
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 28 November 2023
Sec. Nuclear Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1330341
This article is part of the Research Topic Case Reports in PET Imaging 2023 View all 15 articles
Background: Angiosarcoma, a rare endothelial-origin tumor, can develop throughout the body, with the head and neck skin being the most commonly affected areas. It can also originate in other sites such as the breast, iliac artery, and visceral organs including the liver, spleen, and kidneys. Angiosarcoma of the bone is remarkably rare, presenting as either unifocal or multifocal bone lesions and often leading to a grim prognosis. Diagnosing bone angiosarcoma poses a significant challenge. 18F-FDG PET/CT serves as a reliable and indispensable imaging modality for evaluating distant metastases and clinically staging angiosarcomas.
Case report: A 57-year-old woman presented with a 10-day history of dizziness and headaches. Cranial CT scan revealed bone destruction of the parietal bone, accompanied by soft tissue lesions, protruding into the epidural space. MRI examination demonstrated lesions with slightly elevated signal intensity on T2FLAIR, showing moderate enhancement. Furthermore, multiple foci were observed within the T12, L1-5, and S1-2 vertebrae, as well as in the bilateral iliac bones. For staging, 18F-FDG PET/CT was performed. The MIP PET showed multifocal FDG-avid lesions in the sternum, bilateral clavicles, bilateral scapulae, multiple ribs, and pelvic bones. Heterogeneous FDG uptake was observed in multiple bone lesions, including intracranial (SUVmax = 11.3), right transverse process of the T10 vertebra (SUVmax = 5.8), ilium (SUVmax = 3.3), and pubis (SUVmax = 4.7). The patient underwent surgical resection of the cranial lesion. The pathological diagnosis was made with a highly differentiated angiosarcoma.
Conclusion: Angiosarcoma of bone on FDG PET/CT scans is characterized by abnormal FDG uptake along with osteolytic destruction. This case highlights that angiosarcoma of bone can manifest as multicentric FDG uptake, resembling the pattern seen in multiple myeloma. FDG PET/CT can be a useful tool for staging this rare malignant tumor, offering the potential to guide biopsy procedures toward the most metabolically active site. And it should be considered in the differential diagnosis of multiple osteolytic lesions, including metastatic carcinoma, multiple myeloma, and lymphoma of bone.
Angiosarcoma is a rare endothelial-origin tumor that can develop in the whole body (1). The most commonly affected areas are the head and neck skin. Other sites where may originate include the breast, iliac artery, and visceral organs such as the liver, spleen and kidneys (2–7). Angiosarcoma of the bone is remarkably rare, constituting less than 1% of all primary bone sarcomas, predominantly occurring between the ages of 50 and 70 (8, 9). It can manifest as either unifocal or multifocal bone lesions and typically heralds a bleak prognosis (10, 11). Diagnosing angiosarcoma of the bone poses a considerable challenge and represents the malignant end of the CD31/ERG positive vascular tumors spectrum, encompassing hemangiomas, hemangioendotheliomas, as well as well-differentiated and poorly differentiated angiosarcomas (12).
Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) stands as a dependable and indispensable imaging modality for assessing distant metastases and clinically staging angiosarcomas (13). In an effort to enrich comprehension of this rare neoplasm, we present a case study delineating the 18F-FDG PET/CT imaging manifestations in a patient afflicted by multicentric angiosarcomas of the bone, marked by a challenging clinical diagnosis.
A 57-year-old woman presented with a 10-day history of dizziness and headaches. Cranial CT scan revealed bone destruction of the parietal bone, accompanied by soft tissue lesions, with the largest measuring approximately 3.6 × 2.7 × 2.0 cm, protruding into the epidural space (Figures 1A,B). MRI examination demonstrated lesions with slightly elevated signal intensity on T2FLAIR (Figure 1C), showing moderate enhancement (Figure 1D). Furthermore, multiple foci were observed within the T12, L1-5, and S1-2 vertebrae, as well as in the bilateral iliac bones. These exhibited irregular signal patterns on fat-suppressed T2WI and mild enhancement (Figures 1E,F).
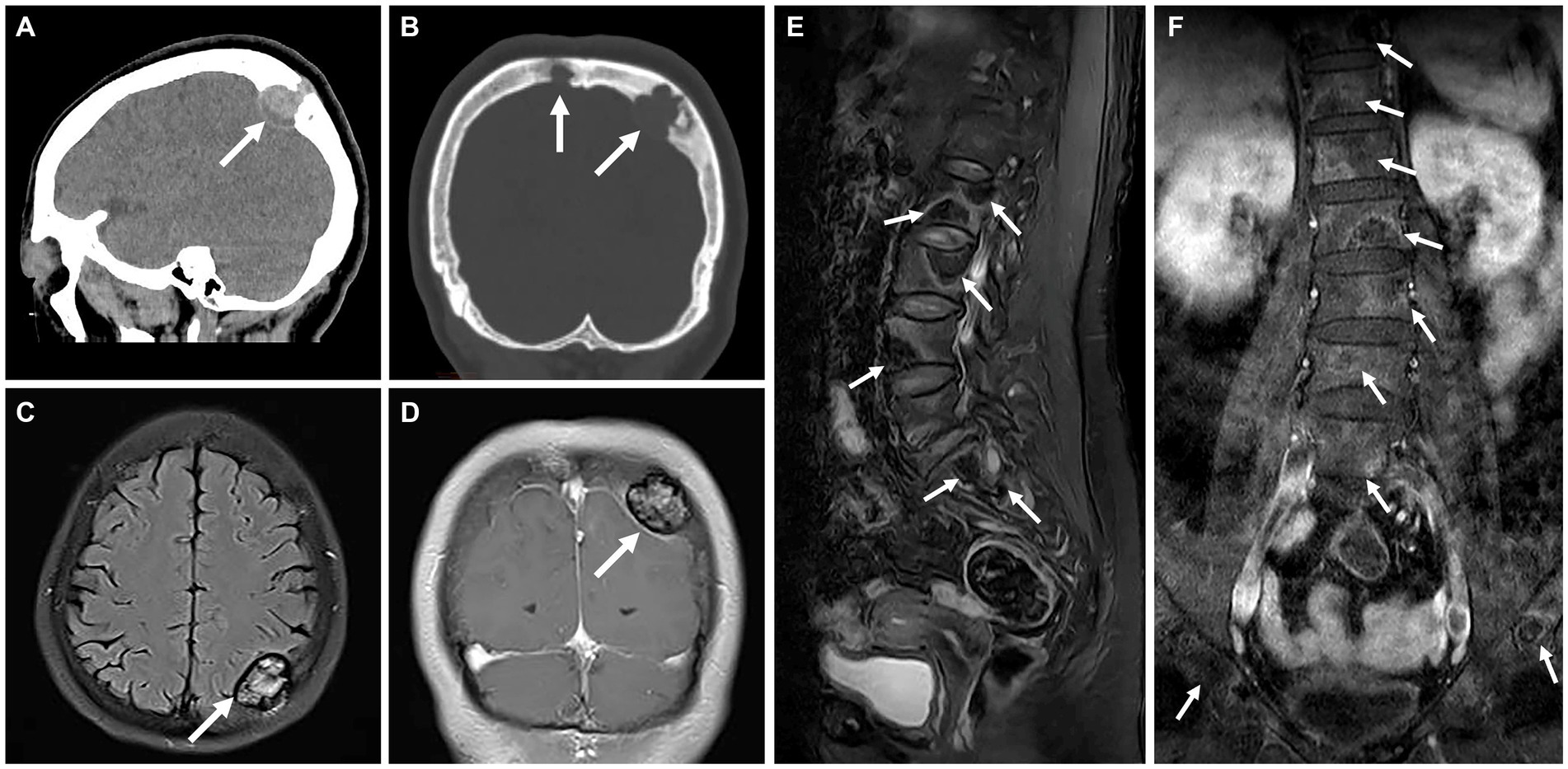
Figure 1. Computed tomography (CT) and magnetic resonance (MR) images of multicentric angiosarcomas of bone. Sagittal and coronal CT image revealed bone destruction of the parietal bone (A), accompanied by soft tissue lesions (long arrows), with the largest measuring approximately 3.6 × 2.7 × 2.0 cm, protruding into the epidural space (B). MRI examination demonstrated lesions with slightly elevated signal intensity on T2FLAIR (C, long arrows), showing moderate enhancement on Fat-sat Gd-T1WI (D). Multiple foci were observed within the T12, L1-5, and S1-2 vertebrae, as well as in the bilateral iliac bones. These exhibited irregular signal patterns on fat-suppressed T2WI (E, long arrows) and mild enhancement on Fat-sat Gd-T1WI (F).
For staging, 18F-FDG PET/CT was performed (Figures 2A–J). The MIP PET showed multifocal FDG-avid lesions in the sternum, bilateral clavicles, bilateral scapulae, multiple ribs, and pelvic bones. Sagittal images of the spine revealed the presence of multiple bone-destroying lesions, with the highest FDG uptake detected in the T6 vertebra (SUVmax = 7.6). Heterogeneous FDG uptake was observed in multiple bone lesions, including intracranial (SUVmax = 11.3), right transverse process of the T10 vertebra (SUVmax = 5.8), ilium (SUVmax = 3.3), and pubis (SUVmax = 4.7).
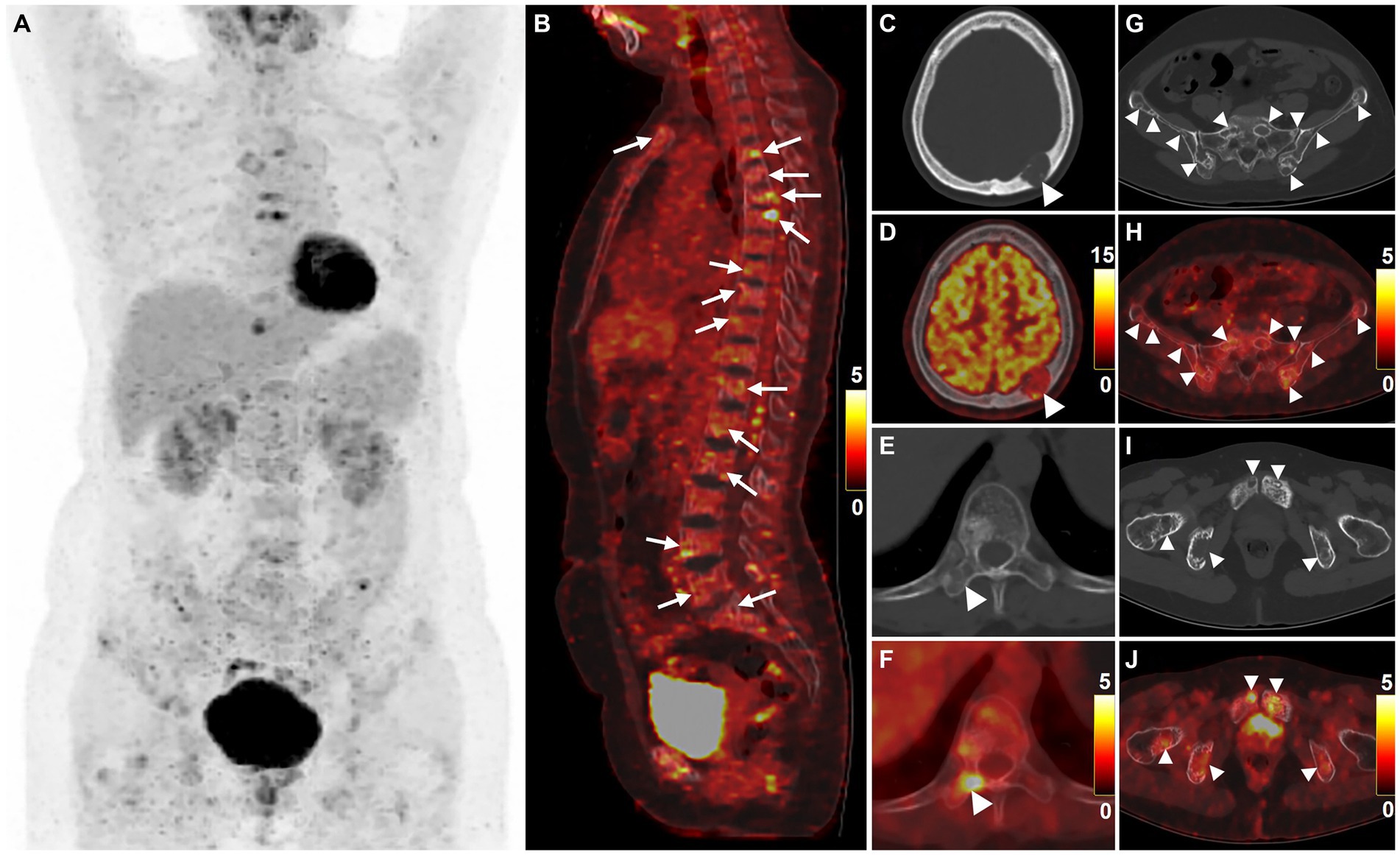
Figure 2. 18F-FDG PET/CT images of multicentric angiosarcomas of bone. The anteroposterior 3-dimensional maximum intensity projection image (MIP) revealed multifocal FDG-avid lesions in the sternum, bilateral clavicles, bilateral scapulae, multiple ribs, and pelvic bones (A). Sagittal images of the spine revealed the presence of multiple bone-destroying lesions (long arrows), with the highest FDG uptake detected in the T6 vertebra (B, SUVmax = 7.6). Heterogeneous FDG uptake was observed in multiple bone lesions (arrowheads), including intracranial (C,D, SUVmax = 11.3), right transverse process of the T10 vertebra (E,F, SUVmax = 5.8), ilium (G,H, SUVmax = 3.3), and pubis (I,J, SUVmax = 4.7).
The patient underwent a bone marrow aspiration biopsy, which showed active proliferation of bone marrow tissue and the presence of granulocyte lineage, thereby ruling out the diagnosis of multiple myeloma. Subsequently, the patient underwent surgical resection of the cranial lesion. Hematoxylin and eosin staining revealed irregular vascular channels and hyperplasia of atypical cells arranged in a diffuse sheet-like growth pattern (Figure 3A). Immunohistochemical staining showed positive expression of CD31 (Figure 3B), CD34 (Figure 3C), ERG, FLI-1 (Figure 3D), and SMA. The pathological diagnosis was made with a highly differentiated angiosarcoma.
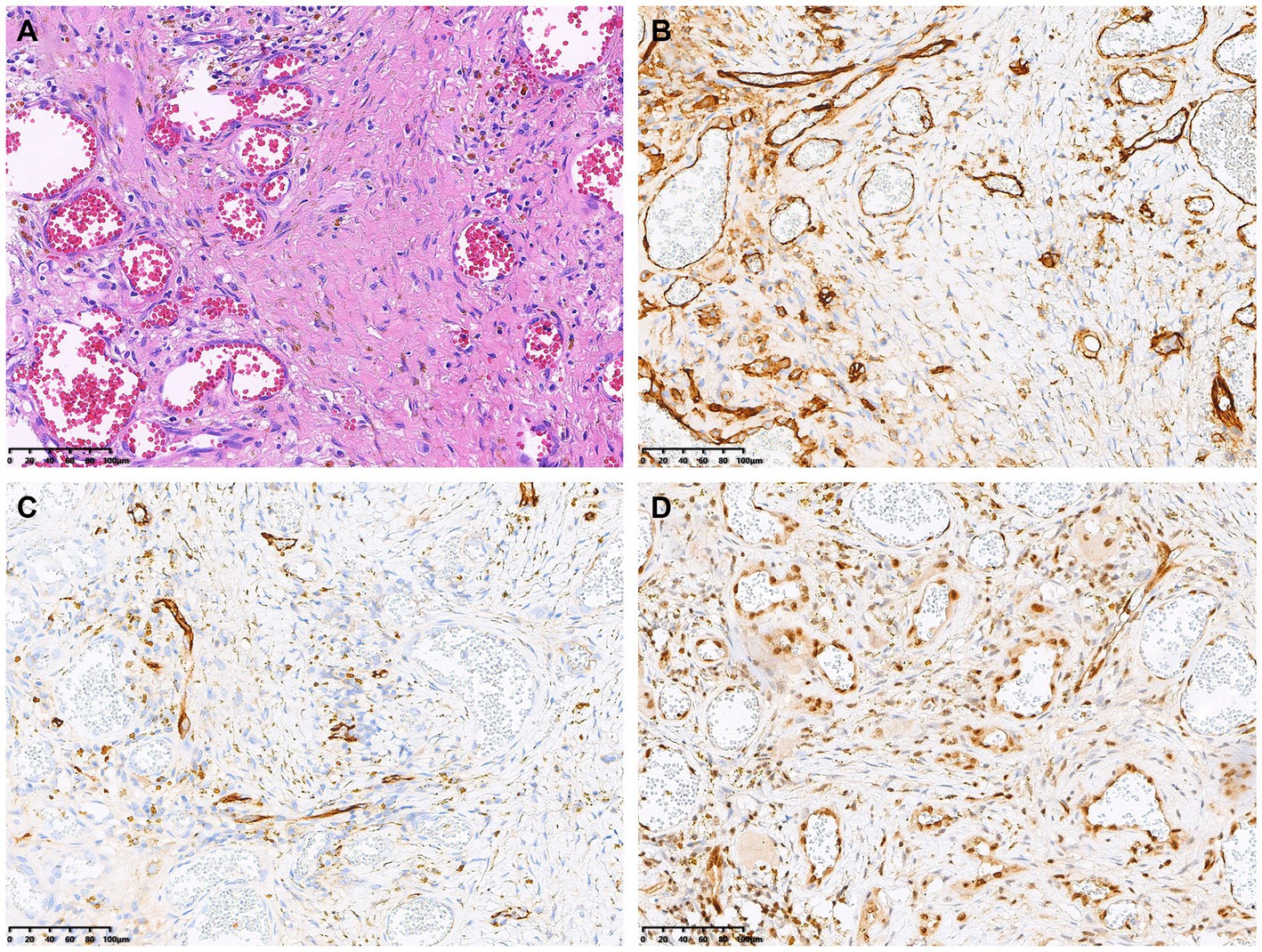
Figure 3. Histopathological and immunohistochemical images. Hematoxylin–eosin (HE) staining (magnification × 200) revealed irregular vascular channels and hyperplasia of atypical cells arranged in a diffuse sheet-like growth pattern (A). Immunohistochemistry showed that the positive expression of CD31 (B), CD34 (C) and FLI-1 (D) of the tumor cells (magnification × 200).
Angiosarcomas, highly aggressive and exceptionally rare within the spectrum of sarcomas, represent less than 1% of all sarcomas (4). These sarcomas predominantly afflict elderly men, with over 50% of cases manifesting in the cutaneous and soft tissues of the head and neck. The remaining occurrences of angiosarcomas may emerge from various sites, including the breast, soft tissues, bones, and visceral organs such as the liver and spleen (1, 4, 14, 15). Long-term sun exposure, radiation exposure, genetic predispositions, environmental factors, and trauma have been implicated in the development of angiosarcoma (16).
Vascular bone tumors encompass a spectrum of clinicopathological entities, ranging from benign hemangiomas at one end to angiosarcomas at the other (8, 17). Compared to hemangiomas, recognized as benign tumors, epithelioid hemangioendothelioma represents a low-grade malignancy, and hemangioendothelioma occupies the intermediate category, while angiosarcoma emerges as a high-grade malignancy (18). Angiosarcoma of bone may present as unifocal or multifocal disease, potentially affecting any bone. In the majority of cases, angiosarcoma of bone occurs in long bones and short tubular bones, most frequently observed in the femur, tibia, and humerus, followed by the pelvis, ribs, and vertebrae (8, 17). It can consist of multiple lesions within a single bone, affecting the same extremity, or spreading throughout the skeleton (8, 10, 11, 19). Clinical manifestations typically involve bone pain, pathologic fractures, and hypercalcemia. In our case, the sternum, bilateral clavicles, bilateral scapulae, multiple ribs, and pelvic bones demonstrated multicentricity of the tumor.
The diagnosis of angiosarcoma heavily relies on biopsy findings, with neoplastic cells demonstrating endothelial differentiation of vascular or lymphatic origin. Angiosarcomas exhibit varying histologic presentations, ranging from well-differentiated to poorly differentiated tumors. Histologic characteristics span from abnormal endothelial cells retaining some degree of well-differentiated vascular architecture to poorly differentiated sheets of abnormal cells with substantial hemorrhage and necrosis (16, 20). The most prevalent histological feature observed was the presence of intracytoplasmic vacuoles, either containing erythrocytes or empty. Additionally, the identification of three or more mitoses per 10 high-power fields (HPF), the presence of a macronucleolus, and fewer than five eosinophilic granulocytes per 10 HPF serve as prognostic risk factors. The presence of all three risk factors in a single lesion decreases the 5-year survival rate to 0% (8). Immunohistochemistry plays a vital role in identifying poorly differentiated tumors. Positive staining for the ERG endothelial marker, factor VIII, CD31, FLI-1, CD99, S-100 protein, STAT6, SMA, and the Ki-67 proliferation marker is characteristic for angiosarcomas. Expression of these markers confirms the endothelial phenotype of malignant vascular tumors. Definitive identification relies on immunohistochemistry demonstrating vascular and endothelial markers, such as CD31, CD34, FLI-1, and ERG. Our patient’s tumor displayed positivity for all these markers, with CD31 often considered the most specific marker for vascular bone tumors (8, 21, 22).
The integrated 18F-FDG PET/CT stands as the foremost functional and metabolic imaging technique, delivering clinicians with comprehensive and precise information at present (23, 24). On FDG PET scans, angiosarcoma typically exhibits increased FDG uptake, and PET/CT is a valuable tool for staging angiosarcoma and assessing distant metastases (5–7, 10, 25–29). Chen et al. (30) conducted a retrospective study involving 19 pathologically diagnosed angiosarcoma cases before treatment, examining the relationship among clinical characteristics, laboratory examinations, 18F-FDG PET/CT parameters, and the prognosis of angiosarcoma. In comparison to conventional imaging, systemic 18F-FDG PET/CT, with its high sensitivity and specificity, presents significant advantages in the evaluation of angiosarcoma, particularly in the detection of occult metastases like those in bone marrow, subcutaneous tissue, liver, and even hydrothorax and ascitic fluid. The SUVmax of angiosarcoma correlates with histopathological tumor grade (27). An increasing SUVmax is associated with a worse prognosis, can be a valuable noninvasive prognostic marker (6). Lee et al. (28) observed that the degree of FDG uptake, as reflected by SUVmax, served as a prognostic indicator for patients with vascular tumors. Using a cutoff SUVmax of 3.0, the 2-year progression-free survival rate was notably higher in the 14 patients with a tumor SUVmax <3.0 (75.0%) compared to the 12 patients with a tumor SUVmax ≥3.0 (0%) (p = 0.0053). In a study of 16 angiosarcoma cases by Kato et al. (27), higher SUVmax, MTV, whole-body TLG, TBR, and whole-body TLG ratio significantly correlated with poorer overall survival in patients evaluated by 18F-FDG PET/CT before treatment. Umemura et al. (29) conducted an early prognosis assessment of 18 cases of cutaneous angiosarcoma using PET/CT, noting that those with higher SUVmax at the initial diagnosis experienced a markedly poorer prognosis compared to those with a lower SUVmax.
The prognosis of angiosarcomas is notably poor (19), with frequent occurrences of both local recurrence and distant metastasis (31, 32), typically resulting in a reported 5-year survival rate of approximately 31–33% (8, 33). Angiosarcoma manifesting in bone exhibits a significantly bleaker prognosis compared to general angiosarcoma, with a 5-year survival rate of 20% and an approximate median survival time of 10 months (34, 35). Wang et al. (11) observed that the prognosis of malignant bone angiosarcoma was notably worse than that of malignant vascular tumors, with a median overall survival (OS) of merely 9 months. Age, stage, and the utilization of surgery stand as independent prognostic factors for patients with bone angiosarcoma (11, 36, 37). Managing angiosarcoma poses a considerable clinical challenge due to both the aggressive nature of the tumor and the limited evidence guiding treatment modalities and agents. Treatment strategies for angiosarcomas are contingent on the stage and location. Surgical resection remains the primary therapeutic approach for localized disease, although achieving negative margins can be challenging due to the infiltrative nature of the ailment. A multidisciplinary approach, involving surgery, radiation, chemotherapy, and potentially recent immune-oncology agents, can yield positive outcomes (38). Studies have suggested that bone angiosarcoma treated with surgery alone or in combination with radiotherapy exhibits better outcomes compared to cases treated with radiotherapy alone or without therapy. Due to the endothelial origin of angiosarcomas, there is a growing interest in employing antiangiogenic agents for this tumor. Research has explored various agents, including bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor-A; TRC105, a monoclonal antibody against endoglin; trebananib, a neutralizing peptibody to angiopoietin-1/2; and vascular endothelial growth factor receptor inhibitors such as pazopanib, sorafenib, and axitinib (16).
In conclusion, we offer a case study detailing the 18F-FDG PET/CT imaging manifestations in a patient affected by multicentric angiosarcomas of the bone. Angiosarcoma of bone on FDG PET/CT scans is characterized by abnormal FDG uptake along with osteolytic destruction. This case highlights that angiosarcoma of bone can manifest as multicentric FDG uptake, resembling the pattern seen in multiple myeloma. FDG PET/CT can be a useful tool for staging this rare malignant tumor, offering the potential to guide biopsy procedures toward the most metabolically active site. And it should be considered in the differential diagnosis of multiple osteolytic lesions, including metastatic carcinoma, multiple myeloma, and lymphoma of bone.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
WH: Writing – original draft, Writing – review & editing. XX: Data curation, Writing – review & editing. YZ: Supervision, Writing – original draft. YP: Data curation, Formal analysis, Writing – original draft. LS: Data curation, Writing – original draft. LL: Data curation, Writing – review & editing. JG: Supervision, Writing – review & editing. LK: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82171970), the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), the Beijing Municipal Science & Technology Commission (Z221100007422027), National High Level Hospital Clinical Research Funding (Interdisciplinary Research Project of Peking University First Hospital, 2023IR17).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lahat, G, Dhuka, AR, Hallevi, H, Xiao, L, Zou, C, Smith, KD, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. (2010) 251:1098–106. doi: 10.1097/SLA.0b013e3181dbb75a
2. Wang, R, Wang, X, Ji, B, Guan, Q, and Chen, B. Primary common iliac artery Angiosarcoma with multiple bone metastases revealed by dual-time point FDG PET/CT imaging. Clin Nucl Med. (2019) 44:232–3. doi: 10.1097/RLU.0000000000002435
3. Cheng, J, and Ou, X. Prominent bone marrow metastases without concurrent intra-chest metastasis in a case of cardiac Angiosarcoma. Clin Nucl Med. (2020) 45:638–9. doi: 10.1097/RLU.0000000000003130
4. Florou, V, and Wilky, BA. Current Management of Angiosarcoma: recent advances and lessons from the past. Curr Treat Options in Oncol. (2021) 22:61. doi: 10.1007/s11864-021-00858-9
5. Zheng, K, Liu, Y, Cui, R, and Li, F. Primary spleen Angiosarcoma with concomitant hepatic hemangiomas on 18F-FDG PET/CT. Clin Nucl Med. (2018) 43:222–3. doi: 10.1097/RLU.0000000000001982
6. Cassou-Mounat, T, Champion, L, Bozec, L, Laurence, V, Huchet, V, Luporsi, M, et al. Primary and secondary breast Angiosarcoma: FDG PET/CT series. Clin Nucl Med. (2019) 44:e33–5. doi: 10.1097/RLU.0000000000002334
7. Yang, J, Dong, A, Nian, S, Peng, Y, and Zuo, C. FDG PET/CT in a case of primary Angiosarcoma of the kidney. Clin Nucl Med. (2023) 48:370–2. doi: 10.1097/RLU.0000000000004586
8. Verbeke, SLJ, Bertoni, F, Bacchini, P, Sciot, R, Fletcher, CDM, Kroon, HM, et al. Distinct histological features characterize primary angiosarcoma of bone. Histopathology. (2011) 58:254–64. doi: 10.1111/j.1365-2559.2011.03750.x
9. Matti, A, Farolfi, A, Frisoni, T, Fanti, S, and Nanni, C. FDG-PET/CT guided biopsy in Angiosarcoma of bone: diagnosis. Staging and Beyond Clin Nucl Med. (2018) 43:e48–9. doi: 10.1097/RLU.0000000000001918
10. Li, F, and Ou, X. A low-grade bone angiosarcoma presented as low to mild 18F-FDG uptake mimicking multiple myeloma. Hell J Nucl Med. (2020) 23:360–1. doi: 10.1967/s002449912217
11. Wang, B, Chen, L-J, and Wang, X-Y. A clinical model of bone Angiosarcoma patients: a population-based analysis of epidemiology, prognosis, and treatment. Orthop Surg. (2020) 12:1652–62. doi: 10.1111/os.12803
12. Palmerini, E, Leithner, A, Windhager, R, Gosheger, G, Boye, K, Laitinen, M, et al. Angiosarcoma of bone: a retrospective study of the European musculoskeletal oncology society (EMSOS). Sci Rep. (2020) 10:10853. doi: 10.1038/s41598-020-66579-5
13. Ariga, A, Matsumoto, S, Tanizawa, T, Hayakawa, K, Minami, Y, Saito, M, et al. Bone metastases with “false negative” findings on 18F-FDG PET/CT in patients with angiosarcoma: a case series with literature review. Medicine (Baltimore). (2023) 102:e34196. doi: 10.1097/MD.0000000000034196
14. Scholsem, M, Raket, D, Flandroy, P, Sciot, R, and Deprez, M. Primary temporal bone angiosarcoma: a case report. J Neuro-Oncol. (2005) 75:121–5. doi: 10.1007/s11060-005-0375-0
15. Conic, RRZ, Damiani, G, Frigerio, A, Tsai, S, Bragazzi, NL, Chu, TW, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population-based study. J Am Acad Dermatol. (2020) 83:809–16. doi: 10.1016/j.jaad.2019.07.024
16. Bernstock, JD, Shafaat, O, Hardigan, A, Fox, BM, Moore, LS, Chagoya, G, et al. Angiosarcoma of the temporal bone: case report and review of the literature. World Neurosurg. (2019) 130:351–7. doi: 10.1016/j.wneu.2019.07.107
17. Baliaka, A, Balis, G, Michalopoulou-Manoloutsiou, E, Papanikolaou, A, and Nikolaidou, A. Primary angiosarcoma of bone. A case report. Hippokratia. (2013) 17:180–2.
18. Boriani, S, Cecchinato, R, Righi, A, Bandiera, S, Dei Tos, AP, Ghermandi, R, et al. Primary vascular bone tumors in the spine: a challenge for pathologists and spine oncology surgeons. Eur Spine J. (2019) 28:1502–11. doi: 10.1007/s00586-019-05930-5
19. Marthya, A, Patinharayil, G, Puthezeth, K, Sreedharan, S, Kumar, A, and Kumaran, CM. Multicentric epithelioid angiosarcoma of the spine: a case report of a rare bone tumor. Spine J. (2007) 7:716–9. doi: 10.1016/j.spinee.2006.08.013
20. Hammon, RJ, Lin, HW, Sadow, PM, Lin, DT, and Rocco, JW. Pathology quiz case 1. Angiosarcoma. Arch Otolaryngol Head Neck Surg. (2011) 137:198–201. doi: 10.1001/archoto.2010.247-a
21. Young, RJ, Brown, NJ, Reed, MW, Hughes, D, and Woll, PJ. Angiosarcoma. Lancet Oncol. (2010) 11:983–91. doi: 10.1016/S1470-2045(10)70023-1
22. O’Neill, JP, Bilsky, MH, and Kraus, D. Head and neck sarcomas: epidemiology, pathology, and management. Neurosurg Clin N Am. (2013) 24:67–78. doi: 10.1016/j.nec.2012.08.010
23. Donnelly, SC. 18F-FDG-PET/CT scanning-clinical usefulness beyond cancer. QJM. (2018) 111:593. doi: 10.1093/qjmed/hcy184
24. Dammacco, F, Rubini, G, Ferrari, C, Vacca, A, and Racanelli, V. 18F-FDG PET/CT: a review of diagnostic and prognostic features in multiple myeloma and related disorders. Clin Exp Med. (2015) 15:1–18. doi: 10.1007/s10238-014-0308-3
25. Yang, Z, Tao, H, Ye, Z, and Yang, D. Multicentric epithelioid angiosarcoma of bone. Orthopedics. (2012) 35:e1293–6. doi: 10.3928/01477447-20120725-39
26. Takahashi, H, Hara, T, Suzuki, H, Hashimoto, R, and Minami, M. FDG-PET/CT demonstrates splenic Angiosarcoma bone marrow metastasis. Clin Nucl Med. (2020) 45:e20–3. doi: 10.1097/RLU.0000000000002717
27. Kato, A, Nakamoto, Y, Ishimori, T, Saga, T, and Togashi, K. Prognostic value of quantitative parameters of 18F-FDG PET/CT for patients with Angiosarcoma. AJR Am J Roentgenol. (2020) 214:649–57. doi: 10.2214/AJR.19.21635
28. Lee, WW, So, Y, Kang, SY, So, M-K, Kim, H, Chung, HW, et al. F-18 fluorodeoxyglucose positron emission tomography for differential diagnosis and prognosis prediction of vascular tumors. Oncol Lett. (2017) 14:665–72. doi: 10.3892/ol.2017.6192
29. Umemura, H, Yamasaki, O, Kaji, T, Hamada, T, Otsuka, M, Asagoe, K, et al. Prognostic value of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with cutaneous angiosarcoma: a retrospective study of 18 cases. J Dermatol. (2017) 44:1046–9. doi: 10.1111/1346-8138.13839
30. Chen, D, Tang, M, Lv, S, Wang, H, Du, W, Zhao, X, et al. Prognostic usefulness of clinical features and pretreatment 18F-FDG PET/CT metabolic parameters in patients with angiosarcoma. Quant Imaging Med Surg. (2022) 12:2792–804. doi: 10.21037/qims-21-563
31. Germans, SK, and Weinberg, OK. Metastatic angiosarcoma in a bone marrow biopsy. Blood. (2022) 140:2001. doi: 10.1182/blood.2022018036
32. Wang, C, Rabah, R, Blackstein, M, and Riddell, RH. Bone marrow metastasis of angiosarcoma. Pathol Res Pract. (2004) 200:551–5. doi: 10.1016/j.prp.2004.05.003
33. Fury, MG, Antonescu, CR, Van Zee, KJ, Brennan, MF, and Maki, RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. (2005) 11:241–7. doi: 10.1097/00130404-200505000-00011
34. Palmerini, E, Maki, RG, Staals, EL, Alberghini, M, Antonescu, CR, Ferrari, C, et al. Primary angiosarcoma of bone: a retrospective analysis of 60 patients from 2 institutions. Am J Clin Oncol. (2014) 37:528–34. doi: 10.1097/COC.0b013e31827defa1
35. Righi, A, Sbaraglia, M, Gambarotti, M, Gibertoni, D, Rovira, MP, Benini, S, et al. Primary vascular tumors of bone: a Monoinstitutional morphologic and molecular analysis of 427 cases with emphasis on epithelioid variants. Am J Surg Pathol. (2020) 44:1192–203. doi: 10.1097/PAS.0000000000001487
36. Lee, KC, Chuang, S-K, Philipone, EM, and Peters, SM. Characteristics and prognosis of primary head and neck Angiosarcomas: a surveillance, epidemiology, and end results program (SEER) analysis of 1250 cases. Head Neck Pathol. (2019) 13:378–85. doi: 10.1007/s12105-018-0978-3
37. Wang, W, Hong, J, Meng, J, Wu, H, Shi, M, Yan, S, et al. Survival analysis of patients with osseous malignant vascular tumors: results of the surveillance, epidemiology, and end results (SEER) database from 1973 to 2015. Med Sci Monit. (2019) 25:5525–35. doi: 10.12659/MSM.914950
Keywords: bone, angiosarcoma, magnetic resonance imaging, computed tomography, 18F-FDG PET/CT, case report
Citation: Huang W, Xiao X, Zhang Y, Peng Y, Song L, Li L, Gao J and Kang L (2023) Case Report: A rare case of multicentric angiosarcomas of bone mimicking multiple myeloma on 18F-FDG PET/CT. Front. Med. 10:1330341. doi: 10.3389/fmed.2023.1330341
Received: 30 October 2023; Accepted: 15 November 2023;
Published: 28 November 2023.
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Corinna Altini, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, ItalyCopyright © 2023 Huang, Xiao, Zhang, Peng, Song, Li, Gao and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Kang, a2FuZ2xlaUBiam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.