- 1Toronto Lung Transplant Program, Ajmera Multi-Organ Transplant Program and Division of Respirology, University Health Network, Tonronto, ON, Canada
- 2Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 3Pneumology, Aduch Cystic Fibrosis and Lung Transplantation Department, Foch Hospital, Suresnes, France
- 4Biostatistics Research Unit, University Health Network, Toronto, ON, Canada
- 5Toronto General Pulmonary Function Laboratory, University Health Network, Toronto, ON, Canada
Introduction: Prior studies assessing outcomes of lung transplants from cigarette-smoking donors found mixed results. Oscillometry, a non-invasive test of respiratory impedance, detects changes in lung function of smokers prior to diagnosis of COPD, and identifies spirometrically silent episodes of rejection post-transplant. We hypothesise that oscillometry could identify abnormalities in recipients of smoking donor lungs and discriminate from non-smoking donors.
Methods: This prospective single-center cohort study analysed 233 double-lung recipients. Oscillometry was performed alongside routine conventional pulmonary function tests (PFT) post-transplant. Multivariable regression models were constructed to compare oscillometry and conventional PFT parameters between recipients of lungs from smoking vs non-smoking donors.
Results: The analysis included 109 patients who received lungs from non-smokers and 124 from smokers. Multivariable analysis identified significant differences between recipients of smoking and non-smoking lungs in the oscillometric measurements R5-19, X5, AX, R5z and X5z, but no differences in %predicted FEV1, FEV1/FVC, %predicted TLC or %predicted DLCO. An analysis of the smoking group also demonstrated associations between increasing smoke exposure, quantified in pack years, and all the oscillometry parameters, but not the conventional PFT parameters.
Conclusion: An interaction was identified between donor-recipient sex match and the effect of smoking. The association between donor smoking and oscillometry outcomes was significant predominantly in the female donor/female recipient group.
1 Introduction
Lung transplantation is a last resort treatment for advanced lung disease, generally reserved for patients whose life expectancy without transplantation is less than 1 year, or who have severe, irreversible impairment in quality of life as a result of their disease.
In most lung transplant centers, access to transplantation is limited primarily by donor supply. The “ideal donor” is considered to be a life-long non-smoker under the age of 55 years, with a clear chest x-ray, no history of chest surgery or trauma, and no evidence of infection (1). However, most centers utilize lungs from donors who fail to meet one or more of these criteria, in order to maximize the donor pool of this limited resource. Lungs from donors with a history of cigarette smoking are frequently used, especially if the estimated total exposure is less than 20 pack-years and there is no documented history of lung disease. Some studies comparing outcomes between recipients of lungs from smoking and non-smoking donors have shown reduced overall survival and increased risk of baseline allograft dysfunction (BLAD, defined as failure to achieve a post-transplant peak FEV1 which is at least 80% of the predicted value) in recipients of lungs from smokers (2–6). A negative impact on early post-transplant outcomes, such as PaO2/FiO2 ratio, duration of ventilation and intensive care unit (ICU) length of stay, has also been reported (7, 8). However, a review of United Network for Organ Sharing (UNOS) data found no significant impact on mortality, freedom from chronic lung allograft dysfunction (CLAD) or all-cause mortality from utilizing donors even with a heavy (≥ 20 pack year) smoking history (9). Furthermore, it has been demonstrated that the overall risk of death is higher in patients remaining on the waitlist if lungs from smoking donors are not used (4). Therefore, the overall risk of a poor outcome needs to be balanced against the risk of death on the waitlist.
Oscillometry is a non-invasive measurement of respiratory impedance that is sensitive to physiology of small airways, a region of the lung not well characterized by conventional pulmonary function tests (cPFT). Commonly investigated oscillometry parameters include the difference between resistance at 5 Hz and 19 or 20 Hz (R5-19 or R5-20), which increases with rising small airway resistance and ventilatory inhomogeneity; reactance at 5 Hz (X5), a value which becomes more negative with increased lung stiffness and loss of elastic recoil; and area of reactance (AX), an integrated area of reactance between X5 and the resonant frequency (Fres), the frequency at which the reactance curve crosses zero. AX provides a potentially more sensitive indication of changes in the elastic properties of the lung than the single-frequency value X5, as it provides a measure of reactance at a range of frequencies (10). A summary of changes in oscillometry parameters associated with different lung function patterns is provided in Table 1.
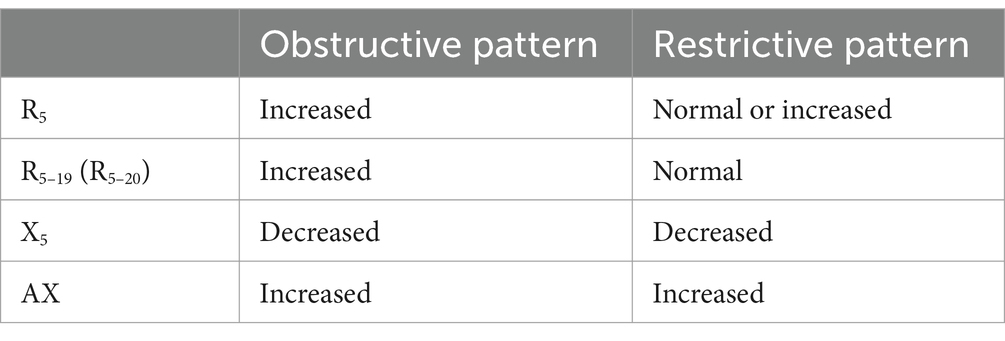
Table 1. Typical changes to oscillometry parameters associated with different obstructive and restrictive lung function patterns.
Oscillometry has been shown to detect abnormalities in people with respiratory symptoms but normal spirometry, and to detect early chronic obstructive lung disease (COPD) prior to changes in cPFT (11–13). R5-20 is higher in smokers than non-smokers, even without a diagnosis of COPD (14), and is correlated with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage and COPD assessment scores (15). A correlation has also been found between the R5-20, Fres and the wall thickness of small airways detected on endobronchial optical coherence tomography in heavy smokers and COPD patients (16). X5 is more negative in smokers than non-smokers, resembling values seen in COPD patients (14).
In lung transplantation, oscillometry has been proposed as a tool for monitoring graft function to complement cPFTs, based on the rationale that many of the pathological processes involved in graft dysfunction, such as acute rejection and bronchiolitis obliterans (BO), affect the small airways (17, 18), which are poorly characterized by spirometry (19, 20). Our group has shown that oscillometry can detect episodes of acute cellular rejection which were not identified by changes in cPFT (21) and that increased intra-subject variability in X5, AX and R5-19 is associated with increased risk of chronic lung allograft dysfunction (CLAD), a key cause of long-term morbidity and mortality in lung transplantation (22). Oscillometry also provides insights into the respiratory mechanics of the different phenotypes of CLAD (23, 24).
It should also be noted that spirometry early post-transplant is likely impacted by deconditioning and recent chest surgery and may not be an accurate representation actual lung tissue function (25–27). Oscillometry, on the other hand, is effort-independent.
The current study aims to examine the differences in oscillometry parameters between recipients of lungs from smoking vs. non-smoking donors. We posit that oscillometry would identify small airway obstruction in smoking lungs even where changes are not detectable on spirometry.
2 Methods
The prospective single-centre observational cohort study was approved by the University Health Network (UHN) Research Ethics Board (REB# 17–5,652) and began in December 2017.
2.1 Study participants
Patients were recruited at their first routine post-transplant visit to the PFT lab, usually within 1 week of discharge from hospital and 3–4 weeks post-transplant. All double lung transplant recipients were eligible. Single lung recipients, patients with known anastomotic issues, and those who remained hospitalized at ≥3 months after transplant were excluded.
2.2 Data collection
Participating patients underwent oscillometry prior to each post-transplant visit to the PFT laboratory. Routine cPFTs except diffusing capacity for carbon monoxide (DLCO) occur weekly for the first 6 weeks, bi-weekly until 3 months post-transplant, then at 6, 9, 12, 18 and 24 months, and annually thereafter. DLCO is generally not performed during the period of frequent PFT lab visits over the first 3 months post-transplant, but is performed at each routine visit thereafter. Additional visits to the PFT laboratory may be prompted by new onset respiratory symptoms or drop in lung function on home monitoring. Oscillometry is performed using the Tremoflo device (model C-100, Thorasys, Montreal, Canada) following published technical, quality control and assurance guidelines (28, 29). Spirometry, body plethysmography and diffusing capacity are performed following international guidelines (30–32).
The primary outcomes of interest included the oscillometry parameters R5, R5-19, X5 and AX, as well as R5z and X5z, defined as the number of standard deviations from the normal population mean for R5 and X5, respectively. R5-19 rather than R5-20 was used, as the Tremoflo, and other devices employing the forced oscillometry technique, measure at prime number frequencies to avoid harmonic amplification. Conventional PFT parameters of interest were those known to be associated with graft function following lung transplant, specifically FEV1 (expressed as % predicted), ratio of FEV1 to forced vital capacity (FEV1/FVC), total lung capacity expressed as % predicted (TLC % predicted) and DLCO. CLAD-free survival was assessed as a secondary outcome.
Clinical characteristics were extracted from the Toronto Lung Transplant Program (TLTP) database, which maintains lifelong records of all patients transplanted by the programme. CLAD was defined according to the 2019 ISHLT consensus statement as a sustained (≥ 3 months) fall in FEV1 to below 80% of the post-transplant baseline value. Baseline is defined as the average of the two highest post-transplant FEV1 readings, measured at least 3 weeks apart (33).
Donor smoking history was extracted from TLTP database. This information was collected from donor families during the donation process, and recorded in the database as a binary variable, with “Yes” indicating any amount of current or prior cigarette smoking at the time of donation, and wherever possible with an estimated number of pack years. We excluded patients if donor smoking history was not recorded, or not quantified. Included recipients had a minimum of 3 months of follow up at the censor date of March 15, 2020, i.e., transplanted before December 15, 2019.
2.3 Statistical analysis
Baseline characteristics were compared using Fisher’s Exact Test for categorical variables and Mann–Whitney U test for continuous variables.
The oscillometry and cPFT outcomes over time were compared between smoking and non-smoking donors, and against smoking severity as quantified by pack-years, using linear mixed effects models. Covariates shown to have a statistically significant univariate association with the outcome were included in the construction of multivariable models.
Multivariable linear regression models were constructed for each outcome of interest. All models included random slopes for each participant to account for repeated measures. The candidate adjustment variables assessed for potential interaction were: recipient age, donor age, recipient body mass index (BMI), primary lung disease, ex vivo lung perfusion (EVLP) use, total ischemic time, sex match, ICU length of stay and transplant hospitalization length of stay. Predictors were assessed for either an additive and/or interactive effect with donor cigarette use as a binary predictor, or total donor cigarette exposure, measured in pack-years, as a continuous predictor.
The detection of additive effect indicates that the predictor has an effect on the outcome of interest, but does not influence the relationship between the outcome and the key predictor being studied, in this case, the donor smoking history. An interaction effect indicates that the variable changes the relationship between smoking history and the outcome.
To the end of obtaining accurate adjusted effect estimates for the donor smoking exposures relative to each outcome variable, great care had to be taken in identifying potential confounders and addressing possible interaction terms; there was no a priori reason to suppose that the same model specification would be adequate/appropriate for all outcome variables. To address this, for each combination of predictor and outcome, we fit both an additive and an interaction model. Using anova F-tests, each predictor was classified as having either an interaction effect, if the interaction model differed significantly from the null model; an additive effect only if the additive model (but not the interaction model) differed from the null model; and none if no significant effects were found.
For each outcome, a final multivariable model was fit using these findings. Heatmaps were created to visualize patterns in covariate inclusion, while varying the outcome variable (Supplementary Figure S1). Predictors were assessed for multicollinearity and those with a generalized variance inflation factor (GVIF1/(2·df)) > 5 were removed in a descending stepwise manner until all GVIF1/(2·df) values were < 5.
Results were expressed as number-of-standard-deviations change in the outcome metric for each standard deviation increase in the predictor variable (continuous variables), with the exception of time post-transplant, where increments of 1 month were used, and donor pack years, which were analysed in 10-pack-year increments. For categorical variables, standard-deviation changes in outcome metrics were shown in comparison to the reference group.
Survival curves were constructed using the Kaplan-Myer method for comparison of CLAD-free survival between the smoking and non-smoking donor groups.
Analysis was performed using R Studio version 4.0.3.
3 Results
3.1 Enrolment and follow-up
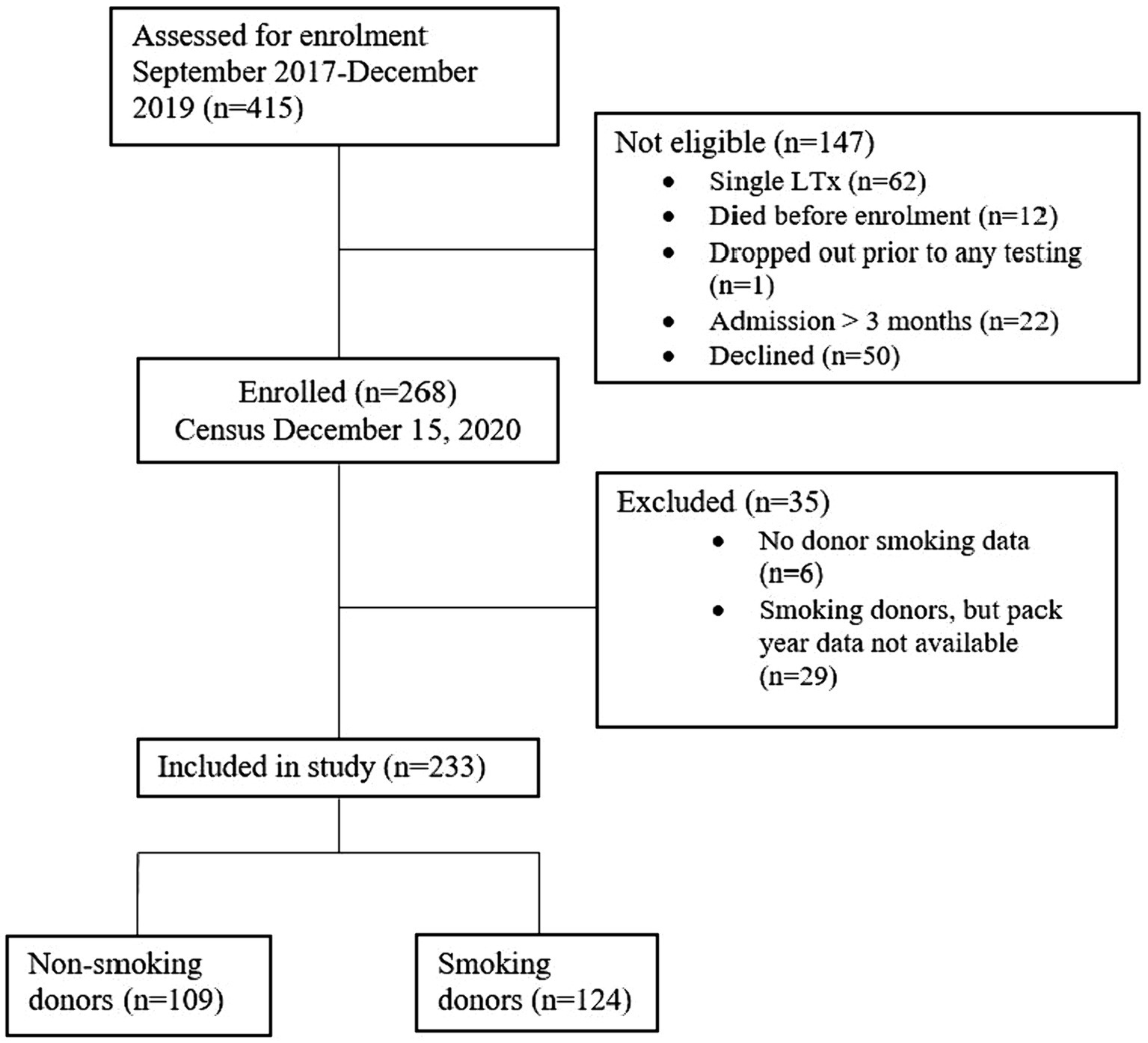
The study enrolled 268 of the 415 recipients transplanted from September 2017 to December 15, 2019. Fifty patients declined the study and 97 did not meet inclusion criteria. Thirty-five patients were excluded due to insufficient donor smoking history data. Of the 233 patients included for analysis, 109 received lungs from non-smoking donors and 124 from smoking donors (Figure 1). Median duration of follow-up was 356 days.
3.2 Baseline characteristics
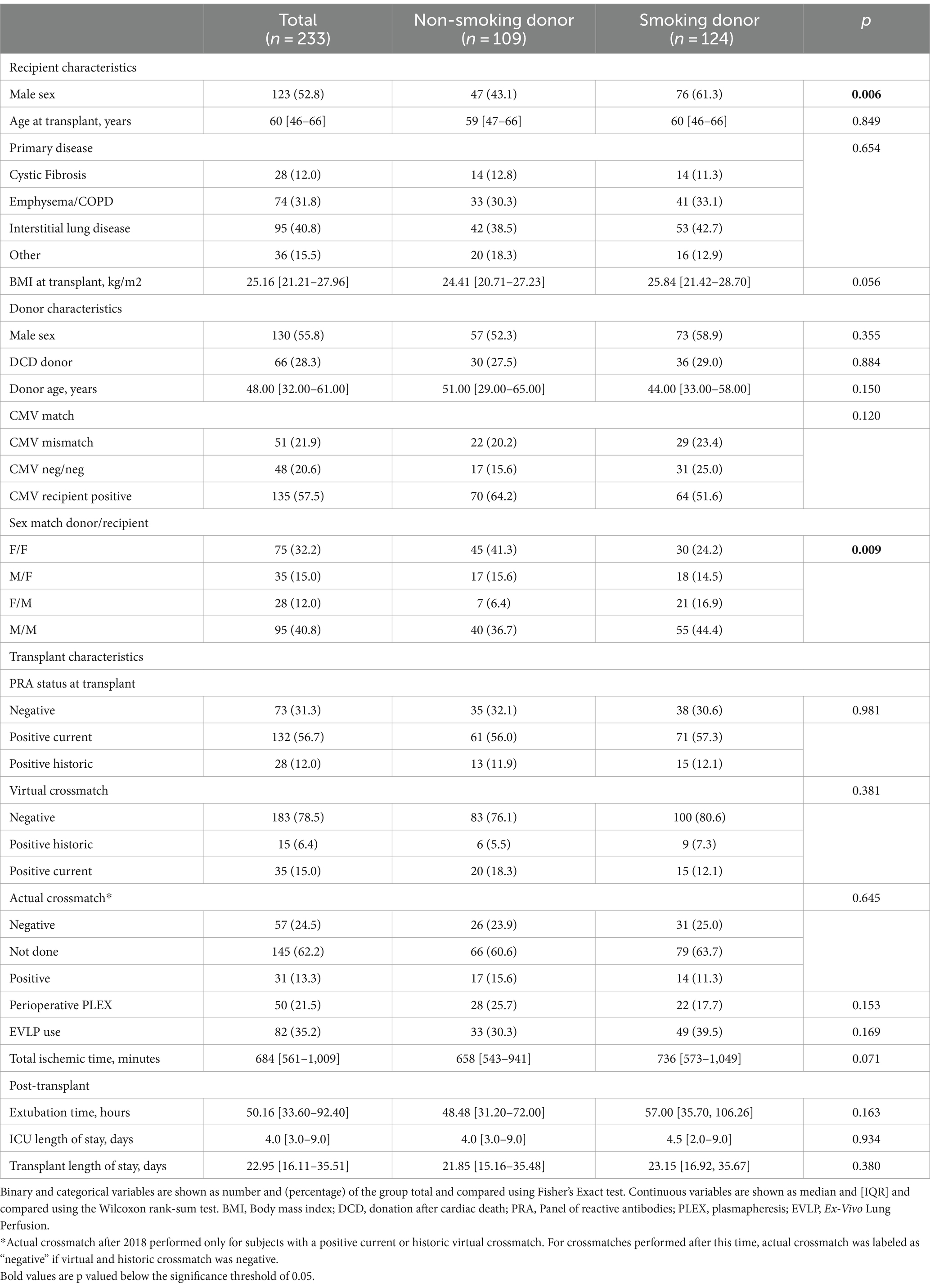
Baseline characteristics were similar between the two groups (Table 2). Notable exceptions include more male recipients in the smoking donor group, and differences in the sex-match distribution between the groups. Specifically, in the non-smoking donor group, there was a higher proportion of F➔F sex-match (female donor and female recipient) and a lower proportion of F➔M. Among the smoking donors, the median estimated number of pack years was 10, with 38 participants receiving lungs from donors with a history of 20 or more pack years.
3.3 Time to best achieved cPFT and oscillometry values
The median times to peak FEV1 and FVC in our cohort were 160 and 186 days, respectively. The median times to best achieved R5, R5-19, X5 and AX were 62, 67, 86 and 93 days, respectively. The best respiratory mechanics are the lowest values for R5, R5-19 and AX, and the highest, or least-negative value for X5. Spaghetti plots showing individual trajectories of R5-19, X5 and FEV1, and the estimated trajectories for the smoking and non-smoking group, are shown in Figure 2.
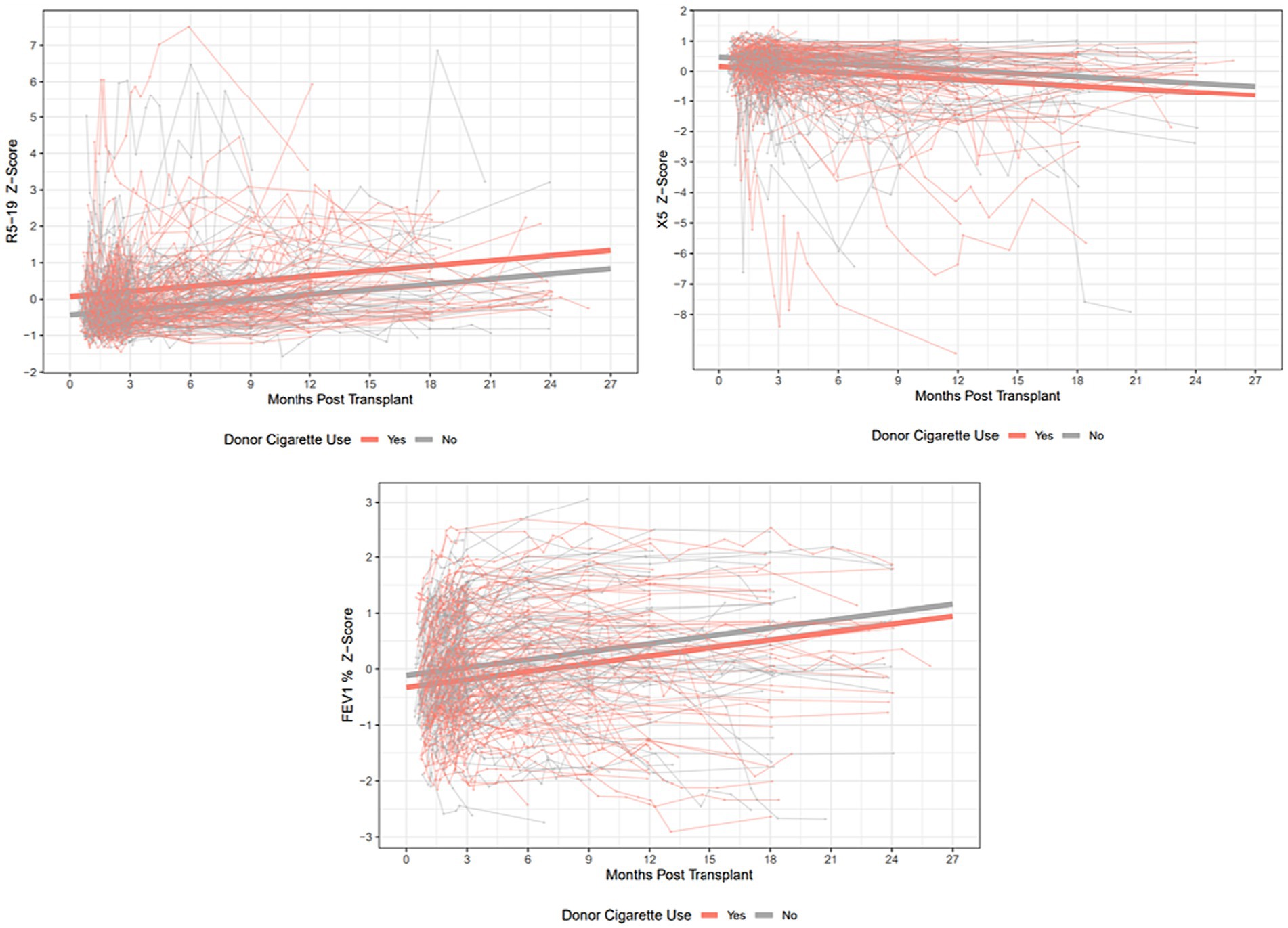
Figure 2. Spaghetti plots showing individual trajectories over time for R5-19, X5 and FEV1% predicted, with group trajectories estimated by mixed linear effects models. Z-scores are plotted to allow ease of comparison between the 3 parameters. R5-19, difference in resistance at 5 and 19 Hz; X5, reactance at 5 Hz; FEV1, forced expiratory volume in 1 s.
3.4 Association between donor smoking history and oscillometry
A total of 2,658 paired oscillometry-cPFT tests were included; 1,213 in non-smoking and 1,445 in smoking donor group. DLCO measurements are only performed at some PFT lab visits, therefore only 622 measurements were available for inclusion in the analysis.
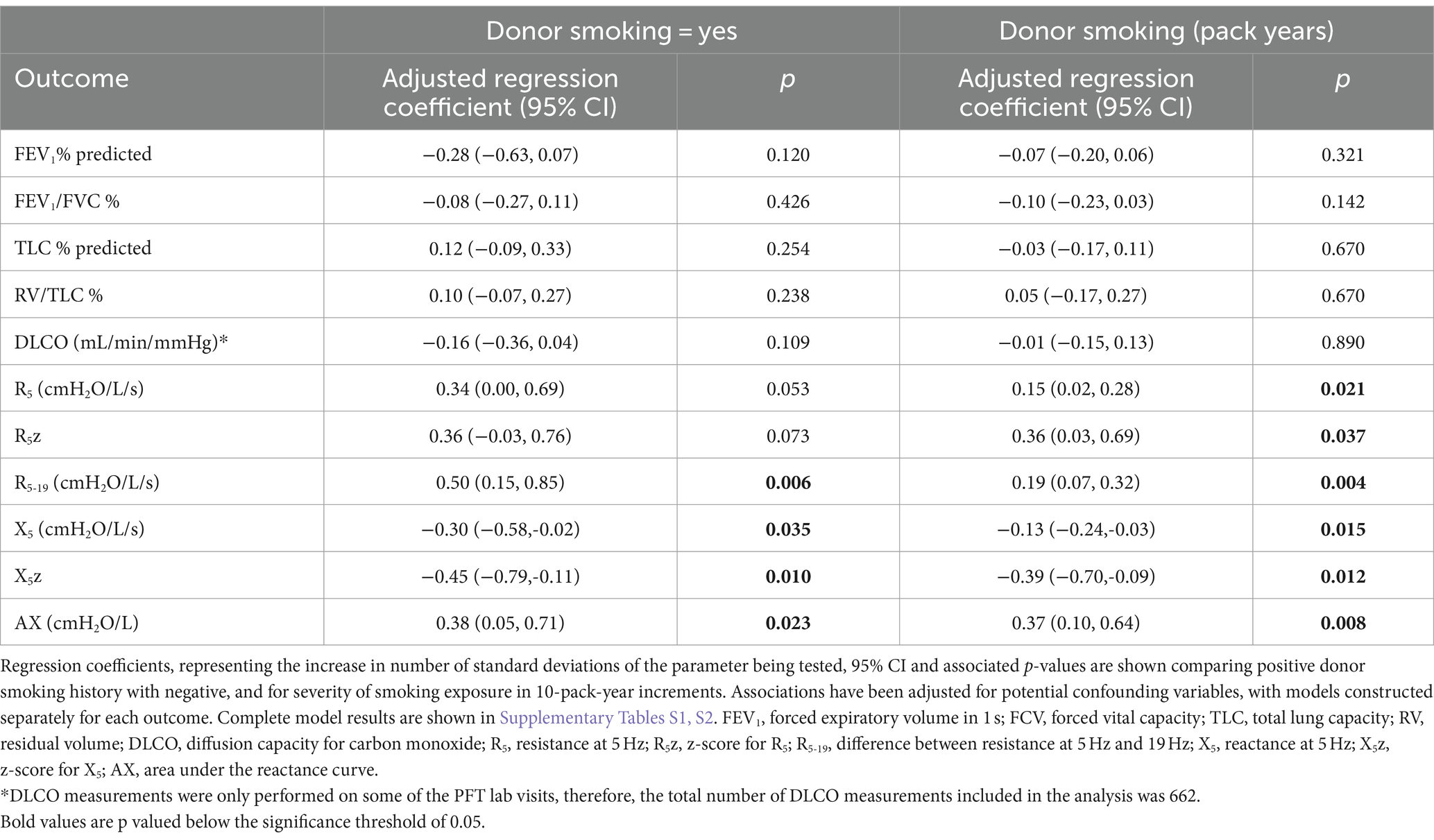
In multivariable analysis, donor smoking compared to no smoking was found to be associated with all the oscillometry parameters examined except R5 and R5z. Donor smoking was associated with a 0.5 standard-deviation (SD) increase in R5-19 (95% CI 0.15–0.85, p = 0.006), a 0.30 SD decrease in X5 (95% CI–0.58 – –0.02, p = 0.035) and a 0.38 SD increase in AX (95%CI 0.05–0.71, p = 0.023). R5 was 0.34 standard deviations higher in the smoking group, but this association did not reach the pre-specified statistical significance level (p = 0.053, 95%CI 0.0–0.69). Donor smoking status did not have a significant association with any cPFT parameters (Table 3). Among the other covariates included in the models, time post-transplant was associated with all outcomes; donor age was associated with FEV1, FEV1/FVC and RV/TLC; underlying diagnosis was associated with all cPFT parameters and with X5; recipient height was associated with DLCO; R5, X5 and AX; and weight was associated with X5. Detailed results of all multivariable models are shown in Supplementary Table S1.
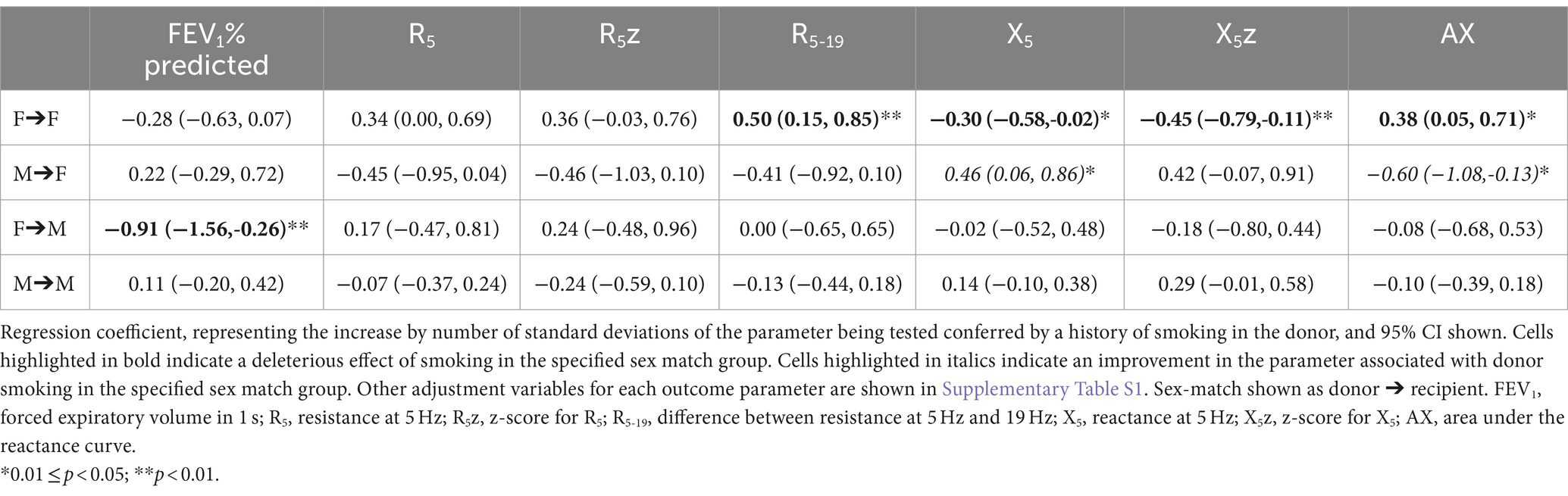
In addition to the above associations, sex match had a significant interaction with donor smoking status, affecting the association between donor smoking status and oscillometry outcomes. The association between smoking and oscillometry metrics was observed in the F➔F group, but was non-significant in the other sex match groups once adjusted for the interaction. The association between donor smoking and X5 and AX was actually reversed in the M➔F group (Table 4).
In the smoking donor group (n = 124), a significant association was seen between increased donor smoking exposure, quantified by pack years, and all oscillometry outcomes, with higher R5, R5-19, R5z and AX, and more negative X5 and X5z. No associations were observed between increasing donor smoking exposure and cPFT outcomes (Table 3).
The interaction with sex match was less prominent when donor smoking was considered as a continuous variable. The sex match interaction was observed for RV/TLC and X5z. The results for each sex-match group, after adjustment for the interaction, are shown in Table 5.

Table 5. Associations between donor smoking as a continuous variable, and outcomes stratified by sex-match group.
3.5 CLAD
Over the study period, 15.6% of patients in the non-smoking group and 16.1% of patients in the smoking group developed CLAD. Kaplan-Myer curves comparing CLAD-free survival between the groups are shown in Figure 3. There was no significant difference between the survival curves (

Figure 3. Kaplan–Meier curves for CLAD-free survival in lung transplant recipients from smoking donors (SD) and non-smoking donors (NSD). Log-rank p-value shown.
4 Discussion
These results demonstrate a clear association between donor smoking history and abnormal oscillometry that reflects abnormal respiratory mechanics of the graft following lung transplantation, with a dose effect from increasing donor smoke exposure. The elevated R5, R5z, R5-19 and AX reflect increases in total respiratory resistance and ventilatory inhomogeneity, while lower X5 and X5z reflect loss of elastic recoil and increased lung stiffness (10, 34, 35). The association was not seen with conventional measures of post-transplant lung function such as FEV1, FEV1/FVC ratio and DLCO.
Oscillometry measures lung mechanics independently of patient effort and is sensitive to ventilatory heterogeneity in the lung periphery, reduced airway caliber and loss of tissue elastance (10, 34, 35). For these reasons, it may be a better indicator of early graft function, since much of the rapid increase in FEV1 and FVC, which is seen in the first few months post-transplant, may be attributable to improvement in physical conditioning rather than tissue quality. This hypothesis is supported by the observation that the baseline FEV1 value is often achieved 12 months or more post-transplant (5, 36), whereas oscillometry values stabilize earlier. In our cohort, the median time to best achieved FEV1 was more than double the time to best R5 and R5-19, and double the time to best X5 and AX. Our study also lends support to the utility of oscillometry for detecting subtle lung function abnormalities in the absence of clinical symptoms, which may be useful for early detection of conditions such as antibody-mediated rejection, which can have long-term consequences for graft function and development of CLAD. This is particularly important in the early post-transplant period when the expected overall upward trajectory of FEV1 can mask potentially significant graft dysfunction.
We did not find a significant difference in CLAD-free survival. However, the follow-up period is short, with a median of 356 days while the median CLAD onset time is 3–5 years post-transplant (37–40). We included patients with a very short duration of follow-up, starting as low as 3 months. As such, many of the included patients would not yet have reached their best post-transplant FEV1. A follow-up period of at least 12–18 months is required for to determine final baseline FEV1, though CLAD can still be diagnosed if an early drop in FEV1 occurs without subsequent recovery (5). The short duration of follow-up means that we were unable to determine the prevalence of BLAD in our cohort. Donor smoking has been identified as a risk factor for BLAD (4, 6), and BLAD patients exhibit a similar pattern of abnormalities to those seen in the smoking donor group in our study, with higher R5-19, R5, R5z and AX, and lower X5 and X5z (23). The role of oscillometry in predicting BLAD is an important subject that warrants further investigation.
The finding of an interaction between donor and recipient sex match and the effect of donor smoking history complicates the results, with an association between smoking and worse oscillometry outcomes seen in the reference (F➔F) group, but attenuated in the other groups, and even reversed for X5 and AX in the M➔F group. This interaction was less prominent when smoking was quantified by pack-years, but still affected R5z, X5z and AX in the F➔F group. The pack-year analysis was conducted in a smaller group of patients, and therefore may lack statistical power to observe the effects for a small sample size. Furthermore, the quantification of donor pack-years is imprecise, as this is estimated by the donor’s family during the donation process.
Female sex increases the deleterious effect of smoking with earlier onset of and worse outcomes from COPD (41, 42). Compared to men, women with COPD report more severe symptoms and poorer quality of life for similar age, FEV1, smoking history and proportion of emphysema on CT (43). This is in keeping with our finding that the deleterious effect of smoking is observed predominantly in female donors with female recipients. While the effect was not observed in the female donor/male recipient group after adjustment for the interaction effect, it must be noted that this was also the smallest of the 4 groups, encompassing just 28 participants.
Previous studies of sex matching and lung transplant outcomes have shown mixed results, and the topic remains controversial. Most studies focused on survival or CLAD. None looked specifically at the interaction between sex match and smoking. Minambres et al. (44) identified a non-significant trend toward increased mortality with female donors in a univariate analysis encompassing 152 transplants. Thabut et al. (45) also reported decreased long-term survival in the F➔M group, with an overall hazard ratio for mortality in female donors of 1.45. Roberts et al. (46) found a survival benefit for sex-mismatched pairs and improved survival for female recipients, while an analysis of ISHLT registry data (47) found that 90-day mortality was higher in the M➔F group and a lower in F➔F. Another ISHLT registry analysis found increase hazard of 10-year mortality F➔M and M➔F compared to F➔F (48). This more recent registry report focused on donor-recipient size mismatch, and identified an association between undersized organs and lower 1 and 5-year survival. As expected, undersizing was more frequent in the F➔M group, but the M➔F group also has worse long-term survival. Size mismatching offers an intuitively simple explanation for any discrepancies found, but the conflicting nature of existing data on sex matching and outcomes indicates this area requires further exploration, and that size matching is not the only factor influencing this relationship. The current study had not set out to explore size matching and this was not taken into account. However, the fact that the association between smoking and poorer oscillometry metrics was seen predominantly in the F➔F group, suggests that size matching is unlikely to be the only explanation for the observed results. A thorough assessment of size mismatch and its relationship with sex match needs to be explored in a future study with a larger cohort. Overall, the results of our study indicate a complex relationship between donor smoking history, sex match, and lung function post-transplant that warrants further investigation.
Our study has several limitations. Due to the challenge of quantifying smoking exposure in a deceased donor, the number of pack years is an approximate value. Second, the current oscillometry parameters do not have established predicted values across the full range of frequencies. Oostveen et al. (49) established impedance values across lower frequencies ≤14 Hz for reactance and all frequencies except 20 and 25 Hz for resistance, with good reproducibility between centers and devices, and provided prediction equations for R5 and X5. This allowed us to examine z-scores for these two parameters. The group comparisons on multivariable analysis were concordant, showing the same significant differences when analysing the raw values and the z-scores for R5 and X5.
Due to the current paucity of information about post-transplant lung mechanics when measured by oscillometry, variable selection for the multivariable analysis posed a significant challenge. We adopted a data-driven approach to variable selection, and some variables which would usually be included in models addressing post-transplant outcomes, such as underlying disease, were excluded from some models, as directed by the backwards stepwise elimination process. We also opted not to correct for multiple comparisons, given the exploratory nature of the study, the modest cohort size and the relatively small number of outcome metrics being tested. However, it is encouraging to note that our results demonstrated associations between donor smoking and the majority of the oscillometry metrics, and no association with cPFT metrics. Future analyses, utilizing larger groups of subjects and longer follow-up, will tease out specific parameters which may have the strongest associations with donor smoking or other potential insults to the allograft.
Our study included 22 subjects whose last cPFT/Oscillometry measurement occurred less than 3 months post-transplant. Although we excluded those patients who died within 3 months of transplant, or who had been transplanted less than 3 months before the census date of March 15, 2020, some patients stopped undergoing testing before the 3-month mark either due to complications preventing further routine testing, or due to temporary closure of the PFT laboratory due to the COVID-19 pandemic. We opted not to exclude these patients, because despite the short duration of follow-up, each of them still contributed several data points to the analysis. Furthermore, as the highest incidence of mild–moderate rejection episodes occurs within the first 3 months (50, 51), these readings provide valuable data on lung function fluctuations during this high-risk period.
4.1 Conclusion
Our study provides further evidence for the utility of oscillometry in detecting subtle lung function abnormalities which are undetectable by conventional pulmonary function testing. Oscillometry is more sensitive to small airways resistance and less influenced by musculoskeletal deconditioning, and can detect abnormalities in the early-post-transplant lung function of recipients of grafts from smoking donors. Furthermore, we have demonstrated a complex interaction between donor and recipient sex match and donor smoking history, a finding which warrants further study. A longer period of follow-up will also be necessary to determine whether these early differences in graft function are associated with long-term survival and CLAD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University Health Network Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AC: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JM: Investigation, Methodology, Supervision, Writing – review & editing. AV: Data curation, Writing – review & editing. JW: Data curation, Methodology, Supervision, Writing – review & editing. RG: Data curation, Resources, Software, Writing – review & editing.TM: Formal analysis, Investigation, Methodology, Writing – review & editing. CR: Data curation, Resources, Supervision, Writing – review & editing. C-WC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study is supported by a grant-in-aid from the Lung Health Foundation, the Pettit Block Term Grants, the CIHR/NSERC Collaborative Health Research Program (grant # 282963) and the Ajmera Foundation Multi-Organ Transplant Innovation Fund. AV was supported by a University of Toronto Open Scholarship and Queen Elizabeth II/Institute of Medical Science Graduate Scholarships in Science & Technology (QEII-GSST).
Acknowledgments
We thank the Registered Pulmonary Technologists at Toronto General Hospital for helping to conduct the study and lab members of C-WC’s research group for collecting the oscillometry data. We thank the many members of the Toronto Lung Transplant Program and the database team who have entered and validated the clinical data and who have performed adjudication of CLAD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1328395/full#supplementary-material
References
1. Orens, JB, Boehler, A, de Perrot, M, Estenne, M, Glanville, AR, Keshavjee, S, et al. Pulmonary Council, International Society for Heart and Lung Transplantation. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. (2023) 22:1183–200. doi: 10.1016/s1053-2498(03)00096-2
2. Schultz, HH, Møller, CH, Zemtsovski, M, Ravn, J, Perch, M, Martinussen, T, et al. Donor smoking and older age increases morbidity and mortality after lung transplantation. Transplant Proc. (2017) 49:2161–8. doi: 10.1016/j.transproceed.2017.09.021
3. Sabashnikov, A, Patil, NP, Mohite, PN, García Sáez, D, Zych, B, Popov, AF, et al. Influence of donor smoking on midterm outcomes after lung transplantation. Ann Thorac Surg. (2014) 97:1015–21. doi: 10.1016/j.athoracsur.2013.11.020
4. Bonser, RS, Taylor, R, Collett, D, Thomas, HL, Dark, JH, and Neuberger, J. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. (2012) 380:747–55. doi: 10.1016/S0140-6736(12)60160-3
5. Liu, J, Jackson, K, Weinkauf, J, Kapasi, A, Hirji, A, Meyer, S, et al. Baseline lung allograft dysfunction is associated with impaired survival after double-lung transplantation. J Hear Lung Transplant. (2018) 37:895–902. doi: 10.1016/j.healun.2018.02.014
6. Paraskeva, MA, Borg, BM, Paul, E, Fuller, J, Westall, GP, and Snell, GI. Abnormal one-year post-lung transplant spirometry is a significant predictor of increased mortality and chronic lung allograft dysfunction. J Hear Lung Transplant. (2021) 40:1649–57. doi: 10.1016/j.healun.2021.08.003
7. Oto, T, Griffiths, AP, Levvey, B, Pilcher, DV, Whitford, H, Kotsimbos, TC, et al. A donor history of smoking affects early but not late outcome in lung transplantation. Transplantation. (2004) 78:599–606. doi: 10.1097/01.TP.0000131975.98323.13
8. Diamond, JM, Cantu, E, Calfee, CS, Anderson, MR, Clausen, ES, Shashaty, MGS, et al. The impact of donor smoking on primary graft dysfunction and mortality after lung transplantation. Am J Respir Crit Care Med. (2023) 209:91–100. doi: 10.1164/rccm.202303-0358OC
9. Taghavi, S, Jayarajan, S, Komaroff, E, Horai, T, Brann, S, Cordova, F, et al. Double-lung transplantation can be safely performed using donors with heavy smoking history. Ann Thorac Surg. (2013) 95:1912–8. doi: 10.1016/j.athoracsur.2012.11.079
10. Kaminsky, DA, Simpson, SJ, Berger, KI, Calverley, P, de Melo, PL, Dandurand, R, et al. Clinical significance and applications of oscillometry. Eur Respir Rev. (2022) 31:210208–19. doi: 10.1183/16000617.0208-2021
11. Crim, C, Celli, B, Edwards, LD, Wouters, E, Coxson, HO, Tal-Singer, R, et al. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med. (2011) 105:1069–78. doi: 10.1016/j.rmed.2011.01.010
12. Kaminsky, DA . Real-world application of Oscillometry: taking the LEAD. Am J Respir Crit Care Med. (2024) 209:356–7. doi: 10.1164/rccm.202311-2127ED
13. Veneroni, C, Valach, C, Wouters, EFM, Gobbi, A, Dellacà, RL, Breyer, MK, et al. Diagnostic potential of Oscillometry: a population-based approach. Am J Respir Crit Care Med. (2024) 209:444–53. doi: 10.1164/rccm.202306-0975OC
14. Shinke, H, Yamamoto, M, Hazeki, N, Kotani, Y, Kobayashi, K, and Nishimura, Y. Visualized changes in respiratory resistance and reactance along a time axis in smokers: a cross-sectional study. Respir Investig. (2013) 51:166–74. doi: 10.1016/j.resinv.2013.02.006
15. Crisafulli, E, Pisi, R, Aiello, M, Vigna, M, Tzani, P, Torres, A, et al. Prevalence of small-airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration. (2016) 93:32–41. doi: 10.1159/000452479
16. Su, ZQ, Guan, WJ, Li, SY, Ding, M, Chen, Y, Jiang, M, et al. Significances of spirometry and impulse oscillometry for detecting small airway disorders assessed with endobronchial optical coherence tomography in COPD. Int J COPD. (2018) 13:3031–44. doi: 10.2147/COPD.S172639
17. Stewart, S, Fishbein, MC, Snell, GI, Berry, GJ, Boehler, A, Burke, MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Hear Lung Transplant. (2007) 26:1229–42. doi: 10.1016/j.healun.2007.10.017
18. Gauthier, JM, Ruiz-Perez, D, Li, W, Hachem, RR, Puri, V, Gelman, AE, et al. Diagnosis, pathophysiology and experimental models of chronic lung allograft rejection. Transplantation. (2018) 102:1459–66. doi: 10.1097/TP.0000000000002250
19. McFadden, E, Kiker, R, Holmes, B, and deGroot, WJ. Small airway disease. An assessment of the tests of peripheral airway function. Am J Med. (1974) 57:171–82. doi: 10.1016/0002-9343(74)90441-0
20. Mead, J . The Lung's quiet zone. N Engl J Med. (1970) 282:1318–9. doi: 10.1056/NEJM197006042822311
21. Cho, E, Wu, JKY, Birriel, DC, Matelski, J, Nadj, R, DeHaas, E, et al. Airway Oscillometry detects Spirometric-silent episodes of acute cellular rejection. Am J Respir Crit Care Med. (2020) 201:1536–44. doi: 10.1164/rccm.201908-1539OC
22. Vasileva, A, Hanafi, N, Huszti, E, Matelski, J, Belousova, N, Wu, JKY, et al. Intra-subject variability in oscillometry correlates with acute rejection and CLAD post-lung transplant. Front Med. (2023):10. doi: 10.3389/fmed.2023.1158870/full
23. Darley, DR, Nilsen, K, Vazirani, J, Borg, BM, Levvey, B, Snell, G, et al. Airway oscillometry parameters in baseline lung allograft dysfunction: associations from a multicenter study. J Hear Lung Transplant. (2023) 42:767–77. doi: 10.1016/j.healun.2022.12.026
24. Fu, A, Vasileva, A, Hana, N, Belousova, N, Wu, J, Rajyam, SS, et al. Characterization of chronic lung allograft dysfunction phenotypes using spectral and intrabreath oscillometry. Front Physiol. (2022) 13:980942. doi: 10.3389/fphys.2022.980942
25. Thompson, BR, Westall, GP, Paraskeva, M, and Snell, GI. Lung transplantation in adults and children: putting lung function into perspective. Respirology. (2014) 19:1097–105. doi: 10.1111/resp.12370
26. Otulana, B, Higenbottam, T, Scott, J, Clelland, C, Igboaka, G, and Wallwork, J. Lung function associated with histologically diagnosed acute lung rejection and pulmonary infection in heart-lung transplant patients. Am Rev Respir Dis. (1990) 142:329–32. doi: 10.1164/ajrccm/142.2.329
27. Theodore, J, Jamieson, SW, Burke, CM, Reitz, BA, Stinson, EB, Van Kessel, A, et al. Physiologic aspects of human heart-lung transplantation. Pulmonary function status of the post-transplanted lung. Chest. (1984) 86:349–57. doi: 10.1378/chest.86.3.349
28. Oostveen, E, MacLeod, D, Lorino, H, Farré, R, Hantos, Z, Desager, K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. (2003) 22:1026–41. doi: 10.1183/09031936.03.00089403
29. Wu, JKY, Dehaas, E, Nadj, R, Cheung, AB, Dandurand, RJ, Hantos, Z, et al. Development of quality assurance and quality control guidelines for respiratory oscillometry in clinic studies. Respir Care. (2020) 65:1687–93. doi: 10.4187/respcare.07412
30. Miller, MR, Crapo, R, Hankinson, J, Brusasco, V, Burgos, F, Casaburi, R, et al. General considerations for lung function testing. Eur Respir J. (2005) 26:153–61. doi: 10.1183/09031936.05.00034505
31. Miller, MR, Hankinson, J, Brusasco, V, Burgos, F, Casaburi, R, Coates, A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
32. Wanger, J, Clausen, JL, Coates, A, Pedersen, OF, Brusasco, V, Burgos, F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. (2005) 26:511–22. doi: 10.1183/09031936.05.00035005
33. Verleden, GM, Glanville, AR, Lease, ED, Fisher, AJ, Calabrese, F, Corris, PA, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment—a consensus report from the pulmonary council of the ISHLT. J Hear Lung Transplant. (2019) 38:493–503. doi: 10.1016/j.healun.2019.03.009
34. Young, HM, Guo, F, Eddy, RL, Maksym, G, and Parraga, G. Oscillometry and pulmonary MRI measurements of ventilation heterogeneity in obstructive lung disease: relationship to quality of life and disease control. J Appl Physiol. (2018) 125:73–85. doi: 10.1152/japplphysiol.01031.2017
35. Smith, HJ, Reinhold, P, and Goldman, MD. Forced oscillation technique and impulse oscillometry. Lung Funct Test. (2005) 31:72–105. doi: 10.1183/1025448x.00031005
36. Azar, M, Krishnan, S, Stump, TE, Gutteridge, D, Roe, DW, and Hage, C. Peak post-transplant lung function in bilateral lung transplant recipients using a prediction model based on donor and recipient demographic characteristics. Respir Med. (2019) 155:29–35. doi: 10.1016/j.rmed.2019.07.009
37. Levy, L, Huszti, E, Tikkanen, J, Ghany, R, Klement, W, Ahmed, M, et al. The impact of first untreated subclinical minimal acute rejection on risk for chronic lung allograft dysfunction or death after lung transplantation. Am J Transplant. (2020) 20:241–9. doi: 10.1111/ajt.15561
38. Chambers, DC, Yusen, RD, Cherikh, WS, Goldfarb, SB, Kucheryavaya, AY, Khusch, K, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report—2017; focus theme: allograft ischemic time. J Hear Lung Transplant. (2017) 36:1047–59. doi: 10.1016/j.healun.2017.07.016
39. Glanville, AR, Aboyoun, CL, Havryk, A, Plit, M, Rainer, S, and Maloufe, MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. (2008) 177:1033–40. doi: 10.1164/rccm.200706-951OC
40. Todd, JL, Jain, R, Pavlisko, EN, Finlen Copeland, CA, Reynolds, JM, Snyder, LD, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. (2014) 189:159–66. doi: 10.1164/rccm.201306-1155OC
41. Aryal, S, Diaz-Guzman, E, and Mannino, DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. (2014) 9:1145–54. doi: 10.2147/COPD.S54476
42. Foreman, MG, Zhang, L, Murphy, J, Hansel, NN, Make, B, Hokanson, JE, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene study. Am J Respir Crit Care Med. (2011) 184:414–20. doi: 10.1164/rccm.201011-1928OC
43. Martinez, FJ, Curtis, JL, Sciurba, F, Mumford, J, Giardino, ND, Weinmann, G, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. (2007) 176:243–52. doi: 10.1164/rccm.200606-828OC
44. Miñambres, E, Llorca, J, Subrviola, B, Ballesteros, MA, Ortiz-Melón, F, and González-Castro, A. Influence of donor-recipient gender mismatch in early outcome after lung transplantation. Transplant Proc. (2008) 40:3076–8. doi: 10.1016/j.transproceed.2008.08.122
45. Thabut, G, Mal, H, Cerrina, J, Dartevelle, P, Dromer, C, Velly, JF, et al. Influence of donor characteristics on outcome after lung transplantation: a multicenter study. J Hear Lung Transplant. (2005) 24:1347–53. doi: 10.1016/j.healun.2004.10.016
46. Roberts, DH, Wain, JC, Chang, Y, and Ginns, LC. Donor - recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Hear Lung Transplant. (2004) 23:1252–9. doi: 10.1016/j.healun.2003.09.014
47. Sato, M, Gutierrez, C, Kaneda, H, Liu, M, Waddell, TK, and Keshavjee, S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry Experience. J Hear Lung Transplant. (2006) 25:634–7. doi: 10.1016/j.healun.2006.01.012
48. Chambers, DC, Cherikh, WS, Harhay, MO, Hayes, D, Hsich, E, Khush, KK, et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart–lung transplantation report—2019; focus theme: donor and recipient size match. J Hear Lung Transplant. (2019) 38:1042–55. doi: 10.1016/j.healun.2019.08.001
49. Oostveen, E, Boda, K, Van Der Grinten, CPM, James, AL, Young, S, Nieland, H, et al. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. (2013) 42:1513–23. doi: 10.1183/09031936.00126212
50. Burton, CM, Iversen, M, Scheike, T, Carlsen, J, and Andersen, CB. Minimal acute cellular rejection remains prevalent up to 2 years after lung transplantation: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. (2008) 85:547–53. doi: 10.1097/TP.0b013e3181641df9
Keywords: lung transplant, oscillometry, donor smoking history, donor selection, lung function
Citation: Belousova N, Cheng A, Matelski J, Vasileva A, Wu JKY, Ghany R, Martinu T, Ryan CM and Chow C-W (2024) Effects of donor smoking history on early post-transplant lung function measured by oscillometry. Front. Med. 11:1328395. doi: 10.3389/fmed.2024.1328395
Edited by:
John-David Aubert, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2024 Belousova, Cheng, Matelski, Vasileva, Wu, Ghany, Martinu, Ryan and Chow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung-Wai Chow, Y2h1bmctd2FpLmNob3dAdWhuLmNh
 Natalia Belousova
Natalia Belousova Albert Cheng2
Albert Cheng2 Clodagh M. Ryan
Clodagh M. Ryan Chung-Wai Chow
Chung-Wai Chow