- 1University Clinic for Nephrology and Hypertension, Diabetes and Endocrinology, Otto-von-Guericke University, Magdeburg, Germany
- 2University Hospital for Hematology and Oncology, Otto-von-Guericke University, Magdeburg, Germany
- 3Institute for Pathology, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder often diagnosed after incidental finding of leukocytosis. Renal involvement is usually clinically silent. Symptomatic renal impairment due to CLL/small lymphocytic lymphoma (SLL) cell infiltration in the kidney tissue is uncommon, and acute kidney injury (AKI) as a presenting feature is rare. In this case report, we describe the case of a patient with AKI caused by CLL/SLL infiltration as a presenting feature. Our report highlights the possibility of kidney injury as the first evident symptom of CLL/SLL. Kidney biopsy is the mainstay in these cases in order to establish a diagnosis. Treatment with zanubrutinib resulted in improved kidney function.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonal lymphoproliferative disorder defined by the progressive accumulation of monoclonal immunocompetent B lymphocytes, primarily involving hematopoietic organs (1, 2). Although CLL and small lymphocytic lymphoma (SLL) are essentially the same diseases, the term SLL is used for cases with fewer than 5 × 109/L circulating monoclonal B cells and nodal/extranodal manifestations (3).
CLL/SLL is more common in men than in women and accounts for ~30% of all types of leukemias in Western countries (4). This disease primarily affects elderly adults with a median age at diagnosis of 71 years (5).
The diagnosis of CLL is often carried out when leukocytosis is incidentally detected during routine analyses of peripheral blood cellular composition. Symptomatic presentations include complaints related to lymphadenopathy/organomegaly, recurrent infections, or classic B symptoms such as fever, weight loss, and night sweats.
B-cell CLL infiltration can affect any organ but predominantly involves lymphoid tissues. Secondary leukemic cell infiltration of the renal parenchyma, often without clinical sequela, commonly occurs in the course of CLL/SLL, as demonstrated by postmortem autopsy studies, where renal infiltration has been proven in 63–90% of all CLL/SLL cases (6–9). Acute kidney injury (AKI) incited by direct cell infiltration is uncommon (10, 11). Nie et al. and Nuguri et al. each reported only one patient with CLL/SLL and kidney injury due to associated infiltrates in recently published retrospective cohort studies of patients with lymphoproliferative disorders (12, 13). AKI as the first indication that prompts the diagnosis of CLL/SLL is extremely rare, with only seven reported cases in the literature (14–20).
Herein, we report an index patient diagnosed with AKI secondary to renal infiltration as the initial manifestation of CLL/SLL that is associated with the histopathological pattern of tubulointerstitial injury and minimal changes glomerulonephritis.
Case presentation
A 77-year-old male Caucasian was admitted to our clinic with deterioration in kidney function alongside a weeklong history of progressive fatigue accompanied by a prior 6-month history of unintended weight loss summing up to ~8% of the total body weight. He did not report having had fever or night sweats. There were no clinical signs for infection by bacteria or viruses. His past medical history included essential arterial hypertension, chronic obstructive lung disease, classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1, due to former nicotine abuse without recent exacerbation, and a history of recurring pulmonary embolism. There was no past medical history of kidney disease. His regular medication, which had not changed recently before this presentation, comprised torasemide, candesartan, and amlodipine to control blood pressure, apixaban for secondary prevention of pulmonary embolism, and pantoprazole to reduce heartburn.
The patient's body temperature and blood pressure were measured at 36.7°C and 135/70 mmHg, respectively. His pulse rate and respiration were within normal limits. Fluid homeostasis was maintained. Physical examination revealed only a slightly pale complexion. Cardiorespiratory and abdominal examinations showed no abnormalities. In particular, no signs of lymphadenopathy or hepatosplenomegaly were found.
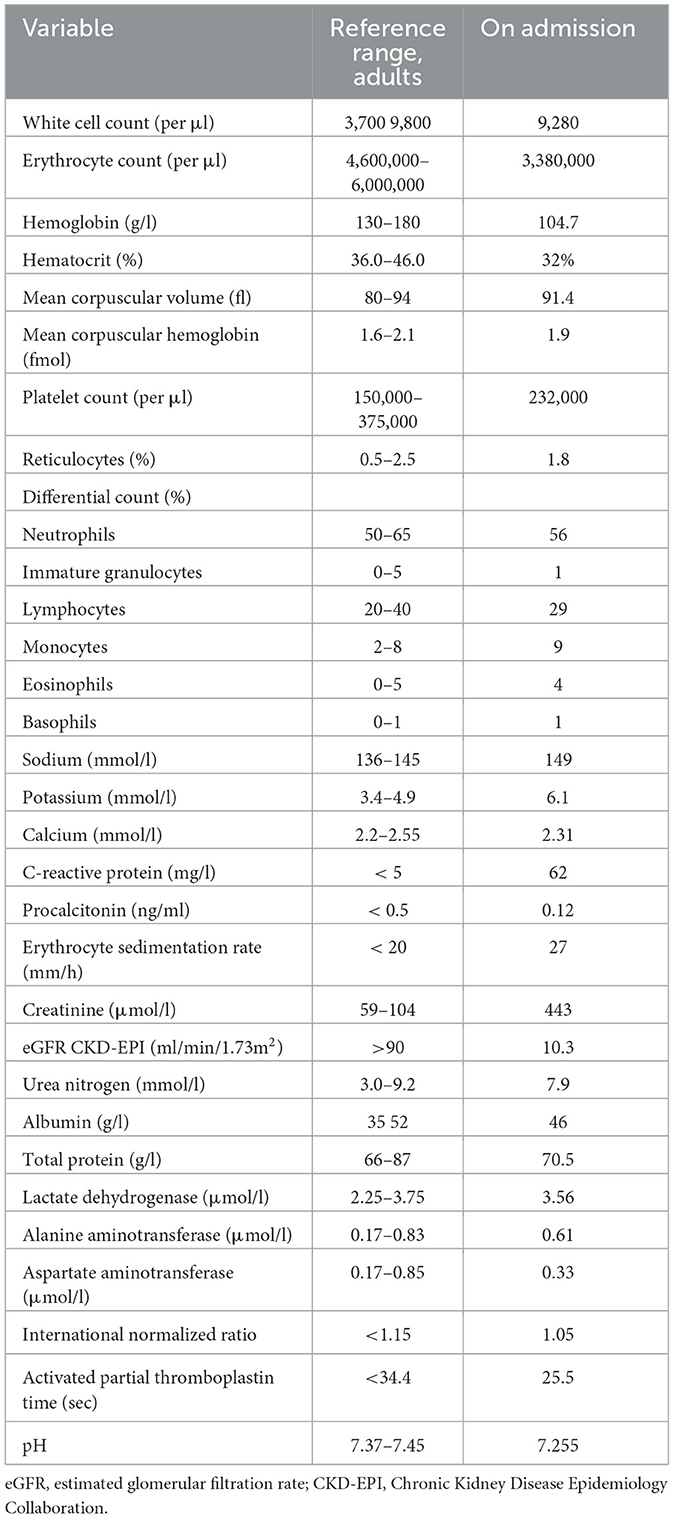
Full blood cell counts revealed normocytic normochromic anemia with hemoglobin values at 104.7 g/l [reference range 130–180 g/l] without leukocytosis or lymphocytosis. The serum biochemical analysis indicated elevated levels of creatinine of 443 μmol/l, which increased from the baseline value of 92 μmol/l over a period of ~10 weeks. Renal impairment presented with metabolic acidosis (pH 7.255) and hyperkalemia (potassium 6.1 mmol/l [ref.range 3.4–4.9 mmol/l]). The serum calcium level was measured at 2.31 mmol/l [ref.range 2.2–2.55 mmol/l]. The relevant laboratory data are presented in Table 1.
Further investigations showed a marginally elevated ANA titer of 1:160, with negative results in the rest of autoimmune serology (ANCA, anti-GBM, anti-dsDNA, and ENA). Serum complement levels (C3 and C4) were quantified within the normal range. Immunoglobulin levels were within the normal range for IgA, IgG, and IgE but decreased for IgM (0.18 g/l [0.4–2.3]). Serum protein electrophoresis yielded normal results. Free light chain detection using serum specimens revealed elevated IgL-kappa level of 42 mg/l [3.3–19.4] and normal IgL-lambda with a kappa-lambda ratio of 1.62. Serum immunofixation depicted the presence of monoclonal IgG kappa. A second serum immunofixation 2 months later showed biclonal IgG kappa and lambda. Immunoglobulin levels were measured with a total IgA of 1.05 g/l [0.7–4], IgM of 0.18 g/l [0.4 2.3], and IgG of 8.83 g/l (7–16). Quantification of the clonal component could not be obtained. Infectious serology was negative for HBsAg, anti-HCV, and HIV-Ag/Ak.
The urinalysis revealed microalbuminuria with an albumine-creatinine ratio of 8.76 mg/mmol [ref. range < 2.5 mg/mmol]) and sterile leukocyturia, excluding erythrocyturia. Additionally, the urine volume indicated marked polyuria with urine output of up to 4.5 l per day.
A computed tomography of the thorax demonstrated no significant lymphadenopathy. An ultrasound of the kidneys illustrated normal-sized kidneys with a swollen parenchyma and no evidence of obstructive nephropathy (Figure 1). Subsequently, a kidney biopsy was performed. Light microscopy showed dense infiltration of the cortical interstitium and capsule by mature monomorphic small lymphocytes (Figures 2A, B). Advanced tubular atrophy reached 50% of the cortical tissue surrounded by interstitial fibrosis. Immunostaining was negative for immunoglobulins and 1+ positive for complement (C3 and C1q). Immunocytochemical staining revealed that the infiltrate consisted mainly of monoclonal B lymphoid cells, which tested positive for CD20 and CD23 (Figures 2C, D). Additionally, aberrant expression of CD5 was observed. The glomeruli showed only minimal changes, as can be observed through electron microscopy, characterized by a global loss of podocyte foot processes.
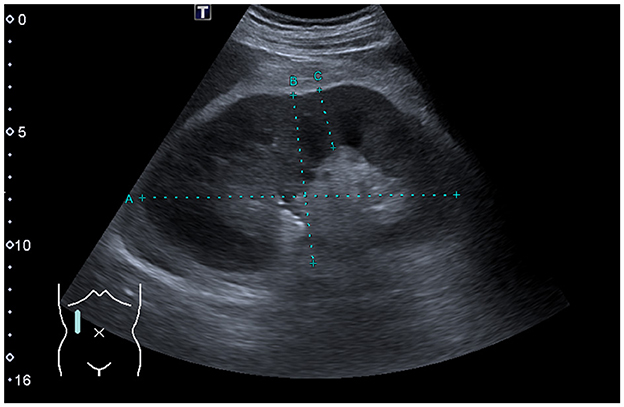
Figure 1. Renal ultrasound findings. Sagittal view of the right kidney showing a normal-sized kidney with swollen parenchyma measuring 26 mm (ref. range: 13–18 mm).
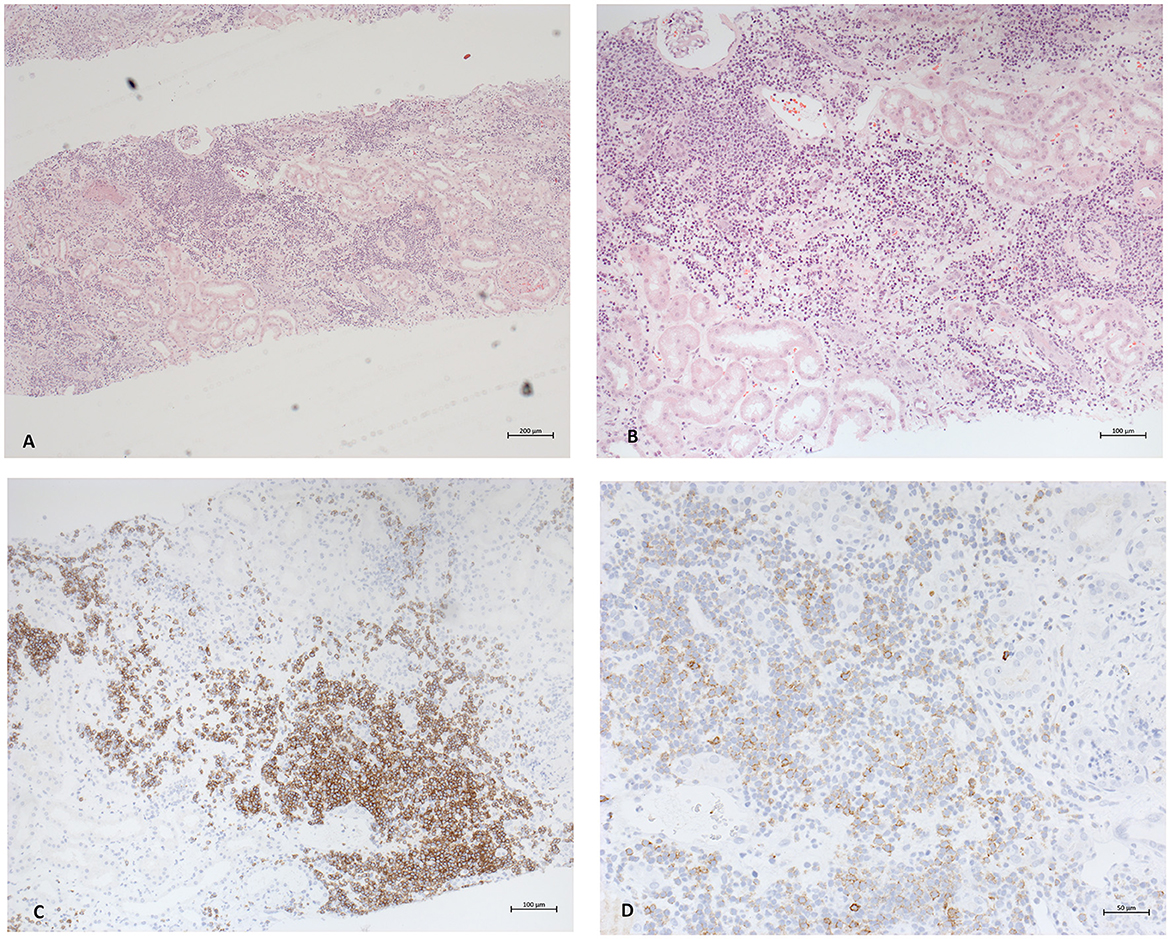
Figure 2. Photomicrographs of the pathological findings in renal biopsy. (A) Light microscopy with hematoxylin and eosin staining 5x. Diffuse dense interstitial infiltration with small lymphocyte-like cells in diffuse pattern. (B) Magnification depicting normal glomeruli and tubular atrophy and fibrosis alongside infiltrates (HE staining 10x). (C, D) Immunocytochemistry. CD20 and CD23 staining proving the presence of lymphoid B cells.
Given the biopsy findings, the patient was referred to the clinic for hematology for further evaluation. There was no evidence of hemolysis. Flow cytometry of peripheral blood confirmed the presence of a CD5 positive monotypic B-cell population expressing CD19, CD20, CD23, and IgM and exhibiting kappa light chain restriction. However, criteria of CLL were not met (monoclonal B cell count < 5 × 109/L). Further analysis showed an immunoglobulin heavy chain (IgHV) mutated status and absence of TP53 mutation/deletion. Bone marrow examination displayed typical monomorphic infiltration in up to 40% of the medullary cavity, with an immunophenotype consistent with CLL/SLL, but without infiltration of plasma cells, ruling out the differential diagnosis of multiple myeloma.
In summary, all the findings clearly present a diagnosis of CLL/SLL. The overall condition of the patient was satisfactory, with an Karnofsky performance score of 80. Treatment with the selective Bruton tyrosine kinase inhibitor, zanubrutinib, at a dosage of 160 mg twice daily together with prednisolone 50 mg, due to the described distinct minimal change glomerulonephropathy pattern, was initiated. Potential nephrotoxic medications including candesartan were temporarily discontinued. Kidney function recovered, electrolytes and metabolism normalized, and polyuria and proteinuria resolved. Moreover, renal replacement therapy was not necessary. At the last follow-up 6 month later, kidney function remained stable with a creatinine level of 170 μmol/l. No clinically visible side effects under zanubrutinib have been observed to date. The overall clinical condition continued to be moderate to good.
Discussion
In previous studies, CLL/SLL-associated kidney disease remains poorly described, thus limiting our understanding. This is primarily due to the infrequent occurrence of kidney biopsy in patients with CLL/SLL (21).
Previous reports depicted a wide spectrum of clinical presentation and histopathological patterns. Patient characteristics and the clinical course of reported cases with AKI resulting from B-cell infiltration as a presenting feature in undiagnosed CLL/SLL are compiled in Table 2.
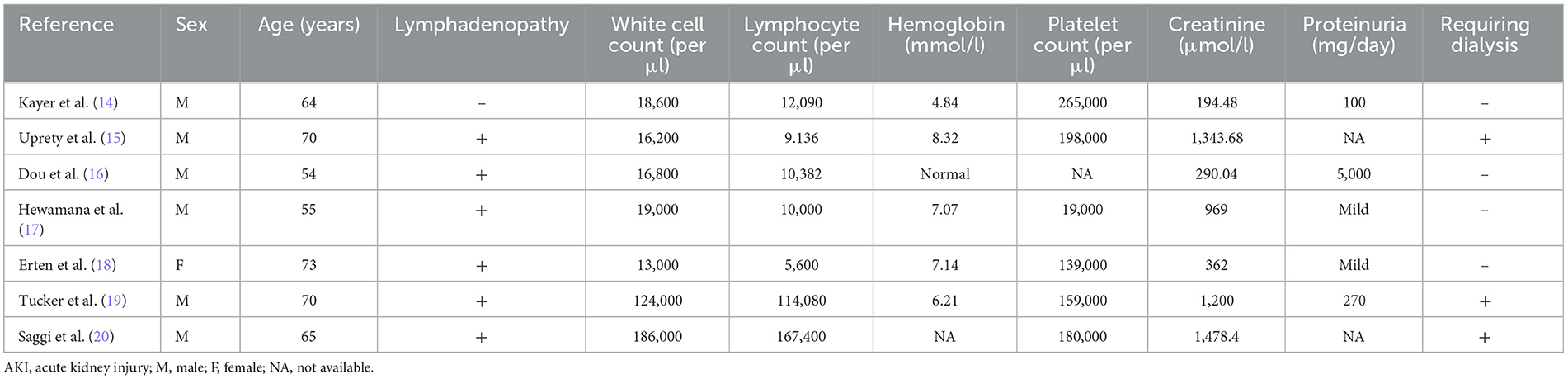
Table 2. Patient characteristics and clinical parameters of reported CLL/SLL cases initially presenting with kidney failure.
Eight cases, including the presented one, were diagnosed with CLL/SLL for the first time through kidney biopsy. Among these cases, seven patients were men (87.5 %), and six (75%) out of eight patients were younger than the median age at a diagnosis of 71 years. To date, all reported cases have indicated peripheral leukocytosis at the time of diagnosis. Mild proteinuria is observed in six cases. Lymphocyte counts and renal impairment, characterized by an increase in serum creatinine levels and proteinuria, did not exhibit correlation. Additionally, the degree of AKI had no correlation with the stage of CLL/SLL. Nevertheless, Wang et al. demonstrated that severe renal insufficiency determined as serum creatinine levels equaling 200 μmol/l or more is associated with ≥ 50% interstitial infiltration, which coincides with our data (22). Kidney enlargement may be present, but this finding is inconsistent and is of low sensitivity and specificity (23–25). Pathomechanistically, it remains unclear how exactly CLL/SLL infiltration causes kidney injury. It has been postulated that infiltration may cause compression of both the tubular system and microvasculature inducing intrarenal obstruction and ischemia (26, 27). However, in most case reports, no such findings could be described. In the biopsy specimen of our index patient, no evidence supporting this theory could be observed (Figure 2). Schwartz et al. reported the accompanying fibrosis, particularly in areas of leukemic infiltration, and postulated an infiltration-associated inflammatory mechanism (8). In accordance with this theory of direct harm are the findings of Wang et al. verifying that CLL/SLL cells can secrete monoclonal immunoglobulins in situ (22).
Usually, infiltration of the renal interstitium is diffuse, occasionally nodular, and develops in the later stage of the illness (28, 29). Typically, it is associated with both glomerular lesions, with membranoproliferative- and minimal change-like patterns appearing to be the most frequent, alongside secondary tubular atrophy (8, 10, 21). It may even directly affect the tubules in selected cases (19, 30, 31). Nevertheless, Corlu et al. and Strati et al. found, independently, an almost even distribution between diffuse and focal infiltration patterns with no difference in renal function among these groups (21, 25). Our patient exhibited diffuse infiltration of the atrophic tubulointerstitium and displayed glomerular minimal changes. However, the latter finding despite the pronounced advanced loss of foot processes in our specimen did not correspond with the clinical presentation, since there was only mild proteinuria detected by urinalysis without clinical signs of nephrotic syndrome, ruling out minimal change disease in our patient.
Administered treatment with zanubrutinib in this case followed the SEQUOIA-trial, recently establishing zanubrutinib as a novel treatment option for untreated CLL/SLL, showing significantly improved progression-free survival rates (32). To our knowledge, we herein describe the first case showing that zanubrutinib could contribute to stabilization of kidney function in a CLL/SLL patient presenting initially with B-cell infiltration-associated acute kidney injury.
Detection of clonal paraprotein, similar to our case, can be found frequently in patients with CLL/SLL and may even precede the diagnosis (33–35). Mozas et al. reported a biclonal serum immunofixation to be detectable in 11% of the cases in a single center study (36). As the origin of this phenomenon is not clear, there are different interpretations of this observation, including clonal evolution and the occurrence of isotype switching (36). Nevertheless, the presence of this feature is associated with poorer overall survival, with biclonal cases appearing to have a worse prognosis (33, 36).
AKI is known to be associated with a less lasting hematological response (37). Kidney disease is a well-established negative prognostic factor for overall survival in CLL/SLL patients (38). Being a major cause of morbidity and mortality, it is important to address renal function to improve long-term outcomes, especially considering that adequate treatment of the hematologic primary disease can improve the kidney function (24, 28, 39–41). For this reason, diagnostic security is to be achieved quickly through kidney biopsy, which is often unavoidable, similar to the index case at hand, for early treatment initiation.
Conclusion
B-cell infiltration should be considered despite its rare clinical manifestation as differential diagnosis of AKI in patients with CLL/SLL. AKI may present as the first clinical feature of CLL/SLL as highlighted in our index case, detectable only after kidney biopsy, which is mandatory in these cases to establish diagnosis and early treatment. Treatment with zanubrutinib can help to improve the kidney function. Close interdisciplinary cooperation between nephrologists and hematooncologists is crucial in managing these patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SB: Conceptualization, Data curation, Writing—original draft, Writing—review and editing. VZ: Writing—review and editing. TW: Visualization, Writing—review and editing. PM: Conceptualization, Supervision, Writing—review and editing. CG: Supervision, Writing—review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Klein U, Dalla-Favera R. New insights into the pathogenesis of chronic lymphocytic leukemia. Sem Cancer Biol. (2010) 20:377–83. doi: 10.1016/j.semcancer.2010.10.012
2. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, supportive management of CLL. Blood. (2018) 131:2745–60. doi: 10.1182/blood-2017-09-806398
3. Weltgesundheitsorganisation. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th edition. In:Swerdlow SH, Campo E, Harris NL, et al., , editors. International Agency for Research on Cancer (2017).
4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA A Cancer J Clinic. (2023) 73:17–48. doi: 10.3322/caac.21763
5. Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological malignancy research network. Br J Cancer. (2011) 105:1684–92. doi: 10.1038/bjc.2011.450
6. Barcos M, Lane W, Gomez GA, Han T, Freeman A, Preisler H, et al. An autopsy study of 1206 acute and chronic leukemias (1958 to 1982). Cancer. (1987) 60:827–37.
7. Xiao JC, Walz-Mattmüller R, Ruck P, Horny HP, Kaiserling E. Renal involvement in myeloproliferative and lymphoproliferative disorders: a study of autopsy cases. Gen Diagn Pathol. (1997) 142:147–53.
8. Schwartz JB, Shamsuddin AM. The effects of leukemic infiltrates in various organs in chronic lymphocytic leukemia. Human Pathol. (1981) 12:432–40. doi: 10.1016/S0046-8177(81)80023-8
9. Kirshbaum JD. Leukemia: a clinical and pathologic study of one hundred and twenty-three fatal cases in a series of 14,400 necropsies. Arch Intern Med (Chic). (1943) 71:777. doi: 10.1001/archinte.1943.00210060038003
10. Kowalewska J, Nicosia RF, Smith KD, Kats A, Alpers CE. Patterns of glomerular injury in kidneys infiltrated by lymphoplasmacytic neoplasms. Human Pathol. (2011) 42:896–903. doi: 10.1016/j.humpath.2010.09.009
11. Da'as N, Polliack A, Cohen Y, Amir G, Darmon D, Kleinman Y, et al. Kidney involvement renal manifestations in non-Hodgkin's lymphoma lymphocytic leukemia: a retrospective study in 700 patients: Renal involvement in CLL NHL. Euro J Haematol. (2001). 67(3):158-164. doi: 10.1034/j.1600-0609.2001.5790493.x
12. Nie G, Sun L, Zhang C. Clinicopathological features and individualized treatment of kidney involvement in B-cell lymphoproliferative disorder. Front Immunol. (2022) 13:903315. doi: 10.3389/fimmu.2022.903315
13. Nuguri S, Swain M, De Padua M, Gowrishankar S. Renal lymphoma diagnosed on kidney biopsy presenting as acute kidney injury. Indian J Nephrol. (2022) 32:342. doi: 10.4103/ijn.ijn_345_21
14. Kayar Y, Ekinci I, Bay I, Bayram Kayar N, Hamdard J, Kazancioglu R. Acute renal failure due to Leukaemic infiltration in chronic lymphocytic leukaemia. Case Rep Med. (2015) 2015:1–3. doi: 10.1155/2015/469136
15. Uprety D, Peterson A, Shah BK. Renal failure secondary to leukemic infiltration of kidneys in CLL—a case report and review of literature. Ann Hematol. (2013) 92:271–3. doi: 10.1007/s00277-012-1547-6
16. Dou X, Hu H, Ju Y, Liu Y, Kang K, Zhou S, et al. Concurrent nephrotic syndrome and acute renal failure caused by chronic lymphocytic leukemia (CLL): a case report and literature review. Diagn Pathol. (2011) 6:99. doi: 10.1186/1746-1596-6-99
17. Hewamana S, Pepper C, Jenkins C, Rowntree C. Acute renal failure as the presenting feature of leukaemic infiltration in chronic lymphocytic leukaemia. Clin Exp Nephrol. (2009) 13:179–81. doi: 10.1007/s10157-009-0129-y
18. Erten N, Saka B, Caliskan YK, Besisik S, Karan MA, Tascioglu C. Acute renal failure due to leukaemic infiltration in chronic lymphocytic leukaemia: case report: acute renal failure due to leukaemic infiltration. Int J Clinic Pract. (2005) 59:53–5. doi: 10.1111/j.1368-504X.2005.00049.x
19. Tucker B, Brown AL, d'Ardenne AJ, Cattell WR. Reversible renal failure due to renal infiltration and associated tubulointerstitial disease in chronic lymphocytic leukaemia. Nephrology Dialysis Transplantation. (1990) 5:616–8. doi: 10.1093/ndt/5.8.616
20. Saggi S, Calandri C, Muhlfelder T, Choi H, Kahn T, Kaji D. Renal failure due to leukaemic infiltration in chronic lymphocytic leukaemia. Nephrol Dial Transplant. (1990) 5:1051–2. doi: 10.1093/ndt/5.12.1051
21. Strati P, Nasr SH, Leung N, Hanson CA, Chaffee KG, Schwager SM, et al. Renal complications in chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis: the Mayo Clinic experience. Haematologica. (2015) 100:1180–8. doi: 10.3324/haematol.2015.128793
22. Wang H, Yu X, Zhang X, Wang S, Zhao M. The pathological features of leukemic cells infiltrating the renal interstitium in chronic lymphocytic leukemia/small lymphocytic lymphoma from a large single Chinese center. Diagn Pathol. (2021) 16:59. doi: 10.1186/s13000-021-01120-4
23. Bach AG, Behrmann C, Holzhausen HJ. Prevalence and patterns of renal involvement in imaging of malignant lymphoproliferative diseases. Acta Radiol. (2012) 53:343–8. doi: 10.1258/ar.2011.110523
24. Poitou-Verkinder A-L, Francois A, Drieux F, Lepretre S, Legallicier B, Moulin B, et al. The Spectrum of Kidney Pathology in B-Cell Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A 25-Year Multicenter Experience. Rastaldi MP, ed. PLoS ONE. (2015). 10:e0119156. doi: 10.1371/journal.pone.0119156
25. Corlu L, Rioux-Leclercq N, Ganard M, Decaux O, Houot R, Vigneau C. Renal dysfunction in patients with direct infiltration by B-Cell Lymphoma. Kidney Int Rep. (2019) 4:688–697. doi: 10.1016/j.ekir.2019.02.008
26. Obrador GT, Price B, O'Meara Y, Salant DJ. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol. (1997) 8:1348–54. doi: 10.1681/ASN.V881348
27. Junglee N, Shrikanth S, Seale J. Rapidly progressive renal failure due to chronic lymphocytic leukemia—response to chlorambucil. Indian J Nephrol. (2012) 22:217. doi: 10.4103/0971-4065.98766
28. Ferreira AC, Brum S, Carvalho D, Pataca I, Carvalho F, Santos MC, et al. Renal dysfunction due to leukemic infiltration of kidneys in a case of chronic lymphocytic leukemia. Hemodialysis Int. (2010) 14:87–90. doi: 10.1111/j.1542-4758.2009.00395.x
29. Norris HJ, Wiener J. The renal lesions in leukemia. Am J Med Sci. (1961) 241:512–8. doi: 10.1097/00000441-196104000-00016
30. Sangala N, Dewdney A, Marley N, Cranfield T, Venkat-Raman G. Progressive renal failure due to renal infiltration by BK polyomavirus and leukaemic cells: which is the culprit? Clinical Kidney J. (2011) 4:46–8. doi: 10.1093/ndtplus/sfq193
31. Rockx MA, Rizkalla K, Clark WF. Acute renal failure and chronic lymphocytic leukaemia. Nephrol Dial Transplant. (2007) 23:770–1. doi: 10.1093/ndt/gfm617
32. Tam CS, Brown JR, Kahl BS, Ghia P, Giannopoulos K, Jurczak W, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. (2022) 23:1031–43. doi: 10.1016/S1470-2045(22)00293-5
33. Xu W, Wang YH, Fan L, et al. Prognostic significance of serum immunoglobulin paraprotein in patients with chronic lymphocytic leukemia. Leukemia Res. (2011) 35:1060–5. doi: 10.1016/j.leukres.2010.12.005
34. Tsai HT, Caporaso NE, Kyle RA. Evidence of serum immunoglobulin abnormalities up to 9.8 years before diagnosis of chronic lymphocytic leukemia: a prospective study. Blood. (2009) 114:4928–32. doi: 10.1182/blood-2009-08-237651
35. Beaume A, Brizard A, Dreyfus B, Preud'homme JL. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B-cell chronic lymphocytic leukemia. Blood. (1994) 84:1216–9.
36. Mozas P, Pineyroa JA, Nadeu F, Magnano L, Rivero A, Rivas-Delgado A, et al. Serum monoclonal component in chronic lymphocytic leukemia: baseline correlations and prognostic impact. Haematol. (2020) 106:1754–7. doi: 10.3324/haematol.2020.263228
37. Canet E, Zafrani L, Lambert J, Thieblemont C, Galicier L, Schnell D, et al. Acute Kidney Injury in Patients with Newly Diagnosed High-Grade Hematological Malignancies: Impact on Remission and Survival. PLoS ONE. (2013) 8:e55870. doi: 10.1371/journal.pone.0055870
38. Strati P, Chaffee KG, Achenbach SJ, Slager SL, Leung N, Call TG, et al. Renal insufficiency is an independent prognostic factor in patients with chronic lymphocytic leukemia. Haematologica. (2017) 102:e22–5. doi: 10.3324/haematol.2016.150706
39. Comerma-Coma M. Reversible renal failure due to specific infiltration of the kidney in chronic lymphocytic leukaemia. Nephrol Dialysis Transplant. (1998) 13:1550–2. doi: 10.1093/ndt/13.6.1550
40. Pagniez DC, Fenauxaaa P, Delvallez L, Dequiedt P, Gosselin B, Tacquet A. Reversible renal failure due to specific infiltration in chronic lymphocytic leukemia. Am J Med. (1988) 85:579–80. doi: 10.1016/S0002-9343(88)80105-0
Keywords: chronic lymphocytic leukemia/small lymphocytic lymphoma, kidney involvement, infiltration, extranodal, zanubrutinib acute kidney injury as initial manifestation of chronic lymphocytic leukemia/small lymphocytic lymphoma, paraprotein
Citation: Bender ST, Zeremski V, Wiech T, Mertens PR and Gross C (2023) Case report: Acute kidney injury as the initial manifestation of chronic lymphocytic leukemia/small lymphocytic lymphoma. Front. Med. 10:1279005. doi: 10.3389/fmed.2023.1279005
Received: 17 August 2023; Accepted: 20 September 2023;
Published: 20 October 2023.
Edited by:
Sergey Brodsky, Ohio State University Hospital, United StatesReviewed by:
Michael Peres, Centre Hospitalier Universitaire de Nancy, FranceCamille Cohen, Assistance Publique Hopitaux De Paris, France
Copyright © 2023 Bender, Zeremski, Wiech, Mertens and Gross. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sascha T. Bender, c2FzY2hhLmJlbmRlckBtZWQub3ZndS5kZQ==
 Sascha T. Bender
Sascha T. Bender Vanja Zeremski
Vanja Zeremski Thorsten Wiech
Thorsten Wiech Peter R. Mertens
Peter R. Mertens Christian Gross
Christian Gross