
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 January 2024
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1322440
This article is part of the Research Topic Pandemic Response: Challenges, Advances, and Lessons Learnt View all 37 articles
 Xihong Zhang1†
Xihong Zhang1† Haoting Zhan2†
Haoting Zhan2† Lijing Wang3†
Lijing Wang3† Yongmei Liu2†
Yongmei Liu2† Xinru Guo4
Xinru Guo4 Chen Li3
Chen Li3 Xiaomeng Li2,5
Xiaomeng Li2,5 Beilei Li3
Beilei Li3 Haolong Li2
Haolong Li2 Yingxia Li3
Yingxia Li3 Qian Chen3
Qian Chen3 Huixia Gao6
Huixia Gao6 Fumin Feng4*
Fumin Feng4* Yongzhe Li2,7*
Yongzhe Li2,7* Erhei Dai6*
Erhei Dai6*Objectives: The COVID-19 pandemic imposed an enormous disease and economic burden worldwide. SARS-CoV-2 vaccination is essential to containing the pandemic. People living with HIV (PLWH) may be more vulnerable to severe COVID-19 outcomes; thus, understanding their vaccination willingness and influencing factors is helpful in developing targeted vaccination strategies.
Methods: A cross-sectional study was conducted between 15 June and 30 August 2022 in Shijiazhuang, China. Variables included socio-demographic characteristics, health status characteristics, HIV-related characteristics, knowledge, and attitudes toward COVID-19 vaccination and COVID-19 vaccination status. Multivariable logistic regression was used to confirm factors associated with COVID-19 vaccination willingness among PLWH.
Results: A total of 1,428 PLWH were included, with a 90.48% willingness to receive the COVID-19 vaccination. PLWH were more unwilling to receive COVID-19 vaccination for those who were female or had a fair/poor health status, had an allergic history and comorbidities, were unconvinced and unsure about the effectiveness of vaccines, were unconvinced and unsure about the safety of vaccines, were convinced and unsure about whether COVID-19 vaccination would affect ART efficacy, or did not know at least a type of domestic COVID-19 vaccine. Approximately 93.00% of PLWH have received at least one dose of the COVID-19 vaccine among PLWH, and 213 PLWH (14.92%) reported at least one adverse reaction within 7 days.
Conclusion: In conclusion, our study reported a relatively high willingness to receive the COVID-19 vaccination among PLWH in Shijiazhuang. However, a small number of PLWH still held hesitancy; thus, more tailored policies or guidelines from the government should be performed to enhance the COVID-19 vaccination rate among PLWH.
It has been approximately 3 years since the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has imposed an enormous disease and economic burden worldwide. Although herd immunity could also be established through natural SARS-CoV-2 infection, it may have devastating consequences for humans (1). A mass COVID-19 vaccination is conducive to induced herd immunity, decreasing morbidity and mortality (2) and is also one of the most cost-effective health interventions to control the COVID-19 pandemic (3, 4). Many clinical trials and real-world studies have shown that COVID-19 vaccines have favorable safety and immunogenicity and are obviously effective in preventing SARS-CoV-2 infection and improving severe disease outcomes in the general population (5–9).
Immunosuppressed individuals may be at higher risk of severe COVID-19 outcomes (10). The human immunodeficiency virus (HIV) primarily attacks the CD4+T cells of the human immune system, resulting in weakened defenses against many infections and virus-related cancers (11). Previous studies have reported that, compared with HIV-negative individuals, people living with HIV (PLWH) have an increased risk of becoming ill with COVID-19 (12), intubation, and in-hospital death rates (13, 14), especially in those with unsuppressed HIV viral replication or lower CD4+T cell counts (15, 16). Moreover, a systematic review indicated that the COVID-19 pandemic impeded access to follow-up, care, and treatment services for PLWH (17), which may result in a greater mental and disease burden for PLWH. Hence, the timely and effective implementation of public health measures to control COVID-19 could be of great benefit to PLWH.
While COVID-19 vaccination is crucial to prevent the severity and lethality of the disease caused by SARS-CoV-2 infection and control the outbreak (4, 18), achieving a high level of vaccination coverage also requires consideration of people’s willingness to be vaccinated, in addition to assessing the effectiveness and safety of the vaccine. Based on previous research, 68.4% of the global population is willing to receive the COVID-19 vaccination (19), and the acceptance of the COVID-19 vaccine varied extensively among different countries, ranging from 54.85% in Russia to 88.62% in China (20). Among PLWH, studies in China (which included eight cities but not Shijiazhuang) (21) and in the Middle East and North Africa region (22) have shown that the proportion of people willing to receive the COVID-19 vaccination was 57.2 and 64.6%, respectively. Therefore, to achieve early herd immunization, it is important to understand people’s willingness to receive the COVID-19 vaccination.
As the closest provincial capital city to the Chinese capital, Shijiazhuang has an important strategic position. There are no relevant studies to evaluate COVID-19 vaccination willingness among PLWH in Hebei Province. Based on the above considerations, this study aimed to investigate COVID-19 vaccination willingness and influencing factors among PLWH in Shijiazhuang so as to make tailored vaccination strategies for this special population.
A cross-sectional study was conducted in the Fifth Hospital of Shijiazhuang (a tertiary referral university hospital for treating SARS-CoV-2 and HIV infections) between 15 June and 30 August 2022. All the individuals were recruited through convenience sampling. PASS 11.0 was used to calculate the minimum sample necessary, assuming a significance level of 0.05 and an allowable error of 2%, with a willingness rate (90%) of COVID-19 vaccination among PLWH (which was higher than previous studies from China (23) and the United States (24)) and a rate (20%) of invalidity considered, resulting in a minimum sample size of 1,142.
Eligible participants would be informed of the study’s purpose, risks, and benefits before completing the questionnaire, and then they would be surveyed via face-to-face interviews or an online investigation platform named Wenjuanxing (www.wjx.cn). As compensation, participants had the opportunity to get a gift worth approximately 25 RMB. The study protocol was approved by the Medical Ethics Committee of the Fifth Hospital of Shijiazhuang and Peking Union Medical College Hospital, and the need for informed consent was waived.
The eligibility criteria were as follows: (1) has a confirmed HIV infection and has been receiving the antiretroviral therapy (ART) regimen; and (2) has consented to participate in the survey. Participants would be excluded if (1) they were under 18 years old; (2) they had been infected with SARS-CoV-2; (3) they were under 180 s to complete the survey for the online version (which we judged to be the minimal reasonable time to complete the questionnaire); (4) they was more than one response per participant; and (5) they provided incorrect COVID-19 vaccination information. The official information about the COVID-19 vaccination of each patient from the CDC is double-checked with the answers from PLWH’s questionnaire. We regarded the unmatched ones as invalid questionnaires and excluded them from enrollment of this study; (6) the relevant HIV infection information of PLWH was not retrieved from the China Information System for Diseases Control and Prevention.
The questionnaire was self-designed based on previous studies (23, 25) and was endorsed by a panel of six experts with medical backgrounds. It involved five sections: socio-demographic characteristics, health status characteristics, HIV-related characteristics, knowledge, and attitudes toward COVID-19 vaccination and COVID-19 vaccination status.
Socio-demographic characteristics were collected, including age, sex, height, weight, ethnicity, marital status, education level, occupation, monthly income, and area of long-term residence. Health status characteristics included present health status, comorbidities, and previous allergic history to food/drug/vaccine/other. HIV-related characteristics were gathered from the Acquired Immune Deficiency Syndrome (AIDS) Comprehensive Prevention and Control Data Information Management System of the Chinese Center for Disease Control and Prevention (CDC), including the route of HIV transmission, last CD4+/CD8+T cells, time living with HIV, the stages of HIV infection, and the symptoms associated with HIV infection in the last 3 months. To evaluate the knowledge and attitudes toward COVID-19 vaccination, we collected beliefs about vaccine effectiveness and safety, understanding of domestic COVID-19 vaccine types, concerns about the impact of COVID-19 vaccination on ART efficacy, and the situation for proactively consulting vaccination information through medical staff. For COVID-19 vaccination status, participants were asked to answer “whether you have been vaccinated or not, the exact time and brand of each vaccine, and the local or systemic adverse reactions after vaccination within 7 days.” Finally, to assess vaccination willingness, we asked participants, “are you willing to receive COVID-19 vaccine?”
There was a potential bias because some of our PLWH patients had already received the COVID-19 vaccination before participating in this cross-sectional study.
A total of 1,577 questionnaires were collected, leaving an analytic sample of 1,428 (the valid response rate was 90.55%). The selection process for valid questionnaires is shown in Figure 1.
According to COVID-19 vaccination willingness, all participants were divided into “willing” or “unwilling” to receive the COVID-19 vaccination group. The continuous variables were summarized as median and interquartile range (IQR) and tested by the Mann–Whitney U-test. The categorical variables were expressed as frequencies and percentages and compared by the chi-square test.
Subsequently, multivariable logistic regression was conducted to confirm factors associated with COVID-19 vaccination willingness among PLWH. The variables identified from the univariate analysis (p < 0.05) were further analyzed in the adjusted logistic regression model. The aforementioned variables associated with COVID-19 vaccination willingness were presented with crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs). A two-tailed p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (IBM, New York, United States), and the bar diagram and pie chart were visualized by GraphPad Prism 9.0 (San Diego, California, United States).
Among 1,428 PLWH, 90.48% (1,292/1,428) were willing to receive the COVID-19 vaccination. The participants included 1,330 males (93.14%), with a median age of 40.39 years (IQR 31.78–52.18). The socio-demographic characteristics showed no statistical difference between those who are willing and unwilling to receive the COVID-19 vaccination group in the majority of variables, including age, body mass index (BMI), ethnic minorities, education level, occupation, and area of residence. However, the proportion of females, marital status (single, divorced, or widowed) and less than 5,000 RMB in monthly income was higher in the unwilling to accept the COVID-19 vaccination group (p < 0.05) (Table 1).
The health status characteristics and HIV-related characteristics were analyzed in the PLWH who are willing and unwilling to accept the COVID-19 vaccination. Compared with the group willing to accept vaccination, the proportion of the PLWH with fair or poor health status, allergic history, and comorbidities was high in the group unwilling to accept vaccination (p < 0.001). The other variables did not differ between the two groups (Table 2).
For PLWH in this study, the most sources of COVID-19 vaccine information were government agencies (50.42%), followed by television or radio (40.20%), social media (35.64%), and medical staff (32.42%), while only a small percentage of information came from friends and families (18.42%) and others (16.45%).
In addition, when comparing the attitudes toward COVID-19 vaccination between the willingness and unwillingness to accept vaccination groups, we observed a significantly higher willingness to accept COVID-19 vaccination in PLWH who believe the vaccine is effective and safe and those who know at least one type of domestic COVID-19 vaccine (p < 0.001). However, PLWH who believe that vaccination will affect ART efficacy had a lower willingness to receive the COVID-19 vaccination (p < 0.001). There was no statistical difference in the attitude toward taking the initiative to consult the medical staff about the COVID-19 vaccination between the two groups (p > 0.05) (Table 3).
A total of 10 variables (p < 0.05) were presented in the multivariable logistic regression analyses. The results showed that PLWH were more unwilling to be vaccinated for those who were female (aOR 2.15, 95% CI 1.02–4.56) or fair/poor health status (fair: aOR 1.77, 95% CI 1.09–2.87; poor: aOR 3.01, 95% CI 1.03–8.86), those who had allergic history (aOR 2.07, 95% CI 1.25–3.45) and comorbidities (aOR 2.18, 95% CI 1.28–3.71), those who were unconvinced and unsure about the effectiveness of vaccines (unconvinced: aOR 6.71, 95% CI 2.01–22.46; unsure: aOR 2.62, 95% CI 1.48–4.63), those who were unconvinced and unsure about the safety of vaccines (unconvinced: aOR 6.01, 95% CI 1.63–22.17; unsure: aOR 4.38, 95% CI 2.49–7.69), those who were convinced and unsure about that COVID-19 vaccination will affect ART efficacy (convinced: aOR 25.42, 95% CI 9.64–67.01; unsure: aOR 7.56, 95% CI 3.25–17.59), those who do not know at least a type of domestic COVID-19 vaccine (aOR 3.59, 95% CI 1.85–6.97) (Table 4).
In a total of 1,428 PLWH, 1328 (93.00%) have received at least one dose of the COVID-19 vaccine, and the largest proportion (68.00%) have received inactivated vaccines. After being vaccinated against COVID-19, 213 PLWH (14.92%) reported at least one adverse reaction within 7 days (Figure 2). The most common local and systemic adverse reactions were local pain (12.06% first dose, 11.80% second dose, and 10.72% third dose) and fatigue (8.04% first dose, 2.41% second dose, and 3.49% third dose), respectively, and no serious adverse events have been reported (Figure 3).
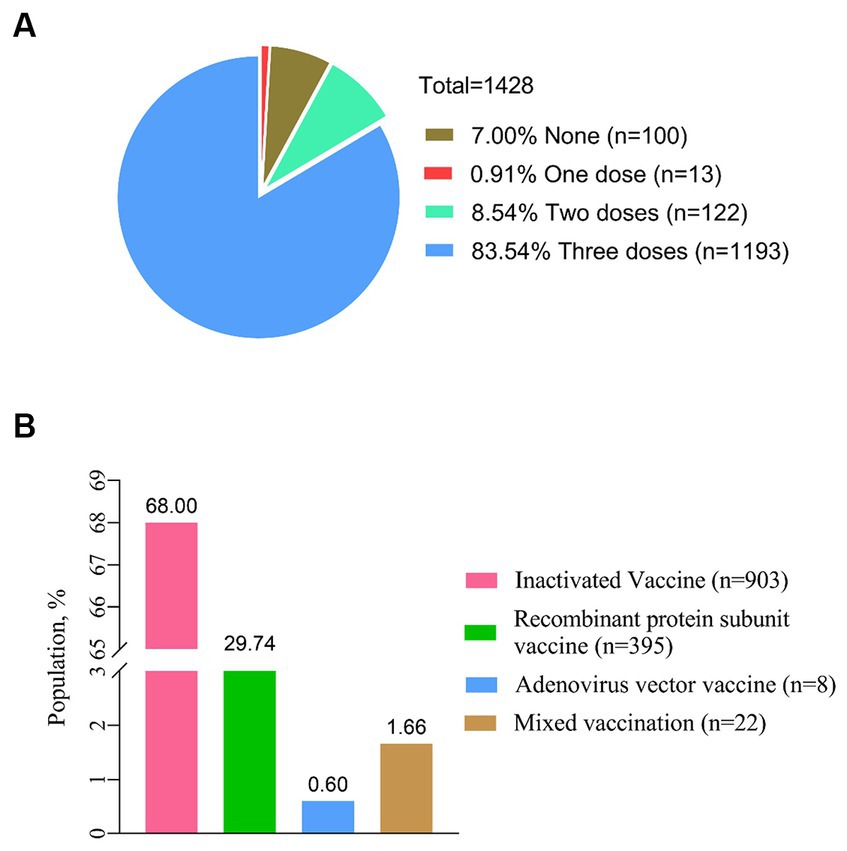
Figure 2. Status of COVID-19 vaccination. (A) Proportion of the different doses for COVID-19 vaccination (n = 1,428). (B) Status of vaccination with different types of COVID-19 vaccines (n = 1,428).
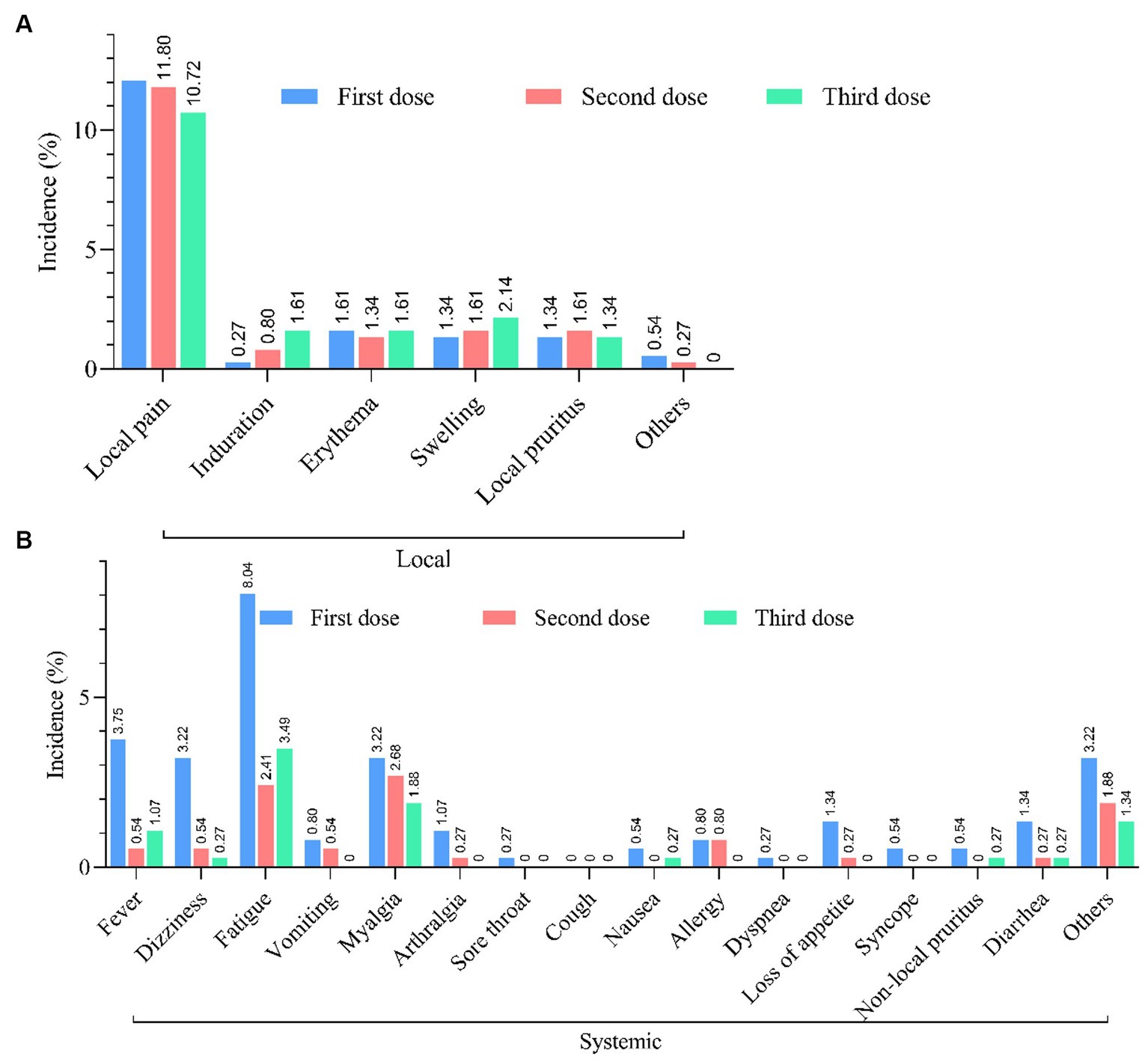
Figure 3. Safety of COVID-19 vaccination. (A) Incidence of local and systemic adverse reactions reported within 7 days after each dose of COVID-19 vaccine in PLWH (n = 213). (B) Incidence of systemic adverse reactions reported within 7 days after each dose of COVID-19 vaccine in PLWH (n = 213).
Vaccination is one of the most effective measures to end the COVID-19 pandemic. According to an expert recommendation on COVID-19 vaccination for PLWH released from China in July 2021, PLWH could also be vaccinated after their HIV-related condition was assessed (26). In addition, authoritative guidance for COVID-19 and people with HIV developed by the Guideline Working Groups of the National Institutes of Health (NIH) Office of AIDS Research Advisory Council (27) suggested that PLWH, regardless of CD4+T cells or viral load, should receive COVID-19 vaccination since the potential benefits outweigh the potential risks. Even so, 31.6% of PLWH reported COVID-19 vaccination unwillingness worldwide (19). Therefore, it is important to understand the willingness of PLWH to receive the COVID-19 vaccination and the factors influencing it.
In the present study, we found a high willingness for COVID-19 vaccination (90.48%) among PLWH in Shijiazhuang, which was almost consistent with the finding in Turin, Italy (92.4%) (28). However, it was significantly higher than that of the general adult population in China (60.4 and 82.6%) (29, 30) and around the world (68.4 and 71.5%) (19, 20). This proportion was higher than that of PLWH in China (57.2%), the United States (83.8%) (24), South Africa (57%) (31), and Northern Nigeria (46.2%) (32). In summary, this study reflected that COVID-19 vaccination willingness among PLWH is relatively high in Shijiazhuang, China. The results of this study should be viewed with caution. We conducted this study on 15 June 2022, which was 17 months later than the launch of the national free vaccination policy (33). At this time, studies in China and Brazil have shown a comparable immune response and safety between PLWH and healthy individuals in response to inactivated COVID-19 vaccine (23, 34), and similar results were also observed in adenovirus vector and messenger RNA (mRNA) COVID-19 vaccines (35, 36), which may be one of the reasons affecting the higher willingness to COVID-19 vaccination. In addition, the propaganda of government agencies plays an important role as it contributes the highest percentage (50.42%) of information sources in COVID-19 vaccines, according to our findings.
The results of multivariable logistic regression analyses indicated that some variables in socio-demographic characteristics, health status characteristics, and knowledge and attitudes toward COVID-19 vaccination were significantly associated with vaccination willingness. For example, the female PLWH had lower COVID-19 vaccination willingness than the male, which was consistent with a prior study in older PLWH (≥50 years) in the Coachella Valley (37). PLWH with fair/poor health status, an allergic history, and comorbidities also reported lower willingness, which suggests that poor health conditions are likely to affect PLWH’s willingness to be vaccinated. A study of the PLWH in French reported that those who are worried about their health status and underlying diseases were more likely to accept COVID-19 vaccination (38). Another study in China also found that PLWH with comorbidities were more willing to be vaccinated (39). An earlier study (from Wuhan, China) showed that PLWH with comorbidities had a higher willingness to receive the COVID-19 vaccination (39), which was diametrically opposed to the results of our study. The reasons for this phenomenon may be related to the late date of our study and the different geographical areas.
In addition, knowledge and attitudes toward COVID-19 vaccination were important factors influencing COVID-19 vaccination willingness. PLWH who were unconvinced and unsure of the effectiveness and safety, those who are convinced and unsure about whether COVID-19 vaccination will affect ART efficacy, and those who do not know at least a type of domestic COVID-19 vaccine had lower COVID-19 vaccination willingness, which is consistent with the findings in China (23, 40), the United States (41), and France (38). The phenomenon is common because vaccine-specific issues are the most frequent determinants of unwillingness to be vaccinated. In addition to COVID-19 vaccines, similar phenomena also existed in other types of vaccines served among PLWH. A study in France has shown that fear of expected effectiveness and adverse effects were the most frequent reasons for refusing vaccination for Streptococcus pneumoniae, influenza, tetanus, and chronic hepatitis among PLWH (42). This suggests that subjective attitudes among PLWH have a strong influence on willingness to be vaccinated because our research found that none of the HIV-related characteristics had a significant effect on the COVID-19 vaccination willingness, including time living with HIV, mode of HIV transmission, clinical stage, the symptoms associated with HIV infection the last 3 months, and the last CD4+/CD8+cells. Therefore, in order to further improve COVID-19 vaccination coverage, it is necessary not only to strengthen research on the efficacy and safety of vaccines but also to emphasize the publicity and popularization of knowledge about vaccination among PLWH.
According to the finding, 93.00% of PLWH have received at least one dose of the COVID-19 vaccine, which is higher than that among PLWH in the United States (64%) (43) as well as in the Middle East and North Africa region (19.3%) (19). The vaccination rates are not comparable between regions due to the fact that the above study was conducted in early 2021. Previous studies have proven that COVID-19 vaccines have a good safety profile among PLWH (44, 45), and similar results were obtained in our study.
This study has several limitations. Because the cross-sectional study design was unable to determine causality, we can only describe associations between COVID-19 vaccination willingness and influencing factors. We recruited an opportunistic sample, which may affect the generalization performance. In addition, information bias is inevitable because part of the data was obtained through online methods, and the health status of patients is subjectively judged by patients themselves according to their own physical conditions, which have not been collected using validated measures. The in-person questionnaire may put limits on the “sincerity” of the answers, but we did not analyze whether there are any differences in willingness according to the way of survey administration. Nevertheless, rigorous data examination was set to exclude ineligible participants and ensure data quality. Another limitation of our study is that we neglected to design-related questions to assess the psychological state of the subjects, which is also a strong predictor of vaccine hesitancy. COVID-19 vaccination willingness will be changing over time due to the multiple influencing factors, so a study considering more aspects will be performed to certify our result.
In conclusion, our study showed that PLWH in Shijiazhuang reported a relatively high willingness to receive the COVID-19 vaccination. There is a lower willingness to receive COVID-19 among PLWH who are female or have a fair/poor health status, those who have an allergic history and comorbidities, those who are unconvinced and unsure about the effectiveness and safety of COVID-19 vaccination, those who are convinced and unsure about whether COVID-19 vaccination will affect ART efficacy, and those who do not know at least a type of domestic COVID-19 vaccine. Consequently, more tailored policies or guidelines from the government should be implemented to enhance COVID-19 vaccination coverage among PLWH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the study was approved by the Ethics Committee of the Fifth Hospital of Shijiazhuang (2022-017-1) and the Medical Ethics Committee of Peking Union Medical College Hospital (JS-2156). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
XZ: Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing, Writing – original draft. LW: Investigation, Project administration, Supervision, Writing – review & editing. YLiu: Formal Analysis, Methodology, Resources, Writing – review & editing. XG: Investigation, Resources, Writing – review & editing. CL: Investigation, Writing – review & editing. XL: Investigation, Supervision, Writing – review & editing. BL: Investigation, Writing – review & editing. HL: Investigation, Methodology, Writing – review & editing. YiL: Project administration, Writing – review & editing. QC: Resources, Writing – review & editing. HG: Investigation, Project administration, Resources, Writing – review & editing. FF: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing. YoL: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. ED: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2018YFE0207300), the Key Research and Development Plan of Hebei Province, the Special Health Innovation Project (22377744D), the Beijing Municipal Science and Technology Commission (Z211100002521021), Science and Technology Program of Shijiazhuang City: Research on the Application Value of HIV Molecular Network in the Precise Prevention and Control of AIDS in Hebei Province (231200103A), and “Clinical Research on Stem Cell Therapy for Advanced AIDS”, Sub-theme of “Research on Clinical Treatment Program for Major Infectious Diseases Based on Stem Cell Technology”, National Key Research and Development Program of the 14th Five-Year Plan (2022YFC2304403).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; HIV, Human immunodeficiency virus; PLWP, People living with HIV; ART, Antiretroviral therapy; AIDS, Acquired immune deficiency syndrome; CDC, Center for Disease Control and Prevention; IQR, Interquartile range; ORs, Odds ratios; CIs, 95% confidence intervals; BMI, Body mass index.
1. Randolph, HE, and Barreiro, LB. Herd immunity: understanding COVID-19. Immunity. (2020) 52:737–41. doi: 10.1016/j.immuni.2020.04.012
2. Brice, Y, Morgan, L, Kirmani, M, Kirmani, M, and Udeh, MC. COVID-19 vaccine evolution and beyond. Neurosci Insights. (2023) 18:26331055231180543. doi: 10.1177/26331055231180543
3. Tregoning, JS, Flight, KE, Higham, SL, Wang, Z, and Pierce, BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. (2021) 21:626–36. doi: 10.1038/s41577-021-00592-1
4. Fiolet, T, Kherabi, Y, MacDonald, CJ, Ghosn, J, and Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. (2022) 28:202–21. doi: 10.1016/j.cmi.2021.10.005
5. Pawlowski, C, Lenehan, P, Puranik, A, Agarwal, V, Venkatakrishnan, AJ, Niesen, MJM, et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. (2021) 2:979–92.e8. doi: 10.1016/j.medj.2021.06.007
6. Chodick, G, Tene, L, Rotem, RS, Patalon, T, Gazit, S, Ben-Tov, A, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. (2022) 74:472–8. doi: 10.1093/cid/ciab438
7. Tanriover, MD, Doğanay, HL, Akova, M, Güner, HR, Azap, A, Akhan, S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. (2021) 398:213–22. doi: 10.1016/s0140-6736(21)01429-x
8. Wu, Z, Hu, Y, Xu, M, Chen, Z, Yang, W, Jiang, Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. (2021) 21:803–12. doi: 10.1016/s1473-3099(20)30987-7
9. Han, B, Song, Y, Li, C, Yang, W, Ma, Q, Jiang, Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. (2021) 21:1645–53. doi: 10.1016/s1473-3099(21)00319-4
10. Fung, M, and Babik, JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. (2021) 72:340–50. doi: 10.1093/cid/ciaa863
11. World Health Organization. HIV/AIDS. (2022). Available at: https://www.who.int/zh/news-room/fact-sheets/detail/hiv-aids (Accessed November 30, 2022).
12. Johnston, R. The first 6 months of HIV-SARS-CoV-2 coinfection: outcomes for 6947 individuals. Curr Opin HIV AIDS. (2021) 16:54–62. doi: 10.1097/coh.0000000000000654
13. Patel, VV, Felsen, UR, Fisher, M, Fazzari, MJ, Ginsberg, MS, Beil, R, et al. Clinical outcomes and inflammatory markers by HIV Serostatus and viral suppression in a large cohort of patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. (2021) 86:224–30. doi: 10.1097/qai.0000000000002578
14. Mellor, MM, Bast, AC, Jones, NR, Roberts, NW, Ordóñez-Mena, JM, Reith, AJM, et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS. (2021) 35:F1–f10. doi: 10.1097/qad.0000000000002836
15. Kanwugu, ON, and Adadi, P. HIV/SARS-CoV-2 coinfection: A global perspective. J Med Virol. (2021) 93:726–32. doi: 10.1002/jmv.26321
16. Yang, X, Sun, J, Patel, RC, Zhang, J, Guo, S, Zheng, Q, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US national COVID cohort collaborative (N3C) data. Lancet HIV. (2021) 8:e690–700. doi: 10.1016/s2352-3018(21)00239-3
17. Mirzaei, H, Moradi, Y, Abbaszadeh, S, Nasiri, N, Mehmandoost, S, Khezri, M, et al. The impact of COVID-19 on disruptions of HIV-related services: A rapid review. Med J Islam Repub Iran. (2022) 36:98. doi: 10.47176/mjiri.36.98
18. Hodgson, SH, Mansatta, K, Mallett, G, Harris, V, Emary, KRW, and Pollard, AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. (2021) 21:e26–35. doi: 10.1016/s1473-3099(20)30773-8
19. Wang, W, Wu, Q, Yang, J, Dong, K, Chen, X, Bai, X, et al. Global, regional, and national estimates of target population sizes for covid-19 vaccination: descriptive study. BMJ. (2020) 371:m4704. doi: 10.1136/bmj.m4704
20. Lazarus, JV, Ratzan, SC, Palayew, A, Gostin, LO, Larson, HJ, Rabin, K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
21. Huang, X, Yu, M, Fu, G, Lan, G, Li, L, Yang, J, et al. Willingness to receive COVID-19 vaccination among people living with HIV and AIDS in China: Nationwide cross-sectional online survey. JMIR Public Health Surveill. (2021) 7:e31125. doi: 10.2196/31125
22. Mohamed, R, White, TM, Lazarus, JV, Salem, A, Kaki, R, Marrakchi, W, et al. COVID-19 vaccine acceptance and associated factors among people living with HIV in the Middle East and North Africa region. South Afr J HIV Med. (2022) 23:1391. doi: 10.4102/sajhivmed.v23i1.1391
23. Su, J, Jia, Z, Wang, X, Qin, F, Chen, R, Wu, Y, et al. Acceptance of COVID-19 vaccination and influencing factors among people living with HIV in Guangxi, China: a cross-sectional survey. BMC Infect Dis. (2022) 22:471. doi: 10.1186/s12879-022-07452-w
24. Wickersham, JA, Meyer, JP, Shenoi, S, Altice, FL, Barakat, LA, Virata, M, et al. Willingness to be vaccinated against COVID-19 among people with HIV in the United States: results from a National Survey. Front Med (Lausanne). (2022) 9:886936. doi: 10.3389/fmed.2022.886936
25. Yang, J, Yu, M, Fu, G, Lan, G, Li, L, Qiao, Y, et al. COVID-19 vaccination uptake among a Nationwide sample of people living with HIV during the early phase of vaccine rollout in China. Front Med (Lausanne). (2022) 9:822680. doi: 10.3389/fmed.2022.822680
26. AIDS and Hepatitis C Professional Group, Chinese Society of Infectious Diseases. Expert recommendation for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in patients with HIV infection. Zhonghua Nei Ke Za Zhi. (2021) 60:615–8. doi: 10.3760/cma.j.cn112138.20210403-00259
27. HIV.gov. Interim guidance for COVID-19 and persons with HIV. (2022). Available at: https://clinicalinfo.hiv.gov/en/guidelines/guidance-covid-19-and-people-hiv/guidance-covid-19-and-people-hiv?view=full (Accessed November 30, 2022).
28. Bert, F, Pivi, A, Russotto, A, Mollero, B, Voglino, G, Orofino, G, et al. COVID-19 vaccination among HIV+ patients: an Italian cross-sectional survey. Vaccines (Basel). (2022) 10:10. doi: 10.3390/vaccines10091438
29. Gan, L, Chen, Y, Hu, P, Wu, D, Zhu, Y, Tan, J, et al. Willingness to receive SARS-CoV-2 vaccination and associated factors among Chinese adults: A cross sectional survey. Int J Environ Res Public Health. (2021) 18:18. doi: 10.3390/ijerph18041993
30. Wang, Z, Xiao, J, Jiang, F, Li, J, Yi, Y, Min, W, et al. The willingness of Chinese adults to receive the COVID-19 vaccine and its associated factors at the early stage of the vaccination programme: a network analysis. J Affect Disord. (2022) 297:301–8. doi: 10.1016/j.jad.2021.10.088
31. Govere-Hwenje, S, Jarolimova, J, Yan, J, Khumalo, A, Zondi, G, Ngcobo, M, et al. Willingness to accept COVID-19 vaccination among people living with HIV in a high HIV prevalence community. BMC Public Health. (2022) 22:1239. doi: 10.1186/s12889-022-13623-w
32. Iliyasu, Z, Kwaku, AA, Umar, AA, Tsiga-Ahmed, F, Nass, NS, Abdullahi, HM, et al. Predictors of COVID-19 vaccine acceptability among patients living with HIV in northern Nigeria: A mixed methods study. Curr HIV Res. (2022) 20:82–90. doi: 10.2174/1570162x19666211217093223
33. The State Council of the People’s Republic of China. COVID-19 vaccination free to Chinese residents: Official. (2021). Available at: http://english.www.gov.cn/statecouncil/ministries/202101/09/content_WS5ff94558c6d0f72576943854.html (Accessed November 30, 2022).
34. Netto, LC, Ibrahim, KY, Picone, CM, Alves, A, Aniceto, EV, Santiago, MR, et al. Safety and immunogenicity of CoronaVac in people living with HIV: a prospective cohort study. Lancet HIV. (2022) 9:e323–31. doi: 10.1016/s2352-3018(22)00033-9
35. Portillo, V, Fedeli, C, Ustero Alonso, P, Petignat, I, Mereles Costa, EC, Sulstarova, A, et al. Impact on HIV-1 RNA levels and antibody responses following SARS-CoV-2 vaccination in HIV-infected individuals. Front Immunol. (2021) 12:820126. doi: 10.3389/fimmu.2021.820126
36. Frater, J, Ewer, KJ, Ogbe, A, Pace, M, Adele, S, Adland, E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. (2021) 8:e474–85. doi: 10.1016/s2352-3018(21)00103-x
37. Davtyan, M, Frederick, T, Taylor, J, Christensen, C, Brown, BJ, and Nguyen, AL. Determinants of COVID-19 vaccine acceptability among older adults living with HIV. Medicine (Baltimore). (2022) 101:e29907. doi: 10.1097/md.0000000000029907
38. Vallée, A, Fourn, E, Majerholc, C, Touche, P, and Zucman, D. COVID-19 vaccine hesitancy among French people living with HIV. Vaccines (Basel). (2021) 9:9. doi: 10.3390/vaccines9040302
39. Wu, S, Ming, F, Xing, Z, Zhang, Z, Zhu, S, Guo, W, et al. COVID-19 vaccination willingness among people living with HIV in Wuhan, China. Front Public Health. (2022) 10:883453. doi: 10.3389/fpubh.2022.883453
40. Zheng, W, Sun, Y, Li, H, Zhao, H, Zhan, Y, Gao, Y, et al. COVID-19 vaccine uptake and hesitancy among HIV-infected men who have sex with men in mainland China: a cross-sectional survey. Hum Vaccin Immunother. (2021) 17:4971–81. doi: 10.1080/21645515.2021.1996152
41. Shrestha, R, Meyer, JP, Shenoi, S, Khati, A, Altice, FL, Mistler, C, et al. COVID-19 vaccine hesitancy and associated factors among people with HIV in the United States: findings from a National Survey. Vaccines (Basel). (2022) 10:10. doi: 10.3390/vaccines10030424
42. Mohseni-Zadeh, M, Rey, D, Batard, ML, Beck Wirth, G, Partisani, ML, Lang, JM, et al. Inadequate vaccination coverage in a French cohort of HIV positive patients. Med Mal Infect. (2010) 40:683–90. doi: 10.1016/j.medmal.2010.06.005
43. Jaiswal, J, Krause, KD, Martino, RJ, D’Avanzo, PA, Griffin, M, Stults, CB, et al. SARS-CoV-2 vaccination hesitancy and behaviors in a National Sample of people living with HIV. AIDS Patient Care STDs. (2022) 36:34–44. doi: 10.1089/apc.2021.0144
44. Liu, Y, Han, J, Li, X, Chen, D, Zhao, X, Qiu, Y, et al. COVID-19 vaccination in people living with HIV (PLWH) in China: A cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines (Basel). (2021) 9:9. doi: 10.3390/vaccines9121458
Keywords: people living with HIV, COVID-19, vaccine, willingness, influencing factors
Citation: Zhang X, Zhan H, Wang L, Liu Y, Guo X, Li C, Li X, Li B, Li H, Li Y, Chen Q, Gao H, Feng F, Li Y and Dai E (2024) COVID-19 vaccination willingness among people living with HIV in Shijiazhuang, China: a cross-sectional survey. Front. Med. 11:1322440. doi: 10.3389/fmed.2024.1322440
Received: 16 October 2023; Accepted: 02 January 2024;
Published: 17 January 2024.
Edited by:
Brent M. Egan, American Medical Association, United StatesReviewed by:
Juan Sebastian Izquierdo-Condoy, University of the Americas, EcuadorCopyright © 2024 Zhang, Zhan, Wang, Liu, Guo, Li, Li, Li, Li, Li, Chen, Gao, Feng, Li and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fumin Feng, Zm1fZmVuZ0BzaW5hLmNvbQ==; Yongzhe Li, eW9uZ3poZWxpcHVtY2hAMTI2LmNvbQ==; Erhei Dai, ZGFpZWgyMDA4QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.