
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med. , 09 February 2024
Sec. Nuclear Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1304718
This article is part of the Research Topic Women in Science - Nuclear Medicine 2024 View all 3 articles
 Marigdalia K. Ramirez-Fort1,2,3*
Marigdalia K. Ramirez-Fort1,2,3* Casey K. Gilman1,4
Casey K. Gilman1,4 Jacob S. Alexander1,4
Jacob S. Alexander1,4 Barbara Meier-Schiesser5*
Barbara Meier-Schiesser5* Arjan Gower3
Arjan Gower3 Mojtaba Olyaie2,3
Mojtaba Olyaie2,3 Neel Vaidya2
Neel Vaidya2 Kiarash Vahidi2
Kiarash Vahidi2 Yuxin Li2,3
Yuxin Li2,3 Christopher S. Lange1,6
Christopher S. Lange1,6 Migdalia Fort1
Migdalia Fort1 Corinne Deurdulian2,3,7
Corinne Deurdulian2,3,7Gender and disease-exclusive language in healthcare may pose a problem in recognition or evaluation of various disease processes. Although more recognition has been placed on the use of proper pronouns in the medical literature for patient care, efforts to utilize inclusive language for biological markers, diagnostic procedures, society guidelines, and other medical terminology should be made. The continued use of gender-exclusive language in oncology and other fields may lead to the exclusion of genotypic female patients from certain therapeutic and diagnostic opportunities that prescribe masculine descriptors. One example highlighted throughout this editorial is “prostate-specific membrane antigen” or “PSMA,” which is encoded by the folate hydrolyase-1 (FOLH1) gene and is expressed in numerous solid tumors present in both male and female patients (1–9). Histopathological findings have been corroborated with molecular imaging (10).
Theragnostic agents targeting folate hydrolase-1 (FOLH1), encoded by the FOLH1 gene, have been approved by the Federal Drug Administration (FDA). FOLH1 is a transmembrane receptor and enzyme that has been linked to disease-free survival, tumor size, metastatic progression, and overall survival in a number of different tumor histologies (1). The clinical potential for targeting FOLH1 was first described in the 1980s; the analogous but membrane-bound upregulation of FOLH1 to prostate-specific antigen in the setting of prostate cancer (PCa) established the term “prostate-specific membrane antigen” at that time. In 1997, Neil H. Bander's team discovered that FOLH1 was also expressed in the neo-endothelium of melanoma, renal cell, urothelial, colon, lung, and breast carcinomas (2). In 1998, the enzyme named “PSMA” was found to be encoded by the FOLH1 gene, indicating that PSMA and FOLH1 were and are the same molecules (1, 11).
Physiologically, FOLH1 is expressed on the apical/luminal surface of the duodenum (12) (Figure 1) by proximal renal tubular cells, the prostate gland, parotid glands (Figure 2), and a subpopulation of astrocytes. It is well-known that salivary glands show avid uptake of PSMA radiopharmaceuticals with molecular imaging studies (Figure 2). Yet, several immunohistochemistry studies observed the absence of PSMA expression in salivary glands (13). Currently, it is hypothesized that PSMA radiopharmaceutical uptake in salivary gland tissue is mostly non-specific (14). Pathologically, FOLH1 is expressed on the surface membrane of PCa cells and adenoid cystic carcinoma cells, intracellularly in a subset of OCT4+ melanoma cells (possible cancer stem cells), and luminally in virtually every solid tumor-associated vasculature (1).
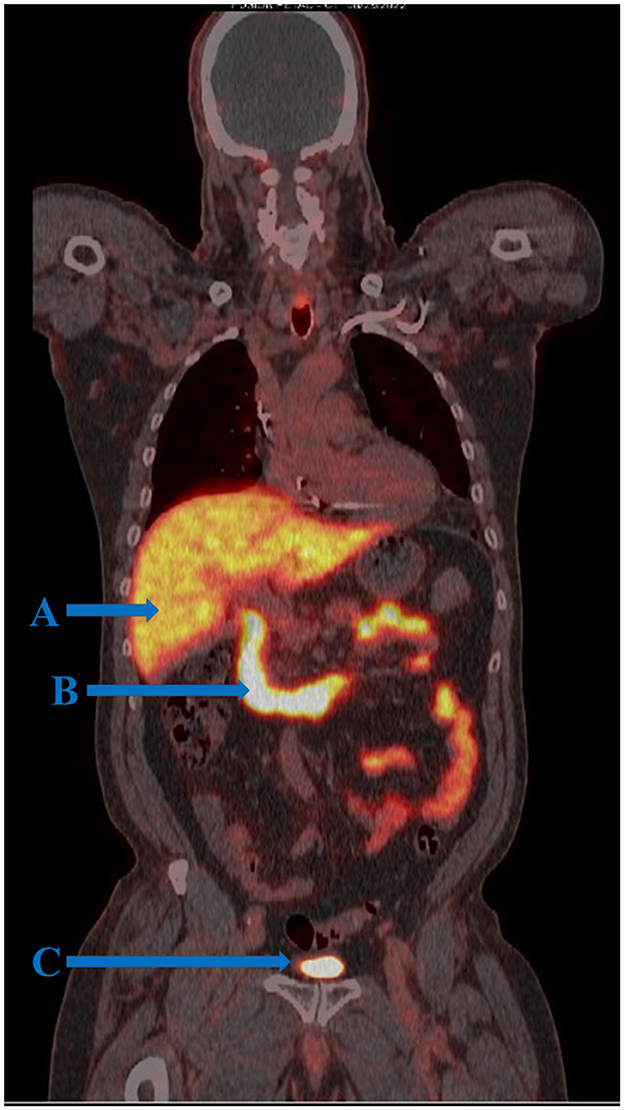
Figure 1. FOLH1 PET/CT of a male patient with prostate cancer. (A) Tracer uptake is demonstrated in the liver where tracer is metabolized and excreted into the gastrointestinal tract. (B) Physiologic tracer uptake is seen in the first three portions of the duodenum and small intestine where FOLH1 regulates intestinal absorption of dietary folate. Although it is known that the duodenum expresses targetable FOLH1, it is not well understood the extent of overlap between physiologic FOLH1-mediated tracer uptake and background positron capture from tracer in transitory bile. (C) Tracer activity in the bladder.
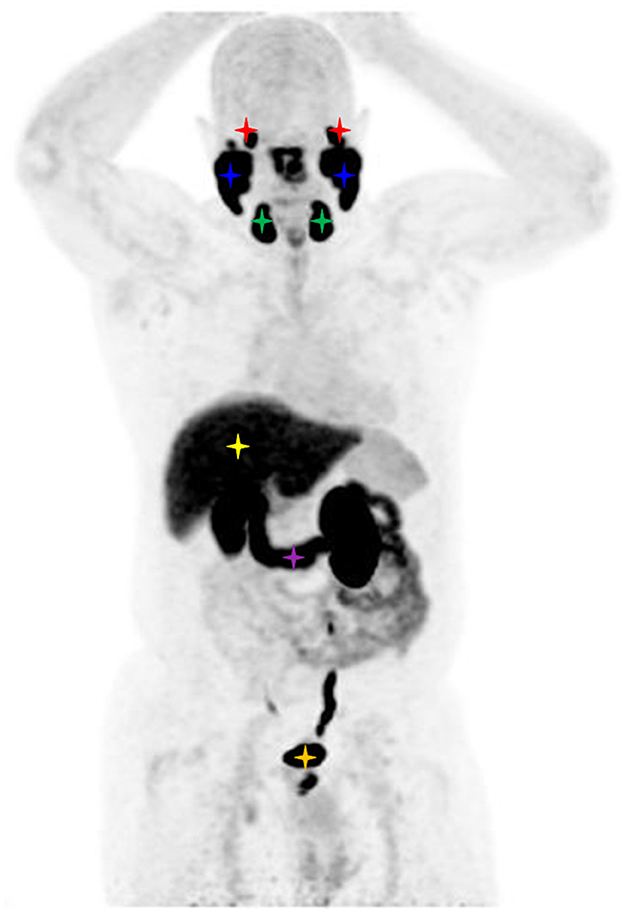
Figure 2. Maximum intensity projection image of a FOLH1 PET/CT of a male patient with prostate cancer. Physiological tracer uptake is seen in the lacrimal glands (red stars), parotid glands (blue stars), submandibular glands (green stars), liver (yellow star), duodenum (purple star), and bladder (orange star).
Nguyen et al. (15) demonstrated that the substrate of solid tumors in vitro induces neoendothelial FOLH1 expression in preclinical solid tumor models containing human umbilical vein endothelial cells. This study demonstrated that FOLH1 is specific to the formation of tumor-associated vessels rather than angiogenic vessels of other etiology (15). Further, our group recently validated FOLH1 as a novel theragnostic target in Merkel cell carcinoma; specifically, prevalent FOLH1 expression within Merkel cell carcinoma-associated neovessels, in 60–77% of patients in an 81-person cohort (3).
Different aliases such as glutamate carboxypeptidase II, N-acetyl-L-aspartyl-L-glutamate peptidase, FOLH1, and PSMA are currently used interchangeably in the medical literature. The foremost names attempt to title the enzyme primarily based on the location and enzymatic function in which it was discovered (i.e., kidney, nervous system glia, duodenum, and the small bowel). For example, glutamate carboxypeptidase II and N-acetyl-L-aspartyl-L-glutamate peptidase are typically described as being found in astrocytes where they cleave terminal glutamate residues to form the neurotransmitter glutamate (1). On the other hand, the use of the PSMA is solely based on its most common contemporary medical use (i.e., FDA-approved PSMA PET/CT and/or 177Lu PSMA molecular brachytherapy).
Decades of oncological research have focused on characterizing FOLH1 expression in PCa; therefore, it is understandable that the most common terminology used to cite this theragnostic target in oncology is “PSMA,” suggesting exclusive enzyme expression to the prostate. The continued use of PSMA, particularly in society guidelines, is problematic and should be considered a misnomer as it falsely implies exclusive expression to the prostate (16) [i.e., the European Association of Nuclear Medicine (EANM) standardized reporting “guidelines v1.0 for PSMA-PET” state “PSMA is a glycoprotein, a membrane-bound metallo-peptidase, encoded by FOLH1 gene on chromosome 11”]. Based on the broader potential of oncological use and clinical significance in a variety of non-prostatic malignancies, accurate nomenclature should be established and standardized with urgency to optimize ongoing translational cancer research and to avoid confusion amongst physicians and patients.
Little effort has been made to standardize the nomenclature to a more representative description that is readily applicable to cancer in other organ systems. For example, groups have reported on FOLH1 expression in the stroma of Merkel cell carcinoma (3) and transitional cell carcinomas of the bladder (4), while other groups reported the same enzyme as “PSMA” expression in melanoma and osteosarcomas (5, 6), demonstrating the lack of consolidated nomenclature in various medical fields managing distinct organ systems. Furthermore, the widely cited PSMA misnomer generates a gender bias that could translate into reduced diagnostic and therapeutic opportunities for genotypic female patients with FOLH1-expressing solid tumors. Misleading nomenclature and related gender bias could adversely impact future FDA approvals for theragnostic indications for all solid tumors that express FOLH1 in the neovasculature, including for gynecological malignancies in patient genotypes that do not have prostates (7).
“FOLH1,” however, is a strong candidate for officiating nomenclature as its name does not imply exclusivity to certain tissues or biological sexes but rather describes its enzymatic function. There is a strong international movement for the use of gender-inclusive language in many aspects of the modern world beyond medicine and science, such as in government and labor (e.g., United Nations Global Sustainable Development Goals). To this end, the U.S. General Services Administration 18F Content Guide strongly suggests that formal writings that include words and phrases that indicate gender bias should be avoided. Similar suggestions should be applied in both science and medicine. Furthermore, the International Union of Biochemistry and Molecular Biology has established that the scientific nomenclature of enzymes should be based on the chemical reaction that they catalyze, as this is a unique feature of each enzyme (17). Inherent to its name, “folate hydrolase-1” utilizes water to break down (i.e., hydrolysis) glutamate residues from dietary folate. Therefore, consolidating the nomenclature into the gene, FOLH1, that encodes the enzyme, is a practical method of standardization.
Regardless of the challenges that a change in nomenclature may pose, if we aspire to create scientific unity, we should collectively consider establishing a common language as delineated by the guidelines set forth by the International Union of Biochemistry and Molecular Biology and the United Nations Global Sustainable Development Goals. The authors recommend establishing gender and disease-inclusive nomenclature for this important biomarker and theragnostic target, encoded by the FOLH1 gene, as “FOLH1.”
MR-F: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. CG: Writing – original draft, Writing – review & editing. JA: Writing – original draft, Writing – review & editing. BM-S: Writing – review & editing, Conceptualization, Supervision, Writing – original draft. AG: Writing – review & editing. MO: Writing – review & editing. NV: Writing – review & editing. KV: Writing – review & editing. YL: Supervision, Writing – review & editing. CL: Supervision, Writing – review & editing, Conceptualization, Formal analysis. MF: Resources, Supervision, Writing – original draft, Writing – review & editing. CD: Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. BioFort and the University Hospital of Zurich Library provided funding for the journal publishing fees. The funders were not involved in the study design, analysis, interpretation of data, the writing of this article or in the decision to submit it for publication.
MR-F and MF are the inventors of U.S. Patent No. 11,707,223 (issued July 25, 2023) that is related to FOLH1 molecular imaging and radiopharmaceutical targeting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ramirez-Fort MK, Mahase SS, Osborne JR, Lange CS. Theragnostic target, prostate-specific membrane antigen-also specific for nonprostatic malignancies. Int J Radiat Oncol Biol Phys. (2018) 101:646–9. doi: 10.1016/j.ijrobp.2018.03.061
2. Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. (1997) 57:3629–34.
3. Ramirez-Fort MK, Meier-Schiesser B, Lachance K, Mahase SS, Church CD, Niaz MJ, et al. Folate hydrolase-1 (FOLH1) is a novel target for antibody-based brachytherapy in Merkel cell carcinoma. Skin Health Dis. (2021) 1:e9. doi: 10.1002/ski2.9
4. Samplaski MK, Heston W, Elson P, Magi-Galluzzi C, Hansel DE. Folate hydrolase (prostate-specific membrane [corrected] antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod Pathol. (2011) 24:1521–9. doi: 10.1038/modpathol.2011.112
5. Snow H, Hazell S, Francis N, Mohammed K, O'Neill S, Davies E, et al. Prostate-specific membrane antigen expression in melanoma metastases. J Cutan Pathol. (2020) 47:1115–22. doi: 10.1111/cup.13774
6. Zeng C, Ke ZF, Yang Z, Wang Z, Yang SC, Luo CQ, et al. Prostate-specific membrane antigen: a new potential prognostic marker of osteosarcoma. Med Oncol. (2012) 29:2234–9. doi: 10.1007/s12032-011-0089-4
7. Wernicke AG, Kim S, Liu H, Bander NH, Pirog EC. Prostate-specific membrane antigen (PSMA) expression in the neovasculature of gynecologic malignancies: implications for PSMA-targeted therapy. Appl Immunohistochem Mol Morphol. (2017) 25:271–6. doi: 10.1097/PAI.0000000000000297
8. Schmidt LH, Heitkotter B, Schulze AB, Schliemann C, Steinestel K, Trautmann M, et al. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS ONE. (2017) 12:e0186280. doi: 10.1371/journal.pone.0186280
9. Khandelwal Y, Singh Parihar A, Sistani G, Ramirez-Fort MK, Zukotynski K, Subramaniam RM. Role of PET/computed tomography in gastric and colorectal malignancies. PET Clin. (2023). doi: 10.1016/j.cpet.2023.12.004 [Epub ahead of print].
10. Rizzo A, Dall'Armellina S, Pizzuto DA, Perotti G, Zagaria L, Lanni V, et al. PSMA radioligand uptake as a biomarker of neoangiogenesis in solid tumours: diagnostic or theragnostic factor? Cancers. (2022) 14:4039. doi: 10.3390/cancers14164039
11. O'Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. (1998) 1443:113–27. doi: 10.1016/S0167-4781(98)00200-0
12. Maraj BH, Aldersley MA, Markham AF. Prostate-specific membrane antigen expression in the duodenum: implications in coeliac disease and immunotherapy for prostate cancer. Lancet. (1998) 351:1559–60. doi: 10.1016/S0140-6736(05)61125-7
13. Rupp NJ, Umbricht CA, Pizzuto DA, Lenggenhager D, Töpfer A, Müller J, et al. First clinicopathologic evidence of a non-PSMA-related uptake mechanism for 68Ga-PSMA-11 in salivary glands. J Nucl Med. (2019) 60:1270–6. doi: 10.2967/jnumed.118.222307
14. Pandit-Taskar N, O'Donoghue JA, Morris MJ, Wills EA, Schwartz LH, Gonen M, et al. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J Nucl Med. (2008) 49:1066–74. doi: 10.2967/jnumed.107.049502
15. Nguyen DP, Xiong PL, Liu H, Pan S, Leconet W, Navarro V, et al. Induction of PSMA and internalization of an anti-PSMA mAb in the vascular compartment. Mol Cancer Res. (2016) 14:1045–53. doi: 10.1158/1541-7786.MCR-16-0193
16. Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. (2021) 48:1626–38. doi: 10.1007/s00259-021-05245-y
Keywords: folate hydrolase-1, prostate-specific membrane antigen, theragnostic target, gender-inclusive language, disease-inclusive language
Citation: Ramirez-Fort MK, Gilman CK, Alexander JS, Meier-Schiesser B, Gower A, Olyaie M, Vaidya N, Vahidi K, Li Y, Lange CS, Fort M and Deurdulian C (2024) Gender and disease-inclusive nomenclature consolidation of theragnostic target, prostate-specific membrane antigen (PSMA) to folate hydrolase-1 (FOLH1). Front. Med. 10:1304718. doi: 10.3389/fmed.2023.1304718
Received: 29 September 2023; Accepted: 31 December 2023;
Published: 09 February 2024.
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Laura Evangelista, Humanitas University, ItalyCopyright © 2024 Ramirez-Fort, Gilman, Alexander, Meier-Schiesser, Gower, Olyaie, Vaidya, Vahidi, Li, Lange, Fort and Deurdulian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marigdalia K. Ramirez-Fort, bWFyaWdkYWxpYUBiaW9mb3J0Lmlv; Barbara Meier-Schiesser, QmFyYmFyYS5NZWllci1TY2hpZXNzZXJAdXN6LmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.