- 1Department of Reproductive Health and Infertility, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Chengdu Xi’nan Gynecological Hospital, Chengdu, China
- 3Sichuan Academy of Medical Sciences of Sichuan Provincial People’s Hospital, Chengdu, China
- 4Chengdu Third People’s Hospital, Chengdu, China
Background: Sequential embryo transfer has been recognized as a strategy to increase pregnancy rates in women undergoing in vitro fertilization and embryo transfer (IVF-ET). However, its impact on assisted reproductive outcomes remains to be substantiated by robust evidence. This systematic review aims to summarize and analyze the available evidence to investigate the effect of sequential embryo transfer on assisted reproductive outcomes.
Methods: A comprehensive literature search was executed across the Pubmed, Cochrane Library, Web of Science, and Scopus databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Data were aggregated utilizing a random effects model, and the resultant outcomes were articulated as odds ratios (ORs) along with their 95% confidence intervals (CIs).
Results: The pooled results revealed a statistically significant enhancement in reproductive outcomes for infertile patients undergoing sequential embryo transfer as evidenced by elevated rates of chemical pregnancy (OR = 1.67, 95% CI = 1.23–2.27), clinical pregnancy (OR = 1.78, 95% CI = 1.43–2.21), and ongoing pregnancy (OR = 1.54, 95% CI = 1.03–2.31). Compared with cleavage-stage embryo transfer, sequential transfer yielded superior outcomes in terms of chemical pregnancy rate (OR = 2.08, 95% CI = 1.35–3.19) and clinical pregnancy rate (OR = 1.78, 95% CI = 1.37–2.31). Furthermore, among the repeated implantation failure (RIF) cohort, sequential embryo transfer surpassed blastocyst-stage transfer, delivering a heightened chemical pregnancy rate (OR = 1.66, 95% CI = 1.19–2.53) and clinical pregnancy rate (OR = 1.65, 95% CI = 1.19–2.27).
Conclusion: Our meta-analysis indicates that sequential transfer may enhance clinical pregnancy rate in a small subgroup of well-selected women. While promising, further evidence from prospective studies is needed.
Introduction
In vitro fertilization and embryo transfer (IVF-ET) is an important treatment option for infertile couples. However, implantation rates of 25–40% mean that many infertile couples require multiple cycles of treatment to achieve a successful pregnancy (1). This makes IVF-ET a time-consuming and costly assisted reproductive technology.
Embryo implantation is a multifaceted process. Success hinges on three pivotal elements: high-quality embryos, a receptive endometrium, and optimal synchronization between the embryo and endometrial lining (2). Among these factors, the endometrium is often deemed the most crucial, accounting for approximately two-thirds of implantation failures (3). Sequential embryo transfer, first introduced by Abramovici et al., (4) comprises two stages of embryo transfer on separate days within the same treatment cycle—specifically, the first transfer occurs on the second day post-oocyte retrieval, and the second on the third-day (4). This approach capitalizes on the “implantation window,” enhancing endometrial receptivity (4). Building on this innovative strategy, Goto et al. refined the technique by initiating the first transfer with a cleavage-stage embryo, followed by a blastocyst for the second transfer (5). This modified protocol mitigates the risk of cycle cancelation due to failed blastocyst formation and yields superior implantation and pregnancy rates compared to conventional transfer methods (5).
Repeated implantation failure (RIF) is typically defined as the inability to achieve a clinical pregnancy after transferring at least four high-quality embryos across a minimum of three fresh or frozen–thawed embryo transfer cycles in women under the age of 40 (6). One of the most critical factors contributing to RIF is likely poor endometrial receptivity (7). As such, many specialists posit that sequential embryo transfer could be an important therapeutic strategy for patients experiencing RIF.
The effectiveness of sequential embryo transfer remains a subject of debate due to insufficient published data. This study aimed to systematically review and synthesize the existing clinical evidence to elucidate the impact of sequential embryo transfer on IVF-ET outcomes, intending to refine embryo transfer strategies.
Materials and methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Literature search and data extraction
A comprehensive literature search was conducted using four established databases: PubMed, Cochrane Library, Web of Science, and Scopus, covering articles from their inception to July 31, 2023. We also manually screened the reference lists of selected articles to identify additional pertinent studies. The primary search terms employed were [“sequential embryo transfer” OR “consecutive embryo transfer” OR “interval double transfer” OR “two-step transfer”] AND [“in vitro fertilization” OR “IVF” OR “assisted reproductive techniques” OR “ART” OR “intracytoplasmic sperm injections” OR “ICSI”]. The search was not limited by language, geographical location, or study type, thereby maximizing the inclusivity and generalizability of our findings.
After conducting the literature search, two authors (WT and HX) independently screened the titles, abstracts, and full-text articles to identify eligible studies. Any disagreements arising during this process were resolved through consultation with a third author (FW), ensuring a consensus was reached. Additionally, the quality of the included studies and the overall quality of evidence were independently evaluated by two authors (WT and HX). Should disagreements occur regarding the type or quality of a given study, a consensus was reached through discussion with the third author (FW).
Eligibility criteria
Studies had to meet three predetermined criteria to be included in this review. Firstly, the study design had to be either a retrospective cohort study, a post hoc analysis, or a randomized controlled trial (RCT), as these study types best align with the objectives of this review. Secondly, the studies were required to compare the outcomes of sequential embryo transfers involving both cleavage-stage and blastocyst-stage embryos against other transfer protocols. Lastly, the clinical pregnancy rates had to be reported as an outcome measure. Exclusion criteria encompassed other study designs such as reviews, case reports, and studies lacking data on clinical pregnancy rates.
Data extraction
For each selected study, we recorded the following variables: the first author, year of publication, country of origin, study design, and patient characteristics—such as age, body mass index (BMI), embryo transfer protocol, and RIF status. Additionally, we documented any outcome metrics reported by the study.
Outcome measures
The primary outcome measure selected for this review was the pregnancy rate, subdivided into chemical pregnancy rate, clinical pregnancy rate, and ongoing pregnancy rate. Other outcomes encompassed implantation rate, multiple pregnancy rate, and miscarriage rate.
Assessment of heterogeneity
We assessed the similarity between the eligible studies in their design and clinical characteristics using the I2 statistic. An I2 > 50% was labeled as marked heterogeneity (8).
Quality and risk of bias assessment
The integrity of the included studies was rigorously evaluated based on the following parameters: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, treatment of incomplete outcome data, and selective reporting. We employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to grade the quality of evidence systematically (9). The evidence quality was downgraded by one level of severe reservations and by two levels for exceedingly grave concerns concerning the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Two authors independently executed this assessment of bias and quality. Any divergences in opinion were resolved either through discussion or consultation with a third author. The findings of this quality assessment are delineated in Table S1.
Data analysis
Studies that satisfied the inclusion criteria were included in the meta-analysis. Data extracted from these studies were aggregated using Review Manager software (Version 5.4; The Cochrane Collaboration, 2020). Pooled odds ratios (OR) with 95% confidence intervals (CI) were calculated employing a random-effects model. Heterogeneity across studies was quantified using the I2 statistic. Subgroup analyses were conducted based on the RIF status of patients and whether the control group involved blastocyst-stage transfers. Additionally, sensitivity analyses were carried out by sequentially omitting studies with high weight to assess their influence on heterogeneity and effect estimates. Finally, the potential for publication bias was evaluated using funnel plots.
Results
Results of the searches
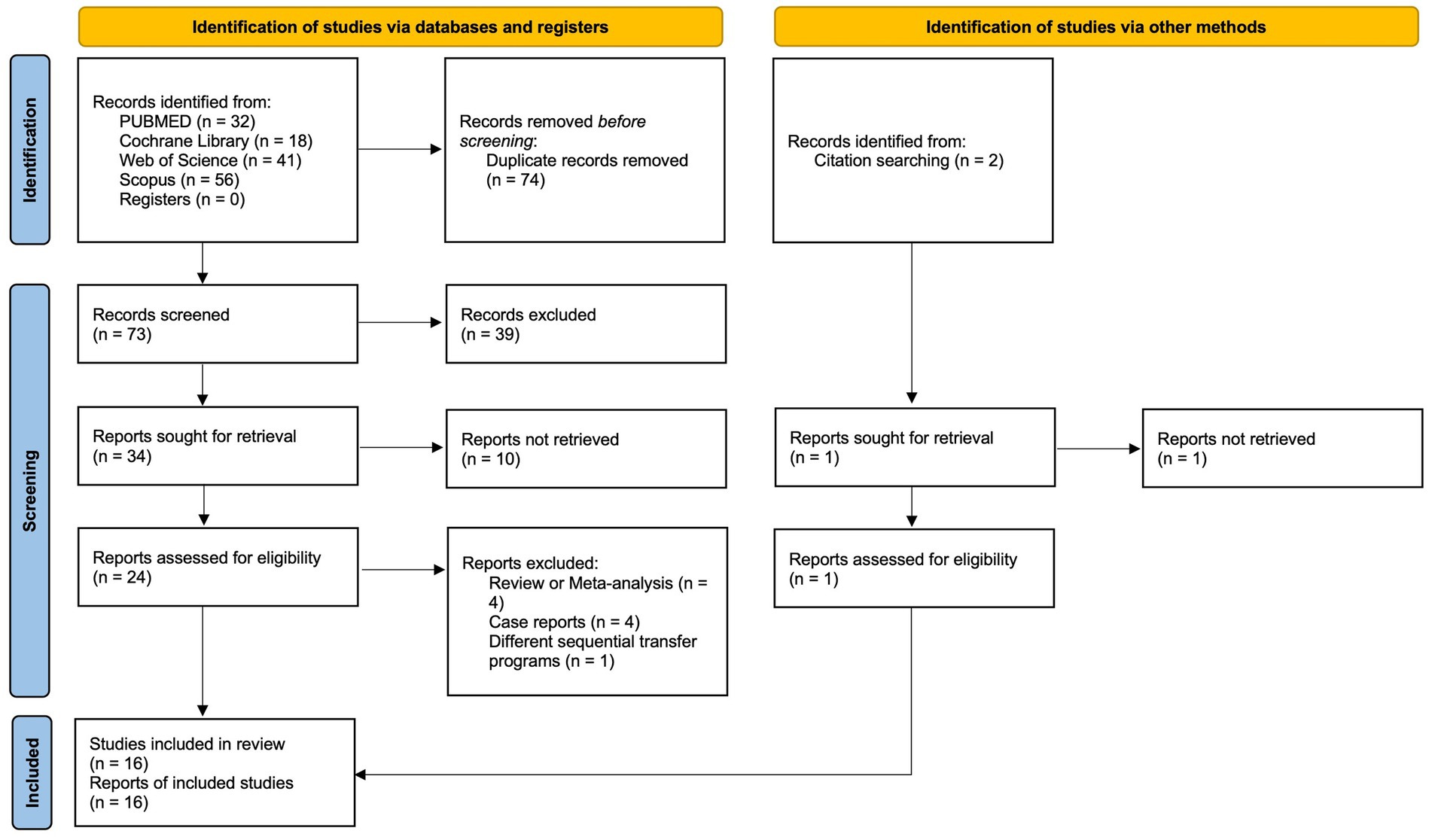
Our initial database search yielded 147 studies, of which 74 were duplicates and subsequently removed. After screening titles and abstracts, 34 studies were deemed eligible for further evaluation. Of these, eight were excluded due to the unavailability of full text or missing information. Detailed examination of the remaining 26 full-text studies led to the inclusion of 15 articles in the meta-analysis. An additional study that met the inclusion criteria was identified through cross-referencing, bringing the final count to 16 studies incorporated into this meta-analysis (5, 10–24). The process for study selection is detailed in the PRISMA flowchart provided in Figure 1.
Included studies
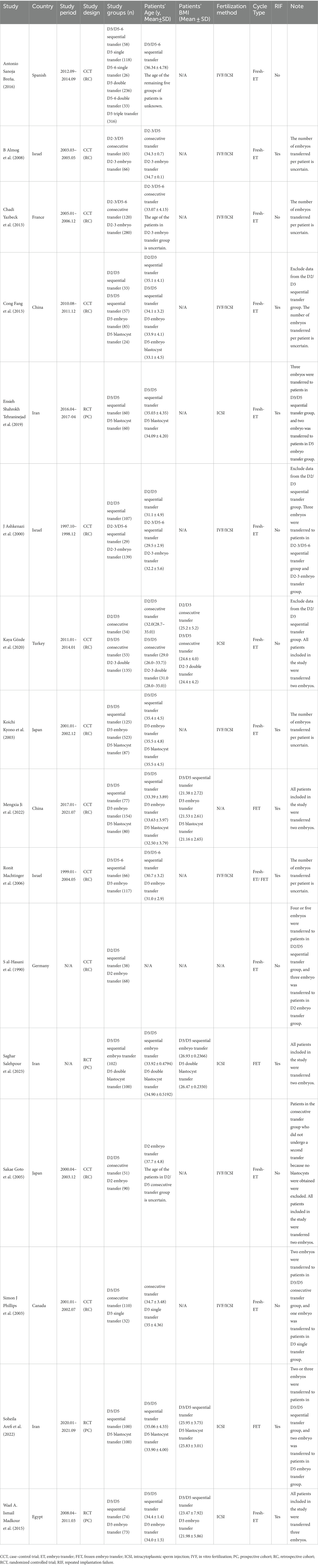
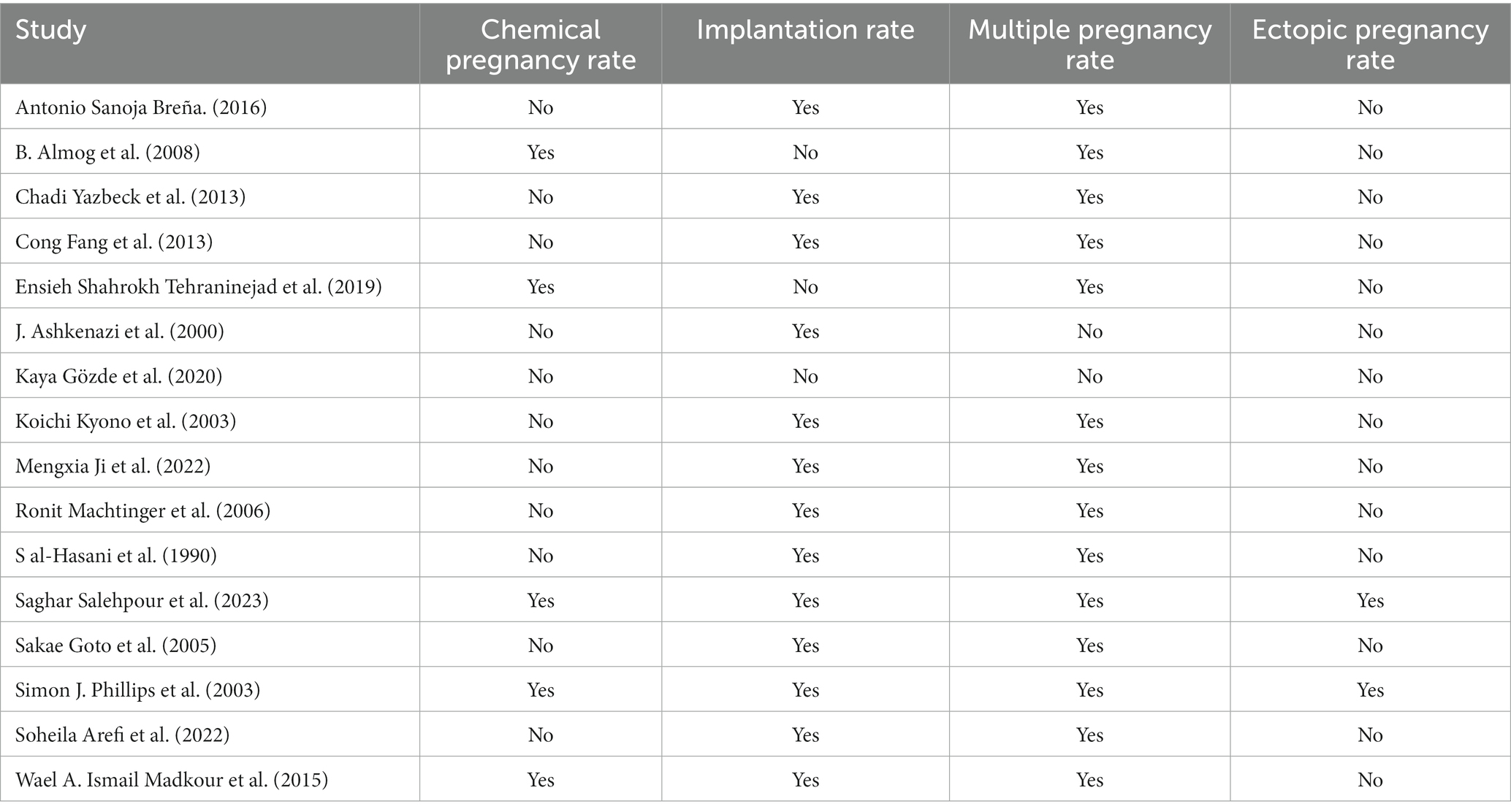
The general characteristics of the 16 studies are shown in Table 1. Four of these were randomized controlled trials (RCTs), while the remaining 12 were case–control trials (CCTs). The studies were geographically diverse, featuring participants from North America, Africa, Asia, and Europe from 1997 to 2021. The cumulative sample consisted of 4,054 participants: 1,185 in the sequential transfer group and 2,869 in the control group. In addition, Table 1 also includes information on the patient’s age, BMI, fertilization method, cycle type, and RIF status. Notably, not all studies reported all the predefined outcomes; the outcomes reported per study are further elaborated in Table 2.
Outcomes
Main outcomes
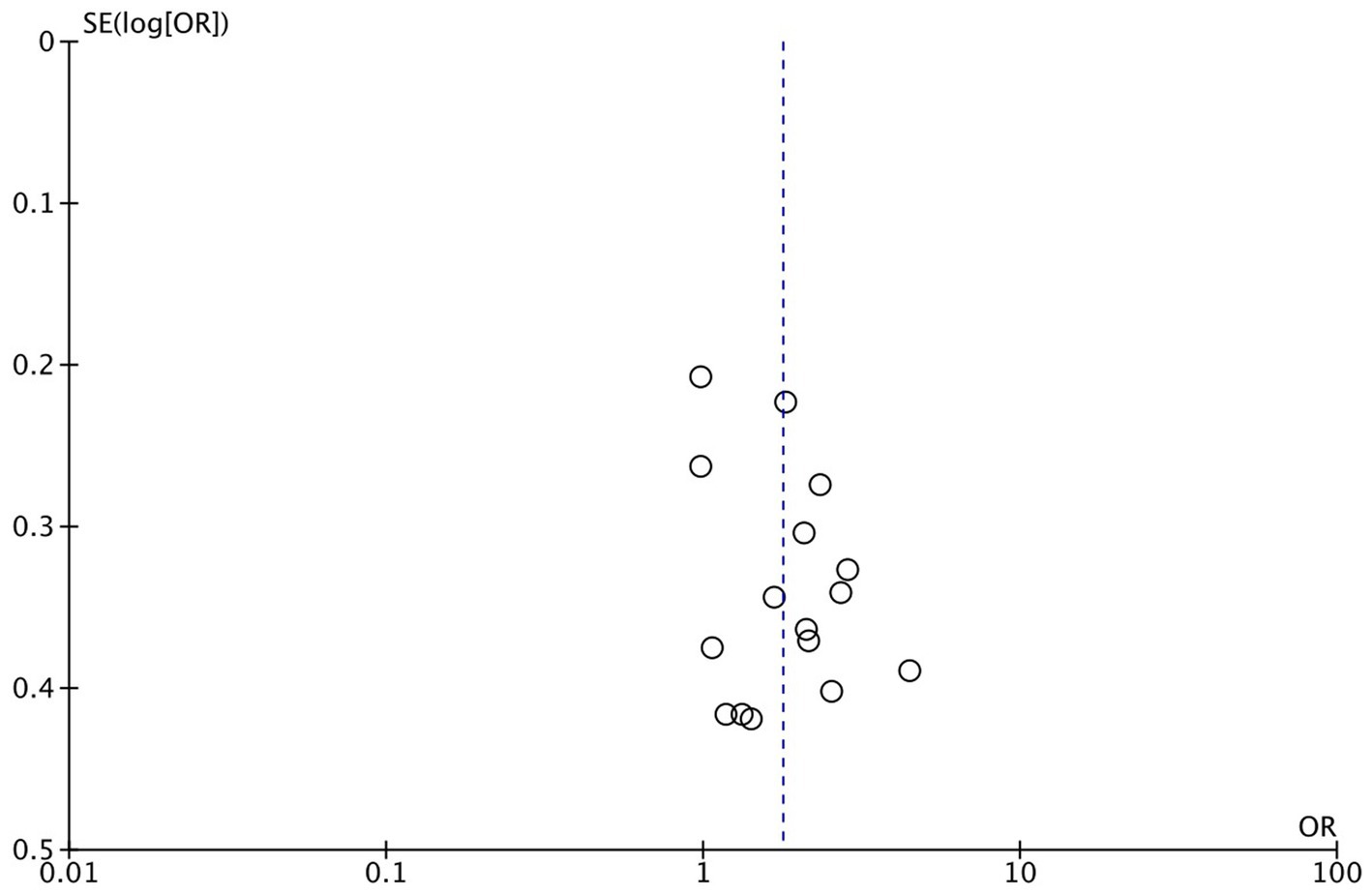
For all patients included in the study, significant improvements were observed in the sequential transfer group compared to the control group for the following outcome measures: chemical pregnancy rate (OR 1.67; 95% CI 1.23–2.27), clinical pregnancy rate (OR 1.78; 95% CI 1.43–2.21), and ongoing pregnancy rate (OR 1.54; 95% CI 1.03–2.31). Conversely, no significant differences were discerned in implantation rate (OR 1.25; 95% CI 0.86–1.81), multiple pregnancy rate (OR 1.14; 95% CI 0.79–1.67), or miscarriage rate (OR 1.42; 95% CI 0.96–2.10) between the two groups (Figure 2). Analysis revealed no significant publication bias among the studies included (Figure 3). Sensitivity analyses were conducted for variables with I2 > 50%, and pooled adjusted ORs yielded estimates congruent with the primary findings (Supplementary Figure S1). Given the high study quality of the RCTs, we performed a separate small subgroup analysis of the four RCTs included in the study, with results similar to the overall analysis (Supplementary Figure S2).
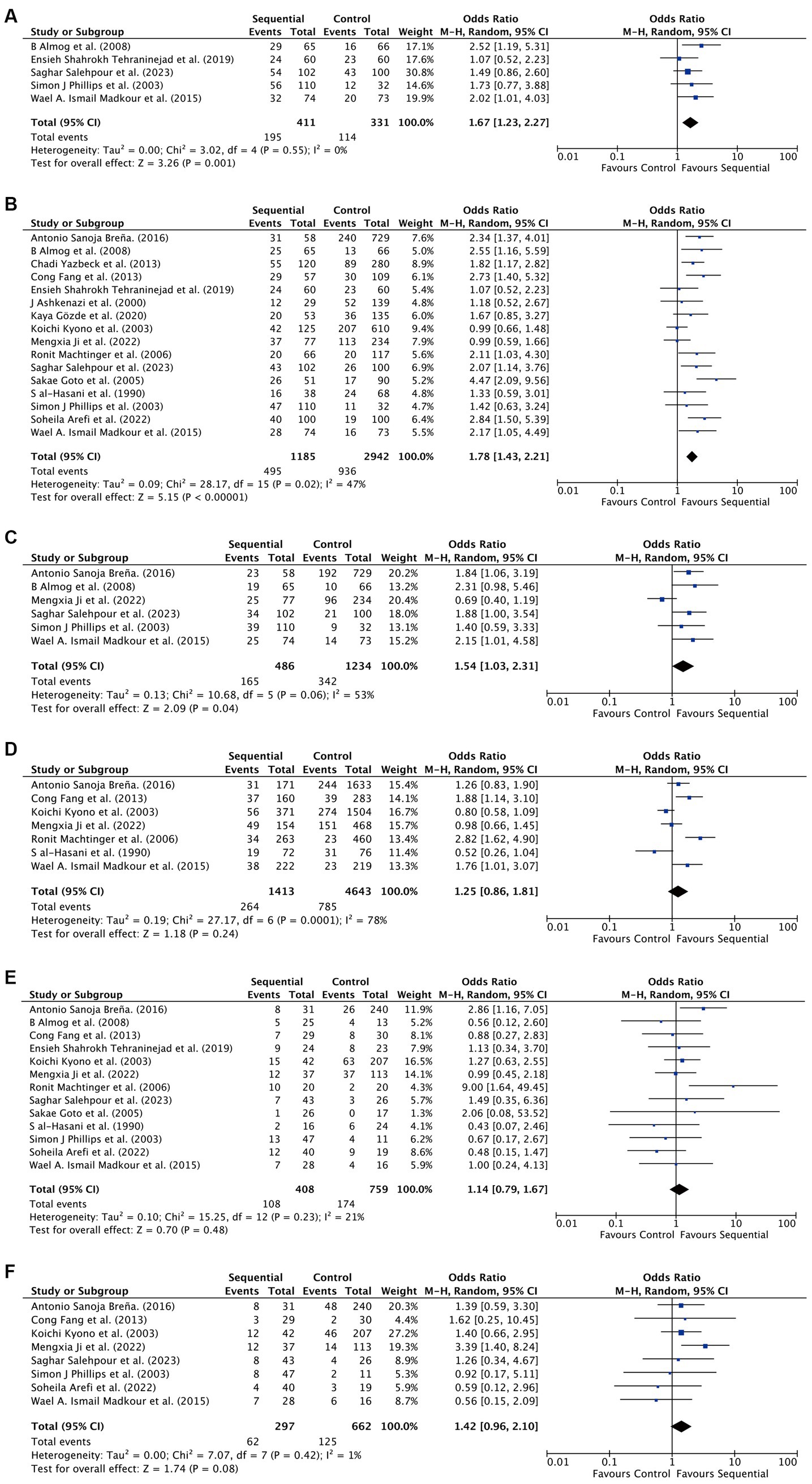
Figure 2. Differences in assisted reproductive outcomes between sequential transfer group and control group. (A) Chemical pregnancy rate. (B) Clinical pregnancy rate. (C) Ongoing pregnancy rate. (D) Implantation rate. (E) Multiple pregnancy rate. (F) Miscarriage rate.
Subgroup analysis
When patients undergoing only cleavage embryo transfers served as the control group, we observed a statistically significant elevation in both the chemical pregnancy rate (OR 2.08; 95% CI 1.35–3.19) and the clinical pregnancy rate (OR 1.78; 95% CI 1.37–2.31) within the sequential transfer group. However, no significant differences were found in ongoing pregnancy rate (OR 1.48; 95% CI 0.94–2.34), implantation rate (OR 1.32; 95% CI 0.86–2.03), multiple pregnancy rate (OR 1.38; 95% CI 0.87–2.18), or miscarriage rate (OR 1.49; 95% CI 0.93–2.37) between the two groups (Table 3 and Supplementary Figure S3). Sensitivity analysis corroborated these findings (Table 3).
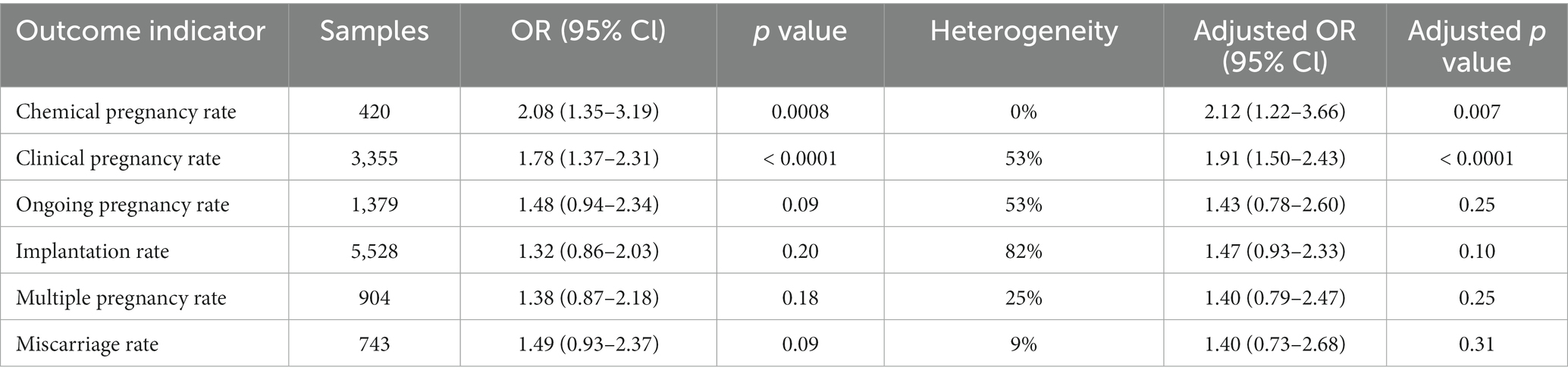
Table 3. Summary of meta-analyses of comparison between sequential transfer and cleavage embryo transfer.
When utilizing patients who underwent only blastocyst transfers as the control, the clinical pregnancy rate (OR 1.68; 95% CI 1.12–2.53) was the sole metric to display a significant difference between the groups. Neither the chemical pregnancy rate (OR 1.32; 95% CI 0.85–2.06), ongoing pregnancy rate (OR 1.59; 95% CI 0.61–4.13), implantation rate (OR 0.98; 95% CI 0.67–1.41), multiple pregnancy rate (OR 0.85; 95% CI 0.50–1.45), nor the miscarriage rate (OR 1.24; 95% CI 0.71–2.19) showed statistically significant differences (Table 4 and Supplementary Figure S4). Sensitivity analyses aligned with these results (Table 4).
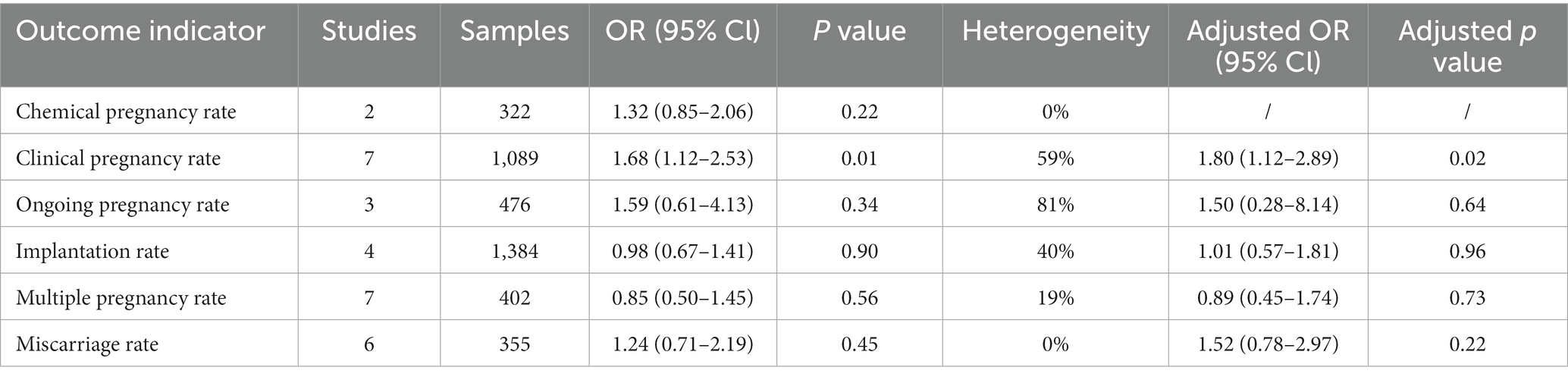
Table 4. Summary of results of meta-analyses of comparison between sequential transfer and blastocyst transfer.
We conducted individualized subgroup analyses on patients diagnosed with RIF. Notably, the chemical pregnancy rate (OR 1.66; 95% CI 1.19–2.32) and the clinical pregnancy rate (OR 1.65; 95% CI 1.19–2.27) were significantly elevated in the sequential transfer group compared to the control group. Conversely, no significant differences emerged in ongoing pregnancy rate (OR 1.53; 95% CI 0.82–2.84), implantation rate (OR 1.44; 95% CI 0.90–2.29), multiple pregnancy rate (OR 1.08; 95% CI 0.72–1.61), or miscarriage rate (OR 1.40; 95% CI 0.80–2.44) between the groups (Table 5 and Supplementary Figure S5). Upon excluding the study conducted by Mengxia Ji et al., the ongoing pregnancy rate significantly improved in the sequential transfer group (OR 2.06; 95% CI 1.35–3.15). Similarly, when omitting the Koichi Kyono et al. study, the implantation rate also showed a significant increase (OR 1.70; 95% CI 1.07–2.70) in the sequential transfer group compared to the control group (Table 5). These findings suggest that caution should be exercised when interpreting these results until further evidence becomes available.
Discussion
Principal findings
Sequential embryo transfers notably enhance pregnancy outcomes in infertile patients, with improvements most evident compared to cleavage embryo transfers. Furthermore, sequential embryo transfers have elevated both chemical and clinical pregnancy rates among patients diagnosed with RIF.
The evidence quality spanned from very low to high. This variance largely stems from the inherent nature of this review, which predominantly relies on observational studies. Additionally, the considerable heterogeneity observed across the studies can likely be attributed to diverse study populations.
Study strengths
Our systematic review is comprehensive, encompassing 4,054 cases identified to date. Beyond merely comparing sequential embryo transfer to cleavage embryo transfer and blastocyst transfer, we also distinctly analyzed subgroups comprising RIF patients. This study adhered to the PRISMA guidelines, underscoring its methodological rigor. Additionally, we meticulously assessed the risk of bias within the included studies. Collectively, these factors considerably bolster the validity of our findings.
Study limitations
Most of the studies incorporated into this review were observational. Significant variability was evident among the included studies, particularly in aspects such as study population, study duration, transfer protocol, patient BMI, cycle type, and the number of embryos transferred, as detailed in Table 1. Our results could have been influenced by the disparate transfer methodologies, the number of embryos transferred, and certain incomplete observations from some studies. A notable limitation was our inability to adjust for relevant confounders in our primary analyses, due to the absence of individual patient data—specifically metrics like obesity levels, hormone concentrations, and endometrial conditions. Furthermore, there was inconsistency in the definitions of observed outcome indicators across studies; notably, the definitions for RIF varied among the included studies.
Interpretation of the results
Transferring embryos from the laboratory to the uterus is a pivotal phase in any ART cycle. Embryos were commonly transferred on day 3, during the cleavage stage; however, the last decade has seen a growing inclination toward day 5 blastocyst transfers (25). This shift to the blastocyst stage more closely mirrors the physiologically natural timing of implantation, fostering improved synchronization between embryonic development and the endometrial environment (25). It’s been documented that women who opt for blastocyst transfers exhibit higher clinical pregnancy and live birth outcomes compared to their counterparts who undergo cleavage stage embryo transfers (26). A caveat to this approach is that extended culture to the blastocyst stage might reduce the number of viable embryos suitable for transfer or cryopreservation, occasionally even leading to cycle cancelation.
Sequential embryo transfer offers a solution to this limitation. Preliminary data showcased a rise in pregnancy outcomes post sequential embryo transfers (4), although subsequent studies yielded mixed results (15). Our research showed that sequential embryo transfers bolstered the rates of chemical pregnancies, clinical pregnancies, and ongoing pregnancies in patients facing infertility. Our subgroup analysis revealed that sequential transfers led to superior chemical and clinical pregnancy rates when juxtaposed against cleavage stage transfers. Interestingly, this disparity diminished when pitted against blastocyst transfers, alluding to the potential benefits of blastocysts in elevating pregnancy outcomes. The heightened clinical pregnancy rates following sequential transfers, instead of mere blastocyst transfers, might be attributable to the nuanced two-step transfer protocol. The endometrium is optimally receptive to embryos within a concise timeframe, dubbed the “window of implantation” (27). It’s theorized that this “window” might oscillate during the luteal phase, thus pinpointing its precise dynamics is integral for embryo transfer scheduling (28). Sequential transfers aptly address this challenge by potentially enhancing the likelihood of embryos coinciding with this “implantation window” (29). Moreover, emerging evidence posits that the preliminary transferred cleavage-stage embryos can modulate the immune response and engage in pivotal interactions with immune cells within the uterus during sequential transfers. This paves the way for a more conducive endometrial setting for the subsequent transfer (30–32). It’s crucial to underscore that the exact definition of ongoing pregnancy rates might vary across studies. Given that ongoing pregnancy rates are intricately tied to various maternal factors—like basal metabolic conditions, hormonal levels, and endometrial health—the credibility of this metric warrants circumspection, especially in the absence of granular patient data. In addition, our study did not have significant data on live birth rates. Results on live birth rate are more valuable but were not reported in any of the included studies.
In our study, there was no significant difference in multiple pregnancy rates. Although logically, the multiple pregnancy rate should be higher for sequential embryo transfer, the multiple pregnancy rate is mainly related to the number of embryos transferred. Of all the included studies, excluding those in which the number of embryos transferred to patients was unclear, only one study showed that two embryos were transferred to patients in the sequential transfer group and one embryo was transferred to patients in the control group, whereas the rest of the studies had essentially the same number of embryos transferred to patients in the sequential transfer group and the control group. Therefore, it makes sense that there is no difference in multiple pregnancy rates.
Previous research has demonstrated that sequential embryo transfer considerably enhances pregnancy outcomes in patients with RIF (24, 33). Contrastingly, the same investigations revealed no marked improvement in clinical pregnancy rates for RIF patients undergoing sequential embryo transfer compared to day 5 blastocyst transfers (14). One plausible explanation for these divergent findings lies in the variations in the definitions of RIF and the inclusion criteria adopted across these studies. In our research, sequential embryo transfer yielded an uptick in both chemical and clinical pregnancy rates for RIF patients, even without distinguishing between cleavage-stage embryos and blastocysts.
Conclusion
Our systematic review and meta-analysis confirm that sequential transfer may enhance clinical pregnancy rate in a small subgroup of well-selected women. Women with an adequate number of embryos will do equally good with blastocyst transfer. Guidance should be developed for clinicians to decide who should be considered for sequential transfer based on grade and number of available embryos and clinical history. Nonetheless, precise definitions for metrics such as the chemical pregnancy rate, ongoing pregnancy rate, and implantation rate need to be standardized, especially when considering patients with RIF. Furthermore, comprehensive data collection, encompassing a broader spectrum of patient information, is pivotal for more in-depth and rigorous analyses in future research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
WT: Writing – original draft, Data curation, Methodology, Software. HX: Writing – original draft, Software. FW: Writing – review & editing, Data curation. YW: Methodology, Validation, Writing–review–&–editing. XM: Data curation, Writing – review & editing. XZ: Conceptualization, Writing – review & editing. XS: Resources, Writing – review & editing. JY: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Science Foundation of Sichuan Medical Association (S21098). Natural Science Foundation of Sichuan Provincial Science and Technology Departmen (2022YFS0058). Transformation Project of Sichuan Provincial Science and Technology Department (2023JDZH0033).
Acknowledgments
The authors acknowledge Dan Zhao and her team for providing medical writing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1303493/full#supplementary-material
References
1. Mitri, F , Nayot, D , Casper, RF , and Bentov, Y . Current tools for the optimization of embryo transfer technique for recurrent implantation failure. Minerva Ginecol. (2016) 68:431–49.
2. Ashary, N , Tiwari, A , and Modi, D . Embryo implantation: war in times of love. Endocrinology. (2018) 159:1188–98. doi: 10.1210/en.2017-03082
3. Liu, C , Yao, W , Yao, J , Li, L , Yang, L , Zhang, H, et al. Endometrial extracellular vesicles from women with recurrent implantation failure attenuate the growth and invasion of embryos. Fertil Steril. (2020) 114:416–25. doi: 10.1016/j.fertnstert.2020.04.005
4. Abramovici, H , Dirnfeld, M , Weisman, Z , Sorokin, Y , Lissak, A , Rofe, A, et al. Pregnancies following the interval double-transfer technique in an in vitro fertilization-embryo transfer program. J In Vitro Fert Embryo Transf. (1988) 5:175–6. doi: 10.1007/BF01131183
5. Goto, S , Shiotani, M , Kitagawa, M , Kadowaki, T , and Noda, Y . Effectiveness of two-step (consecutive) embryo transfer in patients who have two embryos on day 2: comparison with cleavage-stage embryo transfer. Fertil Steril. (2005) 83:721–3. doi: 10.1016/j.fertnstert.2004.07.974
6. Bashiri, A , Halper, KI , and Orvieto, R . Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. (2018) 16:121. doi: 10.1186/s12958-018-0414-2
7. Craciunas, L , Gallos, I , Chu, J , Bourne, T , Quenby, S , Brosens, JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25:202–23. doi: 10.1093/humupd/dmy044
8. Higgins, JP , Thompson, SG , Deeks, JJ , and Altman, DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
9. Atkins, D , Eccles, M , Flottorp, S , Guyatt, GH , Henry, D , Hill, S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE working group. BMC Health Serv Res. (2004) 4:38. doi: 10.1186/1472-6963-4-38
10. Breña, AS . Doble transferencia embrionaria consecutiva D3/D5-6: una alternativa para mejorar las tasas de embarazo en pacientes sometidas a fertilización in vitro/transferencia embrionaria (FIV/TE). Rev Obstet Ginecol Venez. (2016) 76
11. Almog, B , Levin, I , Wagman, I , Kapustiansky, R , Schwartz, T , Mey-Raz, N, et al. Interval double transfer improves treatment success in patients with repeated IVF/ET failures. J Assist Reprod Genet. (2008) 25:353–7. doi: 10.1007/s10815-008-9237-y
12. Yazbeck, C , Jamaa, NB , Hazout, A , Cohen-Bacrie, P , Junca, AM , and Rougier, N . Advantages of the two-step embryo transfer strategy in human IVF/ICSI cycles. Zygote. (2013) 21:77–83. doi: 10.1017/S096719941100058X
13. Fang, C , Huang, R , Li, TT , Jia, L , Li, LL , and Liang, XY . Day-2 and day-3 sequential transfer improves pregnancy rate in patients with repeated IVF-embryo transfer failure: a retrospective case-control study. Reprod Biomed Online. (2013) 26:30–5. doi: 10.1016/j.rbmo.2012.10.004
14. Tehraninejad, ES , Raisi, E , Ghaleh, FB , Rashidi, BH , Aziminekoo, E , Kalantari, V, et al. The sequential embryo transfer compared to blastocyst embryo transfer inin vitrofertilization (IVF) cycle in patients with the three repeated consecutive IVF. A randomized controlled trial. Gynecol Endocrinol. (2019) 35:955–9. doi: 10.1080/09513590.2019.1613639
15. Ashkenazi, J , Yoeli, R , Orvieto, R , Shalev, J , Ben-Rafael, Z , and Bar-Hava, I . Double (consecutive) transfer of early embryos and blastocysts: aims and results. Fertil Steril. (2000) 74:936–40. doi: 10.1016/S0015-0282(00)01549-1
16. Kaya, G , Alyürük, B , Cicek, Y , Senem, O , Köpük, SY , Çakiroğlu, AY, et al. Effect of double cleavage stage versus sequential cleavage and blastocyst stage embryo transfer on clinical pregnancy rates. Asian Pacific J Reprod. (2020) 9:124–8. doi: 10.4103/2305-0500.284269
17. Kyono, K , Fukunaga, N , Chiba, S , Nakajo, Y , Fuchinoue, K , Yagi, A, et al. Two-step consecutive transfer of early embryos and blastocysts. Reprod Med Biol. (2003) 2:133–7. doi: 10.1046/j.1445-5781.2003.00031.x
18. Ji, M , Zhang, L , Fu, X , Xie, W , Wu, X , and Shu, J . The outcomes of sequential embryo transfer in patients undergoing in vitro fertilization with frozen-thawed embryos: a retrospective study. J Obstet Gynaecol Res. (2022) 48:2563–70. doi: 10.1111/jog.15369
19. Machtinger, R , Dor, J , Margolin, M , Levron, J , Baum, M , Ferber, B, et al. Sequential transfer of day 3 embryos and blastocysts after previous IVF failures despite adequate ovarian response. Reprod Biomed Online. (2006) 13:376–9. doi: 10.1016/S1472-6483(10)61442-3
20. al-Hasani, S , van der Ven, H , Diedrich, K , Reinecke, A , Hartje, H , and Krebs, D . Effect of sequential embryo transfer on pregnancy following in vitro fertilization. Geburtshilfe Frauenheilkd. (1990) 50:640–3. doi: 10.1055/s-2008-1026516
21. Salehpour, S , Hosseini, S , Razghandi, Z , Hosseinirad, H , and Ziaee, H . Comparing the effect of sequential embryo transfer versus double blastocyst embryo transfer on pregnancy outcomes in intracytoplasmic sperm injection (ICSI) cycles in patients with repeated implantation failure: a randomized controlled trial. Taiwan J Obstet Gynecol. (2023) 62:264–9. doi: 10.1016/j.tjog.2022.10.009
22. Phillips, SJ , Dean, NL , Buckett, WM , and Tan, SL . Consecutive transfer of day 3 embryos and of day 5-6 blastocysts increases overall pregnancy rates associated with blastocyst culture. J Assist Reprod Genet. (2003) 20:461–4. doi: 10.1023/B:JARG.0000006708.26464.23
23. Arefi, S , Ataei, M , Maleki, N , Yari, N , Razi, S , and Amirajam, S . Sequential (two-step) day 3/day 5 frozen-thawed embryo transfer: does it improve the pregnancy rate of patients suffering recurrent implantation failure? J Med Life. (2022) 15:1365–70. doi: 10.25122/jml-2022-0041
24. Ismail, WA , Madkour, BN , Zaheer, H , Al-Bahr, A , Abdelhamid, AMS , Shaeer, M, et al. Does sequential embryo transfer improve pregnancy rate in patients with repeated implantation failure? A randomized control study. Middle East Fertility Soc J. (2015) 20:255–61. doi: 10.1016/j.mefs.2015.04.002
25. Glujovsky, D , and Farquhar, C . Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. (2016) 106:244–50. doi: 10.1016/j.fertnstert.2016.06.029
26. Eftekhar, M , Mohammadi, B , Tabibnejad, N , and Mortazavi Lahijani, M . Frozen-thawed cleavage stage versus blastocyst stage embryo transfer in high responder patients. Zygote. (2020) 28:511–5. doi: 10.1017/S0967199420000428
27. Enciso, M , Aizpurua, J , Rodríguez-Estrada, B , Jurado, I , Ferrández-Rives, M , Rodríguez, E, et al. The precise determination of the window of implantation significantly improves ART outcomes. Sci Rep. (2021) 11:13420. doi: 10.1038/s41598-021-92955-w
28. Stamenov, GS , Parvanov, DA , and Chaushev, TA . Successful pregnancy following mixed double embryo transfer in a patient with variable window of implantation. Case Rep Obstet Gynecol. (2018) 2018:1687583. doi: 10.1155/2018/1687583
29. Haouzi, D , Entezami, F , Torre, A , Innocenti, C , Antoine, Y , Mauries, C, et al. Customized frozen embryo transfer after identification of the receptivity window with a transcriptomic approach improves the implantation and live birth rates in patients with repeated implantation failure. Reprod Sci. (2021) 28:69–78. doi: 10.1007/s43032-020-00252-0
30. Zhang, J , Wang, C , Zhang, H , and Zhou, Y . Sequential cleavage and blastocyst embryo transfer and IVF outcomes: a systematic review. Reprod Biol Endocrinol. (2021) 19:142. doi: 10.1186/s12958-021-00824-y
31. Mor, G , Aldo, P , and Alvero, AB . The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. (2017) 17:469–82. doi: 10.1038/nri.2017.64
32. Schumacher, A , Sharkey, DJ , Robertson, SA , and Zenclussen, AC . Immune cells at the Fetomaternal Interface: how the microenvironment modulates immune cells to Foster fetal development. J Immunol. (2018) 201:325–34. doi: 10.4049/jimmunol.1800058
33. Torky, H , Ahmad, A , Hussein, A , el-Desouky, ES , Aly, R , Ragab, M, et al. RETRACTION NOTE TO: comparing sequential vs. day 3 vs. day 5 embryo transfers in cases with recurrent implantation failure: randomized controlled trial. JBRA Assist Reprod. (2021) 25:185–92. doi: 10.5935/1518-0557.20200083ra
Glossary
Keywords: sequential embryo transfer, in vitro fertilization-embryo transfer, repeated implantation failure, systematic review, IVF
Citation: Teng W, Xian H, Wang F, Wang Y, Meng X, Zhang X, Shan X and Yi J (2023) Effect of sequential embryo transfer on in vitro fertilization and embryo transfer outcomes: a systematic review and meta-analysis. Front. Med. 10:1303493. doi: 10.3389/fmed.2023.1303493
Edited by:
Simcha Yagel, Hadassah Medical Center, IsraelReviewed by:
Sujata Kar, Ravenshaw University, IndiaPinar Yalcin Bahat, University of Health Sciences, Türkiye
Copyright © 2023 Teng, Xian, Wang, Wang, Meng, Zhang, Shan and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangying Yi, eWlqeTIwMDZAMTI2LmNvbQ==
 Wending Teng
Wending Teng Hong Xian1
Hong Xian1 Xiaojian Zhang
Xiaojian Zhang Xudong Shan
Xudong Shan