- 1School of Traditional Chinese Medicine, Changchun University of Traditional Chinese Medicine, Changchun, Jilin, China
- 2Postdoctoral Research Workstation, Jilin Cancer Hospital, Changchun, Jilin, China
- 3Translational Cancer Research Lab, Jilin Cancer Hospital, Changchun, Jilin, China
- 4Jilin Provincial Key Laboratory of Molecular Diagnostics for Lung Cancer, Jilin Cancer Hospital, Changchun, Jilin, China
- 5Department of Thoracic Oncology, Jilin Cancer Hospital, Changchun, Jilin, China
Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a high-grade neuroendocrine carcinoma (HGNEC) accounting for 3% of primary lung cancer, and characterized by strong invasion, high heterogeneity, and extremely poor prognosis. At present, the diagnosis and treatment of LCNEC remains controversial and refer to therapeutic strategy of small cell lung cancer (SCLC), lacking precise therapy. Recently, the genetic analysis and clinical trials of LCNEC gradually emerged, providing more evidence for precise diagnosis and treatment. Here, we review the diagnosis, molecular characteristics, and treatment of LCNEC based on the existing research and frontier progress to provide a potential direction for future diagnosis and treatment of LCNEC.
1 Introduction
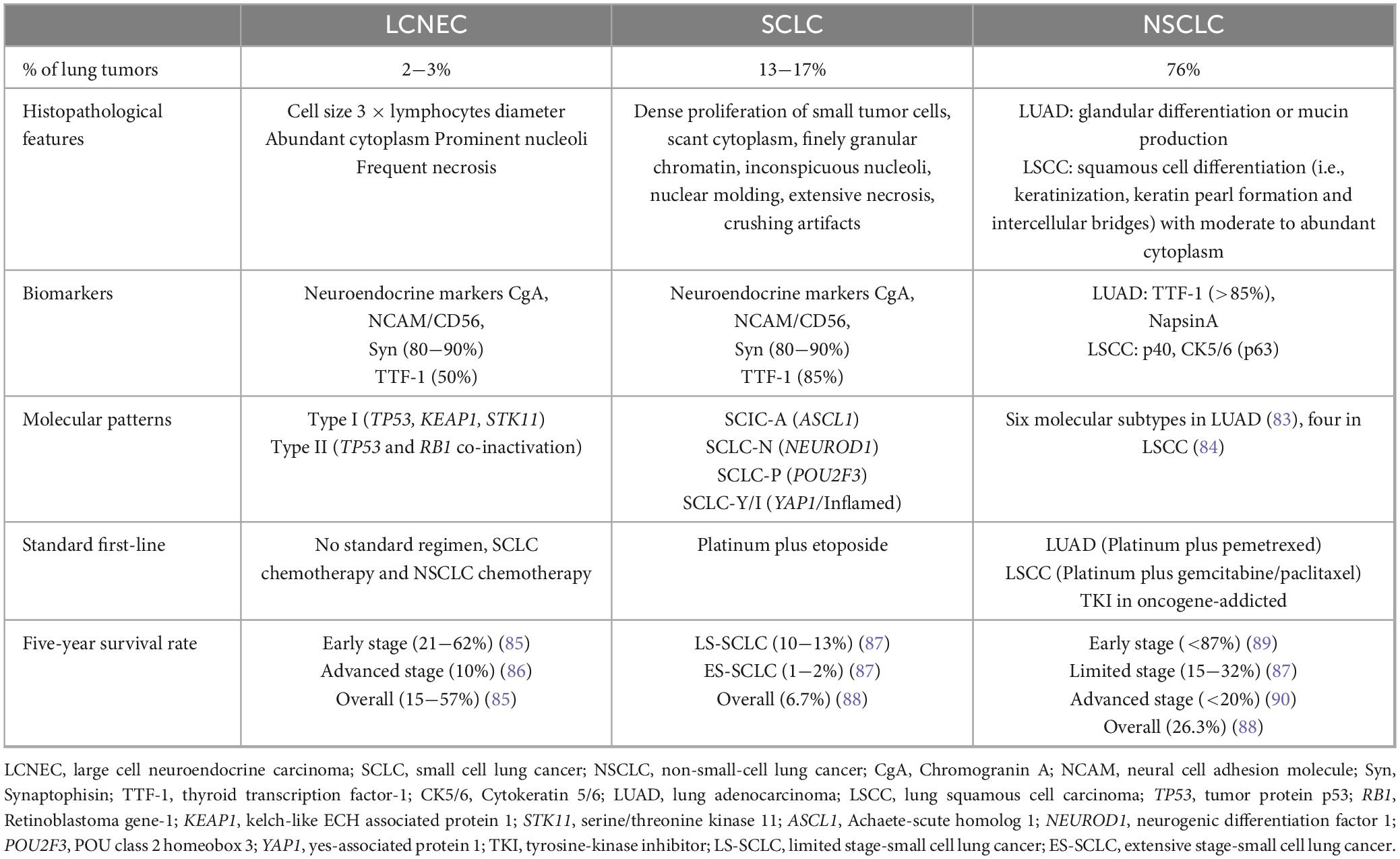
Pulmonary large cell neuroendocrine carcinoma (LCNEC), a rare pathological type of lung cancer, with high invasiveness and poor prognosis, classified into high-grade neuroendocrine carcinomas (HGNEC). Although LCNEC merely accounts for 2.4−3.1% of lung cancer, only 25% of the patients are early stage, while 40−50% are advanced metastatic stage when diagnosis. The median overall survival (mOS) of LCNEC patients are 36 °months, and the 3-year and 5-year survival rates are 49 and 44.7%, respectively, (1). The majority of LCNEC patients are male (about 62.5%), with an average age of about 65°years old and a history of heavy smoking (2). LCNEC was once categorized as a variant of large cell carcinoma, with the increased understanding, it was classified as neuroendocrine carcinoma in 2015 by World Health Organization (WHO). The diagnostic criteria of LCNEC are mainly based on cell morphology and neuroendocrine differentiation. In detail, large cells, low nuclear/plasmic ratio, vacuolated chromatin with obvious nucleoli, organoid, fenestrated and rosette structures, as well as the identification of neuroendocrine morphology and the expression of at least one of the neuroendocrine markers (chromogranin A, synaptophysin, or CD56) or show endocrine characteristics under the electron microscope, are criteria for the diagnosis of LCNEC. However, diagnosis by morphology through neuroendocrine markers is not able to differ LCNEC from other lung neuroendocrine carcinomas, such as small cell lung cancer (SCLC), increasing the risk of underdiagnosis and misdiagnosis of LCNEC. Additionally, the low prevalence rate and small sample size have resulted in finite research on genotype and biomarkers for LCNEC.
Due to the limited understanding and low incidence of LCNEC, there is currently no standard treatment guideline from prospective clinical trials. Surgery is recommended for early-stage LCNEC patients (TNM I-III, AJCC 8th edition), while chemotherapy for patients with medium-term and advanced stage. The chemotherapy regimens include etoposide-cisplatin (EC) and etoposide-platinum (EP), or platinum-gemcitabine/paclitaxel (GEM/TAX) and gemcitabine/paclitaxel regimens, the former is used in SCLC and the latter used in non-small cell lung cancer (NSCLC). However, the high heterogeneity of LCNEC has resulted in contradictory and limited efficacy of therapeutic regimens across different clinical trials (3). Therefore, it is imperative to seek more effective treatment options and potential therapeutic targets and improve prognosis of patients with LCNEC. This review is to provide an overview on the genetic profile and clinical advances of LCNEC to enhance the understanding of its biology, prognostic implications and therapeutic strategies.
2 Diagnosis of LCNEC
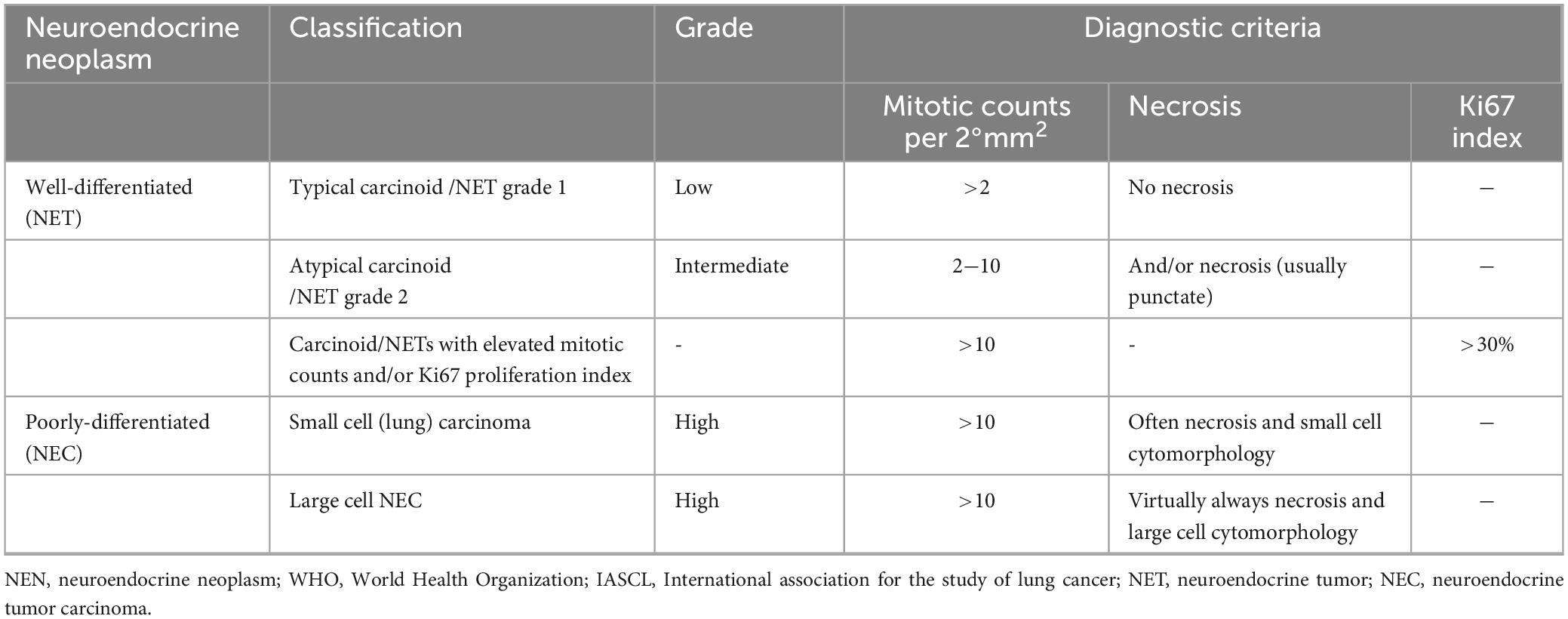
Large cell neuroendocrine carcinoma (LCNEC) was included in HGNEC, which proposed by WHO and the International Association for the Study of Lung Cancer (IASLC) (4, 5) (Table 1), with a neuroendocrine morphology including lobules, organoid or trabecular patterns, rosette-like structures, or peripheral palisading, accompanied by extensive necrosis and a high proliferation rate. Accurate diagnosis of LCNEC requires large surgical specimens and the use of morphological and endocrine markers. However, the histological features of LCNEC overlapped with NSCLC and SCLC, increasing the difficulty of histological diagnosis (6, 7). The result of a phase II clinical trial showed that approximately 27.5% of the subjects initially diagnosed with LCNEC were reclassified as other types of lung cancer, predominantly SCLC. In another study, only 53% (n = 44) of patients were initially correctly classified as LCNEC, while 47% (n = 37) were misdiagnosed as NSCLC (8, 9). Therefore, LCNEC needs to be distinguished from NSCLC and SCLC by clinicopathological and molecular features (Table 2).
Reliable diagnostic biomarkers are essential for LCNEC, which typically shows high expression of multiple neuroendocrine markers, including neural cell adhesion molecule (NCAM/CD56), synaptophysin (Syn), chromogranin A (CgA), etc. Among them, CD56, expressed in 92−98% of LCNEC, may be the most sensitive marker for the diagnosis of LCNEC, but also expressed in nearly 10% of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, leading to low specificity. In contrast, CgA is expressed in nearly 70% of LCNEC and may be the most specific marker, lacking sensitivity conversely. Syn is expressed in 87% of LCNEC, nevertheless, in 10% of adenocarcinomas and 5% of squamous cell carcinomas (10). The combination of the three traditional neuroendocrine markers increased the sensitivity for LCNEC from 47 to 93% on paired biopsy specimens (11). Additionally, other potential diagnostic markers have been investigated. Insulinoma-associated protein 1 (INSM1) is a nuclear transcription factor, expressed in neuroendocrine neoplasms (NENs). Multiple studies had shown that INSM1 was expressed in 68−91.3% of LCNEC (12–14). The sensitivity of INSM1 (75%) in LCNEC was higher than that of CgA (46%), and the specificity (97%) was similar to that of CgA (98%), but higher than that of Syn (90%), and CD56 (87%) (15). Möller et al. (16) found that INSM1 was used as an additional marker, the sensitivity for detecting neuroendocrine differentiation in NENs increased from 96.6% (Syn and CgA) to 97.2% (Syn, CgA, and INSM1). Whether INSM1 could be a complementary marker for LCNEC need more research. Thyroid transcription factor 1 (TTF-1) was positively expressed in 40−50% of LCNEC cases (10). Achaete-scute homolog 1 (ASCL1) could also be detected in most LCNECs (17), suggesting TTF1 and ASCL1 were the potential diagnostic markers for LCNEC, exhibit promising prospects in the field of research. Ye and colleagues conducted IHC staining on adenocarcinoma, squamous cell carcinoma, and various types of pulmonary NENs, including lung typical carcinoid (TC), SCLC, and LCNEC, revealing that the expression of human achaete-scute homolog 1 (hASH1), a regulator of neuroendocrine cells growth, was higher in LCNEC (72.7%) and SCLC (79.2%). It was considered that hASH1 could serve as a potential diagnostic marker for HGENC (18). ISL LIM homobox 1 (ISL1), secretagogin (SECG) and syntaxin were proposed for identifying NENs, while the accuracy needs to be fully validated in large case series (19).
The exploration of diagnostic markers for LCNEC and SCLC (20–22) or NSCLC (23) is also crucial. Bari et al. (24) performed RNA sequencing and IHC staining, revealing significant differential expression of caudal type homeobox 2 (CDX2), Villin 1 (VIL1), and brain-specific angiogenesis inhibitor 3 (BAI3) between the cohorts of LCNEC (n = 71) and SCLC (n = 76). CDX2 and VIL1 in combination had sensitivity and specificity of both 81% for LCNEC while BAI3 showed 89% sensitivity and 75% specificity for SCLC. Therefore, CDX2, VIL1, and BAI3 have the potential to distinguish LCNEC from SCLC. Fan et al. (25) reported that CK7 exhibited negative or weakly positive expression in 88.9% of SCLC cases, whereas 90.8% of LCNEC cases demonstrated partial or complete membrane reported that CK7 exhibited negative or weakly positive expression in 88.9% of SCLC, whereas partial or complete membrane-like positivity with strong intensity in 90.8% of LCNEC, and also had a clear staining pattern in the mixed carcinoma of SCLC and LCNEC. Therefore, CK7 is considered to have a high differential value in SCLC and LCNEC. Stathmin1 (STMN1) mediates cell division and proliferation by regulating microtubule dynamics (26). The expression of STMN1 in HGNEC tissues was found to be significantly higher than NSCLC and showed a high-precision AUC (AUC: 0.984, cutoff: 8.667, sensitivity 92.3%, specificity 95.1%), which could be useful for the differential diagnosis of NSCLC and HGNEC, especially LCNEC (27). The future research should focus on further exploring the gene and protein characteristics of LCNEC, as well as conducting rigorous clinical validation to identify more sensitive and specific diagnostic markers.
3 Molecular characterization and subtypes
3.1 Molecular characteristic
The genetic alteration of LCNEC has been partially elucidated which is crucial for the therapeutic strategies of LCNEC. Results from genetic sequencing revealed a high prevalence of TP53 (46.4−92%) and RB1 (26−42%) gene mutations (23, 28, 29). Bi-allelic alterations in both genes were observed in 40% of LCNEC. STK11(30%), KEAP1(22%), and PTEN (7%) gene mutations were also common (30). Combined with loss-of-heterozygosity (LOH), bi-allelic alterations of serine/threonine kinase 11 (STK11) and kelch-like ECH associated protein 1 (KEAP1) were identified in 37% of the cases. The somatic alterations of RB1 and STK11/KEAP1 were detected in 82% of the cases (n = 49) and occurred in a mutually exclusive pattern (23). Alterations in specific functional genes, such as CREBBP, EP300, NOTCH, MEN1, and ARID1A had also been reported in LCNEC (28, 29, 31–34). Other specific mutations including SMARCA2 (11%), neurotrophic receptor tyrosine kinase 2 (NTRK2) and 3 (NTRK3) (19%) genes (33). FGFR2 was identified as a distinct gene mutation site in LCNEC compared to other NENs (32). Furthermore, pure LCNEC displayed characteristic changes resembling those observed in typical adenocarcinoma, such as EGFR mutation (35, 36), ALK rearrangement (37), RAS pathway mutation, and BRAF mutation (29). This distinguished itself from SCLC as the SCLC lacks these driver gene mutations (38–40). Karlsson et al. (30) used massive parallel sequencing for investigating mutations in 26 cancer-related genes and gene fusions in a cohort of 41 cases of large cell carcinoma (LCC) and 32 cases of LCNEC. The results unveiled TP53 (83%), KRAS (22%), and MET (12%) as the most frequently mutated genes in LCC, while TP53 (88%), STK11(16%), and PTEN (13%) exhibited higher prevalence in LCNEC, no ALK, RET or ROS1 fusions were detected in either LCC or LCNEC.
Gene amplification also occurs frequently in LCNEC with high rates of SRY-box 2 (SOX2) (11%), cyclin E1 (CCNE1) (9%), and MYCN (2%) genes (41). In addition, 4% of LCNEC patients had an increased copy number of ERBB2 and SETBP1 (28, 42). As a downstream target of ASCL1, DLL3 was involved in the regulation of neuroendocrine differentiation (43). Hermans et al. (17) found that expression of DLL3 was positive in 74% (70/94) of LCNEC and higher in 89% (8/9) of TP53 wild-type LCNEC than in 50% (29/58) of mutant-type (P = 0.035), indicating that DLL3 played a potential role in identifying molecular subtypes of LCNEC. Klotho served as a potential tumor suppressor in a variety of tumors, inhibited cell proliferation and induced tumor apoptosis (44). It was found that the overall survival (OS) of LCNEC patients with Klotho positive expression was significantly longer than that of Klotho negative (P = 0.015, HR = 0.37, 95%CI: 0.17−0.86) (45).
Since the difficulty in obtaining and inadequate tissue of LCNEC, cell-free DNA (cfDNA) analysis has demonstrated great potential in genomic profiling. Zhuo et al. (46) applied next-generation sequence (NGS) to detect cfDNA and the genomic analysis of patients with LCNEC, and showed that the mutation landscape of cfDNA were 90% consistent with tumor DNA, suggesting cfDNA sequencing may be a reliable alternative for genome profiling of LCNEC. In addition, cfDNA sequencing is non-invasive and “real-time,” which makes it an ideal tool for monitoring response and investigating genomic evolution during treatment. Designing the prospective clinical trials to guide treatment based on cfDNA sequencing can help accelerate its clinical application.
3.2 Molecular subtypes
As a highly heterogeneous tumor, the categorization of LCNEC based on distinct genomic characteristics holds potential for guiding treatment optimization. Various studies have suggested classification methods for genotyping, thereby determining the response of subtypes to chemotherapy regimens and diverse prognosis (46).
The LCNEC tissues were subjected to NGS sequencing by Rekhtman et al. (33), resulting in the classification of LCNEC into three subtypes. Among the main molecular subtypes, one exhibited SCLC-like features characterized by TP53/RB1 co-mutation or deletion, accompanied by amplification of MYCL, SOX2, and FGFR1 as well as PTEN mutation/deletion, and shared several clinicopathological characteristics with traditional SCLC, including higher proliferation activity and shorter relapse-free survival (RFS). The other subtype displayed NSCLC-like features with intact TP53/RB1 function, NOTCH mutations, and genes commonly found in NSCLC such as STK11/KRAS/TTF1 mutations resembling adenocarcinoma or KEAP1 mutations resembling squamous cell carcinoma, or SOX2/FGFR1 amplification. There was evidence suggesting that SCLC-like subtype of LCNEC demonstrated greater sensitivity to platinum-based chemotherapy compared to other subtypes based on a small subgroup analysis. Furthermore, a third subtype was identified with a lower mutation burden, and shared genetic characteristics similar to TC along with MEN1 gene mutation. Subsequently, George et al. (23) concluded that LCNEC constituted own category within the expression profile domain which differed from NSCLC and TC but bears resemblance to SCLC instead. LCNEC was divided into two independent subtypes, type I was similar to classic SCLC subtype, characterized by alterations in STK11/KEAP1, neuroendocrine phenotype with high expression of ASCL1 and DLL3, down-regulating NOTCH pathway, while type II was characterized by changes in TP53/RB1 displaying predominantly non-neuroendocrine phenotype, with low expression of CgA and Syn, low expression of ASLC1 and DLL3 alongside activation of NOTCH pathway. Zhuo et al. (46) stratified 63 LCNEC patients into SCLC-like and NSCLC-like subtypes based on genomic features derived from tumor DNA and/or cfDNA, and administered chemotherapy regimens of SCLC and NSCLC accordingly. The findings demonstrated varying sensitivities of patients to different chemotherapy regimens, indicating the potential guiding significance of genotyping in treatment selection for improved therapeutic outcomes. However, there is few studies on treatment strategies based on classification and effectiveness, more effective classification methods and corresponding treatment regimens are warranted.
4 Treatment
Currently, there is a lack of prospective clinical trial data to support the optimal treatment approach for LCNEC. Approximately 25% of LCNEC patients are early-stage and primarily undergo surgical treatment, while 20% are locally advanced, and 45−50% are advanced patients requiring multidisciplinary treatment. Local radiotherapy may be considered for post-operative or inoperable locally advanced patients, however, the effectiveness of radiotherapy in LCNEC remains uncertain. Due to most of patients being diagnosed at intermediate or late stages, systemic treatment should be combined to enhance therapeutic outcomes. At present, there is a gradual increase in LCNEC patients benefiting from immunotherapy and targeted therapies, and further confirmation is still warranted.
4.1 Surgery and radiotherapy
The optimal therapeutic approach for patients with early LCNEC is surgical intervention (47). A previous study data from the SEER database including 1619 patients with stage I-III LCNEC to demonstrate that mOS in surgical patients (41.0°months, 95% CI, 34.9−47.1°months) was significantly longer than in non-surgical patients (12.0°months, 95% CI, 10.3−13.7°months) (P < 0.05) (48).
For patients with locally advanced or inoperable LCNEC, local radiotherapy may be considered, whereas the efficacy remains uncertain. The above study retrospectively analyzed patients with stage I-III LCNEC and found that lobectomy had the most favorable impact on OS and lung cancer-specific survival (LCSS) for stage I-II LCNEC. For patients with stage III LCNEC, radiotherapy was significantly associated with an improvement in survival time (48). However, in patients undergoing surgery, post-operative radiotherapy appeared to shorten survival time, suggesting limited additional benefit in improving prognosis.
4.2 Chemotherapy
Chemotherapy combined with surgical treatment has significantly enhanced the survival rate of LCNEC. Several studies have investigated post-operative adjuvant regimens. In a retrospective study, Kujtan et al. (49) analyzed 1232 LCNEC patients, among whom 957 (77.7%) underwent surgical resection alone while 275 (22.3%) received EC regimens combined with surgery. Patients who received chemotherapy had a significantly improved 5-year OS rate compared to those who underwent surgery alone (64.5 vs. 48.4%, P < 0.001). Multivariate Cox model confirmed that chemotherapy was beneficial for the survival of resected patients with stage I (HR = 0.54, 95%CI: 0.43−0.68, P < 0.0001). The efficacy of post-operative adjuvant therapy with chemotherapy regimens for NSCLC/SCLC in patients with LCNEC was retrospectively analyzed by Rossi et al. (8). The results showed a significant prolongation in mOS of LCNEC patients with SCLC regimens (EP) compared to non-treatment (42 vs. 11°months, P < 0.001), suggesting that EP should be preferred as the usual regimen for LCNEC. Because of the similarity of genetic and neuroendocrine features between LCNEC and SCLC, the design of clinical trials for LCNEC have mostly favored SCLC. A prospective study demonstrated the addition of adjuvant chemotherapy EP regimen was superior to surgery alone, resulting in a significant improvement in 2-year disease-free survival (DFS) rates (86.7 vs. 47.8%, P = 0.0133) and OS rates (88.9 vs. 65.2%, P = 0.0252) (50). The phase III clinical trial (51) enrolled 221 patients with post-operative HGNEC, including 30 cases of LCNEC and randomly assigned into EP (n = 111) and irinotecan plus cisplatin (IP, n = 110). The median follow-up time was 24.1°months, and the 3-year RFS rates were 65.4% for EP and 69.0% for IP (P = 0.619), hinting IP was not superior to EP in improving RFS in completely resected HGNEC patients, and EP remained the standard adjuvant therapy option for patients with LCNEC.
The choice of chemotherapy regimens remains controversial for advanced patients with LCNEC. The conventional chemotherapy regimens used for NSCLC and SCLC are also employed in LCNEC, however, the overall efficacy of LCNEC is inferior to that in NSCLC and SCLC (3). The 2023 national comprehensive cancer network (NCCN) guidelines recommend multiple chemotherapy regimens for the systemic treatment of locally unresectable or metastatic pulmonary NEC, including LCNEC. However, these regimens have limited efficacy as second- or subsequent-line therapies, resulting in unsatisfactory patient survival rates and a scarcity of immunotherapy options. According to the 2015 American Society of Clinical Oncology (ASCO) guidelines for systematic treatment of stage IV NSCLC, etoposide plus cisplatin/carboplatin or alternative therapy of non-squamous cell carcinoma was recommended as a first-line approach for patients with advanced LCNEC, nevertheless, the efficacy of this treatment remained uncertain (52). Sun et al. (53) retrospectively analyzed 45 patients with advanced LCNEC, who were divided into SCLC (n = 11) and NSCLC regimens (n = 34) groups according to first-line chemotherapy regimens. The results showed that the median progression-free-survival (mPFS) of SCLC and NSCLC groups were 6.1 and 4.9°months, respectively, (P = 0.41). There was also a considerable difference in the type and efficacy of chemotherapeutic regimens between the two groups, salvage regimens containing irinotecan, platinum, or taxanes had high objective responses in the SCLC group, while frequently used agents in the NSCLC group such as pemetrexed, gefitinib, or erlotinib showed no objective response. Derks et al. (54) conducted a retrospective analysis on 128 patients with advanced LCNEC and categorized them into three groups. First-line use of platinum-based combination chemotherapy, including gemcitabine, docetaxel, paclitaxel, or vinorelbine, was classified as “NSCLC-t,” pemetrexed monotherapy as “NSCLC-pt,” and etoposide chemotherapy as “SCLC-t.” The results demonstrated that the mOS of NSCLC-t chemotherapy was 8.5°months (95% CI: 7.0−9.9), which significantly exceeded the 5.9°months (5.0−6.9; HR = 2.51; 95%CI, 1.39−4.52; P = 0.002) of NSCLC-pt treatment and 6.7°months (5.0−8.5; HR = 1.66; 95% CI, 1.08−2.56; P = 0.020) of SCLC-t chemotherapy, indicating that NSCLC-t exhibited superior efficacy in advanced LCNEC. In a phase II clinical trial conducted by Niho et al. (55), 44 patients with advanced LCNEC were enrolled, and the results demonstrated the efficacy of irinotecan and cisplatin. The response rate (RR) was 54.5% (95% CI: 38.8−69.6%), while the mPFS and mOS were 5.9°months (95% CI: 5.5−6.3) and 15.1°months (95% CI: 11.2−19.), respectively. Zhuo et al. (46) demonstrated that SCLC-like LCNEC patients (n = 15, 24%) treated with EP exhibited superior disease control rates (DCR) compared to those treated with pemetrexed-platinum and GEM/TAX-platinum (100 vs. 20%, P = 0.007). For NSCLC-like LCNEC patients (n = 48, 76%), treatment with EP or pemetrexed-platinum resulted in longer PFS than GEM/TAX-platinum (5.5 vs. 2.5°months, P = 0.045), and there was a trend toward prolonged OS as well (19.6 vs. 9.4°months, P = 0.07). Currently, the treatment of LCNEC relies on retrospective and small-scale studies, yielding inconsistent findings that may be attributed to the differential molecular characteristics of LCNEC. In RB1 wild-type LCNEC, patients treated with the NSCLC regimen (GEM/TAX-platinum) demonstrated significantly improved PFS and OS compared to those receiving the EP regimen (34). In a study by Ito et al. (56), YAP1 expression was detected in 30 patients with LCNEC, with 18/30 (60%) showing YAP1 deletion. Additionally, a significant correlation between YAP1 deletion and neuroendocrine markers expression was observed. Survival analysis revealed that YAP1-negative patients exhibited increased chemotherapy sensitivity. EP regimens are more extensively used in LCNEC, however, prospective large-scale clinical studies are still warranted to provide the improved treatment protocols and the significance of molecular characteristics of LCNEC in guiding precision chemotherapy regimens.
4.3 Immunotherapy
Immunotherapy had demonstrated comparable therapeutic efficacy on NSCLC and SCLC, while limited studies had been conducted on LCNEC, which is currently under clinical trial investigation. LCNEC cells exhibit a high expression level of PD-L1, along with a median tumor mutational burden (TMB) of 9.9 muts/Mb, suggesting the potential effectiveness of immunotherapy in patients with LCNEC. Dudnik et al. (57) reported that the mOS for patients (n = 41) with LCNEC who received immune checkpoint inhibitors (ICIs) from diagnosis or initiation of treatment was 12.4 or 11.0°months, respectively. In contrast, the mOS for non-ICI regimens among 84 patients was 6.0°months. The retrospective study conducted by Komiya et al. (58) revealed that immunotherapy resulted in a significant improvement in OS among patients with stage IV LCNEC (n = 37), compared to those who did not receive immunotherapy (n = 624). The 12-month survival rate was 34.0% versus 24.1%, while the 18-month survival rate stood at 29.1% versus 15.0% in the patients treated with or without immunotherapy. Furthermore, Oda et al. (59) reported a case of obtaining complete response (CR) for up to 4°years after treatment with pembrolizumab. According to the report of Zhang et al. (60), pembrolizumab plus endostar was administered as first-line therapy for a patient diagnosed with LCNEC, the PFS was 2°years. Sherman et al. (61) conducted a clinical trial in which a total of 37 patients with advanced LCNEC were enrolled, patients (n = 21) who received anti-PD-1/PD-L1 immunotherapy demonstrated an ORR of 33%, a complete response rate (CRR) of 11%, a mPFS of 4.2°months (95% CI, 2.4−8.1), and a mOS of approximately 11.8°months (95% CI, 3.7-NR). These findings were consistent with the previous report on NSCLC and LCNEC, indicating similar effectiveness between LCNEC and NSCLC in the context of immunotherapy. The prospective multicenter phase II clinical trial by Patel et al. (62) enrolled 32 patients diagnosed with neuroendocrine tumors to assess the efficacy of the treatment with anti-PD-1 plus anti-CTLA-4 antibodies. Among these cases, 18 (56%) patients were classified as HGNEC. The results showed the ORR was 25%, the 6-month PFS rate was 31% (95% CI, 19−52%), and the mOS was 11°months of the patients with HGNEC, and there was no significant increase in adverse reactions compared with other patients. However, the current study had a short follow-up time and did not evaluate long-term toxic effects such as pulmonary fibrosis and esophageal stenosis or fistula formation.
Nowadays, immunotherapy plus chemoradiotherapy remains the main research direction of LCNEC. Levra et al. (63) conducted a retrospective analysis of 10 patients with stage IIIB-IV LCNEC who received nivolumab or pembrolizumab after first-line treatment with platinum, and revealed an ORR of 60% and a mPFS of 14°months. Song et al. (64) retrospectively analyzed 10 cases of LCNEC treated with pembrolizumab plus chemotherapy as the first-line treatment, the mPFS was 5.5°months (95%CI, 2.3−8.7), mOS was 13.0°months (95%CI, 11.0−15.0), ORR and DCR were 70 and 90%, respectively. In 8 patients who treated with the pembrolizumab with or without chemotherapy as second-line or subsequent treatment, the mPFS was 3.8°months (95%CI, 0.0−7.6) and mOS was not reached, suggesting that pembrolizumab plus chemotherapy may be an attractive first-line treatment strategy for improving survival outcomes in patients with advanced LCNEC. Meanwhile, the use of pembrolizumab plus chemotherapy as first-line therapy was found to be associated with improved PFS in patients with PD-L1 expression ≥50% (P = 0.024). Chauhan et al. (65) reported three LCNEC patients who received nivolumab after progression of platinum-based chemotherapy, all of whom achieved a stable and durable response. Mauclet et al. (66) also reported a case of locally advanced LCNEC achieving complete tumor response after palliative thoracic radiotherapy and nivolumab therapy. Immunotherapy holds promise as a treatment for LCNEC and has the potential to overcome current therapeutic limitations.
Although the above findings indicated that LCNEC patients respond well to immunotherapy, it existed divergent outcomes. The phase I study conducted by Kim et al. (67) included 9 cases of pulmonary HGNEC, the nivolumab plus lutetium-labeled somatostatin analog (lutathera) was administered. Despite exhibiting favorable tolerability, the anti-tumor activity of the drugs demonstrated limitations, necessitating the expansion of sample size. Han et al. (68) reported a case of LCNEC patient treated with post-operative chemotherapy followed by bevacizumab, paclitaxel, and nivolumab. Despite the positive PD-L1 expression, the patient exhibited rapid disease progression. Delayed initiation of immunotherapy may limit efficacy in such cases.
Immunotherapy may overcome existing therapeutic limitations of LCNEC, exploring efficacy and prognostic markers that can identify beneficiary populations or responses and are crucial for achieving precise treatment. For instance, inflammation and immune biomarkers such as the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in peripheral blood may be potential biomarkers for predicting the efficacy of immunotherapy in LCNEC (69). Okui et al. (70) demonstrated that preoperative NLR was an independent prognostic factor for OS in LCNEC (HR = 2.46, 95%CI, 1.508−4.011, P < 0.001). Christopoulos et al. (71) found that mild lymphocyte depletion commonly occurred in LCNEC, while patients who exhibited significant alterations in T-cell repertoire (TCR) subsets and higher lymphocyte counts prior to treatment demonstrated improved outcomes and longer survival time (441 vs. 157°days, P = 0.019). Thus, TCR could be exploited for prognostic, predictive and therapeutic purposes. Chen et al. (72) reported that KEAP1 mutation was a potential therapeutic biomarker in patients undergoing immunotherapy. In other types of lung cancer, immunotherapy efficacy-related markers include PD-L1, TMB, MSI/dMMR, POLE/POLD1 mutations, etc (73). Nevertheless, single and generic predictive markers may not be able to predict the therapeutic efficacy accurately, the specificity of biomarkers could vary depending on tumor type and different ICIs, so relevant predictive markers of efficacy in LCNEC remain to be explored.
4.4 Targeted therapy
Large cell neuroendocrine carcinoma (LCNEC) contains potentially targetable gene alterations, including EGFR mutations ( ± 2%), BRAF mutations ( ± 1%) and FGFR1 amplification ( ± 3%), and these potential therapeutic targets are more frequent in RB1 wild type (84%) than in RB1 mutant type (50%) tumors (23). Gefitinib and icotinib, the first-generation EGFR tyrosine kinase inhibitors (TKIs), were effective in the treatment of LCNEC with EGFR exon 19 deletion, and the response lasted for 6 and 8°months, respectively, (35, 74). Ricco et al. (75) demonstrated that the LCNEC with BRAF V600E (G469R) mutation responded to BRAF/mitogen-activated protein kinase inhibitors. ALK rearrangement rarely occurs in LCNEC (37, 76). LCNEC patients with ALK rearrangement showed regression in both the primary site and multiple metastatic sites after receiving ALK-TKI treatment (76). Therefore, the second or third generation ALK TKIs, such as ceritinib, brigatinib, and loratinib, may be the options of LCNEC patients with ALK rearrangement and secondary central nervous system involvement.
Alteration of genes associated with PI3K-AKT-mTOR pathway was common in LCNEC, and patients with these mutations may respond to targeted therapy. In a clinical trial of phase II (77), 49 patients with metastatic LCNEC were enrolled in the first-line treatment with everolimus plus paclitaxel and carboplatin. The outcomes showed that the ORR was 45% (95%CI, 31−60%), the DCR was 74% (95%CI, 59−85%), the mPFS was 4.4°months (CI, 3−6), the mOS was 9.9°months (CI, 6.9−11.7), and grade 3/4 toxicity occurred in 51% of patients, suggesting that everolimus plus carboplatin and paclitaxel was considered to be the effective and well tolerated first-line treatment for metastatic LCNEC. Rossi et al. (8) conducted a review on 83 LCNEC patients, the histological analysis of LCNEC indicated a strong expression of receptor tyrosine kinases (RTKs), including KIT (62.7%), PDGFRα (60.2%), PDGFRβ (81.9%), and Met (47%), suggesting RTKs could serve as potential therapeutic targets, while no mutation was detected in the exons encoding for the relevant juxta membrane domains. Delta-like ligand 3 (DLL3) is an inhibitory Notch ligand with extremely low expression in normal tissues but high expression in LCNEC and SCLC (78). Saunders et al. (78) demonstrated that the ADC drug SC16LD6.5 (rovalpituzumab tesirine, Rova-T) effectively targeted and eradicated DLL3-expressing initiating cells (TICs) in SCLC and LCNEC patient-derived xenograft (PDX) tumors and was a promising first-in-class ADC for the treatment of HGNEC (79). Although the clinical trial and several other studies investigating ADC agents targeting DLL3 in lung cancer did not meet anticipated outcomes (80, 81) the ongoing clinical investigations on drugs targeting DLL3 are numerous. Subsequently, study on phase I by Morgensztern et al. (82) applied SC-002, a DLL-3 targeted drug, to treat SCLC and LCNEC. The results revealed that SC-002 had systemic toxicity and limited efficacy, similar to the Rova-T ADC, indicating that this drug is unlikely to achieve the requisite level of benefit in this difficult-to-treat patient population. At present, the global pipeline is still working on three projects that activate T cells through CD3 and specifically target cancer cells with high expression of DLL3, which have shown good anti-tumor efficacy in preclinical and clinical stages. The bispecific antibody may be promising in the treatment of LCNEC.
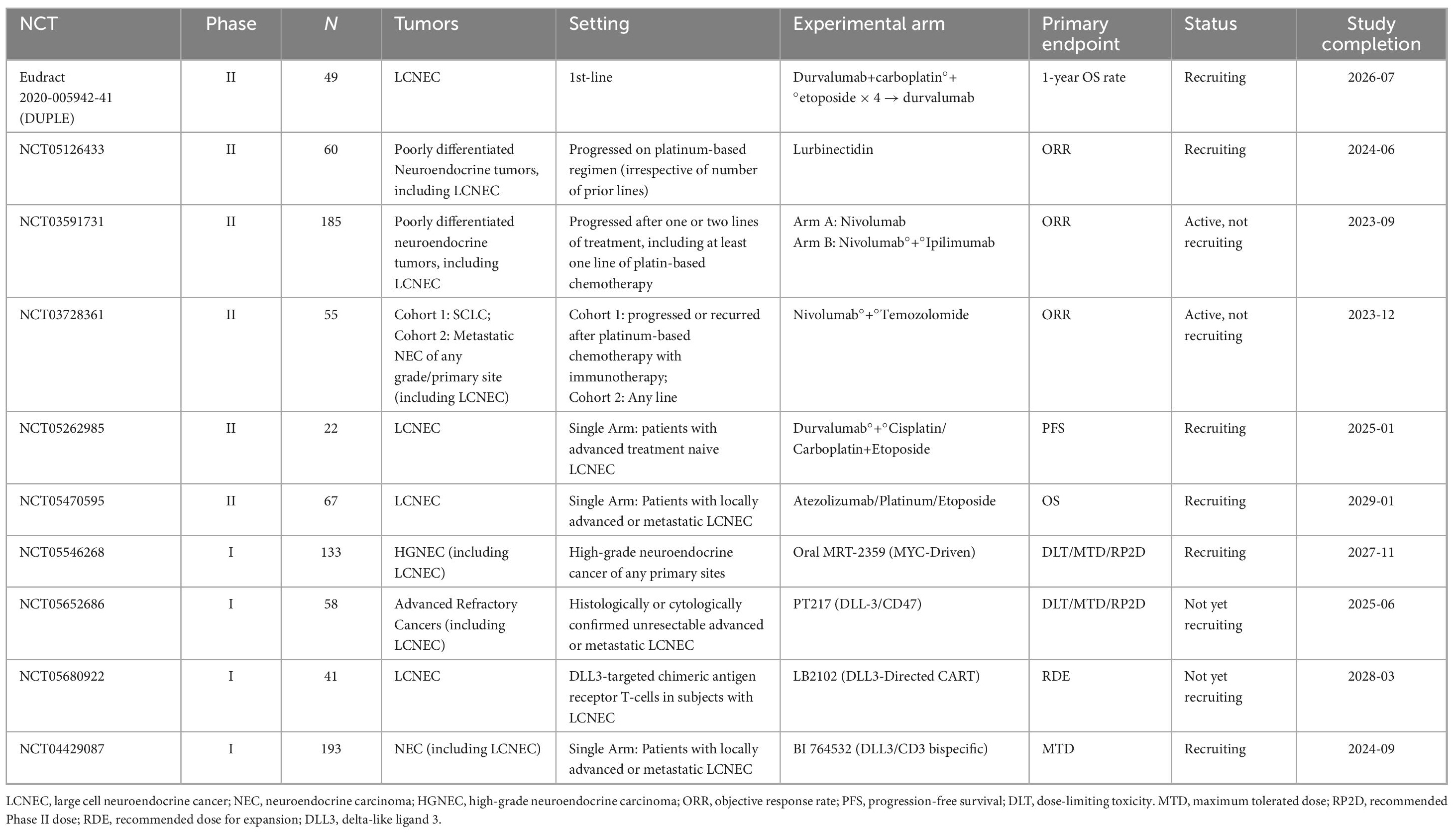
The investigation of immune and targeted therapy in the management of LCNEC has emerged as a prominent area of research. Table 3 presents an overview of ongoing clinical trials focusing on advanced and metastatic LCNEC.
5 Conclusions and perspectives
Large cell neuroendocrine carcinoma (LCNEC) is a rare neuroendocrine carcinoma with high heterogeneity. According to the gene status of TP53 and RB1, researchers classified LCNEC to SCLC-like type and NSCLC-like type, and initially explored the sensitivity of different subtypes to chemotherapy regimen. However, the results still need more clinical data to confirm. Since there is no standard treatment currently, clinical trials of immunotherapy and targeted therapy in LCNEC had exhibited promising outcomes, instilling optimism for enhancing the survival rates of patients.
The focus of future research should be directed toward elucidating the molecular mechanisms and characteristics of LCNEC, with the aim of identifying more effective molecular targets to overcome therapeutic challenges. It is imperative to discover novel treatment modalities by exploring combination strategies involving ICIs, new immunotherapeutic agents, and other innovative approaches. Additionally, researchers should evaluate the efficacy of ICIs in larger patient cohorts and establish correlations between genomic features and treatment responses, thereby facilitating informed decision-making. Furthermore, investigating molecular biomarkers associated with immunotherapy and profiling the molecular characteristics of patients who derive survival benefits will greatly enhance therapeutic outcomes through personalized treatment strategies for LCNEC.
Author contributions
SZ: Writing – original draft. XW: Writing – original draft. HL: Writing – original draft. PZ: Writing – review & editing. JL: Writing – original draft. LZ: Writing – review & editing. YC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This manuscript was supported by the Science and Technology Planning Project of Jilin Province (YDZJ202302CXJD059, YDZJ202202CXJD009, and 20210303002SF); Chinese medicine science and technology project of Jilin Province (2023073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Y, Zhang J, Huang C, Tian Z, Zhou X, Guo C, et al. Survival outcomes of surgery in patients with pulmonary large-cell neuroendocrine carcinoma: A retrospective single-institution analysis and literature review. Orphanet J Rare Dis. (2021) 16:82. doi: 10.1186/s13023-021-01730-7
2. Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ, et al. Clinical features of large cell neuroendocrine carcinoma: A population-based overview. Eur Respir J. (2016) 47:615–24. doi: 10.1183/13993003.00618-2015
3. Fasano M, Della Corte C, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large-cell neuroendocrine carcinoma: From epidemiology to therapy. J Thorac Oncol. (2015) 10:1133–41. doi: 10.1097/JTO.0000000000000589
4. Noonan K, Derks J, Laskin J, Dingemans A-MC. Neuroendocrine tumors of the lung other than small cell lung cancer. IASLC Thorac Oncol. (2018) 2018:555–568.e6.
5. Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens L, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. (2022) 33:115–54. doi: 10.1007/s12022-022-09708-2
6. den Bakker M, Willemsen S, Grünberg K, Noorduijn L, van Oosterhout M, van Suylen R, et al. Small cell carcinoma of the lung and large cell neuroendocrine carcinoma interobserver variability. Histopathology. (2010) 56:356–63. doi: 10.1111/j.1365-2559.2010.03486.x
7. Thunnissen E, Borczuk A, Flieder D, Witte B, Beasley M, Chung J, et al. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. An international reproducibility study in a demanding set of cases. J Thorac Oncol. (2017) 12:334–46. doi: 10.1016/j.jtho.2016.12.004
8. Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. (2005) 23:8774–85. doi: 10.1200/JCO.2005.02.8233
9. Le Treut J, Sault M, Lena H, Souquet P, Vergnenegre A, Le Caer H, et al. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: The GFPC 0302 study. Ann Oncol. (2013) 24:1548–52. doi: 10.1093/annonc/mdt009
10. Ionescu D, Treaba D, Gilks C, Leung S, Renouf D, Laskin J, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation–an entity of no clinical or prognostic significance. Am J Surg Pathol. (2007) 31:26–32. doi: 10.1097/01.pas.0000213319.04919.97
11. Derks J, Dingemans A, van Suylen R, den Bakker M, Damhuis R, van den Broek E, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. (2019) 74:555–66. doi: 10.1111/his.13800
12. Doxtader E, Mukhopadhyay S. Insulinoma-associated protein 1 is a sensitive and specific marker of neuroendocrine lung neoplasms in cytology specimens. Cancer Cytopathol. (2018) 126:243–52. doi: 10.1002/cncy.21972
13. Dermawan J, Mukhopadhyay S. Insulinoma-associated protein 1 (INSM1) differentiates carcinoid tumourlets of the lung from pulmonary meningothelial-like nodules. Histopathology. (2018) 72:1067–9. doi: 10.1111/his.13458
14. Sakakibara R, Kobayashi M, Takahashi N, Inamura K, Ninomiya H, Wakejima R, et al. Insulinoma-associated protein 1 (INSM1) is a better marker for the diagnosis and prognosis estimation of small cell lung carcinoma than neuroendocrine phenotype markers such as chromogranin A, synaptophysin, and CD56. Am J Surg Pathol. (2020) 44:757–64. doi: 10.1097/PAS.0000000000001444
15. Mukhopadhyay S, Dermawan J, Lanigan C, Farver C. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: An immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol. (2019) 32:100–9. doi: 10.1038/s41379-018-0122-7
16. Möller K, Uhlig R, Gorbokon N, Dum D, Menz A, Büscheck F, et al. Comparison of INSM1 immunostaining with established neuroendocrine markers synaptophysin and chromogranin A in over 14,000 neuroendocrine and non-neuroendocrine tumors. Mol Cell Endocrinol. (2024) 581:112106. doi: 10.1016/j.mce.2023.112106
17. Hermans B, Derks J, Thunnissen E, van Suylen R, den Bakker M, Groen H, et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer. (2019) 138:102–8. doi: 10.1016/j.lungcan.2019.10.010
18. Ye B, Cappel J, Findeis-Hosey J, McMahon L, Yang Q, Xiao G, et al. hASH1 is a specific immunohistochemical marker for lung neuroendocrine tumors. Hum Pathol. (2016) 48:142–7. doi: 10.1016/j.humpath.2015.09.019
19. Juhlin C, Zedenius J, Höög A. Clinical routine application of the second-generation neuroendocrine markers ISL1, INSM1, and secretagogin in neuroendocrine neoplasia: Staining outcomes and potential clues for determining tumor origin. Endocr Pathol. (2020) 31:401–10. doi: 10.1007/s12022-020-09645-y
20. Jones M, Virtanen C, Honjoh D, Miyoshi T, Satoh Y, Okumura S, et al. Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet. (2004) 363:775–81. doi: 10.1016/S0140-6736(04)15693-6
21. Cerilli L, Ritter J, Mills S, Wick M. Neuroendocrine neoplasms of the lung. Am J Clin Pathol. (2001) 116:S65–96.
22. Matsumura Y, Umemura S, Ishii G, Tsuta K, Matsumoto S, Aokage K, et al. Expression profiling of receptor tyrosine kinases in high-grade neuroendocrine carcinoma of the lung: A comparative analysis with adenocarcinoma and squamous cell carcinoma. J Cancer Res Clin Oncol. (2015) 141:2159–70. doi: 10.1007/s00432-015-1989-z
23. George J, Walter V, Peifer M, Alexandrov L, Seidel D, Leenders F, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. (2018) 9:1048. doi: 10.1038/s41467-018-03099-x
24. Bari M, Brown H, Nicholson A, Kerr K, Gosney J, Wallace W, et al. BAI3, CDX2 and VIL1: A panel of three antibodies to distinguish small cell from large cell neuroendocrine lung carcinomas. Histopathology. (2014) 64:547–56. doi: 10.1111/his.12278
25. Fan J, Li H, Zhou C, Xiong W, Villamil C, Ionescu D, et al. Classifying pulmonary and urinary high-grade neuroendocrine carcinoma by CK7 immunohistochemistry. Appl Immunohistochem Mol Morphol. (2022) 30:459–68. doi: 10.1097/PAI.0000000000001036
26. Obayashi S, Horiguchi J, Higuchi T, Katayama A, Handa T, Altan B, et al. Stathmin1 expression is associated with aggressive phenotypes and cancer stem cell marker expression in breast cancer patients. Int J Oncol. (2017) 51:781–90. doi: 10.3892/ijo.2017.4085
27. Shimizu K, Goto Y, Kawabata-Iwakawa R, Ohtaki Y, Nakazawa S, Yokobori T, et al. Stathmin-1 is a useful diagnostic marker for high-grade lung neuroendocrine tumors. Ann Thorac Surg. (2019) 108:235–43. doi: 10.1016/j.athoracsur.2019.02.040
28. Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, et al. Gene expression profiling of lung atypical carcinoids and large cell neuroendocrine carcinomas identifies three transcriptomic subtypes with specific genomic alterations. J Thorac Oncol. (2019) 14:1651–61. doi: 10.1016/j.jtho.2019.05.003
29. Miyoshi T, Umemura S, Matsumura Y, Mimaki S, Tada S, Makinoshima H, et al. Genomic profiling of large-cell neuroendocrine carcinoma of the lung. Clin Cancer Res. (2017) 23:757–65. doi: 10.1158/1078-0432.CCR-16-0355
30. Karlsson A, Brunnström H, Lindquist K, Jirström K, Jönsson M, Rosengren F, et al. Mutational and gene fusion analyses of primary large cell and large cell neuroendocrine lung cancer. Oncotarget. (2015) 6:22028–37. doi: 10.18632/oncotarget.4314
31. Karlsson A, Jönsson M, Lauss M, Brunnström H, Jönsson P, Borg Å, et al. Genome-wide DNA methylation analysis of lung carcinoma reveals one neuroendocrine and four adenocarcinoma epitypes associated with patient outcome. Clin Cancer Res. (2014) 20:6127–40. doi: 10.1158/1078-0432.CCR-14-1087
32. Vollbrecht C, Werner R, Walter R, Christoph D, Heukamp L, Peifer M, et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: A comparison of a neglected tumour group. Br J Cancer. (2015) 113:1704–11. doi: 10.1038/bjc.2015.397
33. Rekhtman N, Pietanza M, Hellmann M, Naidoo J, Arora A, Won H, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. (2016) 22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946
34. Derks J, Leblay N, Thunnissen E, van Suylen R, den Bakker M, Groen H, et al. Molecular subtypes of pulmonary large-cell neuroendocrine carcinoma predict chemotherapy treatment outcome. Clin Cancer Res. (2018) 24:33–42. doi: 10.1158/1078-0432.CCR-17-1921
35. De Pas T, Giovannini M, Manzotti M, Trifirò G, Toffalorio F, Catania C, et al. Large-cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gefitinib. J Clin Oncol. (2011) 29:e819–22. doi: 10.1200/JCO.2011.36.2251
36. Aroldi F, Bertocchi P, Meriggi F, Abeni C, Ogliosi C, Rota L, et al. Tyrosine kinase inhibitors in EGFR-mutated large-cell neuroendocrine carcinoma of the lung? A case report. Case Rep Oncol. (2014) 7:478–83. doi: 10.1159/000365413
37. Omachi N, Shimizu S, Kawaguchi T, Tezuka K, Kanazu M, Tamiya A, et al. A case of large-cell neuroendocrine carcinoma harboring an EML4-ALK rearrangement with resistance to the ALK inhibitor crizotinib. J Thorac Oncol. (2014) 9:e40–2. doi: 10.1097/JTO.0000000000000103
38. George J, Lim J, Jang S, Cun Y, Ozretiæ L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. (2015) 524:47–53. doi: 10.1038/nature14664
39. Rudin C, Durinck S, Stawiski E, Poirier J, Modrusan Z, Shames D, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. (2012) 44:1111–6. doi: 10.1038/ng.2405
40. Saghaeiannejad Esfahani H, Vela C, Chauhan A. Prevalence of TP-53/Rb-1 Co-mutation in large cell neuroendocrine carcinoma. Front Oncol. (2021) 11:653153. doi: 10.3389/fonc.2021.653153
41. Peng W, Cao L, Chen L, Lin G, Zhu B, Hu X, et al. Comprehensive characterization of the genomic landscape in Chinese pulmonary neuroendocrine tumors reveals prognostic and therapeutic markers (CSWOG-1901). Oncologist. (2022) 27:e116–25. doi: 10.1093/oncolo/oyab044
42. Przygodzki R, Finkelstein S, Langer J, Swalsky P, Fishback N, Bakker A, et al. Analysis of p53, K-ras-2, and C-raf-1 in pulmonary neuroendocrine tumors. Correlation with histological subtype and clinical outcome. Am J Pathol. (1996) 148:1531–41.
43. Borromeo M, Savage T, Kollipara R, He M, Augustyn A, Osborne J, et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. (2016) 16:1259–72. doi: 10.1016/j.celrep.2016.06.081
44. Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. (2010) 29:99. doi: 10.1186/1756-9966-29-99
45. Brominska B, Gabryel P, Jarmołowska-Jurczyszyn D, Janicka-Jedyńska M, Kluk A, Trojanowski M, et al. Klotho expression and nodal involvement as predictive factors for large cell lung carcinoma. Arch Med Sci. (2019) 15:1010–6. doi: 10.5114/aoms.2018.75889
46. Zhuo M, Guan Y, Yang X, Hong L, Wang Y, Li Z, et al. The prognostic and therapeutic role of genomic subtyping by sequencing tumor or cell-free DNA in pulmonary large-cell neuroendocrine carcinoma. Clin Cancer Res. (2020) 26:892–901. doi: 10.1158/1078-0432.CCR-19-0556
47. Ramirez R, Chauhan A, Gimenez J, Thomas K, Kokodis I, Voros B. Management of pulmonary neuroendocrine tumors. Rev Endocr Metab Disord. (2017) 18:433–42. doi: 10.1007/s11154-017-9429-9
48. Jiang Y, Lei C, Zhang X, Cui Y, Che K, Shen H. Double-edged role of radiotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Cancer. (2019) 10:6422–30. doi: 10.7150/jca.32446
49. Kujtan L, Muthukumar V, Kennedy K, Davis J, Masood A, Subramanian J. The role of systemic therapy in the management of stage i large cell neuroendocrine carcinoma of the lung. J Thorac Oncol. (2018) 13:707–14. doi: 10.1016/j.jtho.2018.01.019
50. Iyoda A, Hiroshima K, Moriya Y, Takiguchi Y, Sekine Y, Shibuya K, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. (2006) 82:1802–7. doi: 10.1016/j.athoracsur.2006.05.109
51. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Ishii G, Nakagawa K, et al. Randomized phase III study of irinotecan plus cisplatin versus etoposide plus cisplatin for completely resected high-grade neuroendocrine carcinoma of the lung: JCOG1205/1206. J Clin Oncol. (2020) 38:4292–301. doi: 10.1200/JCO.20.01806
52. Hanna N, Johnson D, Temin S, Baker S, Brahmer J, Ellis P, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. (2017) 35:3484–515. doi: 10.1200/JCO.2017.74.6065
53. Sun J, Ahn M, Ahn J, Um S, Kim H, Kim H, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: Similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer. (2012) 77:365–70.
54. Derks J, van Suylen R, Thunnissen E, den Bakker M, Groen H, Smit E, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinomas: Does the regimen matter? Eur Respir J. (2017) 49:1601838.
55. Niho S, Kenmotsu H, Sekine I, Ishii G, Ishikawa Y, Noguchi M, et al. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: A multicenter phase II study. J Thorac Oncol. (2013) 8:980–4. doi: 10.1097/JTO.0b013e31828f6989
56. Ito T, Matsubara D, Tanaka I, Makiya K, Tanei Z, Kumagai Y, et al. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci. (2016) 107:1527–38. doi: 10.1111/cas.13013
57. Dudnik E, Kareff S, Moskovitz M, Kim C, Liu S, Lobachov A, et al. Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung. J Immunother Cancer. (2021) 9:e001999. doi: 10.1136/jitc-2020-001999
58. Komiya T, Ravindra N, Powell E. Role of immunotherapy in stage IV large cell neuroendocrine carcinoma of the lung. Asian Pac J Cancer Prev. (2021) 22:365–70. doi: 10.31557/APJCP.2021.22.2.365
59. Oda R, Okuda K, Yamashita Y, Sakane T, Tatematsu T, Yokota K, et al. Long-term survivor of pulmonary combined large cell neuroendocrine carcinoma treated with nivolumab. Thorac Cancer. (2020) 11:2036–9. doi: 10.1111/1759-7714.13471
60. Zhang S, Xiao Y, Chen L, Li Z, Zong Y, Zhu K, et al. Endostar plus pembrolizumab combined with a platinum-based dual chemotherapy regime for advanced pulmonary large-cell neuroendocrine carcinoma as a first-line treatment: A case report. Open Life Sci. (2022) 17:577–85. doi: 10.1515/biol-2022-0062
61. Sherman S, Rotem O, Shochat T, Zer A, Moore A, Dudnik E. Efficacy of immune check-point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung Cancer. (2020) 143:40–6. doi: 10.1016/j.lungcan.2020.03.008
62. Patel S, Othus M, Chae Y, Giles F, Hansel D, Singh P, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. (2020) 26:2290–6. doi: 10.1158/1078-0432.CCR-19-3356
63. Levra M, Mazieres J, Valette C, Molinier O, Planchard D, Frappat V, et al. Efficacy of immune checkpoint inhibitors in large cell neuroendocrine lung cancer: Results from a French retrospective cohort: Topic: Drug treatment alone and in combination with radiotherapy. J Thorac Oncol. (2017) 12:S702–3.
64. Song L, Zhou F, Xu T, Zeng L, Xia Q, Wang Z, et al. Clinical activity of pembrolizumab with or without chemotherapy in advanced pulmonary large-cell and large-cell neuroendocrine carcinomas: A multicenter retrospective cohort study. BMC Cancer. (2023) 23:443. doi: 10.1186/s12885-023-10952-w
65. Chauhan A, Arnold S, Kolesar J, Thomas H, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: Current status. Oncotarget. (2018) 9:14738–40. doi: 10.18632/oncotarget.24553
66. Mauclet C, Duplaquet F, Pirard L, Rondelet B, Dupont M, Pop-Stanciu C, et al. Complete tumor response of a locally advanced lung large-cell neuroendocrine carcinoma after palliative thoracic radiotherapy and immunotherapy with nivolumab. Lung Cancer. (2019) 128:53–6. doi: 10.1016/j.lungcan.2018.12.006
67. Kim C, Liu S, Subramaniam D, Torres T, Loda M, Esposito G, et al. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer. (2020) 8:e000980. doi: 10.1136/jitc-2020-000980
68. Han Y, Pang L, Huang J, Chen J. Large cell neuroendocrine carcinoma of the lungs: Case report and literature review. Ann Palliat Med. (2020) 9:3705–9. doi: 10.21037/apm-20-1667
69. Shi M, Zhao W, Zhou F, Chen H, Tang L, Su B, et al. Neutrophil or platelet-to-lymphocyte ratios in blood are associated with poor prognosis of pulmonary large cell neuroendocrine carcinoma. Transl Lung Cancer Res. (2020) 9:45–54. doi: 10.21037/tlcr.2020.01.17
70. Okui M, Yamamichi T, Asakawa A, Harada M, Saito M, Horio H. Prognostic significance of neutrophil-lymphocyte ratios in large cell neuroendocrine carcinoma. Gen Thorac Cardiovasc Surg. (2017) 65:633–9. doi: 10.1007/s11748-017-0804-y
71. Christopoulos P, Schneider M, Bozorgmehr F, Kuon J, Engel-Riedel W, Kollmeier J, et al. Large cell neuroendocrine lung carcinoma induces peripheral T-cell repertoire alterations with predictive and prognostic significance. Lung Cancer. (2018) 119:48–55. doi: 10.1016/j.lungcan.2018.03.002
72. Chen X, Su C, Ren S, Zhou C, Jiang T. Pan-cancer analysis of KEAP1 mutations as biomarkers for immunotherapy outcomes. Ann Transl Med. (2020) 8:141. doi: 10.21037/atm.2019.11.52
73. Bourreau C, Treps L, Faure S, Fradin D, Clere N. Therapeutic strategies for non-small cell lung cancer: Experimental models and emerging biomarkers to monitor drug efficacies. Pharmacol Ther. (2023) 242:108347. doi: 10.1016/j.pharmthera.2023.108347
74. Wang Y, Shen Y, Ma S, Zhou J. A marked response to icotinib in a patient with large cell neuroendocrine carcinoma harboring an EGFR mutation: A case report. Oncol Lett. (2015) 10:1575–8. doi: 10.3892/ol.2015.3405
75. Ricco G, Seminerio R, Andrini E, Malvi D, Gruppioni E, Altimari A, et al. BRAF V600E-mutated large cell neuroendocrine carcinoma responding to targeted therapy: A case report and review of the literature. Anticancer Drugs. (2023) 34:1076–84. doi: 10.1097/CAD.0000000000001508
76. Zheng Q, Zheng M, Jin Y, Shen X, Shan L, Shen L, et al. ALK-rearrangement neuroendocrine carcinoma of the lung: A comprehensive study of a rare case series and review of literature. Onco Targets Ther. (2018) 11:4991–8. doi: 10.2147/OTT.S172124
77. Christopoulos P, Engel-Riedel W, Grohé C, Kropf-Sanchen C, von Pawel J, Gütz S, et al. Everolimus with paclitaxel and carboplatin as first-line treatment for metastatic large-cell neuroendocrine lung carcinoma: A multicenter phase II trial. Ann Oncol. (2017) 28:1898–902. doi: 10.1093/annonc/mdx268
78. Saunders L, Bankovich A, Anderson W, Aujay M, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. (2015) 7:302ra136. doi: 10.1126/scitranslmed.aac9459
79. Rudin C, Pietanza M, Bauer T, Ready N, Morgensztern D, Glisson B, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. (2017) 18:42–51. doi: 10.1016/S1470-2045(16)30565-4
80. Blackhall F, Jao K, Greillier L, Cho B, Penkov K, Reguart N, et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: Results from the phase 3 TAHOE study. J Thorac Oncol. (2021) 16:1547–58. doi: 10.1016/j.jtho.2021.02.009
81. Malhotra J, Nikolinakos P, Leal T, Lehman J, Morgensztern D, Patel J, et al. A phase 1-2 study of rovalpituzumab tesirine in combination with nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J Thorac Oncol. (2021) 16:1559–69. doi: 10.1016/j.jtho.2021.02.022
82. Morgensztern D, Johnson M, Rudin C, Rossi M, Lazarov M, Brickman D, et al. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: Phase 1 study. Lung Cancer. (2020) 145:126–31. doi: 10.1016/j.lungcan.2020.04.017
83. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. (2014) 511:543–50. doi: 10.1038/nature13385
84. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. (2012) 489:519–25. doi: 10.1038/nature11404
85. Lowczak A, Kolasinska-Cwikla A, Osowiecka K, Glinka L, Palucki J, Rzepko R, et al. Outcomes of patients with pulmonary large cell neuroendocrine carcinoma in I-IV stage. Medicina (Kaunas). (2021) 57:118. doi: 10.3390/medicina57020118
86. Wang Z, Wu Y, Lu T, Xu Y, Chen M, Zhong W, et al. The outcomes of different regimens depend on the molecular subtypes of pulmonary large-cell neuroendocrine carcinoma: A retrospective study in China. Cancer Med. (2024) 13:e6834. doi: 10.1002/cam4.6834
87. Liang M, Chen M, Singh S, Singh S. Identification of a visualized web-based nomogram for overall survival prediction in patients with limited stage small cell lung cancer. Sci Rep. (2023) 13:14947. doi: 10.1038/s41598-023-41972-y
88. Siegel R, Miller K, Wagle N, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
89. Jovanoski N, Bowes K, Brown A, Belleli R, Di Maio D, Chadda S, et al. Survival and quality-of-life outcomes in early-stage NSCLC patients: A literature review of real-world evidence. Lung Cancer Manag. (2023) 12:LMT60. doi: 10.2217/lmt-2023-0003
Keywords: pulmonary large cell neuroendocrine carcinoma, molecular subtype, biomarkers, therapeutic advances, immunotherapy
Citation: Zhu S, Wang X, Li H, Zhao P, Liu J, Zhang L and Cheng Y (2024) Advances in genetic profile and therapeutic strategy of pulmonary large cell neuroendocrine carcinoma. Front. Med. 11:1326426. doi: 10.3389/fmed.2024.1326426
Received: 23 October 2023; Accepted: 12 February 2024;
Published: 27 February 2024.
Edited by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilReviewed by:
Leonard Silva, Oncoclinicas Group, BrazilVera Luiza Capelozzi, University of São Paulo, Brazil
Copyright © 2024 Zhu, Wang, Li, Zhao, Liu, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Cheng, amwuY2hlbmdAMTYzLmNvbQ==
 Siyu Zhu
Siyu Zhu Xinyue Wang
Xinyue Wang Hui Li
Hui Li Peiyan Zhao
Peiyan Zhao Jingjing Liu
Jingjing Liu Liang Zhang
Liang Zhang Ying Cheng
Ying Cheng