- 1Department of Anesthesiology and Pain Clinic, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Ji’nan, China
- 2Department of Respiratory, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Ji’nan, China
Background: Cough variant asthma (CVA) is a chronic inflammatory airway disease characterized by airway hyper-responsiveness (AHR), of which cough is the only symptom. The cough is a result of the contraction of the vocal cords, diaphragm, sternocleidomastoid muscle, and other respiratory related muscles caused by the AHR. Long-term chronic coughing can lead to repetitive contraction and chronic strain of the muscles involved in the head and neck, ultimately contributing to the formation of latent myofascial trigger points (MTrPs). In turn, latent MTrPs can also irritate or compress the nerves around them, triggering cough. The date indicated that latent MTrPs can induce autonomic phenomena and are effective in allergic rhinitis. But their roles in asthma are unclear. In this article, the efficacy and safety of latent MTrPs injection therapy in CVA were investigated.
Methods: This randomized controlled trial was conducted with 110 patients. Patients were assigned to the intervention or control group in a 1:1.5 ratio. Intervention group (n = 44): single injection therapy with latent MTrPs. Control group (n = 66): budesonide-formoterol plus montelukast for 8 weeks. During the 36-week follow up period, the recurrence rate at week 36, cough visual analog scale (VAS), ACT (asthma control test)-scores, ACQ5 (asthma control questionnaire)-scores, AQLQ (asthma quality of life questionnaire)-scores, proportion of using rescue medication, and adverse events were evaluated.
Results: The recurrence rate at week 36 was lower in the intervention group than in the control group (36 weeks, 5.0 vs. 34.55%, p = 0.001). There were significant differences between groups in change from baseline to 36 weeks in VAS [36 weeks, 1.70 (1.49) vs. 3.18 (2.04), p < 0.001]; ACT-score [36 weeks, 21.38 (2.65) vs. 18.53 (3.00), p < 0.001]; ACQ5-score [36 weeks, 0.85 (0.55) vs. 1.52 (0.62), p < 0.001]; AQLQ-score [36w, 174.40 (18.22) vs. 151.69 (24.04), p < 0.001]; proportion of using rescue medication (36 weeks, 5.0 vs. 29.1%, p = 0.003). Fewer adverse events occurred in the two groups.
Conclusion: Latent myofascial trigger points injection therapy provided long-acting, practical, short treatment duration and safety methods for CVA.
Clinical Trials Registration: http://www.chictr.org.cn/index.aspx, Chinese Clinical Trial Registry Center, ChiCTR2100044079.
1. Introduction
Cough variant asthma (CVA) is an atypical form of asthma with cough as the only symptom. It is a common chronic inflammation disease of the airway worldwide, affecting 1–18% of the population in different countries (1). A multicenter prospective observational study shows that approximately one-third of chronic cough is caused by CVA (2, 3). Nevertheless, the mechanisms responsible for the hypersensitivity of the cough reflex are not fully understood. Guidelines recommend that adults or children with CVA regularly use a low dose of inhaled glucocorticoids as maintenance therapy to reduce airway inflammation and AHR (4–6). Pharmacogenomics has shown that genetic polymorphisms can alert the response to bronchodilators in patients with asthma (7). Approximately, 5% of patients with CVA remain poorly controlled despite inhaled glucocorticoids or leukotriene receptor antagonists (8).
Myofascial trigger points (MTrPs) are defined as hyperirritable spots, usually within a taut band of skeletal muscle, that are painful on palpation and can induce referred pain, motor dysfunction, and autonomic phenomena (9). Whether accompanied by spontaneous pain, MTrPs can be divided into active and latent MTrPs (10, 11). In general, active MTrPs cause clinical pain syndromes. Latent MTrPs, on the other hand, can cause non-pain-related symptoms such as depression, anxiety, insomnia, and tinnitus. In addition, in the head and cervical can cause cough, tears, rhinorrhea, salivation, and other autonomic phenomena (12). In a previous study, injection of latent MTrPs has been shown to be effective for the treatment of allergic rhinitis (13).
The pathophysiological mechanisms of CVA remain unclear, however, chronic inflammation and AHR are thought to play an important role. Our previous experience with CVA patients offered two main reasons to explain the possible interaction between CVA and latent MTrPs: (1) palpation of latent MTrPs transiently triggered cough in some patients and (2) significant improvement in cough symptoms within minutes after latent MTrPs injection. Therefore, we suspected that latent MTrPs in the head and neck regions were somewhat correlated to the pathogenesis of CVA. We proposed that latent MTrPs affect the balance of the autonomic nervous system, leading to abnormal contraction of airway smooth muscle. To investigate the efficacy and safety of injection therapy of latent MTrPs in CVA, we conducted an open-label randomized controlled trial.
2. Materials and methods
2.1. Trial design
The study was an open-label, randomized observer-masked controlled trial to compare the effect of latent MTrPs injection therapy vs. budesonide-formoterol plus montelukast therapy in adult CVA patients. Signed informed consent was obtained from all participants. The study took place at Qilu Hospital, Cheeloo College of Medicine of Shandong University, Jinan, China. This study was approved by the Human Research Ethics Committee of Qilu hospital (KYLL-202011-127) and registered in the Clinical Trial Registry Center (ChiCTR2100044079).
The research included a 2-week placebo run-in phase, a treatment process, and an observational period. A total of 110 patients were randomly assigned utilizing computer access in a 1:1.5 ratio to receive a single injection therapy of latent MTrPs, sternocleidomastoid trigger points, medial or lateral pterygoid muscle trigger points, splenius capitis muscle trigger points, and sternoclavicular joint trigger points (intervention group, n = 44) or budesonide-formoterol plus montelukast for 8 weeks (control group, n = 66). If patients have long-term poor asthma control during the study, they may use rescue asthma medication. The rescue medication was salbutamol sulfate (GLAXO WELLCOME, S.A.: 100 μg/treatment via a metered-dose inhaler, maximum usage 400 μg/day as needed). Participants were recruited from the CVA Specialized Outpatient Department of Qilu Hospital, Shandong University. A 36-week of follow up was conducted after treatment.
2.2. Patients
Patients who were 18 years of age or older and had a clinical diagnosis of CVA [Global Initiative for Asthma (GINA) 2018 criteria (14, 15)] for at least 8 weeks were included. The diagnosis of CVA was made by a respiratory physician based on history, symptoms, signs, and pulmonary function test. The other inclusion criteria for patients were as follows: (1) <75 years old. (2) Any gender or ethnicity. (3) All patients signed an informed consent form. The exclusion criteria were as follows: (1) Cough caused by pneumonia, upper respiratory tract infections, chronic obstructive pulmonary disease, interstitial fibrosis, or other extra-pulmonary diseases. (2) Patients with known hypersensitivity to lidocaine, vitamin B12, or betamethasone. (3) Comorbidity, includes chronic pulmonary, cardiovascular, renal, neurologic, or other systemic disease. (4) Long-term use of oral glucocorticoids within the last 3 weeks. (5) Smoking within the past 6 months. (6) Pregnancy.
2.3. Procedures
2.3.1. Intervention group
2.3.1.1. Latent MTrPs injection procedures
The drugs used for latent MTrPs injection containing vitamin B12 (JinYao Corp, Tianjin City, China; 2 ml:1 mg), 2% lidocaine injection (ZhaoHui Corp, Shanghai City, China; 5 ml:100 mg), and compound betamethasone injection (MSD Merck Sharp & Dohme AG, Switzerland; 1 ml: 5 mg betamethasone dipropionate and 2 mg betamethasone sodium phosphate) were diluted to 20 ml with 0.9% saline for a single injection. Injection of latent MTrPs was performed using needle 25 (0.5 mm × 36 mm) and a 20 ml syringe (We Go Corp, Weihai City, China).
The latent MTrPs were mainly found in the sternoclavicular joint, sternocleidomastoid, medial or lateral pterygoid muscles, and splenius capitis muscles by palpation (Figure 1). However, it was difficult to palpate when some trigger points were hidden in muscles, and the final therapeutic effects depend on the accuracy of palpated points (16). Accurate signs of latent MTrPs can be confirmed by the patient showing “jumping signs,” which may include head retraction, fascial (or forehead) wrinkles, verbal responses, or local twitch responses (LTRs) (11, 12). Palpation and injection of latent MTrPs were performed according to Travell and Simons’ “Trigger Point Manual” (17).
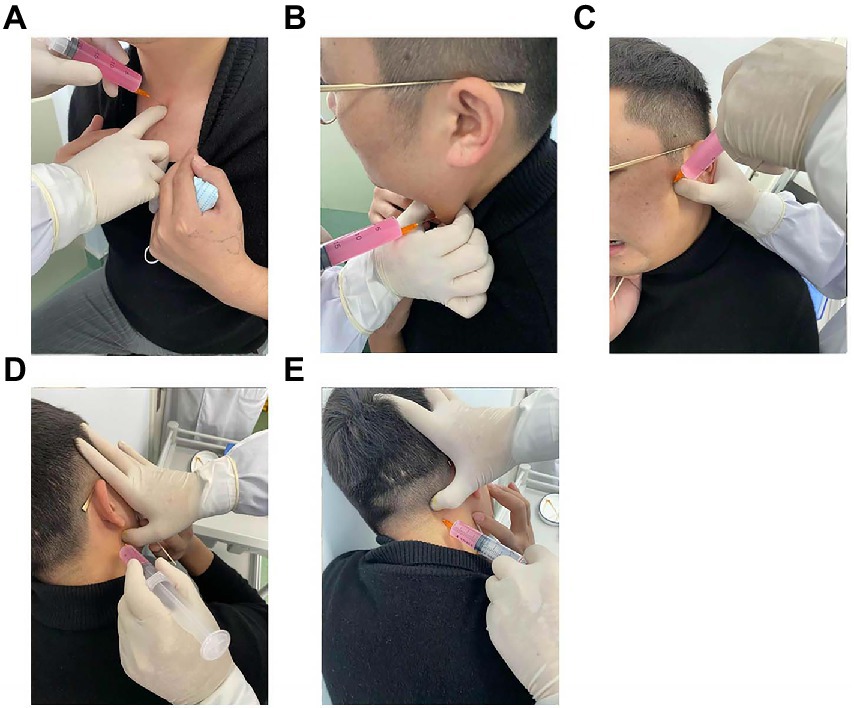
Figure 1. Latent MTrPs injection for CVA. Sternoclavicular joint latent MTrPs injection (A). Sternocleidomastoid muscles latent MTrPs injection (B). Medial or lateral pterygoid muscles latent MTrPs injection (C,D). Splenius capitis muscles latent MTrPs injection (E). MTrPs, myofascial trigger points; CVA, cough variant asthma.
2.3.1.2. Latent MTrPs in the medial or lateral pterygoid muscles
Palpation was performed with progressive and continuous deep pressure with a finger on the skin surface to identify latent MTrPs in the medial or lateral pterygoid muscles. Patients were instructed to remain seated and to immobilized the head and shoulders to maximize relaxation of the muscle being palpated (18). Once the trigger points were identified, the thumb of one hand of the therapist remained in a fixed position on the skin, disinfected the surrounding skin, had the patients keep their mouths open (to prevent the needle from blocking in the temporomandibular joint) and with the other hand inserted the syringe into the muscle, adjusted the depth of the needle, and when the patients felt a referred pain indicating that the needle had been inserted into the latent MTrPs, 3–5 mL of a liquid medication was then injected. There were many small blood vessels near the muscles. To avoid intravascular injection, it was necessary to withdraw before injection. The other side was injected using the same technique.
2.3.1.3. Latent MTrPs in the sternocleidomastoid muscles
For palpation of the sternocleidomastoid muscle, the patient was placed in a sitting position with the head slightly tilted to one side, and the examiner pinched the muscle with the thumb and index finger to identify the latent MTrPs. Using the same technique, both sides of the sternocleidomastoid muscles were injected.
2.3.1.4. Latent MTrPs in the splenius capitis muscles
We had the patients sit on the table with both forearms crossed and place the forehead resting on the forearms to identify the trigger point by palpation. The injection technique was the same as above, with pressure on the injection sites after injection to promote better absorption of the drug.
2.3.1.5. Latent MTrPs in the sternoclavicular joint
When palpating the sternoclavicular joint, we had the patient sit upright and located the trigger point by palpation. Care was taken to avoid accidental injection of medication into the subclavian artery. Palpation and injection were performed by the same professor who has 30 years of experience.
2.3.2. Control group
Budesonide and formoterol fumarate powder for inhalation (AstraZeneca AB: 160 ug budesonide and 4.5 μg of formoterol per inhaler, one inhaler every 12 h) plus montelukast (Merck Sharp & Dohme Ltd.: 10 mg per night) for 8 weeks.
2.4. Outcomes
The primary outcome was recurrence rate at week 36. The secondary outcomes mainly included cough VAS, ACQ-5 scores, ACT scores, and AQLQ scores at each of the measurement times points; the proportion of patients using rescue medication at week 36. Scores were obtained before treatment (baseline) and 1, 2, 4, 8, 12, 24, and 36 weeks after treatment.
The VAS is a validated summative score for cough variant asthma, ranging from 0 to 10 points, with VAS with 1–3, 4–6, and 7–10 points indicate mild, moderate, and severe cough, respectively (19). Recurrence was defined as the patient’s 36 weeks cough VAS score returning to or being higher than the baseline level. The “Asthma Control Questionnaire” (ACQ) is a very common questionnaire used to assess asthma control. The ACQ-5 includes five questions about asthma symptoms in the past week (20). Each score ranges from 0 (no impairment) to 6 points (maximum impairment). The ACT includes five questions about asthma symptoms in the past 4 weeks, with each score ranging from 1 (maximum impairment) to 5 points (no impairment). The AQLQ contains 29 questions about asthma symptoms and social and psychological problems during the past 1 week. Each item is scored on a scale from 1 (severe impairment) to 7 points (no impairment).
Safety was evaluated according to the type and severity of adverse events (A.E.s). Data on A.E.s are collected from the start of treatment until the end of follow-up. Patients were asked to record any local or systemic effects during the observation period.
2.5. Statistical analysis
Prior to conducting the study, a pre-experiment was performed, by which we obtained a 36 weeks recurrence rate of 11.1% in Group I and 45.5% in Group C. Setting α = 0.05, β = 0.1, and an allocation ratio of 1:1.5, we calculated at least 24 patients in group I and 36 patients in group C using the PASS software. Assuming a 20% lost rate, we calculated at least 30 patients in group I and 45 patients in group C. Date were analyzed using independent samples t-tests, paired-samples t-test, Chi-square test or Fisher’s exact test. For statistical analysis, we used SPSS version 22.0 software and GraphPad Prism 8.3, and measured data are described as mean (SD). A p value less than 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patients
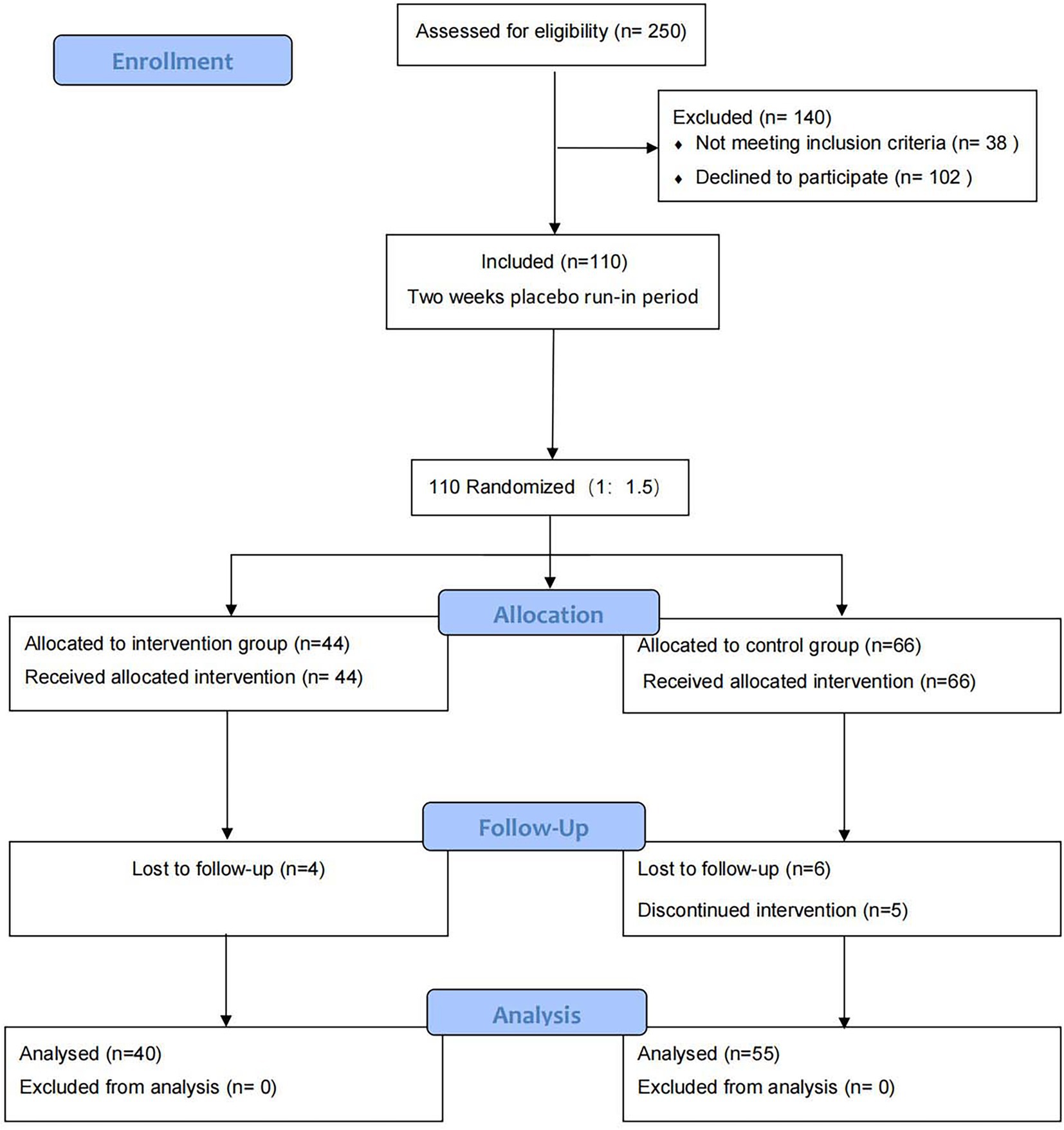
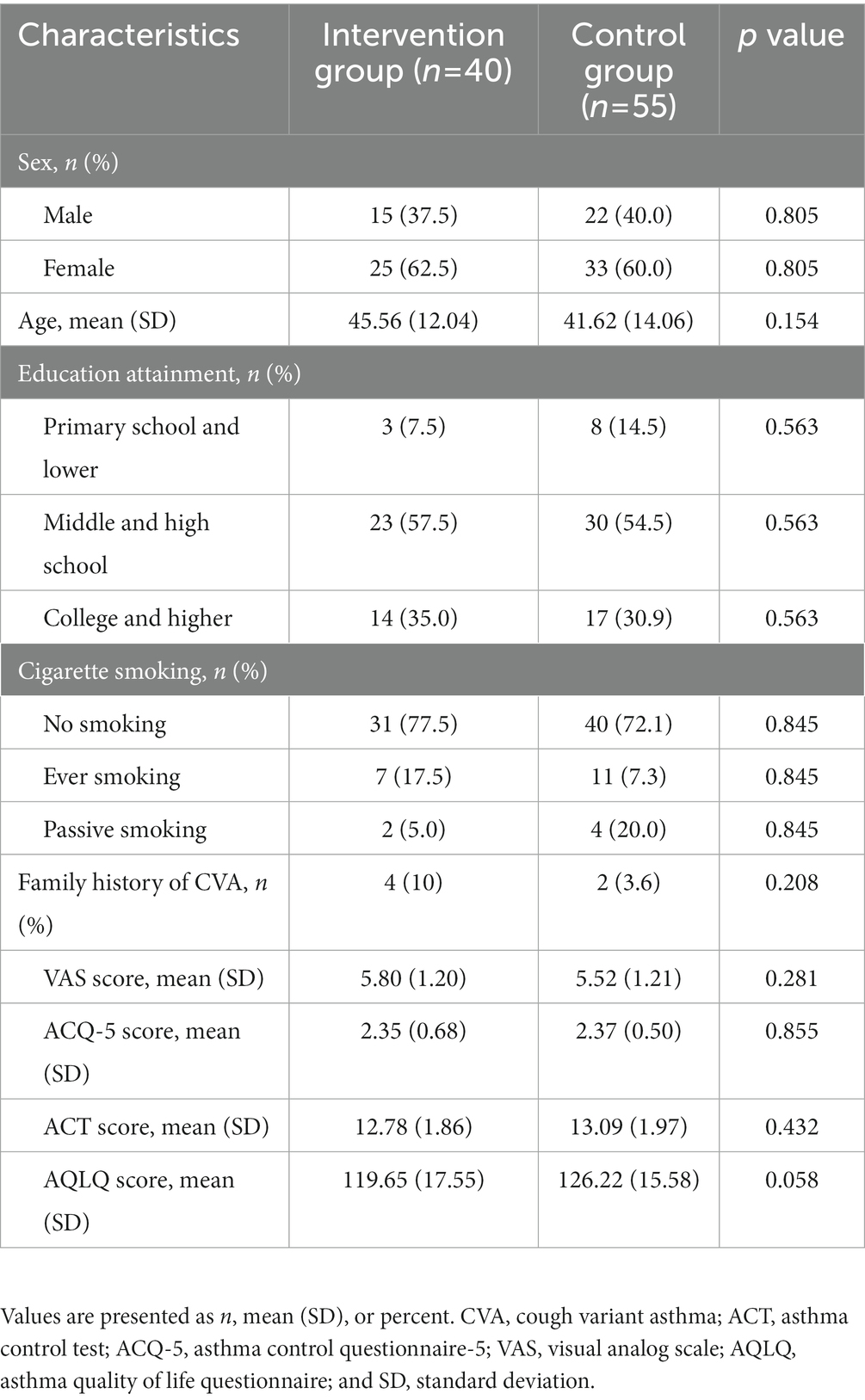
According to the recruitment strategy, a total of 110 patients who had CVA were enrolled in the study. Of them, 95 (86.4%) participants completed the trial and were included in the full analysis. Ten patients were lost to follow up, including 4 (9%) in the intervention group and 6 (9%) in the control group. Five patients failed to persist on budesonide-formoterol. The flow diagram of the participant is shown in Figure 2. The two groups were similar with respect to baseline characteristics (p > 0.05; Table 1).
3.2. Primary outcomes
Overall, recurrence occurred in 21 of 95 participants (22.11%) at week 36, 2 of 40 (5%) in the intervention group vs. 19 of 55 (34.55%) in the control group [36 weeks, −0.296 (95%CI, −0.4388 to −0.1533), p = 0.001; Table 2].
3.3. Secondary outcomes
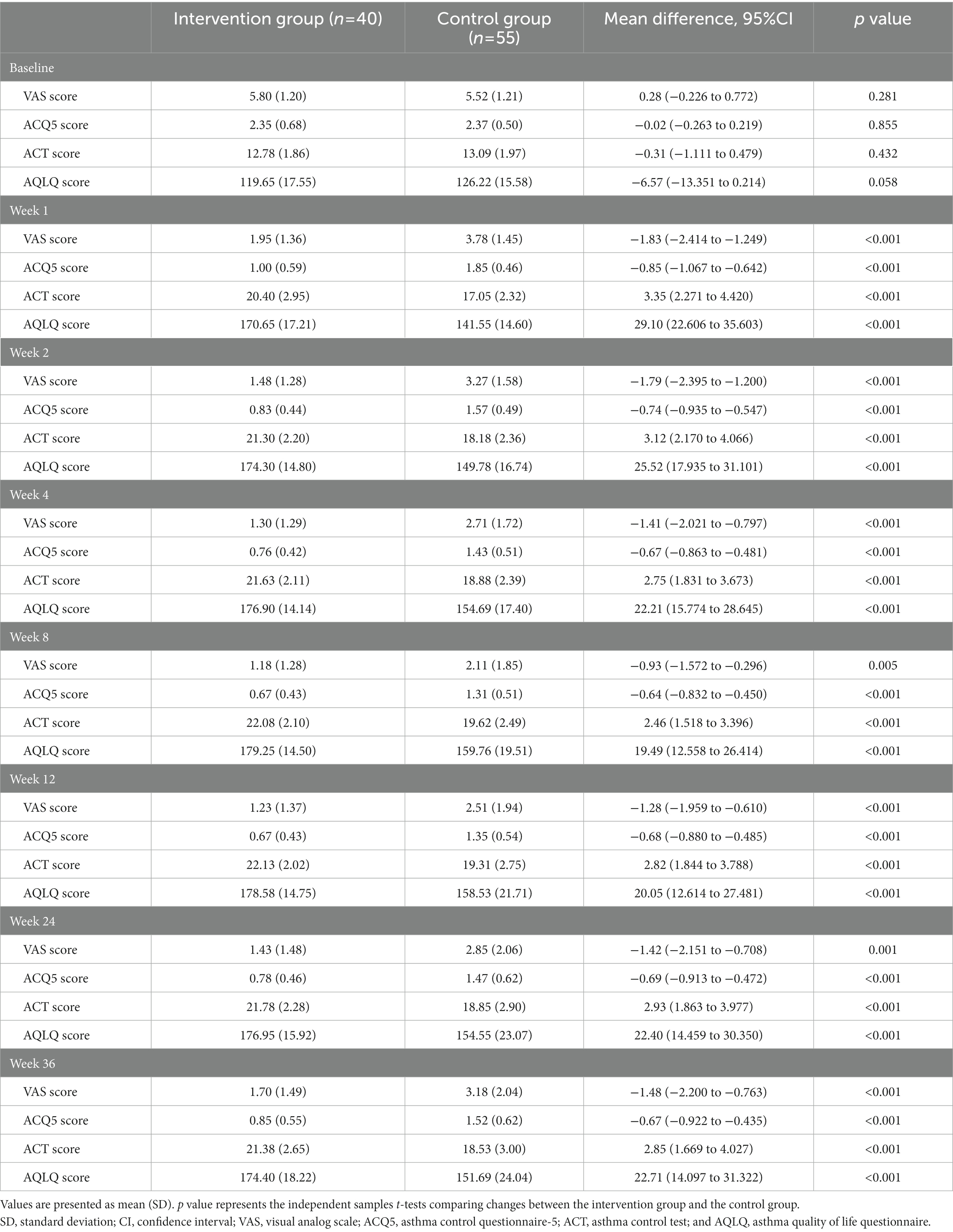
After treatment, the intervention group showed a significant VAS reduction compared with the control group throughout the follow-up period. The improvement in cough symptoms was observed in the first week of treatment and persisted until the end point. The results of efficacy analysis are shown in Table 3 and Figure 3A. From week 8 to the end of the follow-up period, cough VAS tended to increase in both groups, but at week 36, there was a significant improvement in cough symptoms in both the intervention and control groups compared with baseline [−4.10 (1.66) and − 2.35 (1.54), p < 0.001, Figure 4].
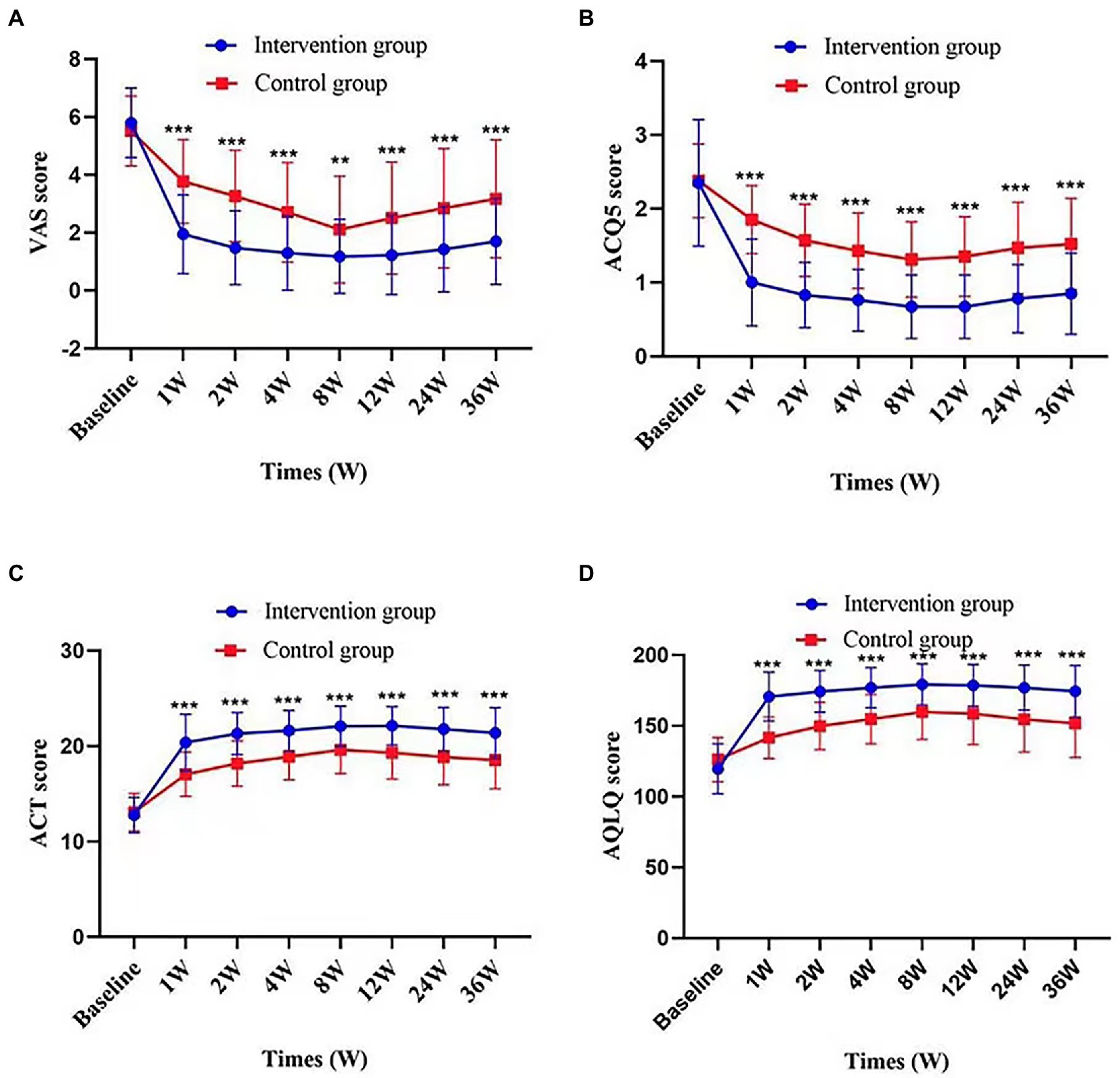
Figure 3. Change in VAS score, ACQ5score, ACT score, and AQLQ score according to times and groups. Figures demonstrate values of VAS score (A), ACQ5 score (B), ACT score (C), and AQLQ score (D) at each time point in intervention group and control group. Values are presented as mean (SD). p value represents the independent samples t-tests comparing changes between the two groups. **p value <0.01; ***p value <0.001, comparison between the two groups.
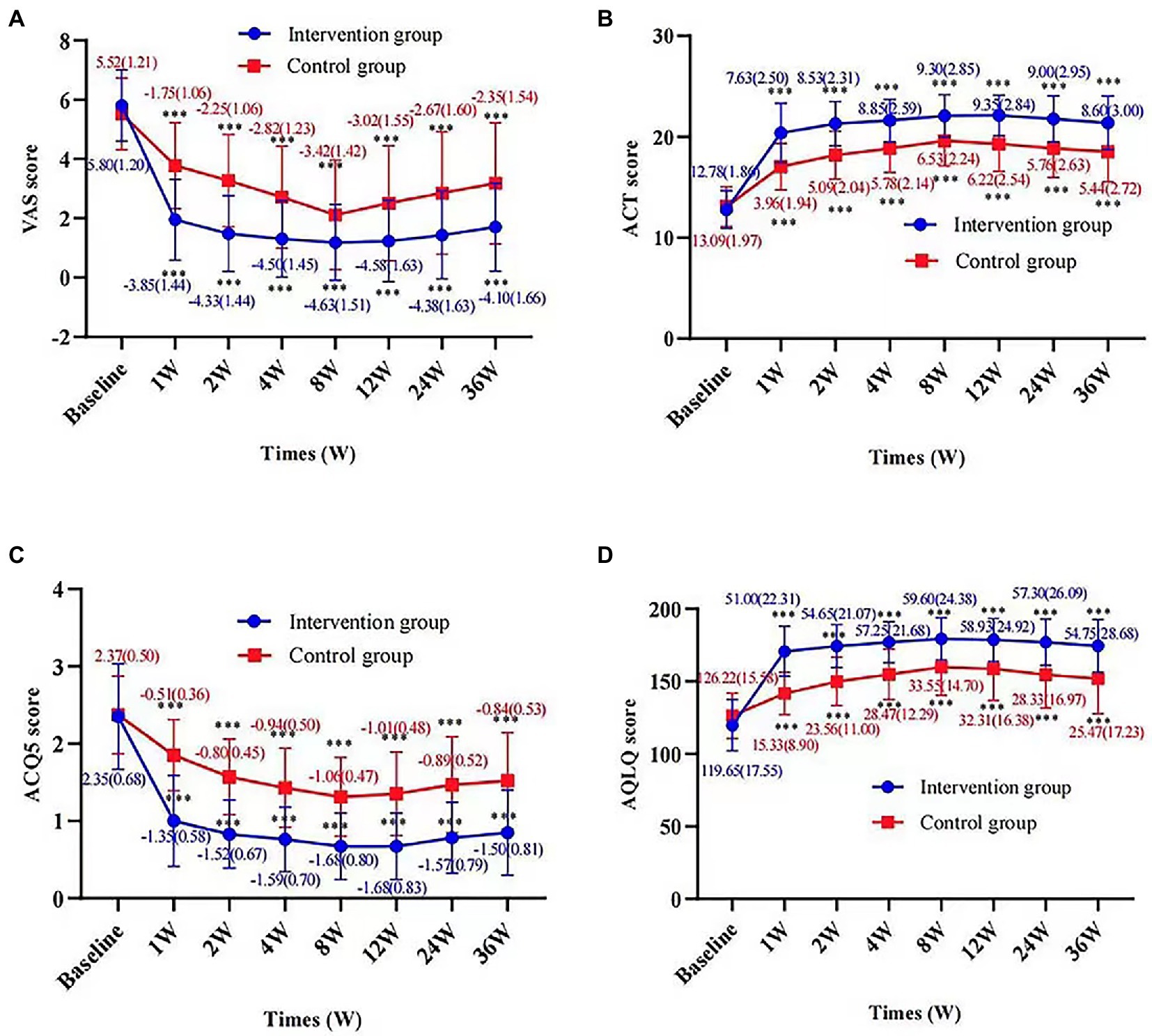
Figure 4. Change in VAS score, ACT score, ACQ5 score, and AQLQ score according to times and groups. Figures show the difference in VAS score (A), ACT score (B), ACQ5 score (C), and AQLQ score (D) at each time point from baseline for the intervention and control groups, respectively. Values are presented as mean (SD). p value represents a paired sample t-test for the change from baseline in the two groups. ***p value <0.001, compared with baseline.
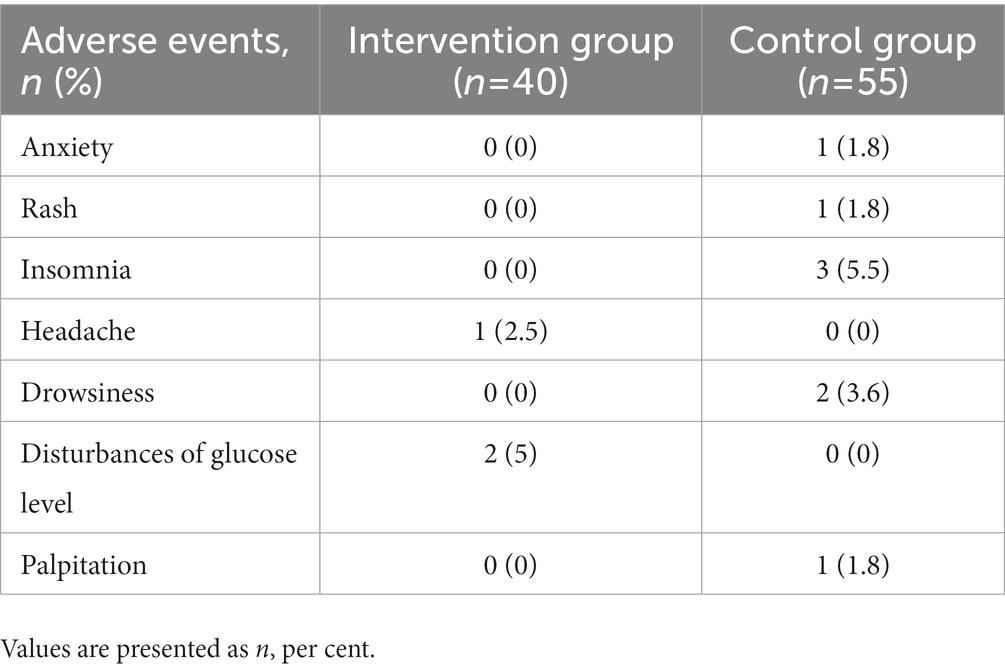
There were also significant differences between groups in change from baseline to 36 weeks in ACQ-5 score, ACT score, AQLQ score (p < 0.001); proportion using rescue medication [36 weeks, −0.241 (95%CI, −0.3787 to −0.1033), p = 0.003]. Changes in these outcomes on the prespecified end points are summarized in Tables 2, 3 and Figure 3. Adverse events were rare and generally not attributed to intervention therapy (Tables 2, 4). A total of 11 A.E.s were classified as mild. Intervention group: disturbances of glucose level (2, 5%); headache (1, 2.5%). Control group: insomnia (3, 5.5%); drowsiness (2, 3.6%); anxiety (1, 1.8%); rash (1, 1.8%); palpitation (1, 1.8%). Two patients developed a transient hyperglycemia after latent MTrPs injection, with blood glucose levels were 13.6 and 14.1 mmol/L, respectively, and this phenomenon was disappeared the day after the injection therapy.
4. Discussion
This study was the first trial to evaluate the efficacy and safety of latent MTrPs injection in CVA. Latent MTrPs injection therapy showed significant improvement in asthma symptoms at all time points, especially at week 8 and week 12. Although budesonide-formoterol plus montelukast therapy is effective to some extent in CVA, cough VAS increased from week 8 and the increase was statistically significant at weeks 12, 24, and 36 of follow-up compared with week 8. This study also suggests that latent MTrPs injection therapy may address patient’s overdependence on or poor adherence to maintenance therapy with inhaled of glucocorticoids. There are no serious adverse events attributable to latent MTrPs injection therapy or budesonide-formoterol plus montelukast therapy; two patients reported a transient hyperglycemia after latent MTrPs injection treatment, but they were alleviated quickly. On the one hand, this phenomenon may be related to betamethasone. On the other hand, it might be associated with the nervous stress of the patient during the needling to make the sympathetic nerves relatively excited (21).
4.1. Relationship between CVA and latent MTrPs
The pathogenesis and pathological process of CVA have not been fully elucidated. Previous studies have shown that transient receptor potential vanilloid 1 (TRPV1) and endogenous neuropeptides are closely associated with the development of CVA (22–26). According to Simons’ theory, latent MTrPs reside in asymptomatic areas and trigger referenced pain and autonomic phenomena only upon stimulation (17). Previous research has established that many inflammatory cytokines are expressed in the vicinity of MTrPs (27), including bradykinin (BK), capsaicin, substances P (SP), α-tumor necrosis factor, serotonin, histamine, norepinephrine, and extracellular fluid. The fluid contains hydrogen ions that can induce the release of calcitonin gene-related peptides (CGRP) via motor terminals and nociceptive muscle receptors (28). Some endogenous substances such as SP, CGRP, BK can not only activate TRPV1 directly, but also phosphorylate the specific structure of TRPV1, lowing its threshold and sensitizing TRPV1 (29, 30). The depolarizing current generated by the activation of TRPV1 is conducted to the cough center of the nucleus solitaire via the vagus nerve (31). The relief of CVA symptoms after injection of latent MTrPs gave us two main hypotheses for a possible interaction between the physiopathology of CVA and latent MTrPs: (1) the rebalancing between sympathetic and parasympathetic nerves and (2) the subsiding of neurogenic inflammations at MTrPs.
We also found that injection of latent MTrPs into the head and neck muscles was more effective than trigger points in the extremities or shoulders in relieving cough symptoms. This may further suggest that neuroinflammation and imbalances in the autonomic nerve network of the head and neck region, especially sympathetic and parasympathetic nerves, are the main mechanisms causing airway smooth muscle contraction and AHR.
4.2. Relief CVA symptoms through latent MTrPs injection
Various neuropeptides released by neurogenic inflammation have different effects on CVA (32). The CGRP can dilate the blood vessels by acting on CGRP receptors in airway blood vessels, causing congestion and edema of the airway mucosa (33). SP acts on the NK1 receptor, increasing airway microvascular permeability and promoting infiltration of plasma proteins and inflammatory cells. In addition, SP stimulates airway smooth muscle and induces AHR. Latent MTrPs injection therapy alleviated the neurogenic inflammations at the MTrP, decreased the production of endogenous peptides such as SP and CGRP, and balanced the autonomic nerve network, especially the cervical sympathetic and vagus nerves, thereby restoring the airway mucosal function.
We also examined whether the improvements in symptoms were related to the drug used for the injection. Lidocaine has been reported to have anti-inflammatory properties (34). Its potential mechanisms may include blocking vagus reflex and inhibiting smooth muscle contraction. Betamethasone is a long-acting corticosteroid with anti-inflammatory and anti-allergic effects, including reduce inflammatory exudation and improve local blood flow. The longest duration of any drugs we used did not last more than 4 weeks. But, in our observations, we have found that the relief of symptoms can persist for tens of weeks. Consequently, we attribute the relief of cough to the alleviation of neurogenic inflammation at the MTrP by the injection of latent MTrPs rather than to the effect of the inject drugs themselves.
4.3. Relationship between latent MTrPs injection and traditional acupuncture
Research has shown that acupuncture can improve lung ventilation and anti-inflammatory activity and regulate the neuroendocrine network (35), however, we consider them to be different. First, acupuncture points are located at specific positions on the meridian. In contrast, latent MTrPs are hyperirritable points, usually within a taut band of skeletal muscle; the location of the point is individual and may vary from person to person. Second, acupuncture points in traditional Chinese medicine have pathological and physiological properties, while trigger points have only pathological properties. Moreover, trigger points injection focus on inducing LTRs.
Although the study has obtained certain research results, there are some limitations of this research. Firstly, the outcome variable may be subject to unmasked administration of therapy. In terms of the implementation of blinding methods, the injection of latent MTrPs is special, and it is difficult to achieve double blindness, it was only a randomized, observer-masked controlled trial. Secondly, outcome indicators are relatively subjective and susceptible to other factors. Thirdly, the long-term side-effects of latent MTrPs injection were not evaluated in this study. In future studies, we would add objective indexes such as FeNO or lung function tests.
5. Conclusion
In the pilot study, we suggested that latent MTrPs injection therapy provided long-acting, practical, short treatment duration and safety methods for CVA. We inferred that the inflammatory of latent MTrPs might play an essential role in the pathogenesis of CVA. The potential mechanism may be that neurogenic inflammations at MTrPs disrupt the balance between sympathetic and parasympathetic nerves in the autonomic nervous system, result in airway smooth muscle contraction and airway hyper-responsiveness.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Human Research Ethics Committee of Qilu hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QL: conceptualization, methodology, validation, resources, writing–original draft, and writing–review and editing. WZ: conceptualization, methodology, investigation, data curation, and writing–original draft. TT: validation, formal analysis, and data curation. YL and HB: conceptualization and methodology. QH: formal analysis, data curation, writing–original draft, and visualization. FQ: conceptualization, methodology, validation, resources, writing-original draft, writing–review and editing, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was funded in part by the National Natural Science Foundation of China (No. 81672250) and the Fundamental Research Funds of Shandong University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CVA, Cough variant asthma; MTrPs, Myofascial trigger points; LTRs, Local twitch responses; AHR, Airway hyperresponsiveness; VAS, Visual analog scale; ACT, Asthma control test; ACQ5, Asthma control questionnaire-5; AQLQ, Asthma quality of life questionnaire; A.E.s, Adverse events; TRPV1, Transient receptor potential vanilloid 1; SP, Substance P; CGRP, Calcitonin gene-related peptide; BK, Bradykinin; NK1, Neurokinin 1.
References
1. Huang, K, Yang, T, Xu, J, Yang, L, Zhao, J, Zhang, X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. (2019) 394:407–18. doi: 10.1016/S0140-6736(19)31147-X
2. Lai, K, Chen, R, Lin, J, Huang, K, Shen, H, Kong, L, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. (2013) 143:613–20. doi: 10.1378/chest.12-0441
3. Liu, W, Chen, H, Zhang, D, Wu, F, and Zhou, L. A retrospective study of clinical features of cough variant asthma in Chinese adults. Allergy, Asthma Clin Immunol. (2019) 15:3. doi: 10.1186/s13223-019-0318-5
4. Cloutier, MM, Dixon, AE, Krishnan, JA, Lemanske, RF Jr, Pace, W, and Schatz, M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and prevention program. JAMA. (2020) 324:2301–17. doi: 10.1001/jama.2020.21974
5. Stanford, RH, Shah, MB, D’Souza, AO, Dhamane, AD, and Schatz, M. Short-acting beta-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. (2012) 109:403–7. doi: 10.1016/j.anai.2012.08.014
6. Morice, AH, Millqvist, E, Bieksiene, K, Birring, SS, Dicpinigaitis, P, Domingo Ribas, C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. (2020) 55:1901136. doi: 10.1183/13993003.01136-2019
7. Lima, JJ. Do genetic polymorphisms alter patient response to inhaled bronchodilators? Expert Opin Drug Metab Toxicol. (2014) 10:1231–40. doi: 10.1517/17425255.2014.939956
8. Bateman, ED, Bousquet, J, Busse, WW, Clark, TJ, Gul, N, Gibbs, M, et al. Stability of asthma control with regular treatment: an analysis of the gaining optimal asthma controL (GOAL) study. Allergy. (2008) 63:932–8. doi: 10.1111/j.1398-9995.2008.01724.x
9. Gerwin, RD. Myofascial trigger point pain syndromes. Semin Neurol. (2016) 36:469–73. doi: 10.1055/s-0036-1586262
10. Turo, D, Otto, P, Shah, JP, Heimur, J, Gebreab, T, Armstrong, K, et al. Ultrasonic tissue characterization of the upper trapezius muscle in patients with myofascial pain syndrome. Annu Int Conf IEEE Eng Med Biol Soc. (2012) 2012:4386–9. doi: 10.1109/EMBC.2012.6346938
11. Gerwin, R. Trigger point diagnosis: at last, the first word on consensus. Pain Med. (2018) 19:1–2. doi: 10.1093/pm/pnx219
12. Bezerra Rocha, CA, Sanchez, TG, and Tesseroli de Siqueira, JT. Myofascial trigger point:a possible way of modulating tinnitus. Audiol Neurootol. (2008) 13:153–60. doi: 10.1159/000112423
13. Liu, Y, Yang, Y, Hu, Q, Badughaish, A, Zhang, H, Qi, F, et al. Latent myofascial trigger points injection reduced the severity of persistent, moderate to severe allergic rhinitis: a randomized controlled trial. Front Med. (2021) 8:731254. doi: 10.3389/fmed.2021.731254
14. Bateman, ED, Hurd, SS, Barnes, PJ, Bousquet, J, Drazen, JM, FitzGerald, JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. (2008) 31:143–78. doi: 10.1183/13993003.51387-2007
15. Bateman, ED, Reddel, HK, O'Byrne, PM, Barnes, PJ, Zhong, N, Keen, C, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. (2018) 378:1877–87. doi: 10.1056/NEJMoa1715275
16. Alvarez, DJ, and Rockwell, PG. Trigger points: diagnosis and management. Am Fam Physician. (2002) 65:653–60.
17. Simons, Travell D, Simons, J, and Lois, S. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Lippincott Williams and Wilkins. (1999).
18. Fernandez-de-Las-Penas, C, and Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: a Delphi study. Pain Med. (2018) 19:142–50. doi: 10.1093/pm/pnx207
19. Miwa, N, Nagano, T, Ohnishi, H, Nishiuma, T, Takenaka, K, Shirotani, T, et al. An open-label, multi-institutional, randomized study to evaluate the additive effect of a leukotriene receptor antagonist on cough score in patients with cough-variant asthma being treated with inhaled corticosteroids. Kobe J Med Sci. (2018) 64:E134–9.
20. Werner, CU, Linde, K, Schaffner, J, Storr, C, and Schneider, A. Weekly self-measurement of FEV1 and PEF and its impact on ACQ (asthma control questionnaire)-scores: 12-week observational study with 76 patients. NPJ Prim Care Respir Med. (2017) 27:64. doi: 10.1038/s41533-017-0064-4
21. Kuo, T, McQueen, A, Chen, TC, and Wang, JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. (2015) 872:99–126. doi: 10.1007/978-1-4939-2895-8_5
22. Smit, LA, Kogevinas, M, Anto, JM, Bouzigon, E, Gonzalez, JR, Le Moual, N, et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir Res. (2012) 13:26. doi: 10.1186/1465-9921-13-26
23. Kenyon, NJ, Morrissey, BM, Schivo, M, and Albertson, TE. Occupational asthma. Clin Rev Allergy Immunol. (2012) 43:3–13. doi: 10.1007/s12016-011-8272-0
24. O'Byrne, PM, Gauvreau, GM, and Brannan, JD. Provoked models of asthma: what have we learnt? Clin Exp Allergy. (2009) 39:181–92. doi: 10.1111/j.1365-2222.2008.03172.x
25. Watanabe, N, Horie, S, Michael, GJ, Spina, D, Page, CP, and Priestley, JV. Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the Guinea pig respiratory system. Pulm Pharmacol Ther. (2005) 18:187–97. doi: 10.1016/j.pupt.2004.12.002
26. McGarvey, LP, Butler, CA, Stokesberry, S, Polley, L, McQuaid, S, Abdullah, H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. (2014) 133:704–712.e4. doi: 10.1016/j.jaci.2013.09.016
27. Jin, F, Guo, Y, Wang, Z, Badughaish, A, Pan, X, Zhang, L, et al. The pathophysiological nature of sarcomeres in trigger points in patients with myofascial pain syndrome: a preliminary study. Eur J Pain. (2020) 24:1968–78. doi: 10.1002/ejp.1647
28. Grosman-Rimon, L, Parkinson, W, Upadhye, S, Clarke, H, Katz, J, Flannery, J, et al. Circulating biomarkers in acute myofascial pain: a case-control study. Medicine (Baltimore). (2016) 95:e4650. doi: 10.1097/MD.0000000000004650
29. Meents, JE, Neeb, L, and Reuter, U. TRPV1 in migraine pathophysiology. Trends Mol Med. (2010) 16:153–9. doi: 10.1016/j.molmed.2010.02.004
30. Melnick, C, and Kaviany, M. Thermal actuation in TRPV1: role of embedded lipids and intracellular domains. J Theor Biol. (2018) 444:38–49. doi: 10.1016/j.jtbi.2018.02.004
31. McMahon, SB, La Russa, F, and Bennett, DL. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat Rev Neurosci. (2015) 16:389–402. doi: 10.1038/nrn3946
32. Barnes, PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). (2017) 131:1541–58. doi: 10.1042/CS20160487
33. Undem, BJ, and Taylor-Clark, T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. (2014) 133:1521–34. doi: 10.1016/j.jaci.2013.11.027
34. Morina, N, Haliti, A, Iljazi, A, Islami, D, Bexheti, S, Bozalija, A, et al. Comparison of effect of leukotriene biosynthesis blockers and inhibitors of phosphodiesterase enzyme in patients with bronchial Hyperreactivity. Open Access Maced J Med Sci. (2018) 6:777–81. doi: 10.3889/oamjms.2018.187
Keywords: cough variant asthma, latent myofascial trigger points, autonomic phenomena, airway hyper-responsiveness, inflammation
Citation: Liu Q, Zhang W, Tian T, Liu Y, Bai H, Hu Q and Qi F (2023) Latent myofascial trigger points injection therapy for adult cough variant asthma: A randomized controlled trial. Front. Med. 10:937377. doi: 10.3389/fmed.2023.937377
Edited by:
Tu Trinh Hoang Kim, Ho Chi Minh City Medicine and Pharmacy University, VietnamReviewed by:
Dongeon Kim, Stanford University, United StatesHuu Duc Minh Nguyen, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam
Copyright © 2023 Liu, Zhang, Tian, Liu, Bai, Hu and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Qi, ✉ MTk4OTYyMDAxMTExQHNkdS5lZHUuY24=
 Qianqian Liu
Qianqian Liu Wenwen Zhang1
Wenwen Zhang1 Tian Tian
Tian Tian Yu Liu
Yu Liu Feng Qi
Feng Qi