
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 15 January 2024
Sec. Obstetrics and Gynecology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1335139
This article is part of the Research Topic Insights in Obstetrics and Gynecology: 2023 View all 19 articles
Background: The use of frozen embryo transfer (FET) has grown exponentially over the past few years. However, in clinical practice, there are no specific criteria as to whether a delay of at least one menstrual cycle is required for an FET after a failed fresh ET or a freeze-all cycle.
Objective: Through the effects on live birth rate (LBR), clinical pregnancy rate (CPR) and pregnancy loss rate (PLR), to determine whether FET requires a delay of at least one menstrual cycle after fresh ET failure or a freeze-all cycle.
Methods: The search was conducted through PubMed, Web of Science, CNKI, and Wanfang databases for terms related to FET timing as of April 2023. There are no restrictions on the year of publication or follow-up time. Women aged 20 to 46 with any indication for in vitro fertilization and embryo transfer (IVF-ET) treatment are eligible for inclusion. Oocyte donation studies are excluded. Except for the case report, study protocol, and abstract, all original studies are included.
Results: In 4,124 search results, 19 studies were included in the review. The meta-analysis includes studies on the adjusted odds ratio (OR) and 95% confidence interval (CI) of reported live birth rate (LBR), clinical pregnancy rate (CPR), and pregnancy loss rate (PLR), 17 studies were retrospective cohort study, and 2 studies were randomized controlled trial, a total of 6,917 immediate FET cycles and 16,105 delayed FET cycles were involved. In this meta-analysis, the combined OR of LBR was [OR = 1.09, 95% CI (0.93–1.28)], the combined OR of CPR was [OR = 1.05, 95% CI (0.92–1.20)], and the combined OR of PLR was (OR = 0.96, 95% CI 0.75–1.22). There was no statistical significance between the two groups.
Conclusion: Overall, delaying FET by at least one menstrual cycle has no advantage in LBR, CPR, or PLR. So, flexible scheduling of FETs is available to both doctors and patients.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42020161648.
The number of FET cycles in assisted reproductive technology (ART) has been increasing yearly, and it is estimated that in 2014, FET accounted for approximately 40% of the approximately 2 million ART treatment cycles per year worldwide (1). In fact, with the advancement and improvement of freezing, thawing, and resuscitation techniques, frozen embryos are almost indistinguishable from fresh embryos in terms of quality and implantation potential (2, 3). In cases where fresh embryo transfers fail or in cases where fresh embryos fail to transfer for various reasons, patients choose FET.
After determining to adopt FET, how far apart does FET need to be performed for optimal clinical outcomes? The use of controlled ovarian stimulation (COS) before IVF is mostly aimed at obtaining more embryos and, consequently, increasing the success rate of the procedure. Nevertheless, concerns have been raised about the adverse effects of supraphysiological hormones used in COS, including embryo-endometrial asymmetry (4) and alteration of the endometrium’s immune system (5), which may adversely affect the pregnancy outcome of subsequent embryo transfers. There are also multiple luteal or luteal cysts after oocyte retrieval and functional cysts may lead to ovulation disorders and increase the cancellation rate of the FET cycle. If immediate FET fails, the pressure and economic burden on patients will be increased. Therefore, in current clinical practice, most ET procedures are delayed, a practice that aims to minimize the possible residual negative effects of COS on the recovery to normal ovulatory cycles and endometrial receptivity.
However, it has not yet been determined whether delaying FET leads to a better outcome. As a social issue, infertility is a major problem that cannot be ignored, and it also causes heavy psychological stress to patients. In addition, negative emotions such as excessive anxiety and depression can have a negative impact on pregnancy outcomes (6, 7). For infertile couples, delayed ET is a challenge and should be further explored to minimize interruptions in treatment. Therefore, the purpose of this study is to determine whether FET should be delayed for at least one menstrual cycle following a failed fresh ET or following a freeze-all cycle.
1. Study design: randomized controlled trial or cohort study.
2. Participants: women who underwent their first FET following failed fresh ET or freeze-all cycle.
3. Outcome measures: CPR, LBR, and PLR are the primary outcomes of interest.
1. Those who have undergone preimplantation genetic diagnosis and screening (PGD/PGS).
2. Patients who have not undergone an ovarian stimulation cycle.
3. Repeated publication, incomplete data, unable to obtain the full text.
4. Studies on oocyte donation.
We searched PubMed, Web of Science, CNKI, Wanfang, and other databases for medical subject titles as of April 2023, as well as text words related to FET timing. In addition, the references of the included literature were searched to supplement the acquisition of relevant information. The search method is a combination of free words and subject words. The search terms included “freeze all,” “fresh embryo transfer,” “infertility,” “frozen embryo transfer” or “frozen-thawed embryo transfer” or “cryopreserved embryo transfer,” “immediate” or “delayed” or “postpone,” “timing” or “time” or “time interval,” “oocyte retrieval” or “ovum pick-up,” “ovarian stimulation,” “IVF” or “Fertilization in Vitro” or “OPU” etc.
For data extraction, the two researchers independently read the literature based on the unified inclusion and exclusion criteria. In case of disagreement, the third researcher will participate in the discussion and decide. Information extracted included first author’s name, year of publication, country of origin, study design, population characteristics, definition of immediate/delayed FET, ovarian stimulation protocol, trigger agent, endometrial preparation protocol, embryonic development stage, and outcome parameters.
The Newcastle–Ottawa scale (NOS) was used to evaluate the methodological quality of the eligible studies. The scale assigns a maximum of 9 points to each study based on three broad dimensions: subject selection and exposure assessment (4 points), comparability of study groups (2 points), and adequacy of outcome ascertainment and follow-up (3 points). studies with a score of 7–9 are of high quality and low risk of bias. The investigators scored each study independently, and discrepancies were resolved by consensus with the third investigator. The Cochrane Handbook was used to evaluate the methodological quality of the eligible studies. The evaluation content consists of 7 items. Each entry was rated as “low risk,” “unknown,” and “high risk.”
Using RevMan 5.4 statistical software. Relative risk (RR) and 95% CI were selected as the statistical variables of binary classification. Mean difference (MD) and its 95% CI were selected as statistical variables for continuity variables. The statistical heterogeneity of the included studies was analyzed and judged by p-value and I2. When p > 0.1 and I2 ≤ 50%, the heterogeneity among the studies was small, and the fixed-effect model was used for meta-analysis. When p ≤ 0.1 or I2 > 50%, it indicates that there is a large heterogeneity among studies, and a random effects model is used. When the heterogeneity was large, sensitivity analysis was carried out by eliminating each study one by one to check whether the results were stable, and descriptive analysis was carried out to explore the possible sources of heterogeneity. Test level α = 0.05.
A total of 19 studies were included in this systematic review (8–26). All 17 studies were retrospective cohort studies and 2 were randomized controlled trials. The studies included a total of 23,111 cycles, of which 6,842 immediate FETs and 16,269 delayed FETs were involved. The flow chart of literature retrieval is shown in Figure 1, and the general information and quality evaluation results of the included literature are shown in Tables 1-3.
A total of 19 literatures with CPRs supported by original data were included. The combined results of these studies showed that there was no statistical significance in CPR between the immediate FET group and the delayed FET group [OR = 1.05, 95% CI (0.92–1.20), p > 0.05] (Figure 2). We believe that immediate FET is not superior to delayed FET in CPR. In addition, the included studies are highly heterogeneous. To determine the source of heterogeneity, we conducted multi-group subgroup analysis. The subgroup analysis of type of triggering (OR 0.97, 95% CI 0.81–1.15), embryo stage at transfer (OR 1.03, 95% CI 0.80–1.32), endometrial preparation (OR 1.04, 95% CI 0.82–1.31), and FET cycle following a freeze-all cycle or fresh ET failure (OR 1.02, 95% CI 0.88–1.19), did not reveal any statistical significance in CPR between the two groups (Figure 3).

Figure 2. Forest plots of the association between immediate FET and delayed FET and clinical pregnancy rates.
A total of 16 publications with original data were included. According to Figure 4, there was no statistically significant difference between the immediate and delayed FET groups on LBR [OR = 1.09, 95% CI (0.93–1.28), p = 0.31], suggesting that the immediate FET was not superior to the delayed FET in LBR. Considering the high heterogeneity, multi-group subgroup analysis was performed, and the combined result remained unchanged when subgroup analysis was performed for FET cycles following fresh ET failure and for FET cycles following freeze-all (OR 1.05, 95% CI 0.99–1.25). Similarly, subgroup analyses of type of trigger (RR 0.96, 95% CI 0.79–1.17), endometrial preparation (RR 0.97, 95% CI 0.73–1.29), and embryo stage (RR 1.12, 95% CI 0.86–1.46) did not reveal any differences (Figure 5).
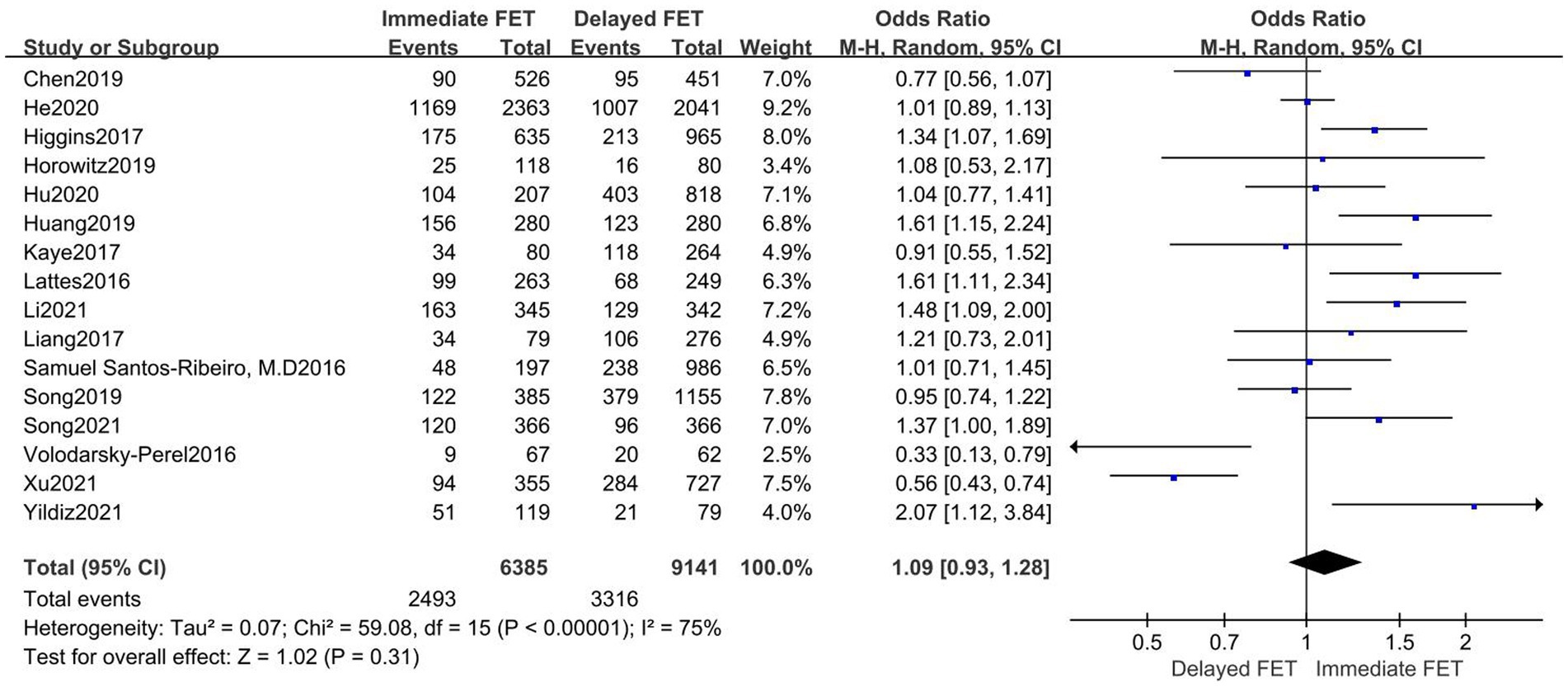
Figure 4. Forest plots of the association between immediate FET and delayed FET and live birth rate.
A total of 12 literatures were included, as shown in the forest diagram in Figure 6. The results of meta-analysis showed that there was no statistical significance (OR = 0.96, 95% CI 0.75–1.22) between immediate FET and delayed FET groups on PLR. To identify the source of heterogeneity, a multi-group subgroup analysis was performed. Type of triggering (OR 0.95, 95% CI 0.74–1.22), endometrial preparation (OR 0.90, 95% CI 0.60–1.35), and embryo stage (RR 0.96, 95% CI 0.67–1.33) were evaluated (Figure 7). However, in the subgroup analysis, after fresh ET failure, delayed FET had a higher rate of pregnancy loss than immediate FET (OR 0.62, 95% CI 0.44–0.87, see Figure 7).
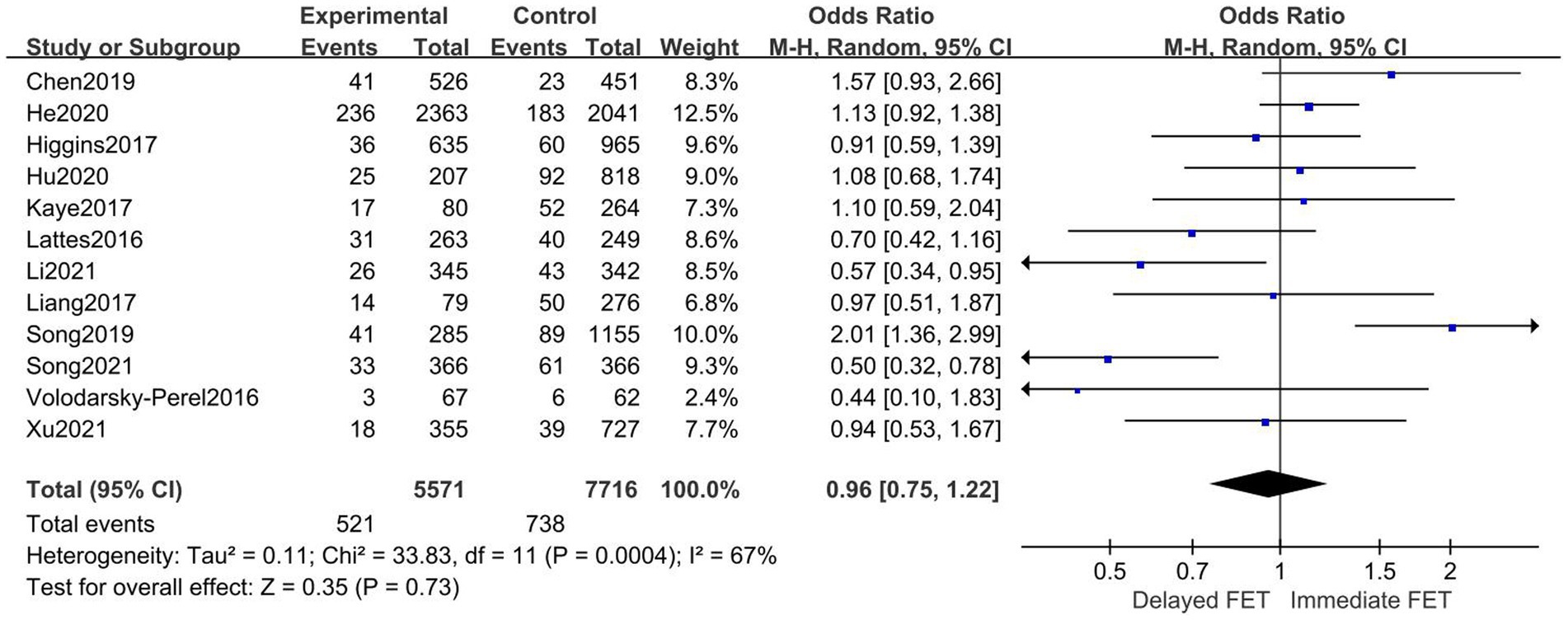
Figure 6. Forest plots of the association between immediate FET and delayed FET and pregnancy loss rate.
In this systematic review and meta-analysis, the effects of FET timing on LBR, CPR, and PLR were summarized. In general, the timing of FET, that is, whether it is performed immediately after fresh ET failure or delayed after freeze-all cycles, LBR, CPR, and PLR was not superior to immediate FET. However, in the FET cycle after fresh ET failure, the PLR with immediate FET is lower than that with delayed FET.
Out of 19 studies, our conclusions are consistent with those of 7 studies (9, 14, 15, 17, 19, 24, 25), regardless of which COS protocol is adopted. While FET is not necessary to delay a menstrual cycle after a freeze-all cycle, Yildiz et al. (24) and Hu et al. (12) both suggest delayed FET may result in a higher birth weight, preeclampsia, and macroia, which may result from the loss of corpus luteum during an artificial cycle and an extended period of isolation and freezing of embryos. On the other hand, the results of He’s et al. (9) study showed that there were no significant differences between immediate and delayed FET cycles in terms of preterm birth, gestational age, birth weight, congenital malformations and sex ratio, and that immediate FET did not improve neonatal risk, which needs more research to be confirmed.
Huang et al. (13) and Higgins et al. (10) have different conclusions with us. In their study, they found that immediate FET has a higher LBR than delayed FET. Most of the patients included in Huang’s study underwent COS with exogenous gonadotrophins by using progestin-primed ovarian stimulation (PPOS) or gonadotropin-releasing hormone agonist (GnRH-a) short protocol, and in the author’s opinion, many luteal products after COS can restore the endometrial blood vessels and improve pregnancy outcomes (13). Nevertheless, Kaye et al. (14) suggests delaying one cycle, as immediate FET cycles can indicate a dysfunctional menstrual cycle.
The optimal timing of FET after a failed fresh ET cycle is a common problem, and after subgroup studies, we found that the PLR of immediate FET after fresh ET failure was lower than that of delayed FET. A large number of follicles develop in COS, and the influence of ovarian superphysiological doses of hormones on endometrial receptivity, resulting in embryo-endometrial dissynchrony (27) may make clinicians more inclined to delay FET after fresh ET failure. However, the study by Horowitz et al. (11), Santos-Ribeiro et al. (18), Song et al. (20), Tian et al. (21), and Peng et al. (26) showed that pregnancy outcomes after fresh ET were better than those after delayed FET, whether in the modified natural cycle or hormone replacement cycle. In Song’s et al. (20) study, the frequency of moderate-to-severe depression and high stress level before FET was significantly higher in the delayed FET group than in the immediate FET group, and high stress level and high stress level had adverse effects on continued pregnancy and live birth rate (28).
In contrast, research by Volodarsky-Perel et al. (22) and Xu et al. (23) found a positive effect of delaying FETs. A long GnRH-a regimen was used by Volodarsky-Perel et al. (22), and the effects of GnRH-a on the endometrium in the ovarian hyperstimulation cycle were found to persist into adjacent menstrual cycles. There are studies showing that, after the full dose of GnRH-a is injected, the effect on the menstrual cycle can last for 11–13 weeks (29). Nevertheless, some studies have evaluated the clinical efficacy of long-acting GnRH agonists in general populations, and have identified a variety of proteins that facilitate embryo implantation in the endometrium, suggesting that long-acting agonists may enhance endometrial receptivity (30). In addition, another study showed that increased levels of GnRH-a directly modulate the expression of enzymes and cytokines and increase the expression of endometrial tolerance markers such as integrin b3 and leukaemia inhibitory factor, improving endometrial tolerance and clinical outcome in patients with intermediate and very thin endometrium (31). Xu’s et al. (23) study used clomiphene citrate (CC) + human menopausal gonadotrophin (HMG) protocol for COS. In clinical practice, CC is widely used as a first-line ovulation-promoting drug. However, due to its anti-estrogen effect, CC occupies endometrial estrogen receptors, inhibits endometrial proliferation, promotes endometrial cell apoptosis, and affects endometrial receptivity through various ways. For example, the study compared the expression of key molecules in the Wnt/β-catenin signaling pathway during the CC expulsion cycle, and CC significantly down-regulated Wnt signaling, which led to thinning of the endometrium (32). Furthermore, due to the prolonged use time of CC during the ovulation induction process, it may take longer for metabolism clearance to be completed (33). Furthermore, this study indicates that embryo implantation rates, CPRs and LBRs during the first menstrual cycle after oocyte retrieval are significantly less than those in other groups (23).
In the selected studies, ovarian hyperstimulation syndrome (OHSS) is a common and potentially risky iatrogenic complication. Especially for women with high ovarian response, the risk of acquiring OHSS is higher, and FET after embryo freezing is the most meaningful strategy for these women (34). A study of 2,060 cases found that delaying the FET cycle did not improve live birth rates in patients who cancelled ET because of high risk of OHSS (35). Patients who opt for a freeze-all policy due to OHSS may have relatively good ovarian reserve function, which may optimize the results of an immediate FET. In addition, differences in embryo quality may be a confounding factor in the comparison of clinical outcomes between the two groups, as embryos with the highest implantation potential are usually transferred first according to morphodynamic criteria, so embryos transferred mid-cycle in the delayed FET group may be of poorer quality than those in the immediate FET group.
Additionally, differences in endometrial preparation protocols between included studies, such as programmed cycle (PC) and natural cycle (NC), may have increased the risk of selection bias. To eliminate potential bias based on the type of endometrial preparation protocol for FETs, we performed a subgroup analysis of PC-FETs, but because most studies in this review were a combination of PC-FETs and NC-FETs, or PC-FETs alone, a subgroup analysis of NC-FETs was not possible. Subgroup analyses of endometrial preparation protocols revealed no significant differences between immediate and delayed PC-FET groups in LBR, CPR, and PRL. PC-FET is a better option for patients with irregular periods, amenorrhoea or poor response to ovulation induction, prolonged persistent anovulation, and recalcitrant polycystic ovary syndrome (PCOS), and PC-FET requires luteal support in the later stage and has strong operability, and patients do not need to be hospitalized for multiple monitoring. NC-FET is a safer and more natural endometrial preparation protocol, in which the timing of embryo transfer is determined by the increased production of luteinizing hormone (LH) or human chorionic gonadotropin (hCG), which induces ovulation. However, women with NC for endometrial preparation must monitor ovulation frequently, and there is a high probability of cycle cancellation, which increases the mental stress and financial costs of the patient. Despite this, studies indicate that NC-FET suffers less complications than PC protocol due to the lack of luteum (36). PC-FET significantly increases the risk of pregnancy-induced hypertension and placental implantation compared to NC-FET. In 2020, Singh et al. (37), summarized recent research on the impact of luteum on FET obstetric outcomes, highlighting the risk for preeclampsia, postpartum hemorrhage, macroia, and overdue labor associated with PC-FET without luteum production, and stating that the luteum plays a crucial role in preventing obstetric complications. In addition to luteal deficiency, Zong’s et al. (38) study found that elevated estrogen levels not only significantly suppressed vascular invasion, but also impaired trophoblast invasion and may be associated with poor maternal and neonatal outcomes. As of now, however, there is no strong evidence supporting which endometrial preparation regimen is more advantageous for women with regular menstrual cycles.
Following fresh ET failure or freeze-all cycles, it may be cumbersome and outdated to delay FET for at least one menstrual cycle in order to minimize the potential negative effects of ovarian stimulation and multiple luteum on the restoration of normal ovulation cycles and the receptive endometrium. Nevertheless, the selected literature does not provide a specific explanation for canceling fresh ET, nor does it provide any explanation for selecting immediate or delayed FET criteria, therefore, in clinical practice, it is imperative that a strict set of delayed FET criteria be established based upon the adverse conditions for immediate FET.
After the development of ART, several studies have demonstrated that the timing of FET following the cancellation of fresh ET does not have a significant impact on pregnancy outcomes. With the advancement in freeze-thaw and resuscitation technology, embryos can be preserved to the maximum extent possible and the quality of freezing and thawing can be improved. In this way, the timing of FET after fresh ET failure or the freeze-all policy has little impact on pregnancy outcomes.
In the present study, it appears that delayed FET may be unnecessary, but caution should be exercised in its interpretation. Important limitations of this review are the retrospective design, including the heterogeneity of the studies. In addition, in some studies, the existence of selection bias is obvious. No article in this systematic review specifically explained the reasons for choosing freeze-all policy instead of fresh ET, the reasons for choosing immediate FET or delayed FET, and the length of time for delayed FET. Therefore, the risk of selection bias is obvious, and the quality of studies is uneven. The results measured in this study included clinical pregnancy, live birth, and preclinical pregnancy loss. Other outcomes, such as preterm birth, birth weight, and fetal development, are not considered, which may also be affected by ovarian stimulation, and therefore by FET timing, and should therefore be considered when applying these results to clinical practice.
Overall, FET immediately or subsequently after fresh ET failure or freeze-all policy had no adverse effects on pregnancy outcomes. Due to the limited number of retrospective cohort studies evaluated, selection bias was evident, and the overall quality of the evidence was low. Therefore, delaying FET may unnecessarily delay pregnancy. Clinical decision-makers can consider patient preferences when selecting an appropriate time for FET after canceling fresh ET and menstruation. However, more future research is needed to confirm this finding.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Y-QG: Writing – original draft. J-YS: Conceptualization, Formal analysis, Writing – review & editing. Z-GS: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1335139/full#supplementary-material
1. De Geyter, C, Calhaz-Jorge, C, Kupka, MS, Wyns, C, Mocanu, E, Motrenko, T, et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open. (2020) 2020:hoz038. doi: 10.1093/hropen/hoz038
2. Cobo, A, de los Santos, MJ, Castello, D, Gamiz, P, Campos, P, and Remohi, J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. (2012) 98:1138–1146.e1. doi: 10.1016/j.fertnstert.2012.07.1107
3. Le, KD, Vuong, LN, Ho, TM, Dang, VQ, Pham, TD, Pham, CT, et al. A cost-effectiveness analysis of freeze-only or fresh embryo transfer in IVF of non-PCOS women. Hum Reprod. (2018) 33:1907–14. doi: 10.1093/humrep/dey253
4. Weinerman, R, and Mainigi, M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. (2014) 102:10–8. doi: 10.1016/j.fertnstert.2014.05.019
5. Evans, J, Hannan, NJ, Edgell, TA, Vollenhoven, BJ, Lutjen, PJ, Osianlis, T, et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. (2014) 20:808–21. doi: 10.1093/humupd/dmu027
6. Ling, H, Ling, J, and Xue, S. Survey and analysis of pregnancy pressure and related factors of pregnant women in vitro of fertilization-embryo trans-plantation. Chin Nurs Res. (2015) 29:3351–3.
7. Facchinetti, F, Tarabusi, M, and Volpe, A. Cognitive-behavioral treatment decreases cardiovascular and neuroendocrine reaction to stress in women waiting for assisted reproduction. Psychoneuroendocrinology. (2004) 29:162–73. doi: 10.1016/S0306-4530(02)00170-1
8. Chen, S, Yao, Y, Luo, Y, Mao, Y, Liu, H, Du, H, et al. Effect of the time for embryo transfer from oocyte retrieval on clinical outcomes in freeze-all cycles: a retrospective cohort study. Arch Gynecol Obstet. (2020) 301:303–8. doi: 10.1007/s00404-019-05405-4
9. He, Y, Zheng, H, Du, H, Liu, J, Li, L, Liu, H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol. (2020) 18:1. doi: 10.1186/s12958-019-0560-1
10. Higgins, C, Healey, M, Jatkar, S, and Vollenhoven, B. Interval between IVF stimulation cycle and frozen embryo transfer: is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol. (2018) 58:217–21. doi: 10.1111/ajo.12696
11. Horowitz, E, Mizrachi, Y, Farhi, J, Shalev, A, Raziel, A, and Weissman, A. Modified natural-cycle cryopreserved embryo transfer: is a washout period needed after a failed fresh cycle? Reprod Biomed Online. (2019) 39:439–45. doi: 10.1016/j.rbmo.2019.05.003
12. Hu, S, Xu, B, Long, R, and Jin, L. Pregnancy and perinatal outcomes in pregnancies resulting from time interval between a freeze-all cycle and a subsequent frozen-thawed single blastocyst transfer. BMC Pregnancy Childbirth. (2020) 20:161. doi: 10.1186/s12884-020-02858-3
13. Huang, J, Lu, X, Xie, Q, Lin, J, Cai, R, and Kuang, Y. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Transl Med. (2019) 7:752. doi: 10.21037/atm.2019.11.74
14. Kaye, L, Marsidi, A, Rai, P, Thorne, J, Nulsen, J, Engmann, L, et al. Frozen blastocyst transfer outcomes in immediate versus delayed subsequent cycles following GnRH agonist or hCG triggers. J Assist Reprod Genet. (2018) 35:669–75. doi: 10.1007/s10815-017-1111-3
15. Lattes, K, Checa, MA, Vassena, R, Brassesco, M, and Vernaeve, V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod. (2017) 32:368–74. doi: 10.1093/humrep/dew306
16. Li, H, Sun, X, Yang, J, Li, L, Zhang, W, Lu, X, et al. Immediate versus delayed frozen embryo transfer in patients following a stimulated IVF cycle: a randomised controlled trial. Hum Reprod. (2021) 36:1832–40. doi: 10.1093/humrep/deab071
17. Santos-Ribeiro, S, Polyzos, NP, Lan, VT, Siffain, J, Mackens, S, Van Landuyt, L, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod. (2016) 31:2541–8. doi: 10.1093/humrep/dew194
18. Santos-Ribeiro, S, Siffain, J, Polyzos, NP, van de Vijver, A, van Landuyt, L, Stoop, D, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril. (2016) 105:1202–1207.e1. doi: 10.1016/j.fertnstert.2015.12.140
19. Song, J, Xiang, S, and Sun, Z. Frozen embryo transfer at the cleavage stage can be performed within the first menstrual cycle following the freeze-all strategy without adversely affecting the live birth rate: a STROBE-compliant retrospective study. Medicine. (2019) 98:e17329. doi: 10.1097/MD.0000000000017329
20. Song, JY, Dong, FY, Li, L, Zhang, XX, Wang, AJ, Zhang, Y, et al. Immediate versus delayed frozen embryo transfer in women following a failed IVF-ET attempt: a multicenter randomized controlled trial. Reprod Biol Endocrinol. (2021) 19:131. doi: 10.1186/s12958-021-00819-9
21. Tian, X, Song, JY, and Sun, ZG. Effects of waiting time between failed fresh cycle embryo transfer and the next frozen embryo transfer on pregnancy outcomes in assisted reproductive technology. Int J Gynaecol Obstet. (2021) 153:248–53. doi: 10.1002/ijgo.13453
22. Volodarsky-Perel, A, Eldar-Geva, T, Holzer, HE, Schonberger, O, Reichman, O, and Gal, M. Cryopreserved embryo transfer: adjacent or non-adjacent to failed fresh long GnRH-agonist protocol IVF cycle. Reprod Biomed Online. (2017) 34:267–73. doi: 10.1016/j.rbmo.2016.11.013
23. Xu, Y-N, Li, L, and Sun, X-X. Effects of the interval between ovulation induction using clomiphene citrate and frozen embryo transfer on pregnancy outcome. Reprod Dev Med. (2021) 5:90–6. doi: 10.4103/2096-2924.320883
24. Yildiz, S, Turkgeldi, E, Kalafat, E, Keles, I, Gokyer, D, and Ata, B. Do live birth rate and obstetric outcomes vary between immediate and delayed embryo transfers following freeze-all cycles? J Gynecol Obstet Hum Reprod. (2021) 50:102224. doi: 10.1016/j.jogoh.2021.102224
25. Liang, L, Lin, H, Pan, P, and Li, Y. The “timing” of the first frozen-thawed embryo transfer after a cancelled fresh embryo transfer. J Sun Yat-sen Univ Med Sci. (2018) 39:540–547. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).20180607.004
26. Peng, C, Chen, D, and Feng, X. Effect of frozen embryo transfer immediately after IVF egg retrieval on clinical outcome. Prog Obstet Gynecol. (2019) 28:219–221. doi: 10.13283/j.cnki.xdfckjz.2019.03.014 (in Chinese).
27. Shapiro, BS, Daneshmand, ST, Garner, FC, Aguirre, M, Hudson, C, and Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertil Steril. (2011) 96:344–8. doi: 10.1016/j.fertnstert.2011.05.050
28. Janštová, Ž, Burkuš, J, Kubandová, J, Fabian, D, Koppel, J, and Čikoš, Š. The effect of maternal stress on blastocyst quality depends on maternal physiological status. Gen Physiol Biophys. (2017) 36:53–63. doi: 10.4149/gpb_2016019
29. Broekmans, FJ, Bernardus, RE, Berkhout, G, and Schoemaker, J. Pituitary and ovarian suppression after early follicular and mid-luteal administration of a LHRH agonist in a depot formulation: decapeptyl CR. Gynecol Endocrinol. (1992) 6:153–61. doi: 10.3109/09513599209015549
30. Xu, B, Geerts, D, Hu, S, Yue, J, Li, Z, Zhu, G, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod. (2020) 35:1306–18. doi: 10.1093/humrep/deaa086
31. Zaat, TR, Kostova, EB, Korsen, P, Showell, MG, Mol, F, and van Wely, M. Obstetric and neonatal outcomes after natural versus artificial cycle frozen embryo transfer and the role of luteal phase support: a systematic review and meta-analysis. Hum Reprod Update. (2023) 29:634–54. doi: 10.1093/humupd/dmad011
32. Mehdinejadiani, S, Amidi, F, Mehdizadeh, M, Barati, M, Pazhohan, A, Alyasin, A, et al. Effects of letrozole and clomiphene citrate on Wnt signaling pathway in endometrium of polycystic ovarian syndrome and healthy women dagger. Biol Reprod. (2019) 100:641–8. doi: 10.1093/biolre/ioy187
33. Hughes, E, Brown, J, Collins, JJ, and Vanderkerchove, P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Rev. (2010) 2010:CD000057. doi: 10.1002/14651858.CD000057.pub2
34. Devroey, P, Polyzos, NP, and Blockeel, C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. (2011) 26:2593–7. doi: 10.1093/humrep/der251
35. Shi, Z, and Li, N. Effect of timing of FET on pregnancy outcome after whole embryo freezing in high risk of OHS. J Reprod Med. (2021) 30:431–5. doi: 10.3969/j.issn.1004-3845.2021.04.003
36. Ginstrom Ernstad, E, Wennerholm, UB, Khatibi, A, Petzold, M, and Bergh, C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol. (2019) 221:126.e1–126.e18. doi: 10.1016/j.ajog.2019.03.010
37. Singh, B, Reschke, L, Segars, J, and Baker, VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. (2020) 113:252–7. doi: 10.1016/j.fertnstert.2019.12.007
Keywords: in vitro fertilization, frozen-thawed embryo transfer, immediate, delayed, live birth
Citation: Gao Y-Q, Song J-Y and Sun Z-G (2024) The optimal timing of frozen-thawed embryo transfer: delayed or not delayed? A systematic review and meta-analysis. Front. Med. 10:1335139. doi: 10.3389/fmed.2023.1335139
Received: 08 November 2023; Accepted: 29 December 2023;
Published: 15 January 2024.
Edited by:
Sarah M. Cohen, Hadassah Medical Center, IsraelReviewed by:
Xian Ling Cao, Fudan University, ChinaCopyright © 2024 Gao, Song and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Yan Song, aGFubGluZ2p1emVpOTFAMTI2LmNvbQ==; Zhen-Gao Sun, c3Vuemhlbmdhbzc3QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.