
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 November 2023
Sec. Rheumatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1286601
This article is part of the Research Topic Updates on Giant Cell Arteritis: Pathogenesis, Diagnosis and Treatment, volume II View all 11 articles
Introduction: Giant cell arteritis (GCA) is the most common vasculitis of the elderly. In recent years, advanced imaging has to a certain extent replaced temporal artery biopsy (TAB) to aid diagnosis in many institutions and helped to identify three major phenotypes of GCA, namely, cranial GCA (c-GCA), large-vessel non-cranial GCA (LV-GCA), and a combination of these two patterns called mixed-GCA, which all show different clinical patterns. Recent 2022 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria respect the changing conception and clinical practice during the last two decades. In this cohort study, we present vasculitis distribution and baseline characteristics using the 2022 ACR/EULAR classification criteria as well as the EULAR core data set.
Methods: In this retrospective study from Southern Norway, we identified all patients diagnosed with GCA between 2006 and 2019 in our single-center fast-track clinic (FTC). We included all patients who were examined using ultrasound (US) of cranial as well as non-cranial large vessels at diagnosis to depict vascular distribution. EULAR core data set, ACR 1990, and 2022 ACR/EULAR classification criteria were used to characterize the cohort.
Results: Seventy-seven patients were diagnosed with GCA at our institution in the aforementioned period. Seventy-one patients (92.2%) were diagnosed with the help of US and included in the further analysis. The 2022 ACR/EULAR classification criteria allocated 69 patients (97.2%), while the ACR 1990 classification criteria allocated 49 patients (69.0%) in our cohort as having GCA. Mixed-GCA was the most common type in 33 patients (46.5%). Weight loss was significantly more common in patients with large-vessel non-cranial vasculitis in LV-GCA and mixed-GCA. Headache, on the other hand, was significantly more common in patients with involvement of cranial vessels.
Conclusion: Mixed GCA was the most common form of GCA in our cohort. In our study, the 2022 ACR/EULAR classification criteria seem to be a more useful tool compared with the old ACR 1990 classification criteria to allocate GCA patients diagnosed and treated at our US-based FTC as having GCA.
Giant cell arteritis (GCA) is the most common form of large-vessel vasculitis in the elderly population (1). If left untreated, it poses a medical emergency due to impending vision loss and stroke risk (2). In certain subpopulations, GCA has also been associated with increased mortality (3, 4). GCA predominates in women and populations of northern European descent (5).
In the last two decades, advanced imaging techniques have changed the understanding of GCA, which seems to be a systemic, rather than a localized vasculitis of cranial arteries (2, 6, 7). Recent studies using positron emission tomography of radioactively labeled glucose (PET) or ultrasound (US) with experienced examiners and extended US protocols identified high rates of large-vessel involvement in GCA (6–9). These findings seem important as they were associated with refractory disease and specific complications such as posterior stroke in vertebral vasculitis or thoracic aortic aneurysm in aortitis (10–14). New classification criteria incorporating these new imaging modalities have recently been published by the American College of Rheumatology (ACR) together with the European Alliance Of Associations For Rheumatology (EULAR) and proved to be applicable to GCA cohorts (15, 16).
The fast-track clinic (FTC) approach incorporating US enables diagnosis and treatment within 48 h and has shown success in reducing vision loss (17, 18). Furthermore, outcome has been improved by new treatment options beyond prednisolone (19–21).
Southern Norway has consistently reported an annual incident rate among the highest in the world, though it shows a declining trend (4). US-based diagnosis was introduced in our rheumatology center on a regular basis in 2010. It has replaced temporal artery biopsy as the first diagnostic modality in diagnosing GCA while US-based FTC algorithms were finally implemented routinely in 2012 (18).
The primary aim of this study was to describe vasculitis distribution in cranial and non-cranial arteries in an FTC using US for diagnosis of GCA. Furthermore, we wanted to characterize our cohort using the 2018 EULAR core data set, the ACR 1990 classification criteria, and the new 2022 ACR/EULAR 2022 classification criteria for GCA (15, 22, 23).
All patients diagnosed with GCA at the central referral FTC in Agder County, Southern Norway, between 2006 and 2019 were retrospectively identified using the International Classification of Disease version 10 (ICD-10) coding system with the codes M31.5 and M31.6 in the central electronic hospital database.
All applicable medical records were thoroughly reviewed manually before the diagnosis was confirmed or rejected based on medical record information. Patients with a sustained diagnosis of GCA on the basis of clinics, imaging results, and temporal artery biopsy (TAB) were identified. Patients without US examinations at diagnosis were excluded for further analysis.
Data were collected in accordance with a structured protocol following the 2018 EULAR recommendations for a core data set to support observational research and clinical care in GCA. However, general disease assessment of patients and examiners was not routinely recorded in most patients prior to 2018 and was therefore not included, while history of cancer was not further stratified (22).
Standard US procedure contained an assessment of both temporal arteries (superficial temporal artery with frontal and parietal branches) in longitudinal and transversal planes with and without color Doppler mode. A positive US test was defined in the presence of hypoechoic vessel wall thickening (halo sign) that was confirmed by the compression sign (24, 25). The axillary and subclavian arteries were assessed in B-mode, and intima–media thickness (IMT) was measured in a longitudinal visualization. A positive test was defined if IMT > 1 mm (2). Other arteries, such as facial-, carotid-, and occipital arteries, were only sporadically assessed and therefore not further analyzed. The US examination was carried out at the FTC, 48 h after referral at the latest. US procedures were conducted by three experienced sonographers (APD, HB, and PMA) using Esaote (Esaote, Genua, Italy) machines up to 2019 and General Electric (General Electric Healthcare, Horten, Norway) Vivid machines in 2018 and 2019. Linear transducers were used with pre-specified settings according to common recommendations (26). Magnetic resonance imaging and PET were not part of a standard assessment and were only used sporadically. TAB was performed by the surgical department at the same hospital, and the specimens were assessed by several local pathologists.
Descriptive statistics were used to characterize the study cohort. Mean and standard deviation were calculated for continuous metric variables and frequencies for nominal and categorical variables.
To compare characteristics between the three major patterns of GCA, the chi-square test was used for categorical variables, and ANOVA and Bonferroni as a post-hoc test for continuous variables. Additionally, a multivariate analysis with multiple comparisons was conducted.
The level of significance of all tests was set at a p-value of ≤ 0.05. The Statistical Package for the Social Sciences (SPSS), version 28 (IBM, Chicago, IL, USA), was used for the statistical analysis.
The study was registered and approved by the local patient data safety council.
Seventy-nine patients were identified, and two patients were excluded as their diagnoses were later changed. Six patients were excluded because of missing US examination at baseline. The resulting 71 patients, 50 women (70.4%), with a confirmed diagnosis of GCA were included. The mean age was 69.7 years (SD: 7.2), range of 56–86 years. Apart from two patients (one Latin American and one from Thailand), all were of Caucasian origin (97.2%).
Characteristics of the cohort in accordance with the EULAR core criteria set are shown in Table 1.
The number of patients in our cohort fulfilling the original ACR 1990 classification criteria was 49 (69.0%), while 69 patients (97.2%) fulfilled the 2022 ACR/EULAR classification criteria. Table 2 shows the absolute number of patients fulfilling the separate criteria for the ACR 1990 classification criteria and the 2022 ACR/EULAR classification criteria. US was crucial for the classification of 27 patients (38.0%), while biopsy was crucial in one patient (1.4%).
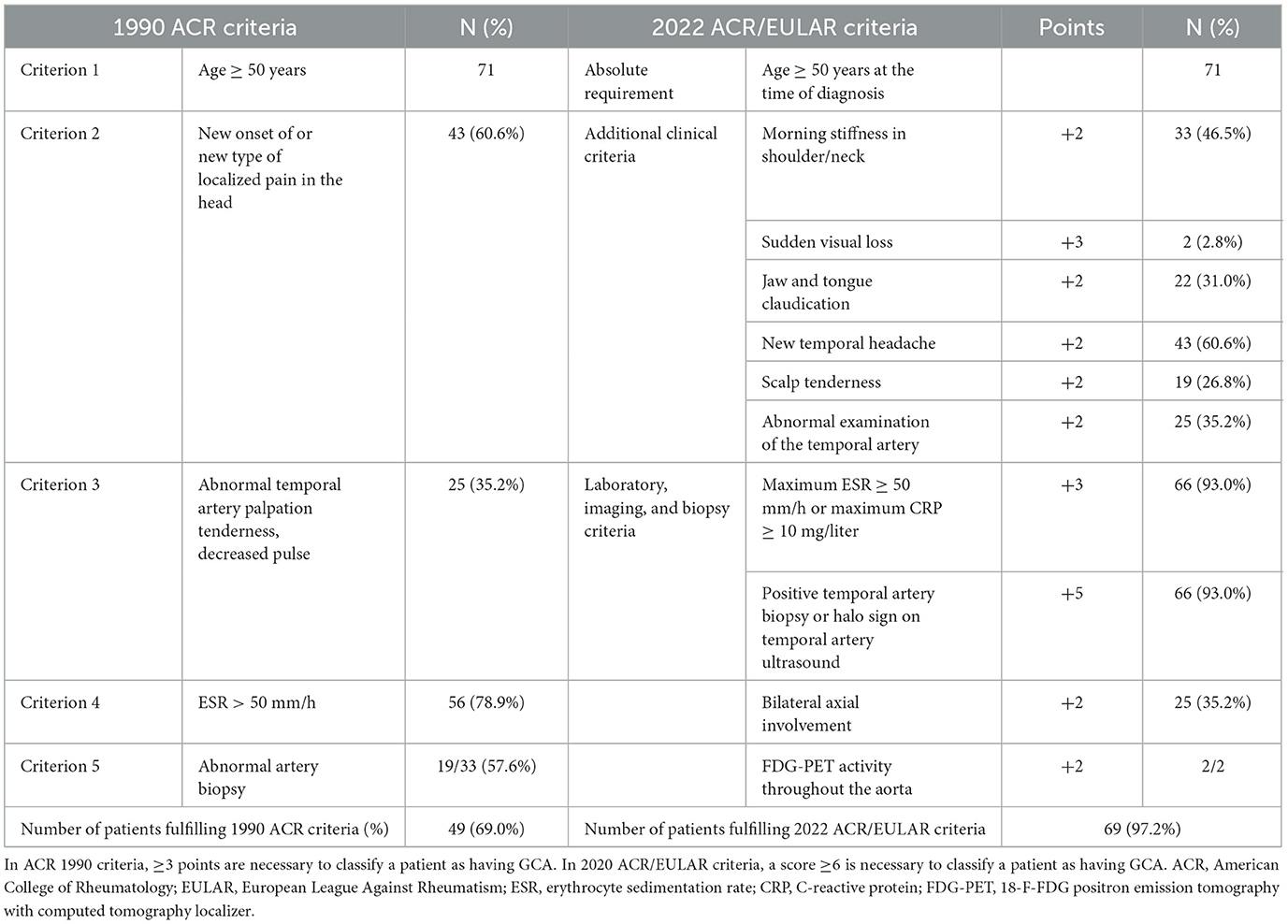
Table 2. Comparison between the 1990 ACR criteria and the new 2022 EULAR/ACR criteria in our cohort of 77 patients diagnosed with GCA on a clinical basis.
Detailed results for vasculitis distribution found by US examination are shown in Table 3.
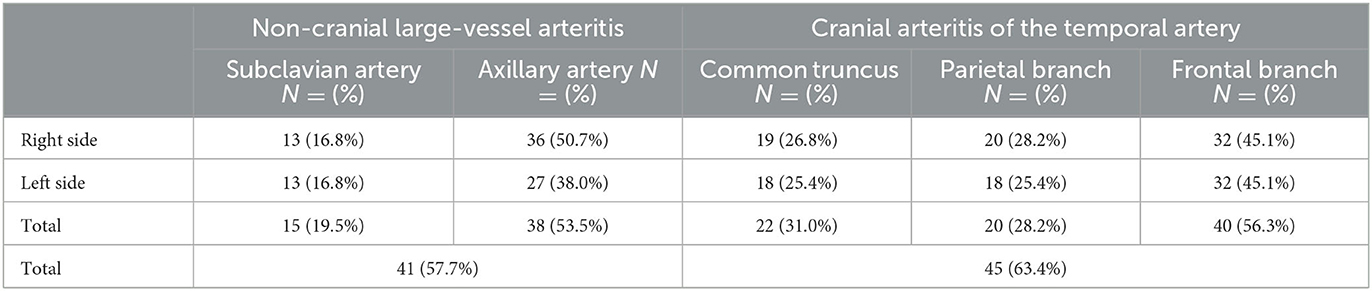
Table 3. Distribution of the reported positive ultrasound vasculitis findings in the 71 patients receiving US at baseline.
Mixed-GCA was observed in 33 patients (46.5%) patients, c-GCA in 22 (28.6%) patients, and LV-GCA in 12 (15.6%) patients. Nine patients had a positive finding at just one site. Five patients had isolated unilateral subclavian vasculitis, and two patients had isolated unilateral frontal artery and superficial artery involvement each.
In five patients (7.0%), the diagnosis was based on clinical grounds only without evidence of vasculitis in ultrasound (all five patients), biopsy (four patients), or magnetic resonance (one patient). The ACR 1990 classification criteria were fulfilled by 14 patients (66.7%) in the c-GCA group, 3 patients (25%) in the LV-GCA group, and 29 patients (87.9%) in the mixed-GCA group. The 2022 ACR/EULAR classification criteria were fulfilled in all patients with positive ultrasound findings, irrespective of the subtype but only in three of the five patients (60%) without evidence of vasculitis in the US examination.
Three ischemic events in two patients were observed. One patient who already received treatment with aspirin for concomitant diagnosis developed a posterior stroke as well as an anterior ischemic optic neuropathy, and another patient without aspirin or oral anticoagulation treatment developed an anterior optic neuropathy. Of the seven patients on oral anticoagulation treatment, none developed ischemic complications. The paucity of ischemic events precluded a further associative analysis.
Weight loss was significantly more frequent in patients with large-vessel non-cranial involvement (p = 0.018), but between mixed-GCA and LV-GCA, no significant difference was found. Headache was significantly more frequent in cranial vasculitis in c-GCA and mixed-GCA compared with LV-GCA (p = 0.003). No significant differences between GCA patterns could be demonstrated for other characteristics from the EULAR core data set nor arthralgia, dry cough, carotidynia, night sweats, and other constitutional symptoms. The three events of new vision loss were seen in two c-GCA patients.
In this study, we present all patients in Agder County who were diagnosed with GCA in the given period and underwent expert ultrasound to characterize the extent of the vasculitis. However, this study comes with relevant shortcomings. Among others, they encompass, that some parts of the vasculature deemed relevant, such as the vertebral-, occipital-, and facial arteries but also the aorta, were inconsequently or never assessed (2, 6, 27). US follow-up data and IMT were not documented (28). Incomplete data were collected in the follow-up regarding medication dose, steroid tapering, steroid toxicity, and relapse. As no data on patients, in which a GCA diagnosis was rejected in the FTC was collected, no conclusion on the performance of the two criteria sets could be made.
Mixed-GCA was the most common form in our cohort, confirming recent findings (6, 9, 29–32). Mixed-GCA was observed in 33 patients (46.5%), c-GCA in 22 patients (28.6%), and LV-GCA in 12 patients (15.6%). Our data highlight the importance of an extended US examination of cranial and non-cranial large arteries for diagnosing GCA in daily clinical care, comparable to other recent literature (6, 7, 9). The US data demonstrated the widespread nature of arterial inflammation in GCA that rarely involves only one site. However, the relatively lower numbers of large-vessel vasculitis compared with other studies may be a consequence of an often-limited US examination executed in this cohort, only occasionally encompassing subclavian, carotid, aortic, vertebral, facial, or occipital arteries (6). Furthermore, the training and experience of sonographers varied as well as US machines. This may also explain why five (7.0%) patients showed no objective vasculitis in the US examination and nine patients were identified with just one single involved vascular site. An US was executed after a maximum of three oral doses of prednisolone. Even though some vasculitic changes, especially in the cranial vasculature, may have vanished by then, in our cohort, LV-GCA showed a trend toward a longer diagnostic delay that did not reach significance (14, 33). Six patients were excluded due to a missing ultrasound at baseline. Only two of these patients underwent TAB and PET. Both modalities showed positive findings in these two patients. The remaining four patients were solely diagnosed by TAB without further assessment of possible large-vessel vasculitis.
Headache was significantly associated with cranial vasculitis. However, no significant difference between c-GCA and mixed-GCA could be demonstrated (7, 17, 18, 29). Weight loss was significantly associated with vasculitis in large non-cranial vessels, but no further significant difference between LV-GCA and mixed-GCA could be shown. In contrast to other studies, neither age, sex, treatment length, nor any laboratory markers differed significantly between the three patterns (14, 29, 33).
The 2022 ACR/EULAR classification criteria allocated a much higher proportion of our US-based FTC cohort as having GCA than the 1990 ACR classification criteria. This is in accordance with other recent cohort studies (16, 34, 35). This was especially true for the LV-GCA subgroup where only 25% of the patients would have been classified as having GCA using the 1990 ACR classification criteria, while all patients fulfilled the 2022 ACR/EULAR classification criteria. As previously demonstrated in FTCs, ischemic complications were few as only two patients (2.8%), both with c-GCA, developed three ischemic events (17, 18). However, diagnostic delay based on retrospective first symptom occurrence to the specialist investigation was 4.6 (SD: 7.7) months despite an established FTC that is set up to see patients on the next working day. This potentially mirrors the unspecific nature of symptoms that both the patient and the primary health service are confronted with in GCA patients. Treatment length, indicated by the last corticosteroid dose, reflected on the one hand the relapsing nature of GCA and on the other hand the need for steroid-sparing strategies. In our small cohort, GCA subgroups by US stratification alone were associated with some clinical features. However, this approach was insufficient to predict the duration of the treatment, indicating the need for better risk stratification using improved imaging parameters or scores as well as laboratory markers (36, 37).
Our study confirms that GCA is a multisite vasculitis with distinct clinical features depending on the involved vessels. This should be considered in any workup procedure. 2022 ACR/EULAR classification criteria allocated a much higher percentage of our GCA cohort (97.2%) as having GCA compared with the 1990 ACR classification criteria (69.0%) and reflected the clinical practice in our FTC better.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by local patient data safety council, Sørlandets Sykehus, Kristiansand, Norway. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
PA: Conceptualization, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing. AD: Investigation, Supervision, Writing—review & editing. GM: Supervision, Writing—review & editing. GH: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. To publish this article financial and organizational support from the Southern Norwegian Hospital Research Trust as well as South-Eastern Norwegian Hospital Research Department and Central Library have been granted.
The authors thank Helle Bitter for documenting clinical and ultrasound findings, and Vilde Haraldstad, Serina Brådland, and Hanne Kalstad Vestaby for assistance with data processing and identification of patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. (2012) 8:509–21. doi: 10.1038/nrrheum.2012.97
2. Andel PM, Chrysidis S, Geiger J, Haaversen ACB, Haugeberg G, Myklebust G, et al. Diagnosing giant cell arteritis: a comprehensive practical guide for the practicing rheumatologist. Rheumatology (Oxford). (2021) 60:4958–71. doi: 10.1093/rheumatology/keab547
3. Barra L, Pope JE, Pequeno P, Gatley JM, Widdifield J. Increased mortality for individuals with giant cell arteritis: a population-based study. Arthr Care Res. (2022) 74:1294–9. doi: 10.1002/acr.24573
4. Andersen JB, Myklebust G, Haugeberg G, Pripp AH, Diamantopoulos AP. Incidence trends and mortality of giant cell arteritis in Southern Norway. Arthritis Care Res (Hoboken). (2021) 73:409–14. doi: 10.1002/acr.24133
5. Haugeberg G, Paulsen PQ, Bie RB. Temporal arteritis in vest agder county in southern norway: incidence and clinical findings. J Rheumatol. (2000) 27:2624–7.
6. Bull Haaversen AC, Brekke LK, Kermani TA, Molberg Ø, Diamantopoulos AP. Extended ultrasound examination identifies more large vessel involvement in patients with giant cell arteritis. Rheumatology. (2023) 62:1887–94. doi: 10.1093/rheumatology/keac478
7. Chrysidis S, Døhn UM, Terslev L, Fredberg U, Lorenzen T, Christensen R, et al. Diagnostic accuracy of vascular ultrasound in patients with suspected giant cell arteritis (eureka): a prospective, multicentre, non-interventional, cohort study. Lancet Rheumatol. (2021) 3:E865–73. doi: 10.1016/S2665-9913(21)00246-0
8. Soussan M, Nicolas P, Schramm C, Katsahian S, Pop G, Fain O, et al. Management of large-vessel vasculitis with fdg-pet: a systematic literature review and meta-analysis. Medicine. (2015) 94:E622. doi: 10.1097/MD.0000000000000622
9. Aschwanden M, Kesten F, Stern M, Thalhammer C, Walker UA, Tyndall A, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis. (2010) 69:1356–9. doi: 10.1136/ard.2009.122135
10. Sugihara T, Hasegawa H, Uchida HA, Yoshifuji H, Watanabe Y, Amiya E, et al. Associated factors of poor treatment outcomes in patients with giant cell arteritis: clinical implication of large vessel lesions. Arthritis Res Ther. (2020) 22:72. doi: 10.1186/s13075-020-02171-6
11. de Mornac D, Espitia O, Néel A, Connault J, Masseau A, Espitia-Thibault A, et al. Large-vessel involvement is predictive of multiple relapses in giant cell arteritis. Ther Adv Musculoskeletal Dis. (2021) 13:1759720X211009029. doi: 10.1177/1759720X211009029
12. De Boysson H, Daumas A, Vautier M, Parienti JJ, Liozon E, Lambert M, et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun Rev. (2018) 17:391–8. doi: 10.1016/j.autrev.2017.11.029
13. Kargiotis O, Psychogios K, Safouris A, Bakola E, Andreadou E, Karapanayiotides T, et al. Cervical duplex ultrasound for the diagnosis of giant cell arteritis with vertebral artery involvement. J Neuroimaging. (2021) 31:656–64. doi: 10.1111/jon.12857
14. Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford). (2015) 54:463–70. doi: 10.1093/rheumatology/keu329
15. Ponte C, Grayson PC, Robson JC, Suppiah R, Gribbons KB, Judge A, et al. American college of rheumatology/eular classification criteria for giant cell arteritis. Ann Rheum Dis. (2022) 81:1647–53. doi: 10.1136/ard-2022-223480
16. Wiberg F, Naderi N, Mohammad AJ, Turesson C. Evaluation of revised classification criteria for giant cell arteritis and its clinical phenotypes. Rheumatology (Oxford). (2021) 61:383–7. doi: 10.1093/rheumatology/keab353
17. Patil P, Williams M, Maw WW, Achilleos K, Elsideeg S, Dejaco C, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol. (2015) 33:103–6.
18. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology (Oxford). (2016) 55:66–70. doi: 10.1093/rheumatology/kev289
19. Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. (2017) 377:317–28. doi: 10.1056/NEJMoa1613849
20. Venhoff N, Schmidt W, Bergner R, Rech J, Unger L, Tony H, et al. Secukinumab in giant cell arteritis: a randomized, parallel-group, double-blind, placebo-controlled, multicenter phase 2 trial. Arthritis Rheum. (2021) 73:9. doi: 10.1186/s13063-021-05520-1
21. Stone JH, Bao M, Han J, Aringer M, Blockmans D, Brouwer E, et al. Op0140 long-term outcome of tocilizumab for patients with giant cell arteritis: results from part 2 of the giacta trial. Ann Rheum Dis. (2019) 78:145–6. doi: 10.1136/annrheumdis-2019-eular.2099
22. Ehlers L, Askling J, Bijlsma HW, Cid MC, Cutolo M, Dasgupta B, et al. Eular recommendations for a core data set to support observational research and clinical care in giant cell arteritis. Ann Rheum Dis. (2018) 78:1160–6. doi: 10.1136/annrheumdis-2018-214755
23. Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology. (2016) 56:506–15. doi: 10.1093/rheumatology/kew273
24. Aschwanden M, Daikeler T, Kesten F, Baldi T, Benz D, Tyndall A, et al. Temporal artery compression sign–a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med. (2013) 34:47–50. doi: 10.1055/s-0032-1312821
25. Schmidt WA, Kraft HE, Völker L, Vorpahl K, Gromnica-Ihle EJ. Colour doppler sonography to diagnose temporal arteritis. Lancet. (1995) 345:866. doi: 10.1016/S0140-6736(95)93005-1
26. Terslev L, Diamantopoulos AP, Døhn UM, Schmidt WA, Torp-Pedersen S. Settings and artefacts relevant for doppler ultrasound in large vessel vasculitis. Arthritis Res Ther. (2017) 19:167. doi: 10.1186/s13075-017-1374-1
27. Tomelleri A, Van Der Geest KSM, Khurshid MA, Sebastian A, Coath F, Robbins D, et al. Disease stratification in gca and pmr: state of the art and future perspectives. Nat Rev Rheumatol. (2023) 19:446–59. doi: 10.1038/s41584-023-00976-8
28. Ponte C, Monti S, Scirè CA, Delvino P, Khmelinskii N, Milanesi A, et al. Ultrasound halo sign as a potential monitoring tool for patients with giant cell arteritis: a prospective analysis. Ann Rheum Dis. (2021) 80:1475–82. doi: 10.1136/annrheumdis-2021-220306
29. Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A, Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford). (2008) 47:96–101. doi: 10.1093/rheumatology/kem322
30. Prieto-González S, Arguis P, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Butjosa M, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. (2012) 71:1170–6. doi: 10.1136/annrheumdis-2011-200865
31. Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res. (2014) 66:113–9. doi: 10.1002/acr.22178
32. Monjo I, Fernández E, Peiteado D, Balsa A, Miguel ED. Op0180 diagnostic validity of ultrasound including extra-cranial arteries in giant cell arteritis. Ann Rheum Dis. (2020) 79:112–112. doi: 10.1136/annrheumdis-2020-eular.5036
33. Schmidt WA, Moll A, Seifert A, Schicke B, Gromnica-Ihle E, Krause A. Prognosis of large-vessel giant cell arteritis. Rheumatology (Oxford). (2008) 47:1406–8. doi: 10.1093/rheumatology/ken258
34. Molina-Collada J, Castrejón I, Monjo I, Fernández-Fernández E, Torres Ortiz G, Álvaro-Gracia JM, et al. Performance of the 2022 ACR/EULAR giant cell arteritis classification criteria for diagnosis in patients with suspected giant cell arteritis in routine clinical care. RMD Open. (2023) 9:e002970. doi: 10.1136/rmdopen-2022-002970
35. Hemmig AK, Aschwanden M, Imfeld S, Berger CT, Daikeler T. A diagnostic performance study of the 2022 American college of rheumatology/eular classification criteria for giant cell arteritis in a cohort of patients presenting with suspected giant cell arteritis. Arthritis Rheumatol. (2023) 75:1075–7. doi: 10.1002/art.42440
36. Van Der Geest KSM, Sandovici M, Van Sleen Y, Sanders JS, Bos NA, Abdulahad WH, et al. Review: what is the current evidence for disease subsets in giant cell arteritis? Arthritis Rheumatol. (2018) 70:1366–76. doi: 10.1002/art.40520
Keywords: large-vessel vasculitis (LVV), giant cell arteritis (GCA), ultrasound, classification criteria, imaging
Citation: Andel PM, Diamantopoulos AP, Myklebust G and Haugeberg G (2023) Vasculitis distribution and clinical characteristics in giant cell arteritis: a retrospective study using the new 2022 ACR/EULAR classification criteria. Front. Med. 10:1286601. doi: 10.3389/fmed.2023.1286601
Received: 31 August 2023; Accepted: 17 October 2023;
Published: 13 November 2023.
Edited by:
Ryu Watanabe, Osaka Metropolitan University, JapanReviewed by:
Maria Sandovici, University Medical Center Groningen, NetherlandsCopyright © 2023 Andel, Diamantopoulos, Myklebust and Haugeberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter M. Andel, UGV0ZXIuTWljaGFlbC5BbmRlbEBzby1oZi5ubw==
†ORCID: Peter M. Andel orcid.org/0000-0002-6176-4165
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.