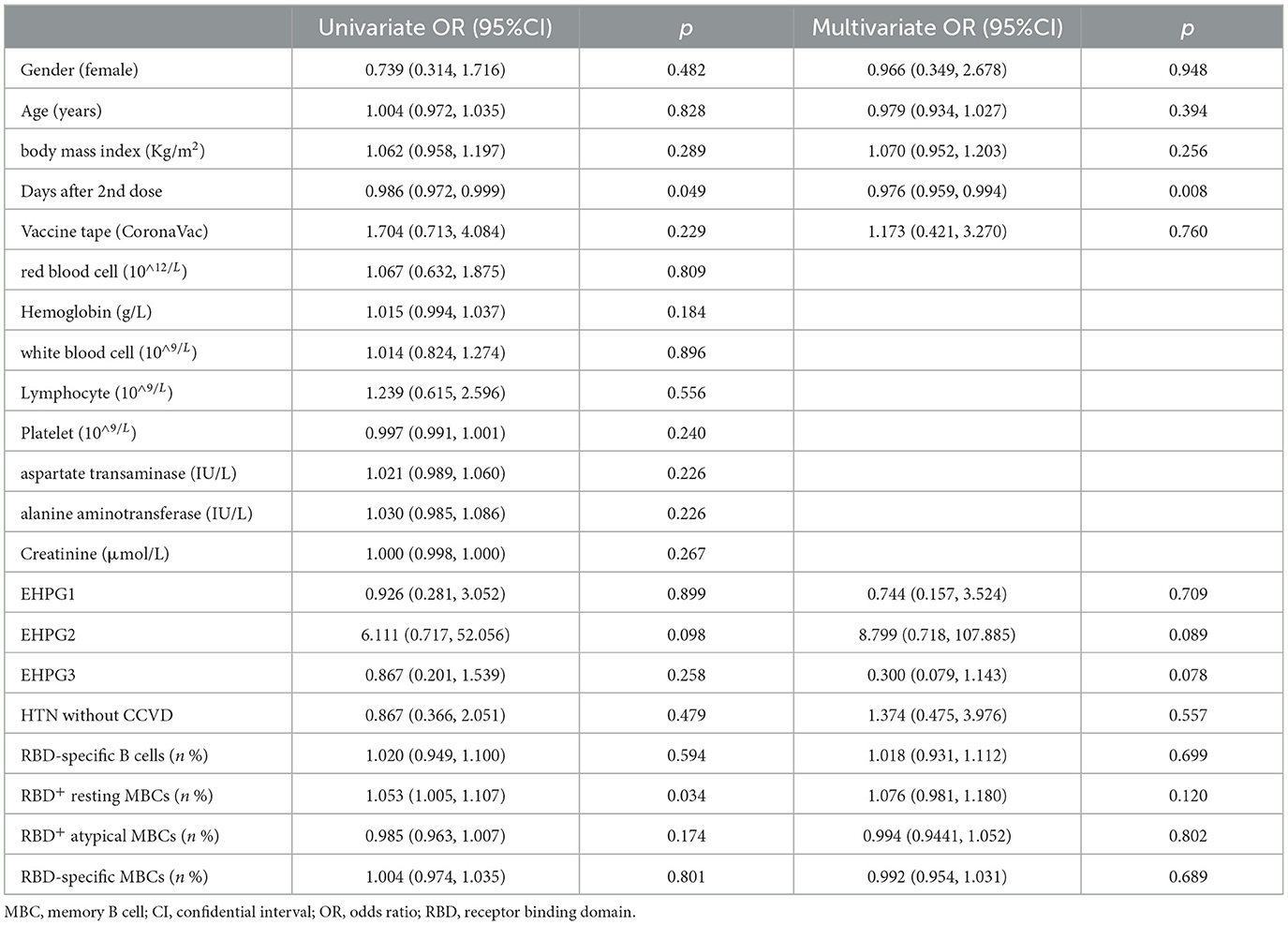
Following that, we discovered that the seropositivity rates of both Abs were lower in HTN patients with or without CCVD than HCs in Table 5. Moreover, the titers of CoV-2 NAb were significantly lower in HTN patients with CCVD than HCs (median [IQR], 0.20 [0.19–0.30] vs. 0.26 [0.28–0.49], p = 0.019) (Figures 2D,E). The frequencies of RBD-specific MBCs were somewhat lower in HTN patients with or without CCVD than HCs, even if there was no statistical difference (Figure 2F).
Humoral immune to inactivated SARS-CoV-2 vaccines in HTN by age
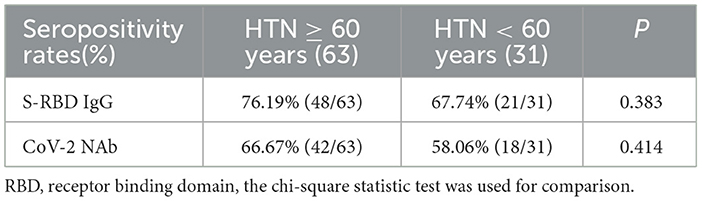
The seropositivity rates of both Abs were similar in HTN patients aged ≥ 60 and < 60 years, as shown in Table 6. Moreover the titers of both Abs were similar in HTN patients aged ≥ 60 and < 60 years (median[IQR], 2.90 [1.09–5.44] vs. 1.84 [0.60–5.52], p = 0.216 and 0.22 [0.12–0.34] vs. 0.19 [0.13–0.30], p = 0.628, respectively) (Figures 3A,B). The frequencies of RBD+ resting MBCs were higher in HTN patients aged ≥60 and < 60 years, and the frequencies of RBD+ atypical MBCs were lower in HTN patients aged ≥60 and < 60 years (median [IQR], 15.10 [8.38–21.10] vs. 1.39 [0.69–2.46], p = 0.000) and 28.70 [21.70–36.00] vs. 51.40 [45.90–69.90], p = 0.000, respectively) (Figure 3C).
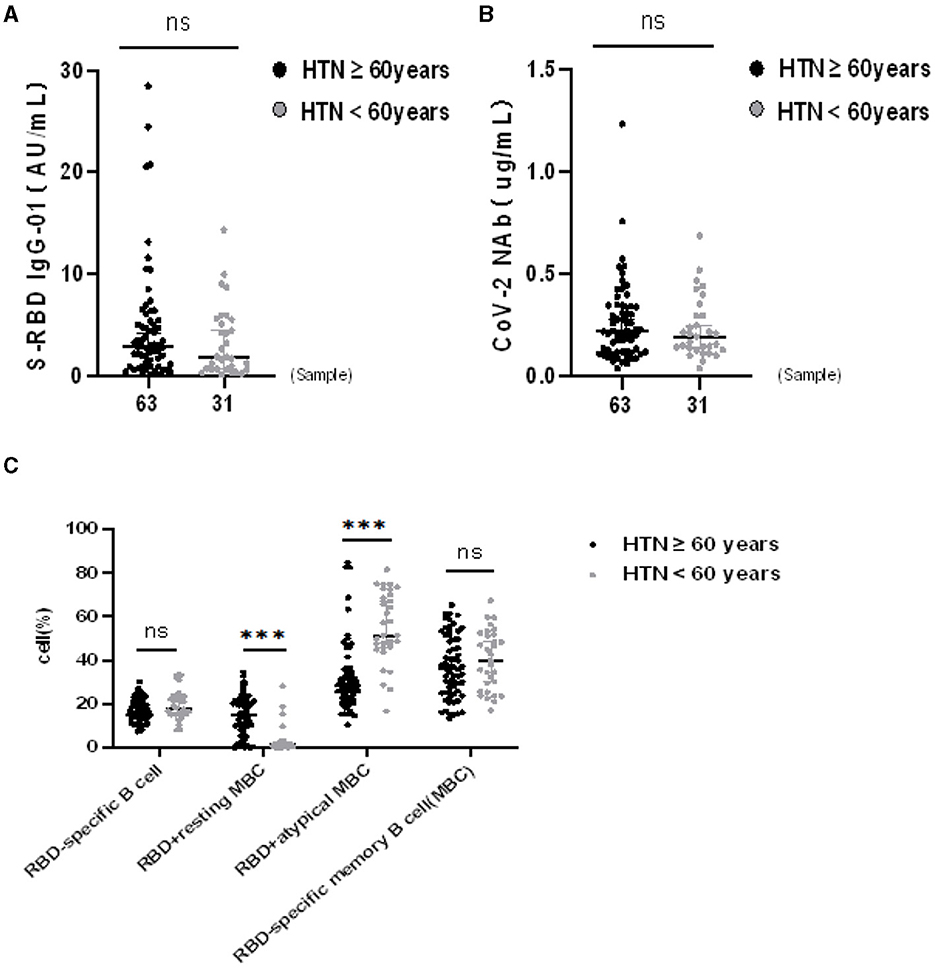
Figure 3. (A–C) Humoral immune responses to inactivated SARS-CoV-2 vaccines in HTN by age. ***p < 0.001.
Humoral immune responses to inactivated SARS-CoV-2 vaccines in HTN over time
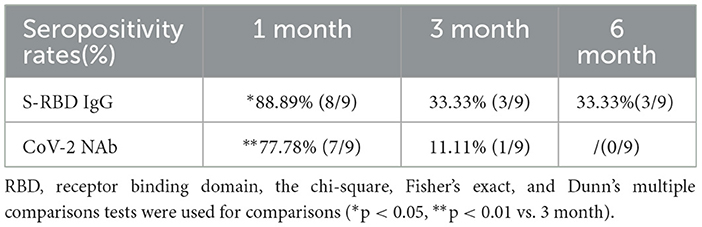
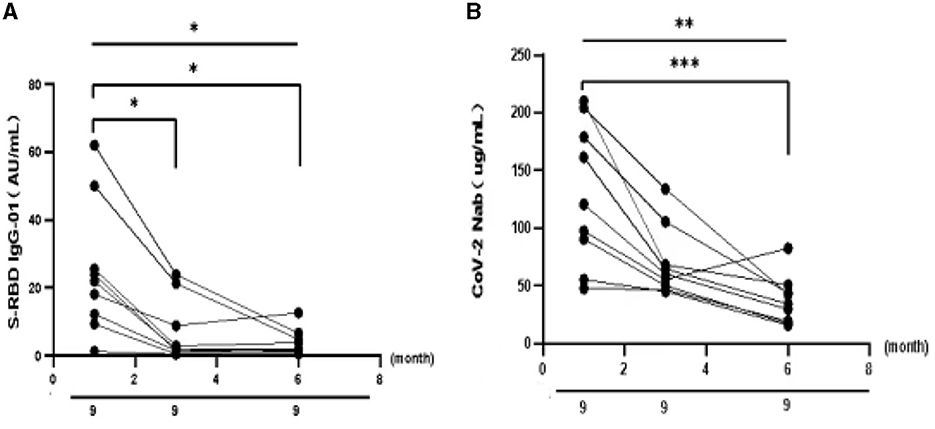
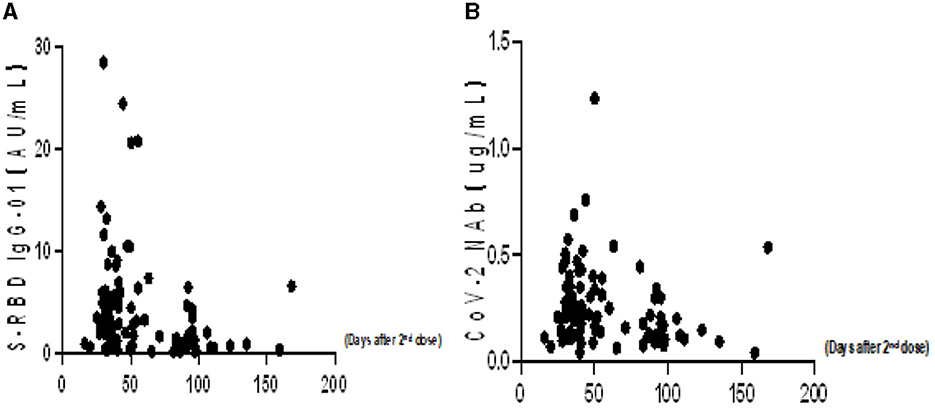
To better understand the variation of humoral immune responses with passing time, we followed up some HTN patients. As expected, the seropositivity rates and titers of both Abs gradually decreased over time in HTN patients, especially from 1 month to 3 months (Table 7, Figures 4A,B). The titers of both Abs were declined with the days after the second dose in HTN patients (Figures 5A,B).
Finally, we are interested in learning more about the variables that affect anti-RBD IgG Abs and CoV-2 NAbs response in HTN patients. The time period following a two-dose vaccination was the key element in Tables 8, 9 that was connected to a poor Ab response.
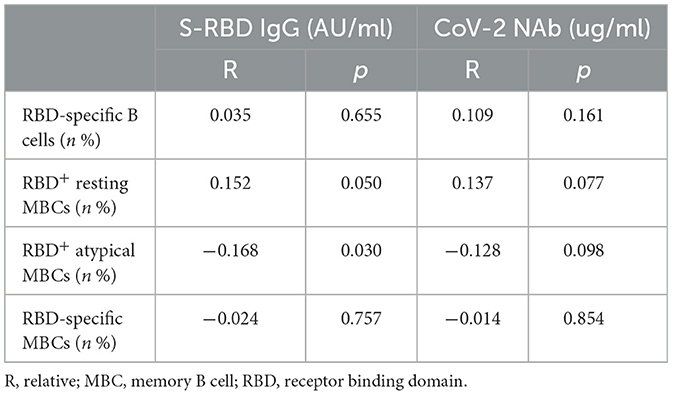
Analysis revealed a negative connection between the titers of anti-RBD IgG Abs and RBD+ atypical MBCs in all participants (R = −0.168, p = 0.030) (Table 10).
Discussion
In this prospective investigation, the safety, Abs response, RBD-specific B cells, and MBCs were evaluated between HTN patients and healthy controls for inactivated SARS-CoV-2 vaccinations. Inactivated vaccinations were well tolerated in HTN, according to our findings. In HTN, both Abs and RBD-specific MBC responses were limited.
According to the most recent data from the Chinese Hypertension Survey, the prevalence of hypertension in people aged 18 years and older in China was 27.9% between 2012 and 2015 (standardized rate: 23.2%) (16) and showed a tendency of rising annually.
In HTN with infected COVID-19, the mortality rate rose (4, 17–19). However, there are just a few safety and immunogenicity trials on inactivated SARS-CoV-2 vaccinations in populations with hypertension. As a result, we initially evaluated the safety of inactivated vaccinations in HTN. We discovered that the overall incidence of adverse events within 7 days and 30 days of vaccination was similar in HTN patients and HCs, respectively, (8.5% vs. 9.5%) and (9.6% vs. 9.5%). However, this finding is significantly lower than that of previous studies, such as phase 1/2 trials of BBIBP-CorV in China (23–29%) (20) and phase 3 trials of Corona Vac in Turkey (18.9%) (21).
Recent data suggest that immune system malfunction may be the cause of hypertension and a subpar immunization response (22). After receiving the SARS-CoV-2 vaccine, the production and maintenance of antigen-specific isotype-switched memory B cells and high-affinity neutralizing antibodies are crucial for the preservation of protective humoral immunity (23). Our findings showed that following immunization, the titers of anti-RBD IgG Abs and CoV-2 NAbs in HTN patients were lower than HCs, which is consistent with earlier research, revealing that these patients had less favorable immune responses to the vaccine (3, 9, 24). Although the titers of anti-RBD IgG Abs were lower in HTN than HCs, the frequencies of RBD+ atypical MBCs were higher in HTN. We found that the titers of anti-RBD IgG Abs were adversely linked with the frequencies of RBD+ atypical MBCs by correlation analysis. Atypical memory B cells are described as fatigued B cells that are inhibiting the production of Abs; however, they typically occur in great numbers in chronic diseases (25–27). Therefore, it is suggested that a possible correlation between an increase in the frequencies of RBD+ and atypical MBC frequencies reduced the titers of anti-RBD IgG Abs.
MBCs are necessary for sustaining long-lasting humoral immunity to pathogenic agents (28). Previous research has demonstrated that RBD-specific MBCs can survive in a vaccinated person for an extended period of time, up to 1 year (29, 30). Another investigation verified that the MBC repertoire produced by mRNA vaccines still gives some protection against the Omicron variant in people who have received vaccination, despite the Omicron variant has a considerable immune escape potential (31). We therefore focus on how MBCs react to inactivated vaccination. In this investigation, RBD-specific MBCs can still be evaluated in HTN patients and HCs after 6 months of receiving the whole course of immunization. Additionally, we discovered that HTN had lower frequencies of RBD-specific MBCs than HCs, which is consistent with earlier research (21) and showed that the diminished humoral immunity in HTN patients may be brought on by an immune system that is not functioning properly. In this investigation, through subgroup analysis, we discovered that the titers of CoV-2 NAbs were lower in HTN patients with CCVD than in healthy controls; in contrast, this result was not found in HTN patients without CCVD. Analysis of the reason may be that the humoral immunity level of these people is low or our sample size was small. We found that the titers of both Abs were similar among SH, EHPG1, EHPG2, and EHPG3; therefore, we hypothesized that patients with different grades of hypertension may experience comparable immunogenicity.
Age is a significant risk factor for hypertension as we are all aware. Previous research has demonstrated that the immunogenicity of the COVID-19 BNT162b2 vaccine results in a weaker antibody response in elderly than in young people (32), and that for people ages > 55 years, the total immune response is low, following a two-dose CoronaVac vaccination (33). However, in clinical trials, the Ab responses of elderly to the mRNA-1273 COVID-19 vaccination and the ChAdOx1nCoV-19 vaccine were comparable with that of young people (34, 35). When the Ab responses in HTN patients ages > 60 were evaluated, it was discovered that these patients were similar levels of Ab responses following two doses of the CoronaVac vaccine, and the audience and the type of vaccine may be to blame for this variation. Therefore, additional real-world data will be required in the future to confirm this. Through univariate and multivariate analyses, we found that the time period, following full-course vaccination, was a factor strongly connected to the levels of both Abs, which is similar to many other studies (36, 37). This implies that Ab titers in inactivated vaccines decreased with time; hence, we might need to deliver a booster dose to maintain Ab levels.
There were some restrictions in the current investigation. First of all, this was a single-center observation research in southwest China with a somewhat modest sample size. Second, T cells of the participants were not detected in this study due to experimental flaws. Third, only a few participants in this study were followed longitudinally. Fourth, antihypertensive drug regimens were not collected for hypertensive patients. Fifth, it is essential to acknowledge that individuals with hypertension may have other conditions, such as diabetes, dyslipidaemia, cardiac, and kidney problems, which could influence the immune response. However, this study also has some benefits: first, HTN patients in China were used to study the immunogenicity of SARS-CoV-2 vaccination. Second, this study thoroughly assessed humoral immune response to SARS-CoV-2. Third, it was once more established that subpar anti-RBD IgG Ab responses were related to the period of time, following the complete vaccination.
In conclusion, the inactivated SARS-CoV-2 vaccine was well tolerated among individuals with hypertension; however, humoral immune response was limited, especially merged CCVD, and declined gradually over time.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Second Affiliated Hospital, Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LY: Writing – original draft. DJ: Writing – review & editing. TZ: Data collection, Writing – review & editing. YL: Data collection, Writing – review & editing. QG: Data collection, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge the support of the National Natural Science Foundation of China (81772198) and the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0389) and supported by the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cooper TJ, Woodward BL, Alom S, Harky A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. (2020) 21:567–77. doi: 10.1111/hiv.12911
2. Xu G, Yang Y, Du Y, Peng F, Hu P, Wang R, et al. Clinical pathway for early diagnosis of COVID-19: updates from experience to evidence-based practice. Clin Rev Allergy Immunol. (2020) 59:89–100. doi: 10.1007/s12016-020-08792-8
3. Rifai A, Wahono CS, Pratama MZ, Handono K, Susianti H, Iskandar A, et al. Association between the effectiveness and immunogenicity of inactivated SARS-CoV2 vaccine (CoronaVac) with the presence of hypertension among health care workers. Clin Exp Hypertens. (2022) 44:233–9. doi: 10.1080/10641963.2021.2022687
4. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. (2020) 21:1470320320926899. doi: 10.1177/1470320320926899
5. Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. (2017) 390:2549–58. doi: 10.1016/S0140-6736(17)32478-9
6. Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, et al. Status of hypertension in China: results from the china hypertension survey, 2012-2015. Circulation. (2018) 137:2344–56. doi: 10.1161/CIRCULATIONAHA.117.032380
7. Soegiarto G, Wulandari L, Purnomosari D, Dhia Fahmita K, Ikhwan Gautama H, Tri Hadmoko S, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine. (2022) 40:4046–56. doi: 10.1016/j.vaccine.2022.05.059
8. Ebinger JE, Joung S, Liu Y, Wu M, Weber B, Claggett B, et al. Demographic and clinical characteristics associated with variations in antibody response to BNT162b2 COVID-19 vaccination among healthcare workers at an academic medical centre: a longitudinal cohort analysis. BMJ Open. (2022) 12:e059994. doi: 10.1136/bmjopen-2021-059994
9. Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. (2022) 38:e3465. doi: 10.1002/dmrr.3465
10. Parthymou A, Habeos EE, Habeos GI, Deligakis A, Livieratos E, Marangos M, et al. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: a longitudinal observational cohort study in western Greece. BMJ Open. (2022) 12:e057084. doi: 10.1136/bmjopen-2021-057084
11. Ogega CO, Skinner NE, Blair PW, Park HS, Littlefield K, Ganesan A, et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J Clin Invest. (2021) 131:7. doi: 10.1172/JCI145516
12. Sakharkar M, Rappazzo CG, Wieland-Alter WF, Hsieh CL, Wrapp D, Esterman ES, et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol. (2021) 6:abg6916. doi: 10.1126/sciimmunol.abg6916
13. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
14. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26. COV2S vaccine against Covid-19. N Engl J Med. (2021) 384:2187–201. doi: 10.1056/NEJMoa2101544
15. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B. 1351 variant. N Engl J Med. (2021) 384:1885–98. doi: 10.1056/NEJMoa2102214
16. Zhang WZ. Intensified, optimized and standardized management of Chinese patients with hypertension: comments on “2018 Chinese guidelines for prevention and treatment of hypertension”. J Geriatr Cardiol. (2019) 16:178–81.
17. Killerby ME, Link-Gelles R, Haight SC, Schrodt CA, England L, Gomes DJ, et al. Characteristics associated with hospitalization among patients with COVID-19 - metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:790–4. doi: 10.15585/mmwr.mm6925e1
18. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. doi: 10.1136/bmj.m1966
19. Maddaloni E, D'Onofrio L, Alessandri F, Mignogna C, Leto G, Pascarella G, et al. Cardiometabolic multimorbidity is associated with a worse COVID-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol. (2020) 19:164. doi: 10.1186/s12933-020-01140-2
20. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
21. Tanriover MD, Doganay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. (2021) 398:213–22. doi: 10.1016/S0140-6736(21)01429-X
22. Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. (2019) 19:517–32. doi: 10.1038/s41577-019-0160-5
23. Haralambieva IH, Monroe JM, Ovsyannikova IG, Grill DE, Poland GA, Kennedy RB. Distinct homologous and variant-specific memory B-cell and antibody response over time after severe acute respiratory syndrome coronavirus 2 messenger RNA vaccination. J Infect Dis. (2022) 226:23–31. doi: 10.1093/infdis/jiac042
24. Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. (2021) 12:752233. doi: 10.3389/fimmu.2021.752233
25. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. (2008) 205:1797–805. doi: 10.1084/jem.20072683
26. Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: an interim report. Cell Immunol. (2017) 321:18–25. doi: 10.1016/j.cellimm.2017.07.003
27. Weiss GE, Crompton PD Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. (2009) 183:2176–82. doi: 10.4049/jimmunol.0901297
28. Brown SL, Bauer JJ, Lee J, Ntirandekura E, Stumhofer JS. IgM(+) and IgM(-) memory B cells represent heterogeneous populations capable of producing class-switched antibodies and germinal center B cells upon rechallenge with P. yoelii J Leukoc Biol. (2022) 112:1115–35. doi: 10.1002/JLB.4A0921-523R
29. Sokal A, Chappert P, Barba-Spaeth G, Roeser A, Fourati S, Azzaoui I, et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. (2021) 184:1201–13.e14. doi: 10.1016/j.cell.2021.01.050
30. Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. (2021) 595:426–31. doi: 10.1038/s41586-021-03696-9
31. Sokal A, Broketa M, Barba-Spaeth G, Meola A, Fernández I, Fourati S, et al. Analysis of mRNA vaccination-elicited RBD-specific memory B cells reveals strong but incomplete immune escape of the SARS-CoV-2 Omicron variant. Immunity. (2022) 55:1096–104.e4. doi: 10.1016/j.immuni.2022.04.002
32. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. (2020) 383:2439–50. doi: 10.1056/NEJMoa2027906
33. Medeiros GX, Sasahara GL, Magawa JY, Nunes JPS, Bruno FR, Kuramoto AC, et al. Reduced T cell and antibody responses to inactivated coronavirus vaccine among individuals above 55 years old. Front Immunol. (2022) 13:812126. doi: 10.3389/fimmu.2022.812126
34. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. (2020) 383:2427–38. doi: 10.1056/NEJMoa2028436
35. Lee SW, Moon JY, Lee SK, Lee H, Moon S, Chung SJ, et al. Anti-SARS-CoV-2 spike protein RBD antibody levels after receiving a second dose of ChAdOx1 nCov-19 (AZD1222) vaccine in healthcare workers: lack of association with age, sex, obesity, and adverse reactions. Front Immunol. (2021) 12:779212. doi: 10.3389/fimmu.2021.779212
36. Barin B, Kasap U, Selçuk F, Volkan E, Uluçkan Ö. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study. Lancet Microbe. (2022) 3:e274–e83. doi: 10.1016/S2666-5247(21)00305-0
Keywords: COVID-19, safety, immunogenicity, inactivated vaccine, hypertension
Citation: Yang L, Zeng T, Li Y, Guo Q and Jiang D (2024) Poor immune response to inactivated COVID-19 vaccine in patients with hypertension. Front. Med. 11:1329607. doi: 10.3389/fmed.2024.1329607
Received: 29 October 2023; Accepted: 03 January 2024;
Published: 02 May 2024.
Edited by:
Soheil Ebrahimpour, Babol University of Medical Sciences, IranReviewed by:
Ritthideach Yorsaeng, Chulalongkorn University, ThailandZhongshan Cheng, St. Jude Children's Research Hospital, United States
Copyright © 2024 Yang, Zeng, Li, Guo and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: DePeng Jiang, Z2RwMTE2QGhvc3BpdGFsLmNxbXUuZWR1LmNu
†These authors share first authorship
 Lei Yang
Lei Yang TingTing Zeng2†
TingTing Zeng2† DePeng Jiang
DePeng Jiang