
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med. , 20 February 2024
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1305184
Even though the global SARS-CoV-2 pandemic is considered to be over for the time being, there are fears of more frequent infections with the virus and new mutations in the coming winter months. In this context, one surprising lesson from the outgoing suffer seems to be related to antidepressants (AD), which might have the potential to be beneficial for prevention of SARS-CoV-2 infections and critical COVID-19 outcomes (1, 2). The vast majority of large-scale retrospective studies evaluating more 20000 patients point into this direction (Table 1). Especially selective serotonin reuptake inhibitors (SSRI) and serotonin–norepinephrine reuptake inhibitors (SNRI) seem to be efficient in this context. It is not clear whether this advantage is specific only for serotonergic AD as these are by far the most commonly prescribed AD or for the whole AD substance class itself, which comprised also AD with no direct serotonergic potential (e.g. bupropion—however being indirectly involved in the regulation of neuronal serotonin activity as found in rat brain) (14). The underlying biological mechanisms are assumed to be more sophisticated. In addition to direct inhibitory effects on several steps of the viral infection process itself (Supplementary Figure 1), the mitigation of the remarkable and sustainable pro-inflammatory cellular response to SARS-CoV-2 (“hyperinflammation”) is favored (1, 2). Within the latter, the most prominent mechanisms are activation of the intracellular sigma-1 receptor – IRE 1-System by SSRI (first demonstrated for fluvoxamine) (15) and the functional inhibition of acid sphingomyelinase (FIASMA) by most AD, including SSRI, SNRI, tricyclics, mirtazapine, trazodone and bupropion (1, 16).
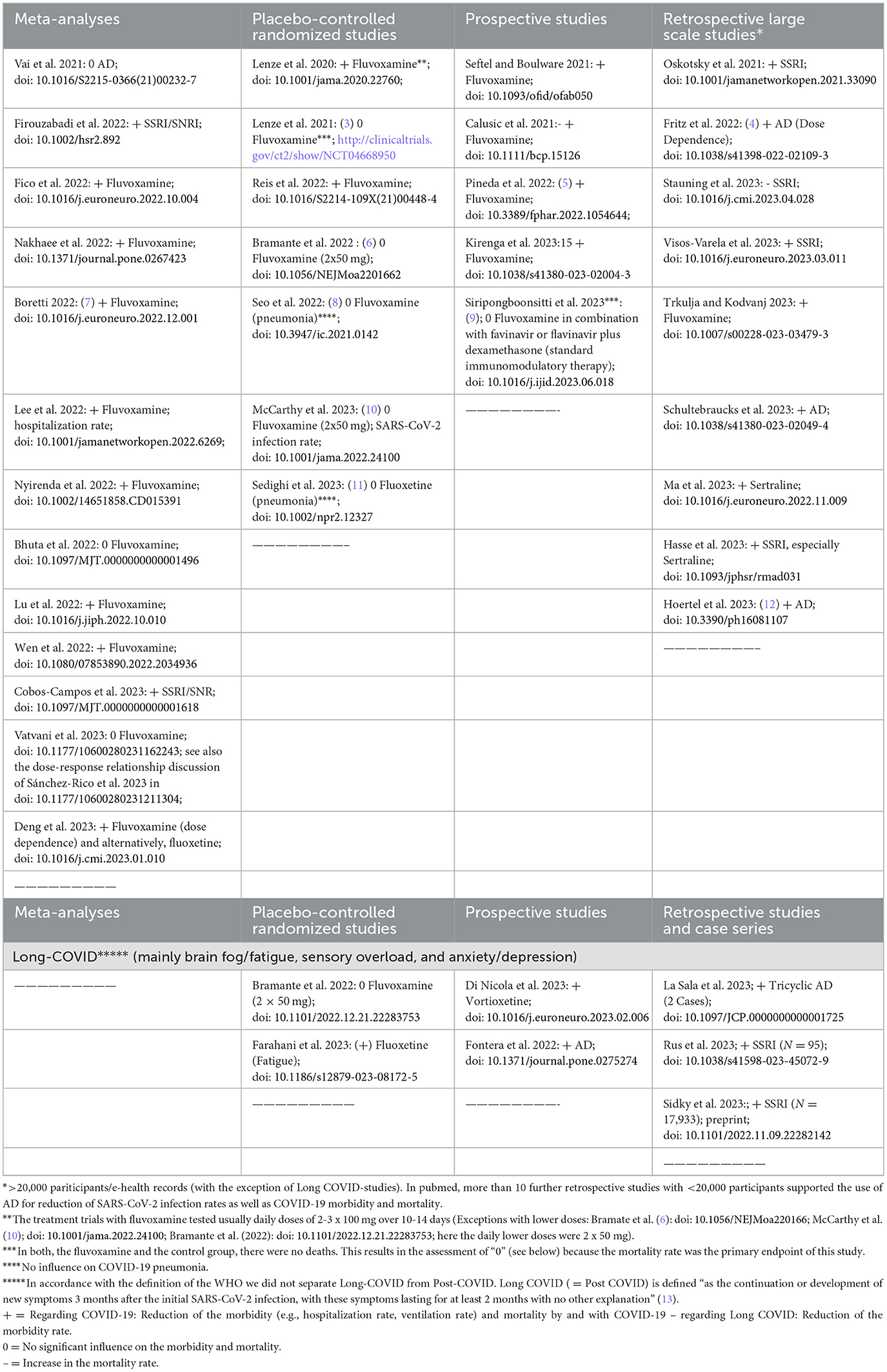
Table 1. Brief overview of clinical studies testing the effectiveness/efficacy of antidepressants (AD) on COVID-19 severity/sequels (as of 09/25/2023 found in pubmed; https://pubmed.ncbi.nlm.nih.gov/).
For further evidence that AD might be beneficial for COVID-19 and also for its aftermath, the post-acute COVID-19 syndrome (PACS = Long COVID syndrome), prospective studies, placebo-controlled randomized controlled studies (RCT) and meta-analyses should be considered above all. As of 09/25/2023, literature research in pubmed revealed five prospective studies (plus 2 for Long COVID), 7 RCT (plus 2 for Long COVID) and 12 meta-analyses in this context (Table 1). Among the meta-analyses (incorporating primarily RCT and prospective studies, mostly with fluvoxamine), only three (one for any AD and two for fluvoxamine) showed no “anti-COVID-19 effect” (see these meta-analyses in bold letters in Table 1), but notably any negative effects on COVID-19 outcomes, which means that AD were not associated with a deterioration of COVID-19. A possible explanation for the lack of a “protective” effect could be following a dose-response relationship, i.e., that in the case of fluvoxamine, its tested dosage (2 x 50 mg/d) (6, 10) was simply too low. This assumption is supported by the results of a large retrospective study exploring the effects of AD on COVID-19 severity and mortality (N = 25 034) (4). Also bearing in mind the assumed mechanisms of action, which make the major effect in particular at the beginning of the infection plausible, the current evidence suggests that the earlier the AD were administered to patients exposed to SARS-CoV-2 or showing first mild-to-moderate COVID-19 symptoms, the more favorable was the outcome regarding SARS-CoV-2 infection rates, COVID-19 severity and related mortality (7, 17). On the other hand, AD seemed to have no relevant impact when they were added to the standard treatment of a full-blown COVID-19 pneumonia (Table 1) (8, 11). The tolerability of the AD (mostly fluvoxamine, sertraline and fluoxetine) usually added to the standard treatment (“add on”) of COVID-19, was reported to be generally well across all prospective, randomized controlled studies and meta-analyses presented in Table 1.
According to Long COVID, the clinical study situation is still limited, however, showing first preliminary promising results for AD (Table 1), even when these drugs were administered the first time during this condition (see retrospective studies and case series in Table 1) and not only specifically within the beginning of the SARS-CoV-2 infection.
In the light of GRADE (18), we think that the quality evidence for “add on” fluvoxamine for prevention of SARS-CoV-2 infections and severe COVID-19 has reached currently the low-to-moderate level. Thus, we support the utilization of this cheaply and easily available drug especially in regions where vaccination and approved “anti-COVID-19” immunomodulatory medications are far from being available. At this juncture, there are first promising results from prospective real-world studies carried out at Honduras (5) and Uganda (19). The same could be true for the whole substance class of AD according to the results of two large scale retrospective studies currently having tested the broadest spectrum of AD (4, 12).
As the pandemic is going to reach its end, it will be rather difficult to provide the next step necessary for further increasing the evidence quality, i.e., performing a prospective randomized controlled trial that compares the efficacy and tolerability of fluvoxamine or another AD with an approved drug for prevention of severe COVID-19. A most recent randomized prospective study found no superiority of fluvoxamine added to immunomodulatory standards (N = 134) (9). However, it should be outlined that in similar to the largest (although still unpublished) placebo-controlled clinical trial (N = 670) (3), there was a very low rate of hospitalization or intensive care and zero mortality in all groups which did not allow any sufficient differentiation according to severe COVID-19 conditions between the study groups (9).
With new mutations constantly emerging and vaccine development lagging behind we think, that the current evidence is sufficient enough to conduct a prospective study comparing an AD alone with a standard immunomodulatory therapy in mild and moderate COVID-19. The very promising, likely positive “anti-COVID-19” effect of AD, perhaps also against or for prevention of Long COVID (first results presented in Table 1) should stimulate further research on this drug-class for the treatment of other infectious diseases threatening the public health. Whether the underlying mechanism of action against Long COVID can also be suspected at an immunological level or is more a “conventional” primary AD effect [e.g., against Long COVID depression (20) or Long COVID fatigue (21)] still remains to be seen. To conclude and clarify, while we think to you have presented enough evidence to show that consideration can be given to trialing AD vs. therapy in mild-moderate COVID-19, there is currently not enough clinical evidence for extrapolating this to other infectious diseases.
There is growing evidence of a close and likely causal relationship between systemic inflammation (e.g., resulting from respiratory infections) and stress-related disorders, especially depressive states (22–25) (supporting the evolutionary concept of a “sickness behavior”) (26, 27). On the other hand, at least in our clinical experience, the susceptibility for infections of people in severe depressive states improved along with the recovery from depression. We are surprised to find no controlled clinical studies on this topic. In the light of the “anti-COVID” experiences with AD it is likely that anti-viral and anti-inflammatory properties of AD itself are crucially involved also in the recovery of those concomitant infections, beyond recovery from depression. In other words, beyond depression treatment, the “hidden” role of favorable immunomodulatory properties of AD in the treatment of moderate to severe depression should be underscored in the light of the above mentioned experiences during the pandemic. AD might be very helpful agents, not only in the treatment of cancer, but also for prevention and, perhaps also in the treatment of post-infection sequels. Besides antiviral properties, the strengthening of the cellular or tissue “resilience” against oxidative stress may play a key role (Supplementary Figure 1) (2). Anti-inflammatory AD properties include autophagy as a conserved strategy governing cellular energy and protein homeostasis, which might support beneficial effects of AD not only in severe depression itself, but also in other “stress-related” (often co-morbid) diseases, e.g., sleeping, anxiety and somatoform disorders, as well as anecdotally, in cancer and neurodegenerative diseases (27, 28). Their role as efflux pump inhibitors counterbalancing the cellular extrusion of antibacterial, anticonvulsant, psychiatric and anticancer medications could pose an additional advantage to limit the development of therapy resistance during the treatment with these drugs (29). Beyond COVID and depression, it is absolutely worth to conduct future repurposing studies on AD at least for further infectious diseases (27, 29, 30). Furthermore, investigating the extent to which a genetic predisposition influences the severity of COVID-19/Long-COVID (31) or other virus infection diseases remains to be challenging. In this regard, further insights on the role of AD in the development and course of those infection diseases could be expected.
UB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft. GJ: Validation, Writing – review & editing. JK: Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1305184/full#supplementary-material
Supplementary Figure 1. Cellular “anti-COVID”-mechanisms of antidepressants (AD): proven inhibitory effects of AD on distinct mechanisms triggering cell dysfunction or cell death in the SARS-COV-297–infection-cell stress response cascade are illustrated in the red boxes, according to Hoertel et al. and Bonnet and Juckel (1, 2).
1. Hoertel N, Sánchez-Rico M, Cougoule C, Gulbins E, Kornhuber J, Carpinteiro A, et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry. (2021) 26:7098–9. doi: 10.1038/s41380-021-01254-3
2. Bonnet U, Juckel G. COVID-19 outcomes: does the use of psychotropic drugs make a difference? Accumulating evidence of a beneficial effect of antidepressants-a scoping review. J Clin Psychopharmacol. (2022) 42:284–92. doi: 10.1097/JCP.0000000000001543
3. Lenze E. Fluvoxamine for Early Treatment of COVID-19: A Fully-Remote, Randomized Placebo Controlled Trial. (2021). Available online at: http://clinicaltrials.gov (accessed 25 October, 2023).
4. Fritz BA, Hoertel N, Lenze EJ, Jalali F, Reiersen AM. Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2. Transl Psychiatry. (2022) 12:341. doi: 10.1038/s41398-022-02109-3
5. Pineda E, Singh J, Pineda MV, Umanzor JG, Baires F, Benitez LG, et al. Impact of fluvoxamine on outpatient treatment of COVID-19 in Honduras in a prospective observational real-world study. Front Pharmacol. (2022) 13:1054644. doi: 10.3389/fphar.2022.1054644
6. Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19. N Engl J Med. (2022) 387:599–610. doi: 10.1056/NEJMoa2201662
7. Boretti A. Effectiveness of fluvoxamine at preventing COVID-19 infection from turning severe. Eur Neuropsychopharmacol. (2023) 67:83–5. doi: 10.1016/j.euroneuro.2022.12.001
8. Seo H, Kim H, Bae S, Park S, Chung H, Sung HS, et al. Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother. (2022) 54:102–13. doi: 10.3947/ic.2021.0142
9. Siripongboonsitti T, Ungtrakul T, Tawinprai K, Nimmol T, Buttakosa M, Sornsamdang G, et al. Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study). Int J Infect Dis. (2023) 134:211–9. doi: 10.1016/j.ijid.2023.06.018
10. McCarthy MW, Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Felker GM, et al. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV)-6 study group and investigators. Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA. (2023) 329:296–305. doi: 10.1001/jama.2022.24100
11. Sedighi F, Zarghami M, Alizadeh Arimi F, Moosazadeh M, Ala S, Ghasemian R, et al. Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non-critical COVID-19 pneumonia: a double-blind randomized, placebo-controlled clinical trial. Neuropsychopharmacol Rep. (2023) 43:202–12. doi: 10.1002/npr2.12327
12. Hoertel N, Rezaei K, Sánchez-Rico M, Delgado-Álvarez A, Kornhuber J, Gulbins E, et al. Medications modulating the acid sphingomyelinase/ceramide system and 28-day mortality among patients with SARS-CoV-2: an observational study. Pharmaceuticals (Basel). (2023) 16:1107. doi: 10.3390/ph16081107
13. WHO (World Health Organization). Post COVID-19 condition (Long COVID). (2022). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed 25 October, 2023).
14. El Mansari M, Ghanbari R, Janssen S, Blier P. Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology. (2008) 55:1191–8. doi: 10.1016/j.neuropharm.2008.07.028
15. Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. (2019) 11:eaau5266. doi: 10.1126/scitranslmed.aau5266
16. Kornhuber J, Hoertel N, Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. (2022) 27:307–14. doi: 10.1038/s41380-021-01309-5
17. Tsiakalos A, Ziakas PD, Polyzou E, Schinas G, Akinosoglou K. Early fluvoxamine reduces the risk for clinical deterioration in symptomatic outpatients with COVID-19: a real-world, retrospective, before-after analysis. Microorganisms. (2023) 11:2073. doi: 10.3390/microorganisms11082073
18. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
19. Kirenga BJ, Mugenyi L, Sánchez-Rico M, Kyobe H, Muttamba W, Mugume R, et al. Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: a prospective interventional open-label cohort study. Mol Psychiatry. (2023). doi: 10.1038/s41380-023-02004-3
20. Fenton C, Lee A. Antidepressants with anti-inflammatory properties may be useful in long COVID depression. Drugs Ther Perspect. (2023) 39:65–70. doi: 10.1007/s40267-022-00975-x
21. Farahani RH, Ajam A, Naeini AR. Effect of fluvoxamine on preventing neuropsychiatric symptoms of post COVID syndrome in mild to moderate patients, a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. (2023) 23:197. doi: 10.1186/s12879-023-08172-5
22. Maeng SH, Hong H. Inflammation as the potential basis in depression. Int Neurourol J. (2019) 23:S63–71. doi: 10.5213/inj.1938226.113
23. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. (2021) 26:7393–402. doi: 10.1038/s41380-021-01188-w
24. Karimi Z, Chenari M, Rezaie F, Karimi S, Parhizgari N, Mokhtari-Azad T. Proposed pathway linking respiratory infections with depression. Clin Psychopharmacol Neurosci. (2022) 20:199–210. doi: 10.9758/cpn.2022.20.2.199
25. Czarny P, Wigner P, Galecki P, Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair andmitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 80:309–21. doi: 10.1016/j.pnpbp.2017.06.036
26. Krapić M, Kavazović I, Wensveen FM. Immunological mechanisms of sickness behavior in viral infection. Viruses. (2021) 13:2245. doi: 10.3390/v13112245
27. Rein T. Is autophagy involved in the diverse effects of antidepressants? Cells. (2019) 8:44. doi: 10.3390/cells8010044
28. Tong RL, Kahn UN, Grafe LA, Hitti FL, Fried NT, Corbett BF. Stress circuitry: mechanisms behind nervous and immune system communication that influence behavior. Front Psychiatry. (2023) 14:1240783. doi: 10.3389/fpsyt.2023.1240783
29. Rácz B, Spengler G. Repurposing antidepressants and phenothiazine antipsychotics as efflux pump inhibitors in cancer and infectious diseases. Antibiotics (Basel). (2023) 12:137. doi: 10.3390/antibiotics12010137
30. Golden SR, Rosenstein DL, Belhorn T, Blatt J. Repurposing psychotropic agents for viral disorders: beyond Covid. Assay Drug Dev Technol. (2021) 19:373–85. doi: 10.1089/adt.2021.014
Keywords: antidepressants, resilience, prevention, COVID-19, Long COVID, Post COVID, PACS
Citation: Bonnet U, Juckel G and Kuhn J (2024) Antidepressants for prevention of severe COVID-19, Long COVID and outlook for other viral diseases. Front. Med. 11:1305184. doi: 10.3389/fmed.2024.1305184
Received: 17 December 2023; Accepted: 05 February 2024;
Published: 20 February 2024.
Edited by:
Daniel Diaz, National Autonomous University of Mexico, MexicoReviewed by:
Velyn Wu, University of Florida, United StatesCopyright © 2024 Bonnet, Juckel and Kuhn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Udo Bonnet, dWRvLmJvbm5ldEB1bmktZHVlLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.