- 1Department of Obstetrics and Gynecology, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, Guangdong Provincial Clinical Research Center for Obstetrics and Gynecology, Guangdong-Hong Kong-Macao Greater Bay Area Higher Education Joint Laboratory of Maternal-Fetal Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Obstetrics and Gynecology, Guangdong Women and Children Hospital, Guangzhou, China
- 3Laboratory for Gynecologic Oncology, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Objective: This study aims to investigate the current status of multiple HPV infection and its association with cervical lesions in the western region of Guangzhou.
Methods: A retrospective analysis of clinical data from cervical cancer screening patients was conducted. The patients were grouped based on HPV genotypes and cervical pathology results to explore the prevalence of high-risk HPV infection and its relationship with cervical lesions in the western region of Guangzhou. The study also analyzed the relationship between high-risk HPV infection and cervical lesions among different age groups.
Results: A total of 13,060 patients were included in the study, with an overall infection rate of 18.46% (2,411/13,060). Among them, the infection rate of HPV genotype 16 was 14.14% (341/2,411), HPV genotype 18 was 5.23% (126/2,411), and other 12 high-risk HPV genotypes accounted for 71.96% (1,735/2,411). When comparing the incidence of HSIL+ (high-grade squamous intraepithelial lesion or worse) among different HPV genotypes, the results showed that the HPV 16 infection group (47.50%) had a higher incidence than the HPV 18 infection group (25.40%) and the other 12 high-risk HPV genotypes group (15.97%; P < 0.05). In the multiple infection groups, the pathogenicity rates were 63.64% (7/11) for the 16+18 HPV infection group, 42.97% (55/128) for the 16+other 12 high-risk HPV genotypes infection group, 26.79% (15/56) for the 18+other 12 high-risk HPV genotypes infection group, and 57.14% (8/14) for the 16+18+other 12 high-risk HPV genotypes infection group. These rates were significantly different compared to the single infection group (P <0.01). Although there was no statistically significant difference in the incidence of cervical cancer between the HPV 16 infection group and the HPV 18 infection group, both groups had a higher incidence compared to the group with other 12 high-risk HPV genotypes infection (P < 0.05). Further analysis suggests that the severity of cervical lesions is not associated with the number of high-risk HPV infections, i.e., the severity of cervical lesions is unrelated to multiple HPV infections but is instead related to the pathogenicity of the HPV genotypes. The infection rate and multiple HPV infection rate of women under 35 years old were higher than those of women aged 35 and above (20% vs. 17.1%; 2% vs. 1.3%; P < 0.05). Moreover, the pathogenicity rate of HSIL+ among high-risk HPV infection increased with age.
Conclusions: In the western region of Guangzhou, the overall infection rate of high-risk HPV is 18.46%. The severity of cervical lesions is unrelated to multiple HPV infections. The fundamental reason is the distinct pathogenicity of different HPV genotypes. The HSIL+ pathogenicity rates, from high to low, are in sequence for HPV 16, HPV 18, and the other 12 HPV types.
1 Introduction
Cervical cancer (CC) is the second most common malignancy among women globally and one of the most prevalent gynecological cancers. According to global cancer data in 2018 (1), out of 18.1 million newly diagnosed cancer cases worldwide, 570,000 were cervical cancer patients, accounting for ~3.2% of all cases. Each year, around 311,000 patients die from cervical cancer, representing ~3.3% of all cancer-related deaths worldwide. These statistics demonstrate the significant threat cervical cancer poses to women's health globally. The etiology of cervical cancer has been a focal point of research among scholars both domestically and internationally. In 1974, German virologist Harold zur Hausen first discovered the Human Papillomavirus (HPV) and confirmed its close association with cervical cancer (2). The International Agency for Research on Cancer stated in 2005 that persistent HPV infection is a direct factor in the development of cervical cancer and precancerous lesions (3).
HPV comprises over 160 different genotypes, with more than 40 genotypes confirmed to be associated with genital tract infections and 13–15 genotypes closely related to cervical squamous epithelial lesions and cervical cancer (4, 5). Due to significant differences in the capsid proteins between different HPV genotypes, cross-protective antibodies cannot be generated in hosts following HPV infection. This inability contributes to the occurrence of multiple and recurrent high-risk HPV infections (6). However, there has been ongoing controversy in the academic community regarding whether multiple high-risk HPV infections exacerbate the severity of cervical lesions or increase the risk of carcinogenesis compared to single high-risk HPV infections (7–9). Some argue that multiple high-risk HPV infections are one of the causes of persistent HPV infection, thereby exacerbating the severity of cervical lesions and increasing the risk of cancer. Others believe that the pathogenicity of HPV genotypes is the key factor determining the progression of cervical lesions and the risk of developing cancer.
The development of cervical cancer from HPV infection is a relatively lengthy process that involves the progression of cervical epithelial neoplasia, which can be categorized into CIN I, CIN II, and CIN III. According to the two-tier classification proposed by the World Health Organization in 2014 (10), benign atypia, mild dysplasia, flat warts, and CIN I are classified as low-grade squamous intraepithelial lesions (LSIL), while moderate dysplasia, severe dysplasia, CIN II, CIN III, and squamous cell carcinoma in situ are classified as high-grade squamous intraepithelial lesions (HSIL). Therefore, regular and effective cervical cancer screening strategies, along with cervical cancer vaccines, have become crucial measures for cervical cancer prevention and control. The Papanicolaou smear staining method, proposed by American scientist Papanicolaou in 1943, initiated the use of cytology as an important means of cervical cancer screening. However, as cytological screening advanced, its limitations became apparent, including low sensitivity, high false-negative rates, and poor reproducibility. Subsequent studies revealed that ~70% of cervical cancer patients are infected with HPV genotype 16 or 18, with 50% associated with genotype 16 infection and 20% associated with genotype 18 infection (11). The detection of high-risk HPV received attention in the academic community. High-risk HPV testing gradually became a co-screening method along with cytology for cervical cancer primary screening in women aged 30 and above, after initially being used as a triage method for patients with ASCUS (atypical squamous cells of undetermined significance) based on cytology results. The ATHENA study (12) confirmed the high sensitivity of HPV testing and its low rate of missed diagnoses for high-grade cervical intraepithelial neoplasia, making it a superior primary screening method compared to other screening methods. As a result, the U.S. FDA approved the Cobas HPV testing method as a primary screening method for cervical cancer in 2014 (13). However, some scholars have raised concerns, suggesting that HPV testing used solely as a primary screening method may detect a large number of individuals with transient HPV infections and potentially lead to missed diagnoses in HPV-negative cervical cancer patients.
Previous epidemiological investigations of high-risk HPV infection in Guangzhou have primarily focused on the eastern region, reporting infection rates of 14.07% (14), 13.94% (15), and 21.66% (16) respectively. Despite large sample sizes, these studies lack data from western Guangzhou and fail to delve into the relationship between multiple infections and cervical lesions.
Being the sole national tertiary Grade A hospital in western Guangzhou, our institution serves the Liwan District and Fangcun area, which collectively represent approximately one-fourth of the urban population of Guangzhou. Consequently, the outcomes derived from opportunistic screening for cervical cancer conducted in our hospital inherently reflect the prevalence of high-risk HPV in the western region of Guangzhou. Thus, our research contributes to addressing this gap by providing additional insights into this geographical area.
This study retrospectively analyzed the distribution of high-risk HPV infections in the western region of Guangzhou and further explored the relationship between multiple high-risk HPV infections and the severity of cervical lesions and the risk of carcinogenesis, aiming to provide evidence for the clinical diagnosis and treatment of cervical lesions.
2 General information
2.1 Study population
Clinical data (age, HPV genotype, liquid-based cytology results) of patients who underwent opportunistic cervical cancer screening at the Third Affiliated Hospital of Guangzhou Medical University from January 1, 2014, to December 31, 2022, were retrospectively analyzed.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
(1) Combined screening using liquid-based cytology and HPV genotyping tests.
(2) Slides reviewed by pathology experts from the Third Affiliated Hospital of Guangzhou Medical University, with final diagnoses of Negative for Intraepithelial Lesion or Malignancy (NILM), Atypical Squamous Cells of Undetermined Significance (ASCUS), Low-Grade Squamous Intraepithelial Lesion (LSIL), High-Grade Squamous Intraepithelial Lesion (HSIL), or cervical cancer.
(3) HSIL patients underwent surgical treatment by specialized doctors (attending physicians or higher positions) at the hospital, and the postoperative specimens were pathologically diagnosed by experts from our hospital.
2.2.2 Exclusion criteria
(1) Patients with acute vaginal inflammation or those who used vaginal medications or engaged in sexual activity within the past 3 days.
(2) Pregnant or lactating women.
(3) Previous history of cervical surgery or hysterectomy.
(4) Recent use of hormonal medications.
2.2.3 Ethical review
This study has obtained ethical approval from the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University and was conducted in accordance with ethical standards (No. 2023C070106).
2.3 Criteria for cervical cancer screening
2.3.1 Cervical liquid-based cytology (TCT) testing
Samples were collected by gynecologists at our hospital and interpreted using the revised 2001 TBS (The Bethesda System) for cervical cytology (17). The results were classified as negative, including normal range and benign reactive cellular changes, or positive, including atypical squamous cells (ASC), ASC of undetermined significance (ASC-US), ASC cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), or squamous cell carcinoma (SCC).
2.3.2 High-risk HPV DNA testing
HPV testing was performed using the COBAS HPV detection method, including genotypes 16 and 18, as well as 12 other high-risk HPV genotypes. The other 12 high-risk genotypes were not included in the multiple high-risk HPV infection or single high-risk HPV infection groups due to the inability to determine if they were caused by a single or multiple HPV genotypes.
2.3.3 Colposcopy-directed punch biopsies (CDB)
Colposcopy-directed punch biopsies were performed by experienced gynecologists at our hospital. Indications for colposcopy examination included abnormal cervical cytology results (LSIL or higher, ASCUS with positive high-risk HPV), suspicious symptoms or signs, positive HPV 16 or 18, suspicious vulvar, vaginal, or cervical lesions, and to assess the cervical transformation zone in postmenopausal women. Biopsy specimens were fixed in 10% formalin, sent immediately for pathological examination, and immunohistochemical staining was performed if necessary.
2.4 Pathological interpretation of results
2.4.1 Pathological diagnosis
Pathological results served as the final basis for determining cervical lesions. Standardized procedures, including fixation, dehydration, transparency, paraffin embedding, serial sectioning, and routine hematoxylin and eosin (HE) staining, were followed to prepare pathological tissue slides. Slides were first reviewed by senior resident physicians at our hospital and then double-checked and finalized by two deputy chief physicians or higher-ranking physicians. In case of disagreement, a third physician with a deputy chief physician or higher-ranking position made the final interpretation. The pathological diagnosis of cervical intraepithelial neoplasia and cervical cancer followed the diagnostic criteria outlined in the second edition of “Obstetrics and Gynecology.”
2.5 Statistical analysis
SPSS version 21.0 statistical software was used for data analysis. Normality and homogeneity of variance tests were conducted for continuous variables. Normally distributed data with homogeneous variances were analyzed using t-tests and presented as mean ± standard deviation (mean ± SD). Non-normally distributed data or data with heterogeneous variances were analyzed using nonparametric Wilcoxon rank-sum tests. Categorical data were analyzed using chi-square tests or Fisher's exact tests. A P-value of < 0.05 was considered statistically significant.
3 Results
3.1 General information
A total of 13,060 patients undergoing opportunistic cervical cancer screening were included in the study. Among them, there were 2,411 cases of high-risk HPV infection, resulting in a prevalence rate of 18.46%. Based on the specific HPV genotypes, there were 341 cases of HPV-16 infection, 126 cases of HPV-18 infection, and 1,735 cases of infection with other high-risk HPV genotypes. Additionally, there were 11 cases of HPV-16+18 co-infection, 128 cases of HPV-16+other high-risk HPV co-infection, 56 cases of HPV-18+other high-risk HPV co-infection, and 14 cases of HPV-16+18+other high-risk HPV co-infection. A total of 10,649 cases were negative for high-risk HPV infection. The pathological examination results showed 10,517 cases of Negative for Intraepithelial Lesion or Malignancy (NILM), 917 cases of inflammatory cytological changes or cervicitis, 385 cases of Atypical Squamous Cells of Undetermined Significance (ASCUS), 676 cases of Low-Grade Squamous Intraepithelial Lesion (LSIL), 465 cases of High-Grade Squamous Intraepithelial Lesion (HSIL), and 106 cases of cervical cancer, Table 1.
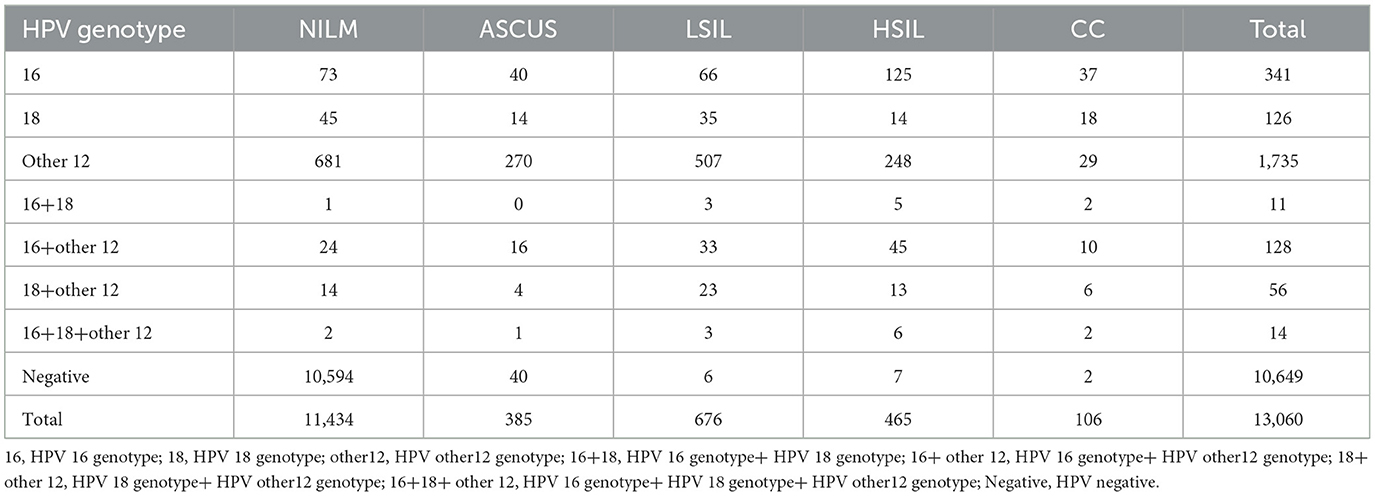
Table 1. Genotypes of high-risk HPV infections and final pathological results in opportunistic cervical cancer screening patients.
3.2 Relationship between cervical lesion severity and genotypes/number of high-risk HPV infections
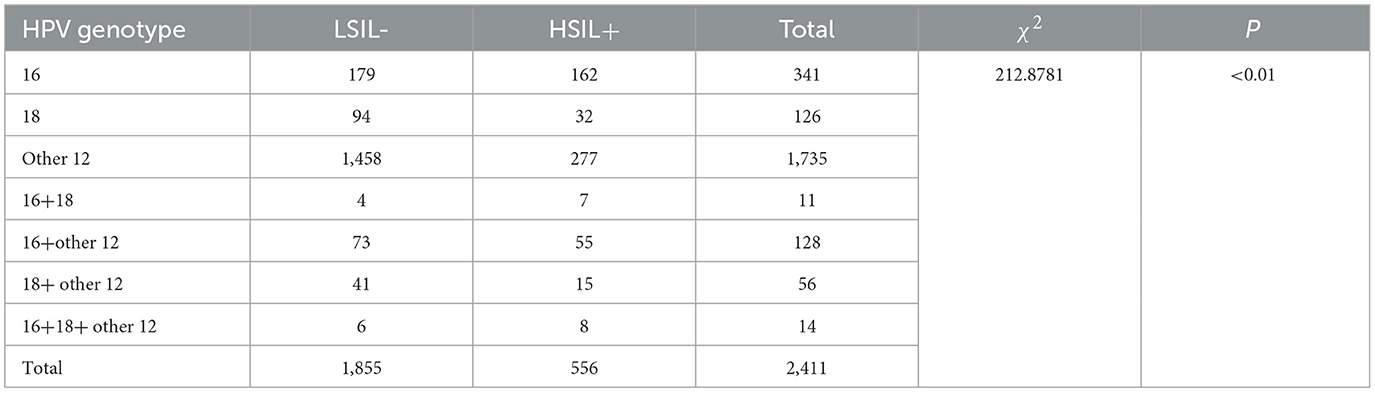
The incidence of high-grade squamous intraepithelial lesions (HSIL+) in different groups based on HPV infection genotypes and combinations, including HPV-16, HPV-18, other high-risk HPV genotypes, HPV-16+18 co-infection, HPV-16+other high-risk HPV co-infection, HPV-18+other high-risk HPV co-infection, and HPV-16+18+other high-risk HPV co-infection, was 47.50% (162/341), 25.40% (32/126), 15.97% (277/1,735), 63.64% (7/11), 42.97% (55/128), 26.79% (15/56), and 57.14% (8/14), respectively. The differences in HSIL+ incidence among these groups were statistically significant (P < 0.01), Table 2.
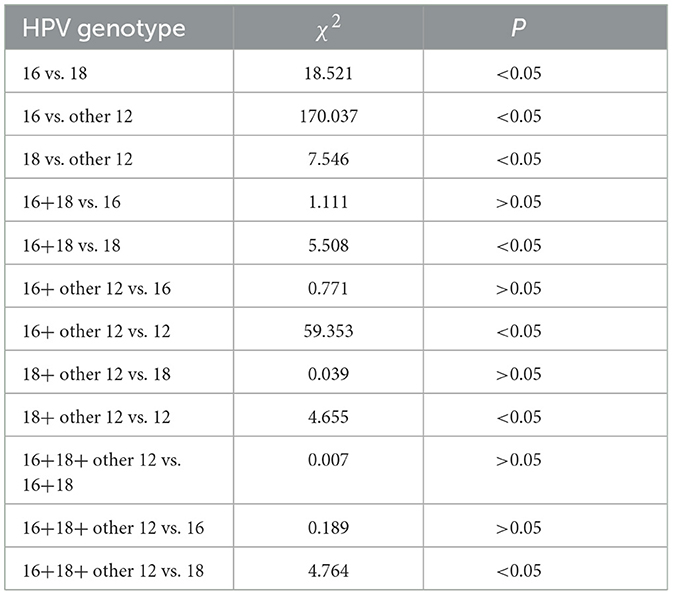
Further intergroup comparisons were conducted among the different groups as follows: the HSIL+ incidence in the HPV-16 infection group was higher than that in the HPV-18 infection group (47.50% vs. 25.40%), with a significant statistical difference (P < 0.01). The HSIL+ incidence in the HPV-16 infection group was higher than that in the other 12 high-risk HPV infection group (47.50% vs. 15.97%), with a significant statistical difference (P < 0.01). The HSIL+ incidence in the HPV-18 infection group was higher than that in the other 12 high-risk HPV infection group (25.40% vs. 15.97%), with a significant statistical difference (P < 0.01). The HSIL+ incidence in the HPV-16+18 co-infection group was higher than that in the HPV-18 infection group (63.64% vs. 25.40%), with statistical significance (P < 0.05). The HSIL+ incidence in the HPV-16+other high-risk HPV co-infection group was higher than that in the other 12 high-risk HPV infection group (42.97% vs. 15.97%), with a significant statistical difference (P < 0.01). The HSIL+ incidence in the HPV-18+other high-risk HPV co-infection group was higher than that in the other 12 high-risk HPV infection group (26.79% vs. 15.97%), with statistical significance (P < 0.05). The HSIL+ incidence in the HPV-16+18+other high-risk HPV co-infection group was higher than that in the HPV-18 infection group (57.14% vs. 25.40%), with statistical significance (P < 0.05).
In addition, when comparing the HPV-16+18 co-infection group to the HPV-16 infection group, the HPV-16+other high-risk HPV co-infection group to the HPV-16 infection group, the HPV-18+other high-risk HPV co-infection group to the HPV-18 infection group, and the HPV-16+18+other high-risk HPV co-infection group to the HPV-16+18 co-infection group, although there were differences in HSIL+ incidence between these pairwise comparisons, the differences were not statistically significant (P > 0.05), Table 3.
3.3 Comparison of the carcinogenicity between multiple high-risk HPV infections and single high-risk HPV infections
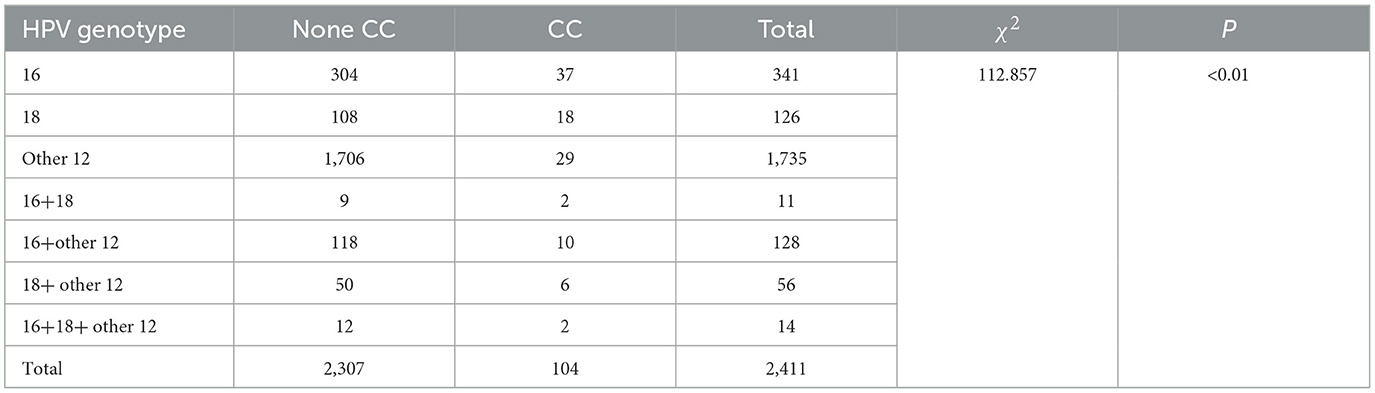
The incidence of cervical cancer in the groups of HPV-16 infection, HPV-18 infection, other 12 high-risk HPV infections, HPV-16+18 co-infection, HPV-16+other high-risk HPV co-infection, HPV-18+other high-risk HPV co-infection, and HPV-16+18+other high-risk HPV co-infection were 10.85% (37/341), 14.29% (18/126), 1.67% (29/1,735), 18.18% (2/11), 7.81% (10/128), 10.71% (6/56), and 0% (2/14), respectively. There were significant statistical differences in the incidence of cervical cancer among the groups (P < 0.01), Table 4.
Further intergroup comparisons were conducted as follows: the HPV-16 infection group had a higher incidence of cervical cancer compared to the other 12 high-risk HPV infection group (10.85% vs. 1.67%), with a significant statistical difference (P < 0.01). The HPV-18 infection group had a higher incidence of cervical cancer compared to the other 12 high-risk HPV infection group (14.29% vs. 1.67%), with a significant statistical difference (P < 0.01). The HPV-16+other high-risk HPV co-infection group had a higher incidence of cervical cancer compared to the other 12 high-risk HPV infection group (7.81% vs. 1.67%), with a significant statistical difference (P < 0.01). The HPV-18+other high-risk HPV co-infection group had a higher incidence of cervical cancer compared to the other 12 high-risk HPV infection group (10.71% vs. 1.67%), with a significant statistical difference (P < 0.01).
In addition, when comparing the HPV-16 infection group to the HPV-18 infection group, the HPV-16+18 co-infection group to the HPV-16 infection group, the HPV-16+18 co-infection group to the HPV-18 infection group, the HPV-16+other high-risk HPV co-infection group to the HPV-16 infection group, the HPV-18+other high-risk HPV co-infection group to the HPV-18 infection group, and the HPV-16+18+other high-risk HPV co-infection group to the HPV-16+18 co-infection group, although there were differences in the incidence of cervical cancer between these pairwise comparisons, the differences were not statistically significant (P > 0.05).
3.4 Analysis of high-risk HPV infection and subsequent cervical lesions in different age groups
A total of 13,060 patients were included in the study, among whom 2,411 had high-risk HPV infection and 10,649 were high-risk HPV negative. The patients were further divided into six age groups: below 25, 25–34, 35–44, 45–54, 55–64, and over 65 years. The high-risk HPV infection status and subsequent cervical lesion conditions were analyzed in each age group, Tables 5, 6.
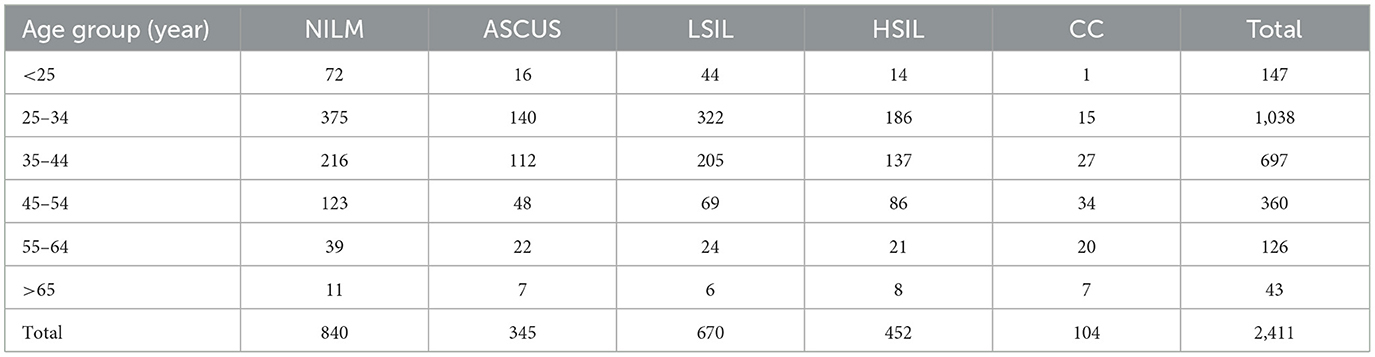
Table 6. Cervical lesion status among individuals with high-risk HPV infection across different age groups.
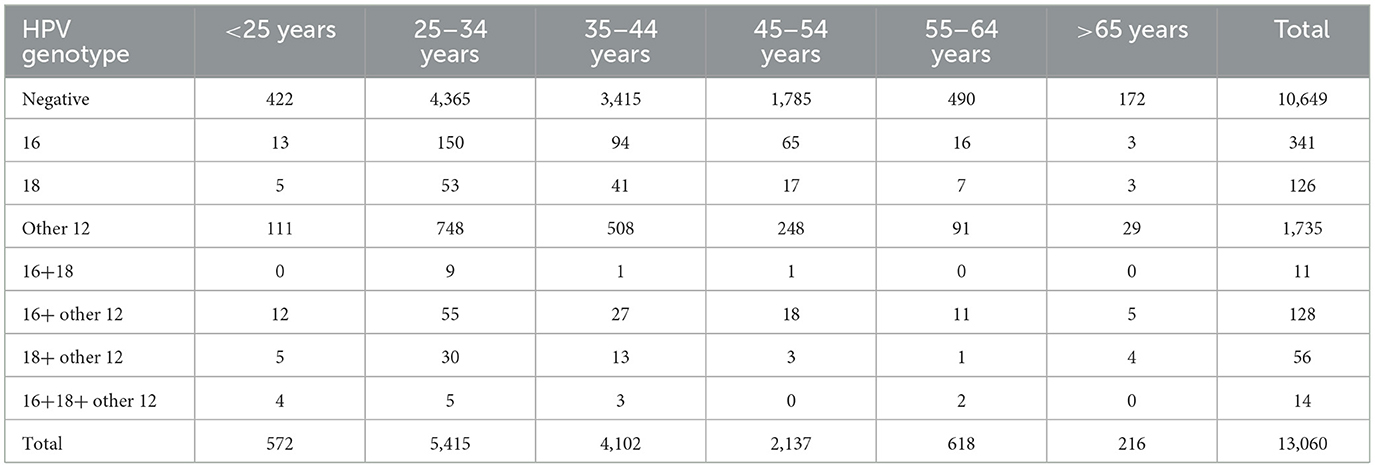
Among the 2,411 patients with high-risk HPV infection, 556 individuals had pathological results of HSIL or above (referred to as HSIL+). The overall prevalence of HSIL+ among high-risk HPV-infected individuals was 23.1% (556/2,411). Moreover, the incidence of HSIL+ showed a progressive increase with age. The rates were 10.2% (15/147) for those below 25 years, 19.36% (201/1,038) for those aged 25–34 years, 23.33% (164/697) for those aged 35–44 years, 33.33% (120/360) for those aged 45–54 years, 32.53% (41/126) for those aged 55–64 years, and 34.88% (15/43) for those over 65 years (Table 6).
Among the 13,060 patients included in the study, there were 5,987 females below the age of 35. Among them, 1,200 individuals were found to have high-risk HPV infection, resulting in a high-risk HPV infection rate of 20%. Additionally, 120 individuals had multiple high-risk HPV infections, corresponding to a multiple high-risk HPV infection rate of 2%. Among the 7,073 females aged 35 and above, 1,211 individuals were identified with high-risk HPV infection, leading to a high-risk HPV infection rate of 17.1%. Furthermore, 89 individuals had multiple high-risk HPV infections, resulting in a multiple high-risk HPV infection rate of 1.3%. The high-risk HPV infection rate and multiple high-risk HPV infection rate were higher in females below the age of 35 compared to those aged 35 and above, with statistically significant differences (P < 0.05), Tables 7, 8.
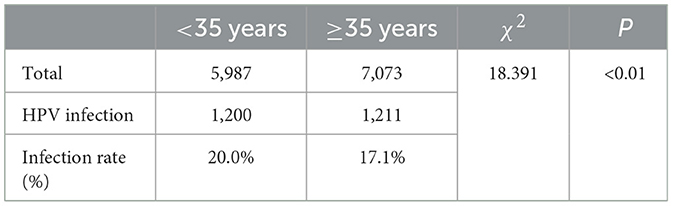
Table 7. Comparison of high-risk HPV infection status between women aged below 35 and women aged 35 and above.
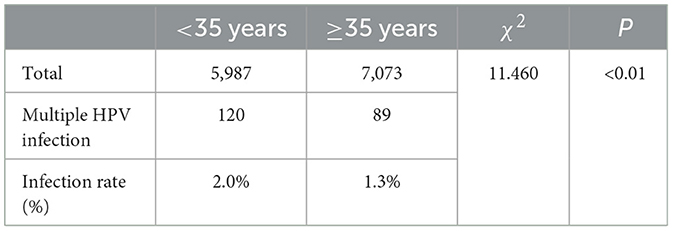
Table 8. Comparison of multiple high-risk HPV infection status between women aged below 35 and women aged 35 and above.
4 Discussion
Cervical squamous intraepithelial neoplasia and cervical cancer are closely associated with persistent infection of high-risk HPV. Currently, over 160 genotypes of HPV have been identified, with more than 40 genotypes related to genital tract infections and 13–15 genotypes closely associated with cervical squamous intraepithelial neoplasia and cervical cancer. Different genotypes of HPV exhibit significant genetic variations in the coding of their capsid proteins, which essentially do not produce cross-protective antibodies after infection. This is one of the important factors leading to multiple and recurrent high-risk HPV infections.
Our study focuses on the population of the western region of Guangzhou, which accounts for approximately one-fourth of the urban population of the city. Previous research has primarily concentrated on data from the eastern region of Guangzhou. Therefore, our study serves to complement the existing literature by addressing this geographical gap and providing a more comprehensive perspective. Our retrospective analysis reveals that the overall prevalence of high-risk HPV infection in the western region of Guangzhou (18.46%) aligns with previous studies conducted in the eastern region (14–16). However, it is lower than the rates reported in Shenzhen (23.2%) (18), Taiwan (29.4%) (19), and certain areas of Hubei Province (44.4%) (20), while higher than those in Shanxi Province (14.8%) (21) and the Nyingchi City of Tibet (13.0%) (22).
Currently, there is no unified conclusion in the academic community regarding whether multiple high-risk HPV infections result in increased severity of cervical lesions or an elevated risk of developing cancer compared to single high-risk HPV infection. Some researchers believe that multiple high-risk HPV infections are one of the causes of persistent HPV infection, further exacerbating the degree of cervical lesions and increasing the risk of cancer (23). On the other hand, some scholars argue that the severity of cervical lesions and the risk of cervical cancer are not related to the number of high-risk HPV infections but rather to the pathogenicity of the genotypes involved (24–31).
Considering the risks and consequences of high-grade squamous intraepithelial lesions (HSIL+) and their importance, this study focuses on the relationship between the genotypes and number of high-risk HPV infections and HSIL+. Among different high-risk HPV infection genotypes, it was found that the incidence of HSIL+ was higher in the HPV 16 infection group compared to the HPV 18 infection group and the other 12 HPV infection group (P-values were all < 0.01). Additionally, the incidence of HSIL+ in the HPV 18 infection group was higher than that in the other 12 HPV infection group (P < 0.01). This indicates that in terms of the risks associated with HSIL+ development, HPV 16 > HPV 18 > other 12 high-risk HPV genotypes. By further comparing the groups, such as the 16+18 HPV infection group vs. the 16 HPV infection group, the 16+other 12 HPV infection group vs. the 16 HPV infection group, the 18+other 12 HPV infection group vs. the 18 HPV infection group, and the 16+18+other 12 HPV infection group vs. the 16+18 HPV infection group, although the number of high-risk HPV infections differed between the groups, there was no statistically significant difference in the risk of causing HSIL+ lesions due to the fact that the groups shared the same highest-risk HPV genotypes. Thus, it can be inferred that the number of high-risk HPV infections is not related to the severity of cervical lesions; instead, the severity of cervical lesions is associated with the pathogenicity of the HPV genotypes. This inference is further supported by observations from the comparison of different groups containing the highest-risk HPV genotypes, such as the 16+18 HPV infection group vs. the 18 HPV infection group, the 16+other 12 HPV infection group vs. the other 12 HPV infection group, the 18+other 12 HPV infection group vs. the other 12 HPV infection group. The group containing HPV genotypes with higher pathogenicity had a correspondingly higher incidence of HSIL+, and the differences were statistically significant (P < 0.05). This further confirms the aforementioned inference. A study by Xiang et al. (32) also found, through retrospective analysis of clinical data from 8,460 patients, that the risk of HSIL caused by HPV 16 and 18 genotypes was significantly higher than other genotypes. Additionally, the incidence of CIN was significantly higher in individuals with single HPV 16 infection compared to those with single HPV 18 infection, with significant statistical differences. This conclusion aligns closely with our study, which found that the risk associated with the development of HSIL+ lesions follows the order of HPV 16 > HPV 18 > other 12 high-risk HPV genotypes. Other reports have indicated that CIN patients infected with HPV 16 or 18 are more likely to experience rapid progression of lesions in the short term (33). Therefore, in cervical cancer screening, it is crucial to focus on the pathogenicity of the HPV genotypes involved in HPV infections, especially in the case of HPV 16 and 18. The number of HPV subgenotype infections is not an indicator for predicting the progression or severity of CIN lesions.
Similarly, whether multiple hrHPV infections increase the risk of cervical cancer compared to single hrHPV infections has been a hot topic of research with ongoing debates in the academic community. Some scholars believe that after the occurrence of multiple hrHPV infections, the presence of different genotypes of HPV may exhibit a certain synergistic effect, making it difficult for the body to completely clear the infection, thus increasing the risk of cervical cancer. On the other hand, another viewpoint suggests that multiple HPV infections are mainly dominated by the manifestation of one subgenotype, such as HPV-16, and an increased number of infections does not necessarily indicate a higher risk of carcinogenesis. In this study, no statistically significant difference was found in the occurrence rates of cervical cancer between the HPV-16 infection group and the HPV-18 infection group (P > 0.05). However, both the HPV-16 infection group and the HPV-18 infection group had significantly higher cervical cancer occurrence rates compared to the other 12 HPV infection groups (P < 0.01). This indicates that in terms of carcinogenicity, both HPV-16 and HPV-18 are higher than the other 12 high-risk HPV genotypes, but there is no significant difference in their carcinogenicity when comparing HPV-16 and HPV-18. Furthermore, when comparing the 16+18 HPV infection group vs. the HPV-16 infection group, the 16+18 HPV infection group vs. the HPV-18 infection group, the 16+other 12 HPV infection group vs. the HPV-16 infection group, the 18+other 12 HPV infection group vs. the HPV-18 infection group, the 16+18+other 12 HPV infection group vs. the 16+18 HPV infection group, the 16+18+other 12 HPV infection group vs. the HPV-16 infection group, and the 16+18+other 12 HPV infection group vs. the HPV-18 infection group, it was observed that although there were different numbers of hrHPV infections between the two groups, an increased number of hrHPV infections did not lead to an increased risk of cervical cancer. Moreover, as the two groups contained the same highest-risk HPV genotypes, there was no statistically significant difference in their ability to cause cervical cancer. Therefore, it can be inferred that there is no relationship between the number of hrHPV infections and the risk of cervical cancer, and the risk of cervical cancer is associated with the carcinogenicity of HPV genotypes.
By comparing the 16+other 12 HPV infection group vs. the other 12 HPV infection group and the 18+other 12 HPV infection group vs. the other 12 HPV infection group, it was found that the two groups being compared contained different highest-risk HPV genotypes, and the group with higher-risk HPV genotypes also had a higher incidence of cervical cancer, with statistically significant differences (P < 0.05). This further confirms that there is no relationship between the number of hrHPV infections and the risk of cervical cancer, and the risk of cervical cancer is associated with the carcinogenicity of HPV genotypes, which is consistent with the monoclonal origin theory of cancer. The higher risk of HPV-16 and HPV-18 may also be related to their longer duration of infection and lower clearance rates. Richardson et al. (34) found in a 2-year follow-up study of 621 patients that HPV-16 infection had the longest duration, with an average duration of 18.3 months. In conclusion, the number of HPV subgenotype infections cannot be considered as a predictor of the risk of cervical cancer. Clinically, for patients with multiple high-risk HPV infections, more attention should be given to the individual carcinogenic risks of specific genotypes.
Previous literature reports have suggested that women under 30 years of age are more likely to have multiple HPV infections compared to those over 30 years old (35, 36). In our study, we found that the prevalence of high-risk HPV infections and multiple high-risk HPV infections was higher in women under 35 years of age compared to those over 35 years old (20% vs. 17.1%; 2% vs. 1.3%), and these differences were statistically significant (P < 0.05). The higher prevalence among younger women can be attributed to factors such as poor sexual habits, including frequent sexual activity and multiple sexual partners. Additionally, the development of genotype-specific HPV antibodies requires exposure to HPV through sexual activity. Women under 35 years of age are still in the process of developing immunity against HPV infections, and their immune systems need time to mature. In contrast, women over 35 years of age have already developed immune systems capable of combating various HPV infections. Further analysis of 2,411 patients with high-risk HPV infections showed an increasing trend in the incidence of high-grade squamous intraepithelial lesions (HSIL+) with age. We propose the following explanations: (1) Persistent infection with high-risk HPV is the direct cause of cervical cancer and precancerous lesions, and the development of HSIL and higher-grade lesions requires a prolonged process. Therefore, the increasing trend in the incidence of HSIL+ with age reflects this progression. (2) Although older women have more mature immune systems to fight against various HPV infections, their immune response gradually decreases with age, making it difficult to completely clear high-risk HPV infections, leading to persistent infection. In conclusion, women under 35 years of age are more prone to high-risk HPV infections and even multiple infections compared to women over 35 years of age. The incidence rate of HSIL+ shows a progressive increase with the age of the infected individuals.
According to data published on the website of the National Health Commission of the People's Republic of China, the national routine immunization report shows that the reported HPV vaccine doses administered in various regions of the country were 3.417 million, 6.753 million, and 12.279 million doses for the years 2018, 2019, and 2020, respectively. While the number of vaccine doses administered has been increasing annually, the vaccination rates in the population remain relatively low. Currently, HPV vaccination is not included in the national immunization program and is administered on a voluntary and self-funded basis. The commission is actively promoting the inclusion of HPV vaccination in local public welfare policies where conditions permit. Since Guangzhou only introduced HPV vaccines in 2016, initially offering only the bivalent vaccine, the percentage of women in Guangzhou receiving HPV vaccines was extremely low (specific data for Guangzhou has not been disclosed). Therefore, the number of HPV vaccine recipients in this study was < 0.5% of the total screening population. This inadequate representation makes it impossible to fully assess the impact of HPV vaccination on HPV infection rates and cervical lesions in this study. Consequently, the vaccination status regarding HPV vaccines was not included in this research, representing a significant limitation and regrettable aspect of this study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study has obtained ethical approval from the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University and was conducted in accordance with ethical standards.
Author contributions
JL, XS, and YZ: Conceptualization and funding acquisition. JL: Formal analysis. JL and HL: Methodology. JL, HL, and DZ: Writing – original draft. HQ, XS, and YZ: Writing – review & editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Science and Technology Planning Project of Guangzhou (Funding No. 202102010003); the Project for Key Medicine Discipline Construction of Guangzhou Municipality (No. 2021-2023-17) and Guangzhou Health Science and Technology Project (No. 20231A011091).
Acknowledgments
Thanks to the obstetrician and gynecologist of the Third Affiliated Hospital of Guangzhou Medical University for providing the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW. Attempts to detect virus-specific DNA in human tumors. II. Nucleic acid hybridizations with complementary RNA of human herpes group viruses. Int J Cancer. (1974) 13:657–64. doi: 10.1002/ijc.2910130510
3. Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. (2005) 89:S4–12. doi: 10.1016/j.ijgo.2005.01.009
4. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. (2004) 324:17–27. doi: 10.1016/j.virol.2004.03.033
5. WHO. WHO Guideline for Screening and Treatment of Cervical Pre-cancer Lesions for Cervical Cancer Prevention [EB/OL]. Geneva: WHO (2022).
6. Bello BD, Spinillo A, Alberizzi P, Cesari S, Gardella B, D'Ambrosio G, et al. Cervical infections by multiple human papillomavirus (HPV) genogenotypes: prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol. (2009) 81:703–12. doi: 10.1002/jmv.21429
7. Salazar KL, Zhou HS, Xu J, Peterson LE, Schwartz MR, Mody DR, et al. Multiple human papilloma virus infections and their impact on the development of high-risk cervical lesions. Acta Cytol. (2015) 59:391–8. doi: 10.1159/000442512
8. Dickson EL, Vogel RI, Geller MA, Downs LS Jr. Cervical cytology and multiple type HPV infection: a study of 8182 women ages 31-65. Gynecol Oncol. (2014) 133:405–8. doi: 10.1016/j.ygyno.2014.03.552
9. Kim M, Park NJ, Jeong JY, Park JY. Multiple Human Papilloma Virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. (2021) 13:1342. doi: 10.3390/v13071342
10. Stewart CJ, Crook ML. PAX2 and cyclin D1 expression in the distinction between cervical microglandular hyperplasia and endometrial microglandular-like carcinoma: a comparison with p16, vimentin, and Ki67. Int J Gynecol Pathol. (2015) 34:90–100. doi: 10.1097/PGP.0000000000000107
11. Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. (2006) 98:303–15. doi: 10.1093/jnci/djj067
12. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. (2015) 136:189–97. doi: 10.1016/j.ygyno.2014.11.076
13. Cuzick J, Wheeler C. Need for expanded HPV genotyping for cervical screening. Papillomavirus Res. (2016) 2:112–5. doi: 10.1016/j.pvr.2016.05.004
14. Huiyun J, Jie D, Huan W, Yuebo Y, Xiaomao L. Prevalence and characteristics of cervical human papillomavirus genotypes and cervical lesions among 58630 women from Guangzhou, China. J Infect Public Health. (2023) 16:1531–6. doi: 10.1016/j.jiph.2023.07.013
15. Li S, Zhang K, Yang L, Wu J, Bhargava N, Li Y, et al. Distribution patterns of human papillomavirus genotypes among women in Guangzhou, China. Infect Agent Cancer. (2023) 18:67. doi: 10.1186/s13027-023-00541-8
16. Yang X, Li Y, Tang Y, Li Z, Wang S, Luo X, et al. Cervical HPV infection in Guangzhou, China: an epidemiological study of 198,111 women from 2015 to 2021. Emerg Microbes Infect. (2023) 12:e2176009. doi: 10.1080/22221751.2023.2176009
17. Nayar R, Solomon D. Second edition of ‘The Bethesda System for reporting cervical cytology'-atlas, website, and Bethesda interobserver reproducibility project. Cytojournal. (2004) 1:4. doi: 10.1007/978-1-4612-2042-8
18. Ren T, Liao Y, Huang T, Wang J, Xiao X, Wei W, et al. Detection and infection of HPV infection in women of Shenzhen, Guangdong province. J Pract Med. (2020) 36:969–73. doi: 10.3969/j.issn.1006-5725.2020.07.027
19. Strong C, Zou H, Ko NY, Liang YL, Ku WW, Lee CW. Prevalence and risk factors of anogenital human papillomavirus infection in a community sample of men who have sex with men in Taiwan: baseline findings from a cohort study. Sex Transm Infect. (2020) 96:62–6. doi: 10.1136/sextrans-2018-053629
20. Weipeng W. Genogenotype distribution of high-risk human papillomavirus in women with cervical infection in our hospital. J Pract Med. (2007) 23:2040–1. doi: 10.3969/j.issn.1006-5725.2007.13.040
21. Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, et al. Human papillomavirus infection in Shanxi Province, People's Republic of China: a population-based study. Br J Cancer. (2006) 95:96–101. doi: 10.1038/sj.bjc.6603208
22. Li J, Li X, Sheng X. Four-year analysis of high-risk human papillomavirus infection among women in rural areas of Nyingchi City, Tibet. Front Public Health. (2023) 11:1251440. doi: 10.3389/fpubh.2023.1251440
23. Bachtiary B, Obermair A, Dreier B, Birner P, Breitenecker G, Knocke TH, et al. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int J Cancer. (2002) 102:237–43. doi: 10.1002/ijc.10708
24. Lee EH, Um TH, Chi HS, Hong YJ, Cha YJ. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. (2012) 27:1091–7. doi: 10.3346/jkms.2012.27.9.1091
25. Sarkar K, Pal R, Bal B, Saha B, Bhattacharya S, Sengupta S, et al. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. (2011) 11:72. doi: 10.1186/1471-2334-11-72
26. Sun C, Zhang L, Yue T. Compare of the influence on cervical lesion between single and multiple HPV genogenotype infection. J Int Obstet Gynecol. (2013) 40:77–80.
27. Wang C, Wang K, Chen Y, Yang Z, Luo N, Zhou K, et al. Analysis of the relationship between HPV multiple infection and cervical lesions. Exp Lab Med. (2012) 30:166–8. doi: 10.3969/j.issn.1674-1129.2012.02.021
28. Huang B, Li R, Wu R, Tang H, Liu Z, Wu L, et al. Screening of cervical cancer by combined detection of papillomavirus L1 capsid protein and human telomerase RNA component. Acta Med Univ Sci Technol Huazhong. (2010) 39:554–7, 561. doi: 10.3870/j.issn.1672-0741.2010.04.030
29. Lee SA, Kang D, Seo SS, Jeong JK, Yoo KY, Jeon YT, et al. Multiple HPV infection in cervical cancer screened by HPVDNAChip. Cancer Lett. (2003) 198:187–92. doi: 10.1016/S0304-3835(03)00312-4
30. Li Y, Liu J, Wan C, Du Y. Study on the relationship between multiple infection of HPV and cervical dysplasia. Mod Med J China. (2011) 13:41–2. doi: 10.3969/j.issn.1672-9463.2011.09.015
31. Wu D, Guo C, Yu J, Liu Q, Miao H, Huang R, et al. Association of multiple high-risk human papillomavirus infection and cervical carcinoma and precancerous lesion. China Med. (2011) 6:604–6. doi: 10.3760/cma.j.issn.1673-4777.2011.05.040
32. Xiang L, Hong Z, Wen D. Relationship between Human Papillomavirus Genogenotypes and Cervical Intraepithelial Neoplasia. J Pract Obstet Gynecol. (2012) 28:937–41. doi: 10.3969/j.issn.1003-6946.2012.11.014
33. Hwang TS, Jeong JK, Park M, Han HS, Choi HK, Park TS. Detection and typing of HPV genogenotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol Oncol. (2003) 90:51–6. doi: 10.1016/S0090-8258(03)00201-4
34. Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, et al. The natural history of genotype-specific human papillomavirusinfections in female university students. Cancer Epidemiol Biomarkers Prev. (2003)12:485–90.
35. Liu X, Xu Y, Wang L, Wang X. Study on the relationship between HPV mixed genotype infection and cervical lesions. Prog Obstet Gynecol. (2010) 19:214–5.
Keywords: high-risk HPV, cervical lesions, multiple infections, age, Canton (Guangzhou)
Citation: Li J, Lai H, Qin H, Zhou D, Zhao Y and Sheng X (2024) Current status of high-risk HPV infection and correlation with multiple infections in cervical lesions in Western Guangzhou. Front. Med. 11:1252073. doi: 10.3389/fmed.2024.1252073
Received: 14 July 2023; Accepted: 01 April 2024;
Published: 17 April 2024.
Edited by:
Long Sui, Fudan University, ChinaReviewed by:
Nuchsupha Sunthamala, Mahasarakham University, ThailandLeabaneng Tawe, Botswana-UPenn Partnership (BUP), Botswana
Copyright © 2024 Li, Lai, Qin, Zhou, Zhao and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujie Sheng, MjAwODY5MTE1MEBnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
 Jianqi Li
Jianqi Li He Lai2†
He Lai2† Yang Zhao
Yang Zhao Xiujie Sheng
Xiujie Sheng