- 1College of Traditional Chinese Medicine, Xinjiang Medical University, Urumqi, Xinjiang Uygur Autonomous Region, China
- 2Department of Pharmacy, Ankang Hospital of Traditional Chinese Medicine, Ankang, China
Objective: To systematically evaluate the clinical efficacy and safety of Shenkang injection (SKI) combined with alprostadil in the treatment of chronic renal failure (CRF).
Method: Randomized controlled trials (RCTs) of Shenkang injection combined with alprostadil in CRF treatment were investigated by retrieving a total of 7 databases including CNKI, Wanfang database, VIP, CBM, PubMed, Embase and Cochrane Library, with the search time ranging from 2012 to now. Revman 5.2 software was used for data analysis, and Cochrane bias risk tool was used to evaluate the quality of the included literature. The final results were represented by relative risk (RR), mean difference (MD) and 95% confidence interval (95% CI).
Results: A total of 20 RCTs and 1,573 patients were included in this study. Meta-analysis showed that the overall response rate (ORR) of the treatment group was superior to the control group [RR = 0.20, 95% CI (0.16, 0.25), P < 0.00001]. Compared with the control group, the treatment group achieved favorable improvement in terms of the creatinine clearance rate (Ccr) [MD = 9.48, 95% CI (8.73, 10.24), P < 0.00001], serum creatinine (Scr) [MD = −55.12, 95% CI (−63.42, −46.82), P < 0.00001], quantitative urine protein (Upro) [MD = −0.48, 95% CI (−0.53, −0.43), P < 0.00001], and blood urea nitrogen (BUN) [MD=-3.73, 95% CI (−4.08, −3.3) 7, P < 0.00001]. There was no statistical difference in the incidence of adverse reactions in each group.
Conclusion: Currently, Shenkang injection combined with alprostadil has been widely used in clinical treatment of CRF due to the certain effect superior to other methods. However, its specific efficacy and safety need to be further verified through numerous large-scale clinical trials.
1. Introduction
The clinical-common chronic renal failure (CRF) can be developed from various diseases, such as primary glomerular disease, hypertensive nephropathy, diabetes nephropathy, drug-induced nephropathy, etc. (1–3). The annually growing prevalence of CRF mainly derives from the change of people's lifestyle, increasing number of diabetes and hypertension patients, as well as aging patients (4, 5). CRF is mainly manifested as disturbance of water and electricity medium and acid-base balance, abnormal endocrine function, retention of metabolites, etc. (6). The inevitable and reversible pathogenesis of CRF will eventually progress into uremia in the later stage, with dismal prognosis, which requires kidney transplantation or renal replacement therapy for life support, resulting in high expenditure and multiple complications. Currently, there is no specific drug to treat CRF (7).
Alprostadil is a kind of prostate drug aiming to expand renal vessels, significantly alleviating glomerular microcirculation, accelerating renal blood flow and increasing urine excretion, thus further promoting toxin excretion (8). The therapeutic efficacy of alprostadil is unsatisfactory despite its common application in CHF treatment. Recent years have witnessed certain outcomes in the application of integrated Chinese and Western medicine in clinical treatment of CRF (9–11). From the perspective of traditional Chinese medicine (TCM), CRF belongs to the categories of “edema,” “Stranguria syndrome,” and “kidney exhaustion.” CRF derives from its close association with the spleen and kidney, that is, the spleen and kidney deficiency, deficiency of both qi and yin, and the accumulation of dampness and turbidity. Shenkang injection (SKI), a kind of Chinese patent injection composed of rhubarb, salvia miltiorrhiza, astragalus and safflower, has been extensively used to treat CRF (12–14). On the one hand, pharmacological studies have found that SKI can decrease proteinuria by effectively alleviating anemia and renal microcirculation, thus inhibiting the pathological proliferation of kidneys, which is conducive to reducing the damage to the kidneys, promoting the repair of damaged tissues, eventually improving renal function (15). On the other hand, SKI aims to reduce the infiltration of inflammatory cells by alleviating glomerulosclerosis and interstitial fibrosis, thus reducing the level of serum creatinine and blood urea nitrogen (16).
The combination of SKI and alprostadil has been shown to be synergistic, with demonstrable therapeutic effect (17–19). Considering that a single study may not represent the overall effect, comprehensive collection and reference were conducted on data from CRF treatment to objectively evaluate the efficacy of Shenkang injection combined with alprostadil, so as to determine their efficacy and safety, and provide evidence for the evidence-based medicine system.
2. Materials and methods
2.1. Inclusion criteria and exclusion criteria
2.1.1. Research type
Clinical randomized controlled trials (RCTS) of Shenkang injection combined with alprostadil in CRF treatment were included in this study.
2.1.2. Research object
Patients with obvious clinical symptoms such as metabolic disorder, metabolite retention, chronic kidney disease or all diseases involving the kidney, the endogenous creatinine clearance rate (Ccr) < 80 mL/min, and the serum creatinine (Scr) >133 μmol/L according to the diagnostic criteria of the Guidelines for the Diagnosis and Treatment of Chronic Renal Failure (37). Patients diagnosed with deficiency of both qi and yin, presenting with at least 4 symptoms including nausea and vomiting, pale face, bloating, qi deficiency, anorexia, night sweats, bitter mouth, dry tongue, etc. according to the Guidelines for the Diagnosis and Treatment of Chronic Renal Failure by Integrated Traditional Chinese and Western Medicine (38).
2.1.3. Interventions
The control group was administrated with alprostadil, Shenkang injection or routine treatment, while the treatment group was administrated with Shenkang injection combined with alprostadil.
2.1.4. Outcome indicators
The outcome indicators were clinical efficacy, serum creatinine (Scr), blood urea nitrogen (BUN), urinary protein quantification (Upro), creatinine clearance rate (Ccr), aromatase (ArE) and adverse reaction rate.
2.1.5. Exclusion criteria
Non-RCT research, repeated published research, related literature review, research, research with incomplete clinical data and inconsistent outcome indicators, patients undergoing renal replacement therapy such as dialysis.
2.2. Search strategy
A total of 7 databases including CNKI, Wanfang database, VIP, CBM, PubMed, Embase and Cochrane Library were retrieved, with the search time ranging from 2012 to now. The retrieval followed the PICOS principle, that is, P: CRF patients; I: Shenkang injection+alprostadil; C: Alprostadil, Shenkang injection or routine treatment; O: Relevant CRF indexes; S: Randomized controlled trial. We adopted the search strategy of combining Chinese and English keywords. key words: Shenkang injection, alprostadil, chronic renal failure, chronic renal insufficiency, chronic kidney disease.
2.3. Data extraction and quality evaluation of document methodology
According to the previously set inclusion and exclusion criteria, the two researchers used Note Express document management software to independently screen the literature. Any difference was resolved by the third researcher through negotiation. After screening the qualified literature and extracting relevant data, the two researchers used the bias risk assessment tool recommended by Cochrane System Reviewer Manual to evaluate the bias risk of the included RCTs. The evaluation methods included random scheme, concealment of distribution scheme, blind method, integrity of result data, selective report and other biased sources The evaluation of each study was divided into low risk, unclear risk and high risk.
2.4. Evidence quality evaluation
The GRADE system was used to evaluate the quality of evidence from the included literature. The limitations, inconsistency, indirection, inaccuracy and publication bias of the outcome indicators were, respectively evaluated, thus accordingly dividing the quality of evidence (39). Thereinto, the quality of RCT evidence was initially preset as “high.” Of all 5 degradation factors, only one was degraded to “medium,” two to “low,” and three to “extremely low.” The difference between the true value and the estimated value increased from “high level” to “extremely low level.” Methodological quality evaluation and evidence quality grading were independently conducted by two researchers and cross-checked. Any problem was solved by both parties through negotiation.
2.5. Statistical methods
RevMan 5.2 software provided by Cochrane was used for meta-analysis. In outcome indicators, the clinical efficacy and adverse reactions were classified as binary variables. The risk ratio (RR) was used for analysis and statistics, and the combined effect amount of mean difference (MD) was used for continuous variables. Each effect amount was represented by 95% confidence interval (95% confidence interval, CI). P-value and I2 were used to evaluate the heterogeneity among the groups, P < 0.05 was considered to be statistically significant. The fixed effect model was used for analysis under the condition of small data heterogeneity between groups (P > 0.10 or I2 ≤ 50%), otherwise the combined effect amount was calculated using the random effect model under the condition of significant data heterogeneity (P ≤ 0.10 or I2 ≥ 50%). Significant heterogeneity was eliminated by subgroup analysis or sensitivity analysis.
3. Results of literature search
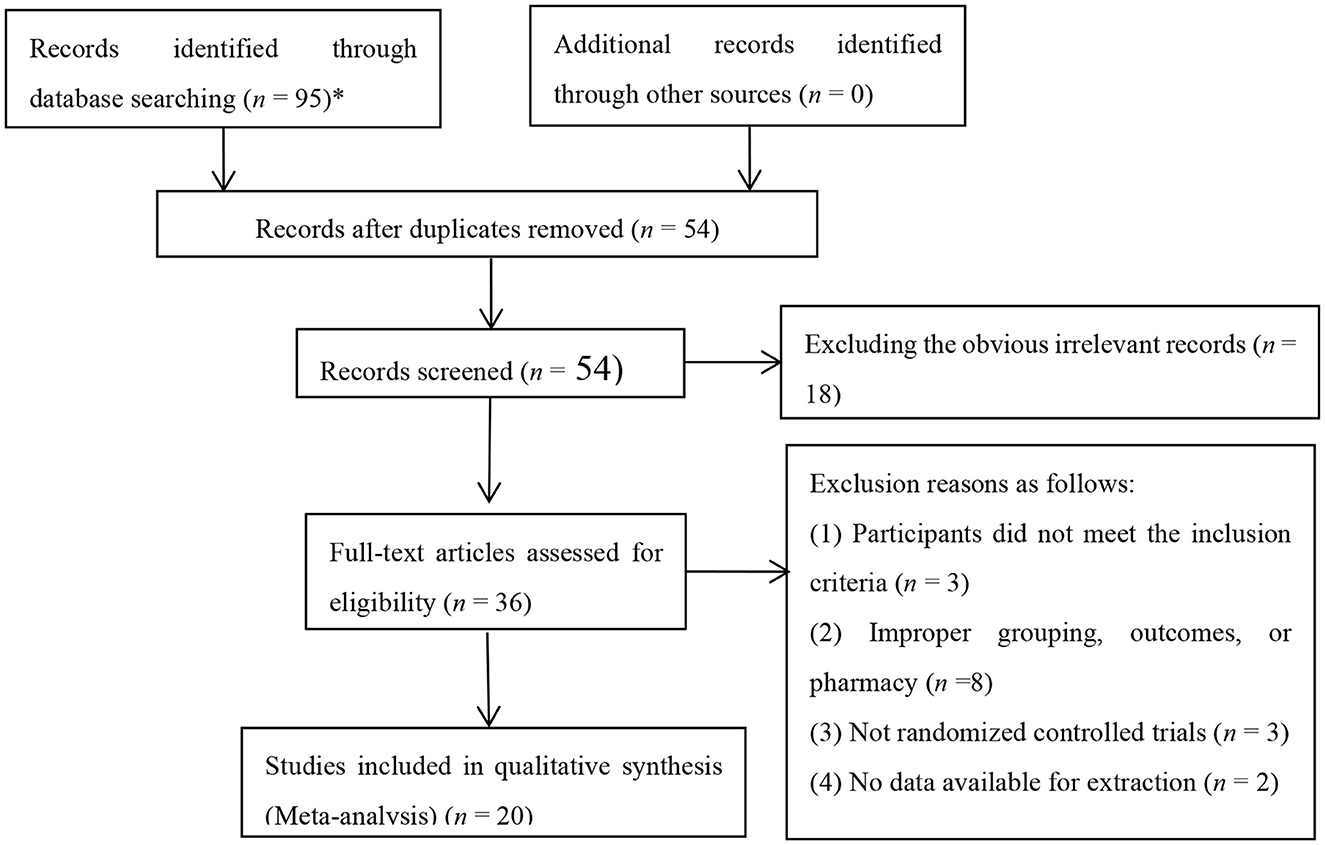
Ninety-five relevant documents were initially retrieved. After eliminating duplicate documents using Note Express, a total of 54 papers were obtained. Non-randomized controlled trials were eliminated after reading the title and abstract, and further the full text. Finally 20 RCTs were included after comparing the inclusion and exclusion criteria, with the published time from 2012 to 2020 (Figure 1).
3.1. Baseline of the included studies
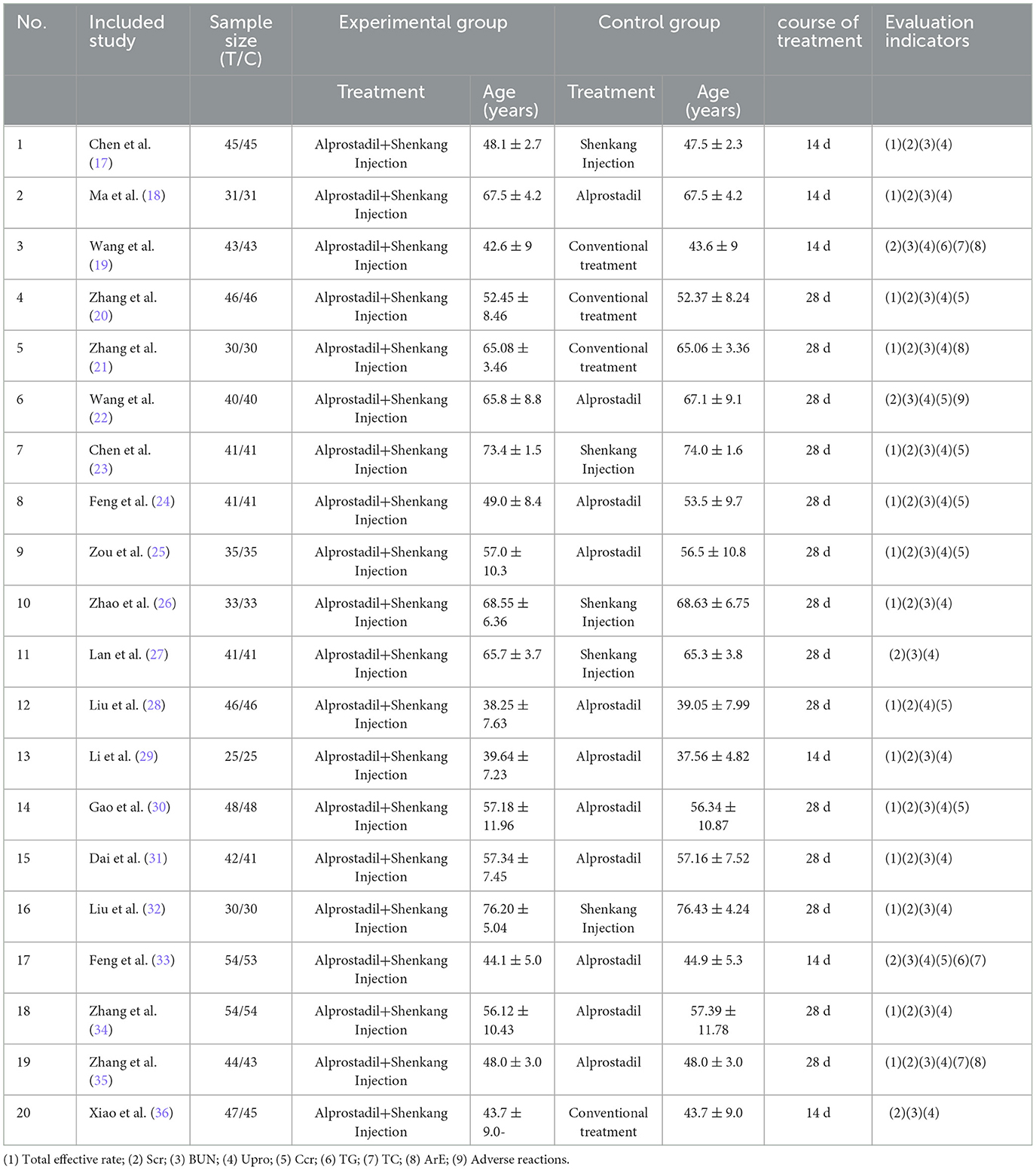
A total of 1,573 patients in 20 papers were included, including 789 cases in the treatment group and 784 cases in the control group, with the shortest course of 14 days and the longest course of 28 days. In terms of treatment measures in the control group, alprostadil accounted for 11 items, Shenkang injection accounted for 5 items, and routine treatment accounted for 4 items. Observation indicators included clinical efficacy, Scr, BUN, UPro, Ccr, ArE, and adverse reactions (Table 1).
3.2. Risk of bias assessment of the included studies
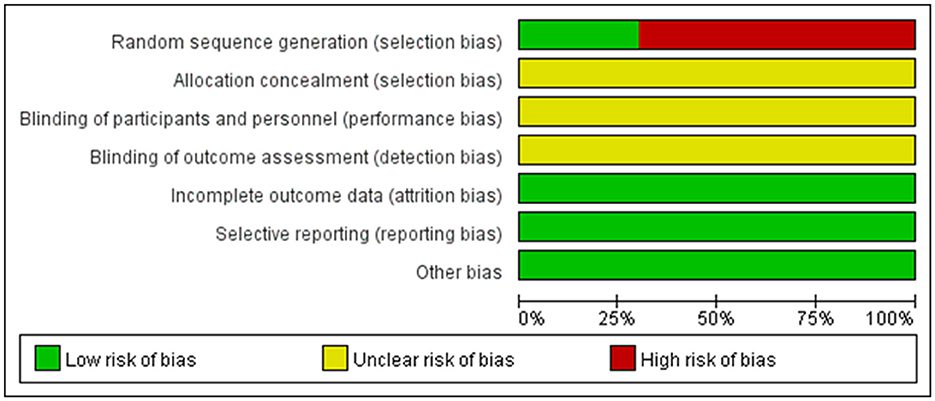
According to the risk of bias assessment method recommended by Cochrane, all studies were randomized trials. In the included 20 articles, 6 of them described specific randomization methods, and the remaining 14 did not specify random allocation methods. None of the research results lacked outcome data, and they were all not selectively reported. However, there was no detailed description of blind method and allocation concealment (Figure 2).
3.3. Results of meta-analysis
3.3.1. Clinical efficacy
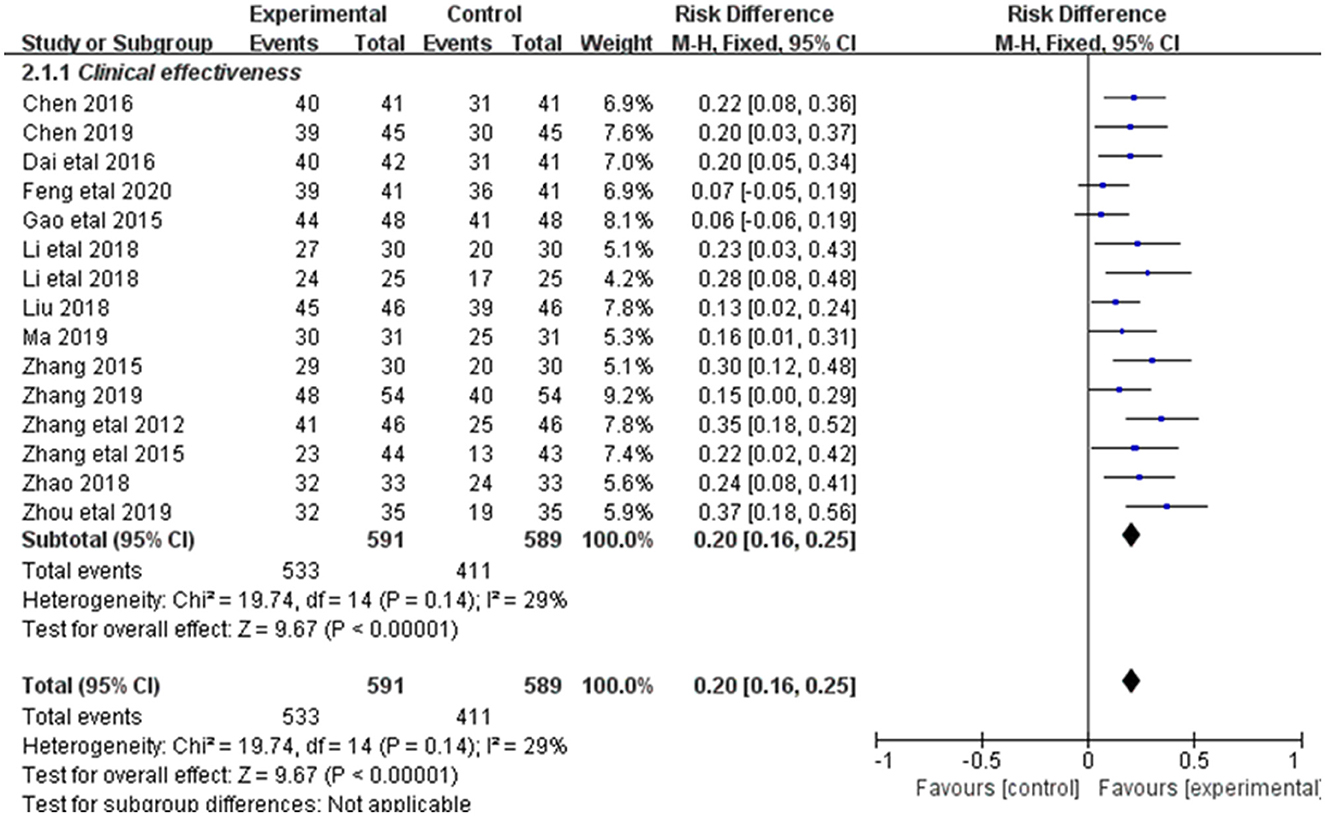
Changes in clinical efficacy were reported in 15 of the 20 included studies (Figure 3). Results showed no heterogeneity among the studies (DF=14, P = 0.14, I2 = 29%). Therefore, the fixed effect model was used for analysis. The overall response rate (ORR) of the combined treatment group was significantly higher than that of the control group [RR = 0.2, 95% CI = (0.16, 0.25)], with statistical significance (P < 0.00001).
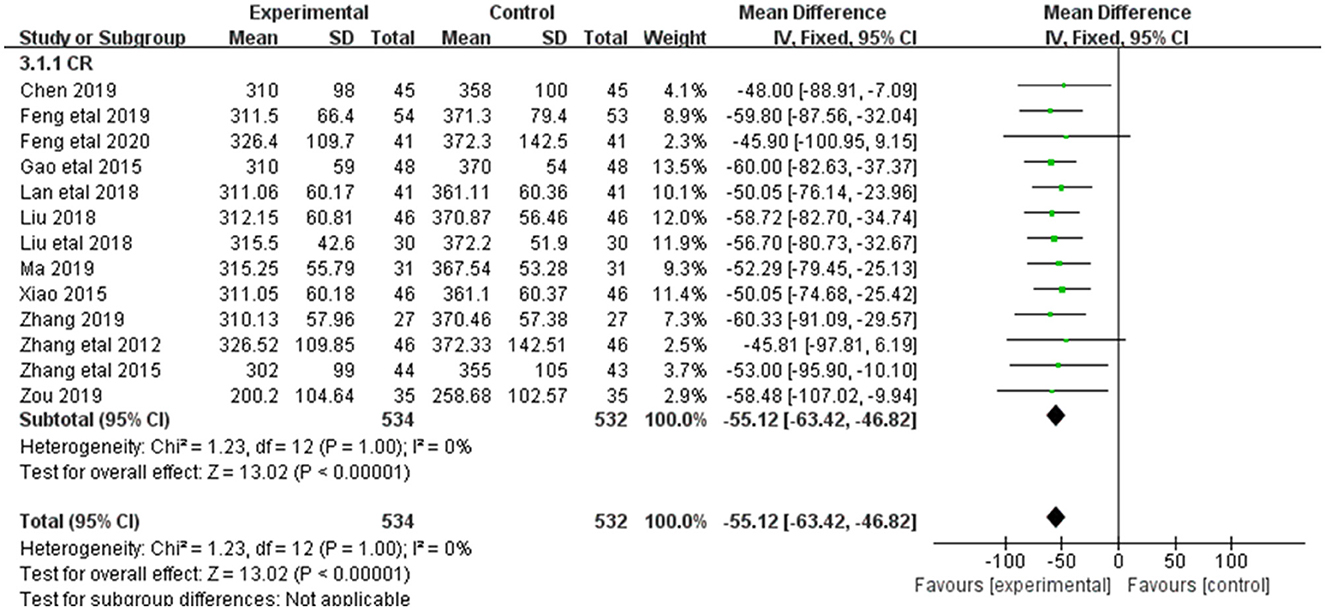
3.3.2. SCR
Alleviation in SCR was reported in 13 of the 20 included studies (Figure 4). The research results showed no heterogeneity among the studies (DF = 12, P = 1, I2 = 0%). Therefore, the fixed effect model was used for analysis. The effect of Shenkang injection combined with alprostadil in treating CRF was superior to the control group [MD = 9.48, 95% CI (8.73, 10.24)], with statistical significance (P < 0.00001).
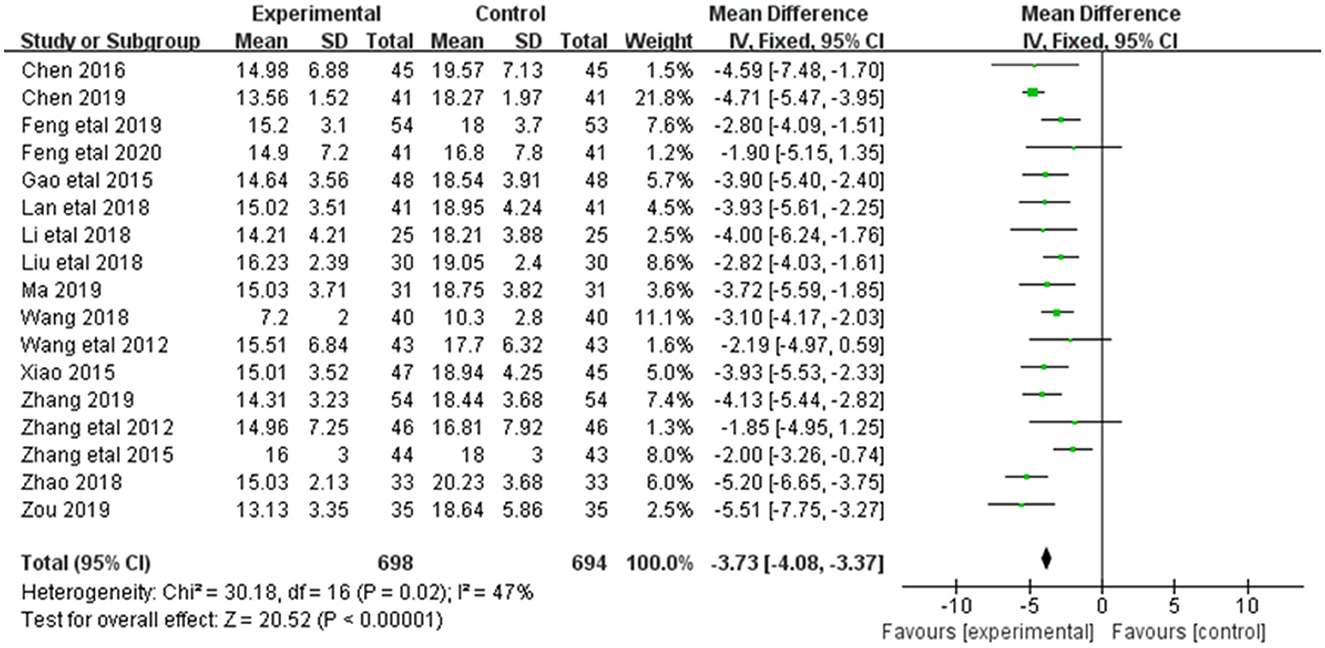
3.3.3. BUN
Alleviation in BUN was reported in 17 of the 20 included studies (Figure 5). The research results showed no heterogeneity among the studies (DF = 16, P = 0.02, I2 = 47%). Therefore, the fixed effect model was used for analysis. The alleviation of BUN in the combined treatment group was superior to the control group [MD = −3.73, 95%CI = (−4.08, −3.37)], with statistical significance (P < 0.00001).
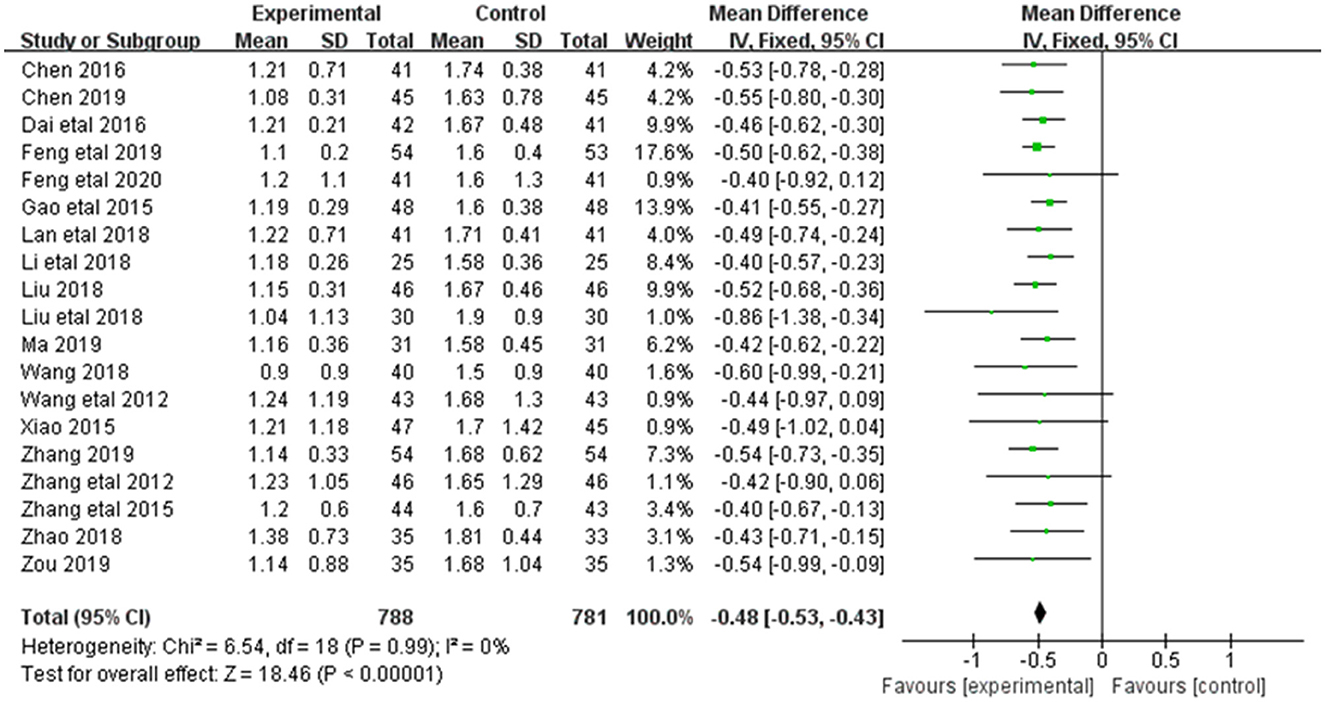
3.3.4. Upro
Alleviation in Upro was reported in 19 of the 20 included studies (Figure 6). The research results showed no heterogeneity among the studies (DF = 18, P = 0.99, I2 = 47%). Therefore, the fixed effect model was used for analysis. The alleviation of Upro in the combined treatment group was superior to the control group [MD = −0.48, 95%CI = (−0.53, −0.43)], with statistical significance (P < 0.00001).
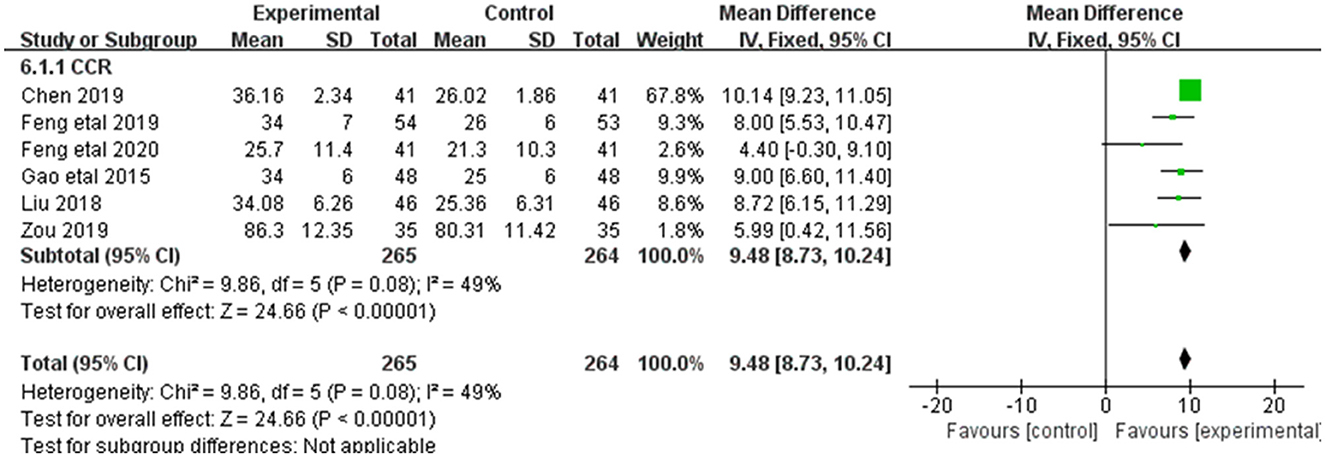
3.3.5. Ccr
Alleviation in Ccr was reported in 6 of the 20 included studies (Figure 7). The research results showed no heterogeneity among the studies (DF = 5, P = 0.08, I2 = 49%). Therefore, the fixed effect model was used for analysis. The alleviation of Ccr in the combined treatment group was superior to the control group [MD = 9.48, 95%CI = (8.37, 10.24)], with statistical significance (P < 0.00001).
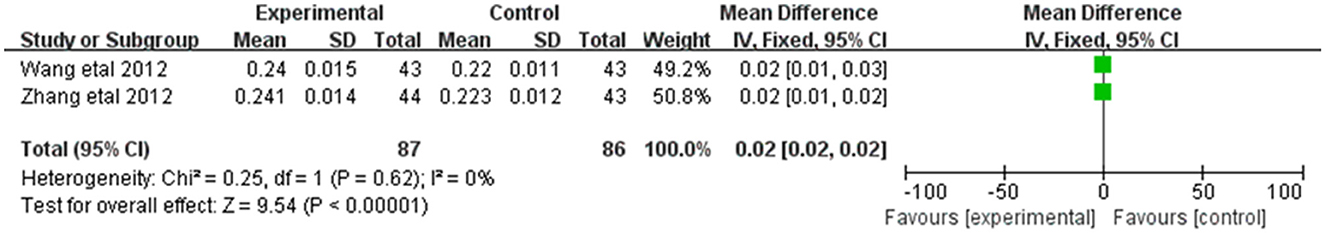
3.3.6. ArE
Alleviation in ArE was reported in 2 of the 20 included studies (Figure 8). The research results showed no heterogeneity among the studies (DF = 1, P = 0.62, I2 = 0%). Therefore, the fixed effect model was used for analysis. The alleviation of ArE in the combined treatment group was superior to the control group [MD = 0.02, 95%CI = (0.02, 0.02)], with statistical significance (P < 0.00001).
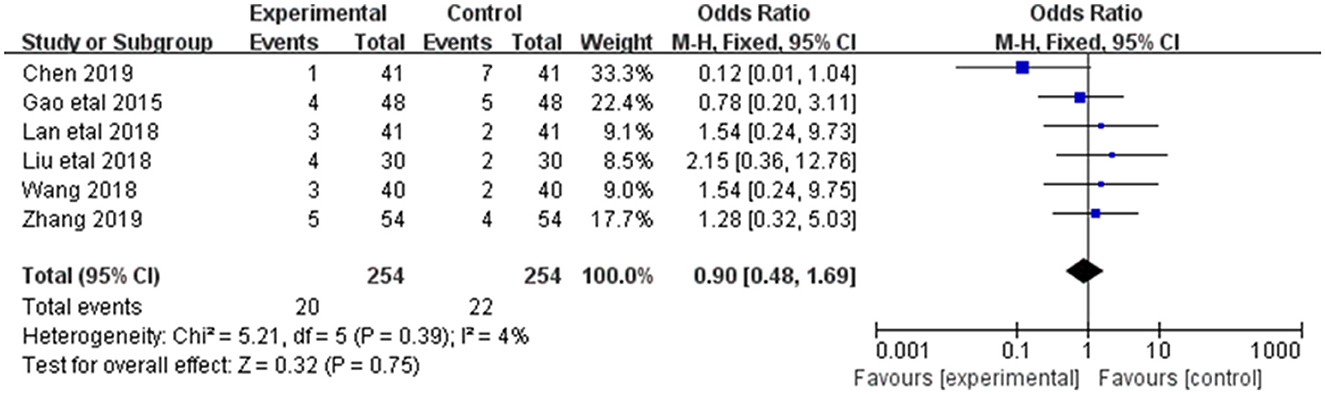
3.3.7. Adverse reactions
Adverse reactions were reported in 6 of the 20 included studies (Figure 9). The research results showed small heterogeneity among the studies (DF = 5, P = 0.30, I2 = 4%). Therefore, the fixed effect model was used for analysis. Meta-analysis showed no statistical significance between the treatment group and the control group [MD = 0.90, 95%CI = (0.48,1.69), P = 0.75].
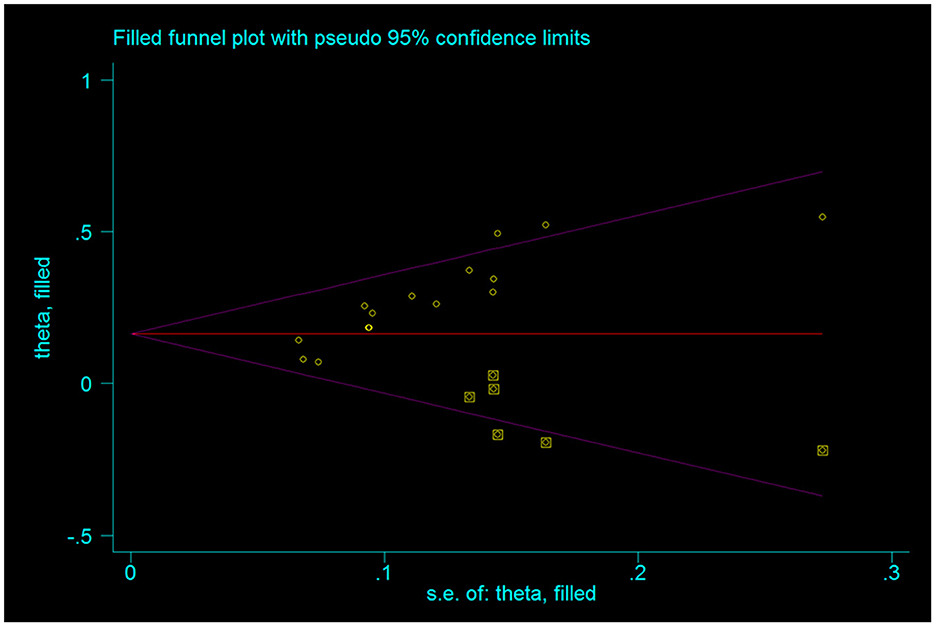
3.3.8. Assessment of publication bias
The publication bias test was conducted for the outcome indicators of more than 10 studies in the report (Figure 10). The relatively asymmetric funnel graph of total effective outcome indicators indicated the existence of certain publication bias. Egger method test showed that the index had a certain publication bias (P = 0.000 < 0.05), and cutting compensation method was used for analysis. The estimated RR point and 95% CI before and after pruning were 1.29 [1.22, 1.37] and 1.177 [1.123, 1.234], respectively. The adjusted statistics and significance were Z = 6.806, P < 0.05, and the adjusted conclusion was not reversed, indicating the relative stability of the conclusion (Figure 11).
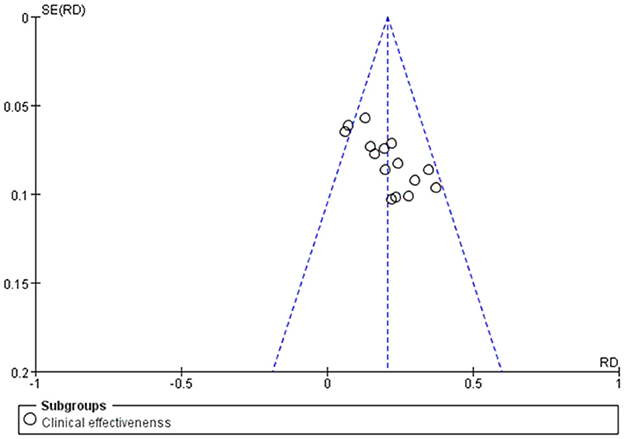
Figure 10. Funnel plot of the total efficacy of Shenkang injection combined with alprostadil and control drugs.
3.3.9. Sensitivity analysis
Using successively eliminating each study, sensitivity analysis was conducted on the remaining results. The Meta-analysis results showed no significant change in each effect value after excluding any study, indicating the stability and reliability of the results.
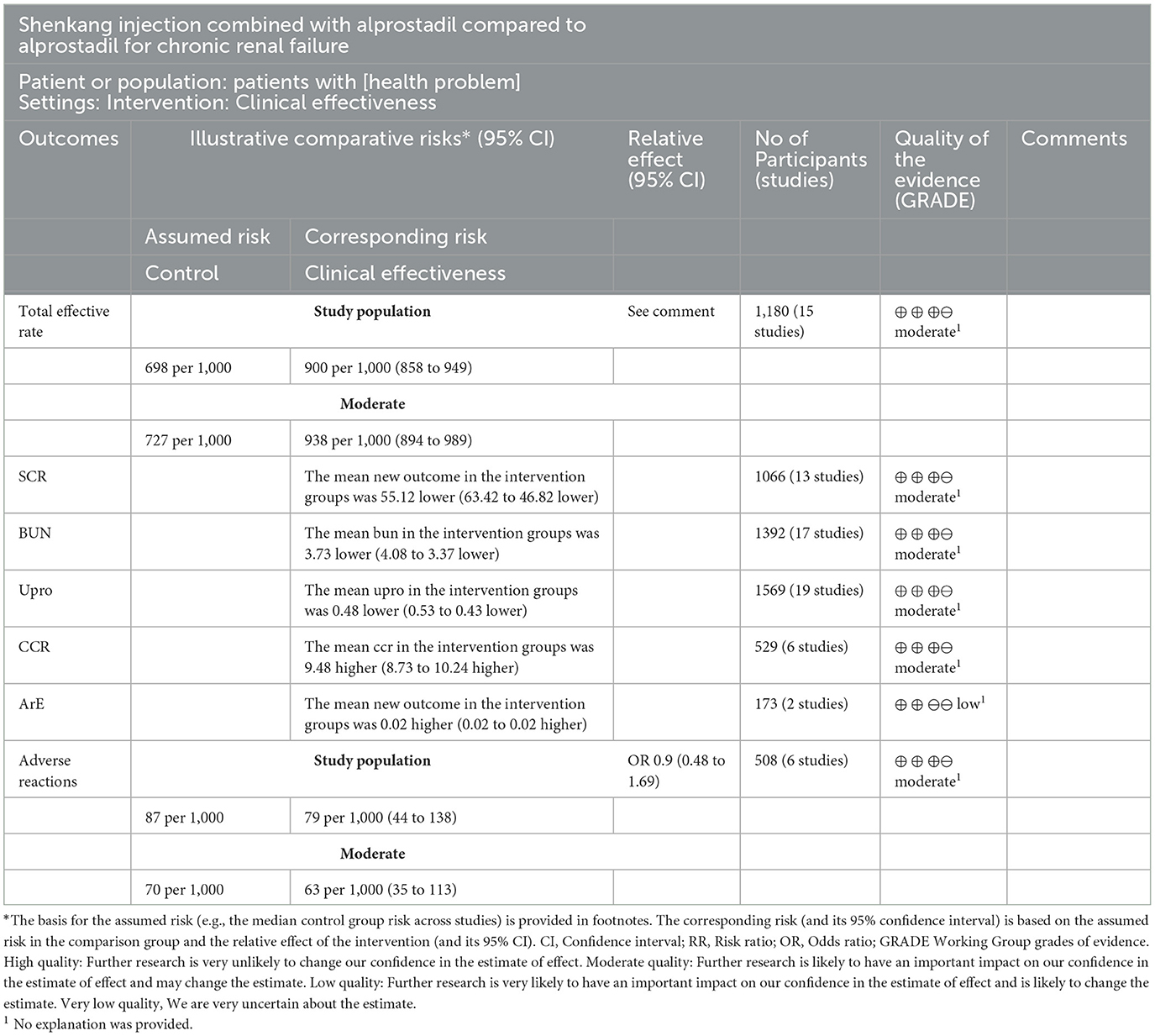
3.4. Results of GRADE evidence quality evaluation
The GRADE system was used to classify the quality of the main outcome indicators of 20 documents (Table 2). All outcome indicators were of medium and low quality, without high-quality evidence, suggesting that the results need to be further confirmed by a large-scale, multi-center RCT.
4. Discussion
4.1. Evaluation clinical efficacy and safety
CRF is a chronic progressive renal parenchymal injury disease with partial or complete loss of renal function due to various reasons such as chronic glomerulonephritis. CRF is characterized by slow progress and irreversibility, and manifested as facial and eyelid edema, nausea, vomiting, abdominal distension, fatigue, dysuria, proteinuria, hematuria, etc., (40–42). CRF will cause a series of complex damage to the kidney, thus inducing chronic systemic inflammation without significant clinical manifestation. This kind of inflammation is classified in immune inflammations, not equivalent to a systemic inflammatory response syndrome (SIRS) or a microbial infection (3). Alprostadil, a kind of prostaglandin E1 preparation, exerts its role in expanding blood vessels and reducing peripheral resistance. On this basis, vascular smooth muscle can be relaxed, and microcirculation can be improved, thus preventing glomerular thrombosis. That will achieve the goal of reducing proteinuria, increasing blood flow, and inhibiting inflammatory mediators. Renal function protection can be achieved through glomerular sclerosis (43, 44). However, alprostadil has certain adverse reactions, such as redness, pain, itching at the injection site, and even causes chest tightness and blood pressure drop. CRF is traditionally considered as blood stasis and water dampness based on the deficiency of kidney and spleen (43). Rhubarb, salvia miltiorrhiza, astragalus, and safflower in SKI have the functions of activating blood circulation and removing stasis, which can clear organs, remove dampness, invigorate qi and promote blood circulation. Modern pharmacological research have shown that Shenkang injection can improve renal tubular reabsorption, inhibit the proliferation of mesangial cells and renal interstitial fibrosis, promote the recovery of renal function, reduce renal tubular injury, and promote Ccr excretion (45). According to relevant studies, the combined treatment of SKI and alprostadil can achieve favorable efficacy in terms of CRF caused by diabetes and chronic kidney disease in elderly patients, with effectively reduced BUN and alleviated condition (46).
In our results showed that the overall response rate (ORR) of the treatment group was superior to the control group [RR = 0.20, 95% CI (0.16, 0.25), P < 0.00001]. Compared with the control group, the treatment group achieved favorable improvement in terms of the creatinine clearance rate (Ccr) [MD = 9.48, 95% CI (8.73, 10.24), P < 0.00001], serum creatinine (Scr) [MD = −55.12, 95% CI (−63.42, −46.82), P < 0.00001], quantitative urine protein (Upro) [MD = −0.48, 95% CI (−0.53, −0.43), P < 0.00001], and blood urea nitrogen (BUN) [MD = −3.73, 95% CI (−4.08, −3.3) 7, P < 0.00001]. There was no statistical difference in the incidence of adverse reactions in each group.
All in all, the combined treatment of SKI and alprostadil exhibited great advantage in CRF. Meanwhile, the results of Meta-analysis remained unchanged after removing some documents, indicating the stablity and reliablity of the results. Adverse reactions were specifically reported in only 6 papers, without serious adverse ones. There was no statistically significant difference between the two groups.
4.2. Limitaitons of the study
(1) Publication bias may exist in this study due to the small number and sample size, uneven course of treatment and Chinese language resources of the included literature; (2) All the studies were RCT, but most of them did not describe the specific random sequence generation method and the implementation scheme of allocation concealment, resulting in some limitations of the research results; (3) The great statistical heterogeneity of BUN indicators cannot be strictly eliminated according to the inclusion and exclusion criteria. (4) Adverse reactions were not reported in most studies. Great importance should be attached to relevant literature at home and abroad to improve the systematic evaluation, so as to more accurately draw conclusion on the efficacy of SKI combined with alprostadil in CRF treatment.
A total of 20 clinical RCTs were included in this study and analyzed through evidence-based medicine. The sound efficacy of SKI combined with alprostadil may shed new light on integrated traditional Chinese and western medicine in CRF treatment. However, there is still a long way to go in CRF treatment with large-scale and high-quality RCTs due to low-quality literature included in this study.
5. Conclusion
The current evidence indicates that therapy of SKI combined with Alprostadil for CHF positive efficacy and high safety. It is also simple, convenient. However, because of the limitations of the follow-up period and publication bias of the included trials, more rigorous clinical studies are necessary to further verify the long-term effects of it.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TZ and FX designed the study, carried out statistical analysis, and revised/reviewed the manuscript. XQ and TZ carried out literature research. ML and PZ acquired data. TZ and PZ edited the manuscript. WL approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Subject Innovation Team of Shaanxi University of Chinese Medicine (2019-QN02), Shaanxi Provincial Department of Education Project (20JC012), Doctoral Foundation of Xinjiang Medical University (No. 2019-1), National Key Research and Development Plan (2017YFC17039001-3), National Key Research and Development Plan (2017YFC17039002-3), National Natural Science Foundation of China (No. 81960771), and Project of Science and Technology Department of Shaanxi Province (No. S2023-JC-YB-2709).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Skalsky K, Shiyovich A, Steinmetz T, Kornowski R. chronic renal failure and cardiovascular disease: a comprehensive appraisal. J Clin Med. (2022) 11:1335. doi: 10.3390/jcm11051335
2. Meola M, Samoni S, Petrucci I. Clinical scenarios in chronic kidney disease: chronic tubulointerstitial diseases. Contrib Nephrol. (2016) 188:108–19. doi: 10.1159/000445473
3. Hung PH, Hsu YC, Chen TH, Lin CL. Recent advances in diabetic kidney diseases: from kidney injury to kidney fibrosis. Int J Mol Sci. (2021) 22:11857. doi: 10.3390/ijms222111857
4. Koushik NS, McArthur SF, Baird AD. Adult chronic kidney disease: neurocognition in chronic renal failure. Neuropsychol Rev. (2010) 20:33–51. doi: 10.1007/s11065-009-9110-5
5. Lai J, Akindavyi G, Fu Q, Li ZL, Wang HM, Wen LH. Research progress on the relationship between coronary artery calcification and chronic renal failure. Chin Med J. (2018) 131:608–14. doi: 10.4103/0366-6999.226066
6. Shiferaw WS, Akalu TY, Aynalem YA. Risk factors for anemia in patients with chronic renal failure: a systematic review and meta-analysis. Ethiop J Health Sci. (2020) 30:829–42. doi: 10.4314/ejhs.v30i5.23
7. Pabst D, Sanchez-Cueva PA, Soleimani B, Brehm CE. Predictors for acute and chronic renal failure and survival in patients supported with veno-arterial extracorporeal membrane oxygenation. Perfusion. (2020) 35:402–8. doi: 10.1177/0267659119889521
8. Chen Y, Wan JX, Jiang DW, Fu BB, Cui J, Li GF. Clinical efficacy and safety of sequential treatment with alprostadil and beraprost sodium for chronic renal failure induced by chronic glomerulonephritis. Nan Fang Yi Ke Da Xue Xue Bao. (2013) 33:1521–4.
9. Zhang L, Miao R, Yu T, Wei R, Tian F, Huang Y, et al. Comparative effectiveness of traditional Chinese medicine and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and sodium glucose cotransporter inhibitors in patients with diabetic kidney disease: a systematic review and network meta-analysis. Pharmacol Res. (2022) 177:106111. doi: 10.1016/j.phrs.2022.106111
10. Wang Z, Zhang S, Zheng X, Zhang L. Efficacy and safety of colonic dialysis combined with traditional Chinese medicine retention enema in the treatment of chronic renal failure: a protocol for systematic review and meta-analysis. Medicine. (2021) 100:e28082. doi: 10.1097/MD.0000000000028082
11. Xi Y, Lu X, Zhu L, Sun X, Jiang Y, He W, et al. Clinical trial for conventional medicine integrated with traditional Chinese medicine (TCM) in the treatment of patients with chronic kidney disease. Medicine. (2020) 99:e20234. doi: 10.1097/MD.0000000000020234
12. Mei J, Yang L, Wang D, Wang H. Efficacy and safety of Shenkang injection in the treatment of chronic renal failure: a protocol of a randomized controlled trial. Medicine. (2021) 100:e27748. doi: 10.1097/MD.0000000000027748
13. Wang Y, Li M, Li C, Xu S, Wu J, Zhang G, et al. Efficacy and safety of Shenkang injection as adjuvant therapy in patients with diabetic nephropathy: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e23821. doi: 10.1097/MD.0000000000023821
14. Zhu J, Yang T, Luo J, Wei M, Li H, Qi Y, et al. Effects of shenkang injection combined with jinshuibao on early diabetic nephropathy and effects on coagulation fibrinolysis system and urinary protein. Evid Based Complement Alternat Med. (2022) 11:2022:3958049. doi: 10.1155/2022/3958049
15. Wei HT, Xu Y, Tan XY, Jing HY, Ma YR. ShenKang injection attenuates renal fibrosis by inhibiting EMT and regulating the Wnt/β-catenin pathway. Evid Based Complement Alternat Med. (2022) 28:9705948. doi: 10.1155/2022/9705948
16. Hao J, Huang X, Guan J, Feng J, Li D, Cao S, et al. Shenkang injection protects against renal fibrosis by reducing perforin expression through the STING/TBK1/IRF3 signaling pathways in natural killer cells. Phytomedicine. (2022) 104:154206. doi: 10.1016/j.phymed.2022.154206
17. Chen Y X. Effect of alprostadil combined with Shenkang injection in the treatment of chronic renal failure. Jilin Med Sci. (2016) 37:2476–7.
18. Ma T. To observe the clinical effect of alprostadil combined with Shenkang injection in the treatment of chronic renal failure in elderly people. The Latest Med Infor Abstr. (2019) 12:161–2.
19. Wang SX, Yao W, Li D. Therapeutic effect of Alprostadil and Shenkang injection in the treatment of chronic renal failure. Henan Med Res. (2012) 21:63–64+67.
20. Zhang QS, Song JP, Luo HP. Therapeutic effect of Alprostadil combined with Shenkang injection in the treatment of chronic renal failure. Int J Med Health. (2012) 18:31–5.
21. Zhang DQ. Clinical effect analysis of alprostadil combined with Shenkang injection in treatment of 60 cases of chronic renal failure in senile patients. Chin J Health and Nutri. (2015) 25:336–7.
22. Wang L. Clinical analysis of alprostadil combined with Shenkang injection in the treatment of chronic renal failure in elderly people. Chin Med Guide. (2018)16:134–5.
23. Chen XH. Clinical effect of alprostadil combined with Shenkang injection on chronic renal failure in elderly patients. Modern Diagnosis and Therapy. (2019) 30:3903–5.
24. Feng DK, Yin G, Zhu JZ. Effect of Alprostadil combined with Shenkang injection on chronic renal failure. Heilongjiang J Tradi Chin Med. (2020) 49:9–10.
25. Zou HZ. Effect of alprostadil combined with Shenkang Injection on chronic renal failure and its effect on renal function. Doctor. (2019) 8:10–2.
26. Zhao JA. Clinical evaluation of alprostadil combined with Shenkang injection in the treatment of chronic renal failure in elderly patients. Abstract Latest Med Infor. (2018) 19:137 + 140.
27. Lan HH, Wang JG. Effect of Alprostadil and Shenkang injection on chronic renal failure in elderly patients. Chin med guide. (2017) 22:59–60.
28. Liu HJ. Clinical observation of Shenkang injection combined with alprostadil in the treatment of chronic renal failure. Mod Chin med applica. (2018)12:87–9.
29. Li WY, Zhang Y, Jiang J, Yang HL. Renal injection combined with high ground, the application effect of the treatment of chronic renal failure study. World's Latest Med Infor Abstract. (2018) 18:78–79.
30. Gao YY, Zhang YQ, Xin XL. Treatment of chronic renal failure by Shenkang injection combined with alprostadil. J Integr Tradi and Western Med. (2015) 24:3388–90.
31. Dai G, Wang DH, Liang Y. Renal injection combined with front row, observation and assessment of the treatment of kidney failure. Chin Mod Drug Applic. (2016) 10:142–4.
32. Liu WL, Huang G, Li PH, Chen ZQ, Pan FL. Clinical effect of Shenkang injection combined with Alprostadil in the treatment of chronic renal failure. Pharmacy Today. (2018) 28:609–11.
33. Feng JF, Wang WJ. Effect of Shenkang Injection combined with Alprostadil injection on chronic renal failure. Hospital Drug Use Eva Anal China. (2019) 42–44 + 47.
34. Zhang YQ. Curative effect of Shenkang injection combined with Alprostadil injection on chronic renal failure. Family Health. (2019) 3:42–44.
35. Zhang R, Li Z D. Effect of Shenkang Injection combined with Western Medicine on chronic renal failure and lipid metabolism disorder. Med Rev. (2015) 21:1322–4.
36. Xiao Wei. Clinical observation of Shenkang Injection combined with Alprostadil in treatment of chronic renal failure in elderly patients. Clin Integrated Trad Chin and Western Med. (2015) 2:13–14.
37. Patel SS, Kimmel PL, Singh A. New clinical practice guidelines for chronic kidney disease: a framework for K/DOQI. Semin Nephrol. (2002) 22:449–58. doi: 10.1053/snep.2002.35973
38. Chen XG, Ni ZH, Liu YN, Xie YS, Sun W. Chinese journal of integrated traditional and western medicine. JAMA. (2015) 35:1029–33.
39. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1 introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
40. Eyeni Sinomono DT, Loumingou R, Gassongo Koumou GC, Mahoungou GH, Mobengo JL. Chronic renal failure in the brazzaville university hospital center: epidemiological, clinical and evolutionary aspects. Saudi J Kidney Dis Transpl. (2021) 32:1450–5. doi: 10.4103/1319-2442.344766
41. Saar-Kovrov V, Zidek W, Orth-Alampour S, Fliser D, Jankowski V, Biessen EAL, et al. Reduction of protein-bound uraemic toxins in plasma of chronic renal failure patients: a systematic review. J Intern Med. (2021) 290:499–526. doi: 10.1111/joim.13248
42. Ito S, Manabe E, Dai Y, Ishihara M, Tsujino T. Juzentaihoto improves adenine-induced chronic renal failure in BALB/c mice via suppression of renal fibrosis and inflammation. J Pharmacol Sci. (2022) 148:172–8. doi: 10.1016/j.jphs.2021.10.009
43. Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol. (2019) 102:312–8. doi: 10.1111/ejh.13208
44. Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. (2015) 39:84–92. doi: 10.1159/000368940
45. Zou JJ, Zhou XT, Chen YK, Liu JL, Wang C, Ma YR, et al. A review on the efficacy and mechanism of action of Shenkang injection against chronic kidney disease. Biomed Pharmacother. (2020) 132:110833. doi: 10.1016/j.biopha.2020.110833
Keywords: Shenkang injection, alprostadil, chronic renal failure, meta analysis, Chinese medicine
Citation: Xie F, Zhang T, Zhang P, Qu X, Li M and Lan W (2023) Shenkang injection combined with alprostadil for chronic renal failure: A systematic review and meta-analysis. Front. Med. 10:982016. doi: 10.3389/fmed.2023.982016
Received: 30 June 2022; Accepted: 27 March 2023;
Published: 06 April 2023.
Edited by:
Zhonglin Chai, Monash University, AustraliaReviewed by:
Valeria Cernaro, University of Messina, ItalyWeifeng Zhang, Shanghai Jiao Tong University, China
Copyright © 2023 Xie, Zhang, Zhang, Qu, Li and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Lan, bGFud2VpNTE2QHNpbmEuY29t
†These authors share first authorship
 Feng Xie
Feng Xie Tiantian Zhang2†
Tiantian Zhang2† Wei Lan
Wei Lan