
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 27 December 2023
Sec. Dermatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1326359
 Francesco Moro1,2*†
Francesco Moro1,2*† Jo Linda Maria Sinagra1,2†
Jo Linda Maria Sinagra1,2† Adele Salemme1
Adele Salemme1 Luca Fania2
Luca Fania2 Feliciana Mariotti1
Feliciana Mariotti1 Anna Pira1
Anna Pira1 Biagio Didona3
Biagio Didona3 Giovanni Di Zenzo1
Giovanni Di Zenzo1Pemphigus is a life-threatening autoimmune blistering disease affecting skin and mucous membranes. Despite its etiopathogenesis remains largely unknown, several trigger and predisposing factors have been reported. Pemphigus is caused by autoantibodies that target desmoglein 1 and desmoglein 3, impacting desmosome function. However, circulating autoantibodies are often the consequence of a precipitating factor that occurs in predisposed individuals. This review aims to describe and discuss almost all trigger and predisposing factors reported as possible or probable cause of the disease. Among the reported trigger factors that may induce or exacerbate pemphigus, we have found of particular interest: drug intake (especially thiol- and phenol-containing compounds), vaccines, infections, as well as some reports about pregnancy, radiations, emotional stress, pesticides and physical trauma. Moreover, we discuss the possible role of food intake in pemphigus onset and particular attention is given to dietary factors containing thiol, phenol and tannin compounds. A trigger factor is “the straw that breaks the camel’s back,” and often acts together with predisposing factors. Here we discuss how pemphigus onset may be influenced by genetic susceptibility and comorbidities like thyroid diseases, malignancies and other autoimmune disorders.
To identify other hitherto unknown trigger and predisposing factors, well designed prospective studies are needed. In this context, future research should explore their connection with the aim to advance our understanding of pemphigus pathogenesis.
Pemphigus disease, including pemphigus vulgaris (PV) and pemphigus foliaceous (PF), belongs to the intraepithelial autoimmune bullous disease (AIBD) group, affecting the skin and mucous membranes. Pemphigus is provoked by an altered desmosome function due to deposition of autoantibodies (autoAbs) directed against desmosomal components: desmoglein (Dsg) 3 and 1, leading to the acantholysis. PV is a potentially life-threatening disease characterized by flaccid cutaneous or mucosal bullae, that easily break and form painful erosions. In PV pathogenesis, autoAbs are directed against Dsg3 or both Dsg3 and Dsg1. PF is a less severe form of pemphigus. In PF the autoAbs are directed to Dsg1 which is localized throughout the epidermis. Dsg3 is expressed in basal and suprabasal layers of keratinocytes and compensates for detachment induced by anti-Dsg1 autoAbs that leads to loss of adhesion in the upper layers. As a consequence, PF is clinically characterized by crusty sores that often begin on the scalp and may also interest the chest, back, and face, while mucous membranes are frequently not involved. On the contrary in PV with only anti-Dsg3 antibodies blister formation occurs deep in the mucous membranes, where Dsg1 does not compensate for loss of Dsg3-mediated adhesion. While in case of developing of both anti-Dsg1 and anti-Dsg3 antibodies, the function of both Dsgs is affected and blisters develop in the skin and mucous membranes (1). Diagnosis is based on clinical assessment, histopathological examination and intercellular deposits of IgG and/or C3 by direct immunofluorescence. Circulating autoAbs assessed by indirect immunofluorescence and/or Dsg3 and/or Dsg1 ELISA have a confirmatory value.
The role of anti-Dsg autoantibodies in PV pathogenesis has been largely demonstrated. Titers and profiles of anti-Dsg antibodies have been correlated with disease activity and clinical phenotype. In addition, passive transfer of IgG from pemphigus patients’ results in pemphigus-like lesions in mice. Moreover, adoptive transfer of splenocytes from Dsg3-knockout mice immunized with murine Dsg3 induced PV phenotype into immunodeficient mice (2). An autoreactive B cell response mainly directed to Dsg 1 and Dsg3 is sustained by a T cell response that is also crucial for pemphigus onset (3). However, the mechanism behind the loss of B and T-cell tolerance is not completely understood so far (4).
Pemphigus is a prototype of an organ-specific autoimmune disease and most agents that favor immune system stimulation may be susceptible to provoke the disease in genetically predisposed individuals.
The etiopathogenesis of pemphigus is largely unknown. Several trigger factors have been described to induce or exacerbate pemphigus, such as drugs, vaccines, pregnancy, radiations, emotional stress, infections, diet or other external factors. A trigger factor is “the straw that breaks the camel’s back,” and acts together with predisposing factors, such as genetic susceptibility and comorbidities.
The aim of the present review is to highlight the trigger and predisposing factors possibly involved in the etiopathogenesis of this AIBD.
Some trigger factors reported in this review are not based on enough evidence, but we choose to report them to possibly inspire other studies that could confirm or disprove their possible role in the onset of pemphigus.
To identify other hitherto unknown trigger and predisposing factors well designed prospective studies should be conducted.
Drugs are considered the most common trigger factors for pemphigus disease (5). They could be divided into three groups according to their dominant chemical structure: thiol drugs, phenol drugs, and non-thiol/non-phenol drugs (5). In a systematic review conducted on 170 patients, the most reported drugs related to pemphigus onset are penicillamine (33.1%), captopril (7.7%), and bucillamine (6.5%). Other involved drugs are: ingenol mebutate, cilazapril, metamizole (dipyrone), imiquimod, penicillin, fosinopril, diazinon, glibenclamide, carbamazepine, lisinopril, nifedipine, rifampin, gold sodium thiomalate, ceftazidime, chloroquine/hydroxychloroquine (4–28) (Table 1). Thiol drugs contain a sulfhydryl group (-SH), and are the most common medications inducing pemphigus. These drugs induce acantholysis by different pathways. On one side the sulfhydryl group involves keratinocytes in a disulfide bond, altering membrane adhesion. On the other one, they activate proteolytic enzymes such as plasmin and inhibit enzymes that promote keratinocytes adhesion (29). Moreover, interacting with Dsg 1 and 3, they could form a neo-antigen, promoting autoimmune response. The most important thiols reported are penicillamine, captopril, and tiopronine (29) (Figure 1).
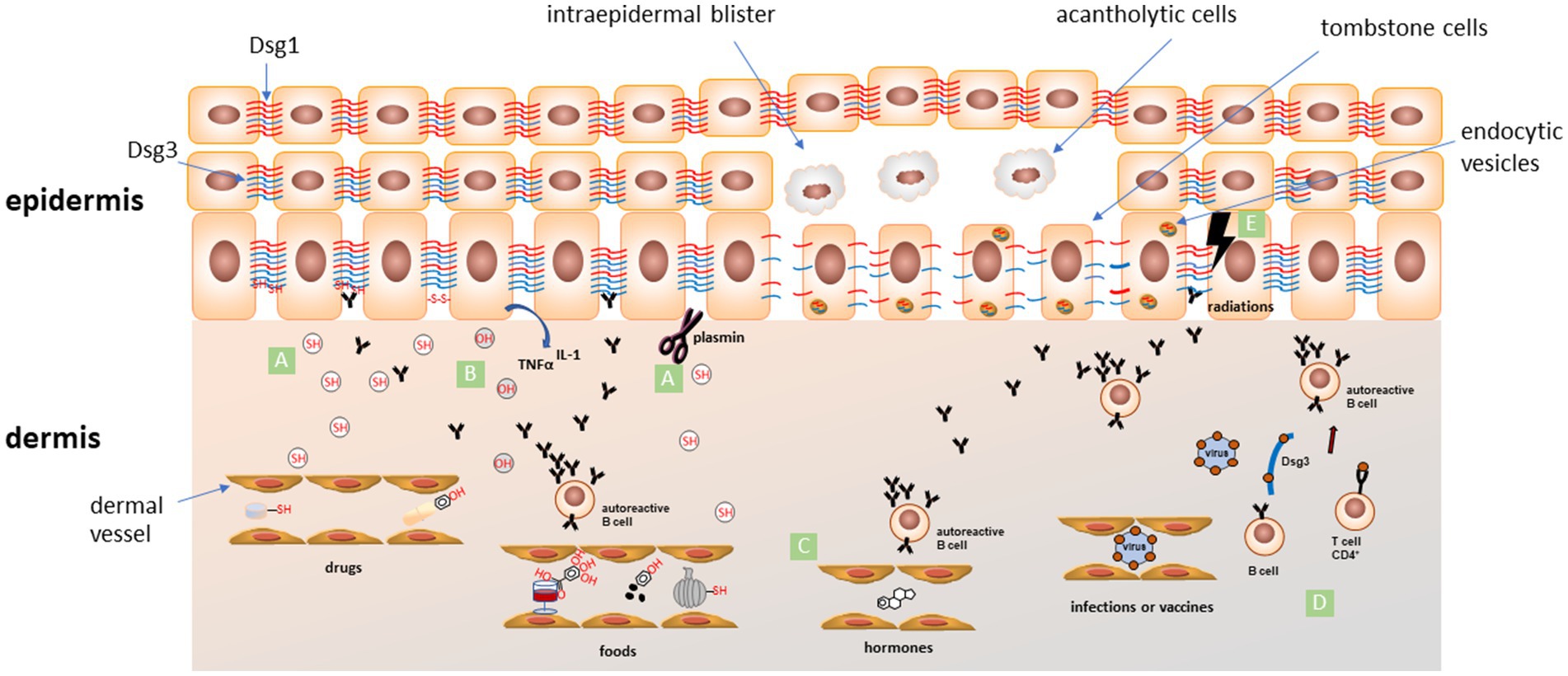
Figure 1. Schematic representation of proposed mechanisms for the most accepted pemphigus trigger factors. (A) The thiol-containing drugs and foods (SH) can induce acantholysis by different pathways: (i) interacting with Dsg 1 and 3, they could form neo-epitopes, promoting an autoimmune response; (ii) sulfhydryl groups involve keratinocytes in a disulfide bond altering membrane adhesion; (iii) activation of proteolytic enzymes such as plasmin that could directly cleave adhesion proteins or indirectly inhibit adhesion process; (B) phenolic compounds (OH) in drugs and foods can induce the release of pro-inflammatory cytokines like TNF-α and IL-1 from keratinocytes. These cytokines are involved in recruitment of immune cells and activation of plasminogen and other proteases involved in the acantholytic process; (C) estrogens levels during pregnancy or menstrual cycle can favor an alteration in Th1/Th2 balance toward a Th2 response, followed by an increase of IgG autoAbs production; (D) an immune response targeting microbial antigens coming from infections or generated after vaccination can cross-react (molecular mimicry) with some epitopes of endogenous molecules (such as Dsg3) leading to activation of CD4+ T cells and initiation of the autoimmune cascade; (E) ionizing radiations could alter skin antigen expression, unmasking hidden epitopes and inducing an autoimmune response in predisposed individuals. All these factors can interfere with normal Dsgs network, induce endocytosis of Dsgs (endocytic vesicles) and activate different intracellular signalling pathways leading to loss of keratinocyte adhesion and acantholysis. Dsg1 in red, Dsg3 in blue.
The chemical structure of phenol drugs is based on an aromatic hydrocarbon group bonded with a hydroxyl group (-OH). These drugs induce the release of pro-inflammatory cytokines like tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1 from keratinocytes. These cytokines are involved in the activation of plasminogen and other proteases and complement, all involved in the acantholytic process (Figure 1). The most important phenol compounds triggering pemphigus are aspirin, heroin, rifampin, and levodopa (29, 30).
Non-thiol/non-phenol drugs could also induce pemphigus through different signalling pathways that include antigen modification, autoAbs induction or immunomodulation. The most noteworthy non thiol/non phenol drugs are non-steroidal anti-inflammatory drugs, and calcium-channel blockers (29).
Drugs can also induce pemphigus through the production of immunoglobulin (Ig) G autoAb against Dsg-1 and 3, provoking an immunologic acantholysis (31).
It has been reported that thiol-containing drugs are more often associated with PF while non-thiol drugs with PV (32). Yoshimura and coworkers in a clinical and histopathological study on17 patients with drug-induced pemphigus, found that most of them showed a PF-type phenotype with antiDsg-1 autoAbs, caused by thiol-containing drugs (33). A paradoxical reaction with disease exacerbation after treatment with rituximab has been reported, but more studies are needed to better elucidate this finding (34, 35).
Hayashida and coworkers reported a drug-induced pemphigus in a patient affected by rheumatoid arthritis treated with secukinumab, an anti-IL17 monoclonal antibody (mAb), then exacerbated after treatment with tocilizumab, an IL-6 receptor antagonist, both belonging to the non-thiol and non-phenol-group (36).
In 2018 Palleria and coworkers reported 3 cases of PV following treatment with ramipril, losartan, irbesartan and hydrochlorothiazide, drugs belonging to the families of angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers. ACEis represent the drugs most frequently associated with PV development (Table 1). Since 1980, a large number of PVs following therapy with ACEi and angiotensin receptor blockers have been reported. In particular, captopril seems to be the most involved, probably because of the sulfhydril group within its molecular structure. Other drugs involved are enalapril, lisinopril, benazepril hydrochloride, fosinopril sodium, quinapril hydrochloride, and ramipril (37) (Table 1).
In 2017, Zhou and colleagues documented a case of PV following treatment with 5-aminolaevulinic acid-based photodynamic therapy (ALA-PDT). While the role of UV radiation in the PV onset has been recognized for some time, this represents, to the best of our knowledge, the first report of PV development after ALA-PDT therapy (38) (Table 1). A case of imiquimod-induced PV has recently been reported. Imiquimod, as toll-like receptor-7 agonist, might induce pemphigus by stimulating dendritic cells and keratinocytes in overproduction of interferon (IFN)-alpha with consequent induction and maintenance of autoreactive B-cells (39).
Furthermore, in women with pemphigus a significantly higher use of oral contraception has been reported compared to the control group (40). This could be explained by the activation of some pathogenic signaling pathways by oral contraceptives. On the other hand, women with pemphigus could use more oral contraceptives compared to healthy individuals to prevent risky pregnancies (5).
Alternative medicine could be also a trigger for pemphigus. Yoo and coworkers reported a case of PF developed after a bee-venom acupuncture treatment and supposed that immunological stimulation by bee venom could have induced PF (41) (Table 1).
Different vaccines against influenza, hepatitis B, rabies, and tetanus have been reported to induce or exacerbate pemphigus. However, considering the higher risk of infection in these patients and the immunosuppressive therapy necessary to treat the disease, vaccination against seasonal influenza, H1N1, tetanus, and pneumococci is still recommended (42–47).
In this context, vaccines against coronavirus disease (COVID)-19 have also been reported to trigger pemphigus. A recent review by Pira and coworkers shows that in the past 3 years, since the start of COVID-19 vaccinations, more than 15 cases of vaccine-induced PV and more than 7 cases of vaccine-induced PF have been reported. Patients of both genders, ages 30 to 89, developed pemphigus 5 to 30 days after the first or second dose of vaccine. The involved vaccines are Comirnaty (BNT162b2), Vaxzevria, Spikevax, ChAdOx1 nCoV-19 vaccine and Sinopharm COVID-19 (BBIBP-CorV). It could be hypothesized that the vaccine acts as a precipitating factor inducing autoimmunity in genetically susceptible individuals by stimulating pre-existing and subclinical autoreactivity against PV targets (48) (Table 1).
Pregnancy could be a trigger factor for pemphigus (49) (Table 1). Many cases of flare-up during pregnancy followed by a remission after delivery have been described (50). This could be explained by the rapid increase in estrogens levels during pregnancy, that favour an alteration in Th1/Th2 balance toward a Th2 response, followed by an increase of IgG autoAbs production (Figure 1). It is very important to manage this autoimmune disease during pregnancy and to prevent its onset in genetically predisposed patients (51). Indeed, control of disease activity, the choice of an appropriate treatment with reasonable few side effects, the strict follow-up of serological parameters and of clinical manifestations, and the study of genetic predisposition are crucial points to guarantee a safe pregnancy in pemphigus female patients.
Development of pemphigus lesions after radiotherapy has been described (Table 1). In a recent case–control study on 365 patients with AIBDs, Hung and coworkers found that 53.4% of the cases had an exposure to radiotherapy before skin disease onset vs. 33.1% of controls, and that this relation was particularly strong in patients with breast cancer (OR 2,986) (52). Unfortunately, authors do not differentiate PV from bullous pemphigoid patients, and do not report specific data about the association between radiotherapy and PV.
Until now, only 30 anecdotal cases were described (53). In reported cases, which have ages ranging from 37 to 92 years (median age: 62 years), lesions seem to appear within variable times (1 week to a year, mean time 3 months) after radiotherapy (52, 54). In 90% of patients lesions appear on irradiated area, and progress to non-irradiated skin in 80% (53). No relationship with radiation dose was observed, as the minimum reported inducing dose was 38G (54). As a pathogenetic hypothesis, ionizing radiation could alter skin antigen expression, unmasking certain epitopes, thus inducing autoimmune response in predisposed individuals (52) (Figure 1). Interestingly, Robbins et al. described a patient with only circulating anti-Dsg3, that developed PV lesions on the irradiated area (53, 55). Intralesional immunomapping of Dsg1, showed an altered expression, suggesting a role for ionizing radiation in altering Dsg1 expression and triggering lesions onset (Figure 1).
Radiotherapy-associated pemphigus seems to have a severe course and usually needs high-dose corticosteroids to get into remission. Rituximab has been successfully adopted in some resistant cases (54).
Ultraviolet (UV) radiation could be another trigger for pemphigus onset. In a recent study on a series of non-endemic PF patients in Turkey, the authors reported a higher incidence of pemphigus onset and relapses during the spring–summer period. This seasonal feature leads the authors to assume that UV could have a role in the pathobiology of the PF disease by inducing the acantholysis (56). An article by Aghassi et al. reported a case of PF following therapy with Psoralen–UV-A (PUVA). Subsequently, a case report was published regarding a case of herpetiform pemphigus that developed following UV-B therapy in a patient with psoriasis. In these patients, there could be a synergistic effect in pemphigus onset, dictated by the underlying condition for which the therapy is administered, such as psoriasis, and the therapeutic intervention with PUVA and UV-B (57, 58).
Traumas have been reported as a triggering factor for pemphigus in a limited number of patients (59) (Table 1). The largest series, described by Daneshpazhooh and coworkers includes 36 patients that developed pemphigus lesions after traumatic events like surgical (dental, orthopaedic or abdominal) procedures or accidental traumas. Thirteen patients had a new-onset pemphigus. Lesions developed after a short time (from 1 week to 1.5 month) from trauma exposure (59). Electrical injury has also been reported as a possible triggering factor. In a case report PV developed with recurrent oral ulcerations 1 year after the electrical injury (60). This could be explained as a gradual alteration in the self-antigen recognitions. Bee stings could be another possible triggering factor due to the cytokine concentration in the sting site (61).
Pesticides could have a critical role in pemphigus onset (40, 62–66). Pietkiewicz and coworkers in 2017 reported 3 cases of pemphigus in a cluster population living near a wastewater treatment plant. They hypothesized a possible role of topical absorption of chemical compounds and emotional stress on the pemphigus onset (67) (Table 1). Alteration of estrogen dependent pathways induced by organochlorine pesticides could promote the production of Th2 cytokines, leading to B-cell mediated autoimmunity (68), (69). Another suggested pathway is the loss of cell adhesion as a consequence of decreased skin muscarinic and nicotinic receptors expression (70). In a recent study, authors measured pesticides in hair samples of pemphigus patients and healthy controls confirming different distribution of contamination with organophosphates and/or organochlorines in hair samples in PV and PF and controls (6.3, 25.6, and 11.9%, respectively; p = 0.0437) (71). More conclusive data come from a very recent systematic review and meta-analysis from Chang and coworkers. They have demonstrated that exposure to pesticides was significantly associated with pemphigus development. Suggested mechanism includes direct damage to cell adhesion molecules by pesticides, and the balance of Th1 and Th2 cells skewed towards the Th2 profile, with generation of autoAbs (72).
There are several evidences supporting the role of contact dermatitis caused by chemicals, photographic developing liquids, dry cleaning, industrial solvents and other molecules in pemphigus onset (73).
Specifically, it has been shown that contact with phenols can induce pemphigus topically. Pemphigus developed in a 66-years-old woman after a cosmetic skin procedure in which phenol-containing chemical peels were used (74). In a more recent case report, pemphigus developed in a 32-years-old woman as a result of exposure to a nonyl phenol containing cleaning agent (75). The authors reported also several other studies with cases of pemphigus induced by contact with various substances such as garlic, benzoin tincture, basochrome, diclofenac.
Finally, an investigative study has shown increased levels of Lachnospiracea incertae sedis, Coprococcus, and Flavonifractor in the gut of pemphigus patients suggesting a role of specific microorganisms in the induction of the disease (76).
Emotional stress in patients with family history of pemphigus or genetic susceptibility could lead to the onset or the exacerbation of blistering (77–79) (Table 1). Emotional stress could induce the initiation of different signalling pathways such as glucocorticoid, leading to an alteration of cytokine production. In 1961 Perry and Brunsting described for the first time the association of emotional stress and pemphigus (80). Actually, several reports discussed the role of emotional stress in the induction or exacerbation of pemphigus disease, mainly in genetically predisposed patients (77–79). This could be due to an alteration of the signalling pathways provoked by stress, such as the glucocorticoid hormone secretion that lead to an alteration of the cytokine secretion. Recently, Wei and colleagues reported 24 cases of pemphigus, of which about 1 in 6 were found to have post-traumatic stress disorder, a prevalence comparable to that of post-traumatic stress disorder following a cancer diagnosis and three times higher than that of the general population. This study highlights how the relationship between pemphigus and emotional stress may exist in both directions. (81). Therefore, a psychiatric assessment in pemphigus patients could be recommended to prevent a worsening of the autoimmune disease (82). However, besides pemphigus, emotional stress could have a role in other autoimmune diseases (83).
Association between pemphigus and viral infections is discussed by many authors (62, 84–90). Viral infections can complicate the treatment of pemphigus, postponing the immunosuppressive therapy to avoid a reactivation of the infectious disease (Table 1). There are many ways a viral infection can induce cutaneous autoimmunity. The first and more reasonable is the molecular mimicry between the viral proteins and the molecules expressed by epidermal cells. After the infection, antigen presenting cells process the viral fragments inducing an immunologic overactivation against self-antigens (Figure 1). Inflammation resulting from infection as well as the viral infection itself can induce cell modification and tissue damage, leading to the unveiling of previous unknown epitopes that can stimulate an autoimmune response. Virus can directly infect B cell and induce polyclonal activation, proliferation and increasing antibodies production. Virus can also influence T lymphocytes inducing their polyclonal activation with superantigens. Superantigens bind both major histocompatibility complex (MHC) class II and T cell receptors and affect signalling pathways resulting in cytokines production and polyclonal T cell proliferation that could result in an autoimmune response. Moreover, silent autoreactive T-cells can be stimulated by inflammation and cytokines during a viral infection and their proliferation can lead to an autoimmune response (89). While contemplating viruses and viral diseases as a possible trigger factor for pemphigus Ruocco and coworkers also consider their pharmacological treatment (91). In fact, immunomodulatory therapies such as IFN and other cytokines have been associated with AIBDs onset and reactivation or exacerbation of an autoimmune disease like pemphigus in genetic susceptible subjects (91). Human herpes virus (HHV) is the virus family more often associated with pemphigus onset. Considerable evidence linking herpes simplex virus (HSV) infection and pemphigus has been highlighted by many authors studying patient serology for HSV and finding HSV DNA in skin lesions (83, 84, 88–103). HHV-8 has been linked to pemphigus disease also in patients without human immunodeficiency virus (HIV) or Kaposi sarcoma, finding specific HHV-8 DNA in patients skin biopsy and specific IgG in patients serum (104, 105). However other authors did not find this linkage between HHV-8 and pemphigus not being able to demonstrate the presence of HHV-8 DNA in patients skin lesions (106–108). The development of PF after citomegalovirus infection has been described in a case report of a child with genetic susceptibility for the disease (109). A possible explanation of interaction between infection and PV is the molecular mimicry. In this context, Cho et al., identified two cross-reactive VH1-46 Abs that both disrupt keratinocyte adhesion and inhibit rotavirus VP6 replication suggesting that in the B cell population some clone, through somatic mutation, may become specific to both Dsg3 and VP6 (110). They also showed that Dsg3-specific memory B cells collected in a PV patient prior to disease diagnosis presented an activated phenotype and signs of ongoing affinity maturation. This gradual process could be at the base of induction of clinical visible disease and could start also from a immune response to viral infection (111). The association between Epstein–Barr virus (EBV) and pemphigus has been investigated by some authors (84, 112, 113). EBV DNA has been found in pemphigus patients skin biopsy and elevated EBV IgG titers in peripheral blood, suggesting a link between EBV infections and the onset of pemphigus (84). Several authors investigated the possible link between hepatitis B and C virus and pemphigus (90, 114–116). In a population based study, Kridin and coworkers found that pemphigus patients have a higher prevalence of hepatitis B virus chronic infection than the controls, although no significant difference was detected for hepatitis C virus chronic infection (90). However, a retrospective study including 62 pemphigus patients and 50 controls detected no significant association between hepatitis virus infections and pemphigus (114).
PV associated with HIV infection has been described in 6 case reports (117). In four of six HIV preceded PV (118–121), in one HIV and PV were diagnosed concurrently (122) and in another one PV preceded HIV diagnosis (123). Ruocco and coworkers reported a case of PV onset 2 weeks after a coxsackievirus infection treated with cefixime (cephalosporin) (124). Pemphigus onset was described as a paraviral eruption as a consequence of both virus and drug effects on the immune response (91, 124).
Finally as for bacterial infections Mortazavi and coworkers showed that untreated PV patients had significantly higher IgG positivity to Helicobacter pylori compared with the healthy individuals (79.3% vs. 59.5%, p = 0.004) suggesting that these pathogenic agents may contribute to PV pathogenesis (125). Cutaneous manifestation of staphylococcal scalded skin syndrome could mimic PF due to a toxin, produced by the Staphylococcus aureus, which targets Dsg1 in the skin layer (126).
A growing body of evidence shows that some nutrients are involved in modulating immune responses and contribute to the pathogenesis of several cutaneous disorders, including bullous diseases (127, 128) (Table 1). A variety of dietary factors has been proposed to play important roles in the onset, progression, exacerbation and treatment of these diseases.
Dietary factors have been suggested to be involved in pemphigus induction based on the similarity of their chemical structure to drugs recognized as possible trigger factors (129, 130). Clinical evidence supports the role of dietary factors in the maintenance and exacerbation of pemphigus (131, 132). Suspected dietary factors contain thiol compounds (garlic, leek, chives, onion, shallot), phenols (black pepper, red chillies, mango, cashew), tannins (tea, red wine, spices, raspberry, cranberry, blackberry), isothiocyanates (mustard, horseradish, cauliflower) and phycocyanins (Spirulina platensis alga) (127–132).
In a case report, heavy garlic consumption worsened pemphigus in a 49-year-old man (131): a garlic-free diet coincided with remission for a few months, followed by recurrence after unintentional ingestion of a garlic-spiced meal. In another case report, oral lesions of pemphigus were induced by ingestion of leek (132) (Table 1). A leek-free diet resulted in oral lesions improvement and antibody titers decrease, while a leek challenge induced oral lesions along with increased antibody titers (132). An in vitro study showed that three compounds of garlic (allylmercaptan, allylmethylsulfide and allylsulfide) induced acantholysis in skin specimens in four of seven donors cultured in the presence of each of the allyl compounds for 3 days (133).
Since thiol compounds in food have been suggested to be involved in the induction of pemphigus based on the similarity of their chemical structure to drugs, a number of mechanisms have been suggested for the effect of thiols: direct biochemical effect by formation of thiol-cysteine bonds instead of cysteine-cysteine bonds, and disturbance to cell adhesion; inhibition of enzymes that aggregate keratinocytes; activation of enzymes that disaggregate keratinocytes like plasminogen activator; immunological reaction with a formation of a neoantigen (134). The antibodies against the new complex have a cross reaction with desmosomes and provoke pemphigus disease (135).
The early age of onset and high incidence of pemphigus in Indian patients might be explained by the high consumption of foods that contain large amounts of phenols, such as mango, cashew nuts and black peppers (128, 136) (Table 1). However, it must be considered, when examining specific populations, the potential impact of genetic factors on the development of the pathology, factors that could downscale the relevance of external risk factors, such as those related to specific dietary habits (137).
Possible mechanisms of phenol-induced pemphigus include the induction of IL-1α and TNF-α release by keratinocytes (138). These cytokines have been shown to be relevant in the regulation and synthesis of complement and proteases, like plasminogen activator, which has been implicated in the pathogenesis of acantholysis in PV (139).
Several foods and drinks contain tannins such as mango, cassava, yucca, guarana, betel nut, raspberry, cranberry, blackberry, avocado, peach, ginger, ginseng, tea and red wine. Although there have been no reports of tannin-containing foods directly inducing pemphigus, some in vitro studies have linked tannins with skin acantholysis (140, 141). In one report, tannic acid added to in vitro cultured human breast skin explants from five different donors without any bullous disease, produced different cytotoxic effects, including suprabasal cleavage and intraepidermal acantholysis (140); the concentrations required to achieve these effects varied significantly between different samples, suggesting high variability in susceptibility to tannin acantholysis. Feliciani and coworkers used high-performance liquid chromatography (HPLC) to measure the levels of tannic acid in the skin blister fluid of four group of patients, subdivided according to their dietary habits (141). Patients with a tannin-rich diet had increased tannin metabolites in their skin. In the same study, in a keratinocyte cell culture experiment, tannic acid was capable of inducing acantholysis, effect that decreased when anti-IL-1α and anti-TNF-α antibodies were added (141). Foods with large amounts of tannins are also heavily consumed in India, another area with a high incidence of pemphigus (128).
No cases of pemphigus induction or aggravation through the consumption of foods containing isothiocyanates, derived from hydrolysis of glucosinolates have been reported so far. These foods may contain an allyl, benzyl or phenyl group and can be immunologically active or lead to non-immunologic skin acantholysis, like thiol-containing drugs. Allyl isothiocyanate is the primary constituent of mustard oil, a known irritant that can cause blistering of mucous membranes (142). Tur and Brenner noted that mustard oil is widely used in India, where pemphigus has a high incidence, not only as a food but also for topical application on scalp hair and for body massage. Contact with this oil may cause antigen exposure leading to pemphigus or other local effects (136). Mustard is a member of the Cruciferae family; other members include horseradish, winter cress, turnip, radish, cabbage, cauliflower and broccoli, but to date no clinical cases are reported.
Phycocyanin is the blue pigment protein in blue-green algae such as Spirulina platensis, which is sold in food stores as a dietary supplement. Two reports have suggested a link between intake of herbal supplements and bullous disorders. In the first report, the authors have described two cases. The first case is a 57-year-old man with chronic PV, which was controlled with azathioprine and prednisone, who experienced a severe flare after intake of a mixture of dietary supplements containing S. platensis; the flare resolved in 2 weeks, upon discontinuation of these supplements, which had never been taken before this episode, and an increased in his prednisone dose (143). The other case was a 55-year-old man with PV treated and controlled with prednisone, dapsone and azathioprine, who developed blisters on his trunk, head and oral mucosa within 1 week of starting to take an Echinacea supplement daily. After discontinuing the use of the Echinacea supplement, partial disease control, but never complete remission, was achieved with prednisone, azathioprine and dapsone (143). In these two patients the temporal relationship of a PV flare occurring within days of ingesting these herbal supplements is highly suggestive of a causal relationship, but it cannot exclude the possibility that these patients may have experienced a flare in conjunction with a standard prednisone taper.
In the second report, a mixed immunobullous disorder with features of both PF and bullous pemphigoid developed in an 82-year-old healthy woman 1 year after she started a food supplement containing the blue-green alga S. patensis (144). The patient’s symptoms and signs resolved 3 months after completion of a prednisone treatment and avoidance of the dietary supplement.
In a report, two patients with new-onset pemphigus, one with IgA pemphigus and one with PF, started a gluten-free diet with subsequent complete remission of their symptoms and signs (145). Both patients had serologic markers of gluten-sensitive enteropathy (IgA and IgG versus antigliadin antibodies) without any manifestations of celiac disease, suggesting that they had silent gluten sensitivity. From these findings, it is plausible to hypothesize an association between gluten intake and pemphigus, and how individuals with pemphigus and serologic markers of gluten-sensitive enteropathy may benefit from a gluten-free diet.
In a recent study, it has been hypothesized that walnut antigens can trigger autoAb development in patients with PV through a “hit-and-run” mechanism (146). Revertant/germline mAbs of 8 anti-Dsg3 pathogenic mAbs from PV patients were tested for reactivity against a panel of possible allergens, including food, epithelia, insects, pollens, and fungi antigens. Lin and coworkers showed that all tested germline PV mAbs reacted to walnut antigen extract, specifically to two protein components, Jug-R2 and uncharacterized 85-kDa protein, regardless of their reactivity to Dsg3 autoantigen. This suggests that walnut proteins might be exogenous antigens that activate naive B cells in subjects genetically predisposed to PV, leading to subsequent autoAb development and disease onset. So walnut antigen might be the initial stimulus and selection of autoreactive B cells is subsequently driven by reactivity to Dsg3. Indeed, IgG1 and IgG4 antibodies against walnut antigens are present at much lower levels than those against Dsg3 in sera from patients with PV (146).
Pemphigus, like many other autoimmune diseases, is strictly related to immune responses that could be altered via gene polymorphisms (Table 2). Differences in the incidence of pemphigus in diverse ethnic populations, some of which could present a higher incidence or endemic distribution (i.e., PV in Ashkenazi Jewish), strongly suggest a role of genetic factors. Furthermore, familial pemphigus cases have even been reported. MHC genes, called human leucocyte antigens (HLA) in humans, are the most involved genetic factors in pemphigus (147, 148). Several studies regarding genetic predisposition reported the association of pemphigus with the class II HLA alleles in specific ethnic groups (149–151). Among Italian, Spanish, French, Slovak, North American, and Brazilian PV populations, the most common associated alleles are DQA1*01:04, 03:01, DQB1*05:03 and DRB1*04:02, 14:01 (152–159). Yan and coworkers reported in a meta-analysis that HLA-DRB1*04, HLA-DRB1*14, and HLA-DRB1*08 HLA are statistically important susceptibility factors for PV, while in the Jewish population, an association between PV and HLA-DRB1*04:02, and DQB1*03:02 has been reported (148, 149). MHC region includes also some long non-coding RNA (lncRNA) genes in the HLA complex group (HCG): recently, Salviano-Silva and coworkers, found an association between HCG lncRNA alleles and pemphigus susceptibility, suggesting their role in pemphigus pathogenesis (160) (Table 2).
On the other hand, it has been demonstrated, through a genome-wide association study in the Jewish population, an association between single nucleotide polymorphisms (SNPs) of the ST18 gene and PV (161). ST18, a regulator of apoptosis and inflammation overexpressed in the skin of PV patients compared to healthy individuals, has been hypothesized to have a role in the pathogenesis of pemphigus provoking an elevated production of TNF-α, IL-1α, and IL-6 and favoring a PV IgG-induced cell–cell altered adhesion (162) Although in Egyptian, Jewish, and Iranian populations SNPs of ST18 and PV association was reported, in Italian (163), Chinese and German population no linkage between ST18 SNPs and PV was demonstrated (161, 164, 165). In addition, increased CD59 transcriptional levels have been associated with gene expression, mainly in female subjects, in PF (166). In this context, a study underlines the importance of a SNP in forkhead box P3 (FOXP3) gene for the development and function of regulatory T-cell, describing SNP association with the clinical course and prognosis of PF (167) (Table 2).
Furthermore, polymorphisms of genes encoding several cytokines, such as IL-4, IL-6, IL-10, TNF-α, and transforming growth factor-β have been reported in pemphigus patients compared to healthy controls (168–174), suggesting a potential involvement in the pathogenesis of this disease.
Comorbidities can be considered as a predisposing factor because they can represent the context in which the disease develops. Alternatively, they can be generated by a cause that is also at the basis of pemphigus, such as dysregulation of the immune system. In some cases, they could represent a trigger factor that induce epitope spreading phenomena leading to an autoimmune response.
Association between pemphigus and thyroid diseases has largely been reported, but published data are controversial. Some authors found that changes in the serum thyroid profile, positive titers of anti-thyroid autoAbs (anti-thyroid peroxidase and anti-thyroglobulin autoAbs) and Hashimoto’s thyroiditis were more frequent in PV patients than controls (175, 176) (Table 2). According to Parameswaran and coworkers, frequency of autoimmune thyroid disease was significantly higher in PV patients than in controls, and in PV patients’ relatives than in the control counterpart (177), in accordance with Heelan and coworkers that found a higher risk of hypothyroidism in PV subjects (178), mainly among women. However, the presence of laboratory thyroid alterations in PV patients not always correlates with clinical disease (176, 179). Recently, a large-scale study conducted on a cohort of 1985 PV patients found a significant association with Hashimoto’s thyroiditis in male patients but not in females, whereas no association was found between pemphigus and Grave’s disease and thyroid cancer (180).
Several reports seem to indicate an association of non-paraneoplastic pemphigus with oncologic diseases, in particular with hematologic malignancies (181–184) (Table 2). According to Schulze and coworkers the percentage of pemphigus patients with hematologic malignancies was 3.9% (182). These findings were confirmed by Kridin and coworkers that found a higher prevalence of chronic leukemia (0.9% vs. 0.4%), multiple myeloma (0.8% vs. 0.4%) and non-Hodgkin lymphoma (1.8% vs. 1.2%) in pemphigus patients than in controls (183). While no definitive explanation has been suggested for this association, it has been proposed that the development of hematologic malignancies could be the result of chronic inflammatory stimulation or due to drug-induced immunosuppression. In this view, this disease could be a consequence rather than a trigger factor for pemphigus. In 1995, Ogawa and coworkers found a 5% prevalence of internal malignancies among PV patients, significantly higher than in the Japanese population (0.6%), according to their data, lung cancer was the most frequent form (185). More recently, Schulze and coworkers found a higher prevalence of oropharyngeal, gastrointestinal and colon cancer in PV patients than in controls (182). Large cohort studies confirmed the high prevalence of oesophageal and laryngeal cancers (respectively 3-fold and 2-fold higher in PV patients than in controls) but not of gastrointestinal and colon cancer (184) or lung cancer (186). As a pathogenetic hypothesis, solid malignant tumors could induce tissue alterations that lead to mucosal antigen unmasking and could favour the development of autoimmune response.
Kaposi’s sarcoma (KS) and pemphigus associations have been reported in the last decades as case reports or epidemiological studies (187–189). These studies lead to the hypothesis that HHV-8 could be a trigger for the onset of the autoimmune blistering disease. Furthermore, some authors described KS after the beginning of immunosuppressive therapy for pemphigus. Even if the link between the two diseases is still unclear, we may suppose that KS could be triggered by pemphigus immunosuppressive therapy and that pemphigus, such as other autoimmune blistering disease, could be triggered by the presence of HHV-8 in the skin cells.
A high number of pemphigus patients has a familial history of autoimmune diseases. Several reports show a possible association of pemphigus with a second autoimmune disorder (5) (Table 2). This could be explained by the activity of the primary immune disease that could lead to the alteration of regulatory immune response inducing a second autoimmune condition. Moreover, autoimmune diseases could share the same signalling pathways, so the upregulation in one of these pathways could represent a predisposition to several autoimmune diseases.
A population-based large scale study by Kridin and coworkers stated that the prevalence of psoriasis was significantly greater in the patients with pemphigus than in the controls (respectively 3.3% vs. 1.2%) (190). Furthermore a population-based case–control study in Taiwan showed a greater prevalence rate of pemphigus in a population of 51,800 patients affected by psoriasis (191). The link between these two diseases is still unclear.
More reports link pemphigus to other autoimmune diseases such as lichen planus, systemic lupus erythematosus, pemphigoid, patchy alopecia areata and alopecia areata universalis, rheumatoid arthritis and autoimmune thyroiditis (5, 192–195).
Although the understanding of pathogenic mechanisms of AIBDs has increased tremendously, there is still much to learn about factors affecting their onset, course, and therapy. Predisposing factors for pemphigus include genetic predisposition and comorbidities.
Precipitating factors, such as drugs, vaccines, pregnancy, radiations, emotional stress, infections, diet or other external factors, could induce pemphigus disease in the presence, but also in the absence of predisposing factors. However, in the majority of patients no conclusive trigger can be evaluated. In fact, most triggers reported in this review are not mechanistically confirmed. Thus, future studies should establish appropriate disease models and also investigate the relationship between trigger and predisposing factors with the aim to improve knowledge on pemphigus pathogenesis.
FMo: Conceptualization, Writing – original draft. JS: Conceptualization, Writing – original draft. AS: Writing – original draft. LF: Writing – original draft. FMa: Writing – review & editing. AP: Writing – review & editing. BD: Writing – review & editing. GZ: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This article was partially supported by the “Progetto Ricerca Corrente” of the Italian Ministry of Health.
IDI-IRCCS is a healthcare provider of the European Reference Network (ERN)-Skin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Dsg, desmoglein; PV, pemphigus vulgaris; PF, pemphigus foliaceous; autoAbs, autoantibodies; AIBD, autoimmune bullous disease; TNFα, tumor necrosis factor alpha; IL, interleukin; Ig, immunoglobulin; ACEi, angiotensin converting enzyme inhibitors; mAb, monoclonal antibody; IFN, interferon; UV, ultraviolet; MHC, major histocompatibility complex; HIV, human immunodeficiency virus; EBV, Epstein–Barr virus; HLA, human leucocyte antigens.
1. Udey, MC, and Stanley, JR. Pemphigus--diseases of antidesmosomal autoimmunity. JAMA. (1999) 282:572–6. doi: 10.1001/jama.282.6.572
2. Di Zenzo, G, Amber, KT, Sayar, BS, Müller, EJ, and Borradori, L. Immune response in pemphigus and beyond: progresses and emerging concepts. Semin Immunopathol. (2016) 38:57–74. doi: 10.1007/s00281-015-0541-1
3. Didona, D, Scarsella, L, Hudemann, C, Volkmann, K, Zimmer, CL, Beckert, B, et al. Type 2 T cell responses against distinct epitopes of the desmoglein 3 ectodomain in pemphigus vulgaris. J Invest Dermatol. (2023). doi: 10.1016/j.jid.2023.07.025
4. Ujiie, H, Yamagami, J, Takahashi, H, Izumi, K, Iwata, H, Wang, G, et al. The pathogeneses of pemphigus and pemphigoid diseases. J Dermatol Sci. (2021) 104:154–63. doi: 10.1016/j.jdermsci.2021.11.003
5. Tavakolpour, S . Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. (2018) 310:95–106. doi: 10.1007/s00403-017-1790-8
6. Bauza, A, Del Pozo, LJ, Saus, C, and Martin, A. Pemphigus-like lesions induced by imiquimod. Clin Exp Dermatol. (2009) 34:e60–2. doi: 10.1111/j.1365-2230.2008.03181.x
7. Brenner, S, Bialy-Golan, A, and Crost, N. Dipyrone in the induction of pemphigus. J Am Acad Dermatol. (1997) 36:488–90. doi: 10.1016/S0190-9622(97)80238-1
8. Lo Schiavo, A, Sangiuliano, S, Puca, RV, Brunetti, G, Ruocco, E, and Cozzi, R. Contact pemphigus: A side-effect of imiquimod therapy. Int J Dermatol. (2008) 47:765–7. doi: 10.1111/j.1365-4632.2008.03533.x
9. Orion, E, Barzilay, D, and Brenner, S. Pemphigus vulgaris induced by diazinon and sun exposure [5]. Dermatology. (2000) 201:378–9. doi: 10.1159/000051564
10. Orion, E, Gazit, E, and Brenner, S. Pemphigus vulgaris possibly triggered by Cilazapril [9]. Acta Derm Venereol. (2000) 80:220. doi: 10.1080/000155500750043078
11. Parodi, A, Cozzani, E, Milesi, G, Drosera, M, and Rebora, A. Fosinopril as a possible pemphigus-inducing drug. Dermatology. (2002) 204:139–41. doi: 10.1159/000051833
12. Patterson, CRS, and Davies, MG. Carbamazepine-induced pemphigus [6]. Clin Exp Dermatol. (2003) 28:98–9. doi: 10.1046/j.1365-2230.2003.01156_6.x
13. Patterson, CRS, and Davies, MG. Pemphigus foliaceus: an adverse reaction to lisinopril. J Dermatolog Treat. (2004) 15:60–2. doi: 10.1080/09546630310013379
14. Pellicano, R, Iannantuono, M, and Lomuto, M. PEMPHIGUS ERYTHEMATOSUS INDUCED BY CEFTAZIDIME. Int J Dermatol. (1993) 32:675–6. doi: 10.1111/j.1365-4362.1993.tb04026.x
15. Russo, I, Ferrazzi, A, and Alaibac, M. Relapse of pemphigus vulgaris after topical application of ingenol mebutate. Clin Exp Dermatol. (2016) 41:664–6. doi: 10.1111/ced.12875
16. Wilkinson, SM, Smith, AG, Davis, MJ, Hollowood, K, and Dawes, PT. Rheumatoid arthritis: an association with pemphigus foliaceous. Acta Derm Venereol. (1992) 72:289–91. doi: 10.2340/0001555572289291
17. Zone, J, Ward, J, Boyce, E, and Schupbach, C. Penicillamine-induced pemphigus. JAMA J Am Med Assoc. (1982) 247:2705. doi: 10.1001/jama.1982.03320440053036
18. Butt, A, and Murge, SM. Pemphigus vulgaris induced by captopril. Br J Dermatol. (1995) 132:315–6. doi: 10.1111/j.1365-2133.1995.tb05038.x
19. Goldberg, I, Sasson, A, Gat, A, Srebrnik, A, and Brenner, S. Pemphigus vulgaris triggered by glibenclamide and cilazapril. Acta Dermatovenerol Croat. (2005) 13:153–5.
20. Goldberg, I, Ingher, A, and Brenner, S. Pemphigus vulgaris triggered by rifampin and emotional stress. Skinmed. (2004) 3:294. doi: 10.1111/j.1540-9740.2004.03343.x
21. Ghaedi, F, Etesami, I, Aryanian, Z, Kalantari, Y, Goodarzi, A, Teymourpour, A, et al. Drug-induced pemphigus: A systematic review of 170 patients. Int Immunopharmacol. (2021) 92:107299. doi: 10.1016/j.intimp.2020.107299
22. Buzon, E, Perez-Bernal, AM, De la Pena, F, Rios, JJ, and Camacho, F. Pemphigus foliaceus associated with cilazapril [7]. Acta Derm Venereol. (1998) 78:227. doi: 10.1080/000155598441639
23. Fujita, H, Iguchi, M, Watanabe, R, and Asahina, A. Pemphigus foliaceus induced by bucillamine [7]. Eur J Dermatol. (2007) 17:98–9. doi: 10.1684/ejd.2007.0180
24. Ghaffarpour, G, Jalali, MHA, Yaghmaii, B, Mazloomi, S, and Soltani-Arabshahi, R. Chloroquine/hydroxychloroquine-induced pemphigus [15]. Int J Dermatol. (2006) 45:1261–3. doi: 10.1111/j.1365-4632.2006.03075.x
25. Heymann, AD, Chodick, G, Kramer, E, Green, M, and Shalev, V. Pemphigus variant associated with penicillin use: A case-cohort study of 363 patients from Israel. Arch Dermatol. (2007) 143:704–7. doi: 10.1001/archderm.143.6.704
26. Kaplan, RP, Potter, TS, and Fox, JN. Drug-induced pemphigus related to angiotensin-converting enzyme inhibitors. J Am Acad Dermatol. (1992) 26:364–6. doi: 10.1016/0190-9622(92)70057-M
27. Kim, SC, Won, JH, and Ahn, SK. Pemphigus foliaceus induced by nifedipine. Acta Derm Venereol. (1993) 73:210–1. doi: 10.2340/000155555573210211
28. Lin, R, Ladd, DJ, Powell, DJ, and Way, BV. Localized pemphigus foliaceus induced by topical imiquimod treatment [6]. Arch Dermatol. (2004) 140:889–90. doi: 10.1001/archderm.140.7.889
29. Brenner, S, and Goldberg, I. Drug-induced pemphigus. Clin Dermatol. (2011) 29:455–7. doi: 10.1016/j.clindermatol.2011.01.016
30. Pietkiewicz, P, Gornowicz-Porowska, J, Bowszyc-Dmochowska, M, and Dmochowski, M. A retrospective study of antihypertensives in pemphigus: a still unchartered odyssey particularly between thiols, amides and phenols. Arch Med Sci. (2015) 11:1021–7. doi: 10.5114/aoms.2015.54857
31. Feng, S, Zhou, W, Zhang, J, and Jin, P. Analysis of 6 cases of drug-induced pemphigus. Eur J Dermatology. (2011) 21:696–9. doi: 10.1684/ejd.2011.1428
32. Brenner, S, Bialy-Golan, A, and Anhalt, GJ. Recognition of pemphigus antigens in drug-induced pemphigus vulgaris and pemphigus foliaceus. J Am Acad Dermatol. (1997) 36:919–23. doi: 10.1016/S0190-9622(97)80273-3
33. Yoshimura, K, Ishii, N, Hamada, T, Abe, T, Ono, F, Hashikawa, K, et al. Clinical and immunological profiles in 17 Japanese patients with drug-induced pemphigus studied at Kurume University. Br J Dermatol. (2014) 171:544–53. doi: 10.1111/bjd.12925
34. Feldman, RJ . Paradoxical worsening of pemphigus vulgaris following rituximab therapy. Br J Dermatol. (2015) 173:858–9. doi: 10.1111/bjd.13823
35. Sharma, VK, Bhari, N, Gupta, S, Sahni, K, Khanna, N, Ramam, M, et al. Clinical efficacy of rituximab in the treatment of pemphigus: A retrospective study. Indian J Dermatol Venereol Leprol. (2016) 82:389. doi: 10.4103/0378-6323.174379
36. Hayashida, MZ, Pinheiro, JRS, Enokihara, MM, and Vasconcellos, MR. Biologic therapy-induced pemphigus. An Bras Dermatol. (2017) 92:591–3. doi: 10.1590/abd1806-4841.20176481
37. Palleria, C, Bennardo, L, Dastoli, S, Iannone, LF, Silvestri, M, Manti, A, et al. Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers induced pemphigus: A case series and literature review. Dermatol Ther. (2019) 32:e12748–20. doi: 10.1111/dth.12748
38. Zhou, Q, Wang, P, Zhang, L, Wang, B, Shi, L, Keyal, U, et al. Pemphigus vulgaris induced by 5-aminolaevulinic acid-based photodynamic therapy. Photodiagn Photodyn Ther. (2017) 19:156–8. doi: 10.1016/j.pdpdt.2017.05.014
39. Moro, F, Ciccone, D, Fania, L, Mariotti, F, Salemme, A, Rahimi, S, et al. Case report: A rare case of imiquimod-induced atypical pemphigus vulgaris. Front Med. (2022) 9:1054544. doi: 10.3389/fmed.2022.1054544
40. Valikhani, M, Kavusi, S, Chams-Davatchi, C, Daneshpazhooh, M, Barzegari, M, Ghiasi, M, et al. Pemphigus and associated environmental factors: A case-control study. Clin Exp Dermatol. (2007) 32:256–60. doi: 10.1111/j.1365-2230.2007.02390.x
41. Yoo, SA, Park, HE, and Kim, M. A case of newly developed Pemphigus Foliaceus and possible association with alternative bee-venom therapy. Ann Dermatol. (2021) 33:467–9. doi: 10.5021/ad.2021.33.5.467
42. Berkun, Y, Mimouni, D, and Shoenfeld, Y. Pemphigus following hepatitis B vaccination-coincidence or causality? Autoimmunity. (2005) 38:117–9. doi: 10.1080/08916930400027078
43. De Simone, C, Caldarola, G, D’Agostino, M, Zampetti, A, Amerio, P, and Feliciani, C. Exacerbation of pemphigus after influenza vaccination. Clin Exp Dermatol. (2008) 33:718–20. doi: 10.1111/j.1365-2230.2008.02835.x
44. Korang, K, Ghohestani, R, Krieg, T, Uitto, J, and Hunzelmann, N. Exacerbation of pemphigus foliaceus after tetanus vaccination accompanied by synthesis of auto-antibodies against paraneoplastic pemphigus antigens [13]. Acta Derm Venereol. (2002) 82:482–3. doi: 10.1080/000155502762064755
45. Mignogna, MD, and Lo, ML. Pemphigus induction by influenza vaccination. Int J Dermatol. (2000) 39:795–800. doi: 10.1046/j.1365-4362.2000.00051-5.x
46. Yalçin, B, and Alli, N. Pemphigus vulgaris following antirabies vaccination [6]. J Dermatol. (2007) 34:734–5. doi: 10.1111/j.1346-8138.2007.00373.x
47. Hertl, M, Jedlickova, H, Karpati, S, Marinovic, B, Uzun, S, Yayli, S, et al. S2 guideline for diagnosis and treatment - guided by the European dermatology forum (EDF) in cooperation with the European academy of dermatology and venereology (EADV). J Eur Acad Dermatol Venereol. (2015) 29:405–14. doi: 10.1111/jdv.12772
48. Pira, A, Sinagra, JLM, Moro, F, Mariotti, F, and Di Zenzo, G. Autoimmune bullous diseases during COVID-19 pandemic: 2022 update on rituximab and vaccine. Front Med. (2023) 10:1112823. doi: 10.3389/fmed.2023.1112823
49. Daneshpazhooh, M, Chams-Davatchi, C, Valikhani, M, Aghabagheri, A, Ali Mortazavizadeh, SM, Barzegari, M, et al. Pemphigus and pregnancy: A 23-year experience. Indian J Dermatol Venereol Leprol. (2011) 77:534. doi: 10.4103/0378-6323.82404
50. Tavakolpour, S, and Rahimzadeh, G. New insights into the Management of Patients with autoimmune diseases or inflammatory disorders during pregnancy. Scand J Immunol. (2016) 84:146–9. doi: 10.1111/sji.12453
51. Tavakolpour, S, Mirsafaei, HS, and Delshad, S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. (2017) 77:12601. doi: 10.1111/aji.12601
52. Hung, TL, Chen, YL, Lin, KT, Chiang, CP, Chung, CH, Hung, CT, et al. Risk of radiotherapy-associated autoimmune bullous disease among Taiwanese patients with breast cancer: a case–control study. Arch Dermatol Res. (2020) 312:69–75. doi: 10.1007/s00403-019-01985-y
53. Schauer, F, Ishii, N, Mockenhaupt, M, Bruckner-Tuderman, L, Hashimoto, T, and Kiritsi, D. Radiation-associated Pemphigus vulgaris in a patient with preceding malignancy: treatment with rituximab as a valuable option. Front Immunol. (2020) 10:3116. doi: 10.3389/fimmu.2019.03116
54. Hee Won, JANG, Seung Hyun, CHUNJML, Jiehyun, JEONTH, and Kim, I-H. Radiotherapy-induced pemphigus vulgaris. Breast J. (2017) 23:747–9. doi: 10.1111/tbj.12910
55. Robbins, AC, Lazarova, Z, Janson, MM, and Fairley, JA. Pemphigus vulgaris presenting in a radiation portal. J Am Acad Dermatol. (2007) 56:S82–5. doi: 10.1016/j.jaad.2006.10.956
56. Kılıç Sayar, S, and Küçükoğlu, R. Evaluation of non-endemic pemphigus foliaceus in a large series of patients: a single-center retrospective study from Turkey focuses on the relapses. An Bras Dermatol. (2021) 96:422–8. doi: 10.1016/j.abd.2020.12.009
57. Aghassi, D, and Dover, JS. Pemphigus Foliaceus induced by psoralen–UV-A. Arch Dermatol. (1998) 134:1300–1. doi: 10.1001/archderm.134.10.1300-a
58. Sanchez-Palacios, C, and Chan, LS. Development of pemphigus herpetiformis in a patient with psoriasis receiving UV-light treatment. J Cutan Pathol. (2004) 31:346–9. doi: 10.1111/j.0303-6987.2004.0188.x
59. Daneshpazhooh, M, Fatehnejad, M, Rahbar, Z, Balighi, K, Ghandi, N, Ghiasi, M, et al. Trauma-induced pemphigus: a case series of 36 patients. J Dtsch Dermatol Gesellschaft. (2016) 14:166–71. doi: 10.1111/ddg.12738
60. Tan, SR, McDermott, MR, Castillo, CJ, and Sauder, DN. Pemphigus vulgaris induced by electrical injury. Cutis. (2006) 77:161–5.
61. Gül, U, Gönül, M, Cakmak, SK, and Kiliç, A. Pemphigus vulgaris induced by honeybee sting? Acta Derm Venereol. (2006) 86:467–8. doi: 10.2340/000155555-0136
62. Ruocco, V, and Ruocco, E. Pemphigus and environmental factors. G Ital Dermatol Venereol. (2003) 138:299–309.
63. Wohl, Y, and Brenner, S. Pemphigus in Israel - an epidemiologic analysis of cases in search of risk factors. Isr Med Assoc J. (2003) 5:410–2.
64. Wohl, Y, Goldberg, I, Shirazi, I, and Brenner, S. Chlorpyrifos exacerbating pemphigus vulgaris: a preliminary report and suggested in vitro immunologic evaluation model. Skinmed. (2006) 5:111–3. doi: 10.1111/j.1540-9740.2006.04767.x
65. Brenner, S, Tur, E, Shapiro, J, Ruocco, V, D’Avino, M, Ruocco, E, et al. Pemphigus vulgaris: environmental factors. Occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol. (2001) 40:562–9. doi: 10.1046/j.1365-4362.2001.01266.x
66. Fisher, KR, Higginbotham, R, Frey, J, Granese, J, Pillow, J, and Skinner, RB. Pesticide-associated pemphigus vulgaris. Cutis. (2008) 82:51–4.
67. Pietkiewicz, P, Gornowicz-Porowska, J, Bartkiewicz, P, Bowszyc-Dmochowska, M, and Dmochowski, M. Reviewing putative industrial triggering in pemphigus: cluster of pemphigus in the area near the wastewater treatment plant. Postep Dermatologii i Alergol. (2017) 34:185–91. doi: 10.5114/ada.2017.67840
68. Cai, Y, Zhou, J, and Webb, DC. Estrogen stimulates Th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int Arch Allergy Immunol. (2012) 158:252–60. doi: 10.1159/000331437
69. Gaido, K, Dohme, L, Wang, F, Chen, I, Blankvoort, B, Ramamoorthy, K, et al. Comparative estrogenic activity of wine extracts and organochlorine pesticide residues in food. Environ Health Perspect. (1998) 106:1347–51. doi: 10.1289/ehp.98106s61347
70. Corsini, E, Liesivuori, J, Vergieva, T, Van Loveren, H, and Colosio, C. Effects of pesticide exposure on the human immune system. Hum Exp Toxicol. (2008) 27:671–80. doi: 10.1177/0960327108094509
71. La Serra, L, Salathiel, AM, Lanaro, R, de Martinis, B, and Roselino, AM. Measurement of pesticides in hair samples from pemphigus foliaceus and pemphigus vulgaris patients in southeastern Brazil. An Bras Dermatol. (2023) 98:644–50. doi: 10.1016/j.abd.2022.10.010
72. Chang, H-C, and Tsai, T-Y. Pesticide exposure is associated with pemphigus: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. (2022) 36:e733–5. doi: 10.1111/jdv.18258
73. Ruocco, V, Ruocco, E, Lo Schiavo, A, Brunetti, G, Guerrera, LP, and Wolf, R. Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clin Dermatol. (2013) 31:374–81. doi: 10.1016/j.clindermatol.2013.01.004
74. Kaplan, RP, Detwiler, SP, and Saperstein, HW. Physically induced pemphigus after cosmetic procedures. Int J Dermatol. (1993) 32:100–3. doi: 10.1111/j.1365-4362.1993.tb01445.x
75. Goldberg, I, Sasson, O, and Brenner, S. A case of phenol-related contact pemphigus. Dermatology. (2001) 203:355–6. doi: 10.1159/000051793
76. Huang, S, Mao, J, Zhou, L, Xiong, X, and Deng, Y. The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand J Immunol. (2019) 90:e12799. doi: 10.1111/sji.12799
77. Brenner, S, and Bar-Nathan, EA. Pemphigus vulgaris triggered by emotional stress. J Am Acad Dermatol. (1984) 11:524–5. doi: 10.1016/S0190-9622(84)80380-1
78. Morell-Dubois, S, Carpentier, O, Cottencin, O, Queyrel, V, Hachulla, E, Hatron, PY, et al. Stressful life events and pemphigus. Dermatology. (2008) 216:104–8. doi: 10.1159/000111506
79. Tamir, A, Ophir, J, and Brenner, S. Pemphigus vulgaris triggered by emotional stress [2]. Dermatology. (1994) 189:210. doi: 10.1159/000246837
80. Perry, HO, and Brunsting, LA. Pemphigus Foliaceus: further observations. Arch Dermatol. (1965) 91:10. doi: 10.1001/archderm.1965.01600070016002
81. Wei, EX, Li, SJ, and Mostaghimi, A. Post-traumatic stress disorder in patients with autoimmune blistering diseases. Br J Dermatol. (2020) 182:1044–5. doi: 10.1111/bjd.18548
82. Wohl, Y, Mashiah, J, Kutz, A, Hadj-Rabia, S, and Cohen, AD. Pemphigus and depression comorbidity: A case control study. Eur J Dermatology. (2015) 25:602–5. doi: 10.1684/ejd.2015.2649
83. Stojanovich, L, and Marisavljevich, D. Stress as a trigger of autoimmune disease. Autoimmun Rev. (2008) 7:209–13. doi: 10.1016/j.autrev.2007.11.007
84. Sagi, L, Baum, S, Agmon-Levin, N, Sherer, Y, Katz, BSP, Barzilai, O, et al. Autoimmune bullous diseases. The spectrum of infectious agent antibodies and review of the literature. Autoimmun Rev. (2011) 10:527–35. doi: 10.1016/j.autrev.2011.04.003
85. Ahmed, AR, and Rosen, GB. Viruses in Pemphigus. Int J Dermatol. (1989) 28:209–17. doi: 10.1111/j.1365-4362.1989.tb04805.x
86. Brenner, S, Sasson, A, and Sharon, O. Pemphigus and infections. Clin Dermatol. (2002) 20:114–8. doi: 10.1016/S0738-081X(01)00254-1
87. Tufano, MA, Baroni, A, Buommino, E, Ruocco, E, Lombardi, ML, and Ruocco, V. Detection of herpesvirus DNA in peripheral blood mononuclear cells and skin lesions of patients with pemphigus by polymerase chain reaction. Br J Dermatol. (1999) 141:1033–9. doi: 10.1046/j.1365-2133.1999.03201.x
88. Ruocco, V, Wolf, R, Ruocco, E, and Baroni, A. Viruses in pemphigus: A casual or causal relationship? Int J Dermatol. (1996) 35:782–4. doi: 10.1111/j.1365-4362.1996.tb02972.x
89. Senger, P, and Sinha, AA. Exploring the link between herpes viruses and pemphigus vulgaris: literature review and commentary. Eur J Dermatology. (2012) 22:728–35. doi: 10.1684/ejd.2012.1836
90. Kridin, K, Zelber-Sagi, S, Comaneshter, D, and Cohen, AD. Is there an association between pemphigus and hepatitis viruses? A population-based large-scale study. Immunol Res. (2017) 65:1083–8. doi: 10.1007/s12026-017-8950-y
91. Ruocco, E, Ruocco, V, Lo Schiavo, A, Brunetti, G, and Wolf, R. Viruses and pemphigus: an intriguing never-ending story. Dermatology. (2014) 229:310–5. doi: 10.1159/000365845
92. Esmaili, N, Hallaji, Z, Soori, T, and Davatchi, CC. Bullous pemphigoid in Iranian patients: a descriptive study on 122 cases. Acta Med Iran. (2012) 50:335–8.
93. Marzano, AV, Tourlaki, A, Merlo, V, Spinelli, D, Venegoni, L, and Crosti, C. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. (2009) 22:781–6. doi: 10.1177/039463200902200324
94. Hocar, O, Zidane, W, Laissaoui, K, Akhdari, N, and Amal, S. L’infection herpétique au cours du pemphigus. Med Mal Infect. (2009) 39:64–5. doi: 10.1016/j.medmal.2008.06.032
95. Belgnaoui, FZ, Senouci, K, Chraibi, H, Aoussar, A, Mansouri, F, Benzekri, L, et al. Predisposition toinfection inpatients with pemphigus. Retrospective study of141cases. Press Medicale. (2007) 36:1563–9. doi: 10.1016/j.lpm.2006.12.034
96. Merlant, M, Seta, V, Bernard, P, Fourati, S, Meritet, JF, Wolkenstein, P, et al. Pemphigus and herpes: multicentre survey and literature review. Ann Dermatol Venereol. (2018) 145:477–85. doi: 10.1016/j.annder.2018.03.169
97. Krain, LS . Pemphigus: epidemiologic and survival characteristics of 59 patients, 1955-1973. Arch Dermatol. (1974) 110:862. doi: 10.1001/archderm.110.6.862
98. Seta, V, Fichel, F, Méritet, JF, Bouam, S, Franck, N, Avril, MF, et al. Dermatoses et surinfection herpétique: étude rétrospective de 34 cas. Ann Dermatol Venereol. (2017) 144:176–81. doi: 10.1016/j.annder.2017.01.011
99. Ghalayani, P, Rashidi, F, and Saberi, Z. Assessment of IgG antibodies against HSV1, HSV2, CMV and EBV in patients with pemphigus vulgaris versus healthy people. J Dent (Tehran). (2015) 12:835–40.
100. Kurata, M, Mizukawa, Y, Aoyama, Y, and Shiohara, T. Herpes simplex virus reactivation as a trigger of mucous lesions in pemphigus vulgaris. Br J Dermatol. (2014) 171:554–60. doi: 10.1111/bjd.12961
101. Oliveira-Batista, DP, Janini, MER, Fernandes, NC, and Santos, N. Laboratory diagnosis of herpesvirus infections in patients with pemphigus vulgaris lesions. Intervirology. (2013) 56:231–6. doi: 10.1159/000349889
102. Lehman, JS, Khunger, M, and Lohse, CM. Infection in autoimmune bullous diseases: A retrospective comparative study. J Dermatol. (2013) 40:613–9. doi: 10.1111/1346-8138.12175
103. Esmaili, N, Mortazavi, H, Noormohammadpour, P, Boreiri, M, Soori, T, Vasheghani Farahani, I, et al. Pemphigus vulgaris and infections: A retrospective study on 155 patients. Autoimmune Dis. (2013) 2013:1–5. doi: 10.1155/2013/834295
104. Memar, OM, Rady, PL, and Tyring, SK. Human herpesvirus-8: detection of novel herpesvirus-like DNA sequences in Kaposi’s sarcoma and other lesions. J Mol Med. (1995) 73:603–9. doi: 10.1007/BF00196354
105. Wang, GQ, Xu, H, Wang, YK, Gao, XH, Zhao, Y, He, C, et al. Higher prevalence of human herpesvirus 8 DNA sequence and specific IgG antibodies in patients with pemphigus in China. J Am Acad Dermatol. (2005) 52:460–7. doi: 10.1016/j.jaad.2004.10.882
106. Dupin, N, Marcelin, AG, Calvez, V, and Andre, C. Absence of a link between human herpesvirus 8 and pemphigus [4]. Br J Dermatol. (1999) 141:159–60. doi: 10.1046/j.1365-2133.1999.02942.x
107. Bezold, G, Messer, G, Peter, RU, Flaig, MJ, and Sander, CA. Quantitation of human herpes virus 8 DNA in paraffin-embedded biopsies of HIV-associated and classical Kaposi’s sarcoma by PCR. J Cutan Pathol. (2001) 28:127–30. doi: 10.1034/j.1600-0560.2001.028003127.x
108. Cohen, SS, Weinstein, MD, Herndier, BG, Anhak, GJ, and Blauvelt, A. No evidence of human herpesvirus 8 infection in patients with paraneoplastic pemphigus, pemphigus vulgaris, or pemphigus foliaceus. J Invest Dermatol. (1998) 111:781–3. doi: 10.1046/j.1523-1747.1998.00384.x
109. Ruocco, V, Rossi, A, Satriano, RA, Sacerdoti, G, Astarita, C, and Pisani, M. Pemphigus foliaceus in a haemophilic child: cytomegalovirus induction? Acta Derm Venereol. (1982) 62:534–7. doi: 10.2340/0001555562534537
110. Cho, MJ, Ellebrecht, CT, Hammers, CM, Mukherjee, EM, Sapparapu, G, Boudreaux, CE, et al. Determinants of VH1-46 cross-reactivity to Pemphigus vulgaris autoantigen Desmoglein 3 and rotavirus antigen VP6. J Immunol. (2016) 197:1065–73. doi: 10.4049/jimmunol.1600567
111. Cho, A, Caldara, AL, Ran, NA, Menne, Z, Kauffman, RC, Affer, M, et al. Single-cell analysis suggests that ongoing affinity maturation drives the emergence of Pemphigus vulgaris autoimmune disease. Cell Rep. (2019) 28:909–922.e6. doi: 10.1016/j.celrep.2019.06.066
112. Markitziu, A, and Pisanty, S. Pemphigus vulgaris after infection by Epstein-Barr virus. Int J Dermatol. (1993) 32:917–8. doi: 10.1111/j.1365-4362.1993.tb01419.x
113. Barzilai, O, Sherer, Y, Ram, M, Izhaky, D, Anaya, JM, and Shoenfeld, Y. Epstein-Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci. (2007) 1108:567–77. doi: 10.1196/annals.1422.059
114. Demirci, GT, Aydingoz, IE, Mansur, AT, Atis, G, and Altunay, IK. Hepatitis C and hepatitis B virus infections in the etiopathogenesis of pemphigus. An Bras Dermatol. (2014) 89:423–6. doi: 10.1590/abd1806-4841.20142566
115. Marinho, RT, Johnson, NW, Fatela, NM, Serejo, FS, Glória, H, Raimundo, MO, et al. Oropharyngeal pemphigus in a patient with chronic hepatitis C during interferon alpha-2a therapy. Eur J Gastroenterol Hepatol. (2001) 13:869–72. doi: 10.1097/00042737-200107000-00017
116. Jang, H, Jin, YJ, Yoon, CH, Kim, CW, and Kim, L. Bullous pemphigoid associated with chronic hepatitis C virus infection in a hepatitis B virus endemic area: A case report. Med (United States). (2018) 97:e0377. doi: 10.1097/MD.0000000000010377
117. Min, MS, Damstetter, E, and Chen, AYY. Autoimmune blistering disorders in the setting of human immunodeficiency virus infection. Int J Women’s Dermatology. (2018) 4:159–65. doi: 10.1016/j.ijwd.2018.02.002
118. Polansky, M, Patel, N, and Feldman, R. Complete remission after rituximab therapy in an HIV-positive patient with pemphigus vulgaris. Br J Dermatol. (2015) 173:1557–9. doi: 10.1111/bjd.13990
119. Hodgson, TA, Fidler, SJ, Speight, PM, Weber, JN, and Porter, SR. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. (2003) 49:313–5. doi: 10.1067/S0190-9622(03)00413-4
120. Splaver, A, Silos, S, Lowell, B, Valenzuela, R, and Kirsner, RS. Case report: Pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDs. (2000) 14:295–6. doi: 10.1089/10872910050046304
121. Capizzi, R, Marasca, G, De Luca, A, Tacconelli, E, Cauda, R, and Rotoli, M. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient [8]. Dermatology. (1998) 197:97–8.
122. Marfatia, Y, Patel, S, Makrandi, S, and Sharma, P. Human immunodeficiency virus and pemphigus vulgaris: an interesting association [5]. Indian J Dermatol Venereol Leprol. (2007) 73:354–5. doi: 10.4103/0378-6323.35745
123. Mignogna, MD, Fedele, S, Lo Russo, L, Bonadies, G, Nappa, S, and Lo, ML. Acute cyclosporine nephrotoxicity in a patient with oral pemphigus vulgaris and HIV infection on antiretroviral therapy [5]. J Am Acad Dermatol. (2005) 53:1089–90. doi: 10.1016/j.jaad.2005.07.054
124. Ruocco, E, Lo Schiavo, A, Baroni, A, Sangiuliano, S, Puca, RV, Brunetti, G, et al. Pemphigus vulgaris after coxsackievirus infection and cephalosporin treatment: A paraviral eruption? Dermatology. (2008) 216:317–9. doi: 10.1159/000113944
125. Mortazavi, H, Hejazi, P, Khamesipour, A, Mohebali, M, Ehsani, AH, Mohammadi, Y, et al. Frequency of seropositivity against infectious agents amongst pemphigus vulgaris patients: a case-control study on Strongyloides stercoralis, Helicobacter pylori, toxoplasma gondii, Leishmania major, and Epstein-Barr virus. Int J Dermatol. (2015) 54:e458–65. doi: 10.1111/ijd.12869
126. Amagai, M, Matsuyoshi, N, Wang, ZH, Andl, C, and Stanley, JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med. (2000) 6:1275–7. doi: 10.1038/81385
127. Ruocco, V, Peluso, G, and Pisani, M. Pemphigus vulgaris in only one of two monozygotic twins. J Am Acad Dermatol. (1985) 12:587–9. doi: 10.1016/S0190-9622(85)80104-3
128. Fedeles, F, Murphy, M, Rothe, MJ, and Grant-Kels, JM. Nutrition and bullous skin diseases. Clin Dermatol. (2010) 28:627–43. doi: 10.1016/j.clindermatol.2010.03.036
129. Brenner, S, and Wolf, R. Possible nutritional factors in induced pemphigus. Dermatology. (1994) 189:337–9. doi: 10.1159/000246874
130. Tur, E, and Brenner, S. Diet and pemphigus: in pursuit of exogenous factors in pemphigus and fogo selvagem. Arch Dermatol. (1998) 134:1406–10. doi: 10.1001/archderm.134.11.1406
131. Ruocco, V, Brenner, S, and Lombardi, ML. A case of diet-related pemphigus. Dermatology. (1996) 192:373–4. doi: 10.1159/000246417
132. Chorzelski, TP, Hashimoto, T, Jablonska, S, Amagai, M, Ishii, K, Olszewska, M, et al. Can pemphigus vulgaris be induced by nutritional factors? Eur J Dermatol. (1996) 6:284–6.
133. Brenner, S, Ruocco, V, Wolf, R, de Angelis, E, and Lombardi, ML. Pemphigus and dietary factors: in vitro acantholysis by allyl compounds of the genus allium. Dermatology. (1995) 190:197–202. doi: 10.1159/000246684
134. Brenner, S, Srebrnik, A, and Goldberg, I. Pemphigus can be induced by topical phenol as well as by foods and drugs that contain phenols or thiols. J Cosmet Dermatol. (2003) 2:161–5. doi: 10.1111/j.1473-2130.2004.00098.x
135. Ruocco, V, De Angelis, E, and Lombardi, ML. Drug-induced pemphigus. II. Pathomechanisms and experimental investigations. Clin Dermatol. (1993) 11:507–13. doi: 10.1016/0738-081X(93)90158-9
136. Tur, E, and Brenner, S. Contributing exogenous factors in pemphigus. Int J Dermatol. (1997) 36:888–93. doi: 10.1046/j.1365-4362.1997.00334.x
137. Kanwar, AJ, and De, D. Pemphigus in India. Indian J Dermatol Venereol Leprol. (2011) 77:439–49. doi: 10.4103/0378-6323.82396
138. Newby, CS, Barr, RM, Greaves, MW, and Mallet, AI. Cytokine release and cytotoxicity in human keratinocytes and fibroblasts induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. (2000) 115:292–8. doi: 10.1046/j.1523-1747.2000.00056.x
139. Feliciani, C, Toto, P, Amerio, P, Mohammad Pour, S, Coscione, G, Amerio, P, et al. In vitro and in vivo expression of interleukin-1α and tumor necrosis factor-α mRNA in pemphigus vulgaris: interleukin-1α and tumor necrosis factor-α are involved in acantholysis. J Invest Dermatol. (2000) 114:71–7. doi: 10.1046/j.1523-1747.2000.00835.x
140. Brenner, S, Ruocco, V, Ruocco, E, Russo, A, Tur, E, Luongo, V, et al. In vitro tannin acantholysis. Int J Dermatol. (2000) 39:738–42. doi: 10.1046/j.1365-4362.2000.00938.x
141. Feliciani, C, Ruocco, E, Zampetti, A, Toto, P, Amerio, P, Tulli, A, et al. Tannic acid induces in vitro acantholysis of keratinocytes via IL-1α and TNF-α. Int J Immunopathol Pharmacol. (2007) 20:289–99. doi: 10.1177/039463200702000209
142. Gaul, LE . Contact dermatitis from synthetic oil of mustard. Arch Dermatol. (1964) 90:158–9. doi: 10.1001/archderm.1964.01600020026004
143. Lee, AN, and Werth, VP. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch Dermatol. (2004) 140:723–7. doi: 10.1001/archderm.140.6.723
144. Kraigher, O, Wohl, Y, Gat, A, and Brenner, S. A mixed immunoblistering disorder exhibiting features of bullous pemphigoid and pemphigus foliaceus associated with Spirulina algae intake. Int J Dermatol. (2008) 47:61–3. doi: 10.1111/j.1365-4632.2007.03388.x
145. Drago, F, Cacciapuoti, M, Basso, M, Parodi, A, and Rebora, A. Pemphigus improving with gluten-free diet [13]. Acta Derm Venereol. (2005) 85:84–5. doi: 10.1080/00015550410022258
146. Lin, L, Moran, TP, Peng, B, Yang, J, Culton, DA, Che, H, et al. Walnut antigens can trigger autoantibody development in patients with pemphigus vulgaris through a “hit-and-run” mechanism. J Allergy Clin Immunol. (2019) 144:720–728.e4. doi: 10.1016/j.jaci.2019.04.020
147. Gough, SCL, and Simmonds, MJ. The HLA region and autoimmune disease: associations and mechanisms of action. Curr Genomics. (2007) 8:453–65. doi: 10.2174/138920207783591690
148. Yan, L, Wang, JM, and Zeng, K. Association between HLA-DRB1 polymorphisms and pemphigus vulgaris: A meta-analysis. Br J Dermatol. (2012) 167:768–77. doi: 10.1111/j.1365-2133.2012.11040.x
149. Gazit, E, and Loewenthal, R. The immunogenetics of pemphigus vulgaris. Autoimmun Rev. (2005) 4:16–20. doi: 10.1016/j.autrev.2004.05.002
150. Tunca, M, Musabak, U, Sagkan, RI, Koc, E, and Akar, A. Association of human leukocyte antigen class II alleles with pemphigus vulgaris in a Turkish population. J Dermatol. (2010) 37:246–50. doi: 10.1111/j.1346-8138.2009.00743.x
151. Ahmed, AR, Yunis, EJ, Khatri, K, Wagner, R, Notani, G, Awdeh, Z, et al. Major histocompatibility complex haplotype studies in Ashkenazi Jewish patients with pemphigus vulgaris. Proc Natl Acad Sci U S A. (1990) 87:7658–62. doi: 10.1073/pnas.87.19.7658
152. Lombardi, ML, Mercuro, O, Ruocco, V, Lo, SA, Lombari, V, Guerrera, V, et al. Common human leukocyte antigen alleles in pemphigus vulgaris and pemphigus foliaceus Italian patients. J Invest Dermatol. (1999) 113:107–10. doi: 10.1046/j.1523-1747.1999.00626.x
153. Carcassi, C, Cottoni, F, Floris, L, Vacca, A, Mulargia, M, Arras, M, et al. HLA haplotypes and class II molecular alleles in Sardinian and Italian patients with pemphigus vulgaris. Tissue Antigens. (1996) 48:662–7. doi: 10.1111/j.1399-0039.1996.tb02689.x
154. González-Escribano, MF, Jiménez, G, Walter, K, Montes, M, Perez-Bernal, AM, Rodríguez, MR, et al. Distribution of HLA class II alleles among Spanish patients with pemphigus vulgaris. Tissue Antigens. (1998) 52:275–8. doi: 10.1111/j.1399-0039.1998.tb03043.x
155. Loiseau, P, Lecleach, L, Prost, C, Lepage, V, Busson, M, Bastuji-Garin, S, et al. HLA class II polymorphism contributes to specify desmoglein derived peptides in Pemphigus vulgaris and Pemphigus foliaceus. J Autoimmun. (2000) 15:67–73. doi: 10.1006/jaut.2000.0388
156. Párnická, Z, Švecová, D, Javor, J, Shawkatová, I, and Buc, M. High susceptibility to pemphigus vulgaris due to HLA-DRB1*14:54 in the Slovak population. Int J Immunogenet. (2013) 40:471–5. doi: 10.1111/iji.12052
157. Brochado, MJF, Nascimento, DF, Campos, W, Deghaide, NHS, Donadi, EA, and Roselino, AM. Differential HLA class I and class II associations in pemphigus foliaceus and pemphigus vulgaris patients from a prevalent southeastern Brazilian region. J Autoimmun. (2016) 72:19–24. doi: 10.1016/j.jaut.2016.04.007
158. Lee, E, Lendas, KA, Chow, S, Pirani, Y, Gordon, D, Dionisio, R, et al. Disease relevant HLA class II alleles isolated by genotypic, Haplotypic, and sequence analysis in north American Caucasians with Pemphigus vulgaris. Hum Immunol. (2006) 67:125–39. doi: 10.1016/j.humimm.2005.09.003
159. Salathiel, AM, Brochado, MJF, Kim, O, Deghaide, NHS, Donadi, EA, and Roselino, AM. Family study of monozygotic twins affected by pemphigus vulgaris. Hum Immunol. (2016) 77:600–4. doi: 10.1016/j.humimm.2016.05.005
160. Salviano-Silva, A, Becker, M, Augusto, DG, Busch, H, Adelman Cipolla, G, Farias, TD-J, et al. Genetic association and differential expression of HLAComplexGroup lncRNAs in pemphigus. J Autoimmun. (2021) 123:102705. doi: 10.1016/j.jaut.2021.102705
161. Sarig, O, Bercovici, S, Zoller, L, Goldberg, I, Indelman, M, Nahum, S, et al. Population-specific association between a polymorphic variant in ST18, encoding a pro-apoptotic molecule, and pemphigus vulgaris. J Invest Dermatol. (2012) 132:1798–805. doi: 10.1038/jid.2012.46
162. Vodo, D, Sarig, O, Geller, S, Ben-Asher, E, Olender, T, Bochner, R, et al. Identification of a functional risk variant for Pemphigus vulgaris in the ST18 gene. PLoS Genet. (2016) 12:e1006008. doi: 10.1371/journal.pgen.1006008
163. Fania, L, Moro, F, De Paolis, E, Provini, A, Salemme, A, Mariotti, F, et al. Pemphigus vulgaris in two pairs of siblings from two unrelated Italian families: human leukocyte antigen genotypes, ST18 mutation and immunological profile. J Dermatol. (2021) 48:211–4. doi: 10.1111/1346-8138.15656
164. Yue, Z, Fu, X, Chen, M, Wang, Z, Wang, C, Yang, B, et al. Lack of association between the single nucleotide polymorphism of ST18 and pemphigus in Chinese population. J Dermatol. (2014) 41:353–4. doi: 10.1111/1346-8138.12363
165. Etesami, I, Seirafi, H, Ghandi, N, Salmani, H, Arabpour, M, Nasrollahzadeh, A, et al. The association between ST18 gene polymorphism and severe pemphigus disease among Iranian population. Exp Dermatol. (2018) 27:1395–8. doi: 10.1111/exd.13778
166. Salviano-Silva, A, Petzl-Erler, ML, and Boldt, ABW. CD59 polymorphisms are associated with gene expression and different sexual susceptibility to pemphigus foliaceus. Autoimmunity. (2017) 50:377–85. doi: 10.1080/08916934.2017.1329830
167. Ben Jmaa, M, Abida, O, Bahloul, E, Toumi, A, Khlif, S, Fakhfakh, R, et al. Role of FOXP3 gene polymorphism in the susceptibility to Tunisian endemic Pemphigus Foliaceus. Immunol Lett. (2017) 184:105–11. doi: 10.1016/j.imlet.2017.02.005
168. Dar, SA, Akhter, N, Haque, S, Singh, T, Mandal, RK, Ramachandran, VG, et al. Tumor necrosis factor (TNF)- α −308G/A (rs1800629) polymorphism distribution in North India and its association with pemphigus: case-control study and meta-analysis. Autoimmunity. (2016) 49:179–87. doi: 10.3109/08916934.2015.1134512
169. Eberhard, Y, Burgos, E, Gagliardi, J, Vullo, CM, Borosky, A, Pesoa, S, et al. Cytokine polymorphisms in patients with pemphigus. Arch Dermatol Res. (2005) 296:309–13. doi: 10.1007/s00403-004-0528-6
170. Javor, J, Chmurova, N, Parnicka, Z, Ferencik, S, Grosse-Wilde, H, Buc, M, et al. TNF-α and IL-10 gene polymorphisms show a weak association with pemphigus vulgaris in the Slovak population. J Eur Acad Dermatology Venereol. (2010) 24:65–8. doi: 10.1111/j.1468-3083.2009.03260.x
171. Mosaad, YM, Fathy, H, Fawzy, Z, and El-Saied, MA. Tumor necrosis factor-α -308 G>A and interleukin-6 -174 G>C promoter polymorphisms and pemphigus. Hum Immunol. (2012) 73:560–5. doi: 10.1016/j.humimm.2012.02.001
172. Pereira, NF, Hansen, JA, Lin, MT, Roxo, VMMS, Braun, K, and Petzl-Erler, ML. Cytokine gene polymorphisms in endemic pemphigus foliaceus: A possible role for IL6 variants. Cytokine. (2004) 28:233–41. doi: 10.1016/j.cyto.2004.08.006
173. Torzecka, JD, Narbutt, J, Sysa-Jedrzejowska, A, Borowiec, M, Ptasinska, A, Woszczek, G, et al. Tumour necrosis factor-α polymorphism as one of the complex inherited factors in pemphigus. Mediat Inflamm. (2003) 12:303–7. doi: 10.1080/09629350310001619735
174. Toumi, A, Abida, O, Ben Ayed, M, Masmoudi, A, Turki, H, and Masmoudi, H. Cytokine gene polymorphisms in Tunisian endemic pemphigus foliaceus: A possible role of il-4 variants. Hum Immunol. (2013) 74:658–65. doi: 10.1016/j.humimm.2013.01.009
175. Kavala, M, Kural, E, Kocaturk, E, Zindanci, I, Turkoglu, Z, and Can, B. The evaluation of thyroid diseases in patients with pemphigus vulgaris. Sci World J. (2012) 26:2913–9. doi: 10.1007/s12206-012-0709-8
176. Pitoia, F, Moncet, D, Glorio, R, Diaz, AG, Rodriguez Costa, G, Carbia, S, et al. Prevalence of thyroid autoimmunity in patients with pemphigus vulgaris. Medicina (B Aires). (2005) 116:A13–8. doi: 10.1097/ALN.0b013e31825dd7ac
177. Parameswaran, A, Attwood, K, Sato, R, Seiffert-Sinha, K, and Sinha, AA. Identification of a new disease cluster of pemphigus vulgaris with autoimmune thyroid disease, rheumatoid arthritis and type i diabetes. Br J Dermatol. (2015) 172:729–38. doi: 10.1111/bjd.13433
178. Heelan, K, Mahar, AL, Walsh, S, and Shear, NH. Pemphigus and associated comorbidities: A cross-sectional study. Clin Exp Dermatol. (2015) 40:593–9. doi: 10.1111/ced.12634
179. Daneshpazhooh, M, Behjati, J, Hashemi, P, Shamohammadi, S, Mortazavi, H, Nazemi, MJ, et al. Thyroid autoimmunity and pemphigus vulgaris: is there a significant association? J Am Acad Dermatol. (2010) 62:349–51. doi: 10.1016/j.jaad.2009.05.024
180. Kridin, K, Khamaisi, M, Comaneshter, D, Batat, E, and Cohen, AD. Autoimmune thyroid diseases and thyroid Cancer in Pemphigus: A big data analysis. Front Med. (2018) 5:1–5. doi: 10.3389/fmed.2018.00159
181. Hsu, DY, Brieva, J, Sinha, AA, Langan, SM, and Silverberg, JI. Comorbidities and inpatient mortality for pemphigus in the U.S.A. Br J Dermatol. (2016) 174:1290–8. doi: 10.1111/bjd.14463
182. Schulze, F, Neumann, K, Recke, A, Zillikens, D, Linder, R, and Schmidt, E. Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol. (2015) 135:1445–7. doi: 10.1038/jid.2014.547
183. Kridin, K, Zelber-Sagi, S, Comaneshter, D, Batat, E, and Cohen, AD. Pemphigus and hematologic malignancies: A population-based study of 11,859 patients. J Am Acad Dermatol. (2018) 78:1084–1089.e1. doi: 10.1016/j.jaad.2017.11.039
184. Kridin, K, Zelber-Sagi, S, Comaneshter, D, and Cohen, AD. Coexistent solid malignancies in pemphigus a population-based study. JAMA Dermatol. (2018) 154:435–40. doi: 10.1001/jamadermatol.2017.6334
185. Ogawa, H, Sakuma, M, Morioka, S, Kitamura, K, Sasai, Y, Imamura, S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. (1995) 9:136–41. doi: 10.1016/0923-1811(94)00371-K
186. Kridin, K, Comaneshter, D, Batat, E, and Cohen, AD. COPD and lung cancer in patients with pemphigus- a population based study. Respir Med. (2018) 136:93–7. doi: 10.1016/j.rmed.2018.02.005
187. Cohen-Barak, E, Sonnenscien, D, Ziv, M, Shani-Adir, A, and Rozenman, D. Kaposi’s sarcoma in a patient with pemphigus vulgaris. Int J Dermatol. (2016) 55:85–8. doi: 10.1111/ijd.12420
188. Balighi, K, Daneshpazhooh, M, Aghazadeh, N, Hejazi, P, Aryanian, Z, Azizpour, A, et al. Pemphigus vulgaris-associated Kaposi’s sarcoma: response to paclitaxel and review of the literature. J Eur Acad Dermatology Venereol. (2014) 28:987–94. doi: 10.1111/jdv.12348
189. Tourlaki, A, Genovese, G, Guanziroli, E, Scoppio, BM, Berti, E, and Brambilla, L. Autoimmune bullous diseases in non-HIV Kaposi’s sarcoma: a retrospective study in a large cohort of patients. J Eur Acad Dermatology Venereol. (2018) 32:1777–83. doi: 10.1111/jdv.15051
190. Kridin, K, Kridin, M, Shalom, G, and Cohen, AD. The coexistence of pemphigus and psoriasis: a systematic review and meta-analysis. Immunol Res. (2019) 67:134–41. doi: 10.1007/s12026-018-9031-6
191. Tsai, TF, Wang, TS, Hung, ST, Tsai, PIC, Schenkel, B, Zhang, M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. (2011) 63:40–6. doi: 10.1016/j.jdermsci.2011.03.002
192. Calebotta, A, Cirocco, A, Giansante, E, and Reyes, O. Systemic lupus erythematosus and pemphigus vulgaris: association or coincidence. Lupus. (2004) 13:951–3. doi: 10.1191/0961203304lu1073cr
193. Gholizadeh, N, Taghavi Zenouz, A, and Eslami, H. Pemphigus vulgaris associated with rheumatoid arthritis in a patient not taking Penicillamine. J Dent Res Dent Clin Dent Prospects. (2012) 6:33–5. doi: 10.5681/joddd.2012.008
194. Iino, Y, Hara, H, Suda, T, Okada, T, Baba, S, and Suzuki, H. Co-existence of pemphigus vulgaris and Hashimoto’s thyroiditis. Eur J Dermatology. (2005) 15:40–2.
Keywords: pemphigus vulgaris, pemphigus foliaceous, autoimmune bullous disease, trigger factors, predisposing factors, etiopathogenesis
Citation: Moro F, Sinagra JLM, Salemme A, Fania L, Mariotti F, Pira A, Didona B and Di Zenzo G (2023) Pemphigus: trigger and predisposing factors. Front. Med. 10:1326359. doi: 10.3389/fmed.2023.1326359
Received: 23 October 2023; Accepted: 29 November 2023;
Published: 27 December 2023.
Edited by:
Ralf J. Ludwig, University of Lübeck, GermanyReviewed by:
Hiroshi Koga, Kurume University School of Medicine, JapanCopyright © 2023 Moro, Sinagra, Salemme, Fania, Mariotti, Pira, Didona and Di Zenzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Moro, Zi5tb3JvQGlkaS5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.