- 1Department of Critical Care Medicine, Nanjing Drum Tower Hospital, Drum Tower Clinical College, Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Critical Care Medicine, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
Purpose: A discussion about the correlation between the level of serum sodium and sepsis-induced coagulopathy (SIC).
Materials and methods: A retrospective analysis was conducted on sepsis patients who were admitted to the Intensive Care Unit (ICU) of Nanjing Drum Tower Hospital from January 2021 to December 2022. Based on the presence of coagulation disorders, the patients were divided into two groups: sepsis-induced coagulopathy (SIC) and non-sepsis-induced coagulopathy (non-SIC) groups. We recorded demographic characteristics and laboratory indicators at the time of ICU admission, and analyzed relationship between serum sodium level and SIC.
Results: One hundred and twenty-five patients with sepsis were enrolled, among which, the SIC and the non-SIC groups included 62 and 63 patients, respectively. Compared to patients in the non-SIC group, the level of serum sodium of those in the SIC was significantly higher (p < 0.001). Multi-factor logistic regression showed serum sodium level was independently associated with SIC (or = 1.127, p = 0.001). Pearson’s correlation analysis indicated that the higher the serum sodium level, the significantly higher the SIC score was (r = 0.373, p < 0.001). Additionally, the mortality rate of patients with sepsis in the ICU were significantly correlated with increased serum sodium levels (p = 0.014).
Conclusion: An increase in serum sodium level was independently associated with an increased occurrence of SIC and also associated with the poor prognosis for patients with sepsis.
1 Introduction
Sepsis-induced coagulopathy (SIC) is a significant component of sepsis-related multiple organ dysfunction syndrome (MODS) and is strongly linked to the woresning of microcirculatory issues and tissue organ damage in patients (1–3). The prevalence of SIC in adults ranges from 50 to 70% (4), and it occurs more frequently in sepsis patients compared to those with sepsis-induced acute kidney injury (SAKI) (26–50%) (5–7) and sepsis-induced acute liver injury (SALI) (30%) (8). The development of SIC is primarily associated with the activation of the coagulation pathway, impairment of the anticoagulant system, suppression of fibrinolysis, and platelet aggregation in sepsis patients (9–11). When the organism was infected, inflammatory mediators of pathogen-associated molecular patterns (PAMPs) and pro-inflammatory substances of damage-associated molecular patterns (DAMPs) are synthesized and released into the blood, which puts the organism in a hypercoagulable state (12–14). At this stage, the anticoagulant mechanism is significantly inhibited, which may cause massive microthrombosis and vascular endothelial damage. In the terminal stage, patients may progress to disseminated intravascular coagulation (DIC), which is closely related to the increased mortality rate of patients with sepsis (15, 16). It has been reported that the mortality rate of patients with sepsis combined with DIC is two times greater than that of patients without DIC (17). Any delayed intervention in sepsis-induced coagulation dysfunction may be harmful (18). The International Society on Thrombosis and Hemostasis currently recommends early to identify of coagulation disorders (19).
Sodium ions (Na+) are the main cations in extracellular fluids and are important for maintaining extracellular fluid volume, regulating acid–base balance, and maintaining normal osmolality and cellular physiological functions. And it is the most effective of all monovalent cations that activate thrombin (20). Nonetheless, as a result of substantial fluid replacement and increased aldosterone secretion, hypernatremia is also more prevalent among sepsis patients. In a study, the occurrence of hypernatremia in ICU-admitted patients varied from 2 to 6%, while the incidence of ICU-acquired hypernatremia reached as high as 26% (21–23). Moreover, Lindner et al. (22) had shown hypernatremia acquired during the ICU was an independent risk factor for patients death. Hypernatremia is also closely associated with sepsis severity, increased rates of organ failure, and increased in-hospital mortality (24, 25).
Na+, a critical thrombin activator, can bind to a specific thrombin site, leading to thrombin activation and stimulation of osmosis (20). These processes regulate the increased expressions of a transcription factor, the nuclear factor of activated T cells 5 (NFAT5), and its binding to the von Willebrand Factor (vWF) promoter, resulting in platelet aggregation (26). Activated thrombin converts fibrinogen into fibrous protein, forming blood clots (27). Additionally, prior research has indicated a connection between serum sodium concentration and damage to vascular endothelial and glycocalyx barriers (17, 28). Excessive sodium concentration leaded to a reduction in the thickness of the endothelial glycocalyx (eGC), a villous layer covering the vascular endothelium (29, 30). However, the integrity of the glycocalyx is important for maintaining normal coagulation function in the body (31).
Presently, there are no clinic studies that have assessed the correlation of serum sodium level with SIC at home and abroad. Thus, this study aimed to retrospectively analyze the relationship between the serum sodium level and SIC in intensive care unit (ICU) patients with sepsis.
2 Materials and methods
2.1 Clinical information
The clinical data about patients with sepsis who were admitted to the ICU of Nanjing Drum Tower Hospital from January 2021 to December 2022 were retrospectively analyzed. The inclusion criteria were: (1) patients aged ≥18 years; (2) ICU stay ≥24 h; (3) patients conforming to the diagnostic criteria 3.0 for sepsis (confirmed or suspected infection and SOFA score ≥ 2 points) (32). The exclusion criteria were: (1) pregnant patients; (2) patients with a history of chronic liver disease; (3) blood dialysis patients; (4) those with chronic kidney disease; (5) patients with hematological diseases and those taking anticoagulant medications; (6) patients with incomplete clinical data. This study was approved by the Ethics Committee of Drum Tower Clinical Medical College Affiliated with Nanjing University (File Number:2022-038-02).
2.2 Data collection
The patient demographic characteristics and laboratory data were collected, based on the patient’s first examination on admission to the ICU. General data: age and gender; underlying diseases: diabetes, hypertension, chronic liver disease, history of chronic kidney disease with hemodialysis, anticoagulation therapy; origins of sepsis; mechanical ventilation (MV); scores: Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE II) score; died in ICU; laboratory indicators: white blood cell (WBC) count, platelet count (PLT), prothrombin time (PT), activated partial prothrombin time (APTT), international normalized ratio (INR), D-Dimer, fibrinogen (FIB), C-reactive protein (CRP), creatinine (Cr), blood urea nitrogen (BUN), albumin (ALB) level, total bilirubin (TB), serum sodium, serum calcium, serum potassium, and serum phosphorus.
2.3 Definition SIC and serum sodium level
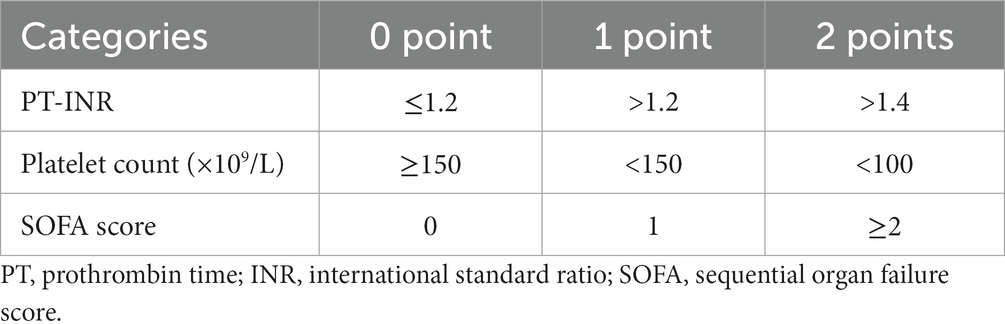
The diagnosis of SIC was based on the SIC score, which were assessed using the PT-INR, PLT, and SOFA score. If the sum of points ≥ 4 and the sum of PT-INR and PLT points > 2 were obtained, the patient was diagnosed with SIC (Table 1). the serum sodium concentration was categorized into three groups according to the definitions of previous studies: hypernatremia (>145 mmol/L), normal sodium level (135–145 mmol/L), and hyponatremia (<135 mmol/L) (33, 34).
2.4 Statistical methods
The SPSS 25.0 software was used for statistical analysis. For comparisons between two groups, independent samples t-tests were used for measurement data that conformed to normal distribution, expressed as mean ± standard deviation; conversely, nonparametric tests were used, expressed as the median (interquartile spacing). Pearson’s chi-square test or continuous calibration chi-square test was used to compare categorical variables, expressed as percentages (%). We analyzed whether the level of serum sodium was independently associated with SIC by utilizing single- and multifactors logistic regression analysis. We included sex, age, and indicators with p-values less than 0.05 in the comparison of baseline data between the two groups in via logistic regression analysis. However, considering the covariance between BUN and Cr, we did not include BUN in the logistic regression analysis. Based on the results we constructed receiver operating characteristic (ROC) curve and calculated the area under the curve (AUC), which was aimed at assessing the efficiency of the serum sodium concentration to predict SIC. One-way ANOVA was used for analysis of variables for more than two groups and for measurement data that conformed to a normal distribution. Correlation between serum sodium levels with SIC scores were analyzed via Pearson’s correlation analysis. Spearman’s correlation analysis was used to process the correlation between serum sodium levels and coagulation parameters The linear-by-linear association was used to analyze whether serum sodium levels were associated with clinical outcomes. A p-value < 0.05 for all the statistical results was considered to indicate statistical significance.
3 Results
3.1 Flowchart of patients meeting inclusion/exclusion criteria for the study
A total of 178 patients were included, of which, 2 pregnant patients, 10 patients with hematological diseases and taking anticoagulant medications, 11 patients with chronic hypohepatia, 7 blood dialysis patients (of whom 5 had been excluded due to a history of chronic liver and kidney diseases), 13 chronic renal insufficiency patients, and 15 patients with incomplete clinical data were excluded. In the end, the data analysis covered 125 patients in total. Sixty two patients were diagnosed with SIC based on the diagnostic criteria, while 63 patients did not meet the criteria (Figure 1).
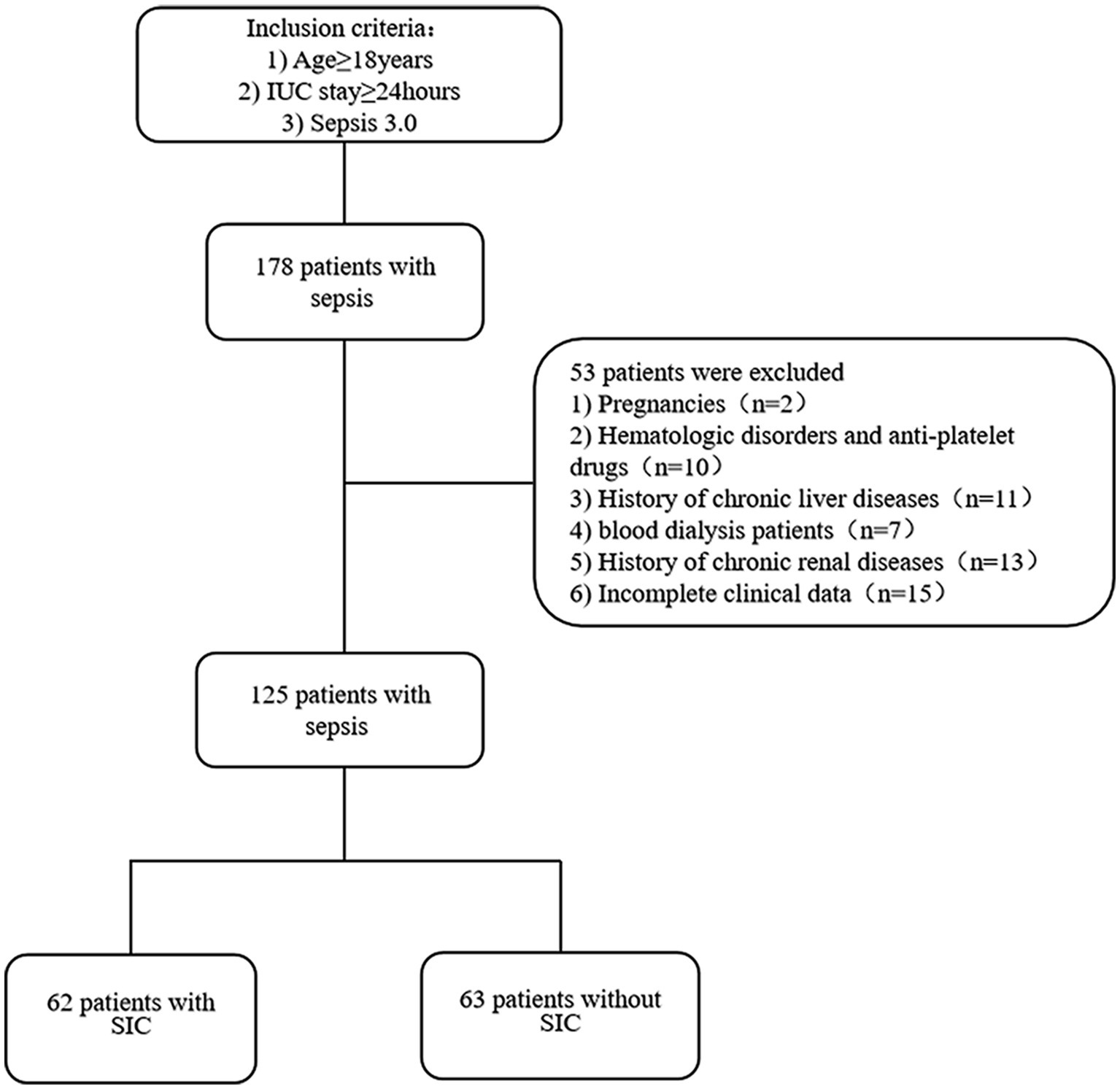
Figure 1. Flow chart of patients with inclusion/exclusion criteria for this study. A total of 178 patients were collected. We excluded 53 patients according to the exclusion criteria. Finally, 125 patients were categorized into SIC (n = 62) or non-SIC groups (n = 63) using the diagnostic criteria for sepsis-related coagulation disorders.
3.2 Comparison of general information between the SIC and non-SIC groups
The result showed no statistical difference was observed between the two groups in age, gender, underlying diseases, origins of sepsis, needing mechanical ventilation, WBC count, CRP, and ALB levels (p > 0.05). However, serum sodium level in the SIC group increased significantly (median, SIC, 144.8 vs. non-SIC, 139.8, p < 0.001), while calcium, potassium, and phosphorus levels exhibited no difference between the two groups. Additionally, Cr and TB levels in the SIC group were significantly higher than those in the non-SIC group (median: Cr; SIC, 103.5 vs. non-SIC, 61, p = 0.001; TB; SIC, 18.85 vs. non-SIC, 12.6, p = 0.014). Moreover, SOFA score and APACHE II score in the SIC group were also significantly higher than those in the non-SIC group (median; SOFA; SIC, 8.5 vs. non-SIC, 6, p = 0.003; APACHEII; SIC, 23.95 vs. non-SIC, 20.43, p = 0.009), which indicated patients in the SIC group had higher severity (Table 2).

Table 2. Comparison of demographic characteristics and laboratory indicators between the SIC and Non-SIC.
3.3 Relationship between serum sodium level and SIC
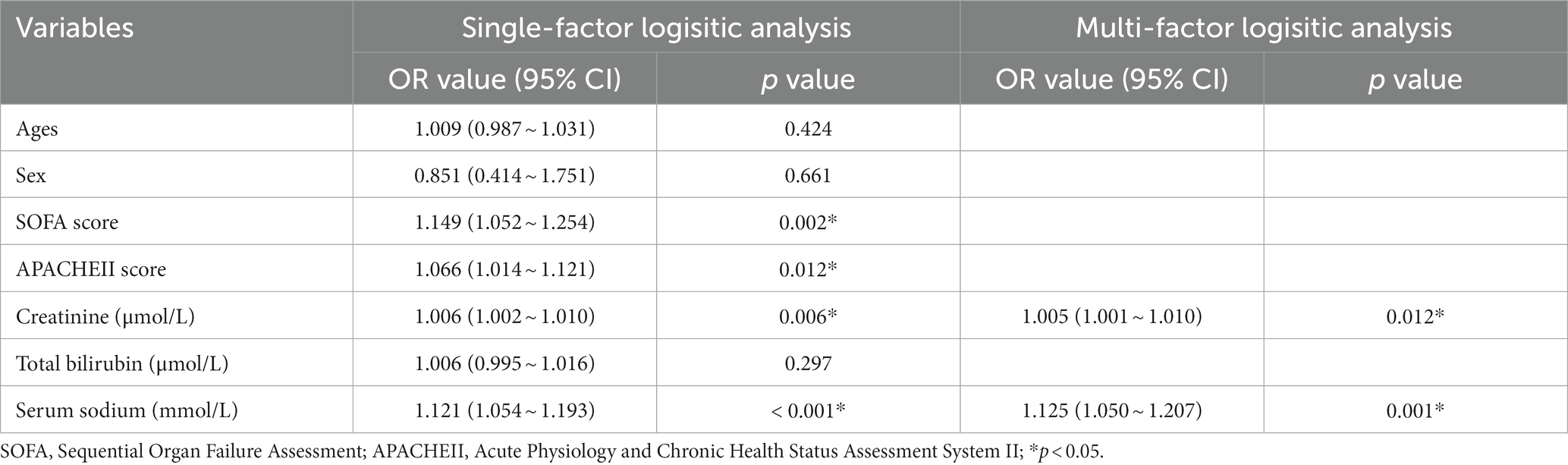
The age and sex, as well as SOFA score, Cr, TB, and Na+ level, were included in the single-factor logistic regression analysis. These indicators were then included in a multi-factor logistic regression analysis. The results showed that Cr (OR, 1.006; 95% CI, 1.010 ~ 1.001; p = 0.011) and serum sodium level (OR, 1.127; 95% CI, 1.051 ~ 1.208; p = 0.001) were independently correlated with SIC (Table 3).
3.4 ROC curve analysis of serum sodium level to predict SIC
We conducted a ROC curve. The result showed that serum sodium level had a predictive value for the occurrence of SIC (AUC = 0.697, 95% CI, 0.605–0.789, p < 0.001). The best cut-off value for predicting SIC was 144.65 mmol/L, with a sensitivity of 53.2% and a specificity of 84.1% (Figure 2).

Figure 2. ROC curve analysis of serum sodium levels to predict SIC. The sensitivity and specificity of serum sodium to predict SIC were 53.2 and 84.1%, respectively; the critical value was 144.65 mmol/L; the area under the curve was 0.697 (p < 0.001); 95% confidence interval: 0.605–0.789.
3.5 Correlation between serum sodium level and SIC score
According to the SIC scores, we created 5 groups (2 points, 3 points, 4 points, 5 points, and 6 points) and compared differences in serum sodium among groups. We also analyzed the correlation between serum sodium level and SIC score. Based on the results, we drew a boxplot and a scatter diagram. The number of patients in each group was 27, 36, 28, 15, and 19, respectively. Compared to the 5 and 6 points groups, the level of serum sodium in 3 points group was significantly lower (mean, 5 points group, 146.41 vs. 3 points group, 143.27, p = 0.026; 6 points group, 147.53 vs. 3 points group, 143.27, p = 0.002), whereas the 2 points group exhibited a statistical difference when compared to serum sodium level in 6 points groups (mean, 6 points group, 147.53 vs. 2 points group, 139.99, p = 0.009) (Figure 3A). There was a correlation between serum sodium level and SIC score (r = 0.373, p < 0.001) (Figure 3B). The higher the SIC score, the higher the serum sodium level.
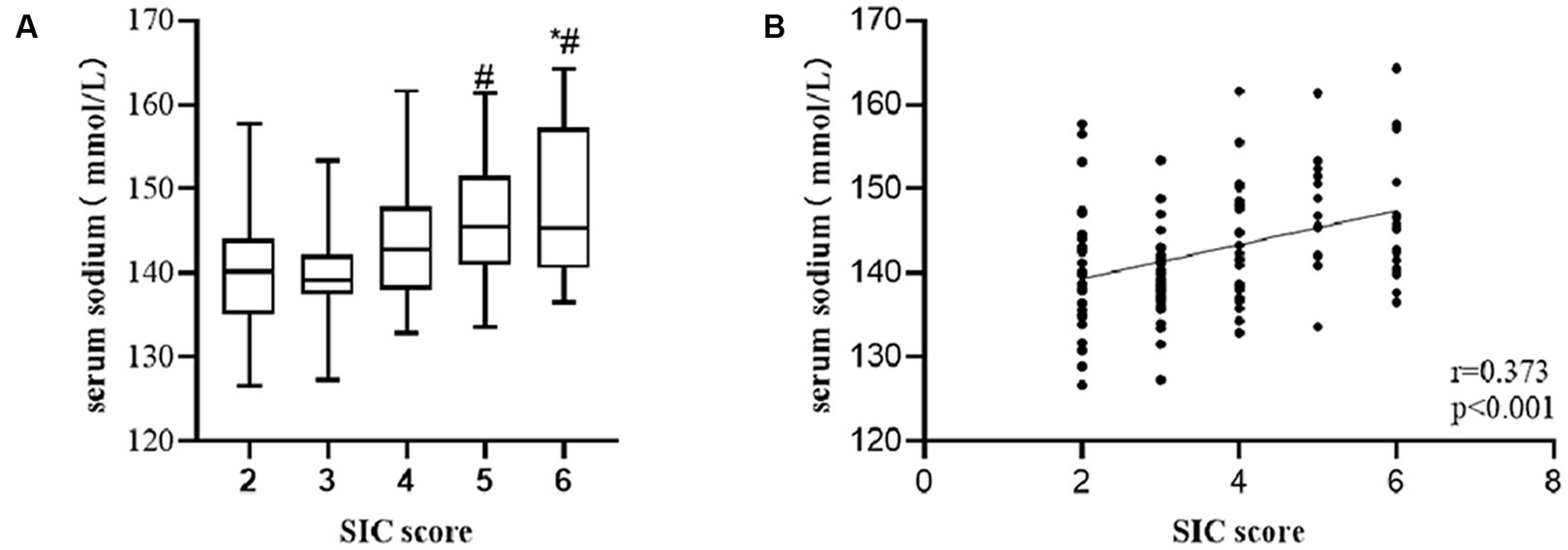
Figure 3. Among patient with sepsis, serum sodium level was significant higher in patients in the group with higher SIC score compared to those in the group with lower SIC score (A) (*p-value < 0.05, comparison vs. 2 points group; #p-value < 0.05, comparison vs. 3 points group). There was a positive correlation of serum sodium level with SIC score (B).
3.6 Correlation between serum sodium level and coagulation parameters
Based on the diagnostic criteria of hypo- and hypernatremia, 125 patients were divided into hyponatremia, normal Na level, and hypernatremia groups. The number of patients in each group was 13, 74, and 38, respectively. A correlation analysis was conducted. The results showed higher serum sodium level displayed a significant correlation with lower PLT level (Figure 4A, r = −0.270, p = 0.002), higher PT level (Figure 4B, r = 0.245, p = 0.006), higher INR level (Figure 4D, r = 0.244, p = 0.007), and lower FIB level (Figure 4F, r = −0.290, p = 0.001), but not with APTT (Figure 4C, r = 0.022, p = 0.808) and D-dimer levels (Figure 4E, r = -0.009, p = 0.924).
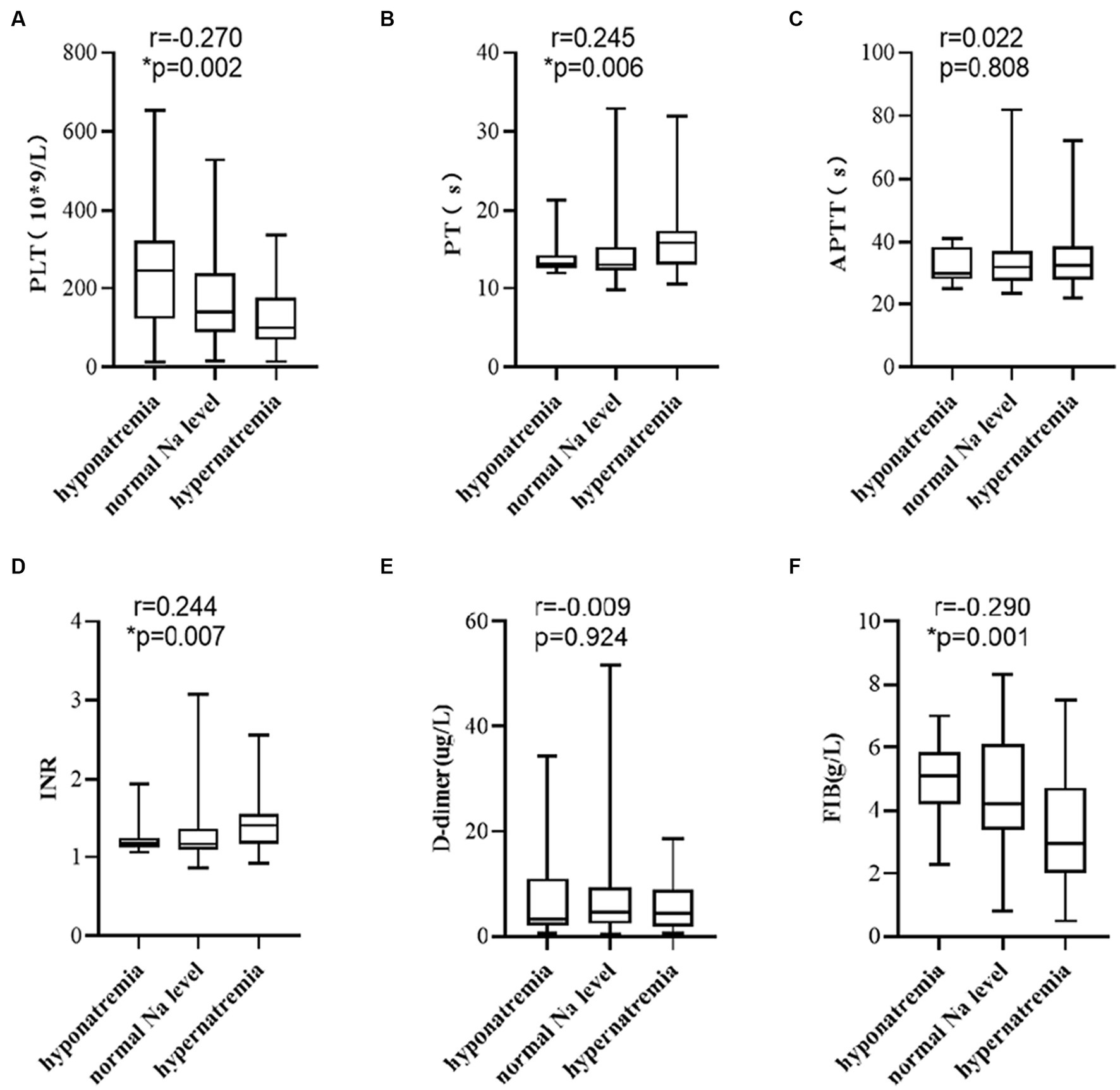
Figure 4. Elevated serum sodium level was associated with (A) PLT, (B) PT, (D) INR, and (F) FIB, but not with (C) APTT, and (E) D-dimer. p-values by Spearman’s correlation analysis test.
3.7 Correlation of serum sodium level with clinical outcomes
The linear-by-linear association was used to evaluate whether the clinical outcomes of patients with sepsis correlate with the serum sodium level. The patients were divided into hyponatremia, normal Na level, and hypernatremia groups as per the serum sodium levels. The in-ICU mortality of each group was 3, 22, and 15, while the number of patients needing mechanical ventilation in each group was 7, 46, and 23, respectively. Our results showed that the in-ICU mortality rate of patients with sepsis correlated with an increased serum sodium level (Figure 5A, p = 0.014), but not with mechanical ventilation (Figure 5B, p = 0.810).

Figure 5. Higher serum sodium levels were associated with (A) an increased in-ICU mortality rate, but not with (B) the needing of mechanical ventilation therapy. p-values by linear-by-linear association test.
4 Discussion
In this study, we explored the correlation between the level of serum sodium and the SIC. We analyzed the relationship between serum sodium and SIC score. The present study showed a positive correlation between serum sodium levels and SIC scores. An increase in serum sodium levels was independently associated with the development of SIC.
Although there are no studies on the relationship between serum sodium and coagulation disorders, studies have shown that serum sodium concentrations are related to the eGC. The serum sodium concentration in the body plays an important role in maintaining eGC stability. The eGC, a layer of negatively charged villus-like structures covering the endothelial surface of blood vessels, attracts circulating Na + ions in the vascular cava (35) and plays a beneficial role in sodium buffering in vivo (36). Consequently, the glycocalyx serves as a significant extrarenal regulator of extracellular sodium and serve as a reservoir for substantial sodium storage (37). When the body experiences sodium overload, it disrupts Na + homeostasis. This alteration causes a transition in endothelial cells from releasing sodium to absorbing sodium (28), leading to damaged in vascular endothelium. This, in turn, results in a reduction in the release of nitric oxide (NO). NO can dilate blood vessels, inhibit platelet activation, prevent platelet aggregation, adhesion, and prevent thrombosis (38, 39). Martin et al. (17) subjected human umbilical vein endothelial cells (HUVECs) to sodium (Na) concentrations of 134 mEq/L (control medium), 150 mEq/L, and 160 mEq/L, respectively. Then they found that excessive sodium concentrations all resulted in a significant increase in the shedding of eGC damage markers. They also measured glycocalyx thickness and found that the thickness of the cellular glycocalyx was significantly reduced by a factor of two under the Na 160 mEq/L concentration compared to the control group under Na 134 mEq/L. A study by Zheng et al. (40) reported that, compared to the normal chow (NC) diet group, the NC diet with 4% salt (NC4%) induced microcirculatory disturbances and glycocalyx degradation in mice. Glycocalyx damage is closely associated with the development of coagulation dysfunction, which has also been reported in several papers (41–43). These findings may laterally indicate that elevated serum sodium levels impair eGC, which in turn affects coagulation.
We investigated the relationship between serum sodium levels and coagulation parameters. The findings revealed that the higher serum sodium levels were associated with activation of the coagulation state, primarily manifesting as reduced platelet counts, prolonged PT, increased INR, and diminished FIB levels. Various mechanisms have been proposed to elucidate the coagulation dysfunction that could be related to increased serum sodium. The blood coagulation factor Xa (FXa) is an important serine protease in the coagulation cascade that plays a vital role in physiological hemostasis. However, excessive thrombin levels lead to the transformation of soluble fibrinogen into the insoluble fibrous protein, thus, resulting in thrombus formation (44–47). Relative studies suggested that thrombin displays better catalysis in the process of clotting in the presence of Na+ (48–51). Rezaie and He (52) also proved that Na+ can effectively activate thrombin which may relate to that it can bind to the 225 s loop residue of Try conformation of FXa. Moreover, Dmitrieva and Burg (26) cultured HUVECs in a high-sodium environment with different osmotic pressures and found that the secretion of vWF displayed a sodium-dependent increase while the high-sodium environment stimulated increased NFAT5 production. The vWF is secreted by endothelial cells and can bind to blood platelets, which is crucial for thrombus formation; the increased NFAT5 activity also contributes to increased vWF production in endothelial cells. Furthermore, Dmitrieva and Burg (26) revealed that when compared to the renal cortex, the vWF proteins and interstitial sodium chloride levels in the renal medulla were significantly higher. This indicated that the elevation of extracellular sodium within the physiological range is sufficient to increase vWF levels, thereby enhancing its coagulation ability and the risk of thrombus formation. Moreover, we found that the increased serum sodium levels were related to the decreased blood platelets and fibrinogen levels, which was consistent with the above-mentioned studies.
We also analyzed the relationship between serum sodium level and clinical outcomes in patients with sepsis. The results showed that the higher the serum sodium level was, the greater the mortality rate was for patients with sepsis in the ICU. In addition, the ROC curve showed that a serum sodium concentration of 144.65 mmol/L had predictive value for the occurrence of SIC. Li et al. (53) found that higher serum sodium level was associated with an increased mortality rate in patients with sepsis in a large-sample, multicenter study. Thongprayoon et al. (54) also reported that borderline hypernatremia (143–147 mmol/L) was associated with an increased hospital mortality rate in a study on serum sodium and the risk of death in hospital patients. All of these support the finding that elevated serum sodium levels are associated with severity in septic patients.
In addition, another new finding of our study was that Cr was also independently associated with SIC. However, the association between Cr and coagulation has not been determined. Cr serves as a predictor of renal function. The kidney is one of the organs most likely to be involved when an organism suffers from an infection. Therefore, we speculate that Cr may be associated with sepsis complicated by acute kidney loss. One study showed that coagulation function was significantly abnormal in patients with SAKI compared with patients without AKI, as evidenced by thrombocytopenia, elevated INR, and prolonged PT (55). This may be related to the fact that coagulation activation crosstalks with an inflammatory response to form extensive microthrombi, resulting in renal ischemic injury (56).
To the best of our knowledge, this study is not only the first study to investigate the correlation between serum sodium level and coagulation dysfunction in sepsis patients, but also the first study on the relationship between serum sodium level and coagulation function. However, this study has several limitations: first, it was a retrospective single-center study with a small sample size; second, our patient population was skewed toward elderly patients; and third, the causality and mechanism of action could not be proven. Therefore, a large sample size is needed to validate our findings further. Further studies are needed in the future to reveal the specific mechanism of action involved in the relationship between serum sodium level and SIC.
In conclusion, our retrospective analysis results suggested that an increase in the serum sodium level was independently associated with an increased occurrence of SIC and was also associated with an increase in-ICU mortality rate in septic patients. Higher serum sodium levels may lead to glycocalyx injury and exacerbate coagulation dysfunction. Therefore, we should pay attention to the serum sodium level in sepsis patients and further explore the molecular mechanisms underlying the relationship between the serum sodium and coagulation function to provide potential targets for improving coagulation function in sepsis patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Nanjing Drum Tower Hospital, The Affiliated Hospital School of Nanjing University Medical School, Nanjing, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this is a retrospective analysis.
Author contributions
YH: Conceptualization, Methodology, Visualization, Writing – original draft. JD: Conceptualization, Methodology, Visualization, Writing – original draft. MC: Supervision, Visualization, Writing – review & editing. SH: Data curation, Writing – review & editing. BZ: Data curation, Writing – review & editing. YW: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. XL: Project administration, Writing – review & editing. WY: Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the State Key Laboratory of Pharmaceutical Biotechnology, Department of Critical Care Medicine, Nanjing Drum Tower Hospital, The Affiliated Hospital School of Nanjing University Medical School [grant numbers 81927808]; Jiangsu Funding Program for Excellent Postdoctoral Talent Program [grant numbers 2022ZB689]; Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University [grant numbers 2021-LCYJ-PY-40].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yu, S, Ma, X, and Li, X. Phenotype-oriented anticoagulant therapy for sepsis: still a work in progress. Int J Hematol. (2022) 116:48–54. doi: 10.1007/s12185-022-03337-5
2. Ziesmann, MT, and Marshall, JC. Multiple organ dysfunction: the defining syndrome of Sepsis. Surg Infect. (2018) 19:184–90. doi: 10.1089/sur.2017.298
3. Gando, S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. (2010) 38:S35–42. doi: 10.1097/CCM.0b013e3181c9e31d
4. Simmons, J, and Pittet, JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. (2015) 28:227–36. doi: 10.1097/aco.0000000000000163
5. Bagshaw, SM, George, C, and Bellomo, R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. (2008) 12:R47. doi: 10.1186/cc6863
6. Yue, S, Li, S, Huang, X, Liu, J, Hou, X, Wang, Y, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers. (2022) 2022:1–12. doi: 10.1155/2022/9367873
7. Kolhe, NV, Stevens, PE, Crowe, AV, Lipkin, GW, and Harrison, DA. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC case mix Programme database. Crit Care. (2008) 12 Suppl 1:S2. doi: 10.1186/cc7003
8. Li, J, Xia, K, Xiong, M, Wang, X, and Yan, N. Effects of sepsis on the metabolism of sphingomyelin and cholesterol in mice with liver dysfunction. Exp Ther Med. (2017) 14:5635–40. doi: 10.3892/etm.2017.5226
9. Ma, R, Xie, R, Yu, C, Si, Y, Wu, X, Zhao, L, et al. Phosphatidylserine-mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci Rep. (2017) 7:4978. doi: 10.1038/s41598-017-04773-8
10. Puskarich, MA, Cornelius, DC, Bandyopadhyay, S, McCalmon, M, Tramel, R, Dale, WD, et al. Phosphatidylserine expressing platelet microparticle levels at hospital presentation are decreased in sepsis non-survivors and correlate with thrombocytopenia. Thromb Res. (2018) 168:138–44. doi: 10.1016/j.thromres.2018.06.017
11. Levi, M, and Poll, T. Coagulation in patients with severe sepsis. Semin Thromb Hemost. (2015) 41:009–15. doi: 10.1055/s-0034-1398376
12. Iba, T, Nisio, MD, Levy, JH, Kitamura, N, and Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. (2017) 7:e017046. doi: 10.1136/bmjopen-2017-017046
13. Masuda, T, and Shoko, T. Clinical investigation of the utility of a pair of coagulation-fibrinolysis markers for definite diagnosis of sepsis-induced disseminated intravascular coagulation: a single-center, diagnostic, prospective, observational study. Thromb Res. (2020) 192:116–21. doi: 10.1016/j.thromres.2020.05.009
14. Kinasewitz, GT, Yan, SB, Basson, B, Comp, P, Russell, JA, Cariou, A, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. (2004) 8:R82–90. doi: 10.1186/cc2459
15. Iba, T, Ito, T, Maruyama, I, Jilma, B, Brenner, T, Müller, MC, et al. Potential diagnostic markers for disseminated intravascular coagulation of sepsis. Blood Rev. (2016) 30:149–55. doi: 10.1016/j.blre.2015.10.002
16. Levi, M, van der Poll, T, and ten Cate, H. Tissue factor in infection and severe inflammation. Semin Thromb Hemost. (2006) 32:033–9. doi: 10.1055/s-2006-933338
17. Martin, JV, Liberati, DM, and Diebel, LN. Excess sodium is deleterious on endothelial and glycocalyx barrier function: a microfluidic study. J Trauma Acute Care Surg. (2018) 85:128–34. doi: 10.1097/ta.0000000000001892
18. Iba, T, Gando, S, and Thachil, J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. (2014) 12:1010–9. doi: 10.1111/jth.12596
19. Iba, T, Levy, JH, Yamakawa, K, Thachil, J, Warkentin, TE, and Levi, M. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. (2019) 17:1265–8. doi: 10.1111/jth.14482
20. Kahler, U, Kamenik, AS, Kraml, J, and Liedl, KR. Sodium-induced population shift drives activation of thrombin. Sci Rep. (2020) 10:1086. doi: 10.1038/s41598-020-57822-0
21. Funk, GC, Lindner, G, Druml, W, Metnitz, B, Schwarz, C, Bauer, P, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. (2010) 36:304–11. doi: 10.1007/s00134-009-1692-0
22. Lindner, G, Funk, GC, Schwarz, C, Kneidinger, N, Kaider, A, Schneeweiss, B, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. (2007) 50:952–7. doi: 10.1053/j.ajkd.2007.08.016
23. Stelfox, HT, Ahmed, SB, Khandwala, F, Zygun, D, Shahpori, R, and Laupland, K. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care. (2008) 12:R162. doi: 10.1186/cc7162
24. Ni, HB, Hu, XX, Huang, XF, Liu, KQ, Yu, CB, Wang, XM, et al. Risk factors and outcomes in patients with hypernatremia and Sepsis. Am J Med Sci. (2016) 351:601–5. doi: 10.1016/j.amjms.2016.01.027
25. Shirazy, M, Omar, I, Abduljabbar, D, Bousselmi, K, Alkhaja, M, Chaari, A, et al. Prevalence and prognostic impact of hypernatremia in Sepsis and septic shock patients in the intensive care unit: a single Centre experience. J Crit Care Med (Targu Mures). (2020) 6:52–8. doi: 10.2478/jccm-2020-0001
26. Dmitrieva, NI, and Burg, MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci U S A. (2014) 111:6485–90. doi: 10.1073/pnas.1404809111
27. Bando, M, Matsushima, A, Hirano, J, and Inada, Y. Thrombin-catalyzed conversion of fibrinogen to fibrin. J Biochem. (1972) 71:897–9. doi: 10.1093/oxfordjournals.jbchem.a129840
28. Oberleithner, H, Peters, W, Kusche-Vihrog, K, Korte, S, Schillers, H, Kliche, K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. (2011) 462:519–28. doi: 10.1007/s00424-011-0999-1
29. Belousoviene, E, Kiudulaite, I, Pilvinis, V, and Pranskunas, A. Links between endothelial Glycocalyx changes and microcirculatory parameters in septic patients. Life (Basel). (2021) 11:10.3390/life11080790. doi: 10.3390/life11080790
30. Dogné, S, and Flamion, B. Endothelial Glycocalyx impairment in disease: focus on Hyaluronan shedding. Am J Pathol. (2020) 190:768–80. doi: 10.1016/j.ajpath.2019.11.016
31. Britten, MW, Lümers, L, Tominaga, K, Peters, J, and Dirkmann, D. Glycocalyx components affect platelet function, whole blood coagulation, and fibrinolysis: an in vitro study suggesting a link to trauma-induced coagulopathy. BMC Anesthesiol. (2021) 21:83. doi: 10.1186/s12871-021-01300-1
32. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
33. Lindner, G, Schwarz, C, Haidinger, M, and Ravioli, S. Hyponatremia in the emergency department. Am J Emerg Med. (2022) 60:1–8. doi: 10.1016/j.ajem.2022.07.023
34. Muhsin, SA, and Mount, DB. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metab. (2016) 30:189–203. doi: 10.1016/j.beem.2016.02.014
35. Oberleithner, H, and Wilhelmi, M. Vascular glycocalyx sodium store – determinant of salt sensitivity? Blood Purif. (2015) 39:7–10. doi: 10.1159/000368922
36. Korte, S, Wiesinger, A, Straeter, AS, Peters, W, Oberleithner, H, and Kusche-Vihrog, K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. (2012) 463:269–78. doi: 10.1007/s00424-011-1038-y
37. Titze, J, and Machnik, A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens. (2010) 19:385–92. doi: 10.1097/MNH.0b013e32833aeb3b
38. Radomski, MW, Palmer, RM, and Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. (1987) 330:1057–8. doi: 10.1016/s0140-6736(87)91481-4
39. Gkaliagkousi, E, Ritter, J, and Ferro, A. Platelet-derived nitric oxide signaling and regulation. Circ Res. (2007) 101:654–62. doi: 10.1161/circresaha.107.158410
40. Zheng, X, Deacon, C, King, AJ, and Machin, DR. Microcirculatory and glycocalyx properties are lowered by high-salt diet but augmented by Western diet in genetically heterogeneous mice. Am J Physiol Heart Circ Physiol. (2022) 322:H328–35. doi: 10.1152/ajpheart.00656.2021
41. Abdullah, S, Ghio, M, Cotton-Betteridge, A, Vinjamuri, A, Drury, R, Packer, J, et al. Succinate metabolism and membrane reorganization drives the endotheliopathy and coagulopathy of traumatic hemorrhage. Sci Adv. (2023) 9:eadf6600. doi: 10.1126/sciadv.adf6600
42. Murphy, LS, Wickersham, N, McNeil, JB, Shaver, CM, May, AK, Bastarache, JA, et al. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann Intensive Care. (2017) 7:102. doi: 10.1186/s13613-017-0325-y
43. van Zyl, N, Milford, EM, Diab, S, Dunster, K, McGiffin, P, Rayner, SG, et al. Activation of the protein C pathway and endothelial glycocalyx shedding is associated with coagulopathy in an ovine model of trauma and hemorrhage. J Trauma Acute Care Surg. (2016) 81:674–84. doi: 10.1097/ta.0000000000001190
44. Bode, W. The structure of thrombin: a janus-headed proteinase. Semin Thromb Hemost. (2006) 32:016–31. doi: 10.1055/s-2006-939551
45. Davie, EW, Fujikawa, K, and Kisiel, W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. (1991) 30:10363–70. doi: 10.1021/bi00107a001
46. Davie, EW, and Kulman, JD. An overview of the structure and function of thrombin. Semin Thromb Hemost. (2006) 32:003–15. doi: 10.1055/s-2006-939550
48. Bush, LA, Nelson, RW, and Di Cera, E. Murine thrombin lacks Na+ activation but retains high catalytic activity. J Biol Chem. (2006) 281:7183–8. doi: 10.1074/jbc.M512082200
49. Dang, QD, Guinto, ER, and di Cera, E. Rational engineering of activity and specificity in a serine protease. Nat Biotechnol. (1997) 15:146–9. doi: 10.1038/nbt0297-146
50. De Cristofaro, R, Picozzi, M, Morosetti, R, and Landolfi, R. Effect of sodium on the energetics of thrombin-thrombomodulin interaction and its relevance for protein C hydrolysis. J Mol Biol. (1996) 258:190–200. doi: 10.1006/jmbi.1996.0242
51. Orthner, CL, and Kosow, DP. Evidence that human alpha-thrombin is a monovalent cation-activated enzyme. Arch Biochem Biophys. (1980) 202:63–75. doi: 10.1016/0003-9861(80)90406-3
52. Rezaie, AR, and He, X. Sodium binding site of factor Xa: role of sodium in the prothrombinase complex. Biochemistry. (2000) 39:1817–25. doi: 10.1021/bi992006a
53. Li, W, Li, S, Yuan, J, Chen, X, Chen, Q, Xiao, C, et al. Association between serum sodium and 28-day mortality in sepsis patients: a secondary data analysis from three large critical illness cohorts. Ther Apher Dial. (2023). doi: 10.1111/1744-9987.14066
54. Thongprayoon, C, Cheungpasitporn, W, Yap, JQ, and Qian, Q. Increased mortality risk associated with serum sodium variations and borderline hypo- and hypernatremia in hospitalized adults. Nephrol Dial Transplant. (2020) 35:1746–52. doi: 10.1093/ndt/gfz098
55. Xin, Q, Xie, T, Chen, R, Zhang, X, Tong, Y, Wang, H, et al. A predictive model based on inflammatory and coagulation indicators for Sepsis-induced acute kidney injury. J Inflamm Res. (2022) 15:4561–71. doi: 10.2147/jir.S372246
Keywords: sodium, sepsis, coagulation disorders, ICU, hypernatremia
Citation: Han Y, Duan J, Chen M, Huang S, Zhang B, Wang Y, Liu J, Li X and Yu W (2024) Relationship between serum sodium level and sepsis-induced coagulopathy. Front. Med. 10:1324369. doi: 10.3389/fmed.2023.1324369
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Andrea Glotta, Istituto Cardiocentro Ticino, SwitzerlandAlexandros Rovas, University Hospital Münster, Germany
Copyright © 2024 Han, Duan, Chen, Huang, Zhang, Wang, Liu, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyao Li, bGl4aWFveWFvbmp1QDE2My5jb20=; Wenkui Yu, eXVkcm5qMkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Yanyu Han1†
Yanyu Han1†