- 1Department of Dermatology, Peking University Shenzhen Hospital, Shenzhen, Guangdong, China
- 2Shenzhen Key Laboratory for Translational Medicine of Dermatology, Institute of Dermatology, Shenzhen Peking University - The Hong Kong University of Science and Technology Medical Center, Shenzhen, Guangdong, China
Background: Bowen’s disease (BD) is a slow-growing precancerous skin condition, often concurrent with other diseases, with a high misdiagnosis rate. Previous studies show that patients with BD in different populations have differentiated characteristics.
Materials and methods: A retrospective study was conducted in a tertiary hospital in Shenzhen, China. Data about demographic information, diagnosis and treatment, clinical and pathological characteristics, and comorbidities of 50 patients with BD were collected and analyzed.
Results: Clinical data of onset age and disease course of 43 patients with BD were available, the average onset age of male and female patients are 55.1 (standard deviation (SD) = 15.29) and 58.2 (SD = 15.59) years old, respectively; the average disease course of male and female patients are 25.3 (SD = 28.63) and 33.9 (SD = 49.65) months, respectively. The onset age (p = 0.52) and disease course (p = 0.49) between male and female patients are not significantly different. Interestingly, there is a negative correlation between onset age and disease course (r = −0.245, p = 0.11). The correct rate of clinical diagnosis is relatively low (54.00%); Some patients with BD are misdiagnosed as Bowenoid papulosis (10.00%), actinic keratosis (8.00%), basal cell carcinoma (8.00%), seborrheic keratosis (6.00%), and pigmented naevus (4.00%). Trunk and limbs are the most common distribution sites of BD lesions, and 94.00% patients with BD are treated with surgical resection; 66.00% patients with BD had comorbidities, including skin diseases (48.48%), cardiovascular diseases (39.39%), gastrointestinal diseases (30.30%), respiratory diseases (27.27%), and tumors (18.18%). The most commonly observed histopathological characteristics of BD are squamous-cell hyperplasia (86.00%), disordered maturation with atypical keratinocytes (74.00%), atypical mitoses (60.00%), hyperkeratosis with hypokeratosis (48.00%), dermal inflammatory cell infiltration (36.00%), and koilocytosis (22.00%).
Conclusion: BD often occurs in middle-aged and elderly people and is easily misdiagnosed. The onset age and disease course of patients with BD are not significantly different between males and females, whereas there is a negative correlation between the onset age and disease course. BD is more likely to occur in trunk and limbs in the Chinese population, and most patients with BD are concurrent with comorbidities.
1 Introduction
Bowen’s disease (BD), also known as squamous cell carcinoma (SCC) in situ, is a slow-growing precancerous skin condition, and 3%–5% BD may progress to invasive tumors (1). The incidence of BD varies due to the difference in sun exposure in different latitudes and climates (2, 3). The etiology of BD is not entirely clear; it may be related to irradiation, carcinogens, human papillomavirus (HPV), and others (such as chronic injury, dermatoses, and heredity) (4–6). BD presents as a well-demarcated, asymptomatic, erythematous hyperkeratotic plaque with irregular margins that is more likely to occur on the exposed site in light-skinned people (5), which is difficult to differentiate from Bowenoid papulosis, actinic keratosis, and seborrheic keratosis. Clinically, patients with BD are concurrent with other diseases, but few studies have focused on the comorbidities of BD. In addition, there are differences in the characteristics of patients with BD in different populations. In order to improve the understanding of BD, we conducted a retrospective study in a tertiary hospital in China, the data about demographic information, clinical and pathological characteristics, diagnosis and treatment, and comorbidities of 50 patients with BD were collected and analyzed.
2 Materials and methods
2.1 Study design and participants
A retrospective study was conducted in a tertiary hospital (Peking University Shenzhen Hospital) in Shenzhen, China. In total, 50 patients with BD from outpatient or inpatient department were confirmed by histopathological examination from January 2016 to August 2023, including 26 males and 24 females. Demographic information, distribution of lesions, clinical diagnosis, onset age, disease course, treatment methods, histopathological characteristics, and comorbidities of BD were collected. The chronological age at the visit or admission time and the disease course were available in the majority of patients with BD; here, we obtained the onset age by chronological age minus the disease course. All patients with BD were called to recall whether there were some predisposing factors present at that time, including sun exposure, radiation, carcinogens, HPV infection, chronic injury, and genetic history. Meanwhile, all patients were called to inquire information about the recurrence of BD.
2.2 Division of the body sites and definition of comorbidities
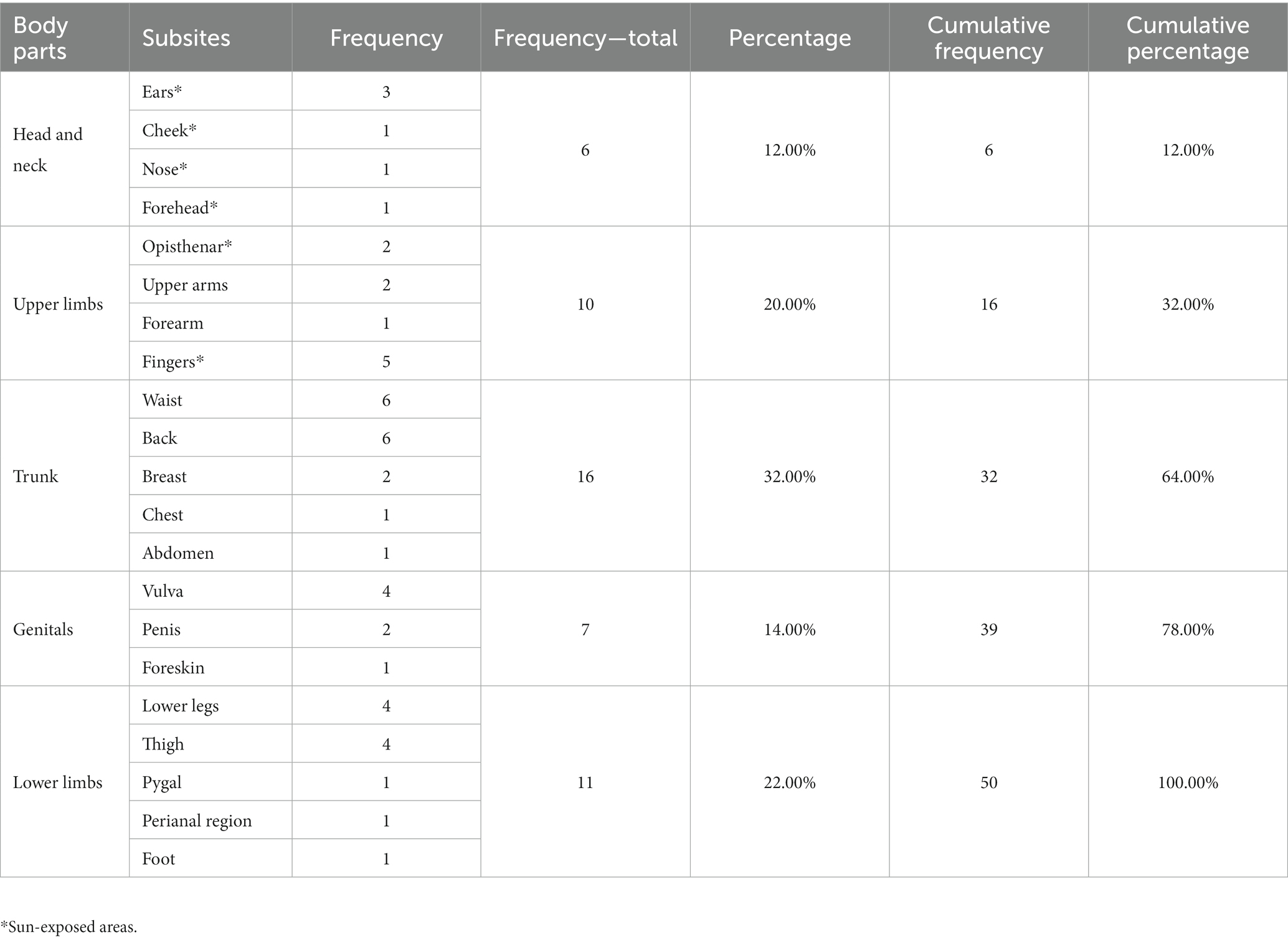
In this study, the lesion distribution of BD in different parts of the body was studied. According to the conventional division method of the body, we divided the body into five parts: head and neck, upper limbs, trunk (including armpit and groin), lower limbs (including buttocks), and genital parts. The sun-exposed parts include ears, cheek, nose, forehead, opisthenar, and fingers. In addition, the comorbidities of patients with BD were collected for further analysis. Here, we defined “comorbidities” as those diseases that occurred before or during the course of BD; the diseases that occurred after the treatment time were not included.
2.3 Statistical analysis
Quantitative data were described by mean and standard deviation (SD), while qualitative data were described by number (N) and percentage (%). SPSS V22.0 (IBM Corp., Armonk, NY) and GraphPad Prism V9.0 (San Diego, CA) were used for statistical analysis. Q-Q plot was used to test whether the dataset followed a normal distribution or not. Continuous variables were evaluated by t-test or t-test with Welch’s correction according to the distribution of the dataset. The relationship between the onset age and disease course of patients with BD was calculated using Pearson’s correlation analysis. p-value <0.05 was considered statistically significant.
3 Results
3.1 Onset age, disease course, and skin lesion distribution of Bowen’s disease
Clinical data of 50 BD patients were collected. The patients’ chronological age ranged from 22 to 86 years old, with a mean age of 59.9 (SD = 14.66) years old. The onset age and disease course of 43 patients with BD were available for further analysis (Figure 1). The average onset age of 43 patients with BD is 56.7 (SD = 15.35) years old and that of male and female patients with BD are 55.1 (SD = 15.29) and 58.2 (SD = 15.59) years old, respectively. The disease course of 43 patients with BD ranges from 1 to 240 months, and the disease course of most patients is distributed in 12 to 24 months. The average disease course of 43 patients with BD is 29.9 (SD = 40.99) months and that of male and female patients are 25.3 (SD = 28.63) and 33.9 (SD = 49.65) months, respectively. The distribution of onset age is in accordance with normal distribution, and the distribution of disease course is approximately normal (Figure 1). The onset age of male and female patients is not significantly different by unpaired t-test (F = 1.04, p = 0.94; t = 0.65, p = 0.52). The difference in disease course between male and female patients is not significant by unpaired t-test with Welch’s correction (F = 3.01, p = 0.02; t = 0.70, p = 0.49). The correlation between onset age and disease course of patients with BD is negative (Pearson’s r = −0.245, p = 0.11). For the lesion distribution of BD (Table 1), trunk and limbs are the most commonly observed distribution sites; there are 26.00% patients with lesions in sun-exposed parts. In particular, 5 patients developed lesions on their fingers and 1 patient developed lesion on her feet.
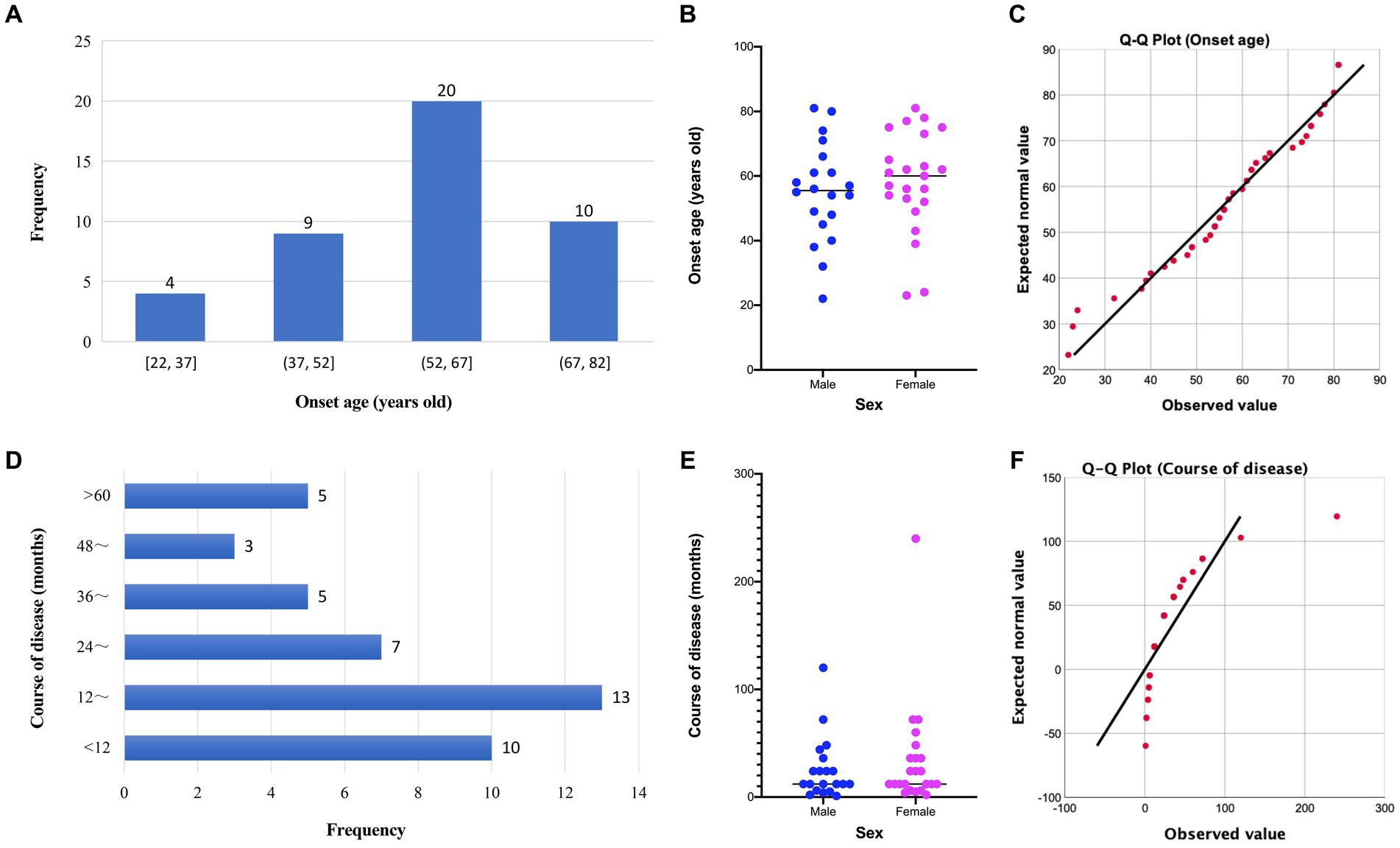
Figure 1. Distribution and comparison of onset age and disease course of patients with Bowen’s disease. (A) Distribution of onset age of patients with Bowen’s disease; (B) comparison of onset age of patients with Bowen’s disease between males and females; (C) Q-Q plot of onset age; (D) distribution of disease course of patients with Bowen’s disease; (E) comparison of disease course of patients with Bowen’s disease between males and females; (F) Q-Q plot of disease course.
3.2 Clinical diagnosis and treatment methods of Bowen’s disease
The correct rate of clinical diagnosis is 54.00%. Some patients with BD are misdiagnosed as Bowenoid papulosis (10.00%), actinic keratosis (8.00%), basal cell carcinoma (8.00%), seborrheic keratosis (6.00%), and pigmented naevus (4.00%). One case each was diagnosed as verruca vulgaris, infectious granuloma, eczema, Paget’s disease, fibrous rash, glomus tumor, and SCC. For the treatment, 94.00% patients with BD received surgical treatment, and imiquimod cream was applied in 1 patient. 2 patients were treated with combined therapy, including surgery combined with photodynamic therapy (PDT) or liquid nitrogen cryotherapy. There were 30 patients with BD who gave feedback on information about recurrence, and 3 patients had a recurrence in the treatment area.
3.3 Predisposing factors and comorbidities of Bowen’s disease
In total, 30 patients with BD gave feedback on predisposing factors, and 5 patients with BD had trauma at the lesion locations. For the comorbidities of 50 patients with BD, 33 patients had comorbidities: 48.48% (16 of 33) patients were comorbid with skin diseases, including allergic dermatosis (n = 7), infectious dermatosis (n = 4), keloid (n = 2), hemangioma (n = 2), actinic keratosis (n = 2), and other skin diseases; 39.39% (10 of 33) patients were comorbid with cardiovascular diseases, of which hypertension was the most common comorbidity (n = 9); 30.30% (10 of 33) patients were comorbid with gastrointestinal disease, mainly chronic gastritis or proctitis (n = 7); 27.27% (9 of 33) patients were comorbid with respiratory diseases, including allergic rhinitis (n = 4), pharyngitis (n = 3), and bronchitis (n = 3); 18.18% (6 of 33) patients were comorbid with tumors. In addition, patients with BD were also comorbid with some other diseases, such as lumbar disk herniation, diabetes mellitus, benign prostatic hyperplasia or prostatitis, and hyperlipidemia; 21.21% patients with BD had 1 kind comorbidity, followed by 3 and 4 kinds of concomitant diseases with the same proportion (18.18%). It is worth noting that more than three-quarters of patients with BD had more than 2 kinds of comorbidities.
3.4 Histopathological characteristics of Bowen’s disease
The histopathological characteristics of 50 patients with BD were summarized, including squamous cell hyperplasia (86.00%), disordered maturation with atypical keratinocytes (74.00%), atypical mitoses (60.00%), hyperkeratosis with hypokeratosis (48.00%), dermal inflammatory cell infiltration (36.00%), and koilocytosis (22.00%). In addition, it also includes some other pathological characteristics, such as dyskeratosis, elastic fiber degeneration, pigment deposition, and scattered pigment incontinence.
4 Discussion
BD often occurs in middle-aged and elderly people; it is slightly more common in women (5). The sex ratio of male and female patients with BD was approximately 1:1 in our study. Of 50 patients with BD, the clinical data of onset age and disease course were available in 43 patients. The average onset age and average disease course of female patients with BD are higher than male patients, but the differences are not significant. We explored the correlation between onset age and disease course of patients with BD, result showing that these two with negative correlation, it means the elderly patients with BD had shorter disease course, it is possible that elderly people are more concerned about their own physical health.
BD is mostly involved in sun-exposed areas of the body and lesions often appear on the head, face, and limbs (7); more recent studies suggest BD has a predilection for the head and neck (8, 9). Trunk and limbs were the most common distribution, while lesions in the head and neck with the lowest proportion in our study, which is slightly different from previous findings (7–9). Interestingly, the lesions of 46.00% patients with BD located on non-sun-exposed sites, such as the trunk and genitals, suggest that we should pay more attention to rashes in non-sun-exposed sites. In particular, skin lesions of BD occurred on the fingers in 5 patients and on the feet in 1 patient. Previous studies indicated that HPV infection may be a potential risk factor for BD (10), multiple BD on the finger associated with HPV-34 (11) and HPV-16 (12), and BD on the dorsum of the foot associated with HPV-16 (13). Therefore, it is necessary to test HPV for BD occurring in the fingers and feet.
In clinical practice, the diagnosis of BD is usually made on the basis of clinical manifestations. Dermoscopy, as a non-invasive tool, is increasingly used in the clinical auxiliary diagnosis of BD (14). Ultrasound biomicroscopy (UBM) and high-frequency ultrasound (HFUS) also have potential as diagnostic tools for BD (15). Skin biopsy is often necessary to arrive at an accurate diagnosis of BD; all patients in our study underwent pathological examination. The correct rate of clinical diagnosis of BD before pathological examination is relatively low; BD is most easily misdiagnosed as Bowenoid papulosis, followed by actinic keratosis, basal cell carcinoma keratosis, seborrheic keratosis, and pigmented naevus. Interestingly, BD and seborrheic keratosis could be correctly identified by the deep learning model (area under the curve score > 0.97), the results of which should be confirmed by qualified histopathologists (16). Hopefully, more diagnostic methods with high accuracy will be developed in future.
The treatment methods for BD include topical therapies (5-fluorouracil (5-FU), imiquimod), cryotherapy (cryosurgery), curettage with cautery, PDT, standard surgical excision, Mohs micrographic surgery, laser (CO2 laser, non-ablative neodymium: YAG), radiotherapy, systemic treatments, and combination therapy (5). Some novel treatment approaches, such as pembrolizumab (a humanized monoclonal anti-PD-1 antibody for the treatment of melanoma and other malignancies) (17, 18) and thermotherapy (19), have also been tried for the treatment of BD. Previous studies reported that the complete clearance rate can reach 94.40% when the surgical margin is 5 mm (20). In this study, more than 90.00% patients with BD received surgical treatment, and two patients were treated with combined therapy, including surgery combined with PDT or cryotherapy. A previous study showed that PDT is a relatively effective treatment modality for BD, the overall clearance rate was 63.40% (21). An observational study compared different therapies: cryotherapy with the longest average treatment period, followed by imiquimod, PDT, and excision; surgical excision with the highest and PDT with the lowest therapeutic efficacy; imiquimod with recurrence rate was the highest (22). The efficacy of PDT in the treatment of BD varies among different studies, a meta-analysis shows that PDT treats BD with better efficacy, less recurrence, and better cosmetic outcomes than cryotherapy and 5-FU (23). Although a variety of methods have been applied to the treatment of BD, more therapeutic targets and methods need to be explored to enrich the treatment of BD in future.
More and more attention is being paid to the coexistence of diseases, but few studies focus on the comorbidity of BD. Previous studies reported that Merkel cell carcinoma (24, 25), cutaneous pseudolymphoma (26), breast cancer (27), and extramammary Paget’s disease (28), were concurrent with BD. In this study, 66.00% BD patients had comorbidities, mainly including skin diseases (48.48%), cardiovascular diseases (39.39%), gastrointestinal diseases (30.30%), respiratory diseases (27.27%), and tumors (18.18%). It is noticed that more than three-quarters of patients with BD had more than 2 kinds of comorbidities, and 1 patient had 14 kinds of comorbidities. Patients with so many kinds of comorbidities, which may be related to that most of the patients with BD are elderly people who have more underlying diseases. Therefore, it is necessary to pay attention to the comorbidities of patients with BD in diagnosis and treatment.
BD exhibits the histopathological features of full-thickness cell atypia, intact stratum basale, widened and elongated epidermal processes, and lymphocyte inflammatory cell infiltration around the superficial dermis vessels. In our study, the most common histopathological characteristic of BD is squamous cell hyperplasia, followed by disordered maturation with atypical keratinocytes, atypical mitoses, hyperkeratosis with hypokeratosis, dermal inflammatory cell infiltration, and koilocytosis. The summarization of these pathological characteristics is helpful for the diagnosis of BD in the Chinese population. Although there are some auxiliary diagnostic methods for the diagnosis of BD, histopathological examination is still necessary for the diagnosis of BD. The combination of fluorescence lifetime imaging microscopy (FLIM) and phasor approach (phasor-FLIM) is a screening tool for the differential diagnosis of BD, actinic keratosis, and basal cell carcinoma based on histopathological analysis (29). More diagnostic pathological characteristics need to be found in future.
5 Conclusions
BD often occurs in middle-aged and elderly people and is easily misdiagnosed clinically. The onset age and disease course of BD are not significantly different between males and females, whereas there is a negative correlation between the onset age and disease course of BD. BD is more likely to occur in the trunk and limbs in the Chinese population, and most BD patients are diagnosed with comorbidities. The associations between BD and its comorbidities should be further studied.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Peking University Shenzhen Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CZ: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. BJ: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. KZ: Data curation, Writing – original draft, Writing – review & editing. JW: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. CH: Data curation, Writing – original draft, Writing – review & editing. NX: Data curation, Writing - review & editing. TY: Resources, Writing – review & editing. BC: Resources, Supervision, Writing – review & editing. BY: Resources, Supervision, Writing – review & editing. YZ: Conceptualization, Resources, Supervision, Writing – review & editing. CS: Conceptualization, Data curation, Resources, Supervision, Writing – original draft, Writing - review & editing.
Funding
The author(s) declare financial support is received for the research, authorship, and/or publication of this article. This study was funded by the Shenzhen Sanming Project (No.: SZSM201812059) and the Shenzhen Key Medical Discipline Construction Fund (No.: SZXK040).
Acknowledgments
The authors thank the efforts and contributions of the study samples and all the clinical staff in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mohandas, P, Lowden, M, and Varma, S. Bowen’s disease. BMJ. (2020) 368:m813. doi: 10.1136/bmj.m813
2. Jansen, MHE, Özhan-Hasan, H, Nelemans, PJ, Winnepenninckx, VJ, and Mosterd, K. Trends in the incidence of Bowen disease based on a single-center study in the Netherlands. Dermatol Surg. (2019) 45:1353–8. doi: 10.1097/dss.0000000000001980
3. Sun, L, Lu, J, Zhang, M, Yang, X, Wu, W, and Liu, Q. Clinical and pathological characteristics of 755 patients with skin cancers in Hainan, China: a 12-year retrospective study. Clin Cosmet Investig Dermatol. (2022) 15:43–50. doi: 10.2147/ccid.S343274
4. Molina-García, M, Malvehy, J, Granger, C, Garre, A, Trullàs, C, and Puig, S. Exposome and skin. Part 2. The influential role of the Exposome, beyond UVR, in actinic keratosis, Bowen’s disease and squamous cell carcinoma: a proposal. Dermatol Ther. (2022) 12:361–80. doi: 10.1007/s13555-021-00644-3
5. Sharma, A, Birnie, AJ, Bordea, C, Cheung, ST, Mann, J, Morton, CA, et al. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma in situ (Bowen disease) 2022. Br J Dermatol. (2023) 188:186–94. doi: 10.1093/bjd/ljac042
6. Kim, YS, Park, GS, Bang, CH, and Chung, YJ. Genomic landscape of multiple Bowen’s disease using whole-exome sequencing. J Dermatol. (2023) 50:397–00. doi: 10.1111/1346-8138.16584
7. Palaniappan, V, and Karthikeyan, K. Bowen’s disease. Indian Dermatol Online J. (2022) 13:177–89. doi: 10.4103/idoj.idoj_257_21
8. Tokez, S, Wakkee, M, Louwman, M, Noels, E, Nijsten, T, and Hollestein, L. Assessment of cutaneous squamous cell carcinoma (cSCC) in situ incidence and the risk of developing invasive cSCC in patients with prior cSCC in situ vs the general population in the Netherlands, 1989-2017. JAMA Dermatol. (2020) 156:973–81. doi: 10.1001/jamadermatol.2020.1988
9. Foo, CC, Lee, JS, Guilanno, V, Yan, X, Tan, SH, and Giam, YC. Squamous cell carcinoma and Bowen’s disease of the skin in Singapore. Ann Acad Med Singap. (2007) 36:189–93. doi: 10.47102/annals-acadmedsg.V36N3p189
10. Namgoong, S, Kim, J, Jeong, KM, Jeon, J, Song, HJ, and Baek, YS. Association of human papillomavirus and extra-genital Bowen disease (squamous cell carcinoma in situ): a systematic review. J Am Acad Dermatol. (2021) 84:822–5. doi: 10.1016/j.jaad.2020.09.059
11. Yonekura, S, Egawa, G, Komori, T, and Kabashima, K. Multiple Bowen’s disease on the finger associated with human papillomavirus type 34. Skin Health Dis. (2023) 3:e238. doi: 10.1002/ski2.238
12. Wen, P, Liu, H, Xue, S, Li, W, Li, F, and Wang, L. Bowen disease of the nail unit associated with HPV-16 infection in a pemphigus patient following rituximab treatment. Eur J Dermatol. (2022) 32:270–2. doi: 10.1684/ejd.2022.4226
13. Murao, K, Yamasaki, K, Agawa, H, Nakajima, M, Iwasaka, M, and Kubo, Y. Bowen disease on the dorsum of the foot associated with human papillomavirus type 16. J Med Investig. (2022) 69:152–4. doi: 10.2152/jmi.69.152
14. Behera, B, Kumari, R, Thappa, DM, Gochhait, D, Srinivas, BH, and Ayyanar, P. Dermoscopy of Bowen’s disease: a case series of five patients. Indian J Dermatol Venereol Leprol. (2021) 87:576–80. doi: 10.25259/ijdvl_987_20
15. Li, MX, Wang, Q, Li, XL, Zhao, CK, Zhu, RZ, Chen, J, et al. Imaging findings of Bowen’s disease: a comparison between ultrasound biomicroscopy and conventional high-frequency ultrasound. Skin Res Technol. (2020) 26:654–63. doi: 10.1111/srt.12849
16. Jansen, P, Baguer, DO, Duschner, N, Le’Clerc Arrastia, J, Schmidt, M, Wiepjes, B, et al. Evaluation of a deep learning approach to differentiate Bowen’s disease and seborrheic keratosis. Cancers (Basel). (2022) 14:518. doi: 10.3390/cancers14143518
17. Delaitre, L, Martins-Héricher, J, Truchot, E, Denis, D, Prophette, B, Maillard, H, et al. Regression of cutaneous basal cell and squamous cell carcinoma under pembrolizumab. Ann Dermatol Venereol. (2020) 147:279–84. doi: 10.1016/j.annder.2019.10.031
18. Zelin, E, Maronese, CA, Dri, A, Toffoli, L, Di Meo, N, Nazzaro, G, et al. Identifying candidates for immunotherapy among patients with non-melanoma skin Cancer: a review of the potential predictors of response. J Clin Med. (2022) 11:364. doi: 10.3390/jcm11123364
19. Kurihara, M, Kimura, U, Hiruma, M, Takamori, K, and Suga, Y. Trial of hyperthermic treatment for Bowen’s disease with disposable chemical pocket warmers: a report of two cases. J Dermatol. (2018) 45:e136–7. doi: 10.1111/1346-8138.14166
20. Westers-Attema, A, van den Heijkant, F, Lohman, BGPM, Nelemans, PJ, Winnepenninckx, V, Kelleners-Smeets, NWJ, et al. Bowen’s disease: a six-year retrospective study of treatment with emphasis on resection margins. Acta Derm Venereol. (2014) 94:431–5. doi: 10.2340/00015555-1771
21. Zaar, O, Fougelberg, J, Hermansson, A, Gillstedt, M, Wennberg-Larkö, AM, and Paoli, J. Effectiveness of photodynamic therapy in Bowen’s disease: a retrospective observational study in 423 lesions. J Eur Acad Dermatol Venereol. (2017) 31:1289–94. doi: 10.1111/jdv.14164
22. Park, HE, Park, JW, Kim, YH, Han, JH, Bang, CH, Park, YM, et al. Analysis on the effectiveness and characteristics of treatment modalities for Bowen’s disease: an observational study. J Clin Med. (2022) 11:741. doi: 10.3390/jcm11102741
23. Xue, WL, Ruan, JQ, Liu, HY, and He, HX. Efficacy of photodynamic therapy for the treatment of Bowen’s disease: a Meta-analysis of randomized controlled trials. Dermatology. (2022) 238:542–50. doi: 10.1159/000519319
24. Swain, M, Yadav, A, Pendharkar, D, and Patnaik, S. Concurrent Merkel cell carcinoma and Bowen’s disease in a young lady. Indian J Dermatol. (2022) 67:761–3. doi: 10.4103/ijd.ijd_926_20
25. Ishida, M, and Okabe, H. Merkel cell carcinoma concurrent with Bowen’s disease: two cases, one with an unusual immunophenotype. J Cutan Pathol. (2013) 40:839–43. doi: 10.1111/cup.12176
26. Uchiyama, A, Kishi, C, Kuriyama, Y, Shimizu, A, Ishikawa, O, and Motegi, SI. A case of coexisting Bowen’s disease and cutaneous pseudolymphoma. Eur J Dermatol. (2020) 30:744–6. doi: 10.1684/ejd.2020.3912
27. Yamaguchi, K, Matsunuma, R, Kumeta, T, Imada, S, Hayami, R, Arai, K, et al. Concurrent Bowen’s disease of the nipple and breast Cancer. Case Rep Oncol. (2020) 13:1410–4. doi: 10.1159/000511565
28. Goyal, T, Varshney, A, and Solanki, R. Co-existence of extramammary Paget’s disease and Bowen’s disease of vulva. Indian J Dermatol Venereol Leprol. (2014) 80:530–3. doi: 10.4103/0378-6323.144170
Keywords: Bowen’s disease, characteristic, diagnosis, treatment, comorbidity, retrospective study
Citation: Zhou C, Jiang B, Zhang K, Wu J, Huang C, Xu N, Ye T, Chen B, Yu B, Zou Y and Shen C (2023) Clinical and histopathological characteristics, diagnosis and treatment, and comorbidities of Bowen’s disease: a retrospective study. Front. Med. 10:1281540. doi: 10.3389/fmed.2023.1281540
Edited by:
Lawrence Chukwudi Nwabudike, National Institute for Diabetes, Nutrition and Metabolic Diseases Prof. Dr. N. Paulescu, RomaniaReviewed by:
Carlo Alberto Maronese, IRCCS Ca ’Granda Foundation Maggiore Policlinico Hospital, ItalyMaryam Nasimi, Tehran University of Medical Sciences, Iran
Copyright © 2023 Zhou, Jiang, Zhang, Wu, Huang, Xu, Ye, Chen, Yu, Zou and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfen Zou, NzMyNzI0NTE5QHFxLmNvbQ==; Changbing Shen, Y2FtYnJpZGdlMjAwOEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Cheng Zhou
Cheng Zhou Bin Jiang1,2†
Bin Jiang1,2† Kaoyuan Zhang
Kaoyuan Zhang Jingwen Wu
Jingwen Wu Cong Huang
Cong Huang Changbing Shen
Changbing Shen