- 1Department of Midwifery, College of Health Sciences, Woldia University, Woldia, Ethiopia
- 2Godere Mission Health Center, Gambela, Ethiopia
Background: Preeclampsia is a multisystem disorder that affects pregnant women. Preeclampsia and its complications are the leading causes of maternal and perinatal morbidity and mortality in developing countries. Studies conducted in Ethiopia have primarily concentrated on preeclampsia’s trends and prevalence rather than its obstetrical and perinatal consequences. Thus, this study aimed to determine the risk of adverse obstetric and perinatal outcomes among women with preeclampsia at Woldia Comprehensive Specialized Hospital, Northeast Ethiopia.
Methods: A retrospective cohort study was conducted among 140 preeclamptic women and 280 normotensive women who gave birth at Woldia Comprehensive Specialized Hospital between 30 December 2020 and 29 December 2022. Maternal records were retrieved using data-extraction tools. The data were entered into EpiData version 4.6.0.6 and analyzed using SPSS version 26. Binary and multivariable logistic regression models were used to test the associations between independent and outcome variables. The adjusted odds ratio (OR) with a 95% confidence interval (CI) and p-values <0.05 were used to measure the strength of the association and declare the level of statistical significance.
Results: The odds of at least one adverse obstetric outcome among preeclamptic women were 2.25 times higher than those among normotensive women [AOR: 2.25, 95% CI: (1.06, 4.77)]. In addition, babies born to preeclamptic women were at a higher risk of perinatal death [AOR: 2.90, 95% CI: (1.10, 8.17)], low birth weight [AOR: 3.11, 95% CI: (1.43, 6.7)], birth asphyxia [AOR: 2.53, 95% CI: (1.15, 5.5)], and preterm birth [AOR: 2.21, 95% CI: (1.02, 4.8)] than babies born to normotensive women.
Conclusion: More adverse obstetric and perinatal outcomes were observed in women with preeclampsia than those in normotensive women. This study highlights the significantly elevated level of at least one adverse obstetric outcome associated with preeclampsia, low hemoglobin level, and rural residents. Moreover, perinatal death, low birth weight, asphyxia, and preterm birth were significantly associated with preeclampsia.
Background
Preeclampsia is a hypertensive disorder that typically occurs in pregnancy after 20 weeks of gestation. It is characterized by the new onset of high blood pressure (hypertension) and the presence of protein in the urine (proteinuria). However, it is important to note that preeclampsia can also manifest without significant proteinuria, and in some cases, it may present with other signs of end-organ dysfunction, such as impaired liver function, kidney dysfunction, blood clotting abnormalities, or neurological symptoms (1, 2).
The exact etiology of preeclampsia is not fully understood. However, this pregnancy-related condition is linked to factors such as placental dysfunction, impaired vascular remodeling, and endothelial dysfunction. These factors lead to reduced blood flow and inflammation, which contribute to the characteristic symptoms of preeclampsia (3). Its hallmark features include high blood pressure (BP) and widespread end-organ injury or damage. However, the clinical presentation can be highly variable and perplexing: elevated BP may precede or follow proteinuria, other symptoms may be masked or absent, and its onset can be gradual, abrupt, or even present in the postpartum period (4).
The incidence of preeclampsia varies across regions; it affects 3–5% of pregnant women worldwide. However, evidence suggests that the prevalence of preeclampsia is higher in low-income countries, with reported rates ranging from 1.8 to 16.7% (5). It is one of the top five leading causes of maternal and perinatal morbidity and mortality worldwide, especially at an early onset (6, 7). Globally, approximately 76,000 mothers and 500,000 babies die annually because of preeclampsia (8). Likewise, in sub-Saharan African countries, hypertensive disorder of pregnancy was the second most common cause of maternal death, accounting for approximately 22.1% (95% CI: 19.9–24.2%) of maternal deaths (9). The overall adverse obstetric and perinatal outcomes in preeclampsia are worse and higher in women with severe preeclampsia and eclampsia (6).
In Ethiopia, the prevalence of preeclampsia ranges from 2.23 to 12.4% (10–13), contributing to 16% of direct maternal mortality (14). A hospital-based study conducted in the Amhara region reported that preeclampsia and eclampsia accounted for 35% of maternal deaths (15). A similar study conducted in Ethiopia revealed that 33.5% of unfavorable maternal outcomes were observed among women with preeclampsia (16). Additionally, studies conducted in different settings in Ethiopia showed that more than half of the perinatal outcomes in preeclamptic women were complicated (16, 17).
Reducing maternal and neonatal mortality is a significant global health priority, prompting countries to adopt diverse strategies in their pursuit of the Sustainable Development Goals. These goals aim to eliminate preventable deaths among newborns, achieve a global neonatal mortality rate of 12:1000 live births or less, and reduce the global maternal mortality ratio to less than 70/100,000 births by 2030 (18).
Numerous studies, both in developed and developing countries, have been conducted to investigate the maternal and perinatal outcomes related to preeclampsia (19–22). However, there are only limited studies that focus on pregnancy outcomes specifically associated with this disease in Ethiopia, especially in Woldia comprehensive specialized hospital (WCSH). Therefore, this study aimed to determine the risk of adverse obstetric and perinatal outcomes among women with preeclampsia at Woldia comprehensive specialized hospital, Northeast Ethiopia.
Methods
Study area, design, and period
The study was conducted at WCSH, found in the Amhara region of northern Ethiopia. WCSH is located in Woldia town, which is approximately 365 km northeast of Bahir Dar and 520 km northeast of Addis Ababa. The hospital serves a population of approximately 3 million residents in the nearby regions and zones. According to the hospital’s annual service reports, it provides care for over 4,500 women who give birth each year. A retrospective cohort study was conducted from 1 April to 1 May 2023.
Exposure ascertainments
We determined the exposure of interest based on the guidelines outlined in the Obstetrics Management Protocol for Hospitals in Ethiopia (2021) and the most recent recommendations from the International Society for the Study of Hypertension in Pregnancy (23). In this study, the main exposure variable was preeclampsia. It is defined as the occurrence of new-onset hypertension and proteinuria after 20 weeks of gestation in women who were previously normotensive. Hypertension is characterized by systolic blood pressure (BP) ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg on at least two occasions, with a time interval of 4 hours. Alternatively, in the absence of proteinuria, new-onset hypertension can be accompanied by thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or new-onset medication-resistant headache. Preeclampsia can be further classified into different types based on its severity, onset, and the presence of specific complications (24). Normotensive women in this study were defined as pregnant individuals whose blood pressure (BP) measurements were below 140/90 mmHg at 20 or more weeks of gestation and who did not develop preeclampsia or proteinuria during their pregnancy (25).
Source and study population
The study included all mothers who gave birth at WCSH, located in Northeast Ethiopia, considered as the source population. The study population comprised all mothers with and without preeclampsia who gave birth at WCSH in Northeast Ethiopia from 30 December 2020 to 29 December 2022.
Eligibility criteria and study variables
Medical records of pregnant mothers with and without preeclampsia who gave birth at WCSH were included in the study. However, mothers diagnosed with chronic hypertension, eclampsia, multiple pregnancies, or gestational diabetes mellitus were excluded from the study. In the study, adverse obstetrical and/or perinatal outcomes were considered the dependent variables, with maternal preeclampsia as the main exposure variable. Sociodemographic covariates included the mother’s age and place of residence. Additionally, obstetric characteristics and reproductive healthcare service utilization-related covariates were assessed, including parity, antenatal care follow-up, number of ANC visits, iron and folic acid supplementation, gestational age at delivery, onset of labor, mode of delivery, duration of labor, and hemoglobin level.
Sample size determination
The sample size for the study was calculated using the double population proportion formula in Epi Info version 7.2.5. The calculation was based on a 95% confidence level, 80% power, and an assumed ratio of 1: 2 between women with preeclampsia and those without preeclampsia. In a study conducted in the Sidama region, Ethiopia, the proportion of cesarean deliveries in pregnant women with preeclampsia and without preeclampsia was 45.1 and 29.9%, respectively (17). After adding 10% of the missing charts, the final sample size was 420 (140 women with preeclampsia and 280 without non-preeclampsia).
Sampling technique and procedures
First, all women who gave birth at the WCSH between 30 December 2020 and 29 December 2022 were identified from the delivery registration logbook, totaling 5,923. Among them, 253 had preeclampsia, while 5,593 did not. The study included mothers who met the eligibility criteria, and separate sampling frames were prepared for both groups using the delivery registration book. The medical record number was used as a frame, and a simple random sampling technique was employed to select a total of 420 records. This comprised 140 records from women with preeclampsia and 280 records from women without preeclampsia.
Data collection instrument and procedures
The data were collected using a structured checklist adapted from previous studies with certain modifications. These included details on sociodemographic characteristics, obstetric and reproductive healthcare service utilization, and obstetric and perinatal outcomes. The data collection instrument was developed in the English language. To collect the data, three BSc midwives and one supervisor with 2 years of professional experience were recruited. The investigators reviewed the data daily to ensure their completeness.
Data quality management
To ensure data quality, data collectors and supervisors underwent a one-day training session in line with the aim of the research, contents of the checklist, obstetric admission-discharge logbook, and issues related to confidentiality. A preliminary review was conducted on a 5% subset of a similar study population, which was not part of the actual sample. This review took place 1 week prior to data collection, and necessary corrections and modifications were made based on the findings from the preliminary review.
Data processing and analysis
After the data were coded, they were entered into EpiData version 4.6.0.6 and then transferred to SPSS version 26 for further analysis. The model fitness was assessed using the Hosmer–Lemeshow goodness-of-fit test, while multicollinearity was checked using the variance inflation factor (VIF). A binary logistic regression model was employed to analyze the variables. Variables that had a p-value of <0.25 in the bivariate analysis were included in the multivariable logistic regression model. Adjusted odds ratios (ORs) with 95% confidence intervals (CI) and p-values of <0.05 were used to determine the strength of the associations and identify statistically significant results.
Operational definitions
• Adverse obstetric outcomes in this study were defined as mothers who experienced at least one of the following complications: blood transfusion, antepartum hemorrhage, postpartum hemorrhage, pulmonary edema, or maternal death (26).
• Favorable (without adverse) perinatal outcomes: Newborns born to women with or without preeclampsia had no perinatal complications (25).
• Adverse perinatal outcomes: newborns born to women with and without preeclampsia had at least one complication (low birth weight, intrauterine growth restriction, preterm birth, low APGAR score at 5 min, perinatal asphyxia, or perinatal death) (21).
Results
Sociodemographic characteristics of the study participants
A total of 420 participant charts (140 preeclamptic women and 280 normotensive women) were reviewed. The mean maternal age was similar between women with preeclampsia and normotensive women, with both groups having a mean age of 27. The standard deviation (SD) for maternal age was ±6.00 for women with preeclampsia and ± 5.00 for normotensive women. The majority of women with and without preeclampsia were in the age range of 20–34 years old. Compared to normotensive women (15.7%), the majority of preeclamptic women (28.6%) were rural inhabitants (Table 1).
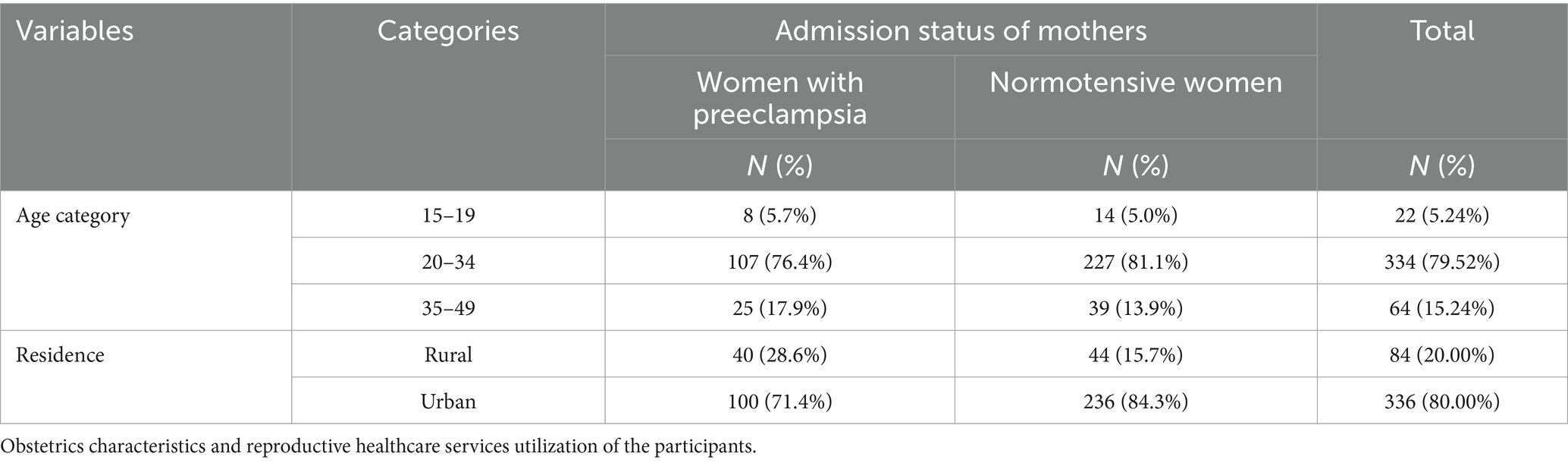
Table 1. Sociodemographic characteristics of the study participants at WCSH, Northeast Ethiopia, 2023.
Obstetrics characteristics and reproductive health care services utilization of the participants
Most of the study participants, 129 (92.1%) and 260 (92.9%) of women with preeclampsia and normotensive women, had at least one ANC follow-up, respectively. Overall, 69 (49.3%) and 125 (44.6%) women with preeclampsia and normotensive, respectively, were nulliparous. Regarding the mode of delivery, 45 (32.1%) preeclamptic women gave birth through cesarean delivery compared to 61 (21.8%) without preeclampsia. Among women with preeclampsia, 41 (29.30%) were initiated by induction, compared to 15 (5.4%) without preeclampsia (Table 2).
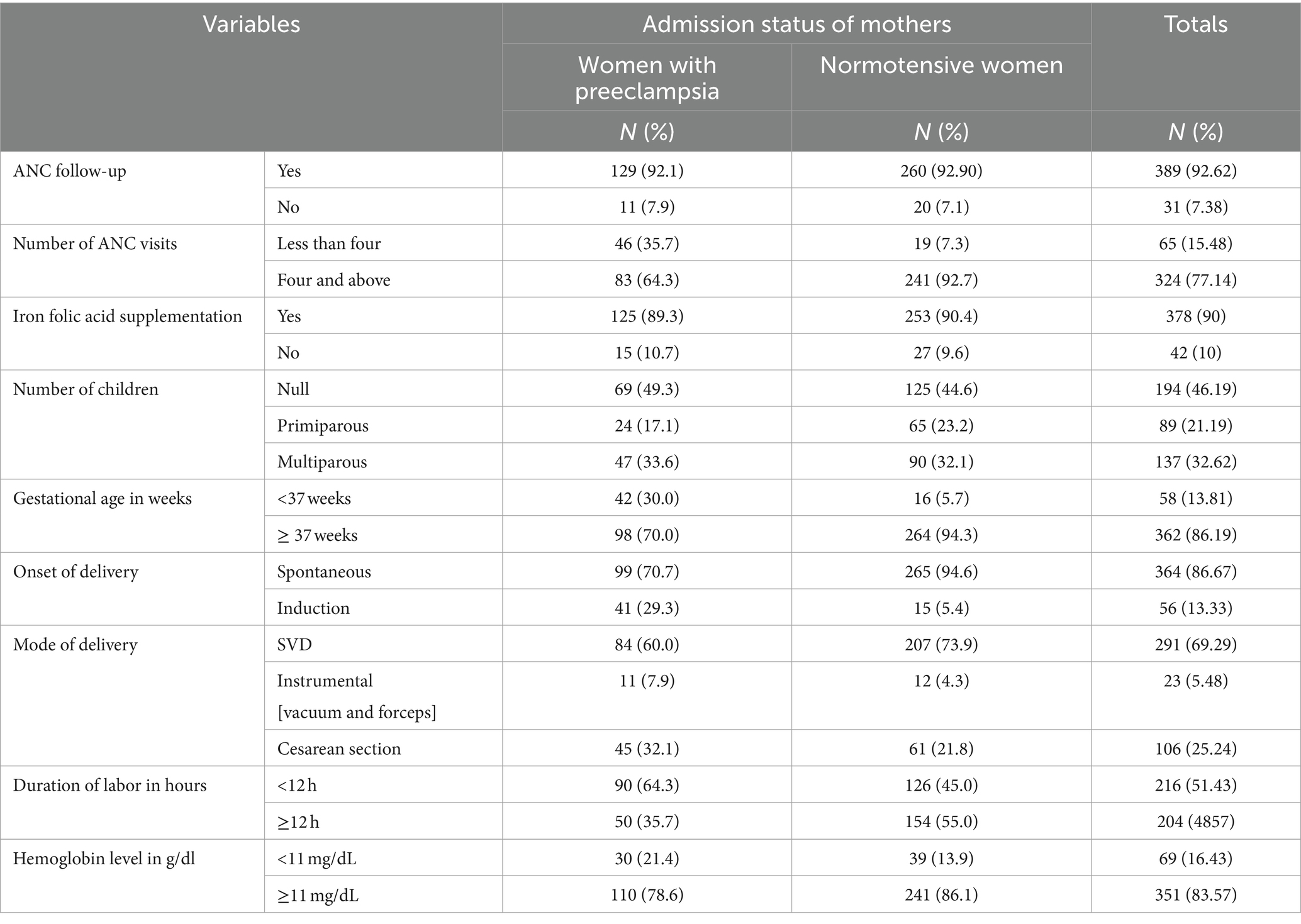
Table 2. Obstetric characteristics and reproductive healthcare services utilization of the study participants at WCSH, Northeast Ethiopia, 2023.
Magnitude of adverse obstetrical and perinatal outcomes
This study’s findings indicated that the prevalence of at least one adverse maternal outcome was higher among women with preeclampsia, with 31 (22.0%) of them experiencing such complications. In comparison, among normotensive women, the prevalence was lower, with 19 (6.80%) encountering adverse maternal outcomes. The proportion of blood transfusion, PPH, APH, and maternal death was higher in women with preeclampsia than in those without preeclampsia (9.3% vs. 2.9, 7.9% vs. 3.2, 5.7% vs. 2.1, and 0.7% vs. 0.0%, respectively). Moreover, the proportion of at least one adverse perinatal outcome among women with and without preeclampsia was 47.90 and 15.30%, respectively. The magnitudes of low birth weight, preterm birth, perinatal death, and perinatal asphyxia in the preeclampsia and normotensive groups were 31.4 and 7.1%, 30.0 and 6.1%, 16.40 and 2.40%, and 18.6 and 5.7%, respectively (Table 3).
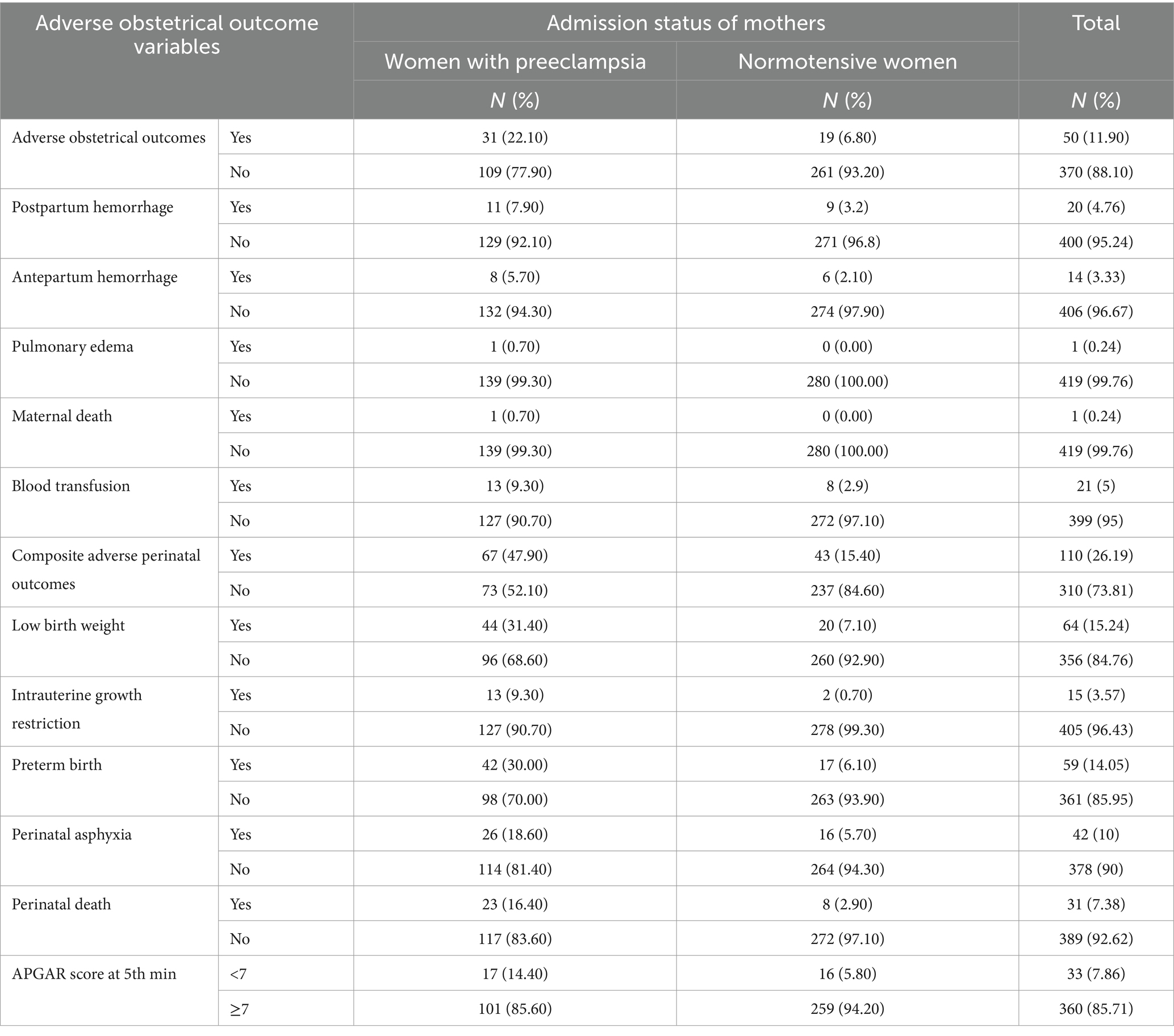
Table 3. Magnitude of adverse obstetrical and perinatal outcomes of the study participant at WCSH, Northeast Ethiopia, 2023.
Association of maternal preeclampsia with adverse obstetrical and perinatal outcomes
Binary logistic regression analysis was conducted to assess the association between various sociodemographic factors, obstetric characteristics, and the utilization of reproductive health services with the occurrence of at least one adverse obstetric outcome. Accordingly, the admission status of mothers, residence, ANC follow-up, the onset of labor, iron foliate supplementation, gestational age at delivery, and hemoglobin level were significant at p < 0.25. After controlling for confounding variables, the odds of adverse obstetric outcomes among women with preeclampsia were 2.25 times higher than those of normotensive women (adjusted odds ratio [AOR: 2.25, 95% CI: (1.06, 4.77)]) (Table 4).
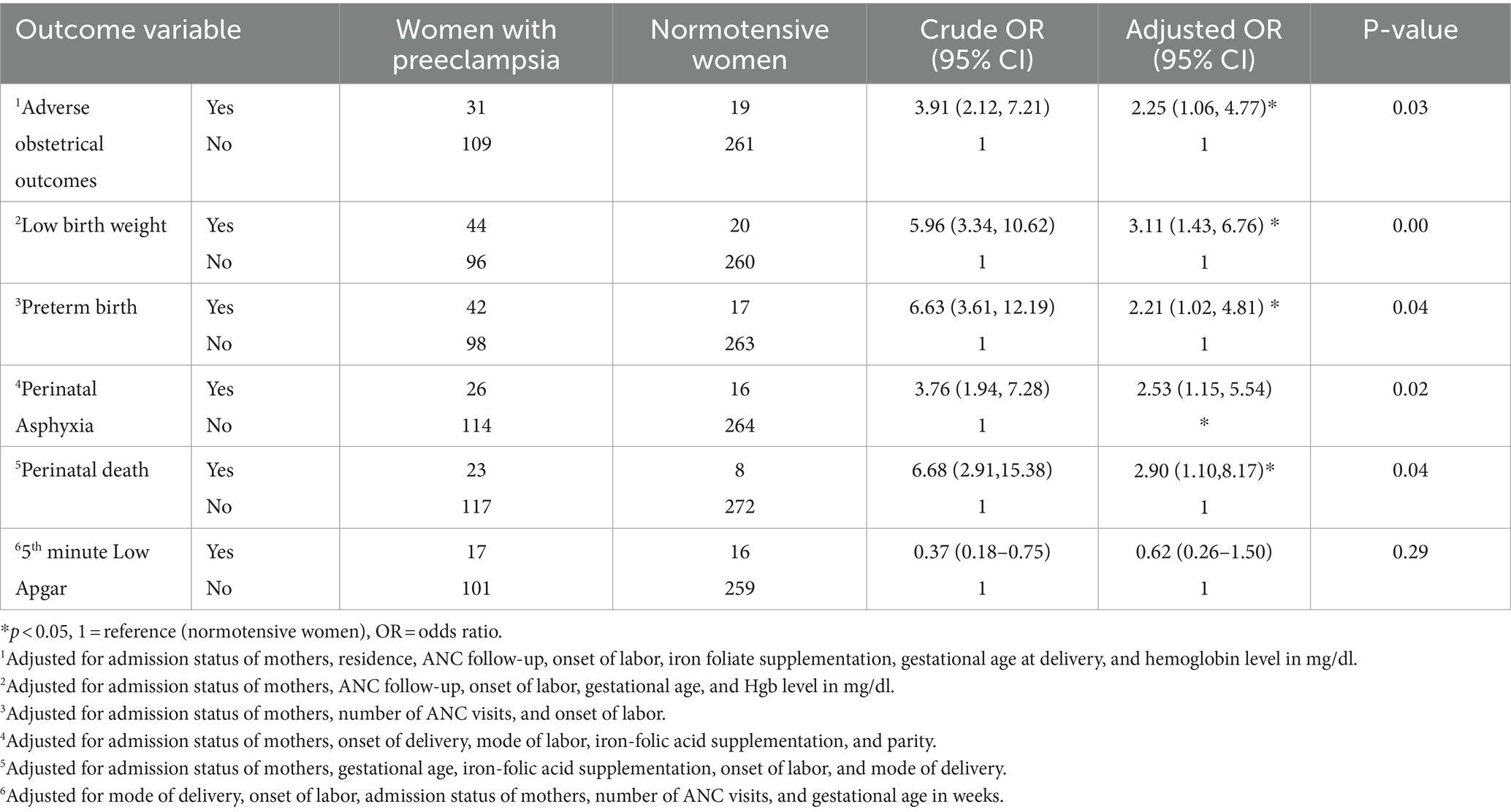
Table 4. Bivariate and multivariate regression analyses of maternal preeclampsia, adverse obstetrical, and perinatal outcomes among women who gave birth at WCSH, Northeast Ethiopia, 2023.
Furthermore, this study revealed that women with preeclampsia had 2.90 times higher odds of perinatal death compared to those without preeclampsia [AOR: 2.90; 95% CI: 1.10, 8.17]. In addition, babies born to women with preeclampsia were 3.11 times more likely to have low birth weight [AOR: 3.11; 95% CI: 1.43, 6.7] and 2.53 times more likely to have birth asphyxia [AOR: 2.53, 95% CI: 1.15, 5.5] than babies born to normotensive women (Table 4).
Discussion
Preeclampsia poses a substantial threat to maternal and perinatal health, contributing significantly to adverse outcomes for both mothers and their infants, particularly in developing nations such as Ethiopia (27). This study also highlights the vulnerability of women with preeclampsia to adverse obstetric and perinatal outcomes compared to normotensive women. The presence of preeclampsia was associated with an increased likelihood of experiencing at least one adverse obstetric outcome. Furthermore, neonates born to preeclamptic women had a higher likelihood of perinatal death, low birth weight, birth asphyxia, and preterm birth than those born to normotensive women.
This study revealed a higher proportion of antepartum hemorrhage, postpartum hemorrhage, and blood transfusions in women with preeclampsia compared to those who were normotensive. Similar findings were observed across diverse geographic settings, including Brazil (28), Kenya (28), Ethiopia (17), and India (29). Similarly, the odds of experiencing at least one adverse obstetric outcome among women with preeclampsia were higher than those among normotensive women. These findings align with previous studies conducted in Ethiopia (19, 21). However, the proportions of these complications varied across the studies. This variation could potentially be attributed to differences in study design. Additionally, variations could be attributed to differences in the guidelines and criteria used for diagnosing postpartum hemorrhage or determining the need for blood transfusions in different healthcare settings. Collectively, these findings paint a concerning picture of the substantial maternal health challenges posed by preeclampsia.
Furthermore, the study revealed that the prevalence of perinatal death, low birth weight, preterm birth, and composite adverse perinatal outcomes was higher in the preeclamptic group than in the normotensive group. This finding is consistent with previous studies conducted in Ethiopia (19, 20, 26, 30, 31), Nigeria (32), India (33, 34), Haiti (35), Bahrain (36), and Ghana (37). The observed neonatal complications may be attributed to the increased emphasis on improving newborn and perinatal care, which aligns with the global efforts to decrease neonatal mortality and morbidity and achieve the Sustainable Development Goals at both the national and international levels. Specific interventions implemented to enhance newborn care including the establishment or strengthening of neonatal intensive care units are vital.
Newborns born to preeclamptic women were found to have a higher likelihood of having low birth weight than those born to normotensive women. This finding aligns with a study conducted in southern Ethiopia (31). Furthermore, this study revealed that neonates born to preeclamptic women had a significantly higher risk of perinatal death, preterm birth, and perinatal asphyxia. Preeclampsia is associated with impaired fetal growth, resulting in low birth weight and related complications (38). Low birth weight is a significant consequence of preeclampsia due to fetal poor nutrition caused by inadequate uteroplacental vascular function. This condition leads to growth retardation and increases the risk of fetal morbidity including premature birth and mortality (39, 40). The consistent evidence from diverse settings emphasizes the urgent need to prioritize the prevention, early identification, and comprehensive management of preeclampsia as a crucial aspect of maternal and newborn health.
Conclusion and recommendations
In this study, adverse obstetric and perinatal outcomes were observed more commonly in women with preeclampsia than in normotensive women. Perinatal death, low birth weight, asphyxia, and preterm birth were significantly associated with preeclampsia. To achieve the Sustainable Development Goals, it is crucial for governments to prioritize the reduction of adverse outcomes related to preeclampsia in mothers and newborns. Healthcare providers play a vital role in promoting early detection, prompt intervention, and prevention of preeclampsia. Additionally, the implementation of calcium supplementation programs should be prioritized, especially in regions with inadequate dietary intake of calcium, as it has been shown to reduce the risk of preeclampsia (41). Furthermore, it is essential to conduct a prospective cohort study to evaluate the impact of various types of hypertensive disorders on adverse maternal and perinatal outcomes during pregnancy.
Limitation of the study
The primary limitation of this study is that it was a single-center study, which may limit the generalizability of the findings to other healthcare settings. Furthermore, this study included only women who attended health institutions. Therefore, the results of this study may not represent the obstetric and perinatal outcomes of women with and without preeclampsia in the community in countries with poor healthcare-seeking behavior, such as Ethiopia.
Ethical considerations
Ethical clearance for the study was obtained from the Institutional Review Board (IRB) of Woldia University. The specific protocol number was WDU/IRB001, and the approval was dated March 06, 2023. However, because the study was conducted by reviewing medical records, confidentiality and anonymity were ensured throughout the study. Further permission for the utilization of charts was obtained from the medical director of the WCSH and the department head of the maternity ward.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Woldia University institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Supervision. DM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing, Resources, Software, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude to Woldia University, School of Public Health, for providing support and facilitation for this study. They also extend their appreciation to the staff of the WCSH and the dedicated data collectors involved in the data collection process. Their contributions and collaboration were instrumental in the successful completion of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANC, Antenatal care; APGAR, Activity–pulse–grimace–appearance–respiration; APH, Antepartum hemorrhage; CI, Confidence interval; GA, Gestational age; HDP, Hypertensive disorders of pregnancy; IUGR, Intrauterine growth restriction; LBW, Low birth weight; PE/E, Preeclampsia–eclampsia; SVD, Spontaneous vaginal delivery; WCSH, Woldia Comprehensive Specialized Hospital.
References
1. Obstetricians ACo, Gynecologists. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. (2020) 135:e237–60. doi: 10.1097/AOG.0000000000003891
2. Brown, MA, Magee, LA, Kenny, LC, Karumanchi, SA, McCarthy, FP, Saito, S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
3. Chaiworapongsa, T, Chaemsaithong, P, Yeo, L, and Romero, R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. (2014) 10:466–80. doi: 10.1038/nrneph.2014.102
4. Chappell, LC, Cluver, CA, Kingdom, J, and Tong, S. Pre-eclampsia. Lancet. (2021) 398:341–54. doi: 10.1016/S0140-6736(20)32335-7
5. Osungbade, KO, and Ige, OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. (2011) 2011:1–6. doi: 10.1155/2011/481095
6. Panda, S, Das, R, Sharma, N, Das, A, Deb, P, Singh, K, et al. Maternal and perinatal outcomes in hypertensive disorders of pregnancy and factors influencing it: A prospective hospital-based study in Northeast India. Cureus. (2021) 13:e13982. doi: 10.7759/cureus.13982
7. Magee, LA, von Dadelszen, P, Stones, W, and Mathai, M. The FIGO textbook of pregnancy hypertension: An evidence-based guide to monitoring, prevention and management. The Global Library of Women’s Medicine, Carlisle. (2016) Available at: http://www.glowm.com/resource_type/resource/textbook/title/the-figo-textbook-of-pregnancy-hypertension/resource_doc/2768
8. Poon, LC, Shennan, A, Hyett, JA, Kapur, A, Hadar, E, Divakar, H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on preeclampsia (PE): a pragmatic guide for first trimester screening and prevention. Int J Gynaecol Obstet. (2019) 145:1–33. doi: 10.1002/ijgo.12802
9. Musarandega, R, Nyakura, M, Machekano, R, Pattinson, R, and Munjanja, SP. Causes of maternal mortality in Sub-Saharan Africa: a systematic review of studies published from 2015 to 2020. J Glob Health. (2021) 11:11. doi: 10.7189/jogh.11.04048
10. Belay, AS, and Wudad, T. Prevalence and associated factors of pre-eclampsia among pregnant women attending anti-natal care at Mettu Karl referal hospital, Ethiopia: cross-sectional study. Clin Hypertens. (2019) 25:1–8. doi: 10.1186/s40885-019-0120-1
11. Andarge, R, Anshebo, A, Halil, H, Kebede, B, and Abdo, R. Prevalence and associated factors of pre-eclampsia among pregnant women at antenatal booking in the Halaba Kullito general hospital, southern Ethiopia. J Women's Health Care. (2020) 9:2167–0420. doi: 10.35248/2167-0420.20.9.496
12. Tessema, GA, Tekeste, A, and Ayele, TA. Preeclampsia and associated factors among pregnant women attending antenatal care in Dessie referral hospital, Northeast Ethiopia: a hospital-based study. BMC Pregnancy Childbirth. (2015) 15:1–7. doi: 10.1186/s12884-015-0502-7
13. Vata, PK, Chauhan, NM, Nallathambi, A, and Hussein, F. Assessment of prevalence of preeclampsia from Dilla region of Ethiopia. BMC Res Notes. (2015) 8:1–6. doi: 10.1186/s13104-015-1821-5
14. Gaym, A, Bailey, P, Pearson, L, Admasu, K, and Gebrehiwot, YTeam ENEA. Disease burden due to pre-eclampsia/eclampsia and the Ethiopian health system's response. Int J Gynecol Obstet. (2011) 115:112–6. doi: 10.1016/j.ijgo.2011.07.012
15. Terefe, W, Getachew, Y, Hiruye, A, Derbew, M, Hailemariam, D, Mammo, D, et al. Patterns of hypertensive disorders of pregnancy and associated factors at Debre Berhan referral hospital, north Shoa, Amhara region. Ethiop Med J. (2015) 57–65.
16. Mulusew, A. Maternal and perinatal management outcome of preeclampsia with severity feature and its associated Facters among pregnant women admitted in Debre Markos comprehensive specialized hospital from January 1 2019 to December 30 2020g (2021).
17. Jikamo, B, Adefris, M, Azale, T, and Alemu, K. The effect of preeclampsia on adverse maternal outcomes in Sidama region, Ethiopia: a prospective open cohort study. Sci Rep. (2022) 12:19300. doi: 10.1038/s41598-022-24034-7
18. Manandhar, M, Hawkes, S, Buse, K, Nosrati, E, and Magar, V. Gender, health and the 2030 agenda for sustainable development. Bull World Health Organ. (2018) 96:644. doi: 10.2471/BLT.18.211607
19. Belay Tolu, L, Yigezu, E, Urgie, T, and Feyissa, GT. Maternal and perinatal outcome of preeclampsia without severe feature among pregnant women managed at a tertiary referral hospital in urban Ethiopia. PLoS One. (2020) 15:e0230638. doi: 10.1371/journal.pone.0230638
20. Melese, MF, Badi, MB, and Aynalem, GL. Perinatal outcomes of severe preeclampsia/eclampsia and associated factors among mothers admitted in Amhara region referral hospitals, north West Ethiopia, 2018. BMC Res Notes. (2019) 12:1–6. doi: 10.1186/s13104-019-4161-z
21. Figa, Z, Temesgen, T, Belekle, E, Ahimed, A, and Tilahun, R. Assessment of maternal outcome among Preeclamptic women at Dilla University referral hospital, Dilla Ethiopia. Int J Biomed Eng Clinic Sci. (2021) 7:65–72. doi: 10.11648/j.ijbecs.20210703.14
22. Mengistu, MD, and Kuma, T. Feto-maternal outcomes of hypertensive disorders of pregnancy in Yekatit-12 teaching hospital, Addis Ababa: a retrospective study. BMC Cardiovasc Disord. (2020) 20:1–10. doi: 10.1186/s12872-020-01399-z
24. Obstetricians ACo, Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. (2019) 133:e1–e25. doi: 10.1097/AOG.0000000000003018
25. Jikamo, B, Adefris, M, Azale, T, and Gelaye, KA. Incidence of adverse perinatal outcomes and risk factors among women with pre-eclampsia, southern Ethiopia: a prospective open cohort study. BMJ Paediatr. Open. (2022) 6:e001567. doi: 10.1136/bmjpo-2022-001567
26. Wassie, AY, and Anmut, W. Prevalence of eclampsia and its maternal-fetal outcomes at Gandhi memorial hospital, Addis Ababa Ethiopia, 2019: retrospective study. Int J Women's Health. (2021) 13:231–7. doi: 10.2147/IJWH.S298463
27. McClure, EM, Saleem, S, Pasha, O, and Goldenberg, RL. Stillbirth in developing countries: a review of causes, risk factors and prevention strategies. J Matern Fetal Neonatal Med. (2009) 22:183–90. doi: 10.1080/14767050802559129
28. Zanette, E, Parpinelli, MA, Surita, FG, Costa, ML, Haddad, SM, Sousa, MH, et al. Maternal near miss and death among women with severe hypertensive disorders: a Brazilian multicenter surveillance study. Reprod Health. (2014) 11:1–11. doi: 10.1186/1742-4755-11-4
29. Gupta, M, Greene, N, and Kilpatrick, SJ. Timely treatment of severe maternal hypertension and reduction in severe maternal morbidity. Preg Hypertens. (2018) 14:55–8. doi: 10.1016/j.preghy.2018.07.010
30. Berhe, AK, Ilesanmi, AO, Aimakhu, CO, and Mulugeta, A. Effect of pregnancy induced hypertension on adverse perinatal outcomes in Tigray regional state, Ethiopia: a prospective cohort study. BMC Pregnancy Childbirth. (2019) 20:7. doi: 10.1186/s12884-019-2708-6
31. Saxena, N, Bava, AK, Nandanwar, YS, and Fentahun, N. Maternal and perinatal outcome in severe preeclampsia and eclampsia. International journal of reproduction, contraception, obstetrics and gynecology. (2016) 5:2171–6.
32. Awoyesuku, PA, John, DH, and Lebara, LB. Maternal and perinatal outcome in severe preeclampsia and eclampsia at the Rivers state university teaching hospital, Nigeria. Int J Reprod Contracept Obstet Gynecol. (2020) 9:4389. doi: 10.18203/2320-1770.ijrcog20204784
33. McKenzie, K-A, and Trotman, H. A retrospective study of neonatal outcome in preeclampsia at the University Hospital of the West Indies: a resource-limited setting. J Trop Pediatr. (2019) 65:78–83. doi: 10.1093/tropej/fmy014
34. Pukale, S, and Patel, A. Maternal and foetal outcome in severe preeclampsiaand eclampsia in A tertiary care rural hospital, Karnataka, India. (2020), 1–4
35. Bridwell, M, Handzel, E, Hynes, M, Jean-Louis, R, Fitter, D, Hogue, C, et al. Hypertensive disorders in pregnancy and maternal and neonatal outcomes in Haiti: the importance of surveillance and data collection. BMC Pregnancy Childbirth. (2019) 19:1–11. doi: 10.1186/s12884-019-2361-0
36. Tabassum, S, AlSada, A, Bahzad, N, Sulaibeekh, N, Qureshi, A, and Dayoub, N. Preeclampsia and its maternal and perinatal outcomes in pregnant women managed in Bahrain’s tertiary care hospital. Cureus. (2022) 14:e24637. doi: 10.7759/cureus.24637
37. Adu-Bonsaffoh, KNM, Obed, SA, and Seffah, JD. Perinatal outcomes of hypertensive disorders in pregnancy at a tertiary hospital in Ghana. BMC Pregnancy Childbirth. (2017) 17:388. doi: 10.1186/s12884-017-1575-2
38. Yilgwan, C, Pam, V, Yilgwan, G, Ige, O, Golit, W, Anzaku, S, et al. Comparing neonatal outcomes in women with preeclampsia and those with normal pregnancy. Nigerian J Paediatric. (2020) 47:258–63. doi: 10.4314/njp.v47i3.11
39. Dimitriadis, E, Rolnik, DL, Zhou, W, Estrada-Gutierrez, G, Koga, K, Francisco, RP, et al. Pre-eclampsia. Nature reviews. Disease primers. (2023) 9:8.
40. Browne, JL, Vissers, KM, Antwi, E, Srofenyoh, EK, Van der Linden, EL, Agyepong, IA, et al., erinatal outcomes after hypertensive disorders in pregnancy in a low resource setting. Tropical medicine & international health (2015) 20:1778–86.
Keywords: preeclampsia, adverse obstetrical outcomes, adverse perinatal outcomes, adverse obstetrical and perinatal outcomes, Woldia
Citation: Kumsa H and Mergiyaw D (2024) Obstetrical and perinatal outcomes of women with preeclampsia at Woldia Comprehensive Specialized Hospital, Northeast Ethiopia. Front. Med. 11:1326333. doi: 10.3389/fmed.2024.1326333
Edited by:
Simcha Yagel, Hadassah Medical Center, IsraelReviewed by:
Sarah M. Cohen, Hadassah Medical Center, IsraelTimothy Abiola Olusesan Oluwasola, University of Ibadan, Nigeria
Copyright © 2024 Kumsa and Mergiyaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henok Kumsa, henokkumsa@gmail.com
 Henok Kumsa1*
Henok Kumsa1* Desalew Mergiyaw
Desalew Mergiyaw