
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 20 March 2024
Sec. Pulmonary Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1289259
This article is part of the Research Topic Future Research Questions for Improving COPD Diagnosis and Care View all 13 articles
Chronic obstructive pulmonary disease (COPD) is a heterogeneous respiratory condition characterized by symptoms of dyspnea, cough, and sputum production. We review sex-differences in disease mechanisms, structure-function-symptom relationships, responses to therapies, and clinical outcomes in COPD with a specific focus on dyspnea. Females with COPD experience greater dyspnea and higher morbidity compared to males. Imaging studies using chest computed tomography scans have demonstrated that females with COPD tend to have smaller airways than males as well as a lower burden of emphysema. Sex-differences in lung and airway structure lead to critical respiratory mechanical constraints during exercise at a lower absolute ventilation in females compared to males, which is largely explained by sex differences in maximum ventilatory capacity. Females experience similar benefit with respect to inhaled COPD therapies, pulmonary rehabilitation, and smoking cessation compared to males. Ongoing re-assessment of potential sex-differences in COPD may offer insights into the evolution of patterns of care and clinical outcomes in COPD patients over time.
Chronic obstructive pulmonary disease (COPD) is a heterogeneous respiratory condition with hallmark chronic symptoms that include dyspnea, cough, and sputum production. It is characterized by airway remodeling and lung parenchymal destruction, resulting from a combination of environmental exposures and individual factors that ultimately alter the trajectory of normal lung development and aging, manifesting in disease (1). COPD is currently defined by the combination of symptoms, risk factors for disease, and airflow obstruction measured on spirometry (1). Dyspnea, reduced exercise capacity, and low quality of life frequently characterize the lives of COPD patients. The estimated global prevalence of COPD is approximately 12% and the prevalence and burden of COPD is increasing (1, 2). Due to multiple factors, the prevalence of COPD in females has increased and the number of females diagnosed with COPD in the United States now outnumbers males (3). COPD has also become the leading cause of death among female smokers (4). In females with COPD, dyspnea severity is greater and associated with increased morbidity compared to males (5–7). By comparison, data are mixed with some studies demonstrating that either males or females may be more likely to have symptoms of cough (8, 9). We review biological mechanisms underpinning sex-differences in COPD, the impacts of dyspnea in females with COPD, and structure-function-symptom interactions uncovered through use of imaging and cardiopulmonary exercise testing. Finally, we consider available evidence of clinical outcomes and treatment in females with COPD.
Sex is defined as the different biological and physiological characteristics of females, males, and intersex persons (10). Gender refers to the socially constructed characteristics of women and men that determine roles and relationships across an individual lifetime (10, 11). Understanding sex- and gender-differences and similarities in COPD is increasingly relevant given the growing focus on “treatable traits” and phenotyping patients (12, 13). There is mounting awareness that COPD is a heterogenous disease and that sex may play a role in the symptoms, clinical presentation, and outcomes of patients. The understanding of COPD in females and women has increased in recent decades, offering insights into observed differences in symptoms and clinical outcomes.
Biological differences between females and males include differences in airway growth leading to differential susceptibility to inhaled substances such as tobacco smoke, alterations in inflammatory responses in the lungs, and hormonal factors (14). Smaller airways relative to lung volume has been described in females (15). As a consequence, particle deposition from noxious substances, including cigarette smoke, may be greater in the proximal airways of females (16). Females experience greater small airways disease compared to males for a similar tobacco smoke exposure (5). Female smokers also experience a faster annual loss of forced expiratory volume in 1 s (FEV1) than male smokers (17). Female smokers tend to present at a younger age and lower pack-year smoking history, compared to males with the same degree of lung function impairment (18). This suggests that females may be more susceptible to the effects of cigarettes compares to males (19). Susceptibility to air contaminants is not limited to cigarettes and often non-smoking COPD patients are female (20). Exposures and risk factors associated with non-smoking COPD vary by geographic region and include exposure to biomass fuels, passive smoking, occupational exposures, infections, and air pollution (20–22).
The greater degree of small airways disease in females and increased emphysema in males may have a basis in sex hormones (11). In humans, testosterone level is associated with higher FEV1 after adjustment for age, height, and smoking (23). In mice models, females develop more small airways disease compared to males who develop more emphysema for the same level of cigarette smoke exposure (24). In female mice treated with tamoxifen or ovariectomy, greater emphysema developed, suggesting that estrogen may contribute to these observed sex-differences in mice (24). The Multi-Ethnic Study of Atherosclerosis Lung Study observed an association between male sex and paraseptal emphysema subtype visualized on computed tomography (CT) chest imaging (25) and an association between male sex and a diffuse emphysema subtype has also been described in a machine learning analysis of the Subpopulations and Intermediate Outcome Measures in COPD Study (26).
Despite the aforementioned insights, defining the role of sex-hormones in COPD and changes throughout the life cycle such as menopause in humans remains complex. Interestingly, early menopause has been associated with a lower risk of airflow obstruction, although other pulmonary function abnormalities such as a non-specific restrictive pattern, may develop in the peri-menopause period (27). Unlike the study of sex in respiratory health and disease, there is limited evidence to date regarding the potential role of gender in lung disease, as many studies to date have not included systematic gender assessment distinct from considering biological sex.
Females with COPD experience more dyspnea compared to males, matched for relative lung function (14). Females less than 65 years of age have a higher exacerbation risk and higher odds of severe airflow obstruction in comparison to males (28). Females with COPD also experience increased depression and anxiety as well as reduced quality of life in comparison to their male counterparts (29–31). Importantly, dyspnea is strongly associated with depression in COPD (29). Anxiety and depression are associated with reduced smoking cessation (32), increased dyspnea (33), and reduced sleep quality (34, 35). Even after controlling for percent predicted lung function, age, smoking history, and extent of emphysema on chest CT, females still experience a greater dyspnea burden, more depression, and reduced quality of life relative to males (5). Female sex, depression, and anxiety are associated with increased risk of exacerbations and mortality (36–39). Recent population-based studies have demonstrated that sex-differences in dyspnea are accounted for by sex-differences in absolute values of lung function measurements [FEV1, forced vital capacity (FVC) or diffusing capacity of the lungs for carbon monoxide (DLCO)] (40–42). The complex interrelationships between dyspnea, mental health symptoms, and clinical outcomes are likely multidirectional. Dyspnea is central to the experience of both females and males with COPD, emphasizing the importance of understanding the underlying mechanisms of this symptom.
Quantitative chest CT scans enable sex-based comparisons of lung structure in people with COPD. Imaging studies using chest CT scans have demonstrated that females with COPD, matched for percentage predicted FEV1, tend to have smaller airways than males as well as a lower burden of parenchymal lung tissue destruction (5). Smaller airways on CT are measured as reduced airway luminal area and diameter, reduced wall thickness, and increased airway wall area (43, 44). Female smokers without established COPD as well as females with severe COPD have less emphysema compared to males (5, 45). Although the precise mechanisms underlying anatomical differences are an area of ongoing research, examining the functional consequences of these structural differences, relative to health, under the stress of exercise with cardiopulmonary exercise testing (CPET), enables integration of structural differences with differences in symptoms, specifically exertional dyspnea.
The current understanding of mechanisms of dyspnea in females with COPD is partially informed by data examining sex-differences in dyspnea in healthy individuals. The Burden of Obstructive Lung Diseases international population-based study describes a greater prevalence of dyspnea in females compared to males, including in a subpopulation of participants without self-reported diseases associated with dyspnea or abnormal spirometry (46). Males have larger conducting airways and lung volumes in comparison to females, even when accounting for differences in height (47–49). Healthy females also have reduced respiratory muscle strength compared to height-matched males (50). These differences may predispose females to developing greater ventilatory abnormalities and increases in both neural respiratory drive, the signal to breathe from the brain to the respiratory muscles, and dyspnea intensity during exercise relative to males (51, 52). There are also sex differences in qualitative descriptors of dyspnea, with healthy females being more likely to select unpleasant dyspnea descriptors related to inspiratory difficulty, shallow breathing, and unsatisfied inspiration than males (52). Sex-differences in dyspnea quality in health are likely a manifestation of relatively greater respiratory mechanical constraints and neural respiratory drive at a given absolute ventilation in females (51, 52).
Dyspnea in COPD is closely related to the physiological consequences of the disease and can be systematically studied using CPET. Airway remodeling and lung parenchymal destruction in COPD leads to airflow limitation, increased lung compliance, and impaired gas exchange. During CPET, the consequences of these abnormalities manifest as dynamic hyperinflation, respiratory muscle weakness, ventilatory inefficiency, and hypoxemia (53–55). Abnormal responses to exercise provide a powerful stimulus for increased neural respiratory drive to the diaphragm in an effort to increase ventilation commensurate with the metabolic demands of exercise (53–55). Neural respiratory drive can be estimated using diaphragm electromyography during CPET and is highly correlated with dyspnea in COPD (53–55).
In a detailed physiology study in females and males with mild COPD, matched for % predicted FEV1, females experienced significantly greater dyspnea intensity ratings during exercise (Figure 1A), constraints on tidal volume expansion (Figure 1C), and a more rapid breathing pattern (Figure 1D) at a given absolute work rate in comparison to males (6). However, when dyspnea was assessed relative to maximum ventilatory capacity (Figure 1B), sex differences in dyspnea between males and females with COPD disappeared, suggesting that increased intensity of exertional dyspnea in females is largely driven by females using a greater fraction of their ventilatory capacity to achieve the same absolute exercise intensity and ventilation compared with males (6).
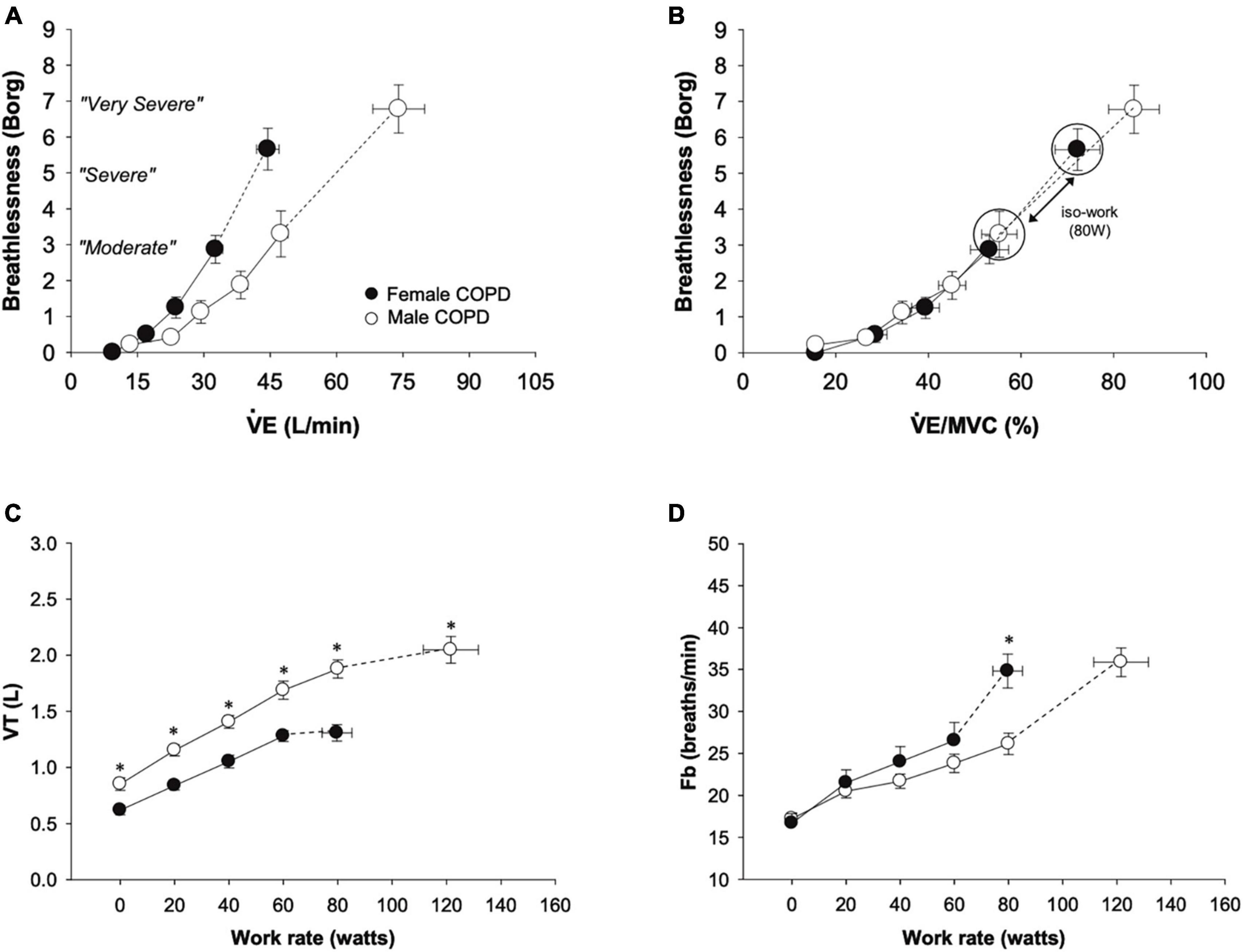
Figure 1. Comparison from rest to peak exercise between males and females with mild COPD in (A) dyspnea, (B) dyspnea relative to ventilation as a percentage of estimated maximum ventilatory capacity, (C) tidal volume, and (D) breathing frequency. Females with COPD experienced greater dyspnea for a given absolute ventilation during exercise and had reduced tidal volume expansion and consequent increased breathing frequency compared to males. At a work rate of 80 W (the highest absolute work rate comparison between males and females with COPD), the VE/MVC ratio and dyspnea were greater in females. Values are mean ± SE. *p < 0.05. Fb, breathing frequency; MVC, maximum ventilatory capacity; VE, minute ventilation; VT, tidal volume. Reprinted from Guenette et al. (6) with permission from Elsevier.
Differences in absolute lung function between males and females in relation to dyspnea has also been examined in several recent population-based studies. The European Community Respiratory Health Survey, a general population-based study, found that dyspnea was twice as common in females and was explained by differences in absolute FEV1 and FVC values, demonstrating that lower absolute lung function accounts for dyspnea in females (41). In the population-based Swedish CArdioPulmonarybioImage Study, similar results were found with respect to absolute diffusing capacity and static lung volumes as well as in obese females (42, 56). Similar findings have been described in individuals with COPD in the COPDGene study, demonstrating that sex-based differences in absolute FEV1 accounted for the difference in dyspnea between females and males (40).
Taken together, detailed physiological studies and larger scale population-based studies demonstrate that differences in exertional dyspnea intensity between females and males are largely explained by differences in absolute measures of lung function and the relatively higher ventilation needed in females to perform a given absolute task compared to males. However, the underlying physiologic explanations for differences in dyspnea quality and unpleasantness across the spectrum of COPD disease severity and any potential association with observed higher anxiety and depression in females with COPD, remains unknown and an important area for future research.
Major classes of inhaled medications for COPD include long-acting muscarinic antagonists (LAMA), long-acting beta agonists (LABA), combination LABA-LAMA, and combination inhaled corticosteroid (ICS)-LABA. A recent systematic review of randomized controlled trials and observational studies found that there is limited data directly comparing treatment effectiveness between sexes in COPD; however, there is evidence from retrospective and sub-group analyses of large randomized controlled trials (57). When clinical trials report sex-differences, they are frequently not found; however, some trials may not be powered to detect differences in treatment response between females and males. Tiotropium demonstrates a similar improvement in exercise capacity, trough FEV1, reduced exacerbations, and improved quality of life in males and females (58, 59). Females experience improvements in dyspnea with LABA-LAMA similar to males in comparison to either LAMA or LABA alone (60, 61). Comparing LABA-LAMA to ICS-LABA, improvement in FEV1 are observed in both males and females to varying degrees (60–62). Only males had a significant reduction in exacerbations (62), but females had better responses with regards to dyspnea and quality of life with LABA-LAMA compared to ICS-LABA (61). Taken together, there is no evidence to support treating females with COPD differently from their male peers in terms of inhaled therapies.
There is similarly no evidence to support differential non-pharmacologic management of individuals with COPD based on sex. Despite greater benefits of smoking cessation with respect to lung function in females compared to males, females find it harder to quit and are more likely to relapse (63). However, upon smoking cessation, the gain in FEV1 in female ex-smokers is greater than males (63). Long term oxygen therapy in females with COPD affords benefits with respect to survival similar to males (64, 65). There are no established sex-differences in exercise capacity and quality of life improvement following pulmonary rehabilitation in females compared to males, although benefits of pulmonary rehabilitation may not be sustained in females (66).
Previously described significant disparities in the accurate diagnosis and clinical outcomes of females with COPD may be changing (67–69). Ongoing prospective re-evaluation of potential sex-differences in females and women with COPD is valuable to understanding how patterns of care may change over time. Females with COPD also present with different comorbidities compared to their male counterparts. Females more often have heart failure, osteoporosis, and diabetes (70). Interestingly, different combinations of comorbidities in COPD are associated with mortality between sexes (71). Healthcare related costs for those living with COPD account for the majority of expenditures in respiratory disease in Europe and are expected to increase in the United States (1). Females incur greater healthcare costs than males and lose more quality-adjusted life years (72). Females with COPD have a higher BODE index (body mass index, airflow obstruction, dyspnea, and exercise capacity) suggesting a worse prognosis (73). Females have also been at greater risk of hospitalization and death (74). Female sex has additionally been identified as a risk factor for acute exacerbations of COPD in the large COPDGene cohort study (39).
Sex and gender have the potential to influence biological mechanisms, symptoms, and clinical outcomes. Several mechanisms may contribute to sex-differences in COPD including differences in airway and lung structure, differential consequent susceptibility to inhaled pollutants, and sex-hormones. Females with COPD experience significantly greater dyspnea compared to males. In mild COPD, females have greater respiratory mechanical constraints at a lower work rate compared to males, which is associated with greater neural respiratory drive and dyspnea (6). However, differences in exertional dyspnea intensity have been demonstrated to be related to differences in absolute measures of lung function and consequent differences in ventilatory capacity between sexes.
Insights into sex-differences and similarities in lung structure, exercise physiology, and responses to mainstay COPD therapies has advanced over the past decade. In contrast, our understanding of gender-differences in COPD remains limited, as studies either frequently neglect to systematically assess gender, or conflate gender related factors with sex. Considering the multidimensional nature of dyspnea, our collective understanding of differences in dyspnea quality between sexes and genders in COPD remains an area of future research.
KM: Conceptualization, Writing – original draft, Writing – review & editing. RM: Writing – review & editing. OF: Writing – review & editing. AH: Writing – review & editing. JG: Conceptualization, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. KM received funding support from Michael Smith Health Research British Columbia and the Academic Enhancement Fund from the Division of Respiratory Medicine at The University of British Columbia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vestbo J, Hurd S, Agustí A, Jones P, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2023) 187:347–65. doi: 10.1164/rccm.201204-0596PP
2. Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
3. Aryal S, Diaz-Guzman E, Mannino D. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. (2014) 9:1145–54. doi: 10.2147/COPD.S54476
4. Thun M, Carter B, Feskanich D, Freedman N, Prentice R, Lopez A, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. (2013) 368:351–64. doi: 10.1056/NEJMsa1211127
5. Martinez F, Curtis J, Sciurba F, Mumford J, Giardino N, Weinmann G, et al. National emphysema treatment trial research group. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. (2007) 176:243–52. doi: 10.1164/rccm.200606-828OC
6. Guenette J, Jensen D, Webb K, Ofir D, Raghavan N, O’Donnell D. Sex differences in exertional dyspnea in patients with mild COPD: Physiological mechanisms. Respir Physiol Neurobiol. (2011) 177:218–27. doi: 10.1016/j.resp.2011.04.011
7. de Torres J, Casanova C, Hernandez C, Abreu J, Aguirre-Jaime A, Celli B. Gender and COPD in patients attending a pulmonary clinic. Chest. (2005) 128:2012–6. doi: 10.1378/chest.128.4.2012
8. Zhang H, Wu F, Yi H, Xu D, Jiang N, Li Y, et al. Gender differences in chronic obstructive pulmonary disease symptom clusters. Int J Chron Obstruct Pulmon Dis. (2021) 16:1101–7. doi: 10.2147/COPD.S302877
9. Lamprecht B, Vanfleteren L, Studnicka M, Allison M, McBurnie M, Vollmer W, et al. Sex-related differences in respiratory symptoms: Results from the BOLD Study. Eur Respir J. (2013) 42:858–60. doi: 10.1183/09031936.00047613
11. Somayaji R, Chalmers J. Just breathe: A review of sex and gender in chronic lung disease. Eur Respir Rev. (2022) 31:210111. doi: 10.1183/16000617.0111-2021
12. Cazzola M, Rogliani P, Barnes P, Blasi F, Celli B, Hanania N, et al. An update on outcomes for COPD pharmacological trials: A COPD investigators report – reassessment of the 2008 American thoracic society/European respiratory society statement on outcomes for COPD pharmacological trials. Am J Respir Crit Care Med. (2023) 208:374–94. doi: 10.1164/rccm.202303-0400SO
13. Stolz D, Mkorombindo T, Schumann D, Agusti A, Ash S, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet commission. Lancet. (2022) 400:921–72. doi: 10.1016/S0140-6736(22)01273-9
14. Gut-Gobert C, Cavailles A, Dixmier A, Guillot S, Jouneau S, Leroyer C, et al. Women and COPD: Do we need more evidence? Eur Respir Rev. (2019) 28:180055. doi: 10.1183/16000617.0055-2018
15. Sheel A, Guenette J, Yuan R, Holy L, Mayo J, McWilliams A, et al. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol. (2009) 107:1622–8. doi: 10.1152/japplphysiol.00562.2009
16. Bennett W, Zeman K, Kim C. Variability of fine particle deposition in healthy adults: Effect of age and gender. Am J Respir Crit Care Med. (1996) 153:1641–7. doi: 10.1164/ajrccm.153.5.8630615
17. Gan W, Man S, Postma D, Camp P, Sin D. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir Res. (2006) 7:52. doi: 10.1186/1465-9921-7-52
18. de Torres J, Casanova C, Montejo de Garcini A, Aguirre-Jaime A, Celli B. Gender and respiratory factors associated with dyspnea in chronic obstructive pulmonary disease. Respir Res. (2007) 8:18. doi: 10.1186/1465-9921-8-18
19. Amaral A, Strachan D, Burney P, Jarvis D. Female smokers are at greater risk of airflow obstruction than male smokers. UK Biobank. Am J Respir Crit Care Med. (2017) 195:1226–35. doi: 10.1164/rccm.201608-1545OC
20. Yang I, Jenkins C, Salvi S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. (2022) 10:497–511. doi: 10.1016/S2213-2600(21)00506-3
21. Celli B, Halbert R, Nordyke R, Schau B. Airway obstruction in never smokers: Results from the third national health and nutrition examination survey. Am J Med. (2005) 118:1364–72. doi: 10.1016/j.amjmed.2005.06.041
22. Siddharthan T, Grigsby M, Goodman D, Chowdhury M, Rubinstein A, Irazola V, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low– and middle-income country settings. Am J Respir Crit Care Med. (2018) 197:611–20. doi: 10.1164/rccm.201709-1861OC
23. Lenoir A, Fuertes E, Gomez-Real F, Leynaert B, van der Plaat D, Jarvis D. Lung function changes over 8 years and testosterone markers in both sexes: UK Biobank. ERJ Open Res. (2020) 6:00070–2020. doi: 10.1183/23120541.00070-2020
24. Tam A, Churg A, Wright J, Zhou S, Kirby M, Coxson H, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2016) 193:825–34. doi: 10.1164/rccm.201503-0487OC
25. Smith B, Austin J, Newell J Jr., D’Souza B, Rozenshtein A, Hoffman E, et al. Pulmonary emphysema subtypes on computed tomography: The MESA COPD study. Am J Med. (2014) 127:94.e7–23. doi: 10.1016/j.amjmed.2013.09.020
26. Angelini ED, Yang J, Balte P, Hoffman E, Manichaikul A, Sun Y, et al. Pulmonary emphysema subtypes defined by unsupervised machine learning on CT scans. Thorax (2023) 78:1067–79. doi: 10.1136/thorax-2022-219158
27. Hong Y, Park H, Chang Y, Jang E, Zhao D, Kim S, et al. Stages of menopause and abnormal lung function: A cross-sectional study of middle-aged women. Menopause. (2021) 28:811–8. doi: 10.1097/GME.0000000000001779
28. DeMeo D, Ramagopalan S, Kavati A, Vegesna A, Han M, Yadao A, et al. Women manifest more severe COPD symptoms across the life course. Int J Chron Obstruct Pulmon Dis. (2018) 13:3021–9. doi: 10.2147/COPD.S160270
29. Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: The roles of gender and disease severity. Respir Med. (2006) 100:1767–74. doi: 10.1016/j.rmed.2006.01.026
30. Laurin C, Lavoie K, Bacon S, Dupuis G, Lacoste G, Cartier A, et al. Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with COPD. Chest. (2007) 132:148–55. doi: 10.1378/chest.07-0134
31. de Torres J, Casanova C, Hernandez C, Abreu J, Montejo de Garcini A, Aguirre-Jaime A, et al. Gender associated differences in determinants of quality of life in patients with COPD: A case series study. Health Qual Life Outcomes. (2006) 4:72. doi: 10.1186/1477-7525-4-72
32. Hanania N, Mullerova H, Locantore N, Vestbo J, Watkins M, Wouters E, et al. Evaluation of COPD longitudinally to identify predictive surrogate endpoints (ECLIPSE) study investigators. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. (2011) 183:604–11. doi: 10.1183/09031936.00111707
33. Jimenez-Ruiz C, Andreas S, Lewis K, Tonnesen P, van Schayck C, Hajek P, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J. (2015) 46:61–79. doi: 10.1183/09031936.00092614
34. Scharf S, Maimon N, Simon-Tuval T, Bernhard-Scharf B, Reuveni H, Tarasiuk A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2010) 6:1–12. doi: 10.2147/COPD.S15666
35. Budhiraja R, Parthasarathy S, Budhiraja P, Habib M, Wendel C, Quan S. Insomnia in patients with COPD. Sleep. (2012) 35:369–75. doi: 10.5664/jcsm.4540
36. McGarvey L, Lee A, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. (2015) 109:228–37. doi: 10.1016/j.rmed.2014.12.006
37. Fan V, Ramsey S, Giardino N, Make B, Emery C, Diaz P, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. (2007) 167:2345–53. doi: 10.1001/archinte.167.21.2345
38. Divo M, Cote C, de Torres J, Casanova C, Marin J, Pinto-Plata V, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2012) 186:155–61. doi: 10.1164/rccm.201201-0034OC
39. Foreman M, Zhang L, Murphy J, Hansel N, Make B, Hokanson J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. (2011) 184:414–20. doi: 10.1164/rccm.201011-1928OC
40. Ekstrom M, Bornefalk-Hermansson A, Wysham N, Currow D, MacIntyre N COPDGene Investigators: Core Untis. Spirometric volumes and breathlessness across levels of airflow limitation: The COPDGene study. Am J Respir Crit Care Med. (2018) 198:678–81. doi: 10.1164/rccm.201803-0594LE
41. Ekstrom M, Schioler L, Gronseth R, Johannessen A, Svanes C, Leynaert B, et al. Absolute values of lung function explain the sex difference in breathlessness in the general population. Eur Respir J. (2017) 49:1602047. doi: 10.1183/13993003.02047-2016
42. Ekstrom M, Sundh J, Schioler L, Lindberg E, Rosengren A, Bergstrom G, et al. Absolute lung size and the sex difference in breathlessness in the general population. PLoS One. (2018) 13:e0190876. doi: 10.1371/journal.pone.0190876
43. Kim Y, Schroeder J, Lynch D, Newell J, Make B, Friedlander A, et al. Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD. (2011) 8:285–92. doi: 10.3109/15412555.2011.586658
44. Li Y, Dai Y, Yu N, Guo Y. Sex-related differences in bronchial parameters and pulmonary function test results in patients with chronic obstructive pulmonary disease based on three-dimensional quantitative computed tomography. J Int Med Res. (2018) 46:135–42. doi: 10.1177/0300060517721309
45. Sverzellati N, Calabro E, Randi G, La Vecchia C, Marchiano A, Kuhnigk J, et al. Sex differences in emphysema phenotype in smokers without airflow obstruction. Eur Respir J. (2009) 33:1320–8. doi: 10.1183/09031936.00109808
46. Gronseth R, Vollmer W, Hardie J, Olafsdottir I, Lamprecht B, Buist A, et al. Predictors of dyspnoea prevalence: Results from the BOLD study. Eur Respir J. (2014) 43:1610–20. doi: 10.1183/09031936.00036813
47. Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. (1980) 121:339–42. doi: 10.1164/arrd.1980.121.2.339
48. Bellemare F, Jeanneret A, Couture J. Sex differences in thoracic dimensions and configuration. Am J Respir Crit Care Med. (2003) 168:305–12. doi: 10.1164/rccm.200208-876OC
49. Martin T, Castile R, Fredberg J, Wohl M, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol. (1987) 63:2042–7. doi: 10.1183/20734735.000318
50. Sheel A, Guenette J. Mechanics of breathing during exercise in men and women: Sex versus body size differences? Exerc Sport Sci Rev. (2008) 36:128–34. doi: 10.1097/JES.0b013e31817be7f0
51. Schaeffer M, Mendonca C, Levangie M, Andersen R, Taivassalo T, Jensen D. Physiological mechanisms of sex differences in exertional dyspnoea: Role of neural respiratory motor drive. Exp Physiol. (2014) 99:427–41. doi: 10.1113/expphysiol.2013.074880
52. Cory J, Schaeffer M, Wilkie S, Ramsook A, Puyat J, Arbour B, et al. Sex differences in the intensity and qualitative dimensions of exertional dyspnea in physically active young adults. J Appl Physiol. (2015) 119:998–1006. doi: 10.1152/japplphysiol.00520.2015
53. Faisal A, Alghamdi B, Ciavaglia C, Elbehairy A, Webb K, Ora J, et al. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am J Respir Crit Care Med. (2016) 193:299–309. doi: 10.1164/rccm.201504-0841OC
54. Jolley C, Luo Y, Steier J, Rafferty G, Polkey M, Moxham J. Neural respiratory drive and breathlessness in COPD. Eur Respir J. (2015) 45:355–64. doi: 10.1183/09031936.00063014
55. Guenette J, Chin R, Cheng S, Dominelli P, Raghavan N, Webb K, et al. Mechanisms of exercise intolerance in global initiative for chronic obstructive lung disease grade 1 COPD. Eur Respir J. (2014) 44:1177–87. doi: 10.1183/09031936.00034714
56. Ekstrom M, Blomberg A, Bergstrom G, Brandberg J, Caidahl K, Engstrom G, et al. The association of body mass index, weight gain and central obesity with activity-related breathlessness: The Swedish cardiopulmonary bioimage study. Thorax. (2019) 74:958–64. doi: 10.1136/thoraxjnl-2019-213349
57. Rogliani P, Cavalli F, Ritondo B, Cazzola M, Calzetta L. Sex differences in adult asthma and COPD therapy: A systematic review. Respir Res. (2022) 23:222. doi: 10.1186/s12931-022-02140-4
58. Tashkin D, Celli B, Kesten S, Lystig T, Decramer M. Effect of tiotropium in men and women with COPD: Results of the 4-year UPLIFT trial. Respir Med. (2010) 104:1495–504. doi: 10.1016/j.rmed.2010.03.033
59. O’Donnell D, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. (2004) 23:832–40. doi: 10.1183/09031936.04.00116004
60. D’Urzo A, Singh D, Donohue J, Kerwin E, Ribera A, Molins E, et al. Efficacy of aclidinium/formoterol 400/12 microg, analyzed by airflow obstruction severity, age, sex, and exacerbation history: Pooled analysis of ACLIFORM and AUGMENT. Int J Chron Obstruct Pulmon Dis. (2019) 14:479–91. doi: 10.2147/COPD.S185502
61. Tsiligianni I, Mezzi K, Fucile S, Kostikas K, Shen S, Banerji D, et al. Response to Indacaterol/Glycopyrronium (IND/GLY) by sex in patients with COPD: A pooled analysis from the IGNITE Program. COPD. (2017) 14:375–81. doi: 10.1080/15412555.2017.1324837
62. Wedzicha J, Singh D, Tsiligianni I, Jenkins C, Fucile S, Fogel R, et al. Treatment response to indacaterol/glycopyrronium versus salmeterol/fluticasone in exacerbating COPD patients by gender: A post-hoc analysis in the FLAME study. Respir Res. (2019) 20:4. doi: 10.1186/s12931-019-0972-7
63. Connett J, Murray R, Buist A, Wise R, Bailey W, Lindgren P, et al. Changes in smoking status affect women more than men: Results of the lung health study. Am J Epidemiol. (2003) 157:973–9. doi: 10.1093/aje/kwg083
64. Miyamoto K, Aida A, Nishimura M, Aiba M, Kira S, Kawakami Y. Gender effect on prognosis of patients receiving long-term home oxygen therapy. The respiratory failure research group in Japan. Am J Respir Crit Care Med. (1995) 152:972–6. doi: 10.1164/ajrccm.152.3.7663812
65. Franklin K, Gustafson T, Ranstam J, Strom K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease–gender differences. Respir Med. (2007) 101:1506–11. doi: 10.1016/j.rmed.2007.01.009
66. Foy C, Rejeski W, Berry M, Zaccaro D, Woodard C. Gender moderates the effects of exercise therapy on health-related quality of life among COPD patients. Chest. (2001) 119:70–6. doi: 10.1378/chest.119.1.70
67. Chapman K, Tashkin D, Pye D. Gender bias in the diagnosis of COPD. Chest. (2001) 119:1691–5. doi: 10.1378/chest.119.6.1691
68. Watson L, Vestbo J, Postma D, Decramer M, Rennard S, Kiri V, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med. (2004) 98:1207–13. doi: 10.2147/COPD.S302877
69. Akbarshahi H, Ahmadi Z, Currow D, Sandberg J, Vandersman Z, Shanon-Honson A, et al. No gender-related bias in COPD diagnosis and treatment in Sweden: A randomised, controlled, case-based trial. ERJ Open Res. (2020) 6:00342–2020. doi: 10.1183/23120541.00342-2020
70. Almagro P, Lopez Garcia F, Cabrera F, Montero L, Morchon D, Diez J, et al. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir Med. (2010) 104:253–9. doi: 10.1016/j.rmed.2009.09.019
71. Trudzinski F, Jorres R, Alter P, Walter J, Watz H, Koch A, et al. Sex-specific associations of comorbidome and pulmorbidome with mortality in chronic obstructive pulmonary disease: Results from COSYCONET. Sci Rep. (2022) 12:8790. doi: 10.1038/s41598-022-12828-8
72. Zafari Z, Li S, Eakin M, Bellanger M, Reed R. Projecting long-term health and economic burden of COPD in the United States. Chest. (2021) 159:1400–10. doi: 10.1016/j.chest.2020.09.255
73. Roche N, Deslee G, Caillaud D, Brinchault G, Court-Fortune I, Nesme-Meyer P, et al. Impact of gender on COPD expression in a real-life cohort. Respir Res. (2014) 15:20. doi: 10.1186/1465-9921-15-20
Keywords: chronic obstructive pulmonary disease, dyspnea, exercise physiology, sex-differences, exercise testing
Citation: Milne KM, Mitchell RA, Ferguson ON, Hind AS and Guenette JA (2024) Sex-differences in COPD: from biological mechanisms to therapeutic considerations. Front. Med. 11:1289259. doi: 10.3389/fmed.2024.1289259
Received: 05 September 2023; Accepted: 29 February 2024;
Published: 20 March 2024.
Edited by:
Azmy Faisal, Manchester Metropolitan University, United KingdomReviewed by:
Magnus Ekström, Lund University, SwedenCopyright © 2024 Milne, Mitchell, Ferguson, Hind and Guenette. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathryn M. Milne, a2F0aHJ5bi5taWxuZUB2Y2guY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.