- Department of Gynecology, Guangdong Women and Children Medical Hospital, Guangzhou, China
Currently, more than 170 modifications have been identified on RNA. RNA modification mainly regulates RNA splicing, intracellular transport, degradation, translation, and stability. Gynecologic cancer (GC) mainly includes cervical cancer (CCA), ovarian cancer (OC), Endometrial cancer (EMC), among others, is the leading cause of cancer-related death. At present, there is still a lack of effective means to eradicate such diseases, so it is important to conduct more in-depth research on gynecological cancers. Numerous studies have shown that a series of epigenetic changes occur during the development of gynecologic cancer. This article reviews the latest findings on the functional significance of RNA modification in gynecologic cancer and discusses the therapeutic potential of RNA modification-related inhibitors in the treatment of gynecologic cancer.
1 Introduction
Epigenetics is a discipline that specializes in the study of heritable gene expression or cell phenotypic changes without changing the nucleotide sequence of inherited genes (1), and known epigenetic phenomena include DNA methylation, RNA modification, protein acetylation, protein methylation (1–3). Among them, RNA modification has been a research hotspot in the field of epigenetics in recent years, and more than 170 types of RNA modification have been identified in organisms (4). Among these RNA modification types, the main ones are N6-methyladenosine(m6A),5-methylcytosine(m5C), N1-methyladenosine(m1A), N7-Methylguanosine(m7G), Pseudouridine(ψ) and adenosine-to-inosine(A-to-I) editing modifications (5). More and more research data show that RNA modification plays an important role in the occurrence and progression of cancer, such as breast cancer (6), liver cancer (7), bladder cancer (8), lung cancer (9). Gynecological cancers mainly include vulvar cancer, vaginal cancer, cervical cancer, uterine body cancer, ovarian cancer, of which cervical cancer, uterine body cancer and ovarian cancer are the three most common types of gynecological cancer.
According to the 2020 Global Cancer Statistics Report, around 1.4 million women were diagnosed with gynecological cancers in 2020, resulting in approximately 671,875 deaths (10). This imposes a significant burden on women worldwide, both physically and psychologically. Although current treatment methods for gynecological cancer primarily involve surgery, radiotherapy, and chemotherapy, these approaches still fail to completely address issues such as metastasis and recurrence. The current development of medical technology still has no effective way to cure gynecological cancer, therefore, it is necessary and urgent to further deepen the research on the unknown mechanism of gynecological cancer. Here, we summarize the recent research progress of RNA modification in the field of gynecological cancer, to provide new ideas for the early diagnosis and treatment of gynecological cancer.
2 m6A modification in gynecological cancer
m6A is the most common internal modification mode of eukaryotic mRNA, which is the methylation of adenosine at the 6th position (11). m6A methyltransferase (writer) mediates methylation modification on mRNA through m6A, while demethylase (eraser) can reverse this modification. Additionally, the m6A recognition protein (reader) participates by identifying methylation modification information on mRNA, thereby influencing splicing, degradation, stability, and translation processes (11, 12). Abnormalities in RNA modification processes are closely related to the pathogenesis and progression of gynecological cancers.
2.1 Writer
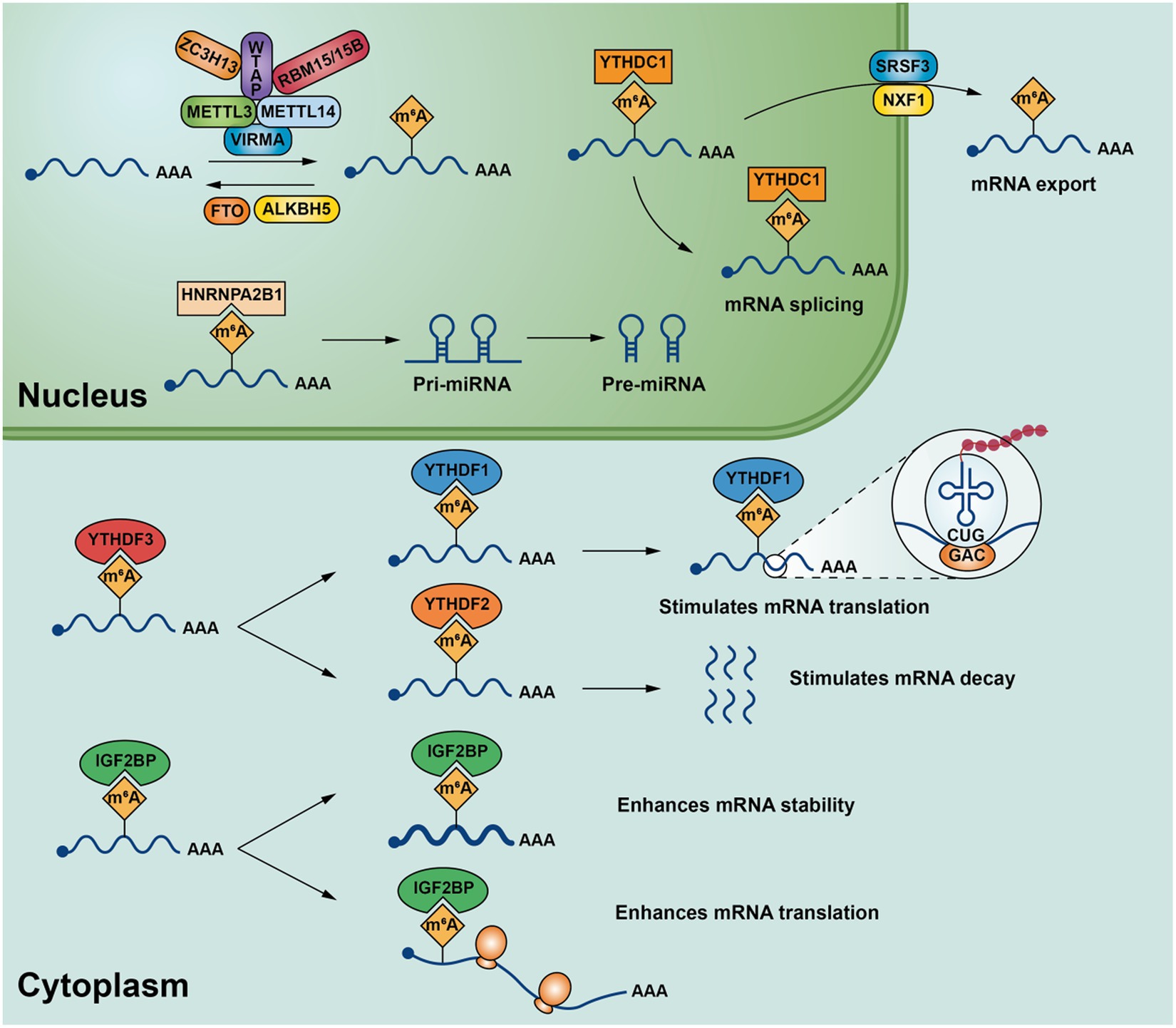
According to current studies, METTL3(methyltransferase-like 3), METTL14(methyltransferase-like 14) (13), WTAP(Wilms’ tumor 1-associating protein) (14), KIAA1429(vir-Like m6A methyltransferase associated) (15), RBM15/15B(RNA-binding motif protein 15/RNA-binding motif protein 15B) (16), ZC3H13(Zinc finger CCCH-type containing 13) (17) belong to m6A methyltransferase. METTL3 and METTL14 are the core components of m6A methyltransferase, and METTL3 is often associated with METTL14 binding forms a stable heterodimeric core complex form involved in the deposition of m6A in mammalian nuclear RNA (18). METTL3 it is the only catalytic subunit that binds to the methyl donor S-adenosylmethionine (SAM) and catalyzes methyl transfer, while METTL14 is often required for METTL3 activation and is used to identify substrates RNA plays a key role (13). WTAP is the main component involved in the regulation of the m6A methylation complex, which interacts with METTL3 and METTL14,assisting METTL3 and METTL14 are localized to nuclear spots rich in precursor mRNA and is required for the catalytic activity of m6A methyltransferase in vivo (18). KIAA1429, also known as VIRMA, can recruit METTL3/METTL14/WTAP, the core component of m6A to regulate region-selective methylation, VIRMA plays a key role in the specific deposition of m6A on 3’UTR (19). Zinc finger protein ZC3H13 also plays an important role in mRNA’s m6A methylation modification, which is coordinated with WTAP, Virilizer and Hakai combine to form complexes that allow WTAP to be accurately localized in the nucleus to promote m6A methylation (17). RBM15 and/or RBM15B often bind to U-rich sites. During m6A modification of mRNA, they bind to WTAP-METTL3 enabling the WTAP-METTL3 complex to bind to specific mRNA and promote further adenosine methylation (16) (Figure 1).
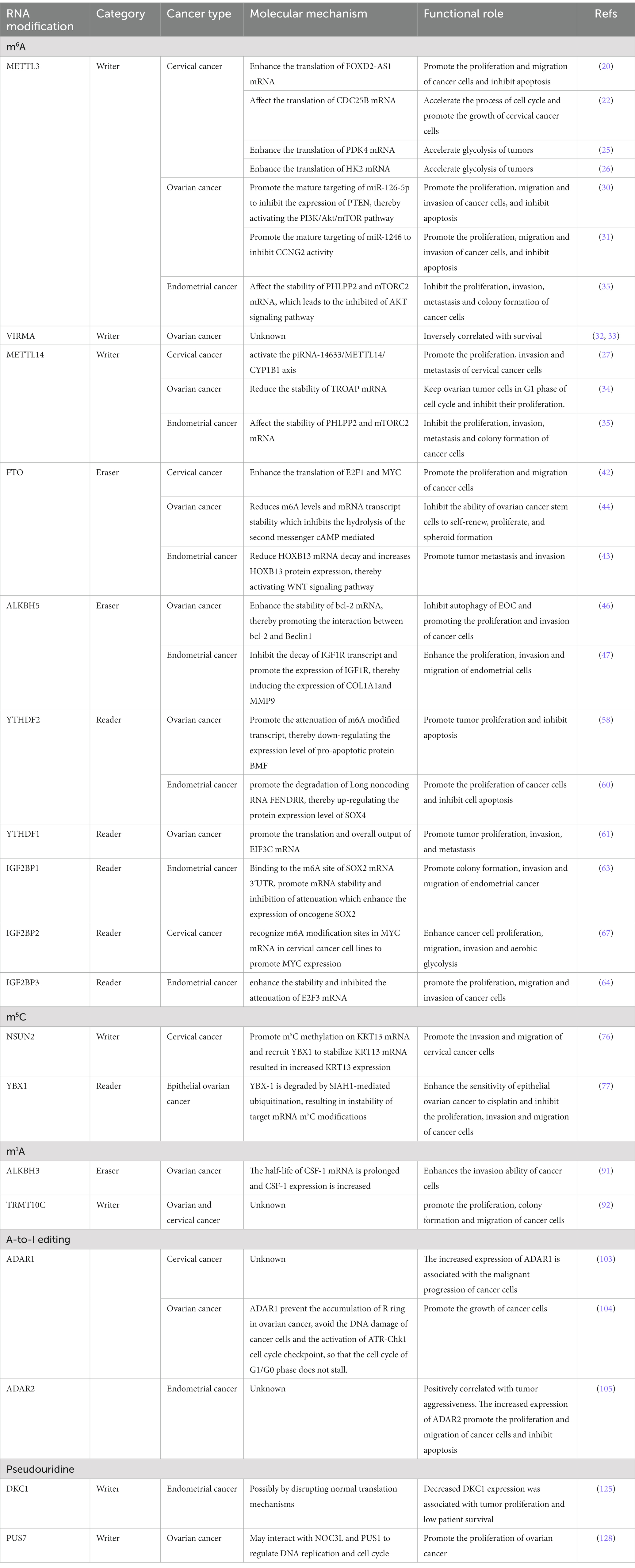
Abnormal modification of m6A methyltransferase is associated with the development and progression of gynecologic cancer, and its components have been implicated as both cancer promoters and cancer suppressors. In cervical cancer, METTL3 plays an important role in the different biological processes of cervical cancer by regulating the transcription and translation levels of different oncogenic targets. For example, in the process of cell cycle, METTL3 can enhance the translation of FOXD2-AS1 (lncRNA FOXD2 adjacent opposite strand RNA 1) mRNA, recruit the key transcription silencing factor LSD1 (lysine-specific demethylase 1) to the promoter of p21 to silence the transcription of cell cycle inhibitor p21, thus promoting the proliferation and migration of cervical cancer and inhibiting the apoptosis of cancer cells (20); In addition, CDC25B (cell division cyclin 25B) is an important factor affecting the activation of cyclin-dependent kinases (21). METTL3 can also affect the translation of CDC25B mRNA which will accelerate the process of cell cycle and promote the growth of cervical cancer cells (22). During the aerobic glycolysis of cancer cells, Pyruvate dehydrogenase kinase 4 (PDK4) is one of the most important factors which can direct carbon flux into glycolysis from oxidative phosphorylation (OXPHOS) (23) and HK2 (hexokinase 2) is a key rate-limiting enzyme in aerobic glycolysis (24). The modification of its coding region by METTL3 can enhance its translational efficiency, thereby facilitating glycolysis in cervical cancer and promoting the progression of the disease (25, 26). METTL14, also an oncogene in cervical cancer, enhanced its m6A methylation levels by being mediated by piRNA-14633 (PIWI-interacting RNA-14633) to activate the piRNA-14633/METTL14/CYP1B1 axis which promoting the proliferation, invasion and metastasis of cervical cancer cells (27). The importance of the m6A pathway in cervical cancer is well established and, as a general effect, m6A methylation is necessary to maintain high levels of translation of key transcripts in cervical cancer.
Surprisingly, in ovarian cancer, m6A methyltransferase appears to play a contradictory role. It is well known that microRNAs (miRNAs) play an important role in tumor-related diseases (28, 29). Studies have shown that METTL3 can inhibit the expression of PTEN (phosphatase and tensin homolog) by promoting the maturation of pre-miRNA-126-5p (pre-microRNA-126-5p) to deactivate PI3K/Akt/mTOR pathway (30). Or inhibiting CCNG2 (cyclin G2) activity by promoting the maturation of pre-miR-1246 (pre-microRNA-146) (31). Thus, they all ultimately led to the enhancement of the proliferation, migration and invasion ability of ovarian cancer cells, and inhibited cell apoptosis. In addition, VIRMA has also been found to be highly expressed in ovarian cancer tissues, and its expression level is negatively correlated with the survival rate (32, 33). Although the specific mechanism of action has not been clarified, some researchers have considered that VIRMA may be related to the WNT signaling pathway process in ovarian cancer (32). Unlike these two, METTL14 has the opposite effect in ovarian cancer. Overexpression of METTL14 can reduce the mRNA stability of target gene by mediating m6A methylation modification which will reduce the expression of trophinin-associated protein (TROAP), thus placing ovarian tumor cells in the G1 phase of the cell cycle and inhibiting their proliferation (34).
In endometrial cancer, METTL3 and METTL14 exhibit a tumor suppressor function. Either the mutation of METTL14 or the downregulation of METTL3 expression can impact on the mRNA stability of PHLPP2 (PH domain and leucine rich repeats protein phosphatase 2) and mTORC2 (Mechanistic target of rapamycin kinase complex 2) that the key members within the AKT pathway which leads to the activation of the AKT signaling pathway and promotes the proliferation, invasion, metastasis, and colony formation of endometrial cancer cells (35).
In summary, m6A methyltransferase has important oncogenic and tumor suppressor effects in different types of gynecological cancer. The expression of its major functions is likely to be mainly dependent on the downstream target of its specific action (Table 1).
2.2 Eraser
The m6A methylation modification process of RNA in the nucleus is dynamic and reversible, and it can be reversed by m6A demethylase. FTO (fat-mass and obesity-associated protein) and ALKBH5(a-ketoglutarate-dependent dioxygenase alkB homolog 5) are the two most important m6A demethylases currently known. Among them, FTO was the first m6A demethylase to be discovered, which is mainly made by Fe (II) and α- Ketoglutaric acid (αKG)-dependent demethylation of m6A in substrate mRNA (36). ALKBH5 is a member of the ALKB family as the second known m6A demethylase, mainly by making nuclear RNA (mainly m6A demethylation on mRNA), thereby affecting nuclear RNA output and metabolism (37). Abnormal expression of FTO and ALKBH5 is closely associated with the occurrence of various diseases, such as leukemia (38), infertility (39), and obesity (40), bladder cancer (41), etc. m6A demethylase also plays an important role in gynecological cancers.
FTO acts as an oncogene in cervical cancer. Its demethylation enhances the translation of E2F1 (E2 promoter binding factor 1) and Myc which promotes the proliferation and migration of cervical cancer cells (42). Besides, FTO also plays an oncogene role in endometrial cancer. It can reduce HOXB13 (Germline homeobox B13) mRNA decay by catalyzing the demethylation of HOXB13 mRNA 3’UTR region to eliminate the recognition of m6A modification by YTHDF2 protein which increases the expression of HOXB13 protein. Thus, the WNT signaling pathway is activated, and the expression of WNT pathway-related proteins c-myc, snail, MMP2, MMP7 and MMP7 is increased, which enhances the ability of tumor metastasis and invasion (43). However, in ovarian cancer, overexpression of FTO reduces m6A levels and mRNA transcript stability which inhibits the hydrolysis of the second messenger cAMP mediated by two phosphodiesterases, PDE1C (phosphodiesterases 1C) and PDE4B (phosphodiesterases 4B). The increase of cAMP level inhibited the ability of ovarian cancer stem cells to self-renew, proliferate and spheroid formation (44). This may be related to the epigenetic reprogramming caused by CAMP-induced PKA activation, which promotes differentiation of tumor stem cells and loss of tumor initiation ability (45).
ALKBH5, another m6A demethylase, is highly expressed in human epithelial ovarian cancer (EOC) (46). ALKBH5 demethylated the mRNA of Bcl-2 (B-cell lymphoma-2), which inhibits apoptosis, and increased its stability, thus promoting the interaction between Bcl-2 and Beclin1, inhibiting the autophagy of EOC and promoting the proliferation and invasion of cancer cells. In endometrial carcinoma, demethylase activity of ALKBH5 can inhibit the decay of IGF1R transcript and promote the expression of IGF1R (insulin-like growth factor 1 receptor), thereby inducing the expression of COL1A1(collagen type I alpha 1 chain) and MMP9(matrix metallopeptidase 9) which enhances the proliferation, invasion, and migration of endometrial cells (47).
All these reports indicate that the function of FTO and ALKBH5 in gynecologic cancer mainly depends on the demethylation of m6A, and they play an important role in the occurrence and development of gynecologic cancer.
2.3 Reader
m6A modification of RNA is dynamically and reversibly regulated by methyltransferases and demethylases, while m6A recognition protein participates in downstream target gene biology processes by recognizing m6A methylation modification information in mRNA. m6A recognition proteins are mainly YT521-B homology (YTH) domain-containing family proteins, including YTHDC1 (YTH domain containing 1), YTHDC2 (YTH domain containing 2), YTHDF1 (YTH domain family protein 1), YTHDF2 (YTH domain family protein 2) and YTHDF3 (YTH domain family protein 3). In addition, insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs, including IGF2BP1/2/3), heterogeneous nuclear ribonucleoprotein family (HNRNP family, including HNRNPA2B1/HNRNPC/HNRNPG) and eIF3 (eukaryotic translation initiation factor 3) are also part of the m6A recognition protein (48). They can be achieved by influencing RNA degradation (49), translation and decay (50), splicing (51), nuclear transport (52), stability (53) or facilitating the processing of precursor miRNAs (54) plays an important role. m6A binding proteins play an important role in the development of a variety of cancers (55–57) by specifically recognizing changes in m6A modification levels on target mRNA and regulating specific target genes after modification.
YTHDF2 is highly expressed in human ovarian cancer, and its expression level is positively correlated with the development of ovarian cancer (58). High levels of YTHDF2 can down-regulate the expression of BMF, a pro-apoptotic protein (59), by promoting the attenuation of m6A modified transcript, thus promoting the proliferation of ovarian cancer cells, and inhibiting cell apoptosis. On the other hand, in endometrial cancer, YTHDF2 can also promote the degradation of tumor suppressor gene Long noncoding RNA FENDRR, thereby up-regulating the protein expression level of SOX4(SRY-related HMG box transcription factor 4), promoting the proliferation of cancer cells, and inhibiting cell apoptosis (60).
YTHDF1 is also abnormally high expressed in human ovarian cancer. As an oncogene, YTHDF1 can affect the overall protein translation in ovarian cancer cells by promoting the translation and overall output of protein translation initiation factor EIF3C mRNA, thus promoting the proliferation, migration, and invasion of ovarian cancer cells (61).
As another important family member of m6A recognition proteins, IGF2BP1, IGF2BP2 and IGF2BP3 are mainly composed of two major domains. The K homology domain is mainly responsible for RNA binding, while the RNA recognition motif domain is responsible for stabilizing the IGF2BP-RNA complex (62). IGF2BP preferentially recognizes m6A-modified mRNAs and promotes the stability and translation of the target mRNA in an m6A-dependent manner (53). For example, in endometrial cancer, IGF2BP1, by binding to the m6A site of SOX2 mRNA 3’UTR, promotes mRNA stability and inhibition of attenuation which enhances the expression of oncogene SOX2(sex-determining region Y-box 2), thus promotes the colony formation, invasion and migration of endometrial cancer (63). In addition, IGF2BP3 can also promote the proliferation, migration, and invasion of cancer cells in human endometrial carcinoma by enhancing the stability and inhibiting the attenuation of E2F3(E2F transcription factor 3) mRNA (64). Recent studies have found that MYC is a key regulator of aerobic glycolysis and is associated with malignant progression of tumors as an oncogene (65, 66). IGF2BP2 can recognize m6A modification sites in MYC mRNA in cervical cancer cell lines to promote MYC expression, thereby enhancing cancer cell proliferation, migration, invasion, and aerobic glycolysis (67).
In summary, these proteins usually exist as carcinogenic components in different gynecological cancers, and they are involved in the malignant progression of cancer by affecting the stability, translation, decay, or output and so on of target mRNA by relying on the recognition of m6A.
3 m5C modification in gynecological cancer
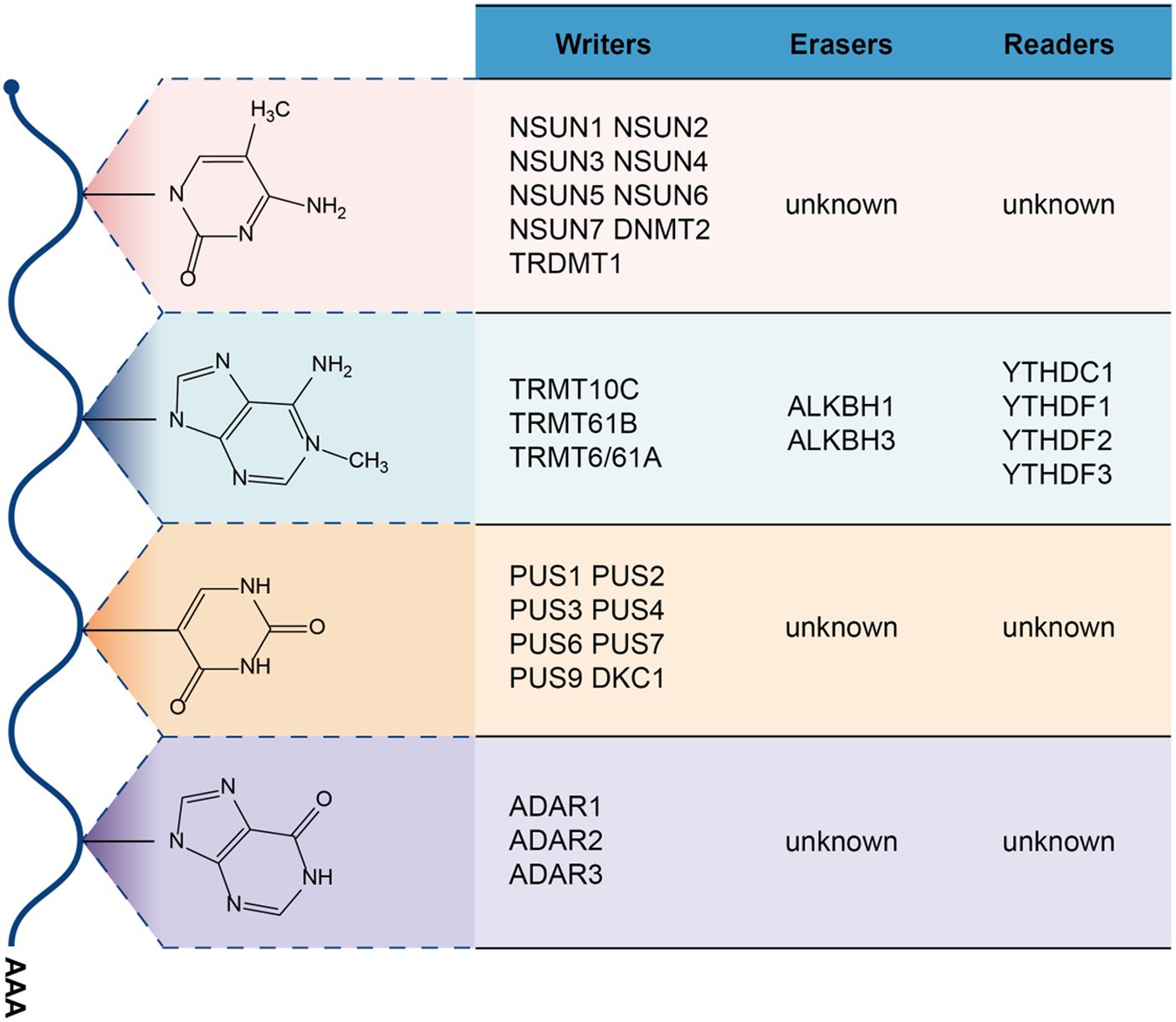
m5C modifications are ubiquitous in RNA, including mRNA, tRNA, and rRNA, and are currently used for DNA or the determination of m5C levels in RNA can generally be quantified by bisulfite sequencing (68). A variety of enzymes associated with 5-cytosine methylation modification have been identified, including NOL1/NOP2/sun family (including NSUN1/2/3/4/5/6/7) and DNMT2/TRDMT1 (DNA methyltransferase member 2/tRNA methyltransferase 1), but no clear studies have found enzymes that demethylate m5C (69), and m5C’s recognition protein is predominantly ALYREF(Aly/REF export factor, an mRNA transport adaptor) (70) and YBX1 (Y-box binding protein 1) (71) (Figure 2). Among m5C methylation modification enzymes, NSUN1 and NSUN5 are responsible for m5C methylation of cytoplasmic 28S rRNA, NSUN3 and NSUN4 are responsible for methylate mitochondrial tRNA and rRNA, and NSUN7 is responsible for methylate enhancer RNA (eRNA) (72). NSUN2, NSUN6 and DNMT2 are mainly responsible for methylate cytoplasmic tRNA, besides, NSUN2 can also methylate mRNA and vault RNA (VtRNA) (72). In the m5C recognition protein, ALYREF mainly recognizes the m5C modification site on mRNA edited by NSUN2, which is promoted The mRNA is output from the nucleus to the cytoplasm (70), while YBX1 is output by recognizing m5C on the mRNA moddifications thereby recruiting Pabpc1a enhance mRNA stability (71). Recently, many studies have shown that abnormal m5C modification promotes cancer proliferation, invasion or metastasis, such as hepatocellular carcinoma (73), bladder cancer (74), Squamous cell carcinoma of the esophagus (75). In gynecologic cancers, abnormalities in the RNA m5C modification are also associated with the progression of its malignancy.
Some studies have found that NSUN2 is highly expressed in human cervical cancer tissues, and by enhancing the methylation modification of m5C of oncogene KRT13 (keratin 13) mRNA and recruiting YBX1 to further stabilize KRT13 mRNA, the expression of KRT13 is increased, and the invasion and migration of cervical cancer cells are promoted (76). In addition, in epithelial ovarian cancer (EOC), SIAH1 (seven in absentia homolog 1), a ubiquitinating ligase, ubiquitinates YBX1 at K304 via the RING domain, making EOC sensitive to cDDP and inhibiting cancer cell proliferation, invasion, and migration (77). It may be that ubiquidization of YBX1 leads to instability of m5C modification of E2F5, YY1 and RCC2 mRNAs, thus accelerating the degradation of these target mRNAs.
At present, the research on m5C modification in gynecological cancer is very limited, what role other m5C modification related enzymes play in the development of gynecological cancer, and whether the potential role of NSUN2 in cancer can be mediated by tRNA modification still need to be further explored and studied.
4 m1A modification in gynecologic cancer
Modification of m1A occurs mainly in cytoplasmic and mitochondrial tRNAs, with 1-methyladenylation usually occurring at positions 58 and 9 of the tRNAs (78, 79). The modification can also occur on mRNA and rRNA. According to current studies, it is known that m1A methyltransferases mainly include TRMT10C(tRNA methyltransferase 10 homolog C) (80), TRMT61B(tRNA methyltransferase 61 homolog B) (79), TRMT6/61A(tRNA methyltransferase 6/tRNA methyltransferase 61 homolog A) (81), m1A main demethylases are ALKBH1(alkylation repair homolog 1) (82), ALKBH3(alkylation repair homolog 3) (83) and the recognition proteins are mainly YTHDF1-3,YTHDC1 (84). m1A modification on tRNA has been shown to promote tRNA structure stability and induce correct tRNA folding (85), and in mitochondrial mRNA, it mainly inhibits mRNA translation through TRMT61B methyltransferase (81). Abnormal expression of m1A modification on RNA is also closely related to the pathogenesis of the disease (85, 86).
CSF-1, a macrophage colony stimulating factor, regulates the migration, proliferation, differentiation and survival of macrophages and their precursors by activating its receptor CSF-1R (87). Overexpression of CSF-1/CSF-1R plays an important role in the malignant development of cancer (88–90). In ovarian cancer, ALKBH3 can extend the half-life of CSF-1 mRNA and enhance the expression of CSF-1 by making CSF-1mRNA near the origin of translation 5’UTR region m1A demethylation, thereby improving the invasion ability of ovarian cancer cells (91). In addition, TRMT10C is highly expressed in cervical cancer and ovarian cancer tissues, promoting the proliferation, colony formation and migration of ovarian cancer and cervical cancer cells, which may be related to the regulation of C-MYC-related pathways (92).
This evidence supports the important role of m1A modification in the development of gynecological cancers, but the information on the key players in the regulation of m1A modification is very limited, and there are still unknown potential key enzymes of m1A modification that have yet to be discovered.
5 A-to-I editing in gynecological cancer
RNA editing events were first identified in the sequence of COXII. transcripts in trypanosomes mitochondria (93). In the animal world, A-to-I RNA editing is the most common, and it is mainly mediated by ADAR enzymes to convert double-stranded RNA (dsRNA) substrate in adenosine is converted to inosine (94). Among them, ADAR1(Adenosine deaminase acting on RNA 1) (95) and ADAR2(Adenosine deaminase acting on RNA 2) enzymes (96) have been shown to have A-to-I editing activity. A-to-I editing modifications in RNA may lead to the production of nonsynonymous codons, leading to the diversity of translation proteins, associated with the development of diseases such as Aicardi-Goutières syndrome (97) and cancer (98, 99). Besides, A-to-I editing works by altering dsRNA in pri-miRNA structure, thereby inhibiting the processing and maturation of pri-miRNA by Drosha (100).
In recent years, the role of A-to-I editing enzymes in gynecological cancers has gradually begun to be discovered. ADAR1 prevents type I interferon in human cells by inhibiting the production of endogenous double-stranded DNA (dsRNA) in the host. The loss of ADAR1 can lead to the accumulation of dsRNA, induce Type I interferon (IFN), promote apoptosis and growth arrest (101). Therefore, in tumors, the high expression of ADAR1 is conducive to the immune escape of cancer cells. Recent studies have also confirmed that the loss of ADAR1 in tumors enhances tumor sensitivity to immunotherapy, thereby overcoming resistance to immune checkpoint blocking (102). In gynecological cancers, ADAR1 mainly acts as an oncogene. In cervical squamous cell carcinoma, the increased expression of ADAR1 is associated with the malignant progression of cancer cells, and its specific molecular mechanism needs to be further studied (103). In addition, ADAR1 is also highly expressed in human ovarian cancer tissues and is inversely associated with progression-free survival. The presence of ADAR1 maintains normal dsRNA editing, prevents R ring accumulation in ovarian cancer, avoids cancer cell DNA damage and ATR-Chk1 cell cycle checkpoint activation, prevents G1/G0 phase cell cycle stagnation, and promotes the growth of cancer cells (104). This study revealed a new ADAR1/R-loop/ATR pathway that is crucial for ovarian cancer progression, and the exploration of ADAR1 inhibitors and ATR inhibitors may be a good direction for future treatment of ovarian cancer.
The mechanism of action of ADAR2 in gynecological cancer has not been well described. Existing studies have found that ADAR2 is overexpressed in endometrial cancer, and its expression level is positively correlated with tumor aggressiveness. The increased expression of ADAR2 promote the proliferation and migration of cancer cells and inhibit apoptosis (105).
Overall, these results demonstrate that adenosine to inosine editing is an important regulatory mechanism in the development of gynecologic cancers, and targeting this pathway, specifically ADAR1, is a promising possibility for gynecologic cancer treatment.
6 Pseudouridine nucleoside modification in gynecological cancer
Pseudouridine nucleoside modification occurs after transcription and is one of the most common modifications in RNA (106), and it was first discovered in yeast (107). Pseudouridine nucleoside modification plays an important role in the functional output of tRNA and rRNA. Loss of pseudouridine nuclear modification will lead to decreased expression level of rRNA and enhanced read through activity of stop codon (108), and also affect the translation output of tRNA (109). In addition, pseudouracil nucleosidation modification can also be found in Mt-tRNA, snRNA, miRNA, and lncRNA (110). There is currently no clear research evidence for whether mRNA has naturally occurred pseudouridine modifications, but studies have found that artificially pseudouridation of mRNA can promote non-canonical base pairing at the ribosome decoding center and change the genetic code, resulting in increased protein diversity (111, 112). The main method to identify whether RNA contains pseuduridine modification is the specific binding of pseuduracil nucleoside to n-cyclohexyl-N ‘-(2-morpholine ethyl) carbodiimine methyl-p-toluenesulfonate (CMCT) (113). At present, it is known that enzymes involved in pseudouridine modification mainly include PUS1(pseudouridine synthases 1), PUS2(pseudouridine synthases 2), PUS3(pseudouridine synthases 3), PUS4(pseudouridine synthases 4), PUS6(pseudouridine synthases 6), PUS7(pseudouridine synthases 7), PUS9(pseudouridine synthases 9), DKC1(Dyskerin pseudouridine synthase 1) et al. (113, 114). The abnormalities of their modifier enzymes are involved in straightening the development process of bowel cancer (115), lung adenocarcinoma (116), neurodevelopmental disorders (117), congenital dyskeratosis (118) and other diseases. There are no known erasers or readers for pseudouridine nucleoside modification in eukaryotic cells (119).
Dyskerin protein is a pseudouracil synthetase encoded by DCK1 gene, which can convert specific uracil on ribosomal RNAs (rRNAs), nuclear RNAs (snRNAs) and messenger RNAs (mRNAs) into pseudouracil (120), and participate in many biological processes, including protecting telomere integrity and maintaining ribosomal biogenesis (121). The abnormal expression of Dyskerin is related to the poor prognosis of patients in most cancer research at present (122, 123), and it is considered that Dyskerin maintains high telomerase activity and promotes the proliferation of cancer cells (124). Different from the previous research results, Dyskerin is low in endometrial carcinoma, and the decrease of Dyskerin expression is related to the proliferation of endometrial carcinoma and the low survival rate of patients (125). It is considered that the loss of abnormal Dyskerin may promote the development of endometrial cancer by disturbing the normal translation mechanism (126, 127). In addition, some studies have also found that PUS7 is highly expressed in human ovarian cancer tissues. Through the analysis of gene pathway data, it is considered that PUS7 may interact with NOC3L and PUS1 to promote the proliferation of ovarian cancer by regulating DNA replication and cell cycle (128).
At present, the research progress of pseudouridine modification in gynecological cancer is very limited, and the specific molecular mechanism of pseudouridine synthase in gynecological cancer has not been studied in detail in known studies. Further research is needed to fully explore the role of pseudouridine modification in gynecological cancer in the future.
7 RNA-modifying enzyme-related inhibitors and gynecologic cancer
At present, the exploration of small molecule inhibitors of m6A has made great progress. In the study of m6A methyltransferase inhibitors, some researchers discovered STM2457 as a candidate specific inhibitor catalyzed by METTL3 can cause damage to AML mouse model modeling and prolong the survival time of mice (129). In addition, through drug library screening, quercetin can also act as an inhibitor of METTL3 to inhibit the proliferation of pancreatic and liver cancer cells (130). A newly synthesized compound, UZH2(lead compound 22) (131), has also been shown to have the inhibitory activity of the METTL3 enzyme. Inhibitors of m6A demethylase, such as meclofenamic acid (MA) (132), Rhein (133), R-2HG (134), FTO-04 (135), FB23 and FB23-2 (136), have been discovered in recent years. They all have FTO enzyme inhibitory activity and can be used in AML (134, 136), Glioblastoma (135) and other cancers have shown significant antitumor activity. MV1035 (137) and ALK-04 (138) as inhibitors of ALKBH5 also show tumor suppressor effects in glioblastoma and melanoma. For inhibitors of the m6A-recognition protein, a compound called “7,773” has been found to bind IGF2BP1 and inhibit its interaction with KRAS RNA (139), thereby reducing the expression of KRAS protein and downstream signaling, inhibiting the cancer-promoting activity of IGF2BP1. BTYNB (140) inhibited the malignant process of ovarian cancer by weakening the stability of IGF2BP1 on E2F1 mRNA, thus limiting the protein translation of E2F1. MO-460 (141), a newly synthesized similar based on (R)-(−)-Moracin-O, acts on the glycine-rich C-terminal domain of hnRNPA2B1 and inhibits binding of hnRNPA2B1 to the 3′-untranslated region of HIF-1αmRNA. The inhibition of HIF-1α translation can play an anticancer role.
As for inhibitor studies of other RNA-modifying enzymes, RUS0207-A006, RUS0202-G005 and JK0395-B007 have been found to have inhibitory activity of YBX1 recognition protein (142), and RUS0207-A006 and JK0395-B007 inhibited malignant melanoma cells, Proliferation of colon cancer cells and breast cancer cells. In addition, azacytidine (143) has also been found to act as an inhibitor of the RNA methyltransferase DNMT2, inhibiting the main substrate of DNMT2, tRNA (Asp) Methylation of the C38 site. and 2,3-diaryl indenones (144) as ALKBH3, a selective inhibitor that plays a significant inhibitory role in the proliferation of lung cancer cells.
More and more RNA-modifying enzyme-related inhibitors are gradually being synthesized or discovered, and their anti-cancer effects in a variety of tumors are gradually being shown to us, but their specific tumor suppression mechanisms need to be further explored.
8 Future perspectives
With the discovery and functional study of RNA modification types and various RNA modification-related regulators, the understanding of RNA modification has been greatly improved. Despite tremendous progress, there are still many unknowns about our understanding of RNA modifications and their specific regulatory mechanisms in gynecologic tumors. From a macro perspective, many of the authors, readers, and erasers of modifications are still unknown, which hinders further research into the functional mechanisms of these modifications. In addition, the mechanisms by which many known writers, readers, and erasers can selectively identify, install, or remove modifications, as well as the mechanisms by which their specific environments and spatiotemporal manipulations are regulated, remain unknown. The full range of regulatory and biological roles of mRNA modification remains largely unexplored. In some cases, one enzyme produces opposite effects in different cancer types, or even in the same cancer type. Given the diversity of RNA modifications and the sheer number of modified coding RNAs and ncrRNAs, it may not seem surprising that this contradiction sometimes arises. Therefore, more relevant studies are needed to further validate and explain the specific mechanism of RNA methylation in cancer, and to explain more rationally some of the existing conflicting studies. In addition, RNA modifications have greatly contributed to the development of therapeutics, including antisense oligonucleotides, RNA aptamers, and short-interfering RNA drugs. Given the great success of Ψ-modified mRNA vaccines for infectious diseases, the development of modified vaccines for the treatment of cancer has bright prospects. In addition, we believe that the development of small molecule inhibitors targeting RNA modification sites and RNA-modifying enzymes is very promising, which will provide a new and more targeted approach to cancer treatment. Specific to the field of gynecologic oncology, we believe that there are still many issues that need to be clarified: (1) the changes of RNA modification in different stages of gynecological cancer development are not clear; (2) Does the same RNA modification between different types of RNA (mRNA, rRNA, tRNA, lncRNA) produce different biological functions? (4) The carcinogenic and tumor suppressor functions of RNA modification regulators sometimes play opposite roles in different cancers, and their complex regulatory mechanisms need to be further studied; (5) it would be interesting to elucidate crosstalk or competition between different types of RNA modifications; (6) Whether the changes in RNA modification can be used as a predictor of treatment response in patients with gynecologic tumors needs to be studied; (7) Although drugs targeting RNA-modified enzymes have shown potential to promote the efficacy of chemotherapy or immunotherapy in preclinical studies, related research is still in its infancy, and rigorous clinical trials are needed to prove their efficacy in the future. (8) The prediction of prognostic RNA modification-related molecules is often based on bioinformatics and database mining, and further experimental verification and prospective clinical trials are needed.
Author contributions
WH: Writing – original draft. XH: Conceptualization, Writing – original draft. GC: Data curation, Writing – original draft. XL: Investigation, Writing – review & editing. YL: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangzhou Science and Technology Foundation (No. 202002030174).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goldberg, AD, Allis, CD, and Bernstein, E. Epigenetics: a landscape takes shape. Cell. (2007) 128:635–8. doi: 10.1016/j.cell.2007.02.006
2. Peixoto, P, Cartron, PF, Serandour, AA, and Hervouet, E. From 1957 to nowadays: a brief history of epigenetics. Int J Mol Sci. (2020) 21:7571. doi: 10.3390/ijms21207571
3. Chao, YL, and Pecot, CV. Targeting epigenetics in lung Cancer. Cold Spring Harb Perspect Med. (2021) 11:a038000. doi: 10.1101/cshperspect.a038000
4. Wiener, D, and Schwartz, S. The epitranscriptome beyond m(6)a. Nat Rev Genet. (2021) 22:119–31. doi: 10.1038/s41576-020-00295-8
5. Barbieri, I, and Kouzarides, T. Role of RNA modifications in cancer. Nat Rev Cancer. (2020) 20:303–22. doi: 10.1038/s41568-020-0253-2
6. Peng, F, Xu, J, Cui, B, Liang, Q, Zeng, S, He, B, et al. Oncogenic AURKA-enhanced N(6)-methyladenosine modification increases DROSHA mRNA stability to transactivate STC1 in breast cancer stem-like cells. Cell Res. (2021) 31:345–61. doi: 10.1038/s41422-020-00397-2
7. Lan, T, Li, H, Zhang, D, Xu, L, Liu, H, Hao, X, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. (2019) 18:186. doi: 10.1186/s12943-019-1106-z
8. Han, J, Wang, JZ, Yang, X, Yu, H, Zhou, R, Lu, HC, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. (2019) 18:110. doi: 10.1186/s12943-019-1036-9
9. Qian, X, Yang, J, Qiu, Q, Li, X, Jiang, C, Li, J, et al. LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J Hematol Oncol. (2021) 14:112. doi: 10.1186/s13045-021-01123-0
10. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
11. Zaccara, S, Ries, RJ, and Jaffrey, SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. (2019) 20:608–24. doi: 10.1038/s41580-019-0168-5
12. He, L, Li, H, Wu, A, Peng, Y, Shu, G, and Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. (2019) 18:176. doi: 10.1186/s12943-019-1109-9
13. Wang, P, Doxtader, KA, and Nam, Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell. (2016) 63:306–17. doi: 10.1016/j.molcel.2016.05.041
14. Ping, XL, Sun, BF, Wang, L, Xiao, W, Yang, X, Wang, WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. (2014) 24:177–89. doi: 10.1038/cr.2014.3
15. Schwartz, S, Mumbach, MR, Jovanovic, M, Wang, T, Maciag, K, Bushkin, GG, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. (2014) 8:284–96. doi: 10.1016/j.celrep.2014.05.048
16. Patil, DP, Chen, CK, Pickering, BF, Chow, A, Jackson, C, Guttman, M, et al. M(6)a RNA methylation promotes XIST-mediated transcriptional repression. Nature. (2016) 537:369–73. doi: 10.1038/nature19342
17. Wen, J, Lv, R, Ma, H, Shen, H, He, C, Wang, J, et al. Zc3h13 regulates nuclear RNA m(6)a methylation and mouse embryonic stem cell self-renewal. Mol Cell. (2018) 69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015
18. Liu, J, Yue, Y, Han, D, Wang, X, Fu, Y, Zhang, L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. (2014) 10:93–5. doi: 10.1038/nchembio.1432
19. Yue, Y, Liu, J, Cui, X, Cao, J, Luo, G, Zhang, Z, et al. VIRMA mediates preferential m(6)a mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. (2018) 4:10. doi: 10.1038/s41421-018-0019-0
20. Hu, Y, Li, Y, Huang, Y, Jin, Z, Wang, C, Wang, H, et al. METTL3 regulates the malignancy of cervical cancer via post-transcriptional regulation of RAB2B. Eur J Pharmacol. (2020) 879:173134. doi: 10.1016/j.ejphar.2020.173134
21. Aressy, B, and Ducommun, B. Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med Chem. (2008) 8:818–24. doi: 10.2174/187152008786847756
22. Li, H, Zhong, Y, Cao, G, Shi, H, Liu, Y, Li, L, et al. METTL3 promotes cell cycle progression via m(6)a/YTHDF1-dependent regulation of CDC25B translation. Int J Biol Sci. (2022) 18:3223–36. doi: 10.7150/ijbs.70335
23. Stacpoole, PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) Axis in Cancer. J Natl Cancer Inst. (2017) 109:djx071. doi: 10.1093/jnci/djx071
24. Feng, J, Li, J, Wu, L, Yu, Q, Ji, J, Wu, J, et al. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. (2020) 39:126. doi: 10.1186/s13046-020-01629-4
25. Li, Z, Peng, Y, Li, J, Chen, Z, Chen, F, Tu, J, et al. N(6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. (2020) 11:2578. doi: 10.1038/s41467-020-16306-5
26. Wang, Q, Guo, X, Li, L, Gao, Z, Su, X, Ji, M, et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. (2020) 11:911. doi: 10.1038/s41419-020-03071-y
27. Xie, Q, Li, Z, Luo, X, Wang, D, Zhou, Y, Zhao, J, et al. piRNA-14633 promotes cervical cancer cell malignancy in a METTL14-dependent m6A RNA methylation manner. J Transl Med. (2022) 20:51. doi: 10.1186/s12967-022-03257-2
28. Patel, A, Patel, S, Patel, P, Mandlik, D, Patel, K, and Tanavde, V. Salivary Exosomal miRNA-1307-5p predicts disease aggressiveness and poor prognosis in Oral squamous cell carcinoma patients. Int J Mol Sci. (2022) 23:10639. doi: 10.3390/ijms231810639
29. Han, B, Chu, C, Su, X, Zhang, N, Zhou, L, Zhang, M, et al. N6-methyladenosine-dependent primary microRNA-126 processing activated PI3K-AKT-mTOR pathway drove the development of pulmonary fibrosis induced by nanoscale carbon black particles in rats. Nanotoxicology. (2020) 14:1–20. doi: 10.1080/17435390.2019.1661041
30. Bi, X, Lv, X, Liu, D, Guo, H, Yao, G, Wang, L, et al. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther. (2021) 28:335–49. doi: 10.1038/s41417-020-00222-3
31. Bi, X, Lv, X, Liu, D, Guo, H, Yao, G, Wang, L, et al. METTL3 promotes the initiation and metastasis of ovarian cancer by inhibiting CCNG2 expression via promoting the maturation of pri-microRNA-1246. Cell Death Dis. (2021) 7:237. doi: 10.1038/s41420-021-00600-2
32. Fan, L, Lin, Y, Lei, H, Shu, G, He, L, Yan, Z, et al. A newly defined risk signature, consisting of three m(6)a RNA methylation regulators, predicts the prognosis of ovarian cancer. Aging (Albany NY). (2020) 12:18453–75. doi: 10.18632/aging.103811
33. Li, Q, Ren, CC, Chen, YN, Yang, L, Zhang, F, Wang, BJ, et al. A risk score model incorporating three m6A RNA methylation regulators and a related network of miRNAs-m6A regulators-m6A target genes to predict the prognosis of patients with ovarian Cancer. Front Cell Dev Biol. (2021) 9:703969. doi: 10.3389/fcell.2021.703969
34. Li, Y, Peng, H, Jiang, P, Zhang, J, Zhao, Y, Feng, X, et al. Downregulation of methyltransferase-like 14 promotes ovarian Cancer cell proliferation through stabilizing TROAP mRNA. Front Oncol. (2022) 12:824258. doi: 10.3389/fonc.2022.824258
35. Liu, J, Eckert, MA, Harada, BT, Liu, SM, Lu, Z, Yu, K, et al. M(6)a mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. (2018) 20:1074–83. doi: 10.1038/s41556-018-0174-4
36. Jia, G, Fu, Y, Zhao, X, Dai, Q, Zheng, G, Yang, Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. (2011) 7:885–7. doi: 10.1038/nchembio.687
37. Zheng, G, Dahl, JA, Niu, Y, Fedorcsak, P, Huang, CM, Li, CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. (2013) 49:18–29. doi: 10.1016/j.molcel.2012.10.015
38. Wang, J, Li, Y, Wang, P, Han, G, Zhang, T, Chang, J, et al. Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling Axis. Cell Stem Cell. (2020) 27:81–97.e8. doi: 10.1016/j.stem.2020.04.001
39. Tang, C, Klukovich, R, Peng, H, Wang, Z, Yu, T, Zhang, Y, et al. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3'-UTR mRNAs in male germ cells. Proc Natl Acad Sci U S A. (2018) 115:E325–33. doi: 10.1073/pnas.1717794115
40. Loos, RJ, and Yeo, GS. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. (2014) 10:51–61. doi: 10.1038/nrendo.2013.227
41. Tao, L, Mu, X, Chen, H, Jin, D, Zhang, R, Zhao, Y, et al. FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clin Transl Med. (2021) 11:e310. doi: 10.1002/ctm2.310
42. Zou, D, Dong, L, Li, C, Yin, Z, Rao, S, and Zhou, Q. The m(6)a eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. (2019) 19:321. doi: 10.1186/s12935-019-1045-1
43. zhang, L, Wan, Y, Zhang, Z, Jiang, Y, Lang, J, Cheng, W, et al. FTO demethylates m6A modifications in HOXB13 mRNA and promotes endometrial cancer metastasis by activating the WNT signalling pathway. RNA Biol. (2021) 18:1265–78. doi: 10.1080/15476286.2020.1841458
44. Huang, H, Wang, Y, Kandpal, M, Zhao, G, Cardenas, H, Ji, Y, et al. FTO-dependent N (6)-Methyladenosine modifications inhibit ovarian Cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res. (2020) 80:3200–14. doi: 10.1158/0008-5472.CAN-19-4044
45. Pattabiraman, DR, Bierie, B, Kober, KI, Thiru, P, Krall, JA, Zill, C, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. (2016) 351:aad3680. doi: 10.1126/science.aad3680
46. Zhu, H, Gan, X, Jiang, X, Diao, S, Wu, H, and Hu, J. ALKBH5 inhibited autophagy of epithelial ovarian cancer through miR-7 and BCL-2. J Exp Clin Cancer Res. (2019) 38:163. doi: 10.1186/s13046-019-1159-2
47. Pu, X, Gu, Z, and Gu, Z. ALKBH5 regulates IGF1R expression to promote the proliferation and Tumorigenicity of endometrial Cancer. J Cancer. (2020) 11:5612–22. doi: 10.7150/jca.46097
48. Wang, T, Kong, S, Tao, M, and Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. (2020) 19:88. doi: 10.1186/s12943-020-01204-7
49. Lee, Y, Choe, J, Park, OH, and Kim, YK. Molecular mechanisms driving mRNA degradation by m(6)a modification. Trends Genet. (2020) 36:177–88. doi: 10.1016/j.tig.2019.12.007
50. Shi, H, Wang, X, Lu, Z, Zhao, BS, Ma, H, Hsu, PJ, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. (2017) 27:315–28. doi: 10.1038/cr.2017.15
51. Roundtree, IA, and He, C. Nuclear m(6)a reader YTHDC1 regulates mRNA splicing. Trends Genet. (2016) 32:320–1. doi: 10.1016/j.tig.2016.03.006
52. Roundtree, IA, Luo, GZ, Zhang, Z, Wang, X, Zhou, T, Cui, Y, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. (2017) 6:6. doi: 10.7554/eLife.31311
53. Huang, H, Weng, H, Sun, W, Qin, X, Shi, H, Wu, H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. (2018) 20:285–95. doi: 10.1038/s41556-018-0045-z
54. Alarcón, CR, Goodarzi, H, Lee, H, Liu, X, Tavazoie, S, and Tavazoie, SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. (2015) 162:1299–308. doi: 10.1016/j.cell.2015.08.011
55. Li, Q, Ni, Y, Zhang, L, Jiang, R, Xu, J, Yang, H, et al. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. (2021) 6:76. doi: 10.1038/s41392-020-00453-8
56. Wang, S, Gao, S, Zeng, Y, Zhu, L, Mo, Y, Wong, CC, et al. N6-Methyladenosine reader YTHDF1 promotes ARHGEF2 translation and RhoA signaling in colorectal Cancer. Gastroenterology. (2022) 162:1183–96. doi: 10.1053/j.gastro.2021.12.269
57. Ma, L, Chen, T, Zhang, X, Miao, Y, Tian, X, Yu, K, et al. The m(6)a reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. (2021) 38:101801. doi: 10.1016/j.redox.2020.101801
58. Xu, F, Li, J, Ni, M, Cheng, J, Zhao, H, Wang, S, et al. FBW7 suppresses ovarian cancer development by targeting the N (6)-methyladenosine binding protein YTHDF2. Mol Cancer. (2021) 20:45. doi: 10.1186/s12943-021-01340-8
59. Puthalakath, H, Villunger, A, O'Reilly, LA, Beaumont, JG, Coultas, L, Cheney, RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. (2001) 293:1829–32. doi: 10.1126/science.1062257
60. Shen, J, Feng, XP, Hu, RB, Wang, H, Wang, YL, Qian, JH, et al. N-methyladenosine reader YTHDF2-mediated long noncoding RNA FENDRR degradation promotes cell proliferation in endometrioid endometrial carcinoma. Lab Invest. (2021) 101:775–84. doi: 10.1038/s41374-021-00543-3
61. Liu, T, Wei, Q, Jin, J, Luo, Q, Liu, Y, Yang, Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. (2020) 48:3816–31. doi: 10.1093/nar/gkaa048
62. Wachter, K, Kohn, M, Stohr, N, and Hüttelmaier, S. Subcellular localization and RNP formation of IGF2BPs (IGF2 mRNA-binding proteins) is modulated by distinct RNA-binding domains. Biol Chem. (2013) 394:1077–90. doi: 10.1515/hsz-2013-0111
63. Xue, T, Liu, X, Zhang, M, E, Q, Liu, S, Zou, M, et al. PADI2-catalyzed MEK1 Citrullination activates ERK1/2 and promotes IGF2BP1-mediated SOX2 mRNA stability in endometrial Cancer. Adv Sci (Weinh). (2021) 8:2002831. doi: 10.1002/advs.202002831
64. Wang, C, Kong, F, Ma, J, Miao, J, Su, P, Yang, H, et al. IGF2BP3 enhances the mRNA stability of E2F3 by interacting with LINC00958 to promote endometrial carcinoma progression. Cell Death Dis. (2022) 8:279. doi: 10.1038/s41420-022-01045-x
65. Koppenol, WH, Bounds, PL, and Dang, CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. (2011) 11:325–37. doi: 10.1038/nrc3038
66. Ye, M, Dong, S, Hou, H, Zhang, T, and Shen, M. Oncogenic role of Long noncoding RNAMALAT1 in thyroid Cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. (2021) 23:1–12. doi: 10.1016/j.omtn.2020.09.023
67. Hu, C, Liu, T, Han, C, Xuan, Y, Jiang, D, Sun, Y, et al. HPV E6/E7 promotes aerobic glycolysis in cervical cancer by regulating IGF2BP2 to stabilize m(6)A-MYC expression. Int J Biol Sci. (2022) 18:507–21. doi: 10.7150/ijbs.67770
68. Gilbert, WV, Bell, TA, and Schaening, C. Messenger RNA modifications: form, distribution, and function. Science. (2016) 352:1408–12. doi: 10.1126/science.aad8711
69. Nombela, P, Miguel-Lopez, B, and Blanco, S. The role of m(6)a, m(5)C and psi RNA modifications in cancer: novel therapeutic opportunities. Mol Cancer. (2021) 20:18. doi: 10.1186/s12943-020-01263-w
70. Yang, X, Yang, Y, Sun, BF, Chen, YS, Xu, JW, Lai, WY, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. (2017) 27:606–25. doi: 10.1038/cr.2017.55
71. Yang, Y, Wang, L, Han, X, Yang, WL, Zhang, M, Ma, HL, et al. RNA 5-Methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol Cell. (2019) 75:1188–1202.e11. doi: 10.1016/j.molcel.2019.06.033
72. Bohnsack, KE, Höbartner, C, and Bohnsack, MT. Eukaryotic 5-methylcytosine (m5C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes (Basel). (2019) 10:102. doi: 10.3390/genes10020102
73. Sun, Z, Xue, S, Zhang, M, Xu, H, Hu, X, Chen, S, et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. (2020) 39:6906–19. doi: 10.1038/s41388-020-01475-w
74. Chen, X, Li, A, Sun, BF, Yang, Y, Han, YN, Yuan, X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. (2019) 21:978–90. doi: 10.1038/s41556-019-0361-y
75. Su, J, Wu, G, Ye, Y, Zhang, J, Zeng, L, Huang, X, et al. NSUN2-mediated RNA 5-methylcytosine promotes esophageal squamous cell carcinoma progression via LIN28B-dependent GRB2 mRNA stabilization. Oncogene. (2021) 40:5814–28. doi: 10.1038/s41388-021-01978-0
76. Wang, L, Zhang, J, Su, Y, Maimaitiyiming, Y, Yang, S, Shen, Z, et al. Distinct roles of m(5)C RNA methyltransferase NSUN2 in major gynecologic cancers. Front Oncol. (2022) 12:786266. doi: 10.3389/fonc.2022.786266
77. Gao, W, Chen, L, Lin, L, Yang, M, Li, T, Wei, H, et al. SIAH1 reverses chemoresistance in epithelial ovarian cancer via ubiquitination of YBX-1. Oncogene. (2022) 11:13. doi: 10.1038/s41389-022-00387-6
78. Vilardo, E, Nachbagauer, C, Buzet, A, Taschner, A, Holzmann, J, and Rossmanith, W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase—extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. (2012) 40:11583–93. doi: 10.1093/nar/gks910
79. Chujo, T, and Suzuki, T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. (2012) 18:2269–76. doi: 10.1261/rna.035600.112
80. Safra, M, Sas-Chen, A, Nir, R, Winkler, R, Nachshon, A, Bar-Yaacov, D, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. (2017) 551:251–5. doi: 10.1038/nature24456
81. Li, X, Xiong, X, Zhang, M, Wang, K, Chen, Y, Zhou, J, et al. Base-resolution mapping reveals distinct m(1)a Methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. (2017) 68:993–1005.e9. doi: 10.1016/j.molcel.2017.10.019
82. Liu, F, Clark, W, Luo, G, Wang, X, Fu, Y, Wei, J, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. (2016) 167:816–828.e16. doi: 10.1016/j.cell.2016.09.038
83. Chen, Z, Qi, M, Shen, B, Luo, G, Wu, Y, Li, J, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. (2019) 47:2533–45. doi: 10.1093/nar/gky1250
84. Dai, X, Wang, T, Gonzalez, G, and Wang, Y. Identification of YTH domain-containing proteins as the readers for N1-Methyladenosine in RNA. Anal Chem. (2018) 90:6380–4. doi: 10.1021/acs.analchem.8b01703
85. Shafik, AM, Zhou, H, Lim, J, Dickinson, B, and Jin, P. Dysregulated mitochondrial and cytosolic tRNA m1A methylation in Alzheimer's disease. Hum Mol Genet. (2022) 31:1673–80. doi: 10.1093/hmg/ddab357
86. Esteve-Puig, R, Climent, F, Piñeyro, D, Domingo-Domènech, E, Davalos, V, Encuentra, M, et al. Epigenetic loss of m1A RNA demethylase ALKBH3 in Hodgkin lymphoma targets collagen, conferring poor clinical outcome. Blood. (2021) 137:994–9. doi: 10.1182/blood.2020005823
87. Hume, DA, and MacDonald, KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. (2012) 119:1810–20. doi: 10.1182/blood-2011-09-379214
88. Sossey-Alaoui, K, Pluskota, E, Bialkowska, K, Szpak, D, Parker, Y, Morrison, CD, et al. Kindlin-2 regulates the growth of breast Cancer tumors by activating CSF-1-mediated macrophage infiltration. Cancer Res. (2017) 77:5129–41. doi: 10.1158/0008-5472.CAN-16-2337
89. Menke, J, Kriegsmann, J, Schimanski, CC, Schwartz, MM, Schwarting, A, and Kelley, VR. Autocrine CSF-1 and CSF-1 receptor coexpression promotes renal cell carcinoma growth. Cancer Res. (2012) 72:187–200. doi: 10.1158/0008-5472.CAN-11-1232
90. Hung, JY, Horn, D, Woodruff, K, Prihoda, T, LeSaux, C, Peters, J, et al. Colony-stimulating factor 1 potentiates lung cancer bone metastasis. Lab Invest. (2014) 94:371–81. doi: 10.1038/labinvest.2014.1
91. Woo, HH, and Chambers, SK. Human ALKBH3-induced m1A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim Biophys Acta Gene Regul Mech. (2019) 1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008
92. Wang, Q, Zhang, Q, Huang, Y, and Zhang, J. m1A regulator TRMT10C predicts poorer survival and contributes to malignant behavior in gynecological cancers. DNA Cell Biol. (2020) 39:1767–78. doi: 10.1089/dna.2020.5624
93. Benne, R, van den Burg, J, Brakenhoff, JPJ, Sloof, P, van Boom, JH, and Tromp, MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. (1986) 46:819–26. doi: 10.1016/0092-8674(86)90063-2
94. Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem. (2010) 79:321–49. doi: 10.1146/annurev-biochem-060208-105251
95. Kim, U, Wang, Y, Sanford, T, Zeng, Y, and Nishikura, K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci U S A. (1994) 91:11457–61. doi: 10.1073/pnas.91.24.11457
96. Lai, F, Chen, CX, Carter, KC, and Nishikura, K. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol Cell Biol. (1997) 17:2413–24. doi: 10.1128/MCB.17.5.2413
97. Rice, GI, Kasher, PR, Forte, GMA, Mannion, NM, Greenwood, SM, Szynkiewicz, M, et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet. (2012) 44:1243–8. doi: 10.1038/ng.2414
98. Ma, C, Wang, X, Yang, F, Zang, Y, Liu, J, Wang, X, et al. Circular RNA hsa_circ_0004872 inhibits gastric cancer progression via the miR-224/Smad4/ADAR1 successive regulatory circuit. Mol Cancer. (2020) 19:157. doi: 10.1186/s12943-020-01268-5
99. Ramírez-Moya, J, Miliotis, C, Baker, AR, Gregory, RI, Slack, FJ, and Santisteban, P. An ADAR1-dependent RNA editing event in the cyclin-dependent kinase CDK13 promotes thyroid cancer hallmarks. Mol Cancer. (2021) 20:115. doi: 10.1186/s12943-021-01401-y
100. Song, B, Shiromoto, Y, Minakuchi, M, and Nishikura, K. The role of RNA editing enzyme ADAR1 in human disease. Wiley Interdiscip Rev RNA. (2022) 13:e1665. doi: 10.1002/wrna.1665
101. Chung, H, Calis, JJA, Wu, X, Sun, T, Yu, Y, Sarbanes, SL, et al. Human ADAR1 prevents endogenous RNA from triggering translational shutdown. Cell. (2018) 172:811–824.e14. doi: 10.1016/j.cell.2017.12.038
102. Ishizuka, JJ, Manguso, RT, Cheruiyot, CK, Bi, K, Panda, A, Iracheta-Vellve, A, et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature. (2019) 565:43–8. doi: 10.1038/s41586-018-0768-9
103. Chen, Y, Wang, H, Lin, W, and Shuai, P. ADAR1 overexpression is associated with cervical cancer progression and angiogenesis. Diagn Pathol. (2017) 12:12. doi: 10.1186/s13000-017-0600-0
104. Cui, H, Yi, Q, Tian, M, Yang, HT, Liang, Y, Huang, J, et al. ADAR1 prevents R-loop accumulation-driven ATR pathway activation in ovarian Cancer. J Cancer. (2022) 13:2397–412. doi: 10.7150/jca.72108
105. Altadill, T, Dowdy, TM, Gill, K, Reques, A, Menon, SS, Moiola, CP, et al. Metabolomic and Lipidomic profiling identifies the role of the RNA editing pathway in endometrial carcinogenesis. Sci Rep. (2017) 7:8803. doi: 10.1038/s41598-017-09169-2
106. Hamma, T, and Ferre-D'Amare, AR. Pseudouridine synthases. Chem Biol. (2006) 13:1125–35. doi: 10.1016/j.chembiol.2006.09.009
107. Davis, FF, and Allen, FW. Ribonucleic acids from yeast which contain a fifth nucleotide. J Biol Chem. (1957) 227:907–15. doi: 10.1016/S0021-9258(18)70770-9
108. Liang, XH, Liu, Q, and Fournier, MJ. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell. (2007) 28:965–77. doi: 10.1016/j.molcel.2007.10.012
109. Han, L, Kon, Y, and Phizicky, EM. Functional importance of Psi38 and Psi39 in distinct tRNAs, amplified for tRNAGln(UUG) by unexpected temperature sensitivity of the s2U modification in yeast. RNA. (2015) 21:188–201. doi: 10.1261/rna.048173.114
110. Zhao, BS, and He, C. Pseudouridine in a new era of RNA modifications. Cell Res. (2015) 25:153–4. doi: 10.1038/cr.2014.143
111. Karijolich, J, and Yu, YT. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature. (2011) 474:395–8. doi: 10.1038/nature10165
112. Fernández, IS, Ng, CL, Kelley, AC, Wu, G, Yu, YT, and Ramakrishnan, V. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature. (2013) 500:107–10. doi: 10.1038/nature12302
113. Carlile, TM, Rojas-Duran, MF, Zinshteyn, B, Shin, H, Bartoli, KM, and Gilbert, WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. (2014) 515:143–6. doi: 10.1038/nature13802
114. Heiss, NS, Knight, SW, Vulliamy, TJ, Klauck, SM, Wiemann, S, Mason, PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. (1998) 19:32–8. doi: 10.1038/ng0598-32
115. Kan, G, Wang, Z, Sheng, C, Chen, G, Yao, C, Mao, Y, et al. Dual inhibition of DKC1 and MEK1/2 synergistically restrains the growth of colorectal Cancer cells. Adv Sci (Weinh). (2021) 8:2004344. doi: 10.1002/advs.202004344
116. Kan, G, Wang, Z, Sheng, C, Yao, C, Mao, Y, and Chen, S. Inhibition of DKC1 induces telomere-related senescence and apoptosis in lung adenocarcinoma. J Transl Med. (2021) 19:161. doi: 10.1186/s12967-021-02827-0
117. Nøstvik, M, Kateta, SM, Schönewolf-Greulich, B, Afenjar, A, Barth, M, Boschann, F, et al. Clinical and molecular delineation of PUS3-associated neurodevelopmental disorders. Clin Genet. (2021) 100:628–33. doi: 10.1111/cge.14051
118. Marrone, A, and Mason, PJ. Dyskeratosis congenita. Cell Mol Life Sci. (2003) 60:507–17. doi: 10.1007/s000180300042
119. Penzo, M, Guerrieri, A, Zacchini, F, Treré, D, and Montanaro, L. RNA Pseudouridylation in physiology and medicine: for better and for worse. Genes (Basel). (2017) 8:301. doi: 10.3390/genes8110301
120. Schwartz, S, Bernstein, DA, Mumbach, MR, Jovanovic, M, Herbst, RH, León-Ricardo, BX, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. (2014) 159:148–62. doi: 10.1016/j.cell.2014.08.028
121. Angrisani, A, Vicidomini, R, Turano, M, and Furia, M. Human dyskerin: Beyond telomeres. Biol Chem. (2014) 395:593–610. doi: 10.1515/hsz-2013-0287
122. Sieron, P, Hader, C, Hatina, J, Engers, R, Wlazlinski, A, Müller, M, et al. DKC1 overexpression associated with prostate cancer progression. Br J Cancer. (2009) 101:1410–6. doi: 10.1038/sj.bjc.6605299
123. Liu, B, Zhang, J, Huang, C, and Liu, H. Dyskerin overexpression in human hepatocellular carcinoma is associated with advanced clinical stage and poor patient prognosis. PloS One. (2012) 7:e43147. doi: 10.1371/journal.pone.0043147
124. Khattar, E, Kumar, P, Liu, CY, Akıncılar, SC, Raju, A, Lakshmanan, M, et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J Clin Invest. (2016) 126:4045–60. doi: 10.1172/JCI86042
125. Alnafakh, R, Saretzki, G, Midgley, A, Flynn, J, Kamal, AM, Dobson, L, et al. Aberrant Dyskerin expression is related to proliferation and poor survival in endometrial Cancer. Cancers (Basel). (2021) 13:273. doi: 10.3390/cancers13020273
126. Bellodi, C, Krasnykh, O, Haynes, N, Theodoropoulou, M, Peng, G, Montanaro, L, et al. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. (2010) 70:6026–35. doi: 10.1158/0008-5472.CAN-09-4730
127. Montanaro, L, Calienni, M, Bertoni, S, Rocchi, L, Sansone, P, Storci, G, et al. Novel dyskerin-mediated mechanism of p53 inactivation through defective mRNA translation. Cancer Res. (2010) 70:4767–77. doi: 10.1158/0008-5472.CAN-09-4024
128. Li, H, Chen, L, Han, Y, Zhang, F, Wang, Y, Han, Y, et al. The identification of RNA modification gene PUS7 as a potential biomarker of ovarian Cancer. Biology (Basel). (2021) 10:1130. doi: 10.3390/biology10111130
129. Yankova, E, Blackaby, W, Albertella, M, Rak, J, de Braekeleer, E, Tsagkogeorga, G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. (2021) 593:597–601. doi: 10.1038/s41586-021-03536-w
130. du, Y, Yuan, Y, Xu, L, Zhao, F, Wang, W, Xu, Y, et al. Discovery of METTL3 small molecule inhibitors by virtual screening of natural products. Front Pharmacol. (2022) 13:878135. doi: 10.3389/fphar.2022.878135
131. Dolbois, A, Bedi, RK, Bochenkova, E, Müller, A, Moroz-Omori, EV, Huang, D, et al. 1,4,9-Triazaspiro[5.5] undecan-2-one derivatives as potent and selective METTL3 inhibitors. J Med Chem. (2021) 64:12738–60. doi: 10.1021/acs.jmedchem.1c00773
132. Huang, Y, Yan, J, Li, Q, Li, J, Gong, S, Zhou, H, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. (2015) 43:373–84. doi: 10.1093/nar/gku1276
133. Chen, B, Ye, F, Yu, L, Jia, G, Huang, X, Zhang, X, et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. (2012) 134:17963–71. doi: 10.1021/ja3064149
134. Su, R, Dong, L, Li, C, Nachtergaele, S, Wunderlich, M, Qing, Y, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)a/MYC/CEBPA signaling. Cell. (2018) 172:90–105.e23. doi: 10.1016/j.cell.2017.11.031
135. Huff, S, Tiwari, SK, Gonzalez, GM, Wang, Y, and Rana, TM. M(6)A-RNA demethylase FTO inhibitors impair self-renewal in glioblastoma stem cells. ACS Chem Biol. (2021) 16:324–33. doi: 10.1021/acschembio.0c00841
136. Huang, Y, Su, R, Sheng, Y, Dong, L, Dong, Z, Xu, H, et al. Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. (2019) 35:677–691.e10. doi: 10.1016/j.ccell.2019.03.006
137. Malacrida, A, Rivara, M, di Domizio, A, Cislaghi, G, Miloso, M, Zuliani, V, et al. 3D proteome-wide scale screening and activity evaluation of a new ALKBH5 inhibitor in U87 glioblastoma cell line. Bioorg Med Chem. (2020) 28:115300. doi: 10.1016/j.bmc.2019.115300
138. Li, N, Kang, Y, Wang, L, Huff, S, Tang, R, Hui, H, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci U S A. (2020) 117:20159–70. doi: 10.1073/pnas.1918986117
139. Wallis, N, Oberman, F, Shurrush, K, Germain, N, Greenwald, G, Gershon, T, et al. Small molecule inhibitor of Igf2bp1 represses Kras and a pro-oncogenic phenotype in cancer cells. RNA Biol. (2022) 19:26–43. doi: 10.1080/15476286.2021.2010983
140. Müller, S, Bley, N, Busch, B, Glaß, M, Lederer, M, Misiak, C, et al. The oncofetal RNA-binding protein IGF2BP1 is a druggable, post-transcriptional super-enhancer of E2F-driven gene expression in cancer. Nucleic Acids Res. (2020) 48:8576–90. doi: 10.1093/nar/gkaa653
141. Soung, NK, Kim, HM, Asami, Y, Kim, DH, Cho, Y, Naik, R, et al. Mechanism of the natural product moracin-O derived MO-460 and its targeting protein hnRNPA2B1 on HIF-1alpha inhibition. Exp Mol Med. (2019) 51:1–14. doi: 10.1038/s12276-018-0200-4
142. Trevarton, A, Zhou, Y, Yang, D, Rewcastle, GW, Flanagan, JU, Braithwaite, A, et al. Orthogonal assays for the identification of inhibitors of the single-stranded nucleic acid binding protein YB-1. Acta Pharm Sin B. (2019) 9:997–1007. doi: 10.1016/j.apsb.2018.12.011
143. Schaefer, M, Hagemann, S, Hanna, K, and Lyko, F. Azacytidine inhibits RNA methylation at DNMT2 target sites in human cancer cell lines. Cancer Res. (2009) 69:8127–32. doi: 10.1158/0008-5472.CAN-09-0458
Keywords: gynecological cancer, RNA modification, epigenetics, N6-methyladenosine, 5-methylcytosine
Citation: He W, Hong X, Chen G, Luo X and Lin Y (2024) RNA modifications in gynecological cancer: current status and future directions. Front. Med. 11:1314075. doi: 10.3389/fmed.2024.1314075
Edited by:
A. Seval Ozgu-Erdinc, Ankara City Hospital, TürkiyeReviewed by:
Komal Ramani, Cedars Sinai Medical Center, United StatesAslihan Yurtkal, Kafkas University, Türkiye
Copyright © 2024 He, Hong, Chen, Luo and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Lin, MTM1ODAzMTE3MjZAMTYzLmNvbQ==; Xiping Luo, bHVveGlwaW5nMzMzQDEyNi5jb20=
†These authors have contributed equally to this work
 Wanshan He
Wanshan He Xiaoshan Hong†
Xiaoshan Hong† Guanqiao Chen
Guanqiao Chen Yu Lin
Yu Lin