- 1Department of Pharmacy, Guangzhou Institute of Dermatology, Guangzhou, China
- 2The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, China
Introduction: The association between red blood cell distribution width (RDW) and psoriasis among the US adults is still unknown. We aimed to assess whether RDW is associated with psoriasis in the US adults.
Method: We conducted a cross-sectional study consisting of 14,089 participants from National Health and Nutrition Examination Survey (NHANES) 2009–2014. Psoriasis status were assessed by self-reported questionnaire. We evaluated the association between RDW and risk of psoriasis using multivariate regression models. Subgroup and interaction analysis were performed.
Results: The higher RDW level was associated with an increased risk of psoriasis (OR = 1.10 [95% CI, 1.01, 1.19]; p = 0.025) after adjusting for confounders in female. However, there is no significant association between RDW and risk of psoriasis among male (OR = 0.99 [95% CI, 0.87, 1.15]; p = 0.992). Subgroup and interaction analysis found that the strongest positive association mainly exists in female participants with BMD greater than 29.9 kg/m2 (OR = 1.20 [95% CI, 1.09, 1.32], Pint = 0.004).
Discussion: In conclusion, we found that increased RDW levels were associated with an increased risk of psoriasis in females, which could provide clinicians with auxiliary data for the early diagnosis of psoriasis.
Introduction
Psoriasis is a chronic autoimmune skin disease believed to be caused by a combination of genetic and environmental factors (1). In the United States, approximately 7.5 million adults have psoriasis (2), with variations in prevalence among different age and ethnic groups. Typical symptoms of psoriasis include red, thick patches covered with silvery scales, dry and intensely itchy skin, as well as cracks, pain, and occasional bleeding (3). Currently, psoriasis remains a not curable disease, but symptoms can be relieved through medication and phototherapy (4, 5). Infections, stress, injury, and certain medications may trigger or exacerbate psoriasis (6, 7). Overall, understanding its causes and risk factors is essential for prevention and management of this disease.
Red Cell Distribution Width (RDW) is a parameter that reflects the proportion of red blood cells of different sizes in the blood and is commonly used in clinical testing and diagnosis. The normal range of RDW is usually between 11.5 and 14.5%, an increased value of RDW indicate an uneven distribution of red blood cell size in various diseases, such as anemia, malnutrition, chronic diseases, infections, and bleeding (8). In recent years, many studies reported that high RDW values are associated with an increased risk of malignant tumors, cardiovascular diseases, and pulmonary diseases (9–11). Moreover, it has been proven to be closely related with inflammatory or autoimmune conditions such as rheumatoid arthritis and systematic lupus erythematosus (12, 13). Meanwhile, chronic inflammation has been reported as leading to the development of psoriasis. In this regard, there is ongoing research involve in detecting of the predictive value of RDW for diagnosing psoriasis. Several studies reported that RDW values in psoriasis patients were uncovered higher than healthy matching (14–16). However, another study have detected no association of RDW value with psoriasis (17). In addition, there is limited data on the association between RDW and psoriasis among the representative and large sample of US population.
Therefore, the aim of this study is to analyze the association between RDW levels and psoriasis in US adults, using data from the nationally representative National Health and Nutrition Examination Survey (NHANES) database (2009–2014). We also aim to provide clinicians with auxiliary data for the early diagnosis of psoriasis.
Materials and methods
Study population
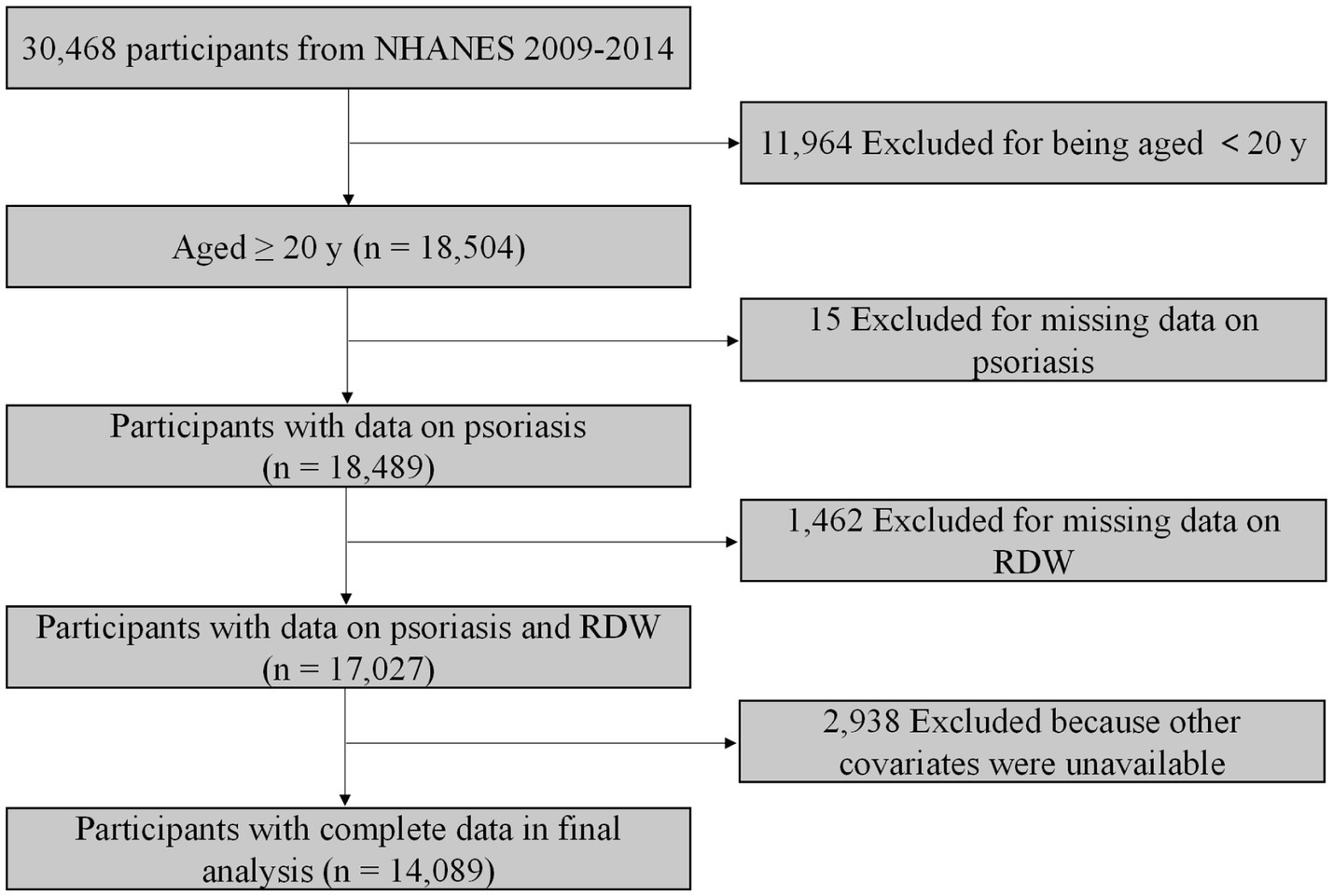
The NHANES database is an ongoing national survey focusing on health and health-related behaviour of the US population. The NHANES database is available publicly at www.cdc.gov/nchs/nhanes. We included data from three 2-year NHANES cycles (2009–2014). In total, 18,504 participants aged greater than 20 years were included. We excluded participants without data on psoriasis (n = 15). After further excluding individuals with missing value for RDW (n = 1,462) and other covariates (n = 2,938), 14,089 participants were included for final analysis (Figure 1).
Assessment of psoriasis and RDW
Psoriasis was defined as an affirmative response to the question, “Have you ever been told by a health care provider that you had psoriasis?” Whole blood samples were collected from all participants, and RBCs were measured using the Beckman automated Coulter method of counting and sizing (Coulter Counter; Coulter Electronics, Luton, United Kingdom) 40. Mean Corpuscular Volume (MCV) was calculated as the average volume of individual erythrocytes derived from the histogram of RBC. Finally, the RDW (%) was calculated as the SD of MCV divided by the mean of MCV, multiplied by 100.
Assessment of covariates
Covariates included age, race/ethnicity (including Non-Hispanic white, Non-Hispanic black, Mexicane American, Other Hispanic, and Other ethnicity), body mass index (BMI), physical activity, alcohol drinking status, smoking status, diabetes, congestive heart failure, and stroke. To assess physical activity, weekly metabolic equivalent (MET) minute aggregated scores were calculated for each participant (18). According to the MET-minutes, participants were categorized into inactive (<600 MET-minute/week), moderately active (600–3,000 MET-minute/week), and highly active (> 3000MET-minute/week). Significant alcohol use was determined by the question, “In any 1 year, have you had at least 12 drinks of any type of alcoholic beverage?” Participants who answered “yes” were defined as alcohol drinkers. Smoking history was determined if the patient responded “yes”to ever smoking at least 100 cigarettes. Covariates relating to medical history such as a history of congestive heart failure, stroke, and diabetes mellitus were gathered from specific, individual questions asking participants whether a physician had ever told them that they had the condition of interest. Information on each variable and acquisition process are publicly available at www.cdc.gov/nchs/nhanes.
Statistical analysis
Data were presented as mean ± standard deviation for continuous variables and as percentages for categorical variables. We grouped participants into quartiles according to hemoglobin levels. We used the ANOVA tests for continuous variables with a normal distribution and Kruskal-Wallis test for continuous variables without a normal distribution. The chi-square tests were performed for categorical variables to assess the characteristics of the participants. Multivariate logistic regression analyses were used to evaluate the relationship between RDW and psoriasis risk with odds ratio (OR) and corresponding 95% confidence interval (CI). We contrasted three models as follows: model 1, no covariate was adjusted; model 2, adjusted for age, and race/ethnicity; model 3, additionally adjusted for BMI, physical activity, alcohol drinking status, smoking status, diabetes, congestive heart failure, and stroke.
In addition, subgroup analyses were also conducted stratified by different age, BMI, race/ethnicity, alcohol drinking status, smoking status, diabetes, congestive heart failure, stroke, and physical activity. A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were conducted using the EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and statistical software packages R (http://www.R-project.org, The R Foundation).
Results
Characteristics of participants
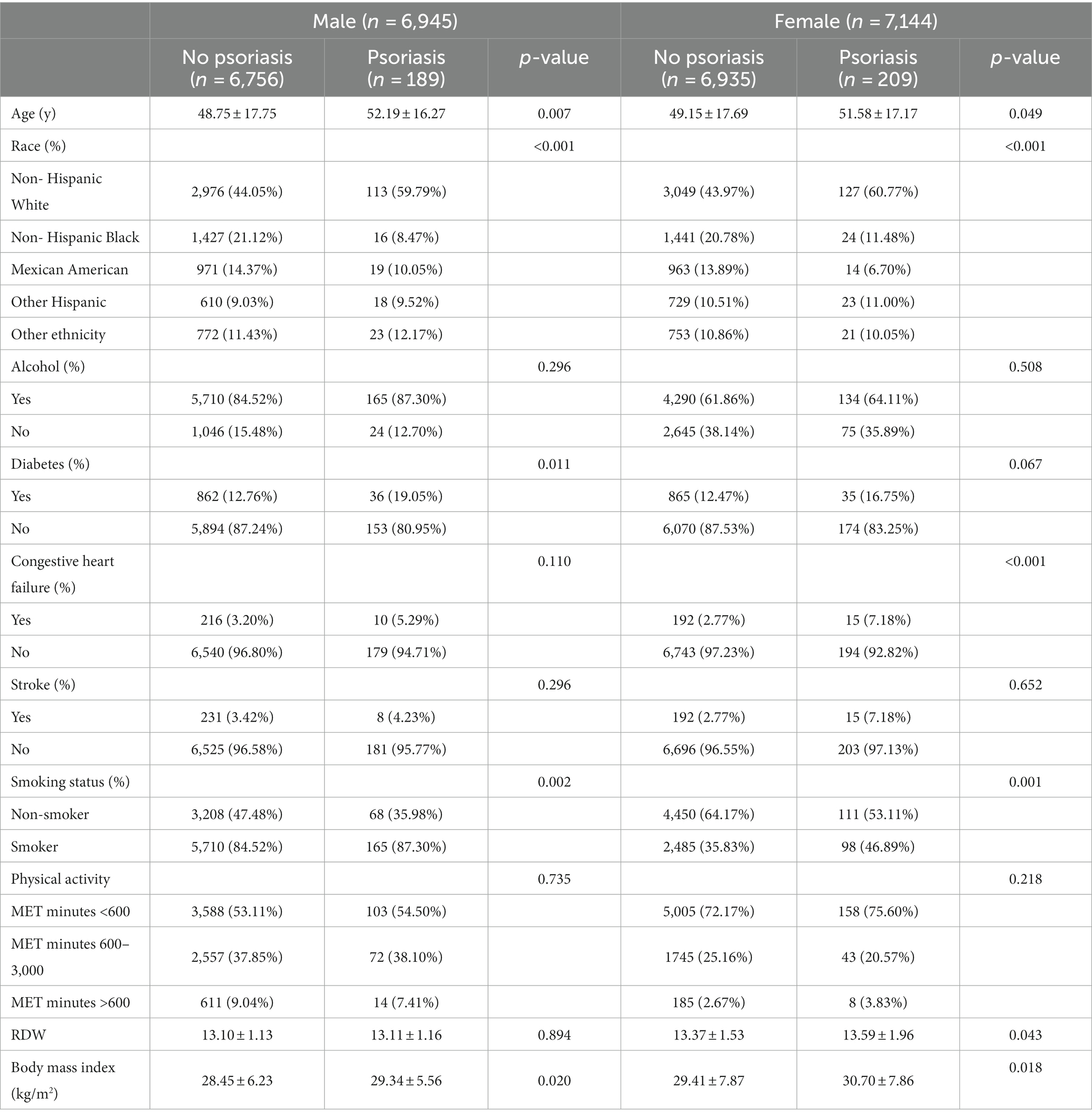
Table 1 presented the characteristics of participant with non-psoriasis and psoriasis. Among males, 189 (2.72%) participants were diagnosed with psoriasis. Participants with psoriasis tend to be older and non-Hispanic White individuals, to have higher mean BMI, and more commonly reported a history of diabetes and smoking. In contrast, 209 (2.93%) participants had psoriasis in females. Participants with psoriasis tend to be older and non-Hispanic White individuals, to have higher mean RDW and BMI, and more commonly reported a history of congestive heart failure and smoking.
Association between RDW and psoriasis risk
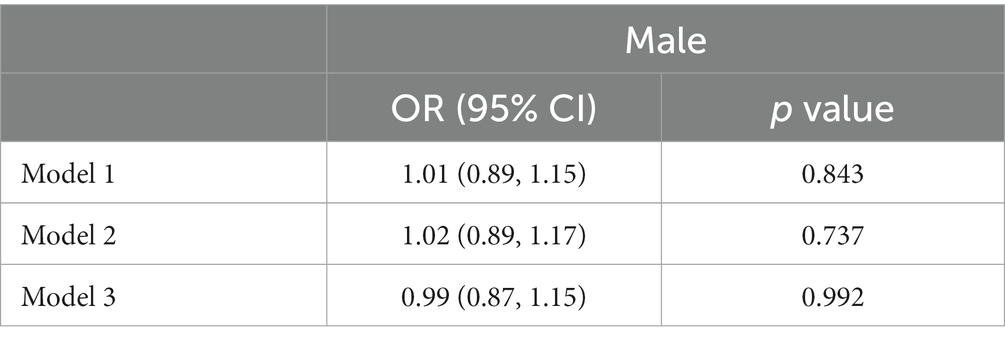
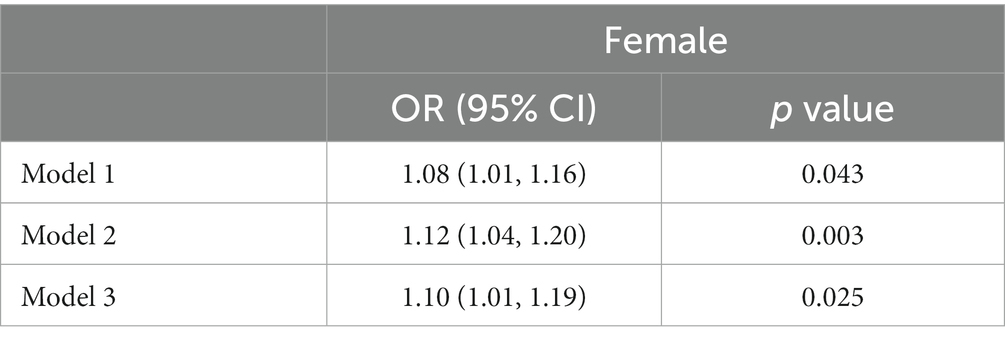
Table 2 showed the association between RDW and psoriasis risk in males group. In the unadjusted model, no significant association between RDW and psoriasis risk was observed (OR = 1.01 [95% CI, 0.89, 1.15]; p = 0.843). After adjusting for confounders, RDW was also not significantly associated with psoriasis risk in model 2 (OR = 1.02 [95% CI, 0.89, 1.17]; p = 0.737) or model 3 (OR = 0.99 [95% CI, 0.87, 1.15]; p = 0.992). Table 3 presented the association between RDW and psoriasis risk in females group. In the unadjusted model, increased RDW levels was significantly correlated with higher psoriasis risk (OR = 1.08 [95% CI, 1.01, 1.16]; p = 0.043). After adjusting for age and race/ethnicity, RDW was positively associated with higher risk of psoriasis (OR = 1.12 [95% CI, 1.04, 1.20]; p = 0.003) in Model 2. After fully adjusting for confounders, RDW was still positively associated with higher risk of psoriasis (OR = 1.10 [95% CI, 1.01, 1.19]; p = 0.025) in Model 3.
The association between RDW and psoriasis risk was not observed in all different subgroups among males, including age, race, BMI, alcohol consumption, diabetes, congestive heart failure, stroke, smoking, and physical activity (Figure 2). We found a significant interaction between RDW and BMI with the risk of psoriasis (p = 0.004 for interaction) in females (Figure 3). The association between RDW and higher risk of psoriasis (OR = 1.20 [95% CI, 1.09, 1.32]) was stronger in populations of female with BMI greater than 29.9 kg/m2.
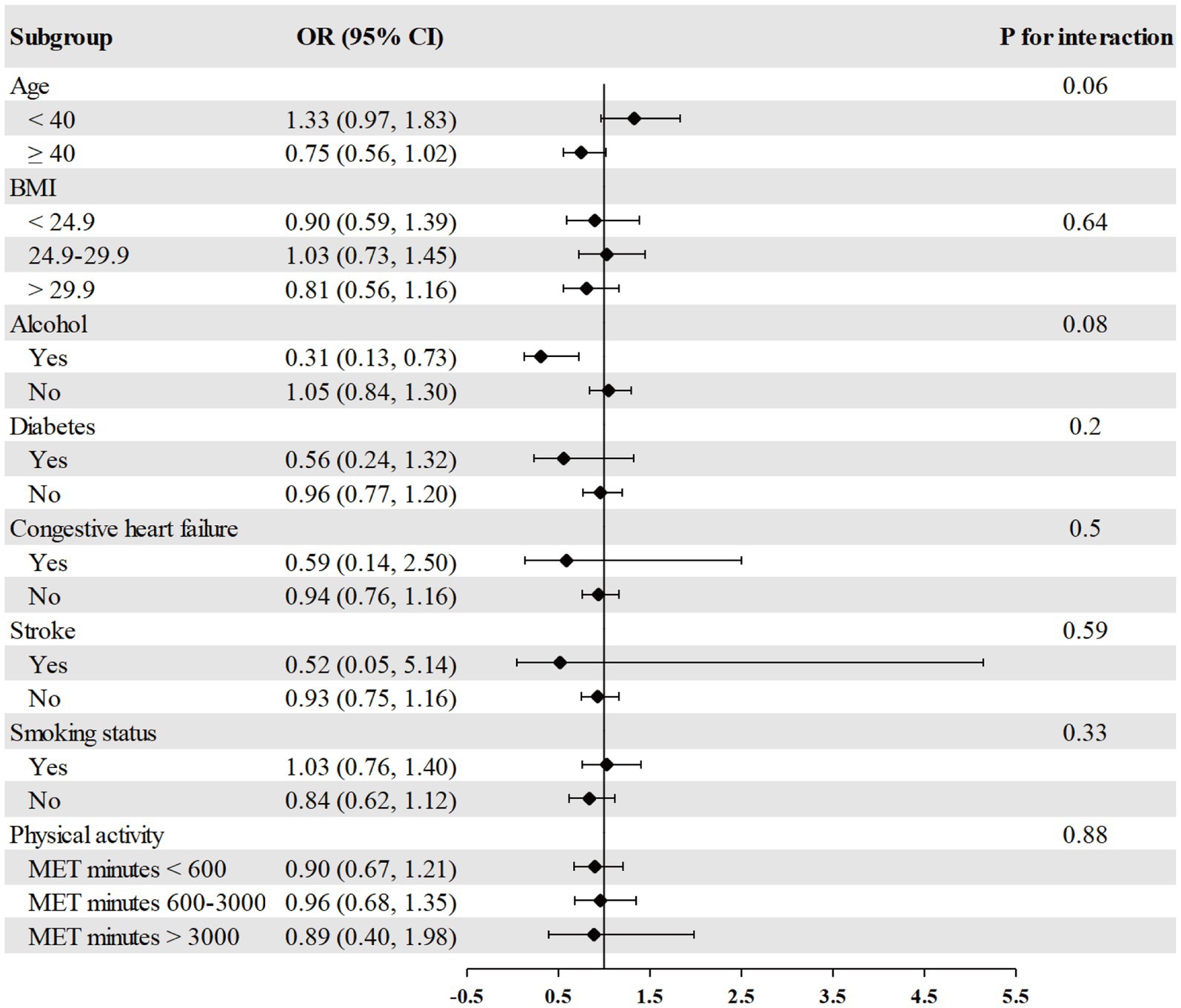
Figure 2. Association between RDW and psoriasis risk in different subgroups among males. Age, race/ethnicity, BMI, physical activity, alcohol drinking status, smoking status, diabetes, congestive heart failure, and stroke were adjusted (the stratified variable was omitted from the model).
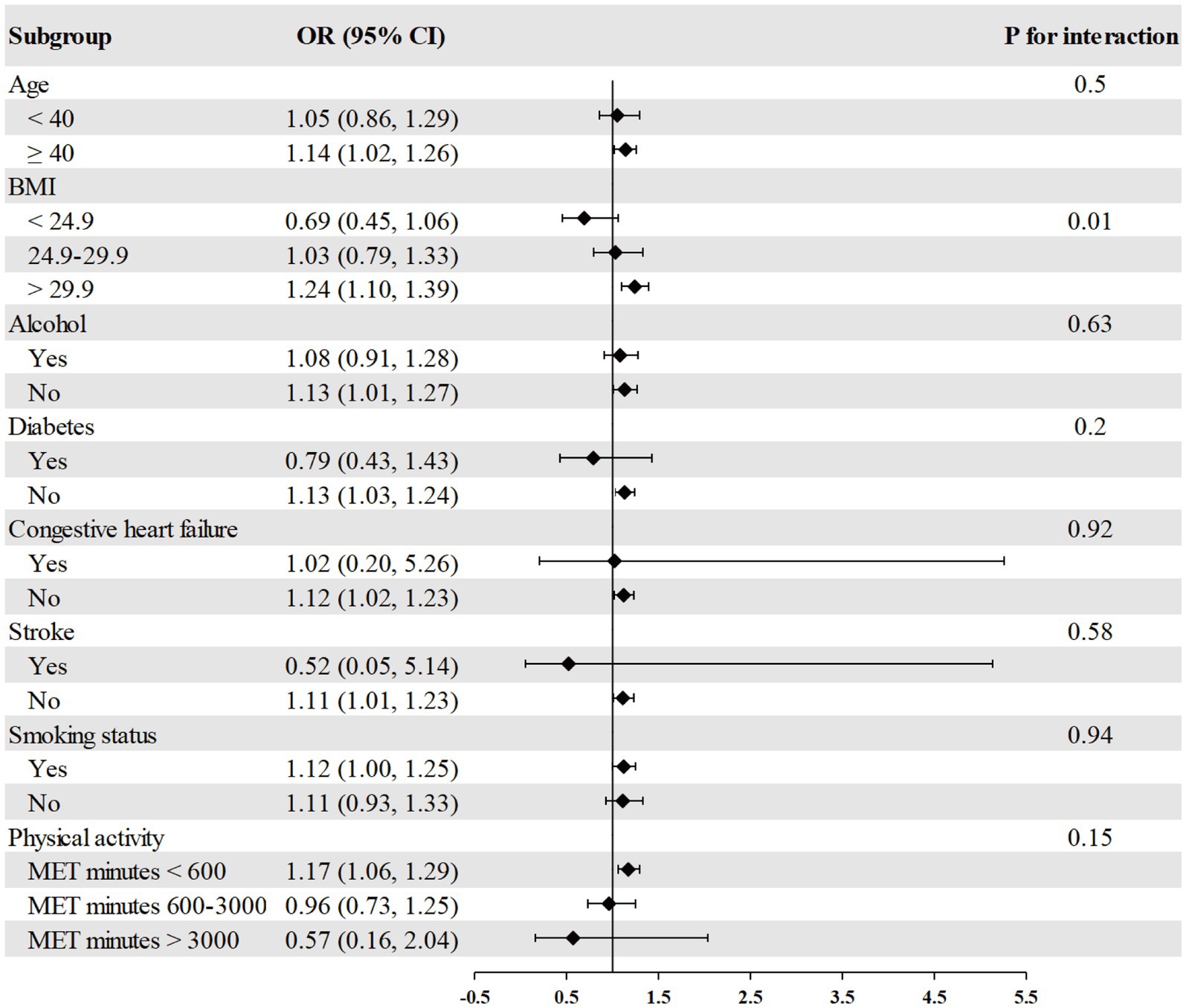
Figure 3. Association between RDW and psoriasis risk in different subgroups among females. Age, race/ethnicity, BMI, physical activity, alcohol drinking status, smoking status, diabetes, congestive heart failure, and stroke were adjusted (the stratified variable was omitted from the model).
Discussion
In the present study, we used the data from population-based national survey to evaluate the association between RDW and psoriasis risk in US adults. We observed a positive association between RDW and psoriasis risk in females after fully adjusting for covariates. No association between RDW and psoriasis risk was found in males. Subgroup analysis indicated that the association between RDW and psoriasis risk was more pronounced in female with BMI greater than 29.9 kg/m2.
Psoriasis is an inflammatory skin disease associated with many other conditions (19). The most obvious complication is psoriatic arthritis, which can be observed in 10–40% of psoriasis patients and usually occurs 10 years after the onset of psoriasis (20, 21). Psoriasis and psoriatic arthritis share some common pathogenic mechanisms and immunological features. Other diseases are more common in psoriasis patients than in the general population, including chronic obstructive pulmonary disease (22), asthma (23), adverse cardiovascular events (MACE) (24), chronic kidney disease (25), and inflammatory bowel disease (IBD) (26). Several retrospective studies reported that RDW can predict the prognosis of chronic obstructive pulmonary disease (27, 28). Conic et al. found that RDW may be a cost-effective clinical tests to identify psoriasis and psoriatic arthritis patients at increased risk of MACE (29). Solak et al. measured RDW value in 367 patients with chronic kidney disease stages 1–5 and observed RDW was independently related to endothelial dysfunction (30). In addition, many studies revealed a closely association between RDW and disease activity in patients with IBD (31, 32). As mentioned above, these psoriasis associated conditions are all related to chronic inflammation and have a certain connection with RDW. Currently, the association between RDW and psoriasis was controversial and remained unclear among the representative and large sample of US population. Here, we detected the association between RDW and psoriasis risk in US adults and observed a positive association between RDW and psoriasis risk in females but not in males. RDW detection technology is mature and widely used as a testing indicator in clinical practice with the advantages of low cost, simplicity, and universality. Therefore, RDW could serve as promising predictive diagnostic biomarkers of psoriasis.
In patients with psoriasis, various biomarkers related to inflammation such as C-reactive protein, erythrocyte sedimentation rate, and platelet activation marker P-selectin are correlated with the severity of psoriasis (33–35). Naik and colleagues have provided evidence that the severity of psoriasis is linked to vascular inflammation detected through 18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) (36), which indicated that psoriatic inflammation can affect blood vessels and leading to inflammation in the vascular wall. Inflammation is an important factor in the formation of erythrocyte anomalies, where abnormalities in the uptake and utilization of iron may impair erythropoiesis and lead to immature red blood cells entering the bloodstream (37). In addition, inflammation can decrease the survival rate of red blood cells, resulting in a higher value of RDW due to mixed numbers of red blood cells in circulation (38). Overall, these findings demonstrate the complex relationship between psoriatic inflammation, vascular inflammation, and erythrocyte anomalies. The results in our study may provide insights into the underlying mechanisms of psoriasis and aid in the development of effective treatments.
Previous study suggested a clear bimodal age pattern in psoriasis onset, and women tend to have a higher incidence of early (39). A questionnaire survey involving 1,979 psoriasis patients in Switzerland revealed that females experienced more severe pruritus symptoms compared to males (40). Our study found a positive association between RDW and psoriasis risk in females but not in males. We speculated that estrogen levels, and subcutaneous fat levels might play a physiological role in females somewhat, although it has not yet been discovered. In addition, subgroup analysis indicated that the association between RDW and psoriasis risk was more pronounced in female with BMI greater than 29.9 kg/m2. Previous study found that BMI was have a significant positive linear correlation with RDW and inflammatory index, such as WBC, neutrophil count, PCT and platelet count (41). A recent research observed a positive association between BMI and RDW in women (42). Given this, further research is needed to verify these results.
The strengths of this study include the use of a large and representative samples of the general adult population and adjustment for potential confounders. There are several limitations need to be acknowledged. Firstly, we can not detected the temporal relationship between RDW and psoriasis due to the nature of the cross-sectional design. Secondly, covariates relating to psoriasis and other medical history were evaluated using self-reported questionnaires, making our data susceptible to recall and information biases. Third, the RDW value can vary over time, and the dynamic value can not obtained from the database, which might interfere the analysis results. In addition, though we adjusted for various potential confounding variables associated with RDW and psoriasis, residual confounding is possible.
Conclusion
In conclusion, our findings suggested that RDW was positively associated with psoriasis risk in adult females in United States. In addition, no association between RDW and psoriasis risk was found in males. The results may benefit patients by providing statistical data for clinicians in the early diagnosis of psoriasis. Future research using multi-center and prospective cohort study designs are needed to assess our results.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes.
Ethics statement
The studies involving humans were approved by the board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YZ: Conceptualization, Writing – original draft. ZL: Formal analysis, Writing – original draft. PP: Data curation, Formal analysis, Methodology, Writing – original draft. TZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Armstrong, AW, Mehta, MD, Schupp, CW, Gondo, GC, Bell, SJ, and Griffiths, CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. (2021) 157:940–6. doi: 10.1001/jamadermatol.2021.2007
3. Griffiths, CE, and Barker, JN. Pathogenesis and clinical features of psoriasis. Lancet. (2007) 370:263–71. doi: 10.1016/S0140-6736(07)61128-3
4. Rendon, A, and Schäkel, K. Psoriasis pathogenesis and treatment. Int J Mol Sci. (2019) 20:1475. doi: 10.3390/ijms20061475
5. Reid, C, and Griffiths, CEM. Psoriasis and treatment: past, present and future aspects. Acta Derm Venereol. (2020) 100:70–80. doi: 10.2340/00015555-3386
6. Kamiya, K, Kishimoto, M, Sugai, J, Komine, M, and Ohtsuki, M. Risk factors for the development of psoriasis. Int J Mol Sci. (2019) 20:4347. doi: 10.3390/ijms20184347
7. Boehncke, WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin N Am. (2015) 41:665–75. doi: 10.1016/j.rdc.2015.07.013
8. Salvagno, GL, Sanchis-Gomar, F, Picanza, A, and Lippi, G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. doi: 10.3109/10408363.2014.992064
9. Chi, G, Lee, JJ, Montazerin, SM, and Marszalek, J. Prognostic value of hemoglobin-to-red cell distribution width ratio in cancer: a systematic review and meta-analysis. Biomark Med. (2022) 16:473–82. doi: 10.2217/bmm-2021-0577
10. Wang, Z, Korantzopoulos, P, Roever, L, and Liu, T. Red blood cell distribution width and atrial fibrillation. Biomark Med. (2020) 14:1289–98. doi: 10.2217/bmm-2020-0041
11. Xing, X, Deng, Y, Zhu, Y, Xu, S, Liu, J, Zhang, C, et al. Red cell distribution width for prognosis in patients with pulmonary embolism: a systematic review and meta-analysis. Clin Respir J. (2020) 14:901–7. doi: 10.1111/crj.13227
12. Wang, J, He, X, Jiang, D, Wang, Z, Xu, D, Chen, J, et al. Evaluation of red blood cell distribution width-platelet ratio as a predictor of adverse pregnancy outcomes and disease severity in systemic lupus erythematosus. Clin Rheumatol. (2022) 41:2987–93. doi: 10.1007/s10067-022-06169-0
13. Al-Rawi, Z, Gorial, F, and Al-Bayati, AA. Red cell distribution width in rheumatoid arthritis. Mediterr J Rheumatol. (2018) 29:38–42. doi: 10.31138/mjr.29.1.38
14. Yi, P, Jiang, J, Wang, Z, Wang, X, Zhao, M, Wu, H, et al. Comparison of mean platelet volume (MPV) and red blood cell distribution width (RDW) between psoriasis patients and controls: a systematic review and meta-analysis. PLoS One. (2022) 17:e0264504. doi: 10.1371/journal.pone.0264504
15. Gisondi, P, Geat, D, Lippi, G, Montagnana, M, and Girolomoni, G. Increased red blood cell distribution width in patients with plaque psoriasis. J Med Biochem. (2021) 40:199–201. doi: 10.5937/jomb0-27237
16. Doğan, S, and Atakan, N. Red blood cell distribution width is a reliable marker of inflammation in plaque psoriasis. Acta Dermatovenerol Croat. (2017) 25:26–31.
17. Sirin, MC, Korkmaz, S, Erturan, I, Filiz, B, Aridogan, BC, Cetin, ES, et al. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. An Bras Dermatol. (2020) 95:575–82. doi: 10.1016/j.abd.2020.02.008
18. Kyu, HH, Bachman, VF, Alexander, LT, Mumford, JE, Afshin, A, Estep, K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the global burden of disease study 2013. BMJ. (2016) 354:i3857. doi: 10.1136/bmj.i3857
19. Griffiths, CEM, Armstrong, AW, Gudjonsson, JE, and Barker, JNWN. Psoriasis. Lancet. (2021) 397:1301–15. doi: 10.1016/S0140-6736(20)32549-6
20. Veale, DJ, and Fearon, U. The pathogenesis of psoriatic arthritis. Lancet. (2018) 391:2273–84. doi: 10.1016/S0140-6736(18)30830-4
21. Van den Bosch, F, and Coates, L. Clinical management of psoriatic arthritis. Lancet. (2018) 391:2285–94. doi: 10.1016/S0140-6736(18)30949-8
22. Ungprasert, P, Srivali, N, and Thongprayoon, C. Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Dermatolog Treat. (2016) 27:316–21. doi: 10.3109/09546634.2015.1107180
23. Östling, J, van Geest, M, Schofield, JPR, Jevnikar, Z, Wilson, S, Ward, J, et al. IL-17-high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol. (2019) 144:1198–213. doi: 10.1016/j.jaci.2019.03.027
24. Yim, KM, and Armstrong, AW. Updates on cardiovascular comorbidities associated with psoriatic diseases: epidemiology and mechanisms. Rheumatol Int. (2017) 37:97–105. doi: 10.1007/s00296-016-3487-2
25. Conti, A, Giovannini, L, Mandel, VD, Odorici, G, Lasagni, C, Bigi, L, et al. Chronic kidney disease in psoriasis: a cohort study. J Dtsch Dermatol Ges. (2020) 18:438–45. doi: 10.1111/ddg.14087
26. Cottone, M, Sapienza, C, Macaluso, FS, and Cannizzaro, M. Psoriasis and inflammatory bowel disease. Dig Dis. (2019) 37:451–7. doi: 10.1159/000500116
27. Lee, JH, Chung, HJ, Kim, K, Jo, YH, Rhee, JE, Kim, YJ, et al. Red cell distribution width as a prognostic marker in patients with community-acquired pneumonia. Am J Emerg Med. (2013) 31:72–9. doi: 10.1016/j.ajem.2012.06.004
28. Braun, E, Domany, E, Kenig, Y, Mazor, Y, Makhoul, BF, and Azzam, ZS. Elevated red cell distribution width predicts poor outcome in young patients with community acquired pneumonia. Crit Care. (2011) 15:R194. doi: 10.1186/cc10355
29. Conic, RR, Damiani, G, Schrom, KP, Ramser, AE, Zheng, C, Xu, R, et al. Psoriasis and psoriatic arthritis cardiovascular disease endotypes identified by red blood cell distribution width and mean platelet volume. J Clin Med. (2020) 9:186. doi: 10.3390/jcm9010186
30. Solak, Y, Yilmaz, MI, Saglam, M, Caglar, K, Verim, S, Unal, HU, et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am J Med Sci. (2014) 347:118–24. doi: 10.1097/MAJ.0b013e3182996a96
31. Song, CS, Park, DI, Yoon, MY, Seok, HS, Park, JH, Kim, HJ, et al. Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig Dis Sci. (2012) 57:1033–8. doi: 10.1007/s10620-011-1978-2
32. Yeşil, A, Senateş, E, Bayoğlu, IV, Erdem, ED, Demirtunç, R, and Kurdaş Övünç, AO. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. (2011) 5:460–7. doi: 10.5009/gnl.2011.5.4.460
33. Strober, B, Teller, C, Yamauchi, P, Miller, JL, Hooper, M, Yang, YC, et al. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br J Dermatol. (2008) 159:322–30. doi: 10.1111/j.1365-2133.2008.08628.x
34. Kanelleas, A, Liapi, C, Katoulis, A, Stavropoulos, P, Avgerinou, G, Georgala, S, et al. The role of inflammatory markers in assessing disease severity and response to treatment in patients with psoriasis treated with etanercept. Clin Exp Dermatol. (2011) 36:845–50. doi: 10.1111/j.1365-2230.2011.04131.x
35. Garbaraviciene, J, Diehl, S, Varwig, D, Bylaite, M, Ackermann, H, Ludwig, RJ, et al. Platelet P-selectin reflects a state of cutaneous inflammation: possible application to monitor treatment efficacy in psoriasis. Exp Dermatol. (2010) 19:736–41. doi: 10.1111/j.1600-0625.2010.01095.x
36. Naik, HB, Natarajan, B, Stansky, E, Ahlman, MA, Teague, H, Salahuddin, T, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. (2015) 35:2667–76. doi: 10.1161/ATVBAHA.115.306460
37. Weiss, G, and Goodnough, LT. Anemia of chronic disease. N Engl J Med. (2005) 352:1011–23. doi: 10.1056/NEJMra041809
38. Kiefer, CR, and Snyder, LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. (2000) 7:113–6. doi: 10.1097/00062752-200003000-00007
39. Iskandar, IYK, Parisi, R, Griffiths, CEM, and Ashcroft, DM, Global Psoriasis Atlas. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol. (2021) 184:243–58. doi: 10.1111/bjd.19169
40. Murer, C, Sgier, D, Mettler, SK, Guillet, C, Maul, JT, Djamei, V, et al. Gender differences in psoriasis: a Swiss online psoriasis survey. Arch Dermatol Res. (2021) 313:89–94. doi: 10.1007/s00403-020-02066-1
41. Furuncuoğlu, Y, Tulgar, S, Dogan, AN, Cakar, S, Tulgar, YK, and Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. (2016) 20:1300–6.
Keywords: red blood cell distribution width, psoriasis, NHANES, cross-sectional study, female
Citation: Zhang Y, Lv Z, Peng P and Zhao T (2023) Association between red blood cell distribution width and psoriasis among the US adults. Front. Med. 10:1290514. doi: 10.3389/fmed.2023.1290514
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Sara Trevisini, Department of dermatology, ItalySerena Bergamo, ULSS2 Marca Trevigiana, Italy
Copyright © 2023 Zhang, Lv, Peng and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tie Zhao, MzM0ODQ0ODgyQHFxLmNvbQ==
 Yunqi Zhang
Yunqi Zhang Zheng Lv2
Zheng Lv2 Peng Peng
Peng Peng