- 1Department of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, China
- 2Department of Spinal Orthopedics, Weifang Hospital of Traditional Chinese Medicine, Weifang, China
- 3Department of Orthopedics, Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, China
Background: The relationship between visceral adipose tissue and osteoarthritis is not clear. The purpose of our study was to explore the relationship between visceral adipose tissue and osteoarthritis.
Methods: We used a two-sample Mendelian randomization method to select single-nucleotide polymorphisms (SNPs) significantly associated with visceral adipose tissue as instrumental variables to explore the relationship between visceral adipose tissue and all osteoarthritis, hand osteoarthritis, hip osteoarthritis, knee osteoarthritis, and spine osteoarthritis. The reliability of the results was tested using sensitivity analysis.
Results: Our findings indicated that visceral adipose tissue was associated with all osteoarthritis, hip osteoarthritis, knee osteoarthritis, and spine osteoarthritis (all osteoarthritis: OR = 1.399, 95% CI: 1.335–1.467, p = 7.95e-44; hip osteoarthritis: OR = 1.399, 95% CI: 1.284–1.524, p = 1.41e-14; knee osteoarthritis: OR = 1.794, 95% CI: 1.662–1.937, p = 1.33e-50; and spine osteoarthritis: OR = 1.445, 95% CI: 1.314–1.589, p = 2.89e-14). Sensitivity analysis demonstrated the reliability of these results.
Conclusion: Our study suggests that genetically predicted visceral adipose tissue is associated with osteoarthritis. Reducing the accumulation of visceral adipose tissue could potentially have an impact on the incidence of osteoarthritis.
1 Introduction
Osteoarthritis (OA) is a chronic degenerative disease characterized by cartilage degeneration, subchondral bone changes, and synovitis, primarily affecting the hip, knee, hand, and other joints (1). It has a high global prevalence (2) and ranks fifth among all causes of disability worldwide, posing a significant threat to human health (3). Treating OA includes early pain management and end-stage joint replacement, but the high cost of treatment imposes a significant burden on society and individuals (4). Although the mechanism and risk factors of OA are not fully understood, it is generally believed to be closely related to age, obesity, and other factors (5).
Obesity has been shown to be closely associated with the pathogenesis of various diseases (6–8). In these studies, obesity is often assessed using human indicators such as BMI, waist-to-hip ratio, waist circumference, and hip circumference. However, due to the heterogeneity of obesity, there are considerable individual differences in body fat distribution and metabolic characteristics, even among individuals with the same body mass index (BMI) (9). Thus, BMI or other general obesity measurement methods may not accurately assess metabolic status and body fat distribution. Visceral adipose tissue, which is considered a marker of ectopic fat deposition and hormonal environmental disorders, is more metabolically active and potentially reflects the natural metabolic abnormalities of obesity (10). It refers to the adipose tissue accumulated in the peritoneal cavity between the organs and the trunk and is a significant component of total body adipose tissue (11). Increased visceral adipose tissue, also known as central obesity, is an important manifestation of obesity. While previous studies have reported a genetic causal relationship between BMI and knee and hip OA (12), there is limited research on the relationship between visceral adipose tissue and OA, and the precise association between them remains unclear (13).
Mendelian randomization is an analytical method that utilizes genetic variation as an instrumental variable to investigate the causal relationship between exposure and outcomes based on the random distribution of genetic variations during conception (14). This method can largely mitigate the influence of reverse causality and confounding factors in observational studies (15), making it increasingly utilized in clinical studies. The purpose of this study is to explore the relationship between visceral adipose tissue and OA using a two-sample Mendelian randomization research method, aiming to provide insights for managing OA.
2 Method and design
2.1 Research design
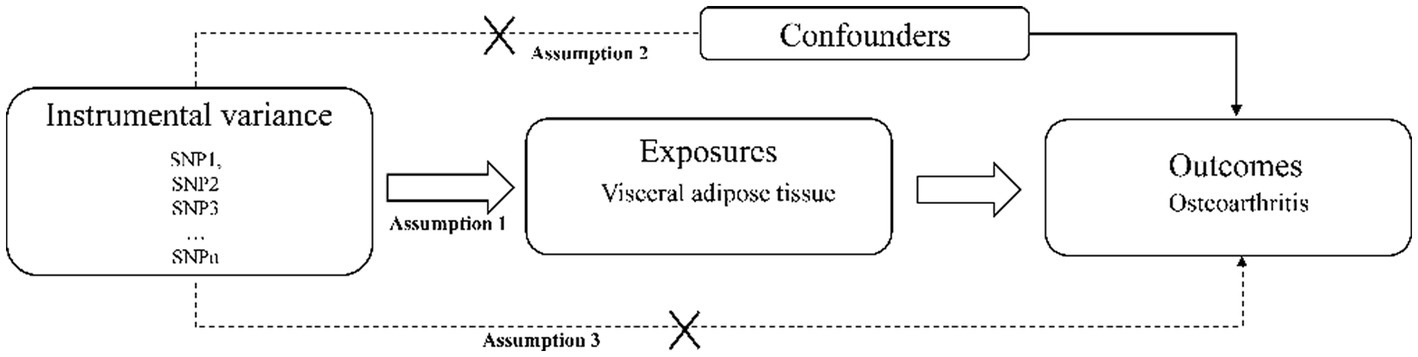
In this study, a two-sample Mendelian randomization analysis was used to select SNPs significantly associated with visceral adipose tissue as instrumental variables to explore the relationship between visceral adipose tissue and all OA, hand OA, hip OA, knee OA, and spine OA. Mendelian randomized research design must meet three assumptions: (1) Instrumental variables are related to exposure factors. (2) Instrumental variables are not related to confounding factors. (3) Instrumental variables can only affect outcomes through exposure factors (16) Figure 1.
2.2 Data sources
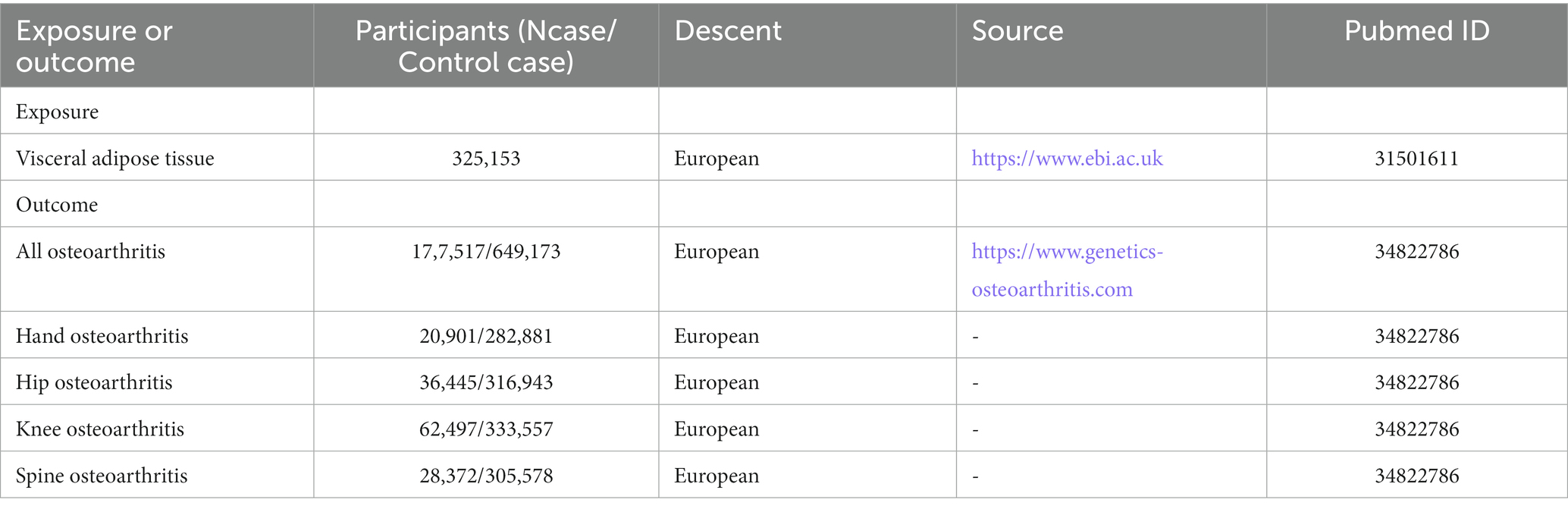
Visceral adipose tissue-related data were obtained from a recent large-scale summary of GWAS by Karlsson et al. (17) which included 325,153 white British subjects. The study consisted of two cohorts and used estimates by dual-energy X-ray absorptiometry (DXA) to create predictive models. Through screening, we selected the single-nucleotide polymorphism (SNP) that was significantly correlated with VAT (p < 5 × 10−8) at the whole-gene level. After removing the linkage imbalance and palindrome sequence, the F-value of each SNP was calculated, and SNPs with an F-value >10 were selected as tool variables. In addition, we removed SNPs associated with confounding factors (apolipoprotein B, low-density lipoprotein, smoking, and osteoporosis) and outcomes through the online website PhenoScanner.1
Data related to OA were obtained from the latest GWAS data (18) of the Osteoarthritis Genetics (GO) Consortium, which included 826,690 samples from 177,517 OA patients. The number of OA cases in the hand, spine, hip, and knee joints was 20,901, 28,372, 36,445, and 62,497, respectively, all of which were European population samples. The basic information included in the data sources is presented in Table 1.
2.3 Statistical analysis
The main effect analysis in this study was the inverse variance weighting (IVW) of random effects, which combined the Wald ratio of results for each SNP and conducted a meta-analysis. In addition, MR-Egger regression and a weighted median estimator (WME) were used to supplement the IVW method. Outliers were screened using the MR PRESSO method. If any outliers were found, they were excluded, and MR analysis was performed again. The reliability of the results was tested using sensitivity analysis methods such as Cochran’s Q, MR-Egger intercept analysis, and funnel plot. Cochran’s Q statistics were used to test for heterogeneity. A p-value of >0.05 indicates no significant heterogeneity in the analysis. To evaluate the bias for gene pleiotropy using MR-Egger intercept analysis, the closer the regression intercept to 0, the less likely the gene pleiotropy would be. We also generated power values for each MR analysis using an online MR power calculation tool2 (19).
All analyses in this study were performed on R 4.2.1 and the MR PERESSO and TwosampleMR packages. After Bonferroni correction, a p-value of <0.01 (0.05/5) was considered significant.
3 Results
3.1 Instrumental variables
After screening, 218 SNPs associated with visceral adipose tissue were identified, explaining approximately 3.38% of the genetic variation. The F-values of the included SNPs were > 10, excluding the possibility of weak instrumental variables. The details of the included SNP are shown in the Supplementary material.
3.2 MR results
The results of the MR analyses are presented in Figure 2. Our results showed that genetically predicted visceral adipose tissue was associated with all OA (OR = 1.399, 95% CI: 1.335–1.467, p = 7.95e-44), which was also directionally consistent and significantly validated in the MR Egger, WME, and MR PRESSO methods. In addition, visceral adipose tissue is also associated with hip OA, knee OA, and spinal OA (hip OA: OR = 1.399, 95% CI: 1.284–1.524, p = 1.41e-14; knee OA: OR = 1.794, 95% CI: 1.662–1.937, p = 1.33e-50; and spine OA: OR = 1.445, 95% CI: 1.314–1.589, p = 2.89e-14), indicating that the visceral adipose tissue is closely associated with OA at multiple sites. The scatter plot of the MR analysis of the visceral adipose tissue and OA can be seen in Figure 3.
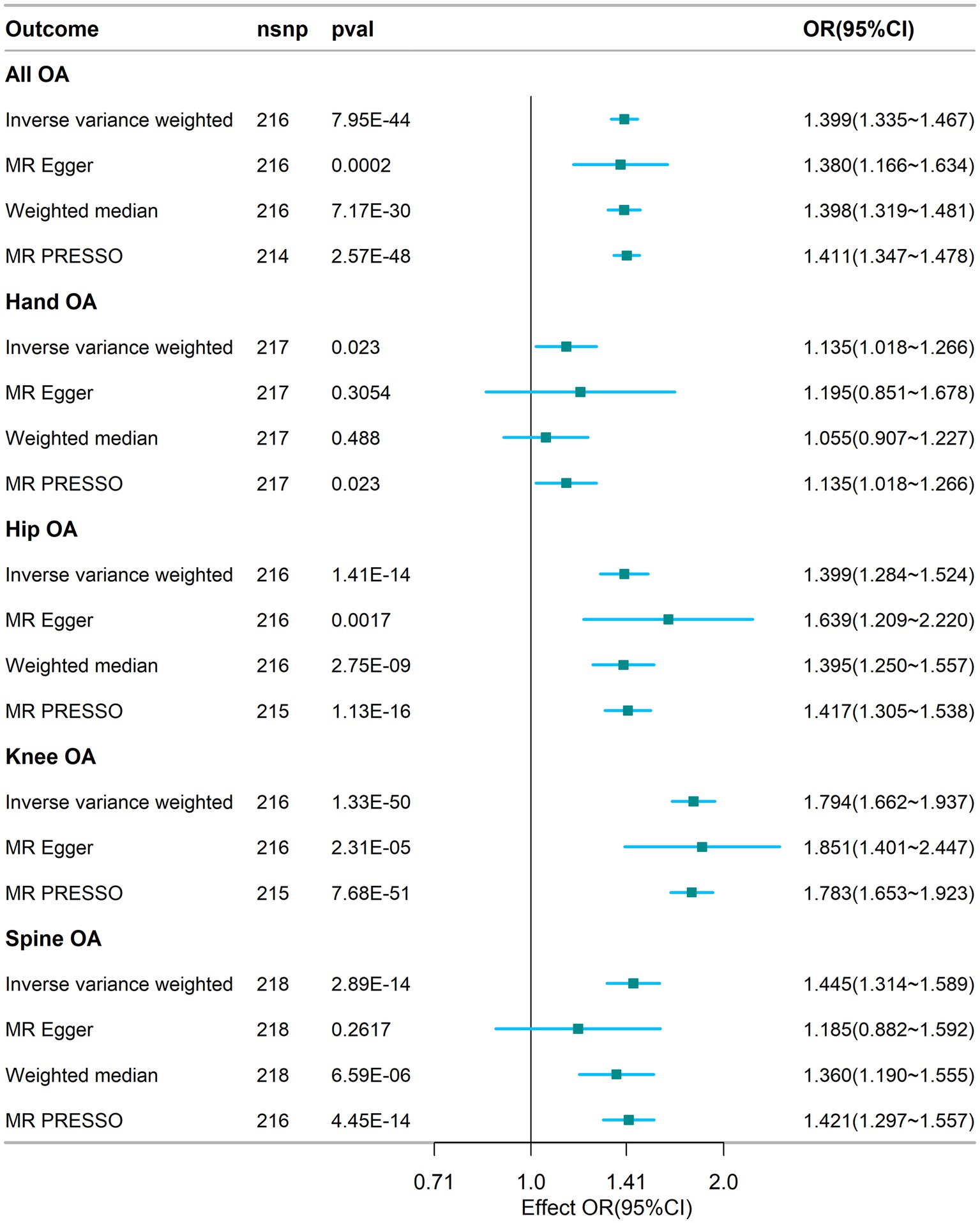
Figure 2. Association between visceral adipose tissue and osteoarthritis based on different methods. OA, osteoarthritis; SNP, single nucleotide polymorphism; IVW, inverse-variance weighted; OR, odds ratio; CI, confidence interval; nsnp, number of snp.
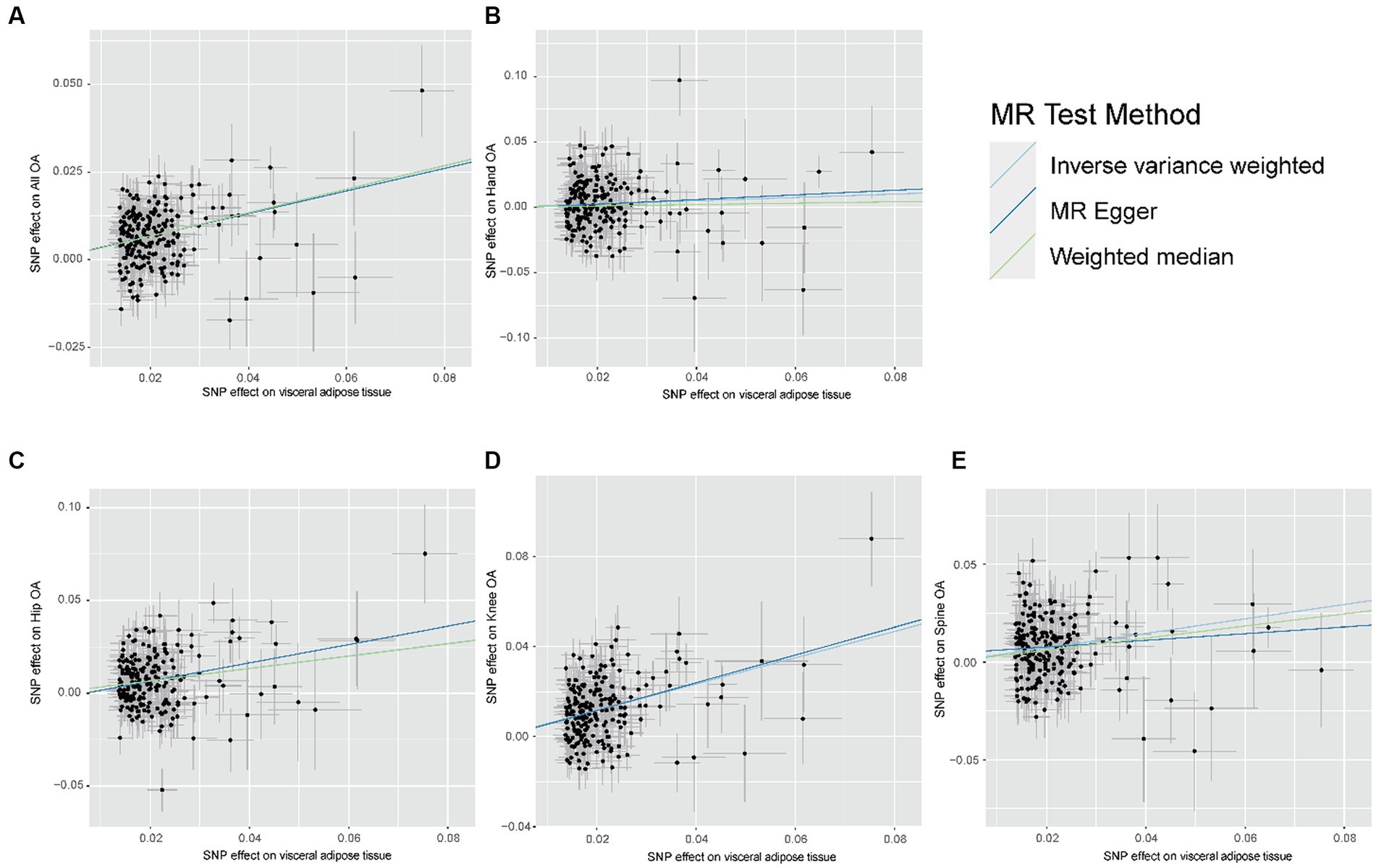
Figure 3. Scatter plot of the relationship between visceral adipose tissue and OA. (A) Scatter plot of the relationship between visceral adipose tissue and All OA. (B) Scatter plot of the relationship between visceral adipose tissue and Hand OA. (C) Scatter plot of the relationship between visceral adipose tissue and Hip OA. (D) Scatter plot of the relationship between visceral adipose tissue and Knee OA. (E) Scatter plot of the relationship between visceral adipose tissue and Spine OA; OA, osteoarthritis.
The results of sensitivity analyses are presented in Table 2, and in sensitivity analyses, the MR Egger intercept test was used to find potential horizontal pleiotropy. No horizontal pleiotropy was found with each MR analysis. The results of Cochran’s Q-test showed extensive heterogeneity. Because we used random effects IVW as the primary outcome, the heterogeneity was acceptable (20). In addition, no significant bias was observed in the funnel plots of each MR analysis (Figure 4).
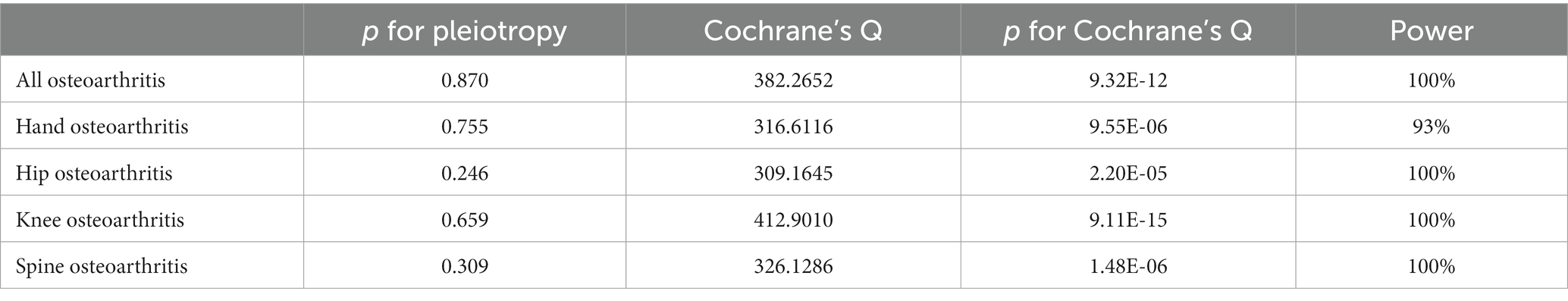
Table 2. Sensitivity analysis results and power value of the correlation between visceral adipose tissue and osteoarthritis.
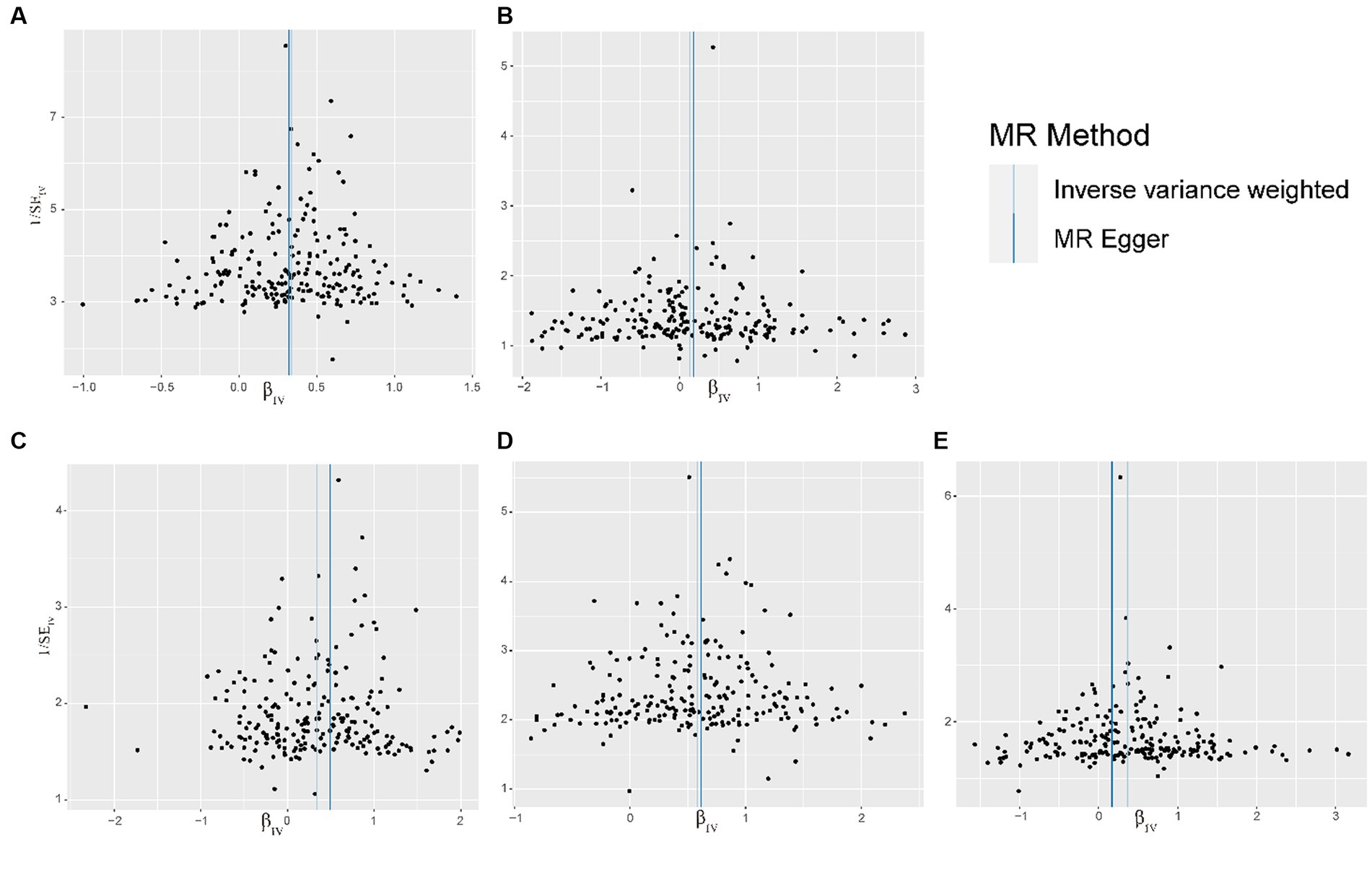
Figure 4. Funnel plot of the relationship between visceral adipose tissue and OA. (A) Funnel plot of the relationship between visceral adipose tissue and All OA. (B) Funnel plot of the relationship between visceral adipose tissue and Hand OA. (C) Funnel plot of the relationship between visceral adipose tissue and Hip OA. (D) Funnel plot of the relationship between visceral adipose tissue and Knee OA. (E) Funnel plot of the relationship between visceral adipose tissue and Spine OA; OA, osteoarthritis.
4 Discussion
Our study has demonstrated a relationship between visceral adipose tissue and OA. Specifically, for every unit increase in visceral adipose tissue, the risk of developing all OA increases by 40%. Moreover, the risk of hip OA increases by 40%, knee OA increases by 79%, and spine OA increases by 45%. These findings may provide new insights into the connection between OA and obesity.
The association between obesity and OA has been extensively explored in previous studies. Reyes et al.’s cohort study highlighted the association between BMI and OA (21). Similarly, Yuan et al.’s Mendelian randomization analysis indicated that elevated BMI increases the risk of hip OA (22). Another study comprehensively evaluated various measures of obesity, such as waist-to-hip ratio, waist circumference, hip circumference, and body fat content, and their effects on knee and hip OA. This study revealed that different measures of obesity have varying impacts on OA (23). However, these studies did not specifically focus on the influence of visceral adipose tissue on OA. Therefore, our study contributes additional evidence to elucidate the relationship between visceral adipose tissue and OA. To the best of our knowledge, this is the first Mendelian randomized study investigating the connection between visceral adipose tissue and OA.
Prior research has suggested that the accumulation of visceral adipose tissue may be more detrimental than adipose tissue in other body locations (24). In a study by Erdal Belen et al., the thickness of epicardial fat in knee OA patients was found to be greater than that in the control group, and this thickness was associated with the severity of knee OA (25). Furthermore, Eric et al. demonstrated that patients with knee OA exhibited excessive fat accumulation in the central region (26). Although there is no direct evidence linking visceral adipose tissue to OA, Li et al. demonstrated an association between visceral adipose tissue and joint pain (27). Additionally, Visser et al.’s epidemiological study (28) on the Dutch population revealed an association between hand OA and visceral adipose tissue, corroborating our findings.
The link between visceral adipose tissue and OA may be influenced by multiple mechanisms. OA, being a degenerative disease, is believed to be associated with inflammatory processes (29). As the primary fat reservoir in the human body, the visceral adipose tissue is thought to secrete various adipokines, including interleukin-6 (IL-6) and tumor necrosis factor (TNF). These adipokines are believed to play a role in the pathogenesis of OA (30, 31). Interleukin 6 is believed to facilitate cartilage degradation in post-traumatic OA by promoting an increase in MMP-13 and aggrecanase expression. Additionally, its effects are influenced by gender (32). In their study, Xue et al. demonstrated that tumor necrosis factor enables the upregulation of mRNA for a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4), which plays a key role in the pathogenesis of OA by promoting cartilage breakdown in humans (33). Furthermore, leptin, an inflammatory adipose factor, has been shown to affect distal joints. It can enhance collagen degradation and regulate the production of metalloproteinases, thus promoting chondrocyte degradation (34, 35). On the other hand, adiponectin may have a protective effect against OA progression (36), but the accumulation of visceral adipose tissue may inhibit adiponectin transcription, thus enhancing its pro-inflammatory effect (37).
Observational studies are bound to be influenced by confounding factors. However, we have minimized the impact of reverse causality and confounding factors as much as possible by using Mendelian randomization methods. This method provides evidence for the connection between visceral adipose tissue and OA at different anatomical sites. This association has also been verified through sensitivity analysis. Nonetheless, our study has certain limitations. First, due to the constraints of the original GWAS data source, our research primarily encompasses the European population, and we have not explored similar associations in other populations. Second, although we did not identify the presence of level pleiotropy in our study, there was significant heterogeneity among SNPs, and we did not undertake further data filtering to reduce heterogeneity. Third, the original data did not provide age stratification, which prevented us from conducting stratified data analysis to assess the impact of age. Fourth, the proportion of genetic variation explained by visceral adipose tissue remains relatively small. Additionally, our MR analysis may reflect the effect of lifelong exposure to high visceral adipose tissue on OA, yet the risk of OA at a specific time may be influenced differently. Finally, the susceptibility of visceral adipose tissue to OA may be influenced by maternal effects. Intrauterine exposure or maternal behavior, influenced by the maternal genetic background, may contribute to the association between offspring genotype and the risk of OA (38).
5 Conclusion
Above all, our study showed that genetically predicted visceral adipose tissue is associated with OA, which also reveals the adverse effects of obesity on human health at the genetic level. Controlling central obesity through intervention is of positive significance for the prevention of OA. However, further large-scale longitudinal studies or randomized controlled trials are needed to further investigate the profound relationship between visceral adipose tissue and the increased risk of OA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Writing – original draft, Data curation, Methodology, Supervision, Writing – review & editing. YW: Writing – review & editing, Conceptualization, Investigation, Writing – original draft. JX: Data curation, Writing – review & editing. ZW: Methodology, Writing – review & editing. WZ: Supervision, Writing – review & editing. CZ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the 2021 project of the Jilin Science and Technology Development Plan (20210303004SF) and the Natural Science Foundation of Jilin Province (YDZJ202201ZYTS216).
Acknowledgments
The authors thank all those involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1324449/full#supplementary-material
Footnotes
References
1. Hunter, DJ, and Bierma-Zeinstra, S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
2. Hunter, DJ, March, L, and Chew, M. Osteoarthritis in 2020 and beyond: a lancet commission. Lancet. (2020) 396:1711–2. doi: 10.1016/S0140-6736(20)32230-3
3. Murray, CJ, Vos, T, Lozano, R, Naghavi, M, Flaxman, AD, Michaud, C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
4. Delanois, RE, Mistry, JB, Gwam, CU, Mohamed, NS, Choksi, US, and Mont, MA. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplast. (2017) 32:2663–8. doi: 10.1016/j.arth.2017.03.066
5. Bijlsma, JW, Berenbaum, F, and Lafeber, FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. (2011) 377:2115–26. doi: 10.1016/S0140-6736(11)60243-2
6. Sommer, A, and Twig, G. The impact of childhood and adolescent obesity on cardiovascular risk in adulthood: a systematic review. Curr Diab Rep. (2018) 18:91. doi: 10.1007/s11892-018-1062-9
7. Marinelli, S, Napoletano, G, Straccamore, M, and Basile, G. Female obesity and infertility: outcomes and regulatory guidance. Acta Biomed. (2022) 93:e2022278. doi: 10.23750/abm.v93i4.13466
8. McGuire, SP, Keller, SL, Maatman, TK, Lewellen, KA, Ceppa, EP, House, MG, et al. Obesity worsens local and systemic complications of necrotizing pancreatitis and prolongs disease course. J Gastrointest Surg. (2022) 26:2128–35. doi: 10.1007/s11605-022-05383-0
9. Chen, L, Sun, X, Han, D, Zhong, J, Zhang, H, and Zheng, L. Visceral adipose tissue and risk of COVID-19 susceptibility, hospitalization, and severity: a Mendelian randomization study. Front Public Health. (2022) 10:1023935. doi: 10.3389/fpubh.2022.1023935
10. Huang, Y, Liu, Y, Ma, Y, Tu, T, Liu, N, Bai, F, et al. Associations of visceral adipose tissue, circulating protein biomarkers, and risk of cardiovascular diseases: a Mendelian randomization analysis. Front Cell Dev Biol. (2022) 10:840866. doi: 10.3389/fcell.2022.840866
11. Ibrahim, MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. (2010) 11:11–8. doi: 10.1111/j.1467-789X.2009.00623.x
12. Funck-Brentano, T, Nethander, M, Moverare-Skrtic, S, Richette, P, and Ohlsson, C. Causal factors for knee, hip, and hand osteoarthritis: a Mendelian randomization study in the UK biobank. Arthritis Rheumatol. (2019) 71:1634–41. doi: 10.1002/art.40928
13. Chang, J, Liao, Z, Lu, M, Meng, T, Han, W, and Ding, C. Systemic and local adipose tissue in knee osteoarthritis. Osteoarthr Cartil. (2018) 26:864–71. doi: 10.1016/j.joca.2018.03.004
14. Holmes, MV, Ala-Korpela, M, and Smith, GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
15. Xu, H, Ling, Y, Jiang, H, Li, Y, and Jiang, M. Osteoarthritis, coronary artery disease, and myocardial infarction: a mendelian randomization study. Front Cardiovasc Med. (2022) 9:892742. doi: 10.3389/fcvm.2022.892742
16. Verduijn, M, Siegerink, B, Jager, KJ, Zoccali, C, and Dekker, FW. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant. (2010) 25:1394–8. doi: 10.1093/ndt/gfq098
17. Karlsson, T, Rask-Andersen, M, Pan, G, Hoglund, J, Wadelius, C, Ek, WE, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. (2019) 25:1390–5. doi: 10.1038/s41591-019-0563-7
18. Boer, CG, Hatzikotoulas, K, Southam, L, Stefansdottir, L, Zhang, Y, Coutinho de Almeida, R, et al. Deciphering osteoarthritis genetics across 826, 690 individuals from 9 populations. Cells. (2021) 184:6003–5. doi: 10.1016/j.cell.2021.11.003
19. Brion, MJ, Shakhbazov, K, and Visscher, PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
20. Burgess, S, Davey Smith, G, Davies, NM, Dudbridge, F, Gill, D, Glymour, MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.1
21. Reyes, C, Leyland, KM, Peat, G, Cooper, C, Arden, NK, and Prieto-Alhambra, D. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. (2016) 68:1869–75. doi: 10.1002/art.39707
22. Yuan, J, Wang, D, Zhang, Y, and Dou, Q. Genetically predicted obesity and risk of hip osteoarthritis. Eat Weight Disord. (2023) 28:11. doi: 10.1007/s40519-023-01538-3
23. Wang, C, Zhu, Y, Liu, Z, Long, H, Ruan, Z, and Zhao, S. Causal associations of obesity related anthropometric indicators and body compositions with knee and hip arthritis: a large-scale genetic correlation study. Front Endocrinol (Lausanne). (2022) 13:1011896. doi: 10.3389/fendo.2022.1011896
24. Fox, CS, Massaro, JM, Hoffmann, U, Pou, KM, Maurovich-Horvat, P, Liu, CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. (2007) 116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355
25. Belen, E, Karaman, O, Caliskan, G, Atamaner, O, and Aslan, O. An indicator of subclinical cardiovascular disease in patients with primary osteoarthritis: epicardial fat thickness. Int J Clin Exp Med. (2015) 8:9491–7.
26. Toussirot, E, Michel, F, Bereau, M, Dehecq, B, Gaugler, B, Wendling, D, et al. Serum adipokines, adipose tissue measurements and metabolic parameters in patients with advanced radiographic knee osteoarthritis. Clin Rheumatol. (2017) 36:2531–9. doi: 10.1007/s10067-017-3789-0
27. Li, S, Schwartz, AV, LaValley, MP, Wang, N, Desai, N, Sun, X, et al. Association of Visceral Adiposity with Pain but not structural osteoarthritis. Arthritis Rheumatol. (2020) 72:1103–10. doi: 10.1002/art.41222
28. Visser, AW, Ioan-Facsinay, A, de Mutsert, R, Widya, RL, Loef, M, de Roos, A, et al. Adiposity and hand osteoarthritis: the Netherlands epidemiology of obesity study. Arthritis Res Ther. (2014) 16:R19. doi: 10.1186/ar4447
29. Yuan, GH, Masuko-Hongo, K, Kato, T, and Nishioka, K. Immunologic intervention in the pathogenesis of osteoarthritis. Arthritis Rheum. (2003) 48:602–11. doi: 10.1002/art.10768
30. Villaret, A, Galitzky, J, Decaunes, P, Esteve, D, Marques, MA, Sengenes, C, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. (2010) 59:2755–63. doi: 10.2337/db10-0398
31. Kraus, VB, McDaniel, G, Huebner, JL, Stabler, TV, Pieper, CF, Shipes, SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr Cartil. (2016) 24:1613–21. doi: 10.1016/j.joca.2016.04.010
32. Liao, Y, Ren, Y, Luo, X, Mirando, AJ, Long, JT, Leinroth, A, et al. Interleukin-6 signaling mediates cartilage degradation and pain in posttraumatic osteoarthritis in a sex-specific manner. Sci Signal. (2022) 15:eabn7082. doi: 10.1126/scisignal.abn7082
33. Xue, J, Wang, J, Liu, Q, and Luo, A. Tumor necrosis factor-alpha induces ADAMTS-4 expression in human osteoarthritis chondrocytes. Mol Med Rep. (2013) 8:1755–60. doi: 10.3892/mmr.2013.1729
34. Massengale, M, Reichmann, WM, Losina, E, Solomon, DH, and Katz, JN. The relationship between hand osteoarthritis and serum leptin concentration in participants of the third National Health and nutrition examination survey. Arthritis Res Ther. (2012) 14:R132. doi: 10.1186/ar3864
35. Hui, W, Litherland, GJ, Elias, MS, Kitson, GI, Cawston, TE, Rowan, AD, et al. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann Rheum Dis. (2012) 71:455–62. doi: 10.1136/annrheumdis-2011-200372
36. Chen, TH, Chen, L, Hsieh, MS, Chang, CP, Chou, DT, and Tsai, SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. (2006) 1762:711–8. doi: 10.1016/j.bbadis.2006.06.008
37. Li, S, Shin, HJ, Ding, EL, and van Dam, RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. (2009) 302:179–88. doi: 10.1001/jama.2009.976
Keywords: obesity, visceral adipose tissue, osteoarthritis, Mendelian randomization, two-sample MR
Citation: Zhang Y, Wang Y, Xu J, Wang Z, Zhao W and Zhao C (2024) Visceral adipose tissue and osteoarthritis, a two-sample Mendelian randomized study. Front. Med. 10:1324449. doi: 10.3389/fmed.2023.1324449
Edited by:
Alberto Marcos Heredia-Rizo, University of Seville, SpainReviewed by:
Jesús Salas González, Sevilla University, SpainLu Liu, Temple University, United States
Lourdes María Fernández-Seguín, Sevilla University, Spain
Copyright © 2024 Zhang, Wang, Xu, Wang, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changwei Zhao, zcw_1980@126.com
†These authors have contributed equally to this work and share first authorship
 Yinzhen Zhang
Yinzhen Zhang