- 1Department of Anesthesiology, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Shandong Institute of Anesthesia and Respiratory Critical Medicine, Jinan, China
- 2Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 3Department of Clinical Laboratory Medicine, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Jinan, China
- 4Yidu Cloud (Beijing) Technology Co. Ltd., Beijing, China
- 5Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University (Shandong Provincial Hospital), Jinan, China
Background: The effect of different non-cardiac surgical methods on islet and renal function remains unclear. We conducted a preliminary investigation to determine whether different surgical methods affect islet function or cause further damage to renal function.
Methods: In this prospective cohort study, the clinical data of 63 adult patients who underwent non-cardiac surgery under general anesthesia were evaluated from February 2019 to January 2020. Patients were divided into the abdominal surgery group, the laparoscopic surgery group, and the breast cancer surgery group. The primary outcome was the difference between the effects of different surgical methods on renal function.
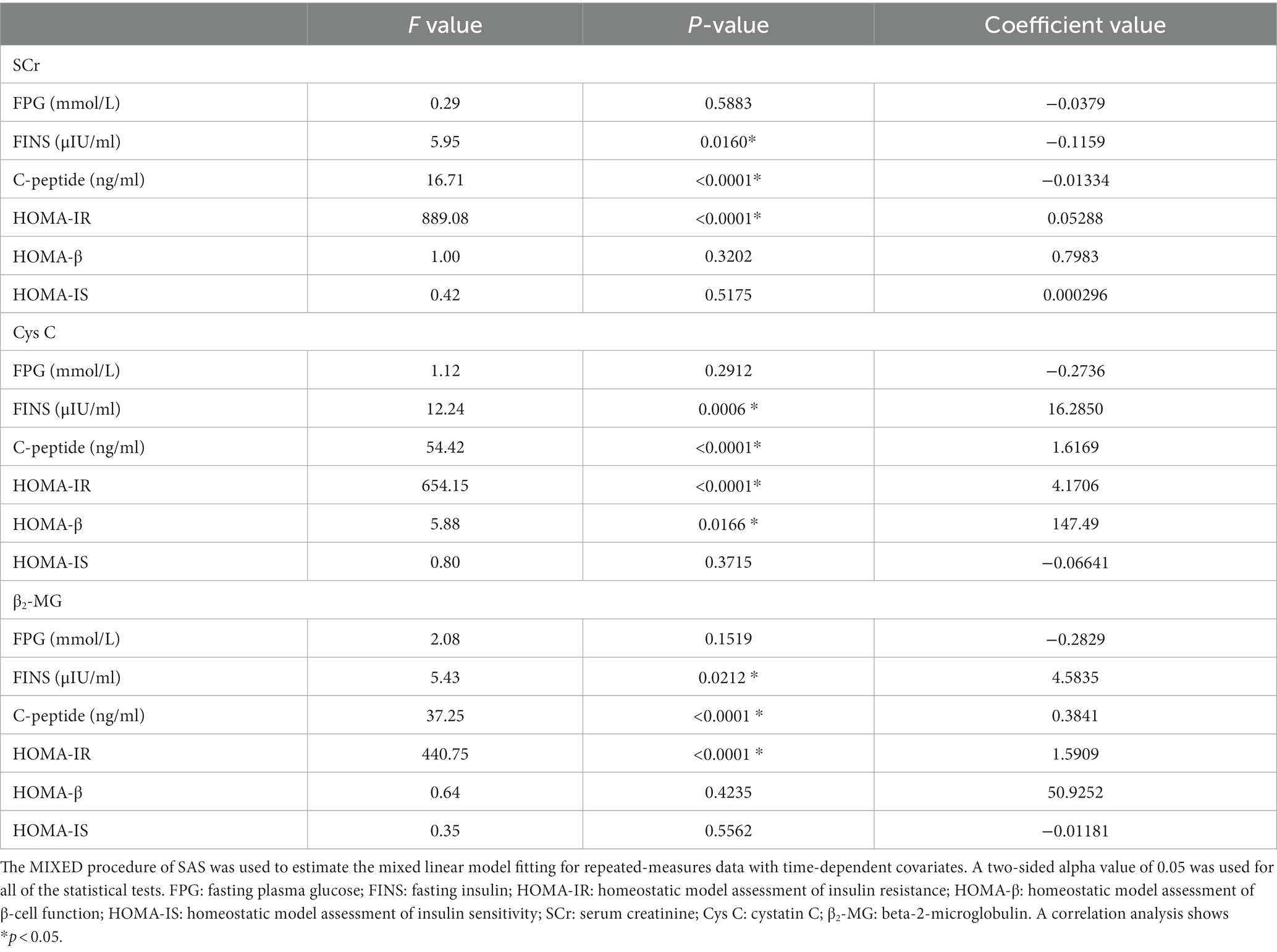
Results: Islet and renal function were not significantly different between the groups. The correlation analysis showed that hematocrit (HCT) and hemoglobin (HB) were negatively correlated with fasting plasma glucose (FPG) (p < 0.05), MAP was positively correlated with C-peptide (p < 0.05), and HCT and Hb were positively correlated with serum creatinine (SCr) (p < 0.05). Fasting insulin (FINS) and C-peptide were negatively correlated with SCr (p < 0.05), and the homeostatic model assessment of insulin resistance (HOMA-IR) was positively correlated with SCr (p < 0.05). FINS, C-peptide, HOMA-IR, and the homeostatic model assessment of β-cell function (HOMA-β) were positively correlated with cystatin C (Cys C) (p < 0.05).
Conclusion: FINS, C-peptide, and HOMA-IR had positive effects on beta-2-microglobulin (β2-MG). FINS, C-peptide, and HOMA-IR were positively correlated with Cys C and β2-Mg. While FINS and C-peptide were negatively correlated with SCr, HOMA-IR was positively correlated with SCr.
1 Introduction
Surgeries stimulate visceral sympathetic nerves, which can increase the synthesis and release of adrenal glucocorticoids through the hypothalamic–pituitary axis, inhibit the utilization of glucose in tissues, antagonize the effect of insulin, and cause stress, insulin resistance (IR), and hyperglycemia, placing the nervous system, endocrine system, and immune system in a state of stress. As a result, the patient’s resistance ability is reduced, and the generation of peroxide and oxygen-free radicals is increased, thereby increasing the risks of postoperative complications and surgery (1, 2). Serum insulin and C-peptide levels may reflect the secretory function of islet β cells. Mechanical stimulation of surgeries and the high-pressure environment of laparoscopic surgeries may stimulate islet β cells and inhibit their secretion. Acute disease, injury, or surgical tissue trauma will lead to changes in glucose metabolism and induce a neuroendocrine stress response, resulting in insulin resistance, increased gluconeogenesis, glycogenolysis, and decreased peripheral glucose uptake (3, 4). The renin-angiotensin system (RAS) involves many physiological and pathophysiological processes. Increasing evidence has supported the involvement of the RAS in expression in human and rodent pancreases (5). In addition, angiotensinogen is the substrate of renin, which is mainly expressed in α cells (which mainly secrete glucagon) (6).
The prevalence of chronic kidney disease (CKD) has increased worldwide, exceeding 10% (7), and it is even higher among patients who undergo surgery. Acute kidney injury (AKI) remains a common perioperative complication that affects the short- and long-term outcomes of patients. Strategies to prevent AKI in high-risk patients include avoiding nephrotoxic drugs and contrast agents, managing hyperglycemia, and closely monitoring renal function (8). Both hyperglycemia and insulin resistance can affect renal function. Diabetic nephropathy (DN) is the most common microvascular complication of diabetes and is the main cause of high mortality and disability in DM patients (9). At present, there are very few relevant studies investigating the effect of surgical methods on islet and renal function. For this reason, we hypothesized that the stress response secondary to surgical anesthesia induces hyperglycemia, which affects islet cell sensitivity and leads to insulin resistance, ultimately resulting in kidney damage.
2 Methods
This was a single-center prospective cohort study. The study was conducted in accordance with the Declaration of Helsinki (World Medical Association 2013) and was approved by the local Ethics Committee of Qianfoshan Hospital, Shandong Province (YXLL-KY-2019 (036)). The study was nationally registered (Chinese Clinical Trials.gov: ChiCTR 1,900,028,686, 30 December 2019). All patients signed informed consent forms prior to enrollment in the study.
2.1 Patients
Patients undergoing non-cardiac surgery, including abdominal surgery or radical mastectomy for breast cancer, in the First Affiliated Hospital of Shandong First Medical University from February 2019 to January 2020 were selected. Abdominal surgery included open surgery and laparoscopic surgery. According to the surgical method, patients were assigned to the abdominal surgery group, the laparoscopic surgery group, or the breast cancer surgery group.
The baseline HR, NBP, SpO2, and bifrequency index (BIS) values (BIS® Monitor, Covidien, Boulder, CO, USA) were routinely monitored and recorded during the patients’ operations. Midazolam 0.05 mg kg−1, etomidate 0.2 mg kg−1, sufentanil 0.4 μg kg−1, and rocuronium 0.6 mg kg−1 were intravenously injected in sequence. TCI propofol 1.5–3.5 μg ml−1 combined with sevoflurane using a minimal alveolar concentration (MAC) of 1.0 and remifentanil pumped at a constant rate of 0.1–1 μg kg−1 min−1 were applied to maintain anesthesia, and rocuronium was used intermittently. EtCO2 was kept between 35 and 45 mmHg, and BIS values were kept between 40 and 60 during surgery. Intraoperative fluids contained no dextrose. Any case of intraoperative hypotension (MAP <65 mmHg) or bradycardia (HR < 50 beats/min) was treated with an intravenous injection of ephedrine 5 mg or atropine 0.4 mg.
2.2 Inclusion criteria
Patients who were between 18 and 70 years of age with an ASA classification of I–II and who underwent level 4 abdominal surgery (including radical gastrectomy, radical resection of rectal cancer, resection of liver tumor, hysterectomy, etc.) or breast cancer surgery were included in the study.
2.3 Exclusion criteria
Patients who had a history of diabetes or had taken hypoglycemic drugs 3 months before surgery; patients with a history of circulatory diseases such as hypertension or heart disease; patients with a history of liver dysfunction or liver surgery; patients with a history of drug allergy; patients who suffered from shock, acidosis, or any other crises during the operation; patients who were treated with blood products or vasoactive drugs for rescue; patients who voluntarily withdrew from the study; patients with water and electrolyte disorders or an acid–base imbalance; and patients who received immunotherapy.
2.4 Primary outcome
The primary outcome was renal function injury. SCr, Cys C, and β2-MG were used to assess renal function. The observation time points were preoperation (baseline), after the induction of anesthesia, and 30 and 60 min after the surgery. Five milliliters of venous blood were collected.
2.5 Secondary outcomes
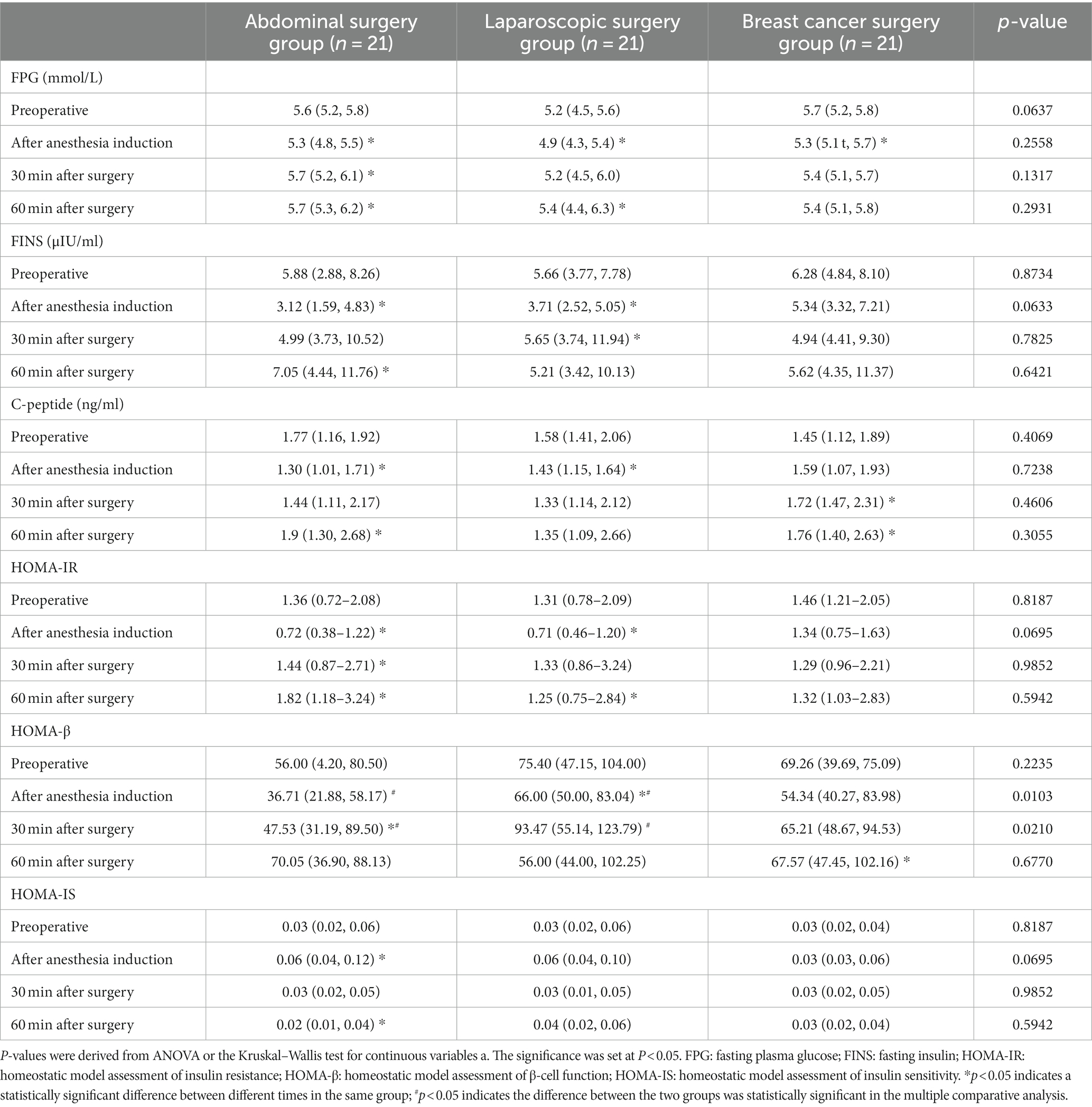
The secondary outcomes included islet function (FPG, FINS, C-peptide, and HOMA-IR, HOMA-β, and HOMA of insulin sensitivity [HOMA-IS]); correlation analysis of intraoperative islet function and renal function; and Hb, HCT, and MAP were obtained from arterial blood gas before surgery, after induction of anesthesia, and 30 min and 60 min after surgery. The incidence of outliers for each indicator induction of anesthesia 30 min and 60 min after surgery (occurrence ≥1, recorded as outliers). HOMA-IR, HOMA-β, and HOMA-IS were used to assess islet function, which was calculated by a homeostasis assessment model with FPG, FINS, and C-peptide, such as HOMA-IR = (FINS × FPG)/22.5, HOMA-β = 20 × FINS/(FPG-3.5), and HOMA-IS = 1/(FPG × FINS).
2.6 Sample size
The sample size was calculated using data from the Long TE study (10), an observational case-control study examining the incidence, risk factors, and mortality in patients with AKI following abdominal surgery, including exploratory laparotomy (12.9%), laparoscopic surgery (3.4%), and minor surgical procedures (4.1%), the sample size was calculated. A sample size of 344 achieves 80% power to detect an effect size (W) of 0.1675 using two degrees of freedom chi-square test with a significance level (alpha) of 0.05 by sample size by PASS 15.0. The study of Long TE observed that the incidence of AKI in all abdominal procedures was 6.8%. Therefore, we assumed that intraoperative AKI was 1/6 of postoperative AKI, and we estimated that the sample size should be 57 cases. With a 10% shedding rate, we needed to include at least 63 subjects (at least 21 in each group).
2.7 Statistical analysis
A comparison of the preoperative baseline data was performed. For continuous variables, the differences between the groups were compared using a one-way ANOVA or the Kruskal–Wallis non-parametric test according to whether the data met a normal distribution. A chi-square test was used to analyze the differences between the groups for categorical variables.
To compare the differences between three groups of patients with the same intraoperative index at the same time, a one-way ANOVA, or the Kruskal–Wallis non-parametric test, was conducted according to whether the data met the normal distribution, and multiple comparisons between the groups were conducted.
Repeated-measures ANOVA was used to compare the changes in the same index at different times in patients within the same group. The trend of the same index over time in the three groups during surgery was compared using a multivariate analysis of variance and Pillai’s trace test, and the variation trend is shown as a broken line graph.
To explore the effects of the different surgical methods on islet and renal function in patients under general anesthesia and to analyze the correlation between islet and renal function, the MIXED procedure of SAS was used to perform a parameter estimation of the MIXED linear model with time-dependent covariate repeated measurement data.
SAS (SAS Institute, Inc., Cary, NC, SAS 9.4 Version) was used for the statistical analysis. Unless otherwise specified, α was 0.05 (two-sided test), and p < 0.05 was defined as statistically significant.
3 Results
3.1 Trial cohort and patient flow
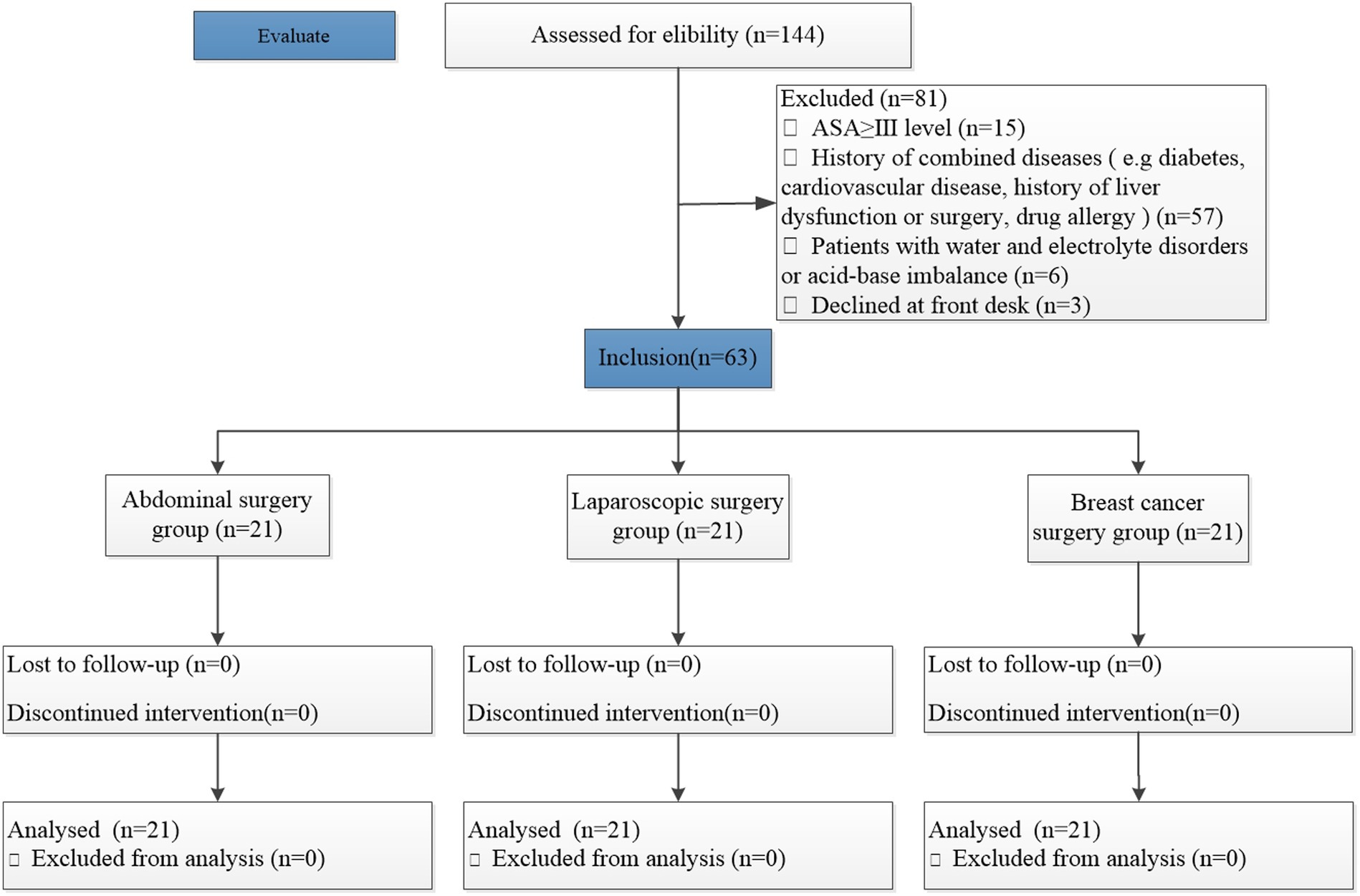
The inclusion and exclusion criteria for the patients are shown in Figure 1. Between February 2019 and January 2020, a total of 144 patients aged 18–70 who underwent abdominal or breast surgery were initially recruited after assessment of the eligibility criteria. However, 81 patients were excluded (the abdominal surgery group included 34 patients, the laparoscopic surgery group included 31 patients, and the breast cancer surgery group included 16 patients); 63 patients were finally included in the study.
3.2 Baseline characteristics
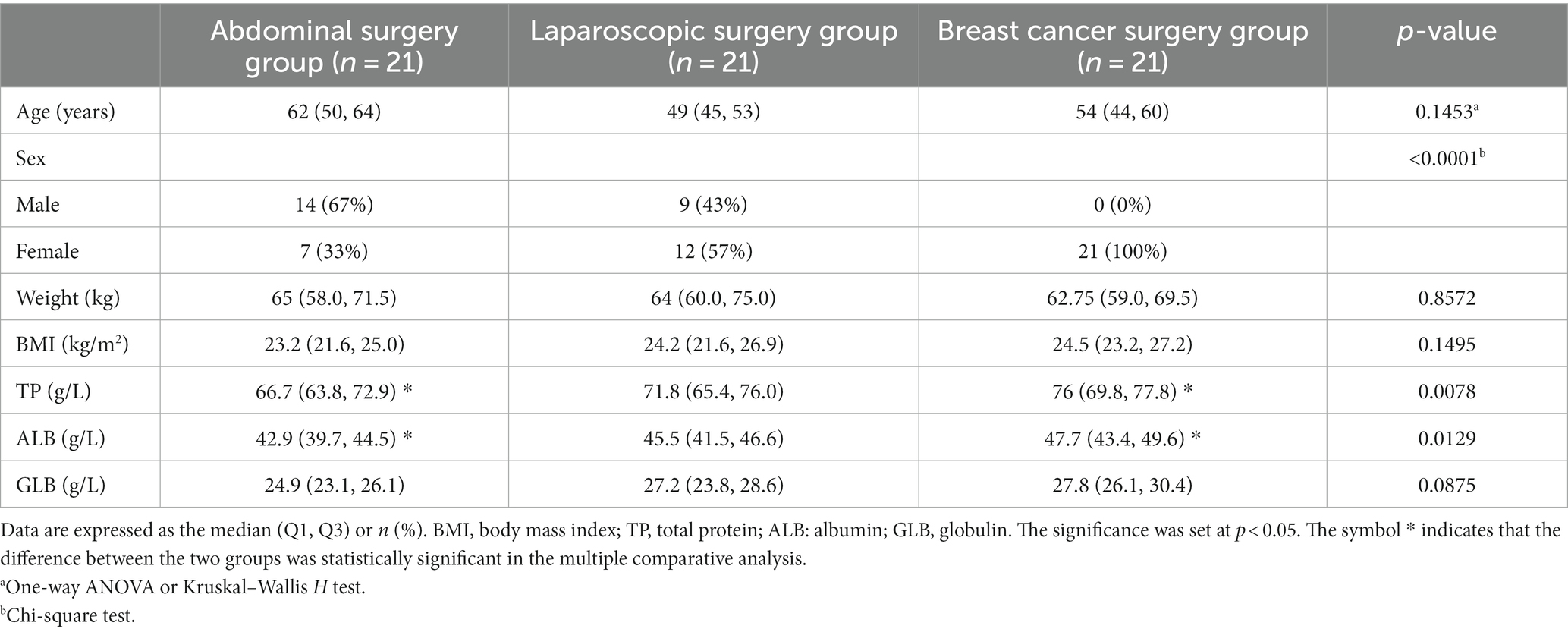
There were no statistically significant differences in age, weight, body mass index (BMI), or globulin (GLB) among the three groups, while there were statistically significant differences in total protein (TP) and albumin (ALB) between the breast group and the open surgery group (p < 0.05). This study compared the breast cancer group with the abdominal surgery group to investigate the differences in islet and renal function between surface surgeries and abdominal surgeries. However, because all the breast cancer patients were female, there was a significant difference between the other two groups of patients. Since islet and renal function are not affected by sex, this difference is not discussed in this study (Table 1).
3.3 Primary outcome
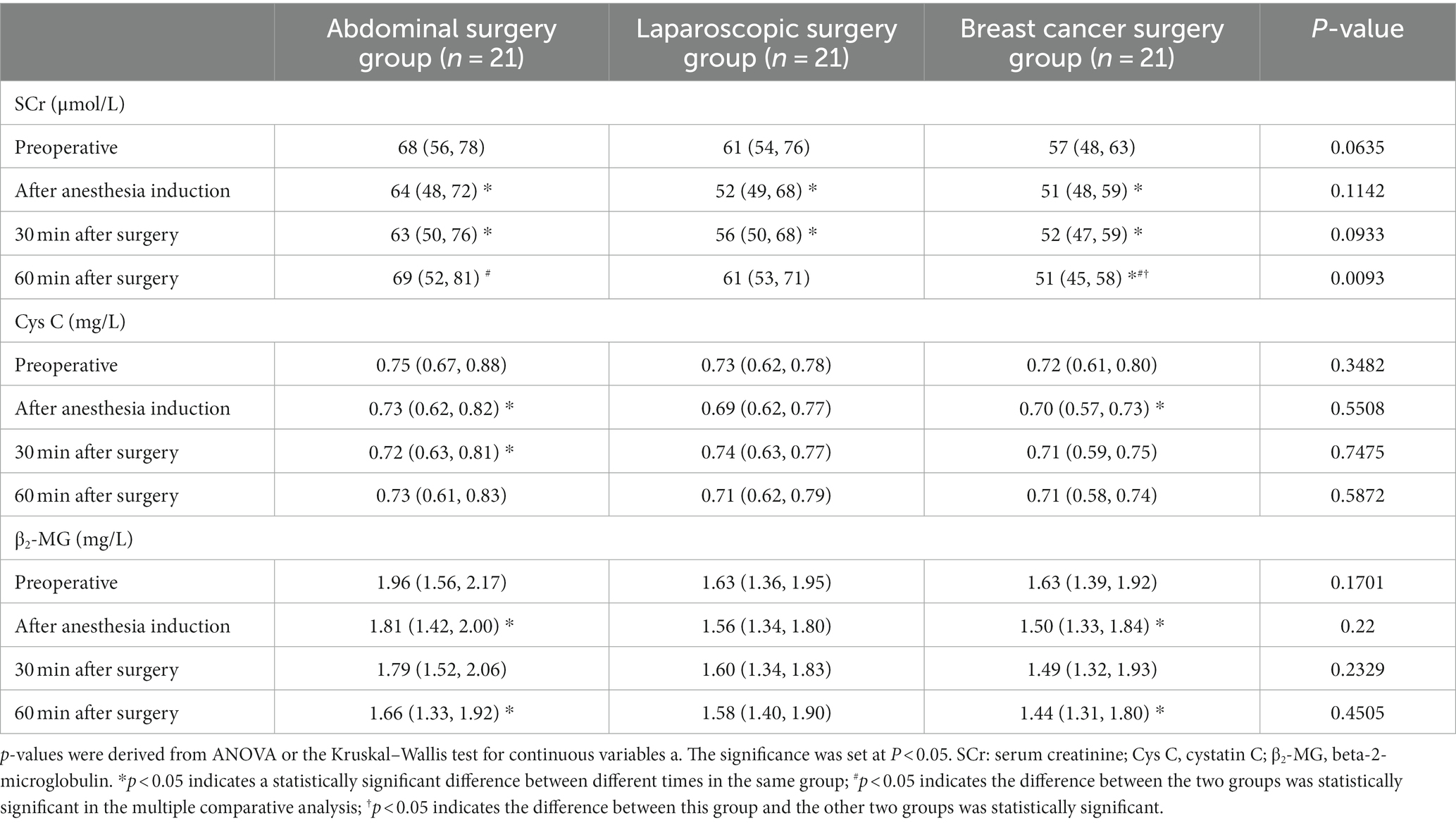
There was no significant difference in β2-MG or Cys C among the three groups at the same time point. At 60 min after surgery, the SCr of the abdominal surgery group and the laparoscopic surgery group was significantly higher than that of the breast cancer surgery group [69 (52, 81) vs. 51 (45, 58), 61 (53, 71) vs. 51 (45, 58), p = 0.0093], and there was no significant difference between the abdominal surgery group and the laparoscopic surgery group (Table 2).
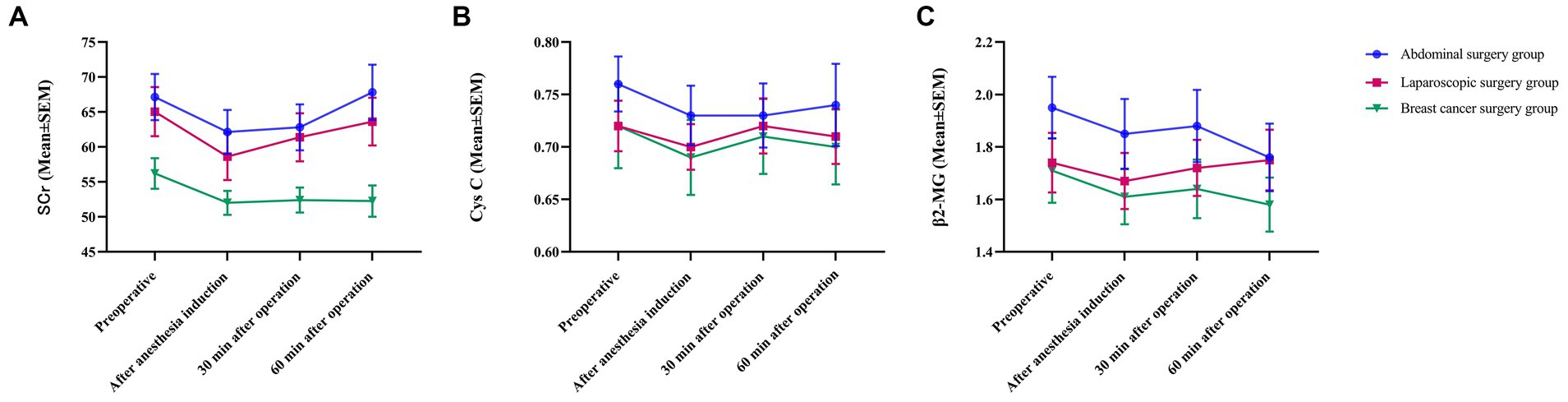
The SCr, Cys C, and β2-MG levels decreased after anesthesia induction. Thirty minutes after surgery, SCr, Cys C, and β2-MG increased to varying degrees and did not exceed their respective values before surgery (Figure 2). Sixty minutes after surgery, the levels of β2-MG in the laparoscopic surgery group continued to increase and exceeded the preoperative level, while the levels of β2-MG in the abdominal surgery group and the breast cancer group continued to decrease and were lower than the preoperative level (Figure 2A). The SCr levels of the patients in the abdominal surgery group and the patients in the laparoscopic surgery group continued to increase and were similar to the preoperative level, while the level of SCr in the patients in the breast cancer group continued to decrease and was lower than the preoperative level (Figure 2B). The level of Cys C in the patients in the abdominal surgery group continued to increase, remaining at the same level as before surgery, while the levels of Cys C in patients in the laparoscopic surgery group and the breast cancer group continued to decrease and were lower than the preoperative level (Figure 2C). There was no significant difference in the results of the different groups at the different time points, and the trends of the three groups were basically the same.
3.4 Secondary outcomes
There was no significant difference in FPG, FINS, C-peptide, HOMA-IR, or HOMA-IS among the three groups at the same time point. At 30 and 60 min after surgery, the HOMA-β of the laparoscopic surgery group was significantly higher than that of the abdominal surgery group [36.71 (21.88, 58.17) vs. 66.00 (50.00, 83.04), p = 0.0103; 47.53 (31.19, 89.50) vs. 93.47 (55.14, 123.79), p = 0.0210] (Table 3).
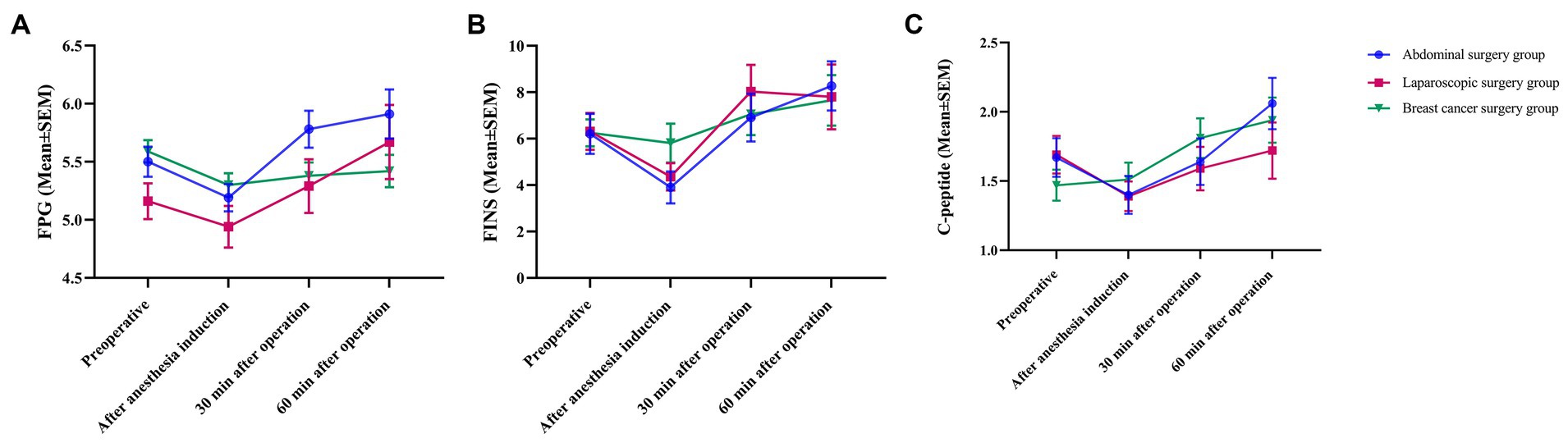
After anesthesia induction, FPG decreased initially and then gradually increased 30 min after surgery. The FPG in the abdominal surgery group and laparoscopic surgery group reached the highest point at 60 min after surgery and was higher than the FPG preoperatively. A decreasing trend during surgery was observed in the breast cancer group (Figure 3A). After anesthesia induction, the FINS decreased first, while the FINS in the breast cancer group decreased slightly, gradually increased 30 min after the surgery, reached the highest point at 60 min after the surgery, and were higher than the FINS at the time before the operation (Figure 3B). In the abdominal surgery group and laparoscopic surgery group, C-peptide showed a downward trend after anesthetic induction and gradually increased 30 min after surgery. Sixty minutes after surgery, the C-peptide level in the abdominal surgery group increased to the highest point, which was higher than the C-peptide level at the time before surgery. In the laparoscopic surgery group, it was lower than the C-peptide level at the time before surgery. The C-peptide levels in the breast cancer group continued to increase during surgery (Figure 3C). There were no significant differences among the different groups at the different time points, and the trends of the three groups were basically the same.
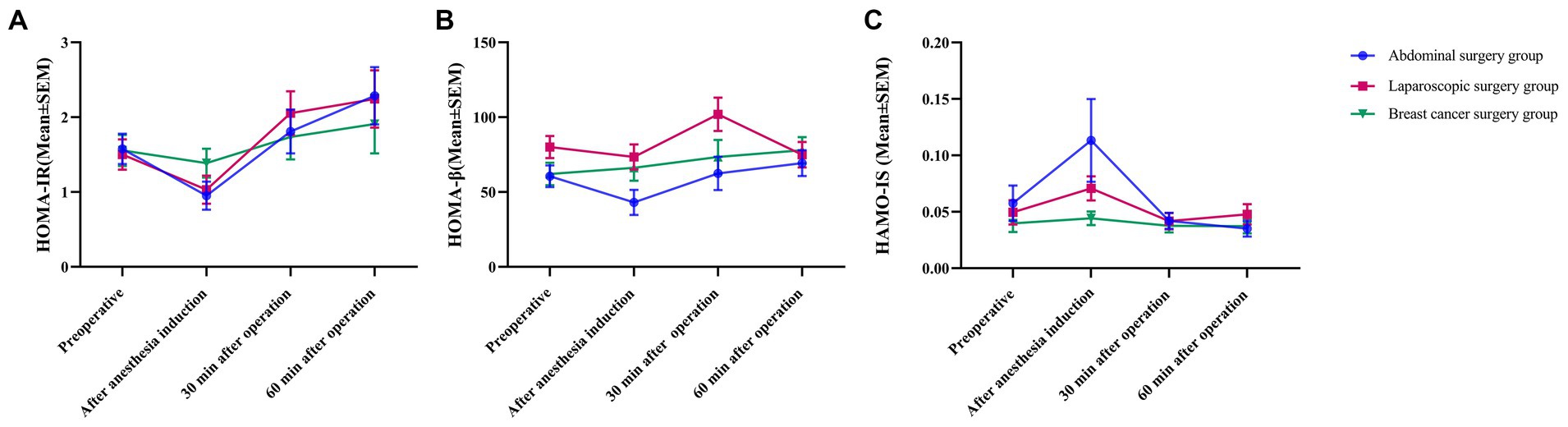
HOMA-IR initially decreased after the induction of anesthesia, gradually increased 30 min after surgery, reached the highest point at 60 min after surgery, and was higher than HOMA-IR before the operation. HOMA-β initially decreased after anesthetic induction in the abdominal surgery group and laparoscopic surgery group and increased gradually 30 min after surgery (Figure 4A). At 60 min after surgery, HOMA-β continued to increase in the abdominal surgery group, while in the laparoscopic surgery group, it decreased and was lower than the preoperative level. In the breast cancer group, HOMA-β continuously increased. HOMA-IS increased to the maximum value after anesthetic induction and decreased to the minimum value at 30 and 60 min after surgery in both the abdominal surgery group and breast cancer group (Figure 4B). In the laparoscopic surgery group, the value decreased to its lowest at 30 min after surgery and gradually increased 60 min after surgery. There was no significant difference in the results of the different groups at the different time points, and the trend of the three groups was basically the same (Figure 4C).
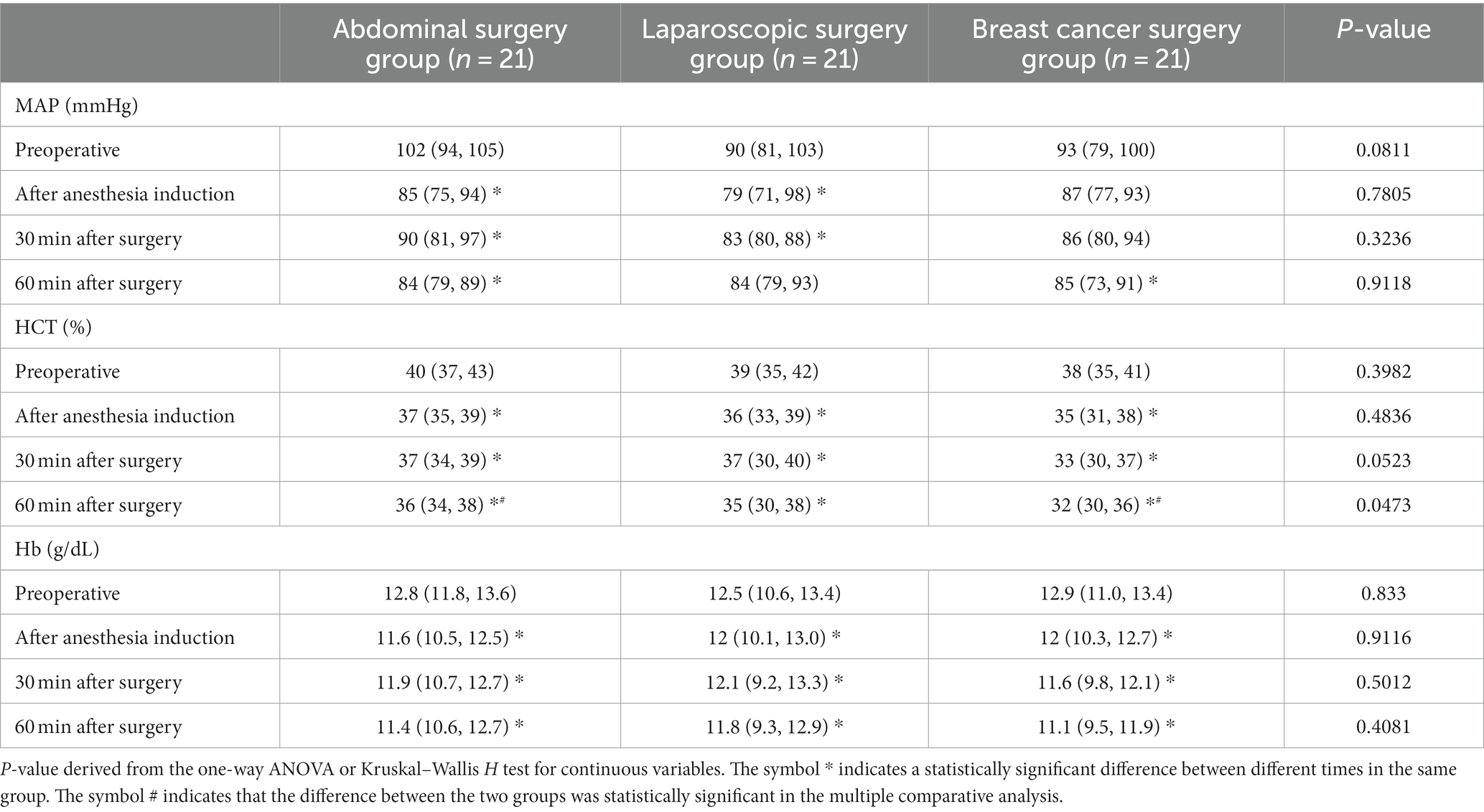
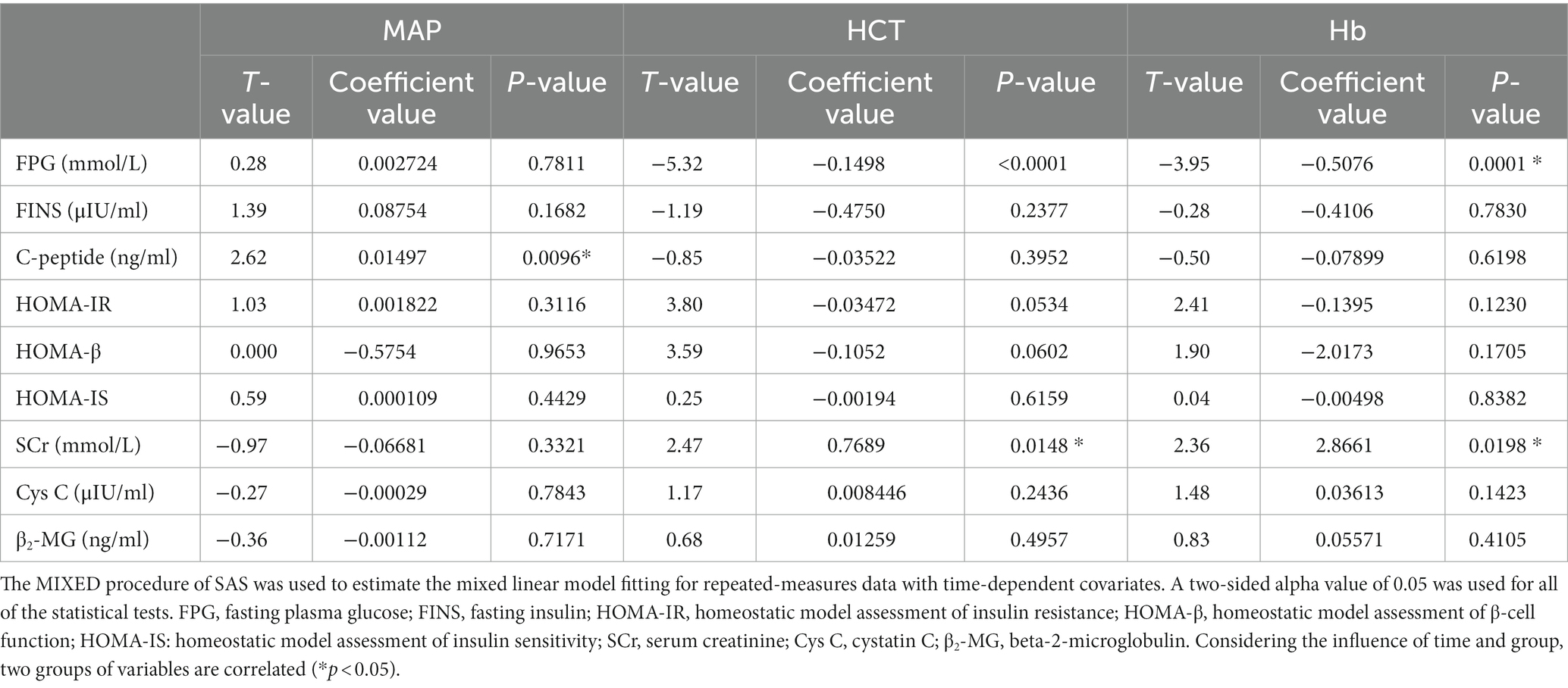
Compared with the preoperative values, MAP, HCT, and Hb decreased to different degrees after anesthetic induction and at 30 and 60 min after surgery, and the differences were statistically significant (p < 0.05). At 60 min after surgery, the HCT of the abdominal surgery group was significantly higher than that of the breast cancer surgery group. The differences were statistically significant [36 (34, 38) vs. 32 (30, 36), p < 0.05], except for the differences in MAP, HCT, and Hb at the same time points between the groups (Table 4).
The correlation analysis of SCr with FINS, C-peptide, and HOMA-IR showed that there was statistical significance (p < 0.05). The time-dependent parameter covariates (the coefficient value) of FINS and C-peptide were negative, and FINS and C-peptide had negative effects on SCr. The coefficient value of HOMA-IR was positive, and it had a positive effect on SCr. The correlation analysis of Cys C with FINS, C-peptide, HOMA-IR, and HOMA-β showed that there was statistical significance (p < 0.05). The coefficient values of FINS, C-peptide, HOMA-IR, and HOMA-β were positive, and they had positive effects on Cys C. The correlation analysis of β2-MG with FINS, C-peptide, and HOMA-IR was statistically significant (p < 0.05). The coefficient values were positive, and FINS, C-peptide, and HOMA-IR had positive effects on β2-MG (Table 5).
The correlation analysis of FPG with HCT and Hb showed that there was statistical significance (p < 0.001). The coefficient values were negative, and HCT and Hb had negative effects on blood glucose. The correlation analysis between C-peptide and MAP showed that there was statistical significance (p < 0.05). The coefficient value was positive, and MAP had a positive effect on C-peptide. The correlation analysis of SCr with HCT and Hb showed that there was statistical significance (p < 0.05). The coefficient values were positive, and HCT and Hb had positive effects on SCr (Table 6).
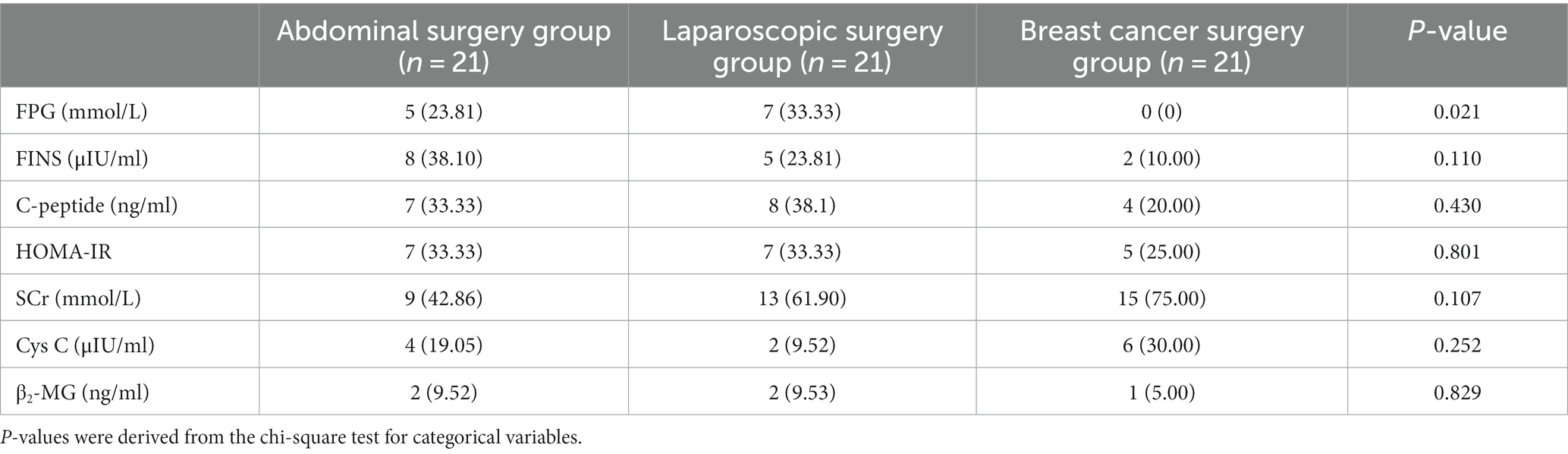
We observed the incidence of intraoperative outliers of FPG, FINS, C-peptide, β2-MG, SCr, and Cys C. The incidence of intraoperative outliers of FPS in the breast cancer surgery group was 0, and there was a significant difference between the other two groups (p < 0.05). The incidence of intraoperative SCr outliers was higher in the laparoscopic surgery group and the breast cancer surgery group, reaching 61.90 and 75.00%, respectively, but there was no statistical significance compared with the abdominal surgery group (Table 7).
4 Discussion
Endogenous hormone secretion, the inflammatory response, and subsequent hyperglycemia are changed due to anesthesia and surgery (such as increases in catecholamines, cortisol, growth hormone, and glucagon) (11). This prospective cohort study shows that there was no significant difference but rather a close correlation between the effects of different surgical methods on islet and renal function in patients who underwent non-cardiac surgery. This conclusion may be related to the limitations of this study. The sample size was small, and the observation time point was limited to 60 min. During the operation, FPG, FINS, and C-peptide gradually decreased after anesthetic induction but gradually increased 30 and 60 min after surgery. At present, there are very few studies on whether changes in islet function affect renal function. Our study found that after anesthetic induction, SCr, Cys C, and β2-MG initially decreased and then gradually increased after surgery. The effects of FINS, C-peptide, and HOMA-IR on Cys C and β2-MG were positive, and HOMA-β had a positive effect on Cys C. The changes in C-peptide had a negative effect on SCr, and there was a significant correlation between islet and renal function.
The islets are made up of five different types of cells: alpha (produces glucagon), beta (produces insulin), delta (produces somatostatin), PP (produces the pancreatic polypeptide), and epsilon (produces ghrelin) (12). FBG, FINS, and C-peptide are indexes reflecting the glucose metabolism function of islets. Reportedly, 20–40% of patients undergoing general surgery (13, 14) and approximately 80% of patients after cardiac surgery will have perioperative hyperglycemia. However, 12–30% of patients with intraoperative and/or postoperative hyperglycemia had no history of diabetes prior to surgery (13), which was described as “stressful high blood sugar” (11). Stress hyperglycemia usually subsides as acute disease symptoms or surgical stress are reduced. Perioperative hyperglycemia will increase the risk of postoperative infection (15). Studies have shown that patients with perioperative blood glucose exceeding 7.97 mmol L−1 have a higher risk of death (16). It is generally believed that only insulin resistance, without β-cell dysfunction, will lead to disturbances in glucose metabolism or diabetes. Impaired β-cell function is a necessary condition for the development of diabetes mellitus. Therefore, any increase in blood sugar means that β cell function is impaired. Surgical stress metabolic disorders are related to the degree of surgical trauma; the more serious the trauma, the more obvious the reactive hyperglycemia (17–19). Controlling blood glucose levels within the normal range during surgery has been shown to improve prognosis (20, 21). Our results in this study show that FPG declines after anesthetic induction, gradually increases after the start of surgery, and exceeds the preoperative level within 1 h. Although FPG was not correlated with MAP, it was negatively correlated with changes in HCT and Hb, which was consistent with the results of previous studies (22, 23). The incidence of FPG outliers in abdominal surgery was higher than that in body surface surgery, which further verified the effect of surgical stress on blood sugar.
Under normal conditions, blood glucose is an important factor that stimulates insulin secretion. However, when islet β cell dysfunction occurs, the curves are separate (3, 24). Consistent with our findings, blood glucose and insulin had the same trend of intraoperative changes, which gradually increased with increasing trauma after the start of the operation; intraoperative glucose levels and glucose–insulin-based metrics do not correlate well with insulin resistance (25). Previous studies have shown that insulin resistance may be an important mechanism in the development of postoperative complications (26). Therefore, we applied the homeostasis model (Homa Model) method in this experiment to further evaluate islet function.
The C-peptide is a negatively charged polypeptide composed of 31 amino acid residues and does not have a tertiary structure under physiological conditions (27). The C-peptide test is considered a powerful method to evaluate the function of pancreatic islets. The C-peptide levels decreased after the induction of anesthesia in patients in the abdominal and laparoscopic surgery groups in this study and gradually increased after the start of surgery. Consistent with the trend of insulin change, patients in the breast cancer group had a persistent elevation in C-peptide. The change in C-peptide was positively correlated with the MAP, and the incidence of abnormal values in the abdominal and laparoscopic surgery groups was significantly higher than that in the breast cancer group, both of which were lower than the normal value. Additionally, islet β cell secretion decreased. It has been further proven that abdominal surgery is more traumatic and that the stress reaction is higher than that of breast cancer surgery.
Hyperglycemia and insulin resistance have been recognized as causes of complications in cardiac surgery (28), surgical ICU (19, 20), and major abdominal surgery (29). The simpler HOMA method (including HOMA-IR, HOMA-β, and HOMA-IS) has been used as an alternative to the hyperinsulinemic normoglycemic clamp in studies of surgery-induced insulin resistance (30). Insulin resistance transiently occurs as a fundamental reaction to injury, including trauma and surgery, but also in response to other types of physical stress, such as fasting (30), pain (31), and immobilization (32). Previous studies reported a 7-fold preoperative change in m-values (a measure of insulin sensitivity) in non-diabetic surgical patients (32). Insulin resistance is closely related to complications in elective surgery (25, 28). This is consistent with our results. HOMA-IR gradually increased with the extension of the operation time.
The HOMA-β index in normal individuals was 100%, which was used to evaluate the individual indicators of islet β cell function (33–35). The better the β cell function, the better the insulin sensitivity, and the lower the blood glucose level. This is consistent with our study; that is, blood glucose levels were negatively correlated with β cell insulin secretion function and insulin sensitivity.
The occurrence of AKI is not uncommon in the perioperative period and remains an underestimated but clinically important complication (36). Even small elevations in creatinine have been associated with increased perioperative mortality and longer lengths of stay (37–39, 40, 41). Therefore, predicting the risk factors and preventing diseases for postoperative AKI are important issues for perioperative patient management (38). In cardiac surgery, a mere 20 mg/dL increase in the intraoperative blood glucose concentration is associated with an approximately 30% increase in pulmonary and renal complications and death (42). The results of our study showed that islet and renal function were highly correlated. FINS and C-peptide had negative effects on SCr and had positive effects on Cys C and β2-MG. HOMA-IR was positively correlated with renal function, and HOMA-β was positively correlated with SCr but did not show a correlation with FPG or a correlation with FPG and HOMA-IS. Our study found that compared to that of the patients in the laparoscopic surgery group, the SCr of the patients in the abdominal surgery group continued to increase and was comparable to the preoperative level 60 min after the start of surgery, whereas the SCr of the patients in the breast cancer group continued to decrease and was lower than that before surgery, indicating that abdominal surgeries have a higher impact on renal function than body surface surgeries. Similar to previous findings, cardiac surgery with CPB had the highest risk of AKI, followed by general surgery, thoracic surgery, orthopedics, vascular surgery, and urologic surgery (43). In addition, the changes in HCT and Hb had a positive effect on SCr, indicating that the occurrence of AKI was closely related to intraoperative hemorrhage (44).
The current clinical diagnostic indicators that reflect early functional damage to the kidney are SCr, blood urea nitrogen (BUN), and creatinine clearance (Ccr). Cys C is a cysteine protease inhibitor produced by all nucleated cells in the body, and it is released at a constant rate (45), is almost completely reabsorbed, and is degraded by the proximal convoluted tubule after passage through the glomerular filtration membrane (46). Therefore, the Cys C concentration in the serum is a very sensitive endogenous marker reflecting glomerular filtration function (47). Similar to Cys C, β2-MG is also a small molecule protein that can freely pass through the glomerular filtration membrane, and approximately 99.9% is reabsorbed and broken down in the proximal tubule. Therefore, β2-MG is elevated when glomerular filtration function declines. β2-MG can be used as an ideal indicator to evaluate the function of glomerular filtration (48).
Because of its constant rate of generation, the plasma Cys C concentration may be a better marker of the GFR than serum creatinine, and it has recently been proposed to replace serum creatinine in the routine assessment of the GFR rather than as a biomarker of AKI (49, 50). The results of our study showed that Cys C in patients in the abdominal surgery group was higher than the preoperative level, whereas Cys C in patients in the laparoscopic surgery group was lower than the preoperative level; however, the levels in the breast cancer group were within the normal range, indicating that there was no early renal function impairment. Cys C had no correlation with the MAP, HCT, or Hb and was not affected by hemodynamic fluctuations or an increased bleeding volume. The effect of the alterations in FINS, C-peptide, HOMA-IR, and HOMA-β on Cys C was positive.
Serum β2-MG is an indicator that reflects glomerular filtration function, and elevations in β2-MG levels in the blood are more sensitive than serum creatinine. Multiple studies have found that the β2-MG level is an early predictor of AKI in critically ill children (51), renal transplant patients (52), and liver transplant patients (53, 54). An additional study found that the concentration of serum β2-MG increased with every 100 μg/L, thereby increasing the prevalence of AKI by 6.4% (55). The results of this study showed that β2-MG decreased after the induction of anesthesia and gradually increased 30 min after surgery. Sixty minutes after surgery, β2-MG continued to increase in patients in the laparoscopic surgery group and exceeded the preoperative level, while in patients in the abdominal surgery group and the breast cancer group, β2-MG continued to decrease and was lower than that before surgery. The incidence of abnormal values of β2-MG at each time point was low, and it was not affected by surgical stress or increased blood loss. However, the changes in FINS, C-peptide, and HOMA-IR have a positive effect on β2-MG.
Compared with cardiac surgery, there are fewer studies on the occurrence of acute kidney injury in non-cardiac and non-vascular surgery, possibly because of its lower overall incidence. At present, related studies on islet and renal function have mainly focused on diabetic nephropathy (56) and the preoperative (57) and postoperative effects of hyperglycemia on renal function (2, 58, 59). This is the first study to explore the influence of different surgical methods on intraoperative islet and renal function. In this study, we were surprised to find that both the gold standard SCr of renal dysfunction and the latest biomarkers, Cys C and β2-MG, suggest a close correlation between islet and renal function. The influence of FINS and C-peptide on SCr is negative, and the influence of HOMA-IR on SCr is positive. This shows that SCr is not sensitive enough to detect early kidney injury, and only after the islet function is impaired can it show a positive effect. Changes in FINS, C-peptide, and HOMA-IR have positive effects on Cys C and β2-MG, indicating that Cys C and β2-MG can be used as ideal indicators for early kidney injury (59–62). It also proves that the design of this study is reasonable and has certain clinical significance.
We acknowledge several limitations of our study. The advantages of this study are the prospective design, detailed patient characteristics and short-term outcome data, low dropout rate, and accurate data. This is the first study to observe the changes in pancreatic islets and renal function during surgery at the same time, and there are few studies on the correlation between the two major organs. The limitations include the relatively small sample size. Due to the limitation of the length of the operation, we had fewer observation time points and did not thoroughly evaluate the incidence of postoperative acute kidney injury and the follow-up postoperative mortality. This study is a preliminary study of the effects of different surgical methods on islet and renal function. The connection between the pancreas and the kidneys has been proven in patients with diabetes, with RAS being involved in expression. Studies on the related mechanisms still need to be carried out to explore whether the two organs are related through the RAS.
5 Conclusion
Surgical stress induces intraoperative changes in pancreatic islet function in patients undergoing non-cardiac surgery. FINS, C-peptide, and HOMA-IR were positively correlated with Cys C and β2-MG. However, FINS and C-peptide were negatively correlated with SCr, HOMA-IR was positively correlated with SCr, and pancreatic islet function was significantly correlated with early renal injury. Whether this correlation would lead to later organ damage needs to be confirmed by further studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Qianfoshan Hospital, Shandong Province. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS and YW provided substantial contributions to the conception or design of the manuscript. XZ, MZ, YG, TS, ML, XG, YL, ZG, LC, and XD contributed to the acquisition, analysis, and interpretation of the data. YS, YW, XZ, MZ, YG, TS, ML, XG, YL, ZG, LC, and XD participated in drafting the manuscript, and YS revised it critically. All the authors have read and approved the final version of the manuscript.
Funding
This research was funded by a grant from the Academic Promotion Programme of Shandong First Medical University (2019QL015), Shandong Provincial Natural Science Foundation (ZR2022MH221), and Shandong Provincial Medical Association Analgesia and Anesthesia Optimization Research Project (YXH2021ZX039).
Acknowledgments
We sincerely appreciate Yidu Cloud (Beijing) Technology Co. Ltd., China, for providing technical support in data processing and statistical analysis.
Conflict of interest
XD was employed by Yidu Cloud (Beijing) Technology Co. Ltd., Beijing, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reudink, M, Huisman, DE, van Rooijen, SJ, Lieverse, AG, Kroon, HM, Roumen, RMH, et al. Association between intraoperative blood glucose and anastomotic leakage in colorectal surgery. J Gastrointest Surg. (2021) 25:2619–27. doi: 10.1007/s11605-021-04933-2
2. Farahani, F, Mirzaei, F, Khodadadi, I, and Abbasi-Oshaghi, E. Importance of hyperglycemia in preoperative, intraoperative and postoperative periods in COVID-19 patients. Int J Surg. (2020) 83:1–2. doi: 10.1016/j.ijsu.2020.08.048
3. Dungan, KM, Braithwaite, SS, and Preiser, JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/s0140-6736(09)60553-5
4. Roca-Ho, H, Palau, V, Gimeno, J, Pascual, J, Soler, MJ, and Riera, M. Angiotensin-converting enzyme 2 influences pancreatic and renal function in diabetic mice. Lab Investig. (2020) 100:1169–83. doi: 10.1038/s41374-020-0440-5
5. Cheng, Q, and Leung, PS. An update on the islet renin-angiotensin system. Peptides. (2011) 32:1087–95. doi: 10.1016/j.peptides.2011.03.003
6. Regoli, M, Bendayan, M, Fonzi, L, Sernia, C, and Bertelli, E. Angiotensinogen localization and secretion in the rat pancreas. J Endocrinol. (2003) 179:81–9. doi: 10.1677/joe.0.1790081
7. Eckardt, KU, Coresh, J, Devuyst, O, Johnson, RJ, Köttgen, A, Levey, AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. (2013) 382:158–69. doi: 10.1016/s0140-6736(13)60439-0
8. Saadat-Gilani, K, Zarbock, A, and Meersch, M. Perioperative Renoprotection: clinical implications. Anesth Analg. (2020) 131:1667–78. doi: 10.1213/ane.0000000000004995
9. Rosas-Martínez, L, Rodríguez-Muñoz, R, Namorado-Tonix, MDC, Missirlis, F, Del Valle-Mondragón, L, Sánchez-Mendoza, A, et al. Hyperglycemic levels in early stage of diabetic nephropathy affect differentially renal expression of claudins-2 and-5 by oxidative stress. Life Sci. (2021) 268:119003. doi: 10.1016/j.lfs.2020.119003
10. Long, TE, Helgason, D, Helgadottir, S, Palsson, R, Gudbjartsson, T, Sigurdsson, GH, et al. Acute kidney injury after abdominal surgery: incidence, risk factors, and outcome. Anesth Analg. (2016) 122:1912–20. doi: 10.1213/ane.0000000000001323
11. Duggan, EW, Carlson, K, and Umpierrez, GE. Perioperative hyperglycemia management: an update. Anesthesiology. (2017) 126:547–60. doi: 10.1097/aln.0000000000001515
12. Shi, J, Dong, B, Mao, Y, Guan, W, Cao, J, Zhu, R, et al. Review: traumatic brain injury and hyperglycemia, a potentially modifiable risk factor. Oncotarget. (2016) 7:71052–61. doi: 10.18632/oncotarget.11958
13. Frisch, A, Chandra, P, Smiley, D, Peng, L, Rizzo, M, Gatcliffe, C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care. (2010) 33:1783–8. doi: 10.2337/dc10-0304
14. Kwon, S, Thompson, R, Dellinger, P, Yanez, D, Farrohki, E, and Flum, D. Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Ann Surg. (2013) 257:8–14. doi: 10.1097/SLA.0b013e31827b6bbc
15. Kheir, MM, Tan, TL, Kheir, M, Maltenfort, MG, and Chen, AF. Postoperative blood glucose levels predict infection after Total joint arthroplasty. J Bone Joint Surg Am. (2018) 100:1423–31. doi: 10.2106/jbjs.17.01316
16. Berbudi, A, Rahmadika, N, Tjahjadi, AI, and Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. (2020) 16:442–9. doi: 10.2174/1573399815666191024085838
17. Norhammar, A, Tenerz, A, Nilsson, G, Hamsten, A, Efendíc, S, Rydén, L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. (2002) 359:2140–4. doi: 10.1016/s0140-6736(02)09089-x
18. Tenerz, A, Nilsson, G, Forberg, R, Ohrvik, J, Malmberg, K, Berne, C, et al. Basal glucometabolic status has an impact on long-term prognosis following an acute myocardial infarction in non-diabetic patients. J Intern Med. (2003) 254:494–503. doi: 10.1046/j.1365-2796.2003.01221.x
19. van den Berghe, G, Wouters, P, Weekers, F, Verwaest, C, Bruyninckx, F, Schetz, M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. (2001) 345:1359–67. doi: 10.1056/NEJMoa011300
20. Finfer, S, Chittock, DR, Su, SY, Blair, D, Foster, D, Dhingra, V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. doi: 10.1056/NEJMoa0810625
21. Marik, PE, and Bellomo, R. Stress hyperglycemia: an essential survival response! Crit Care Med. (2013) 41:e93–4. doi: 10.1097/CCM.0b013e318283d124
22. Li, PF, Chen, JS, Chang, JB, Chang, HW, Wu, CZ, Chuang, TJ, et al. Association of complete blood cell counts with metabolic syndrome in an elderly population. BMC Geriatr. (2016) 16:10. doi: 10.1186/s12877-016-0182-9
23. Jyothsna, P, Suchitra, MM, Kusuma Kumari, M, Chandrasekhar, C, Rukmangadha, N, Alok, S, et al. Effect of Iron deficiency Anemia on glycated albumin levels: a comparative study in nondiabetic subjects with Iron deficiency Anemia. J Lab Physicians. (2023) 15:253–8. doi: 10.1055/s-0042-1757589
24. Baban, B, Thorell, A, Nygren, J, Bratt, A, and Ljungqvist, O. Determination of insulin resistance in surgery: the choice of method is crucial. Clin Nutr. (2015) 34:123–8. doi: 10.1016/j.clnu.2014.02.002
25. Ljungqvist, O, Nygren, J, and Thorell, A. Insulin resistance and elective surgery. Surgery. (2000) 128:757–60. doi: 10.1067/msy.2000.107166
26. Wahren, J, and Larsson, C. C-peptide: new findings and therapeutic possibilities. Diabetes Res Clin Pract. (2015) 107:309–19. doi: 10.1016/j.diabres.2015.01.016
27. Landreh, M, Johansson, J, Wahren, J, and Jörnvall, H. The structure, molecular interactions and bioactivities of proinsulin C-peptide correlate with a tripartite molecule. Biomol Concepts. (2014) 5:109–18. doi: 10.1515/bmc-2014-0005
28. Sato, H, Carvalho, G, Sato, T, Lattermann, R, Matsukawa, T, and Schricker, T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. (2010) 95:4338–44. doi: 10.1210/jc.2010-0135
29. Jackson, RS, Amdur, RL, White, JC, and Macsata, RA. Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg. (2012) 214:68–80. doi: 10.1016/j.jamcollsurg.2011.09.016
30. Bonora, E, Targher, G, Alberiche, M, Bonadonna, RC, Saggiani, F, Zenere, MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. (2000) 23:57–63. doi: 10.2337/diacare.23.1.57
31. Greisen, J, Juhl, CB, Grøfte, T, Vilstrup, H, Jensen, TS, and Schmitz, O. Acute pain induces insulin resistance in humans. Anesthesiology. (2001) 95:578–84. doi: 10.1097/00000542-200109000-00007
32. Stuart, CA, Shangraw, RE, Prince, MJ, Peters, EJ, and Wolfe, RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. (1988) 37:802–6. doi: 10.1016/0026-0495(88)90018-2
33. Li, GW, and Pan, XR. A new insulin-sensitivity index for the population-based study. Zhonghua Nei Ke Za Zhi. (1993) 32:656–60.
34. Matsuda, M, and DeFronzo, RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. (1999) 22:1462–70. doi: 10.2337/diacare.22.9.1462
35. Richards, JE, Hutchinson, J, Mukherjee, K, Jahangir, AA, Mir, HR, Evans, JM, et al. Stress hyperglycemia and surgical site infection in stable nondiabetic adults with orthopedic injuries. J Trauma Acute Care Surg. (2014) 76:1070–5. doi: 10.1097/ta.0000000000000177
36. Li, PK, Burdmann, EA, and Mehta, RL. Acute kidney injury: global health alert. Kidney Int. (2013) 83:372–6. doi: 10.1038/ki.2012.427
37. Gumbert, SD, Kork, F, Jackson, ML, Vanga, N, Ghebremichael, SJ, Wang, CY, et al. Perioperative acute kidney injury. Anesthesiology. (2020) 132:180–204. doi: 10.1097/aln.0000000000002968
38. Goren, O, and Matot, I. Perioperative acute kidney injury. Br J Anaesth. (2015) 115:ii3-14. doi: 10.1093/bja/aev380
39. Thakar, CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. (2013) 20:67–75. doi: 10.1053/j.ackd.2012.10.003
40. Palant, CE, Amdur, RL, and Chawla, LS. Long-term consequences of acute kidney injury in the perioperative setting. Curr Opin Anaesthesiol. (2017) 30:100–4. doi: 10.1097/aco.0000000000000428
41. Hobson, C, Ozrazgat-Baslanti, T, Kuxhausen, A, Thottakkara, P, Efron, PA, Moore, FA, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. (2015) 261:1207–14. doi: 10.1097/sla.0000000000000732
42. Gandhi, GY, Nuttall, GA, Abel, MD, Mullany, CJ, Schaff, HV, Williams, BA, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. (2005) 80:862–6. doi: 10.4065/80.7.862
43. Grams, ME, Sang, Y, Coresh, J, Ballew, S, Matsushita, K, Molnar, MZ, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. (2016) 67:872–80. doi: 10.1053/j.ajkd.2015.07.022
44. Meersch, M, Schmidt, C, and Zarbock, A. Perioperative acute kidney injury: an under-recognized problem. Anesth Analg. (2017) 125:1223–32. doi: 10.1213/ane.0000000000002369
45. Abrahamson, M, Olafsson, I, Palsdottir, A, Ulvsbäck, M, Lundwall, A, Jensson, O, et al. Structure and expression of the human cystatin C gene. Biochem J. (1990) 268:287–94. doi: 10.1042/bj2680287
46. Chew, JS, Saleem, M, Florkowski, CM, and George, PM. Cystatin C--a paradigm of evidence based laboratory medicine. Clin Biochem Rev. (2008) 29:47–62.
47. Gökkuşu, CA, Ozden, TA, Gül, H, and Yildiz, A. Relationship between plasma cystatin C and creatinine in chronic renal diseases and Tx-transplant patients. Clin Biochem. (2004) 37:94–7. doi: 10.1016/j.clinbiochem.2003.10.009
48. Jovanović, D, Krstivojević, P, Obradović, I, Durdević, V, and Dukanović, L. Serum cystatin C and beta 2-microglobulin as markers of glomerular filtration rate. Ren Fail. (2003) 25:123–33. doi: 10.1081/jdi-120017475
49. Lagos-Arevalo, P, Palijan, A, Vertullo, L, Devarajan, P, Bennett, MR, Sabbisetti, V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol. (2015) 30:665–76. doi: 10.1007/s00467-014-2987-0
50. Wasung, ME, Chawla, LS, and Madero, M. Biomarkers of renal function, which and when? Clin Chim Acta. (2015) 438:350–7. doi: 10.1016/j.cca.2014.08.039
51. Herrero-Morín, JD, Málaga, S, Fernández, N, Rey, C, Diéguez, MA, Solís, G, et al. Cystatin C and beta 2-microglobulin: markers of glomerular filtration in critically ill children. Crit Care. (2007) 11:R59. doi: 10.1186/cc5923
52. Schaub, S, Wilkins, JA, Antonovici, M, Krokhin, O, Weiler, T, Rush, D, et al. Proteomic-based identification of cleaved urinary beta 2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am J Transplant. (2005) 5:729–38. doi: 10.1111/j.1600-6143.2005.00766.x
53. Hei, ZQ, Li, XY, Shen, N, Pang, HY, Zhou, SL, and Guan, JQ. Prognostic values of serum cystatin C and beta 2 microglobulin, urinary beta 2 microglobulin and N-acetyl-beta-D-glucosaminidase in early acute renal failure after liver transplantation. Chin Med J. (2008) 121:1251–6. doi: 10.1097/00029330-200807020-00001
54. Foster, MC, Coresh, J, Hsu, CY, Xie, D, Levey, AS, Nelson, RG, et al. Serum β-trace protein and β2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. (2016) 68:68–76. doi: 10.1053/j.ajkd.2016.01.015
55. Wang, R, Hu, H, Hu, S, He, H, and Shui, H. β2-microglobulin is an independent indicator of acute kidney injury and outcomes in patients with intracerebral hemorrhage. Medicine (Baltimore). (2020) 99:e19212. doi: 10.1097/md.0000000000019212
56. Reidy, K, Kang, HM, Hostetter, T, and Susztak, K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. (2014) 124:2333–40. doi: 10.1172/jci72271
57. Thörling, J, Ljungqvist, O, Sköldenberg, O, and Hammarqvist, F. No association between preoperative impaired glucose control and postoperative adverse events following hip fracture surgery - a single-Centre observational cohort study. Clin Nutr. (2021) 40:1348–54. doi: 10.1016/j.clnu.2020.08.023
58. Schwaiger, E, Krenn, S, Kurnikowski, A, Bergfeld, L, Pérez-Sáez, MJ, Frey, A, et al. Early postoperative basal insulin therapy versus standard of Care for the Prevention of diabetes mellitus after kidney transplantation: a multicenter randomized trial. J Am Soc Nephrol. (2021) 32:2083–98. doi: 10.1681/asn.2021010127
59. Wu, LM, Si, HB, Li, MY, Wu, YG, Zeng, Y, and Shen, B. Insulin dependence increases the risk of complications and death in Total joint arthroplasty: a systematic review and Meta-(regression) analysis. Orthop Surg. (2021) 13:719–33. doi: 10.1111/os.12944
Glossary
Keywords: non-cardiac surgery, intraoperative, different operation methods, islet function, renal function
Citation: Sun Y, Zhang X, Zhang M, Guo Y, Sun T, Liu M, Gao X, Liu Y, Gao Z, Chen L, Du X and Wang Y (2024) Preliminary investigation of the effect of non-cardiac surgery on intraoperative islet and renal function: a single-center prospective cohort study. Front. Med. 11:1235335. doi: 10.3389/fmed.2024.1235335
Edited by:
You Shang, Huazhong University of Science and Technology, ChinaCopyright © 2024 Sun, Zhang, Zhang, Guo, Sun, Liu, Gao, Liu, Gao, Chen, Du and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongtao Sun, eXRzdW5Ac2RmbXUuZWR1LmNu; Yuelan Wang, d3lsZGdmQDE2My5jb20=
 Yongtao Sun
Yongtao Sun Xiaoning Zhang1
Xiaoning Zhang1 Yongle Guo
Yongle Guo Zhongquan Gao
Zhongquan Gao Yuelan Wang
Yuelan Wang