- 1National Perinatal Epidemiology Unit, Infectious Disease Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom
- 2Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Centre for Statistics in Medicine, University of Oxford, Oxford, United Kingdom
Background: Integrase strand transfer inhibitor (INSTI) dolutegravir (DTG)-based antiretroviral therapy (ART) is recommended by World Health Organisation as preferred first-line regimen in pregnant women living with human immunodeficiency virus (HIV) (WLHIV). Non-nucleoside reverse transfer inhibitor (NNRTI)-based ART and protease inhibitor (PI)-based ART are designated as alternative regimens. The impact of different ART regimens on perinatal outcomes is uncertain. We aimed to assess the comparative risk of adverse perinatal outcomes in WLHIV receiving different classes of ART.
Materials and methods: A systematic literature review was conducted by searching PubMed, CINAHL, Global Health, and EMBASE for studies published between Jan 1, 1980, and July 14, 2023. We included studies reporting on the association of pregnant WLHIV receiving different classes of ART with 11 perinatal outcomes: preterm birth (PTB), very PTB, spontaneous PTB, low birthweight (LBW), very LBW, term LBW, preterm LBW, small for gestational age (SGA), very SGA (VSGA), stillbirth, and neonatal death. Pairwise random-effects meta-analyses compared the risk of each adverse perinatal outcome among WLHIV receiving INSTI-ART, NNRTI-ART, PI-ART, and nucleoside reverse transfer inhibitor (NRTI)-based ART, and compared specific “third drugs” from different ART classes. Subgroup and sensitivity analyses were conducted based on country income status and study quality.
Results: Thirty cohort studies published in 2006–2022, including 222,312 pregnant women, met the eligibility criteria. Random-effects meta-analyses found no evidence that INSTI-ART is associated with adverse perinatal outcomes compared to NNRTI-ART and PI-ART. We found that PI-ART is associated with a significantly increased risk of SGA (RR 1.28, 95% confidence interval (95% CI) [1.09, 1.51], p = 0.003) and VSGA (RR 1.41, 95% CI [1.08, 1.83], p = 0.011), compared to NNRTI-ART. Specifically, lopinavir/ritonavir (LPV/r) was associated with an increased risk of SGA (RR 1.40, 95% CI [1.18, 1.65], p = 0.003) and VSGA (RR 1.84, 95% CI [1.37, 2.45], p = 0.002), compared to efavirenz, but not compared to nevirapine. We found no evidence that any class of ART or specific “third drug” was associated with an increased risk of PTB.
Conclusion: Our findings support the recommendation of INSTI-ART as first-line ART regimen for use in pregnant WLHIV. However, the increased risks of SGA and VGSA associated with PI-ART, compared to NNRTI-ART, may impact choice of second- and third-line ART regimens in pregnancy.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021248987.
Introduction
In 2022, 39 million people were living with human immunodeficiency virus (HIV) worldwide, including 15.7 million women of childbearing age (1). An estimated 1.3 million women living with HIV (WLHIV) are pregnant each year, with 90% of these women residing in sub-Saharan Africa (1). Sub-Saharan Africa also has the highest rates of neonatal and child mortality (2). Globally, preterm birth (PTB) is the most important cause of neonatal and child mortality and morbidity (3). Babies born small for gestational age (SGA) contribute to 21.9% of neonatal deaths in low-income and middle-income countries (LMICs) (4). PTB and SGA are both causes of low birthweight (LBW), an outcome commonly used in LMICs when gestational age is uncertain (5). The United Nations’ Sustainable Development Goal 3 (SDG3) target 3.2 aspires to decrease neonatal and under-5 mortality to 12 and 25 per 1,000 live births, respectively, by 2030 (6). However, the vast majority of countries in sub-Saharan Africa are not on track to reach these goals (2). There is therefore an urgent need to address adverse perinatal outcomes that contribute to neonatal and child mortality in this region.
Pregnancies in untreated WLHIV are associated with an increased risk of adverse perinatal outcomes, including PTB, LBW, SGA, and stillbirth, compared to HIV-negative women (7). Antiretroviral therapy (ART, i.e., triple drug therapy) is crucial for WLHIV to improve maternal health and to reduce perinatal HIV transmission. In the past, preconception ART was initiated for maternal reasons (i.e., low CD4 count), whereas antenatal ART was initiated for either prevention of vertical HIV transmission (at high CD4 counts) or for maternal reasons (low CD4 count). In 2013 World Health Organization (WHO) recommended that all pregnant WLHIV should receive ART, irrespective of CD4 counts (8). This led to an increase in the global proportion of WLHIV receiving ART during pregnancy, reaching 81% in 2021 (1). Since 2015, WHO recommend that all people living with HIV should initiate lifelong ART, including pregnant women (9). This led to an increase in the proportion of pregnant WLHIV in sub-Saharan Africa who received ART at the time of conception, from 8% in 2010 to 56% in 2020 (10). However, pregnant WLHIV receiving ART remain at increased risk of PTB, spontaneous PTB, LBW, term LBW, SGA, and very SGA (VSGA), compared with HIV-negative women (11). The question as to whether different ART regimens are associated with different risks of adverse perinatal outcomes has long been controversial, with conflicting data reported (12, 13). In particular, protease inhibitors (PIs), specifically lopinavir/ritonavir (LPV/r), have been associated with an increased risk of PTB in some studies (14–16), but not in others (17–19).
ART consists of a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs) combined with a “third drug” of any class, including integrase strand transfer inhibitors (INSTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs, and NRTIs. WHO currently recommends INSTI dolutegravir(DTG)-based ART as preferred first-line regimen for adults, including pregnant women (20). NNRTI efavirenz(EFV)-based ART is an alternative first-line regimen. ART containing PIs, including LPV/r, atazanavir/ritonavir (ATV/r), or darunavir/ritonavir (DRV/r), are designated as second-line or third-line regimens (20). US guidelines recommend DTG-based, or DRV/r-based ART as preferred regimens in pregnancy, with raltegravir(RAL)-based, ATV/r-based, EFV-based or rilpivirin(RPV)-based ART as an alternative regimen (21). European guidelines recommend DTG-based or RAL-based ART or DRV/r-based ART in pregnancy, with EFV-based ART or RPV-based ART as alternative regimens (22).
A network meta-analysis of seven randomized controlled trials (RCTs) compared seven mono-, dual- and triple drug regimens initiated during pregnancy (23). Among the four ART (i.e., triple drug) regimens assessed, zidovudine(ZDV)/lamivudine(3TC)/LPV/r was associated with an increased risk of spontaneous PTB (sPTB) compared to zidovudine/lamivudine/abacavir (ZDV/3TC/ABC; a triple NRTI regimen which is no longer recommended) (24), but no other significant differences in perinatal outcomes between the ART regimens assessed were found. Recent RCTs of ART regimens initiated during pregnancy showed that DTG-based ART had superior virological efficacy compared to EFV-based ART (25, 26). Among regimens with the same backbone, no differences in composite perinatal outcomes were found between DTG-based ART and EFV-based ART, although there was an increase in neonatal death (NND) associated with EFV-based ART (25, 26). A further RCT showed that RAL-based ART also had superior virological efficacy compared to EFV-based ART and no differences in adverse perinatal outcomes were observed (27).
As the number of pregnant WLHIV receiving ART increases, understanding the impact of different ART regimens on perinatal outcomes is crucial. Antiretroviral treatment guidelines cite limited available data concerning pregnancy outcomes associated with antiretroviral drugs (20–22). Few RCTs of ART regimens in pregnancy have been conducted, which enrolled relatively small numbers of women and ART was initiated during the second half of pregnancy, thereby limiting exposure to ART and detection of perinatal outcomes. Observational studies provide important complimentary data, overcome some of the limitations of RCTs, and may provide a more accurate representation of ART regimens, timings of ART initiation, and pregnancy outcomes experienced by pregnant women in the real world. In order to fill this evidence gap, we conducted a systematic review and meta-analysis of observational studies to assess the comparative risk of a range of adverse perinatal outcomes associated with WLHIV receiving INSTI-ART, NNRTI-ART, PI-ART, and NRTI-ART, as well as specific “third drugs” from different ART classes.
Methods
Search strategy
We developed a systematic review and meta-analyses protocol based on the Cochrane guidelines (28). A comprehensive literature search strategy, developed by a specialist librarian (SK), was adapted to four electronic literature databases (PubMed, CINAHL (Ebscohost), Global Health (Ovid), EMBASE (Ovid)) to search for studies published between Jan 1, 1980 and July 14, 2023. Free text and controlled vocabulary search terms for “pregnancy outcomes,” “HIV,” and “antiretroviral drugs” were used. No methodological, country, or language filters were applied, and both full-text articles and abstracts were considered. Full search terms are detailed in Supplementary Appendix 1. Retrieved articles were imported into EndNote reference manager (EndNote X20; Clarivate Analytics, Philadelphia, Pennsylvania, United States) and deduplicated. Reference lists of included studies were assessed for additional relevant studies.
The systematic review is registered online (PROSPERO, CRD42021248987) and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplementary Checklist S1) (29).
Eligibility criteria
Studies were eligible if they included information on the association of pregnant WLHIV receiving different classes of ART with predefined perinatal outcomes. Inclusion criteria were study design (observational studies, i.e., cohort and case control studies), population (pregnant WLHIV), exposure (INSTI-ART, NNRTI-ART, PI-ART, or NRTI-ART) and comparator (different class of ART than the “exposure” group, i.e., INSTI-ART, NNRTI-ART, PI-ART, or NRTI-ART). ART was defined as antiretroviral triple drug therapy. INSTI-ART, NNRTI-ART, PI-ART, and NRTI-ART regimens were defined as two backbone drugs plus any type of INSTI, NNRTI, PI, or NRTI as a “third drug.” Studies were not included if additional treatment was received by one exposure/comparator group only (e.g., anti-tuberculosis treatment), or if less than 95% of WLHIV in an exposure or comparator group conformed to the exposure/comparator definition (e.g., <95% of WLHIV received NNRTI-ART). Preconception and/or antenatal initiation of ART was eligible. Perinatal outcomes assessed were: PTB (birth <37+0 weeks gestation) (30); very PTB (VPTB, birth <32+0 weeks gestation); sPTB (spontaneous birth <37+0 weeks gestation); LBW (<2,500 g) (31); very LBW (VLBW, <1,500 g); SGA (birthweight for gestational age < 10th centile) or very SGA (VSGA, birthweight for gestational age < 3rd centile) according to the reference chart used at the study site (4), stillbirth (delivery of an infant without any signs of life with birthweight ≥1,000 g or gestational age ≥ 24+0 weeks, or body length ≥ 35 cm) (32); and NND (death of an infant in the first 28 days of life) (32). Each perinatal outcome was analyzed as a separate outcome, irrespective of potential overlap between different outcomes (e.g., PTB and LBW). Data for term LBW and preterm LBW were sought, but no relevant data was found.
Study selection
Titles and abstracts of studies retrieved by the literature searches were screened by at least two independent investigators (KB, IC, CP, HS, MK, and ZB) to identify potentially relevant articles. Full text articles of relevant citations were obtained and assessed against the eligibility criteria. Studies were not included if outcomes were not defined or differed from our definitions. If a cohort was reported more than once, the most recent and complete data for each ART comparison and outcome was included. Ambiguities or disagreements regarding inclusion of studies were resolved through discussion with the senior investigator (JH).
Data extraction
From eligible studies data was extracted regarding study and population characteristics, ART exposures and perinatal outcomes by at least two investigators (KB, IC, CP, HS, MK, and ZB). Unadjusted perinatal outcome data according to class of ART exposure (e.g., NNRTI-ART vs. PI-ART), i.e., outcome frequencies according to ART class exposures, were collected.
In addition, reported unadjusted and adjusted risk ratios (RR) and odds ratios (OR) and 95% confidence intervals (CIs) were also extracted from each publication. If reported, perinatal outcome data according to specific “third drugs” from different classes (e.g., EFV-based ART vs. LPV/r-based ART) were also collected. In addition, methods used to adjust for confounders, including regression analysis, risk factor analysis, and matching (Supplementary Appendix 2) were extracted. Ambiguities or disagreements were resolved through discussion with the senior investigator (JH).
Quality assessment
The quality of individual studies was assessed using an adapted version of the Newcastle-Ottawa Scale by at least two investigators (KB, IC, CP, HS, MK, and ZB), and reviewed by the senior investigator (JH). Quality assessment criteria were: Selection of study participants (maximum 4 points), Comparability of comparator groups (maximum 2 points), and Assessment of outcomes of interest, including methods to assess gestational age at birth (maximum 3 points). Studies were classified as “good,” “average,” or “poor” quality according to predefined criteria (Supplementary Appendix 2).
Statistical analysis
Risks of adverse perinatal outcomes were compared between WLHIV receiving INSTI-ART, NNRTI-ART, PI-ART, and NRTI-ART. All analyses were conducted based on frequencies of perinatal outcomes among WLHIV receiving different classes of ART, as extracted from included studies. Pairwise meta-analyses using unadjusted perinatal outcome data from individual studies were carried out if two or more studies reported data for the same ART comparison (e.g., NNRTI-ART vs. PI-ART) and perinatal outcome (e.g., PTB), using a random-effects model to calculate a weighted summary effect estimate (RR), 95% CIs, and p-values. p < 0.05 were considered statistically significant. Meta-analyses were represented in forest plots and the I2 statistic was used to quantify heterogeneity due to clinical and methodological variability between studies. The degree of heterogeneity was classified as none (<25%), low (25–49%), moderate (50–74%), or high (≥75%). There are differences between high income countries (HICs) and low- and middle-income countries (LMICs), including HIV prevalence, environmental factors, genetics, and healthcare systems, which may impact the association between ART and perinatal outcomes. Study quality may also impact these associations by addressing bias and confounding. Pre-specified subgroup analyses were therefore performed to separately assess the associations of ART classes with perinatal outcomes in HICs and LMICs, and in average and poor quality studies. In addition, the interaction of country income status and study quality with the association between ART classes and perinatal outcomes was tested (Supplementary Appendix 4). Sensitivity analyses were carried out to assess the effect of adjustment for confounders. The Peters’ test was utilized to assess small study effects in meta-analyses containing a minimum of 10 studies. All statistical analyses were done with Stata version 17 (College Station, Texas, United States).
Results
The literature search yielded 108,720 citations, of which 30 studies were included that reported outcome data for pregnant WLHIV receiving INSTI-ART, NNRTI-ART, PI-ART, and NRTI-ART (Figure 1). The perinatal outcomes reported were PTB (22 studies), VPTB (8 studies), sPTB (3 studies), LBW (12 studies), VLBW (6 studies), SGA (15 studies), VSGA (5 studies), stillbirth (1 study), and NND (3 studies).
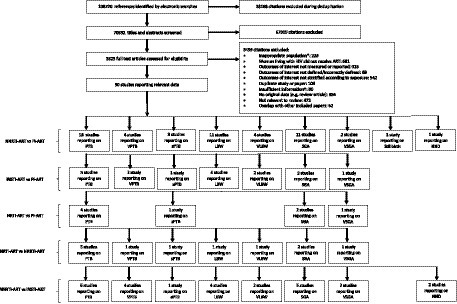
Figure 1. Study selection. *For example, women living with HIV were not pregnant. †For example, paper did not provide relevant outcome data. ART, antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LBW, low birthweight; NND, neonatal death; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor, PTB, preterm birth; SGA, small for gestational age; sPTB, spontaneous preterm birth; VLBW, very low birthweight; VPTB, very preterm birth; VSGA, very small for gestational age.
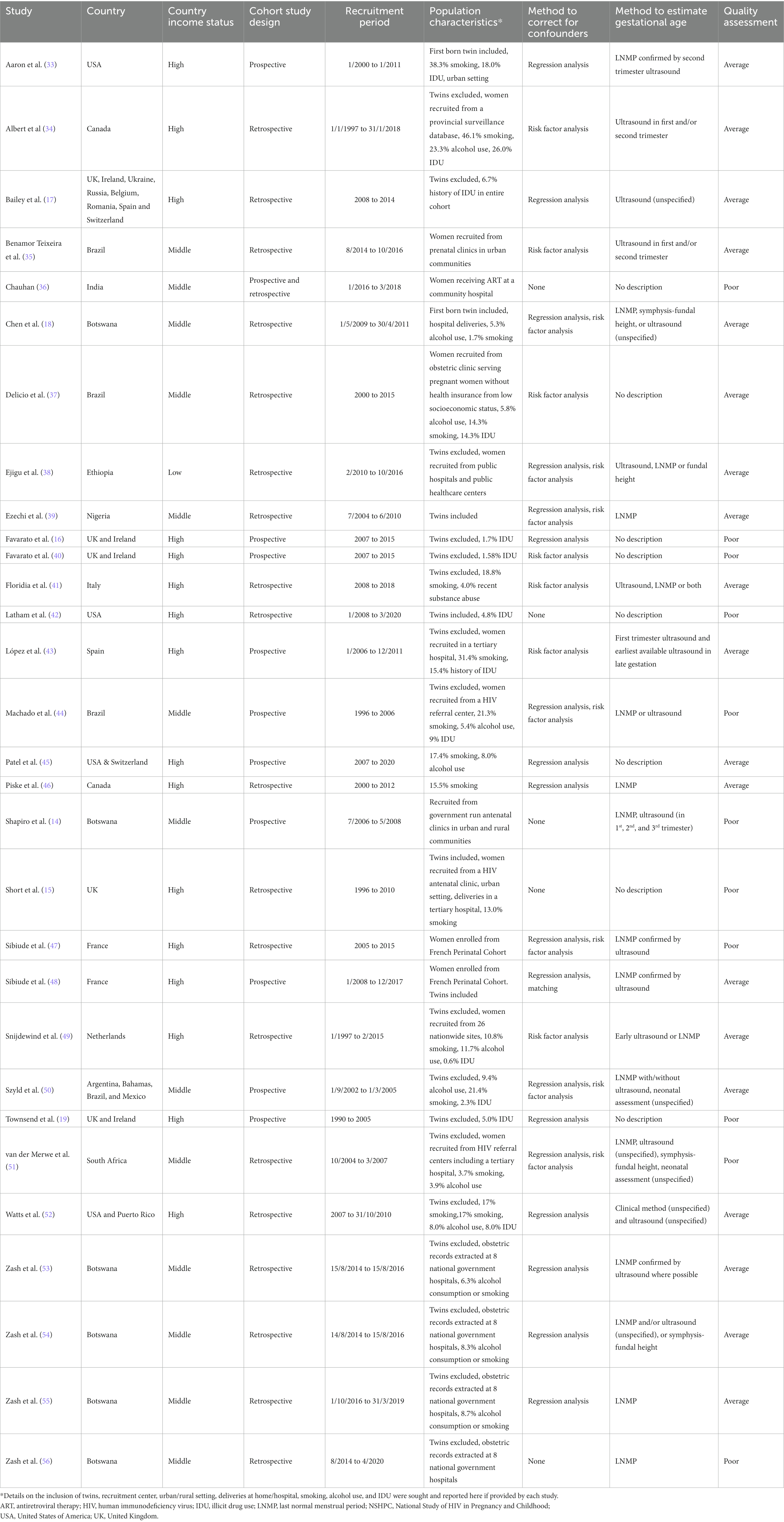
Characteristics of included studies, published in 2006–2022, are summarized in Table 1 (14–19, 33–56). Ten prospective (33%) and 20 retrospective (67%) cohort studies analyzed data from 222,312 pregnant women in 23 countries (Tables 1, 2). No relevant case–control studies were identified. Sixteen studies (53%) with 48,856 (22%) pregnant women were conducted in HICs, and 14 studies (47%) with 173,456 (78%) pregnant women were conducted in LMICs (Tables 1, 2). Twenty two studies (73%) reported the methods used to determine gestational age, with four (13%) studies using first trimester ultrasound, although none universally, the most accurate method of establishing gestational age (Table 1; Supplementary Appendix 2). Twenty five studies (83%) used methods to assess potential confounding factors. Regression analysis was conducted in 17 studies, risk factor analysis was carried out in 14 studies, and matching of participants was carried out in one study (Table 1; Supplementary Appendix 2). Of the 36 comparisons which were adjusted for covariates in individual studies, none resulted in a change in the statistical significance of the effect estimate (Supplementary Appendix 5). Quality assessments classified 11 studies (37%) as poor quality and 19 (63%) as average quality, with no studies deemed good quality (Table 1; Supplementary Appendix 2). Studies from LMICs had quality ratings (64% average, 36% poor quality) comparable to studies from HICs (63% average, 37% poor quality).
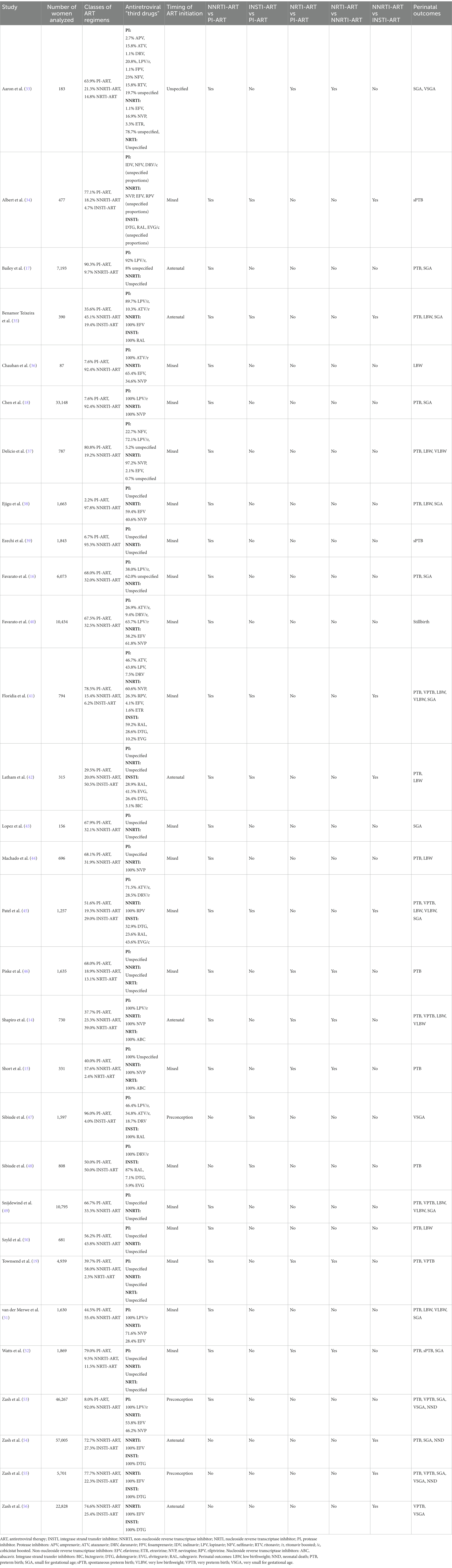
Table 2. Antiretroviral therapies, ART comparisons, and perinatal outcomes reported by studies included in the systematic review and meta-analysis.
ART regimens received by WLHIV, ART class comparisons reported, and perinatal outcomes analyzed are shown for each study in Table 2. ART regimes consisted of two backbone drugs plus a “third drug” that was either INSTI, NNRTI, PI, or NRTI. Twenty (67%) studies reported a mixture of preconception and antenatal ART initiation, with others reporting preconception (3 studies; 10%), antenatal (6 studies; 20%), or unspecified (1 study; 3%) ART initiation (Table 2). Random-effects meta-analyses were conducted to compare perinatal outcomes among WLHIV receiving INSTI-ART, NNRTI-ART, PI-ART, and NRTI-ART. The unadjusted summary effect estimates are presented in Figure 2 and the forest plots in Supplementary Appendix 3. Subgroup analyses were carried out according to country income status and study quality (Supplementary Appendix 4).
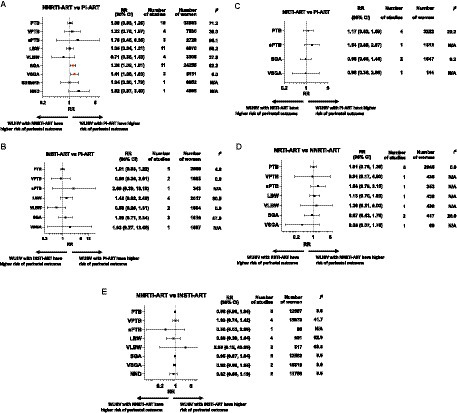
Figure 2. Perinatal outcomes of women living with HIV receiving different ART classes. Random-effects meta-analysis results for perinatal outcomes associated with women living with HIV receiving different classes of ART. Summary effect estimates for associations between each pair of ART classes and each perinatal outcome are shown. Unadjusted risk ratios (RR) and 95% confidence interval (95% CI), p-values, numbers of studies and women included in the analysis of each perinatal outcome are displayed. Forest plots of the meta-analyses, based on unadjusted outcome frequencies of perinatal outcomes according to class of ART exposure, can be found in Supplementary Appendix 3. (A) NNRTI-ART compared to PI-ART. (B) INSTI-ART compared to PI-ART. (C) NRTI-ART compared to PI-ART. (D) NRTI-ART compared to NNRTI-ART. (E) NNRTI-ART compared to INSTI-ART. ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; LBW, low birthweight; NND, neonatal death; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PTB, preterm birth; SGA, small for gestational age; sPTB, spontaneous preterm birth; VLBW, very low birthweight; VPTB, very preterm birth; VSGA, very small for gestational age; WLHIV, women living with HIV.
NNRTI-ART compared to PI-ART
Twenty five studies, including 134,373 pregnant women, reported on nine perinatal outcomes of WLHIV receiving NNRTI-ART compared to PI-ART (Figure 2A; Table 2). In the analysis of 32,883 WLHIV from 18 studies, PI-ART was not significantly associated with PTB compared to NNRTI-ART (RR 1.08, 95% CI [0.93, 1.25], p = 0.332) (Figure 2A). The association remained non-significant in subgroup analyses of studies conducted in LMICs and HICs, and average and poor quality studies (p for interaction = 0.332 and 0.322, respectively) (Supplementary Appendix 4). Specific “third drug” comparisons also did not identify any significant associations (Table 3A).
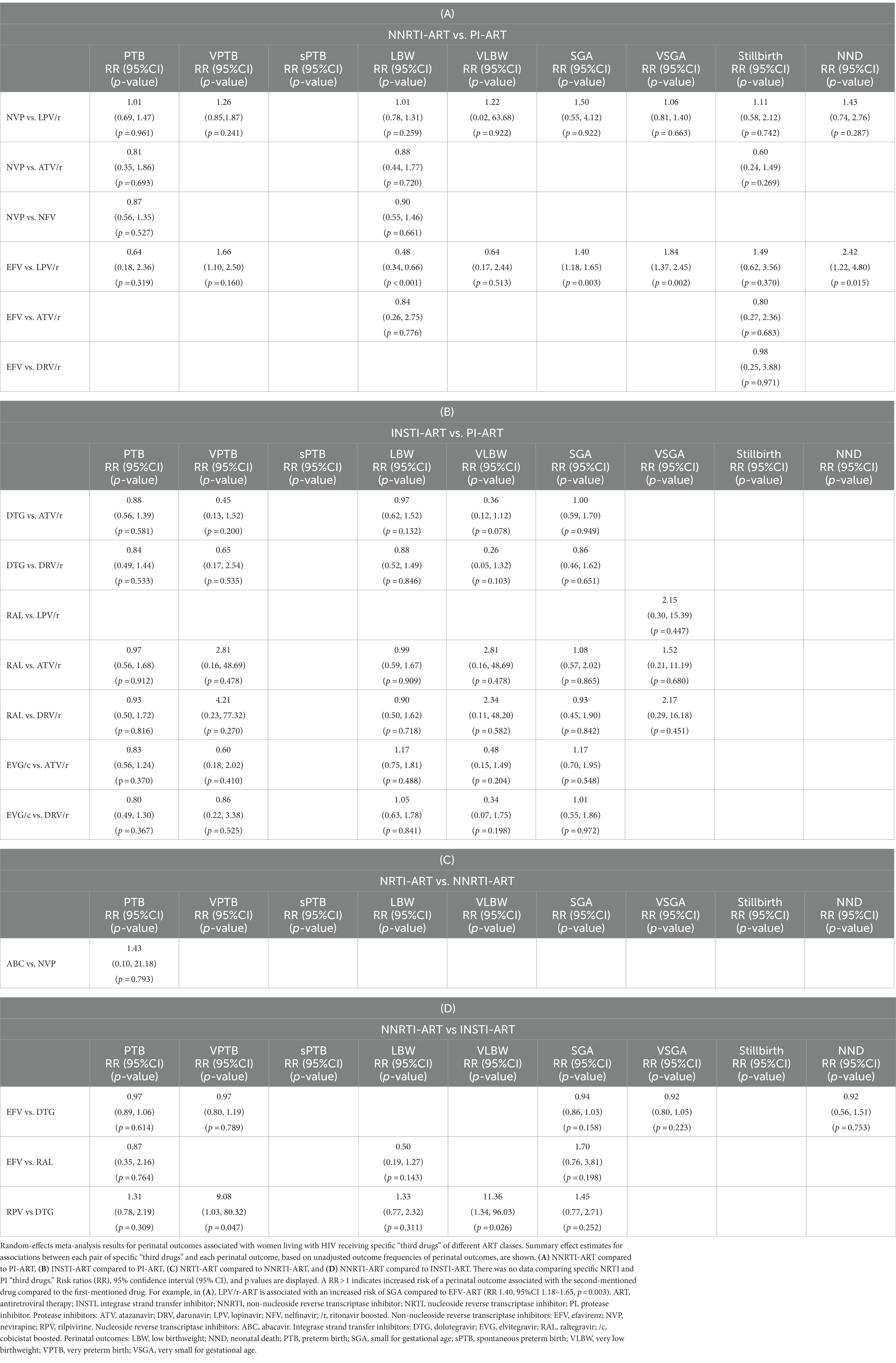
Table 3. Perinatal outcomes of women living with HIV receiving specific “third drugs” of different ART classes.
In the analysis of 7,530 WLHIV from four studies, PI-ART was not significantly associated with VPTB compared to NNRTI-ART (RR 1.22, 95% CI [0.76, 1.97], p = 0.413) (Figure 2A). However, LPV/r-based ART was significantly associated with VPTB compared to EFV-based ART (RR 1.66, 95%CI [1.10, 2.50], p = 0.016), but not NVP-based ART (RR 1.26, 95% CI [0.85, 1.87], p = 0.241) (Table 3A).
In three average quality studies containing 2,729 WLHIV, PI-ART was not significantly associated with sPTB compared to NNRTI-ART (RR 1.70 95% CI [0.45, 6.35], p = 0.388) (Figure 2A). One study analyzing 847 women from a LMIC reported an increased risk of sPTB for PI-ART compared to NNRTI-ART (RR 5.02, 95% CI [3.62, 6.98], p < 0.001) (Supplementary Appendix 4).
In the analysis of 6,870 WLHIV from 11 studies, PI-ART was not significantly associated with LBW compared to NNRTI-ART (RR 1.05, 95% CI [0.84, 1.31], p = 0.671) (Figure 2A). However, LPV/r-based ART, but not ATV/r-based ART, was significantly associated with a decreased risk of LBW compared to EFV-based ART (RR 0.48, 95% CI [0.34, 0.66], p < 0.001). LPV/r, ATV/r, and NFV were not significantly associated with LBW compared to NVP (Table 3A).
Four studies, including 3,308 WLHIV, reported no significant association of PI-ART with VLBW compared to NNRTI-ART (RR 0.71, 95% CI [0.35, 1.43], p = 0.355) (Figure 2A). However, two average quality studies, including 2087 WLHIV, conducted in HICs found that PI-ART was associated with a decreased risk of VLBW compared to NNRTI-ART (RR 0.58, 95% CI [0.34, 0.96], p = 0.034) (p for interaction = 0.335) (Supplementary Appendix 4).
In the analysis of 24,255 WLHIV from 11 studies, PI-ART was associated with a significantly increased risk of SGA compared to NNRTI-ART (RR 1.28, 95% CI [1.09, 1.51], p = 0.003) (Figure 2A). There was moderate heterogeneity (I2 62.0, 95% CI [0.0, 83.8]). In subgroup analysis, 7 studies (16,799 women) in HICs showed an increased risk of SGA (RR 1.20, 95% CI [1.03, 1.40], p = 0.018), whereas no difference in SGA was seen in 4 studies in LMICs (RR 1.34 95% CI [0.89, 2.02], p = 0.157) (p for interaction = 0.620) (Supplementary Appendix 4). The association remained significant in average quality studies (RR 1.19, 95% CI [1.01, 1.41], p = 0.040), but not in poor quality studies (RR 1.51 95% CI [0.91, 2.49], p = 0.107) (p for interaction = 0.380) (Supplementary Appendix 4). LPV/r-based ART was significantly associated with SGA compared to EFV-based ART (RR 1.40 95% CI [1.18, 1.65], p = 0.003), but not compared to NVP-based ART (RR 1.50 95% CI [0.55, 4.12], p = 0.922) (Table 3A).
Similarly, in the analysis of 5,151 women from two average quality studies, PI-ART was associated with a significantly increased risk of VSGA compared to NNRTI-ART (RR 1.41 95% CI [1.08, 1.83], p = 0.011) (Figure 2A). This association remained significant in the study from a LMIC (RR 1.37 95% CI [1.05, 1.80], p = 0.020), but not in the study conducted in a HIC (RR 2.83 95% CI [0.69, 11.72], p = 0.150) (p for interaction = 0.332). LPV/r-based ART was significantly associated with VSGA compared to EFV-based ART (RR 1.84 95% CI [1.37, 2.45], p = 0.002), but not compared to NVP-based ART (RR 1.06 95% CI [0.81, 1.40], p = 0.663) (Table 3A).
In one poor quality study conducted in a HIC analyzing 6,952 women, PI-ART was not associated with stillbirth compared to NNRTI-ART (RR 1.04 95% CI [0.60, 1.79], p = 0.891) (Figure 2A). In one average quality study conducted in an LMIC analyzing 4,495 women, PI-ART was not associated with NND (RR 1.82 95% CI [0.97, 3.40], p = 0.063) (Figure 2A).
INSTI-ART compared to PI-ART
Seven studies, including 5,638 women, reported on seven perinatal outcomes of WLHIV receiving PI-ART compared to INSTI-ART (Figure 2B; Table 2). In the analysis of 2,860 WLHIV from five studies, PI-ART was not significantly associated with PTB compared to INSTI-ART (RR 1.01 95% CI [0.83, 1.22], p = 0.941) (Figure 2B).
In the analysis of 1,685 WLHIV from two average quality studies conducted in HICs, PI-ART was not significantly associated with VPTB compared to INSTI-ART (RR 0.85 95% CI [0.36, 2.01], p = 0.714) (Figure 2B).
One average quality study conducted in a HIC, including 343 WLHIV, found no significant association of PI-ART with sPTB compared to INSTI-ART (RR 2.66 95% CI [0.39, 18.18], p = 0.391) (Figure 2B).
Four studies, including 2,017 WLHIV, reported no significant association of PI-ART with LBW compared to INSTI-ART (RR 1.42 95% CI [0.82, 2.48], p = 0.211) (Figure 2B). However, one study, including 202 WLHIV, conducted in a LMIC found that PI-ART was associated with an increased risk of LBW compared to INSTI-ART (RR 2.67, 95% CI [1.03, 6.91], p = 0.043) (p for interaction = 0.211) (Supplementary Appendix 4).
Two average quality studies conducted in HICs, including 1,654 WLHIV, reported no significant association of PI-ART with VLBW compared to INSTI-ART (RR 0.58, 95% CI [0.26, 1.31], p = 0.190) (Figure 2B).
Three average equality studies, including 1,810 WLHIV, reported no significant association of PI-ART with SGA compared to INSTI-ART (RR 1.29 95% CI [0.71, 2.34], p = 0.408) (Figure 2B).
A single poor quality study of 1,587 WLHIV conducted in a HIC reported no significant association of PI-ART with VSGA compared to INSTI-ART (RR 1.93 95% CI [0.27, 13.66], p = 0.508) (Figure 2B).
The results for perinatal outcomes of WLHIV receiving PI-ART compared to INSTI-ART were reflected in the analyses of “third drugs,” with ATV/r-, DRV/r- or LPV/r-based ART not significantly associated with any adverse perinatal outcomes when compared to DTG-, RAL- or EVG/c-based ART (Table 3B).
NRTI-ART compared to PI-ART
Six studies containing 9,687 women reported on four perinatal outcomes in WLHIV receiving NRTI-ART compared to PI-ART (Figure 2C; Table 2). In four studies including 3,222 women, PI-ART was not significantly associated with PTB compared to NRTI-ART (RR 1.17 95% CI [0.82, 1.69], p = 0.379). Similarly, there was no significant association of PI-ART with sPTB (RR 1.54 95% CI [0.89, 2.67], p = 0.122), SGA (RR 0.98 95% CI [0.66, 1.46], p = 0.906) or VSGA (RR 0.98 95% CI [0.36, 2.68], p = 0.970), compared to NRTI-ART (Figure 2C). There were no data comparing specific NRTI and PI “third drugs.”
NRTI-ART compared to NNRTI-ART
Six studies containing 9,687 women reported on four perinatal outcomes in WLHIV receiving NNRTI-ART compared to NRTI-ART (Figure 2D; Table 2). In five studies including 2,946 women, there was no significant association of NNRTI-ART with PTB compared to NRTI-ART (RR 1.01 95% CI [0.76, 1.36], p = 0.931) (Figure 2D). Similarly, ABC-based ART was not significantly associated with PTB compared to NVP-based ART (RR 1.43 95% CI [0.10, 21.18], p = 0.793) (Table 3C).
In the analysis of 439 women from a single poor quality study from a LMIC, NNRTI-ART was not significantly associated with VPTB compared to NRTI-ART (RR 0.91 95% CI [0.17, 4.90], p = 0.910) (Figure 2D).
A single average quality study with 353 women from a HIC found no significant association of NNRTI-ART with sPTB compared to NRTI-ART (RR 1.58 95% CI [0.79, 3.15], p = 0.196) (Figure 2D).
In the analysis of 439 women from a single poor quality study conducted in a LMIC, NNRTI-ART was not significantly associated with LBW (RR 1.13 95% CI [0.70, 1.83], p = 0.625) or VLBW (RR 1.36 95% CI [0.31, 6.00], p = 0.684) compared to NRTI-ART (Figure 2D).
In the analysis of 417 women from a single average quality study from a HIC, NNRTI-ART was not significantly associated with SGA compared to NRTI-ART (RR 0.87, 95% CI [0.43, 1.78], p = 0.454) (Figure 2D). Likewise, in the analysis of 66 women from a single average quality study from a HIC, NNRTI-ART was not significantly associated with VSGA compared to NRTI-ART (RR 0.35 95% CI [0.07, 1.76], p = 0.201) (Figure 2D).
NNRTI-ART compared to INSTI-ART
Eight studies including 88,767 women reported on eight perinatal outcomes in WLHIV receiving NNRTI-ART compared to INSTI-ART (Figure 2E; Table 2). In 12,687 women from 6 studies, INSTI-ART was not significantly associated with PTB compared to NNRTI-based ART (RR 0.98, 95% CI [0.90, 1.06], p = 0.622) (Figure 2E). Similarly, no specific INSTI drugs (DTG and RAL) were associated with PTB compared to specific NNRTI drugs (EFV and RPV) (Table 3D).
In 15,979 women from four average quality studies, INSTI-ART was not significantly associated with VPTB compared to NNRTI-based ART (RR 1.03, 95% CI [0.74, 1.42], p = 0.879) (Figure 2E). However, DTG-based ART was associated with VPTB compared to RPV-based ART (RR 9.08, 95% CI [1.03, 80.32], p = 0.047), but not compared to EVF-based ART (RR 0.97, 95% CI [0.80, 1.19], p = 0.789) (Table 3D).
In one average quality study from a HIC containing 96 women, INSTI-ART was not associated with sPTB compared to NNRTI-ART (RR 0.36, 95% CI [0.05, 2.60], p = 0.312) (Figure 2E).
In the analysis of 931 women from four studies, INSTI-ART was not significantly associated with LBW compared to NNRTI-ART (RR 0.81, 95% CI [0.40, 1.62], p = 0.551) (Figure 2E).
In the analysis of 517 women from two studies, INSTI-ART was not significantly associated with VLBW compared to NNRTI-ART (RR 2.88, 95% CI [0.19, 43.09], p = 0.443) (Figure 2E). However, DTG-based ART was associated with VLBW compared to RPV-based ART (RR 11.36, 95% CI [1.34, 96.03], p = 0.026) (Table 3D).
In the analysis of 12,582 women from five average quality studies, INSTI-ART was not significantly associated with SGA compared to NNRTI-ART (RR 0.95, 95% CI [0.87, 1.04], p = 0.268)(Figure 2E). Similarly, DTG-based ART and RAL-based ART were not associated with SGA compared to EFV-based ART (Table 3D).
Furthermore, two average quality studies analyzing 15,519 women in LMICs found that.
INSTI-ART was not significantly associated with VSGA compared to NNRTI-ART (RR 0.92, 95% CI [0.80, 1.05], p = 0.223) (Figure 2E).
Two average quality studies conducted in a LMIC including 11,756 women found that INSTI-ART was not significantly associated with NND compared to NNRTI-ART (RR 0.82, 95% CI [0.56, 1.19], p = 0.288) (Figure 2E).
Discussion
Our meta-analysis found no evidence that INSTI-ART is associated with any adverse perinatal outcomes assessed compared to NNRTI-ART and PI-ART. We also found that PI-ART is associated with a significantly increased risk of SGA and VSGA, compared to NNRTI-ART. Specifically, LPV/r was associated with an increased risk of SGA and VSGA, compared to EFV. We found no evidence that any class of ART or specific “third drug” was associated with an increased risk of PTB. Our findings should inform clinical guidelines and support the recommendation of INSTI-ART as first-line ART regimen for use in pregnant WLHIV. However, the increased risks of SGA and VGSA associated with PI-ART, compared to NNRTI-ART, may impact choice of second- and third-line ART regimens in pregnancy.
The lack of association between INSTI-ART and any adverse perinatal outcomes, compared to NNRTI-ART and PI-ART, is reassuring. This confirms the findings from RCTs which showed that, among regimens with the same backbone, no differences in composite perinatal outcomes were found with DTG-based ART or RAL-based ART compared to EFV-based ART, although DTG-based ART was associated with a decrease in NND compared to EFV-based ART (25–27). A regimen containing DTG, emtricitabine (FTC) and tenofovir alafenamide fumarate (TAF) had the lowest rate of composite adverse pregnancy outcomes, compared to DTG/FTC/TDF and EFV/FTC/TDF, and a lower rate of preterm birth compared to EFV/FTC/TDF (26). As both DTG-based ART and RAL-based ART had superior virological efficacy compared to EFV-based ART, these findings support the recommendation of INSTI-based ART as first-line ART regimen for use in pregnant WLHIV (20–22).
The finding that PI-ART is associated with a significantly increased risk of SGA and VSGA, compared to NNRTI-ART, but not compared to INSTI-ART, extends findings from a previous meta-analysis which reported that PI-ART was associated with SGA and VSGA compared to non-PI-ART (57). Furthermore, our findings show that LPV/r is associated with an increased risk of SGA and VSGA, compared to EFV, but not compared to NVP. A previous network meta-analysis of RCTs reported no significant differences in perinatal outcomes in LPV/r-based ART compared to EFV-based ART regimens, which may be due to the small numbers of WLHIV enrolled and/or the initiation of ART late in pregnancy (23). We found no cohort studies comparing the risk of SGA or VSGA for ATV/r or DRV/r, the referred PIs in several guidelines (21, 22), with either EFV or NVP. A previous meta-analysis of cohort studies found no significant differences in perinatal outcomes between ART regimens containing LPV/r, ATV/r, and DRV/r (57), and LPV/r is the only PI analyzed in RCTs conducted in pregnant WLHIV to date (23). Overall, these findings support EFV-based ART in preference of PI-based ART regimens and may impact choice of second- and third-line ART regimen for use in pregnant WLHIV. The increased risk of SGA associated with PI-ART may be a consideration for increased antenatal surveillance of fetal growth to enable timely diagnosis and intervention to improve perinatal outcomes.
We found no evidence that NRTI-ART is associated with any adverse perinatal outcomes compared to NNRTI-ART and PI-ART, and no cohort studies compared NRTI-ART with INSTI-ART. A previous network meta-analysis of RCTs reported that ZDV/3TC/LPV/r was associated with an increased risk of sPTB compared to the NRTI-ART regimen ZDV/3TC/ABC (23, 24). Although NRTI-ART is no longer recommended by most treatment guidelines, NRTI-ART regimens may still be used in settings where recommended ART regimens are not available.
The potential risk of PTB associated with maternal HIV infection and ART has received much attention (12). It is noteworthy and reassuring that we found no evidence that any class of ART or specific “third drug” was associated with an increased risk of PTB. This finding is supported by the largest numbers of studies and WLHIV in our analyses, compared to other perinatal outcomes assessed. However, it remains the case that WLHIV receiving ART are at higher risk of PTB compared to WLHIV receiving ZDV monotherapy and HIV-negative women (11, 58). Our findings indicate that choice of ART regimen does not impact the elevated risk of PTB among pregnant WLHIV and hence other interventions are urgently needed to reduce the burden of PTB among WLHIV.
Our meta-analysis has a number of strengths. To our knowledge, this is the first systematic review and meta-analysis comparing all classes of ART, including INSTI-ART, as well as comparing specific “third drugs” from different ART classes. Our study is the largest to date, assessing a comprehensive range of nine perinatal outcomes in WLHIV receiving different classes of ART, including 222,312 pregnant WLHIV from 30 studies. Our study overcame several methodological limitations of previous studies by conducting quality assessments, subgroup and sensitivity analyses, and assessment of correction for confounders (59–63). In particular, the higher quality studies confirmed our findings in the main analyses. 78% of WLHIV analyzed were from LMICs, lending external validity to our findings. Exposures and outcomes were predefined to minimize selection and misclassification bias and promote consistency across studies. A random-effects meta-analysis model was used to account for different study settings. Where applicable, the Peters’ test confirmed an absence of small study effects.
Studies included in our meta-analysis had a number of limitations. All studies were observational and therefore associated with risks of bias, including indication bias linked to WLHIV receiving second- and third-line regimens being more likely to have failed other regimens. Moreover, indication bias may have played a role in relation to the timing of ART initiation. Prior to the current universal treatment policy, preconception ART was initiated for maternal reasons (i.e., low CD4 count), whereas antenatal ART was initiated for either prevention of vertical HIV transmission (at high CD4 counts) or for maternal reasons (low CD4 count). A recent meta-analysis reported that preconception ART initiation was associated with an increased risk of PTB, but no other outcomes, compared to antenatal ART initiation, which may have impacted some of our analyses (64). Most studies (67%) in our meta-analysis included a mix of preconception and antenatal initiation of ART and in the absence of individual patient data it was not possible for us to compare or stratify outcomes for WLHIV initiating ART preconception and antenatally in these studies. For this reason, we were unable to conduct subgroup analyses according to timing of ART initiation. Chronological bias also may have impacted our results. Included studies recruited WLHIV over the past three decades and over this time there have been overall improvements in nutrition, income level, and medical care, which may have affected results obtained in different time periods, especially since the relatively recent introduction of INSTIs. We could not assess the effect of certain important confounders (e.g., CD4 cell count), because of limited reporting of these confounders in included studies. However, we extensively assessed the methods used to assess potential confounding in each study and found that adjustment for covariates by regression analysis did not result in any changes in the significance of the effect estimates in individual studies. However, residual confounding cannot be excluded. There was no data for the comparison of INSTI-ART with NRTI-ART. The perinatal outcomes VPTB, sPTB, VLBW, VSGA, stillbirth and NND were reported in a limited number of studies (1–4 studies) for each ART class comparison. VPTB, VLBW, and VSGA are subsets of the main outcomes (PTB/LBW/SGA) and these outcomes are therefore not independent of each other. VPTB, VLBW, and VSGA represent more severe outcomes, which occur less frequently and are less frequently reported, but which are associated with higher mortality and morbidity. There were fewer studies reporting perinatal outcomes for ART regimens containing specific “third drugs” and the results from these analyses are therefore less reliable. Some confidence intervals were large, indicating significant uncertainty regarding the true values of some effect estimates and a likelihood that effect estimates may change as more data become available in the future. Our analysis was limited to the “third drugs” in triple drug ART regimens and we did not assess the ART backbone. It is possible that backbone drugs differed between ART classes and drugs compared in our analyses and that our findings in part reflect the backbones used and possible interactions between “third drugs” and backbones (26). Unfortunately, data on perinatal outcomes associated with completely defined ART regimens is very limited and should be improved in future studies. Moreover, no study used a universal first trimester ultrasound, the most accurate method to assess gestational age (65). Imprecise assessment of gestational age may have resulted in misclassification bias for PTB, VPTB, SGA and VSGA. SGA and VSGA were defined according to the charts used at individual study sites, rather than an international reference standard (66), which limits comparability of results from different studies. Finally, differences in populations and settings between studies may have contributed to the heterogeneity observed in some of our analyses.
The mechanisms underlying the association between HIV, ART and adverse perinatal outcomes in general, and the link between PI-ART and SGA/VSGA in particular, remain poorly understood. SGA may be due to fetal growth restriction, which may be secondary to placental dysfunction (67). Placental dysfunction may result from altered placental angiogenesis, maternal or placental vascular malperfusion, or metabolic abnormalities, which have been linked to PI-based ART exposure (68). Pre-eclampsia is an important cause of growth restriction and SGA, but maternal HIV infection does not appear to be associated with an increased risk of pre-eclampsia and evidence regarding ART regimens is inconclusive (69). Given the immunodeficiency associated with HIV infection, an immune mechanism of adverse pregnancy outcomes appears plausible. CD4 depletion and chronic immune activation associated with HIV infection may impact the immunological program of pregnancy (70). Innate immune cells, including innate lymphoid cells, mucosal associated invariant T cells and gamma delta γδ T cells, have been reported to be decimated during early HIV infection and not recover with ART, and may be linked to adverse perinatal outcomes (71–73). WLHIV receiving ART have distinct systemic cytokine profiles throughout pregnancy, which may be associated with SGA (74). A recent review extensively examined the current evidence for the potential effects of PIs on progesterone levels, and effects on placenta and decidua (68). It has been reported that WLHIV receiving PI-ART have lower plasma progesterone levels, which may be due to effects of PIs on placental cytochrome P450 enzymes and/or increase in placental expression of 20-alpha-hydroxysteroid dehydrogenase, which inactivates progesterone (68, 75). In both mouse-models and WLHIV receiving PI, reduced progesterone levels are associated with increased risk of SGA (76). A recent RCT of progesterone supplementation in pregnant WLHIV on ART (mostly NNRTI-ART, only 3% PI-ART) showed that administration of 17-alpha-hydroxyprogesterone had no effect on the primary outcomes of PTB or stillbirth. However, progesterone supplementation was instead associated with a reduction in the risk of VSGA, a finding that requires confirmation in additional studies (77).
Given the limited data available for several ART comparisons and perinatal outcomes, it is clear that more and larger prospective observational pregnancy studies among WLHIV are needed to compare different ART regimens. This is particularly important for new antiretroviral drugs, including long-acting antiretrovirals, such as cabotegravir, dual drug regimens, and monoclonal antibodies, for which very limited data in pregnancy is available (78). Moreover, more data is urgently needed regarding antiretroviral drugs used as part of pre-exposure prophylaxis (PrEP) by pregnant HIV-negative women (79). A full range of perinatal outcomes should be assessed, as it is evident that ART regimens differentially impact distinct perinatal outcomes (80). Long-term follow-up is essential to assess effects of intrauterine ART exposure on growth and neurodevelopment of HIV-exposed uninfected children (81).
ART in pregnancy has important benefits for maternal health, prevention of vertical HIV transmission, and prevention of horizontal HIV transmission (20). It is clear that pregnant WLHIV receiving ART remain at increased risk of adverse perinatal outcomes compared to HIV-negative women (11). Further studies are urgently needed to elucidate the mechanisms underlying the adverse perinatal outcomes and to develop preventative and therapeutic interventions to improve perinatal outcomes of WLHIV.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KB: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. IC: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. CP: Data curation, Methodology, Writing – review & editing. HS: Data curation, Writing – review & editing. MK: Data curation, Writing – review & editing. ZB: Data curation, Writing – review & editing. SK: Data curation, Writing – review & editing, Methodology. JH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1323813/full#supplementary-material
References
2. Sharrow, D, Hug, L, You, D, Alkema, L, Black, R, Cousens, S, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN inter-agency Group for Child Mortality Estimation. Lancet Glob Health. (2022) 10:e195–206. doi: 10.1016/S2214-109X(21)00515-5
3. Perin, J, Mulick, A, Yeung, D, Villavicencio, F, Lopez, G, Strong, KL, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. (2022) 6:106–15. doi: 10.1016/S2352-4642(21)00311-4
4. Lee, AC, Kozuki, N, Cousens, S, Stevens, GA, Blencowe, H, Silveira, MF, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ. (2017) 358:j3677. doi: 10.1136/bmj.j3677
5. Lee, AC, Katz, J, Blencowe, H, Cousens, S, Kozuki, N, Vogel, JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. (2013) 1:e26–36. doi: 10.1016/S2214-109X(13)70006-8
6. United Nations. Transforming our world: The 2030 agenda for sustainable development. Department of Economic and Social Affairs. (2015). Available online at: https://sdgs.un.org/2030agenda. (Accessed February 14, 2024).
7. Wedi, CO, Kirtley, S, Hopewell, S, Corrigan, R, Kennedy, SH, and Hemelaar, J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. (2016) 3:e33–48. doi: 10.1016/S2352-3018(15)00207-6
8. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva: World Health Organisation (2013).
9. WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2nd ed. Geneva: World Health Organisation (2016).
10. Murray, C, Portwood, C, Sexton, H, Kumarendran, M, Brandon, Z, Kirtley, S, et al. Adverse perinatal outcomes attributable to HIV in sub-Saharan Africa from 1990 to 2020: systematic review and meta-analyses. Commun Med. (2023) 3:103. doi: 10.1038/s43856-023-00331-8
11. Portwood, C, Murray, C, Sexton, H, Kumarendran, M, Brandon, Z, Johnson, B, et al. Adverse perinatal outcomes associated with HAART and monotherapy. AIDS. (2022) 36:1409–27. doi: 10.1097/QAD.0000000000003248
12. Mofenson, LM. Antiretroviral therapy and adverse pregnancy outcome: the elephant in the room? J Infect Dis. (2016) 213:1051–4. doi: 10.1093/infdis/jiv390
13. Bailey, H, Zash, R, Rasi, V, and Thorne, C. HIV treatment in pregnancy. Lancet HIV. (2018) 5:e457–67. doi: 10.1016/S2352-3018(18)30059-6
14. Shapiro, RL, Hughes, MD, Ogwu, A, Kitch, D, Lockman, S, Moffat, C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. (2010) 362:2282–94. doi: 10.1056/NEJMoa0907736
15. Short, CE, Douglas, M, Smith, JH, and Taylor, GP. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother-to-child transmission. HIV Med. (2014) 15:233–8. doi: 10.1111/hiv.12083
16. Favarato, G, Townsend, CL, Bailey, H, Peters, H, Tookey, PA, Taylor, GP, et al. Protease inhibitors and preterm delivery: another piece in the puzzle. AIDS. (2018) 32:243–52. doi: 10.1097/QAD.0000000000001694
17. Bailey, H. Nucleoside reverse transcriptase inhibitor backbones and pregnancy outcomes. AIDS. (2019) 33:295–304. doi: 10.1097/QAD.0000000000002039
18. Chen, JY, Ribaudo, HJ, Souda, S, Parekh, N, Ogwu, A, Lockman, S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. (2012) 206:1695–705. doi: 10.1093/infdis/jis553
19. Townsend, CL, Cortina-Borja, M, Peckham, CS, and Tookey, PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS. (2007) 21:1019–26. doi: 10.1097/QAD.0b013e328133884b
20. WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization. (2021).
21. Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States. Department of Health and Human Services. (2024) Available at: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new. (Accessed 14 February 2024).
22. European AIDS Clinical Society. Treatment of pregnant women living with HIV or women considering pregnancy EACS Guidelines (2022).
23. Tshivuila-Matala, COO, Honeyman, S, Nesbitt, C, Kirtley, S, Kennedy, SH, and Hemelaar, J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS. (2020) 34:1643–56. doi: 10.1097/QAD.0000000000002593
24. Powis, KM, Kitch, D, Ogwu, A, Hughes, MD, Lockman, S, Leidner, J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. (2011) 204:506–14. doi: 10.1093/infdis/jir307
25. Kintu, K, Malaba, TR, Nakibuka, J, Papamichael, C, Colbers, A, Byrne, K, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. (2020) 7:e332–9. doi: 10.1016/S2352-3018(20)30050-3
26. Lockman, S, Brummel, SS, Ziemba, L, Stranix-Chibanda, L, McCarthy, K, Coletti, A, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. (2021) 397:1276–92. doi: 10.1016/S0140-6736(21)00314-7
27. João, EC, Morrison, RL, Shapiro, DE, Chakhtoura, N, Gouvèa, MIS, de Lourdes, BTM, et al. Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial. Lancet HIV. (2020) 7:e322–31. doi: 10.1016/S2352-3018(20)30038-2
28. Higgins, J ed. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell Publishing (2008).
29. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
30. Chawanpaiboon, S, Vogel, JP, Moller, AB, Lumbiganon, P, Petzold, M, Hogan, D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
31. Blencowe, H, Krasevec, J, de Onis, M, Black, RE, An, X, Stevens, GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. (2019) 7:e849–60. doi: 10.1016/S2214-109X(18)30565-5
32. GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1725–74. doi: 10.1016/S0140-6736(16)31575-6
33. Aaron, E, Bonacquisti, A, Mathew, L, Alleyne, G, Bamford, LP, and Culhane, JF. Small-for-gestational-age births in pregnant women with HIV, due to severity of HIV disease, not antiretroviral therapy. Infect Dis Obstet Gynecol. (2012) 2012:135030:1–9. doi: 10.1155/2012/135030
34. Albert, AYK, Elwood, C, Wagner, EC, Pakzad, Z, Chaworth-Musters, T, Berg, K, et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS. (2020) 34:719–27. doi: 10.1097/QAD.0000000000002464
35. Benamor Teixeira, ML, Fuller, TL, Da Silveira, F, Gouvêa, MI, Santos Cruz, ML, Ceci, L, et al. Efficacy of three antiretroviral regimens initiated during pregnancy: clinical experience in Rio de Janeiro. Antimicrob Agents Chemother. (2020) 64:e01068–20. doi: 10.1128/AAC.01068-20
36. Chauhan, N, Desai, M, Shah, S, Shah, A, and Gadhavi, R. Treatment outcome of different antiretroviral drug regimens in HIV-positive pregnant women. Perspect Clin Res. (2021) 12:40–7. doi: 10.4103/picr.PICR_74_19
37. Delicio, AM, Lajos, GJ, Amaral, E, Cavichiolli, F, Polydoro, M, and Milanez, H. Adverse effects in children exposed to maternal HIV and antiretroviral therapy during pregnancy in Brazil: a cohort study. Reprod Health. (2018) 15:76. doi: 10.1186/s12978-018-0513-8
38. Ejigu, Y, Magnus, JH, Sundby, J, and Magnus, MC. Pregnancy outcome among HIV-infected women on different antiretroviral therapies in Ethiopia: a cohort study. BMJ Open. (2019) 9:e027344. doi: 10.1136/bmjopen-2018-027344
39. Ezechi, OC, David, AN, Gab-Okafor, CV, Ohwodo, H, Oladele, DA, Kalejaiye, OO, et al. Incidence of and socio-biologic risk factors for spontaneous preterm birth in HIV positive Nigerian women. BMC Pregnancy Childbirth. (2012) 12:93. doi: 10.1186/1471-2393-12-93
40. Favarato, G, Townsend, CL, Peters, H, Sconza, R, Bailey, H, Cortina-Borja, M, et al. Stillbirth in women living with HIV delivering in the United Kingdom and Ireland: 2007-2015. J Acquir Immune Defic Syndr. (1999) 82:9–16. doi: 10.1097/QAI.0000000000002087
41. Floridia, M, Dalzero, S, Giacomet, V, Tamburrini, E, Masuelli, G, Savasi, V, et al. Pregnancy and neonatal outcomes in women with HIV-1 exposed to integrase inhibitors, protease inhibitors and non-nucleoside reverse transcriptase inhibitors: an observational study. Infection. (2020) 48:249–58. doi: 10.1007/s15010-019-01384-5
42. Latham, AH, Nissim, OA, Spitznagel, MC, Kirk, SE, Tarleton, JL, and Lazenby, GB. Impact of integrase Strand transfer inhibitor use during pregnancy on viral suppression at delivery and infant outcomes: a statewide retrospective cohort study. J Acquir Immune Defic Syndr. (2022) 89:448–53. doi: 10.1097/QAI.0000000000002882
43. López, M, Palacio, M, Goncé, A, Hernàndez, S, Barranco, FJ, García, L, et al. Risk of intrauterine growth restriction among HIV-infected pregnant women: a cohort study. Eur J Clin Microbiol Infect Dis. (2015) 34:223–30. doi: 10.1007/s10096-014-2224-6
44. Machado, ES, Hofer, CB, Costa, TT, Nogueira, SA, Oliveira, RH, Abreu, TF, et al. Pregnancy outcome in women infected with HIV-1 receiving combination antiretroviral therapy before versus after conception. Sex Transm Infect. (2009) 85:82–7. doi: 10.1136/sti.2008.032300
45. Patel, K, Huo, Y, Jao, J, Powis, KM, Williams, PL, Kacanek, D, et al. Dolutegravir in pregnancy as compared with current HIV regimens in the United States. N Engl J Med. (2022) 387:799–809. doi: 10.1056/NEJMoa2200600
46. Piske, M, Qiu, AQ, Maan, EJ, Sauvé, LJ, Forbes, JC, Alimenti, A, et al. Preterm birth and antiretroviral exposure in infants HIV-exposed uninfected. Pediatr Infect Dis J. (2021) 40:245–50. doi: 10.1097/INF.0000000000002984
47. Sibiude, JOD, Tubiana, R, Blanche, S, Dollfus, C, Frange, P, Le Chenadec, J, et al. Comparisonof four classical PI- and raltegravir-based regimens during pregnancy. Top. Antiv. Med. (2018):359s.
48. Sibiude, J, Le Chenadec, J, Mandelbrot, L, Dollfus, C, Matheron, S, Lelong, N, et al. Risk of birth defects and perinatal outcomes in HIV-infected women exposed to integrase strand inhibitors during pregnancy. AIDS. (2021) 35:219–26. doi: 10.1097/QAD.0000000000002719
49. Snijdewind, IJM, Smit, C, Godfried, MH, Bakker, R, Nellen, J, Jaddoe, VWV, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS One. (2018) 13:e0191389. doi: 10.1371/journal.pone.0191389
50. Szyld, EG, Warley, EM, Freimanis, L, Gonin, R, Cahn, PE, Calvet, GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS. (2006) 20:2345–53. doi: 10.1097/01.aids.0000253362.01696.9d
51. van der Merwe, K, Hoffman, R, Black, V, Chersich, M, Coovadia, A, and Rees, H. Birth outcomes in south African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. (2011) 14:42. doi: 10.1186/1758-2652-14-42
52. Watts, DH, Williams, PL, Kacanek, D, Griner, R, Rich, K, Hazra, R, et al. Combination antiretroviral use and preterm birth. J Infect Dis. (2013) 207:612–21. doi: 10.1093/infdis/jis728
53. Zash, R, Jacobson, DL, Diseko, M, Mayondi, G, Mmalane, M, Essex, M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. (2017) 171:e172222. doi: 10.1001/jamapediatrics.2017.2222
54. Zash, R, Jacobson, DL, Diseko, M, Mayondi, G, Mmalane, M, Essex, M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. (2018) 6:e804–10. doi: 10.1016/S2214-109X(18)30218-3
55. Zash, R, Holmes, L, Diseko, M, Jacobson, DL, Brummel, S, Mayondi, G, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. (2019) 381:827–40. doi: 10.1056/NEJMoa1905230
56. Zash, R, Caniglia, EC, Diseko, M, Mayondi, G, Mabuta, J, Luckett, R, et al. Maternal weight and birth outcomes among women on antiretroviral treatment from conception in a birth surveillance study in Botswana. J Int AIDS Soc. (2021) 24:e25763. doi: 10.1002/jia2.25763
57. Cowdell, IBK, Portwood, C, Sexton, H, Kumarendran, M, Brandon, Z, Kirtley, S, et al. Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: a systematic review and meta-analysis. EClinicalMedicine. (2022) 46:101368. doi: 10.1016/j.eclinm.2022.101368
58. Portwood, C, Sexton, H, Kumarendran, M, Brandon, Z, Johnson, B, Kirtley, S, et al. Perinatal outcomes associated with combination antiretroviral therapy compared with monotherapy. AIDS. (2023) 37:489–501. doi: 10.1097/QAD.0000000000003432
59. Kourtis, AP, Schmid, CH, Jamieson, DJ, and Lau, J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. (2007) 21:607–15. doi: 10.1097/QAD.0b013e32802ef2f6
60. Mesfin, YM, Kibret, KT, and Taye, A. Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod Health. (2016) 13:30. doi: 10.1186/s12978-016-0149-5
61. Veroniki, AA, Antony, J, Straus, SE, Ashoor, HM, Finkelstein, Y, Khan, PA, et al. Comparative safety and effectiveness of perinatal antiretroviral therapies for HIV-infected women and their children: systematic review and network meta-analysis including different study designs. PLoS One. (2018) 13:e0198447. doi: 10.1371/journal.pone.0198447
62. Saleska, JL, Turner, AN, Maierhofer, C, Clark, J, and Kwiek, JJ. Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with HIV-1 in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr. (2018) 79:1–9. doi: 10.1097/QAI.0000000000001770
63. Pasley, MV, Martinez, M, Hermes, A, d'Amico, R, and Nilius, A. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev. (2013) 15:38–48.
64. Sexton, H, Kumarendran, M, Brandon, Z, Shi, C, Kirtley, S, and Hemelaar, J. Adverse perinatal outcomes associated with timing of initiation of antiretroviral therapy: systematic review and meta-analysis. HIV Med. (2022) 6:13326. doi: 10.1111/hiv.13326
65. Committee opinion no 700: methods for estimating the due date. Obstet Gynecol. (2017) 129:e150–4. doi: 10.1097/AOG.0000000000002046
66. Villar, J, Cheikh Ismail, L, Victora, CG, Ohuma, EO, Bertino, E, Altman, DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. (2014) 384:857–68. doi: 10.1016/S0140-6736(14)60932-6
67. Royal College of Obstetricians and Gynaecologists. The investigation and Management of the Small–for–Gestational–age Fetus. Green-top Guideline No.31. London: Royal College of Obstetricians and Gynaecologists. (2014).
68. Dunk, CE, and Serghides, L. Protease inhibitor-based antiretroviral therapy in pregnancy: effects on hormones, placenta, and decidua. Lancet HIV. (2021) 2:00249–6. doi: 10.1016/S2352-3018(21)00249-6
69. Browne, JL, Schrier, VJ, Grobbee, DE, Peters, SA, and Klipstein-Grobusch, K. HIV, antiretroviral therapy, and hypertensive disorders in pregnancy: a systematic review and meta-analysis. J Acquir Immune Defic Syndr (1999). 70(1):91–98. doi: 10.1097/QAI.0000000000000686
70. Paiardini, M, and Müller-Trutwin, M. HIV-associated chronic immune activation. Immunol Rev. (2013) 254:78–101. doi: 10.1111/imr.12079
71. Akoto, C, Chan, C, Tshivuila-Matala, C, Ravi, K, Zhang, W, Vatish, M, et al. Innate lymphoid cells are reduced in pregnant HIV positive women and are associated with preterm birth. Sci Rep. (2020) 10:13265. doi: 10.1038/s41598-020-69966-0
72. Ravi, K, Chan, CYS, Akoto, C, Zhang, W, Vatish, M, Norris, SA, et al. Changes in the Vα7.2+ CD161++ MAIT cell compartment in early pregnancy are associated with preterm birth in HIV-positive women. Am J Reprod Immunol. (2020) 83:e13240. doi: 10.1111/aji.13240
73. Akoto, C, Chan, CYS, Ravi, K, Zhang, W, Vatish, M, Norris, SA, et al. γδ T cell frequencies are altered in HIV positive pregnant south African women and are associated with preterm birth. PLoS One. (2020) 15:e0235162. doi: 10.1371/journal.pone.0235162
74. Akoto, C, Norris, SA, and Hemelaar, J. Maternal HIV infection is associated with distinct systemic cytokine profiles throughout pregnancy in south African women. Sci Rep. (2021) 11:10079. doi: 10.1038/s41598-021-89551-3
75. Papp, E, Balogun, K, Banko, N, Mohammadi, H, Loutfy, M, Yudin, MH, et al. Low prolactin and high 20-alpha-hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV-infected pregnant women exposed to protease inhibitor-based combination antiretroviral therapy. J Infect Dis. (2016) 213:1532–40. doi: 10.1093/infdis/jiw004
76. Papp, E, Mohammadi, H, Loutfy, MR, Yudin, MH, Murphy, KE, Walmsley, SL, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis. (2015) 211:10–8. doi: 10.1093/infdis/jiu393
77. Price, JT, Vwalika, B, Freeman, BL, Cole, SR, Saha, PT, Mbewe, FM, et al. Weekly 17 alpha-hydroxyprogesterone caproate to prevent preterm birth among women living with HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. (2021) 8:e605–13. doi: 10.1016/S2352-3018(21)00150-8
78. Abrams, EJ, Calmy, A, Fairlie, L, Mahaka, IC, Chimula, L, Flynn, PM, et al. Approaches to accelerating the study of new antiretrovirals in pregnancy. J Int AIDS Soc. (2022) 25:e25916. doi: 10.1002/jia2.25916
79. Joseph Davey, DL, Bekker, LG, Bukusi, EA, Chi, BH, Delany-Moretlwe, S, Goga, A, et al. Where are the pregnant and breastfeeding women in new pre-exposure prophylaxis trials? The imperative to overcome the evidence gap. Lancet HIV. (2022) 9:e214–22. doi: 10.1016/S2352-3018(21)00280-0
80. Eke, AC, Gebreyohannes, RD, and Powell, AM. Understanding clinical outcome measures reported in HIV pregnancy studies involving antiretroviral-naive and antiretroviral-experienced women. Lancet Infect Dis. (2022) 11:00687–9. doi: 10.1016/S1473-3099(22)00687-9
81. Wedderburn, CJ, Weldon, E, Bertran-Cobo, C, Rehman, AM, Stein, DJ, Gibb, DM, et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2022) 6:393–408. doi: 10.1016/S2352-4642(22)00071-2
Keywords: HIV, antiretroviral therapy, protease inhibitor, integrase inhibitor, perinatal outcome, preterm birth, small for gestational age, low birthweight
Citation: Beck K, Cowdell I, Portwood C, Sexton H, Kumarendran M, Brandon Z, Kirtley S and Hemelaar J (2024) Comparative risk of adverse perinatal outcomes associated with classes of antiretroviral therapy in pregnant women living with HIV: systematic review and meta-analysis. Front. Med. 11:1323813. doi: 10.3389/fmed.2024.1323813
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Lars Navér, Karolinska Institutet (KI), SwedenNilesh Chandrakant Gawde, Tata Institute of Social Sciences, India
Copyright © 2024 Beck, Cowdell, Portwood, Sexton, Kumarendran, Brandon, Kirtley and Hemelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joris Hemelaar, am9yaXMuaGVtZWxhYXJAbnBldS5veC5hYy51aw==
†These authors have contributed equally to this work
 Katharina Beck1†
Katharina Beck1† Imogen Cowdell
Imogen Cowdell Clara Portwood
Clara Portwood Joris Hemelaar
Joris Hemelaar