- 1Department of Cardiology, University Hospital of Split, Split, Croatia
- 2Wirral University Teaching Hospital, Wirral, United Kingdom
- 3Department of Research in Biomedicine and Health, Center for Evidence-Based Medicine, University of Split School of Medicine, Split, Croatia
- 4Department of Cardiology, Dubrava University Hospital, Zagreb, Croatia
Introduction: Non-pharmacological invasive interventions in cardiology are complex and often inadequately reported. Template for Intervention Description and Replication (TIDieR) checklist and guide were developed to aid reporting and assessment of non-pharmacological interventions. The aim of our study was to assess the completeness of describing invasive cardiology interventions in clinical trials at the level of trial registration and corresponding journal article publication.
Methodology: We searched for clinical trials in invasive cardiology registered in Clinicaltrials.gov and corresponding journal publications. We used the 10-item TIDieR checklist for registries and 12-item checklist for journal publications.
Results: Out of 7,017 registry items retrieved by our search, 301 items were included in the analysis. The search for corresponding published articles yielded 192 journal publications. The majority of trials were funded by the industry and were medical device trials. The median number of reported TIDieR items was 4.5 (95% CI 4.49–4.51) out of 10, and while the corresponding journal articles reported 6.5 (95% CI 6.0–6.5) out of 12 TIDieR items.
Conclusion: Registration and reporting of invasive cardiology trials is often incomplete and adequate detailed description of the interventions is not provided. TIDieR checklist is an important tool which should be used to ensure rigorous reporting of non-pharmacological interventions in cardiology.
1. Introduction
Non-pharmacological invasive interventions in medicine, such as those in invasive cardiology procedures, are often complex. Their development, adoption, and assessment of their efficacy are challenged by different factors (1). These also include reporting of trials of such interventions, so that the reports ensure adequate presentation of randomized controlled trials (RCT) as minimally biased, highly reliable sources of evidence (2, 3).
The development and evaluation of non-pharmacological interventions have phases that importantly differ from those for pharmacological interventions. In pharmacological research, innovation is tightly controlled in a series of processes, and the majority of those are conducted before the drug is approved for broad human use, that is, its design and adoption are separated (4). For non-pharmacological interventions, innovation of a procedure continues as it is adopted into practice, in stages, as described in the Idea, Development, Exploration, Assessment and Long-term study (IDEAL) Framework and Recommendations in 2009 (5). Any opportunity for formal assessment will thus need to be sought during the early period of adoption of a new surgical operation (4).
Traditionally, those who perform invasive interventions have selected and assessed the outcomes themselves, reporting on short-term clinical outcomes of technical success and harm (6). The reporting of those outcomes is not standardized and often not reproducible, hindering methodological assessment, comparison of interventions and translation to clinical practice (5). Without a complete published description, clinicians and patients cannot reliably implement interventions that are shown to be useful, and other researchers cannot replicate or build on research findings. The quality of description of interventions in publications, however, is remarkably poor (7). Despite calls for surgical and other invasive interventions research to be more rigorous, the overall frequency of RCTs for invasive procedures has been consistently low since the 1970s (8). Since then, reports of non-pharmacological intervention studies still suffer from small sample sizes and reporting bias, with suboptimal registration and lacking the assessment of the quality of intervention (9).
To improve the completeness of reporting of interventions, and non-pharmacological interventions in particular, Template for Intervention Description and Replication (TIDieR) checklist and guide were developed by an international group of experts as an extension of the CONSORT 2010 and the SPIRIT 2013 statement. TIDieR checklist ensures that the most important information is provided about the intervention tested in a trial (7), and is relevant for both the information that has to be registered in a trial registry and in journal publication. Despite the availability of the TIDieR checklist for almost 10 years, the adherence to TIDieR checklist in cardiology interventions is still poor (9) and scientific journals do not require nor endorse the use of this checklist (10).
To our knowledge, no assessment of the completeness of reporting of registry items and publications for non-pharmacological interventions has been conducted for procedures in invasive cardiology. This study aimed the assess the completeness of reporting interventions in clinical trials in invasive cardiology, both at the level of trial registration and corresponding journal publication.
2. Methods
2.1. Study design and setting
This was an observation, cross-sectional study of invasive cardiology clinical trials registered at Clinicaltrials.gov trial registry, as well as matching publications. We defined invasive procedure in cardiology as a complex intervention with deliberate access to the body via an incision or percutaneous puncture, with instrumentation used in addition to the puncture needle (11). We used STROBE (Strengthening the reporting of observational studies in epidemiology) checklist for reporting results (12).
2.2. Sample and inclusion criteria
We developed a search strategy to identify clinical trials in invasive cardiology by searching for completed clinical trials with results, using the following search string: invasive AND (cardiology OR artery OR bypass OR cardiac OR cardiovascular OR coronary OR heart OR myocardial OR stent OR vessel). To be included in the study, registered trials had to: (1) be closed and completed at the time of our search according to Overall Recruitment Status in the registry, (2) with reported study results in Clinicaltrials.gov and (3) had non-pharmacological invasive cardiology intervention noted in one or more following registration fields within the Descriptive Information section of the ClinicalTrials.gov. Studies of unknown status, observational or studies still enrolling participants were excluded. Clinicaltrials.gov was searched on 25 September 2019 (using the classic version of the website)1 and followed up to allow at least 2 years for the publication of trial results in a journal. Trials with registered results were chosen for the study sample because they had higher chance to have a journal publication.
Two authors (VL, HA) independently screened the retrieved items. There were no disagreements. After screening and identifying invasive cardiology trials registered in Clinicaltrials.gov, two authors (VL, MV) independently searched corresponding publications on 26th May 2023, which were identified by screening the following sources: (1) the Publications subheading under the ClinicalTrials.gov Descriptive Information heading (displayed under Tabular view), (2) PubMed/MEDLINE, and (3) Scopus. The manual search used (1) trial unique identification number, and (2) combination of search terms for each trial: intervention name, condition, study phase, and all names under “investigators” field in Clinicaltrials.gov. If there were more than one corresponding journal publication available, we analyzed the first publication, which presented the results related to the primary outcome.
2.3. Data extraction
MV developed a data charting form, which was reviewed by VL and AM. VL and HA independently extracted data for the following items: NCT number, title, acronym (where available), study type, status, study results, conditions, intervention (where applicable), type of intervention (as provided in the Clinicaltrials.gov) comparator (where applicable), outcome measures, funders, sponsors, locations, participant characteristics (gender, age), study phase, enrolment status, participant size, study design, availability of study documents, study start dates, primary completion dates, availability of the results and results dates.
To evaluate the completeness of reporting invasive cardiology interventions, we adapted the 12-item TIDieR checklist. Checklist used in our study included the items from the TIDieR checklist: Item (1) Brief name; Item (2) Why; Item (3) Materials (we separately checked if the manufacturer of the device (3a), type of device used (3b) and specifics of the device (3c) were provided); Item (4) Procedures (we separately checked if the place of entry of the device (4a), preparation for the procedure (4b) and sequence of procedure steps in intervention (4c) were reported); Item (5) Provider (we separately analyzed if the background and job roles of the providers (5a) as well as prior expertise, training and education and competence assessment (5b) was reported); Item (8) When and How much included expected duration of the intervention and if applicable, a number of sessions or intervals of the intervention; Item (9) Tailoring, how the intervention was adapted for individuals in the study; Item (10) Modifications, how the interventions was modified during the study (including changes in the intervention, not the outcomes measured); Item (11) and (12) how well was the intervention adhered to, either planned or actual. Items 10 (Modifications) and 12 (Actual adherence to the intervention procedure) were not analyzed at the level of the registry, as instructed by the TIDieR guide (6). For published articles, a full TIDieR checklist was used.
Completeness of TIDieR checklist item reporting in the Clinicaltrials.gov registry was assessed by two researchers (VL, HA) independently, and a third author (MV) was consulted to resolve discrepancies. HA is a medical doctor, VL is a cardiology resident and a PhD student and MV is a cardiology resident and a researcher with special interest in evidence based medicine and research methodology. The kappa coefficient between the two assessors ranged from 0.72 to 0.94 for individual TIDieR items. Completeness of reporting TIDieR checklist items in the corresponding publications was assessed by two researchers (VL, MV) independently, with no disagreements between the researchers.
2.4. Data analysis
The overall completeness of reporting of interventions was measured as the median of TIDieR checklist items reported. One point was given for complete compliance and no points were given for noncompliance; for checklist items (3) Materials, (4) Procedures, (5) Providers, one point was given if all details were reported and 0.5 points were given for partial reporting if at least half of the subitems were reported, following the methodology from study conducted by Palmer et al. (11). Items 9 (Tailoring), 10 (Modifications), 11 (Planned adherence to the intervention procedure) and 12 (Actual adherence to the intervention procedure) were considered noncompliant unless they were not reported unnecessary or not required by the study. Inadequate reporting for other checklist items were considered non-reporting. Statistical analysis was conducted using MedCalc Statistical Software version 14.8.1 (MedCalc Software, Ostend, Belgium).2 Descriptive statistics were used to present the collected data. Categorical variables were presented as frequencies, absolute values or percentages and continuous variables as mean or median values with 95% confidence intervals, depending on the distribution of the data.
3. Results
The search of Clinicaltrials.gov retrieved 7,017 results (Figure 1). After screening of titles and descriptions of retrieved items, 6,716 were excluded (659 were not reporting invasive procedures, 107 were not interventions in cardiology, 94 were dealing with peripheral artery disease and 36 were dealing with cardiac surgery), leaving 301 items to be included in the analysis. The search for corresponding published articles yielded 192 journal publications.

Figure 1. Study flow diagram for the selection of eligible interventional trials in invasive cardiology.
3.1. General characteristics of registered trials
Characteristics of reported trials are presented in Table 1. The majority of analyzed trials described the interventions in treating arrythmias and conduction disorders (39.8%), followed by coronary artery disease (32.9%), and heart failure (14.3%). The primary purpose for the majority of studies was reported as treatment (80.4%), and the majority of studies were conducted in North America (46.5%). Study types were often open (78.4%) and non-randomized trials (61.4%). The majority of trials were funded by industry (83.4%) and were categorized as device trials (86.7%). There were no studies with more than one registered sponsor in our sample. The majority of interventions were testing devices, and the most registered interventions dealt with arrythmias and conduction disorders, with the most common being arrythmia ablations. The list of most common interventions registered for different conditions is available in the Supplementary Table S1.
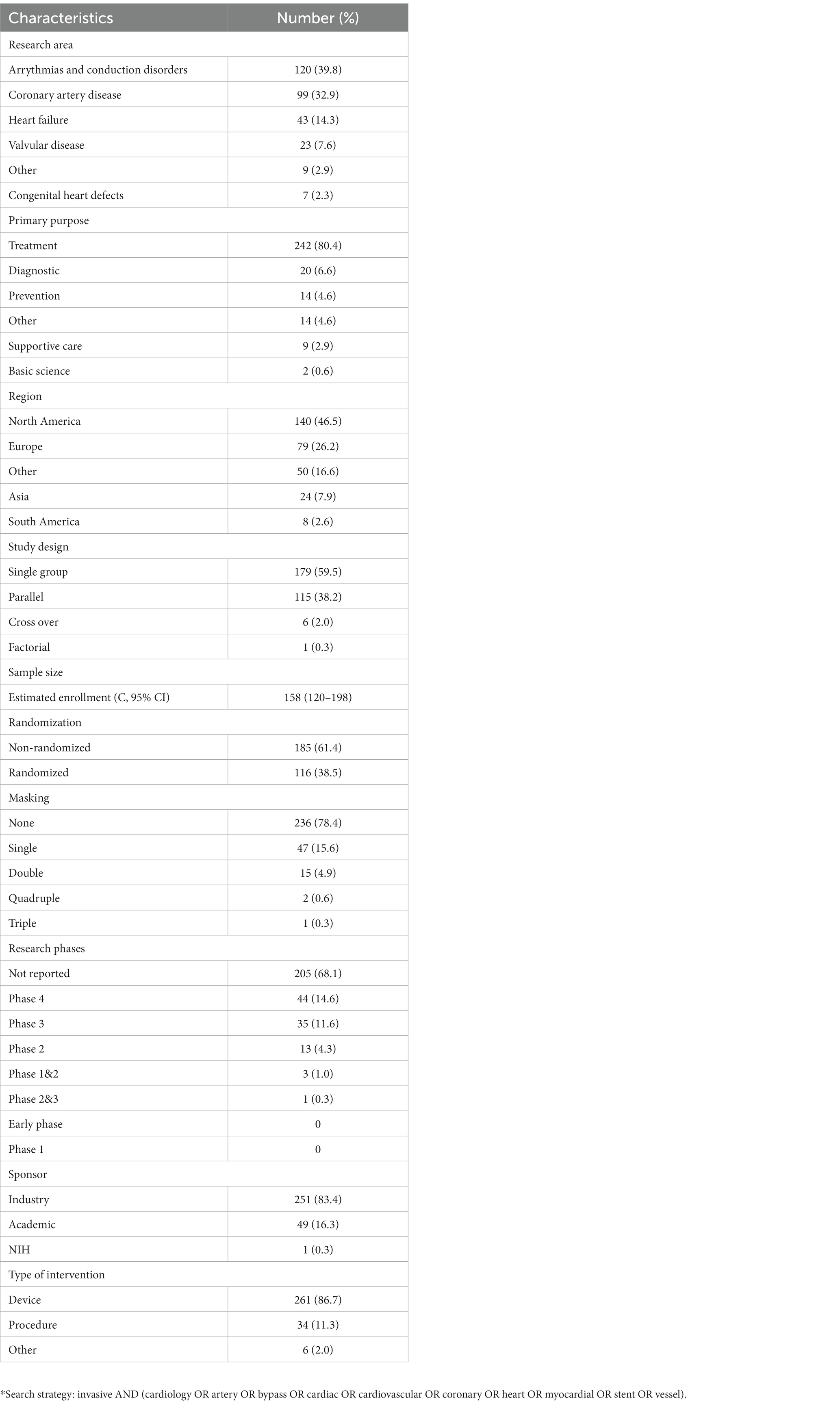
Table 1. Characteristics of the registered trials on invasive cardiology interventions with closed and completed overall recruitment status and with registered results in Clinicaltrials.gov (n = 301).*
3.2. Completeness of intervention descriptions in ClinicalTrials.gov
The trial protocols registered in ClinicalTrials.gov reported a median of only 4.5 (95% CI 4.49–4.51) out of 10 analyzed TIDieR items (ttems 10 and 12 were not analyzed at this step) (Table 2). TIDieR item 1 (Brief name) was present in all 301 trials reviewed. Reporting was also complete (>90%) for TIDieR item 2 (Why), item 3a (Manufacturer of the device) and item 7 (Location). However, the specifics of the device (TIDieR item 3c) were not reported in more than two-thirds of the registered interventions.
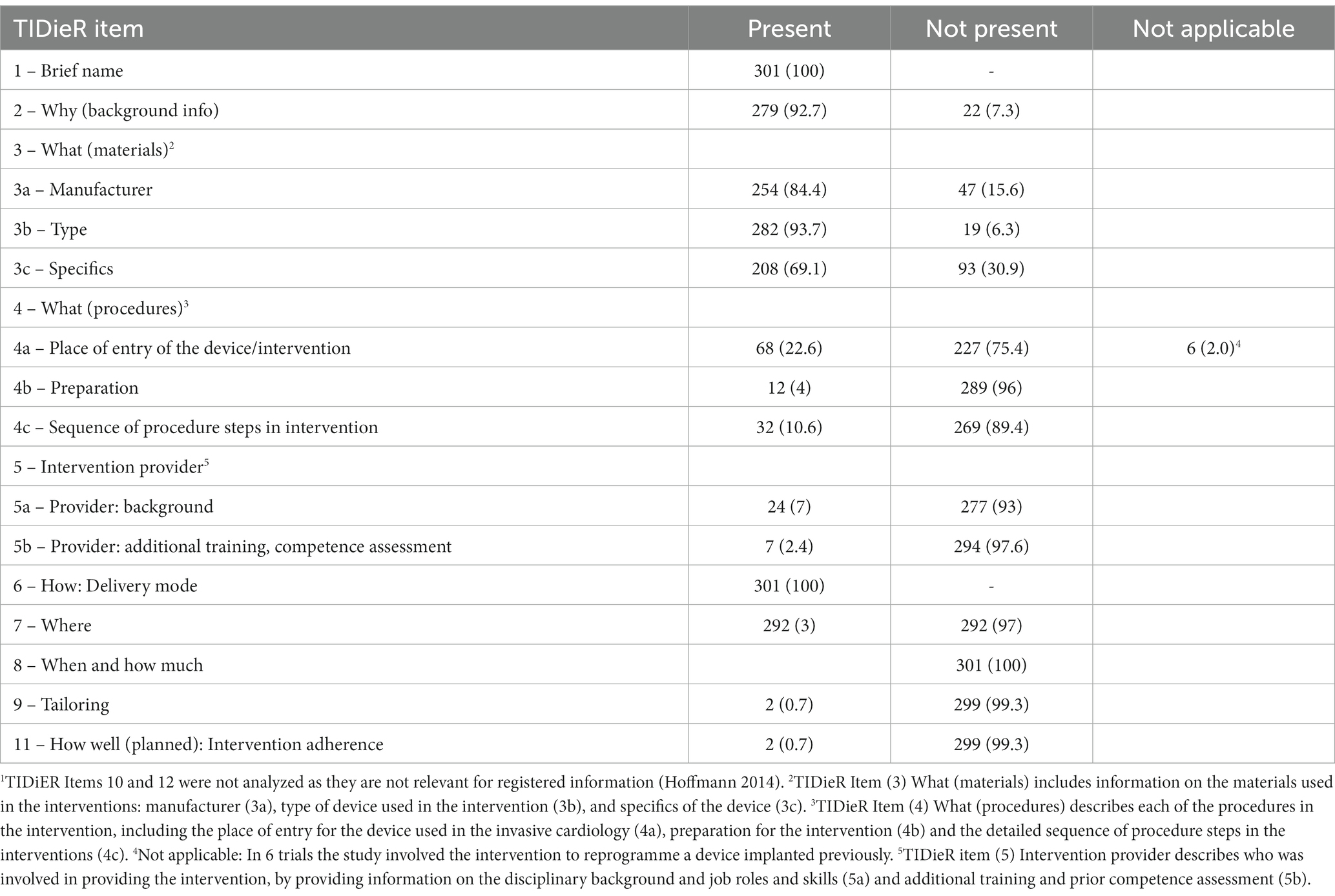
Table 2. The completeness of invasive cardiology intervention descriptions in ClinicalTrials.gov1 (n = 301).
Place of entry of the device (TIDieR item 4a) was not provided in 75.4% of registered trials, information on preparation (TIDieR item 4b) was not present in 96% of trials, and the sequence of procedure (TIDieR item 4c) was not present in 89.4% of trials. Details on providers of the intervention and their previous education or training were not reported for the majority of trials (93, and 97.6%, respectively). TIDieR item 8 (When and how much) was not reported for any of the registered trials. TIDieR item 9 (Tailoring) and item 11 (Planned adherence to the intervention procedure) were not reported for the majority of trials (99.3%).
3.3. Completeness of intervention descriptions in corresponding journal articles
Of 301 trials posted to Clinicaltrials.gov, 191 had the results published in journal articles (Table 3). A median of 6.5 (95% CI 6.0–6.5). TIDieR items were reported in the corresponding journal articles. TIDieR items 1 (Brief name), 2 (Background info), 3 (Manufacturer and specifics of the device), and 7 (Location) were reported most often. TIDieR 5 (Intervention provider) was not provided in more than 60% of publications. TIDieR items 8 (Total duration of the intervention), 9 (Tailoring), 10 (Modifications) and 12 (Actual changes described in adherence to the intervention procedure) were not reported in more than 90% of publications.
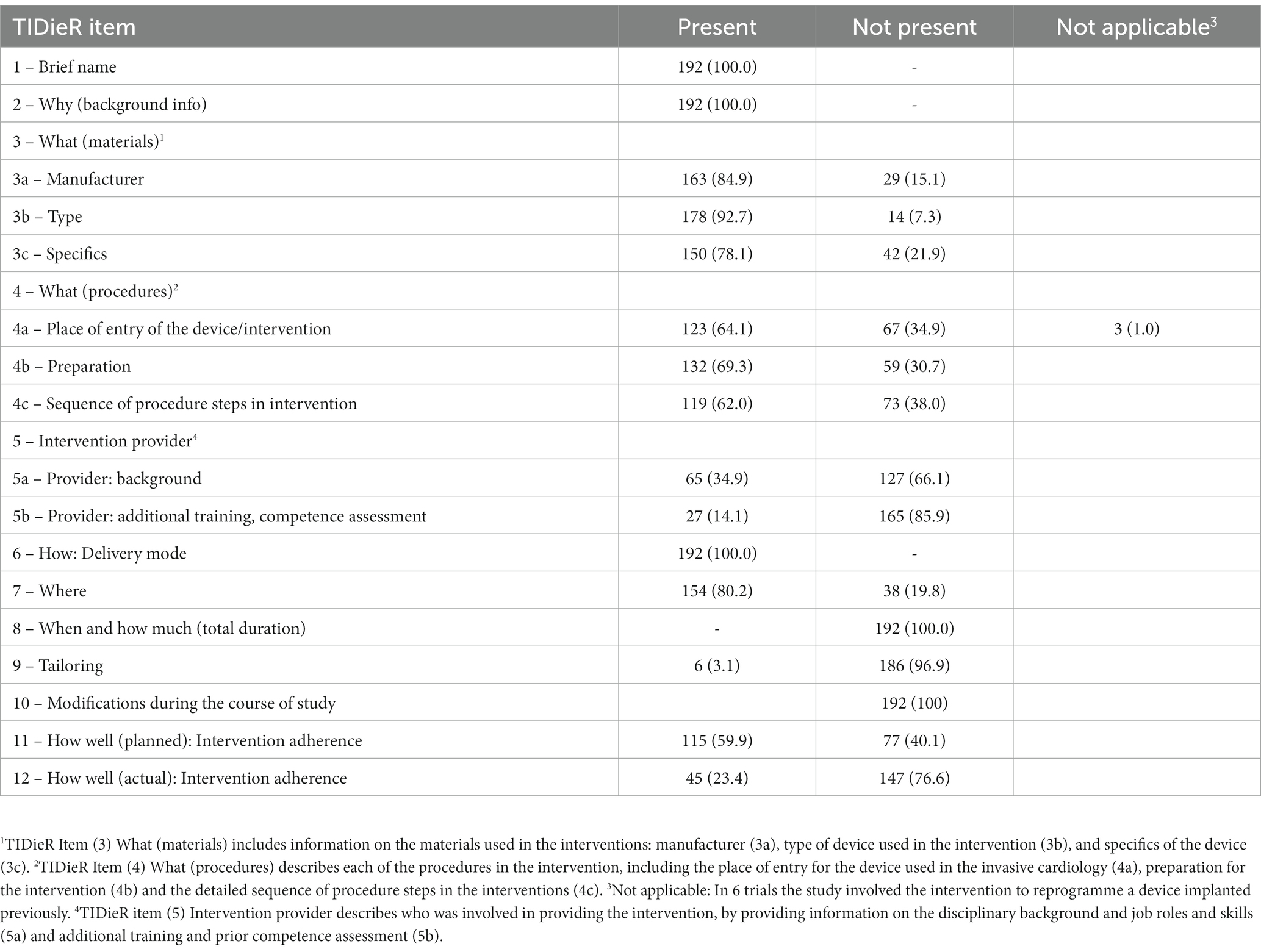
Table 3. The completeness of invasive cardiology intervention descriptions in published articles (n = 192).
3.4. Comparison of intervention descriptions in ClinicalTrials.gov and corresponding journal articles
A comparison of intervention descriptions for trials registered in ClinicalTrials.gov and in corresponding journal articles is presented in Table 4. TIDieR items 1 (Brief name), 2 (Background information) and 7 (Location) were most often present in both Clinicaltrials.gov and in matching publications. The manufacturer of the device (TIDieR 3) was described in both registry and publication for the majority of trials, while the specifics of the device (TIDieR 3), preparation for the procedure and sequence of procedure steps in the intervention (TIDieR 4) were more often reported in published articles. TIDieR item 11 (Planned adherence to the intervention procedure) was reported in both registry and published articles. TIDieR item 5 (Intervention provider), item 8 (When and how much) and item 9 (Tailoring) were mostly unreported both in the registry and the matching publication.
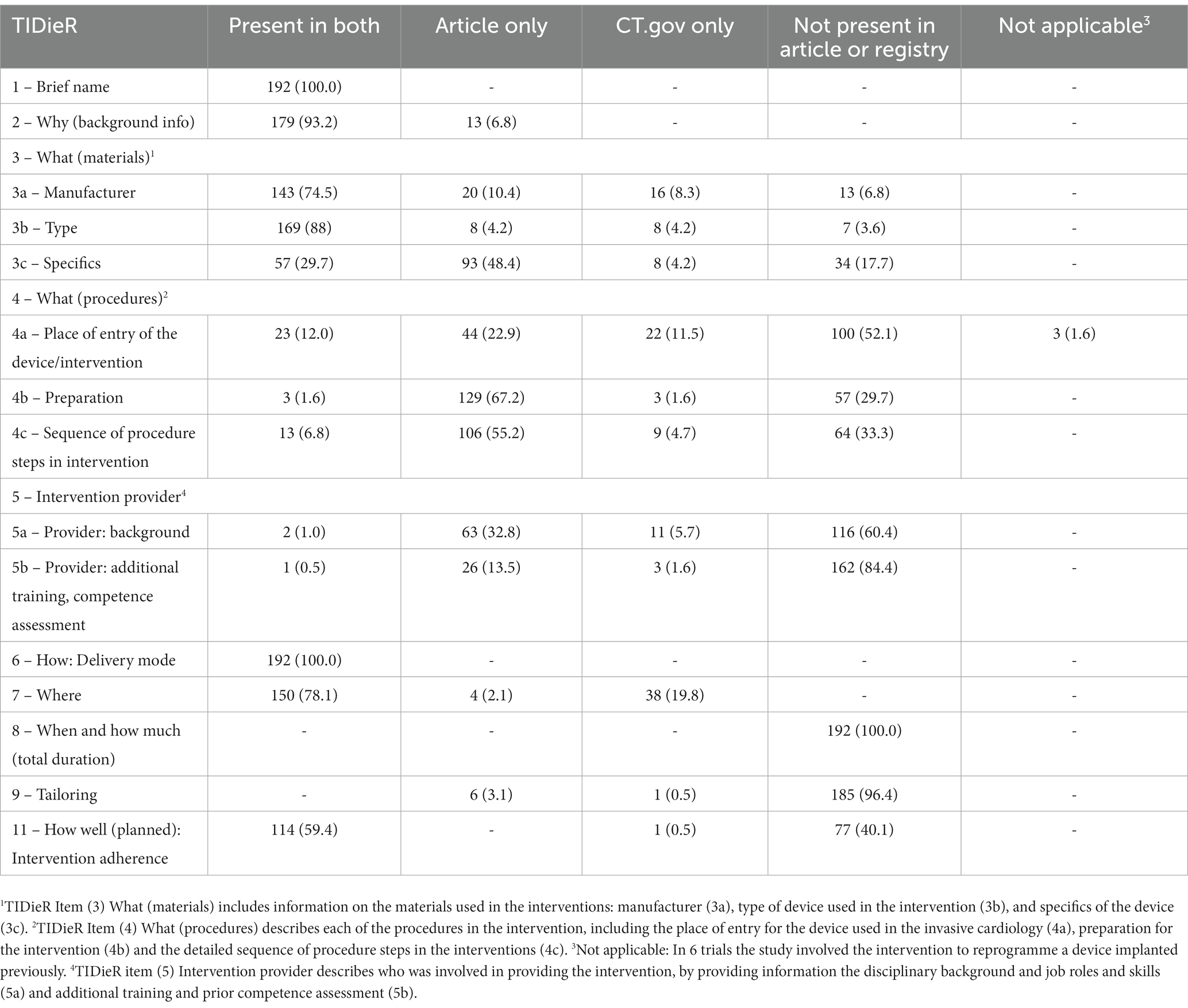
Table 4. Comparison of invasive cardiology intervention descriptions in ClinicalTrials.gov corresponding journal articles (n = 192).
4. Discussion
Our study showed that registration and reporting of invasive cardiology trials are often incomplete, with adequate detailed description of the interventions not provided. Whereas the number of items describing trial intervention increased from the registration to the published data, the information in the published articles often differed from those in matching registry records. This means that it is rather difficult to directly translate new interventions and procedures into clinical cardiology practice.
TIDieR checklist enables precise and structured reporting of complex interventions by facilitating a clear and detailed description of the intervention, regardless of the study design (7, 13). TIDieR checklist can also be used as a quality rating scale (14) and help reviewers during the peer review process (7). TIDieR checklist was originally devised as an extension to the CONSORT reporting guideline, where only one of the 25-item checklists was dedicated to intervention description (15). Low endorsement of the TIDieR checklist is still prevalent, and in a recent call to action, Ryan et al. asked journal editors to update their submission guidelines by making a separate TIDieR checklist mandatory for interventional trials (10). TIDieR checklist enables the implementation and replication of research findings and facilitates transparent reporting of results, supplementing good clinical practice and responsible conduct of research.
TIDieR checklist has previously been used to assess interventions in rehabilitation medicine (16, 17), surgery (18), educational (19) and public health interventions (10), as well as a tool to assess interventions used in systematic reviews (20, 21). In cardiology, a single study looked at adherence to the TIDieR checklist in cardiology journals, which included only higher-impact journals (9). They found higher adherence to the TIDieR checklist (median 8.6 items) than in our study (median 6.5 out of 12 items for published articles). Such differences could be explained by a selective search for high-quality journals which are more likely to adapt and endorse the usage of reporting guidelines (22) and by a higher percentage of pharmacological (drug) interventions in the analyzed sample, which are often better reported (23).
Reporting of non-pharmacological interventions is particularly challenging and the quality of reporting of such trials is lower in comparison to research on pharmacological interventions (24). Quality of reporting of complex interventions is not improving, despite the endorsement of both CONSORT and TIDieR checklists (25). A potential barrier to detailed reporting of interventions could be the word limit for manuscripts in journals. Analysis of RCTs on non-pharmacological interventions in physical therapy and stroke interventions using the TIDieR checklist yielded results similar to our own (16, 17). A previous analysis of reporting of surgical interventions showed poor adherence to the TIDieR checklist (26). A systematic review of non-pharmacological interventions in Crohn’s disease showed that no studies had coverage of all domains of TIDieR (18). In a recently published study on nonsurgical periodontal therapy, adherence to the TIDieR checklist was also low, with discrepancies between registries and published articles (27).
TIDieR items which were most often reported were items 1 (Brief name), 2 (Why) and 7 (Where), both in the clinical trial registry as well as in matching publications. TIDieR item 4 (Procedures), which was not reported in more than two third of the trials, is essential in invasive cardiology. For example, transcatheter aortic valve replacement (TAVR) is usually done using the trans-femoral approach and for a selected population of patients, alternative routes (such as transaortic or transapical) can be used (28) as the choice of access in TAVR seems to be independently associated with an impact on prognosis, transparent reporting of the place of entry is critical.
Previous training and experience of those conducting the intervention were also underreported (item 5). Invasive cardiologists are expected to perform a number of interventions per year in order to maintain proficiency (29). Higher operator volumes are associated with lower in-hospital mortality (30) and adequate reporting of operators’ experience and previous training is important for translation of evidence to different clinical settings (31).
Item 8 (When and how much) was not reported in the majority of trials, both in the registry or the publications. Shortness of intervention in invasive cardiology is associated with the success rate of the intervention and prolonging the intervention is inversely related to the time that has elapsed since its beginning (32) as well as less periprocedural complications (32, 33).
Items 9 (Tailoring), 10 (Modifications), 11 (Planned adherence to the intervention procedure) and 12 (Actual adherence to the intervention procedure) were considered noncompliant if not reported or not clearly considered unnecessary in the study. This might overestimate the level of incompleteness in both registries and publications, but we consider these items of utmost importance for trials in invasive cardiology, where procedures are often modified according to the individual patients.
Item 9 (Tailoring) was not reported for most of analyzed trials, both in the registry and matching publications, even though it is periprocedurally done in everyday clinical practice, for example by choosing the type and dimensions of artificial heart valve (34) or coronary stent size, which directly impacts success rate and number of adverse events (35). Not reporting preparatory steps for the intervention, which enable tailoring to individual patients, impedes the appropriate application of findings.
Modifications during the course of study (Item 10) were not recorded for the majority of trials in both the registry and in the published articles. Reporting of modification of procedures in non-pharmacological research is vital in clinical research to foster safe and efficient innovation, as new procedures and devices undergo a series of improvements during the development before entering clinical practice (36) and clinical trial registries allow providing additional and updated information.
Despite careful planning, changes in interventions are sometimes necessary. Public health crises, such as the COVID-19 pandemic or the Russian invasion of Ukraine led to changes in delivery of interventions (37, 38) by including in-home study visits, distribution of experimental drugs to participants’ homes or implementing other remote monitoring initiatives (39). Our initial search was conducted before the onset of these crises which could explain, to a degree, poor reporting of modifications during studies.
Adherence to interventions, either planned or actual, was not reported for the majority of trials. Adherence is usually linked to pharmacological trials and different approaches to its measurement have been developed (40). Measuring adherence in non-pharmacological interventions is more complex (41), and adherence models have been developed for educational and behavioral interventions (42). Adherence in non-pharmacological, manual studies, such as surgery and invasive cardiology is evaluated through implementation of operative and procedural checklists (43, 44). Ensuring the use of procedural checklists and reporting could reduce postinterventional complications (45) and perhaps enable better adoption of novel interventions in different clinical settings.
More than 80% of trials in our study were industry sponsored. Clinical trials in invasive cardiology have substantial industry involvement (46). Industry-sponsored research is more likely to be published (47) and report favorable study results (48) and these differences cannot be explained by standard risk of bias assessment alone (49). Low adherence to the TIDieR checklist in industry-funded research could be explained by patent policy, especially in the Clinicaltrials.gov registry. While data published in journals remains the most important for informing clinical practitioners, trial registries are an important source of information as well as they are often the only available source of information for unpublished research (27).
Publish or perish in a known dilemma in medical research and publication of scientific papers before patents can lead to patent rejection (50); patentable inventions can often be published fully after the delay necessary to legally protect intellectual ownership. In our study, more TIDieR items were reported in the published corresponding articles than in Clinicaltrials.gov – a finding similar to the other study involving periodontology intervention trials (27), demonstrating that reporting of interventions improves in publications, but is still below the desired level of completeness and transparency. Updating the entries in clinical trial registries is possible and should be done once the patent application process is complete. Even though the publication of scientific research can be delayed, it should not be postponed indefinitely to protect patentable results (51) and our results show that this is still an issue, even after the publication in the scientific journals.
TIDieR checklist is an important tool to ensure rigorous reporting of non-pharmacological interventions. Trial registries serve as a key regulatory tool, and evidence shows that results are often withheld or incompletely reported. Both Food and Drugs Agency and European Medicines Agency have increased their effort to ensure reporting of results within a year of trial completion (52). In the future, adopted TIDieR checklist could be implemented in the clinical trial registry submission forms, to ensure adequate and complete reporting of results.
4.1. Strengths and limitations
This is the first study, to the best of our knowledge, to assess the completeness of reporting of interventions in invasive cardiology. We used a methodologically robust and well-tested TIDieR checklist (6). The strength of our study is also reflected in independent assessments and extraction of the registries and matching publications. The limitation of our study may be that we did not identify all relevant trials. Our search strategy was based around the term ‘invasive cardiology’, and possibly omitted registered trials that did not provide such wording in the title or the registry text. Our strategy, however, retrieved a whole spectrum of invasive cardiology trials (e.g., valvular disease, coronary disease, pacemakers, and electrophysiology studies). We searched a single clinical trial registry and included registry items with results only, thus narrowing our sample size. Clinicaltrials.gov is the largest public clinical trial database with more than 460 thousand registrations (53). Additionally, while Clinicaltrials.gov registry should provide all necessary information regarding the study, the registry cannot be used as a substitute for a protocol and more information were potentially available in the study protocols, which were not analyzed in this study. Finally, although we used a sensitive search strategy and several databases to retrieve all published articles, there is a possibility that we did not identify all available publications.
5. Conclusion
Reporting of interventions in invasive cardiology registered in Clinicaltrials.gov and published in journal articles is low. Endorsement and full implementation of the TIDieR checklist in registration and journal submission policies and procedure and thorough regulatory reforms is necessary to improve the reporting of interventions and thus advance evidence-based patient care.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://osf.io/bx7sc/.
Ethics statement
We analyzed the data publicly registered in ClinicalTrials.gov and published in publicly available scientific journals and an ethics approval is not required.
Author contributions
VL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Investigation. HA: Data curation, Investigation, Methodology, Writing – review & editing. MV: Conceptualization, Methodology, Supervision, Writing – review & editing, Data curation, Formal analysis, Investigation, Writing – original draft. AM: Data curation, Investigation, Methodology, Writing – review & editing, Conceptualization, Funding acquisition, Resources, Supervision, Formal analysis.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Croatian Science Foundation under grant agreement no. IP-2019-04-4882 (Professionalism in Health: Decision making in practice and research, ProDeM). The funder had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest
AM received grant funding for this research from the Croatian Science Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1276847/full#supplementary-material
Footnotes
1. ^ https://classic.clinicaltrials.gov/
2. ^https://www.medcalc.org; 2016.
References
1. McCulloch, P. Developing appropriate methodology for the study of surgical techniques. J R Soc Med. (2009) 102:51–5. doi: 10.1258/jrsm.2008.080308
2. Glasziou, P, Meats, E, Heneghan, C, and Shepperd, S. What is missing from descriptions of treatment in trials and reviews? BMJ. (2008) 336:1472. doi: 10.1136/bmj.39590.732037.47
3. Dunleavy, L, Collingridge Moore, D, Korfage, I, Payne, S, Walshe, C, and Preston, N. What should we report? Lessons learnt from the development and implementation of serious adverse event reporting procedures in non-pharmacological trials in palliative care. BMC Palliat Care. (2021) 20:19. doi: 10.1186/s12904-021-00714-5
4. Barkun, JS, Aronson, JK, Feldman, LS, Maddern, GJ, Strasberg, SM, Balliol Collaboration, et al. Evaluation and stages of surgical innovations. Lancet. (2009) 374:1089–96. doi: 10.1016/S0140-6736(09)61083-7
5. McCulloch, P, Altman, DG, Campbell, WB, Flum, DR, Glasziou, P, Marshall, JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. (2009) 374:1105–12. doi: 10.1016/S0140-6736(09)61116-8
6. Ergina, PL, Cook, JA, Blazeby, JM, Boutron, I, Clavien, PA, Reeves, BC, et al. Challenges in evaluating surgical innovation. Lancet. (2009) 374:1097–104. doi: 10.1016/S0140-6736(09)61086-2
7. Hoffmann, TC, Glasziou, PP, Boutron, I, Milne, R, Perera, R, Moher, D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014):348. doi: 10.1136/bmj.g1687
8. Solomon, MJ, and McLeod, RS. Clinical studies in surgical journals--have we improved? Dis Colon Rectum. (1993) 36:43–8. doi: 10.1007/BF02050300
9. Palmer, W, Okonya, O, Jellison, S, Horn, J, Harter, Z, Wilkett, M, et al. Intervention reporting of clinical trials published in high-impact cardiology journals: effect of the TIDieR checklist and guide. BMJ Evid Based Med. (2021) 26:91–7. doi: 10.1136/bmjebm-2019-111309
10. Ryan, M, Hoffmann, T, Hofmann, R, and van Sluijs, E. Incomplete reporting of complex interventions: a call to action for journal editors to review their submission guidelines. Trials. (2023) 24:176. doi: 10.1186/s13063-023-07215-1
11. Cousins, S, Blencowe, NS, and Blazeby, JM. What is an invasive procedure? A definition to inform study design, evidence synthesis and research tracking. BMJ Open. (2019) 9:e028576. doi: 10.1136/bmjopen-2018-028576
12. Von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
13. Poduval, S, Ross, J, Pal, K, Newhouse, N, Hamilton, F, and Murray, E. Use of the TIDieR checklist to describe an online structured education programme for type 2 diabetes. Digit Health. (2020) 6:2055207620975647. doi: 10.1177/2055207620975647
14. Yamato, TP, Maher, CG, Saragiotto, BT, Catley, MJ, and Moseley, AM. Rasch analysis suggested that items from the template for intervention description and replication (TIDieR) checklist can be summed to create a score. J Clin Epidemiol. (2018) 101:28–34. doi: 10.1016/j.jclinepi.2018.05.014
15. Schulz, KF, Altman, DG, and Moher, D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
16. Alvarez, G, Cerritelli, F, and Urrutia, G. Using the template for intervention description and replication (TIDieR) as a tool for improving the design and reporting of manual therapy interventions. Man Ther. (2016) 24:85–9. doi: 10.1016/j.math.2016.03.004
17. Hoffmann, TC, and Walker, MF. TIDieR-ing up’ the reporting of interventions in stroke research: the importance of knowing what is in the ‘black box. IJS. (2015):657–8. doi: 10.1111/ijs.12524
18. Tyrell, S, Coates, E, Brown, SR, and Lee, MJ. A systematic review of the quality of reporting of interventions in the surgical treatment of Crohn's anal fistula: an assessment using the TIDiER and Blencowe frameworks. Tech Coloproctol. (2021) 25:359–69. doi: 10.1007/s10151-020-02359-7
19. Phillips, AC, Lewis, LK, McEvoy, MP, Galipeau, J, Glasziou, P, Hammick, M, et al. A systematic review of how studies describe educational interventions for evidence-based practice: stage 1 of the development of a reporting guideline. BMC Med Educ. (2014) 14:152. doi: 10.1186/1472-6920-14-152
20. Pool, J, Maissan, F, de Waele, N, Wittink, H, and Ostelo, R. Completeness of the description of manipulation and mobilisation techniques in randomized controlled trials in neck pain; a review using the TiDieR checklist. Musculoskelet Sci Pract. (2020) 45:102098. doi: 10.1016/j.msksp.2019.102098
21. Cotterill, S, Knowles, S, Martindale, AM, Elvey, R, Howard, S, Coupe, N, et al. Getting messier with TIDieR: embracing context and complexity in intervention reporting. BMC Med Res Methodol. (2018) 18:12. doi: 10.1186/s12874-017-0461-y
22. Shamseer, L, Hopewell, S, Altman, DG, Moher, D, and Schulz, KF. Update on the endorsement of CONSORT by high impact factor journals: a survey of journal "instructions to authors" in 2014. Trials. (2016) 17:301. doi: 10.1186/s13063-016-1408-z
23. Hoffmann, TC, Erueti, C, and Glasziou, PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ. (2013) 347:f3755. doi: 10.1136/bmj.f3755
24. Boutron, I, Tubach, F, Giraudeau, B, and Ravaud, P. Methodological differences in clinical trials evaluating nonpharmacological and pharmacological treatments of hip and knee osteoarthritis. JAMA. (2003) 290:1062–70. doi: 10.1001/jama.290.8.1062
25. Candy, B, Vickerstaff, V, Jones, L, and King, M. Description of complex interventions: analysis of changes in reporting in randomised trials since 2002. Trials. (2018) 19:110. doi: 10.1186/s13063-018-2503-0
26. Anderson, JM, Stafford, A, Jellison, S, and Vassar, M. Intervention reporting of published trials is insufficient in Orthopaedic surgery journals: application of the template for intervention description and replication checklist. Arthrosc Sports Med Rehabil. (2021) 3:e619–27. doi: 10.1016/j.asmr.2020.09.019
27. Stazić, P, Jurić, D, Turić, A, Šošić, A, Marušić, A, and Roguljić, M. Reporting characteristics of nonsurgical periodontal therapy trials registered in ClinicalTrials.gov: an observational study. J Comp Eff Res. (2023) 12:e230058. doi: 10.57264/cer-2023-0058
28. Biasco, L, Ferrari, E, Pedrazzini, G, Faletra, F, Moccetti, T, Petracca, F, et al. Access sites for TAVI: patient selection criteria, technical aspects, and outcomes. Front Cardiovasc Med. (2018) 5:88. doi: 10.3389/fcvm.2018.00088
29. Post, PN, Kuijpers, M, Ebels, T, and Zijlstra, F. The relation between volume and outcome of coronary interventions: a systematic review and meta-analysis. Eur Heart J. (2010) 31:1985–92. doi: 10.1093/eurheartj/ehq151
30. Fanaroff, AC, Zakroysky, P, Wojdyla, D, Kaltenbach, LA, Sherwood, MW, and Roe, MT. Relationship between operator volume and long-term outcomes after percutaneous coronary intervention. Circulation. (2019) 139:458–72. doi: 10.1161/CIRCULATIONAHA.117.033325
31. Reed, GW, Hantz, S, Cunningham, R, Krishnaswamy, A, Ellis, SG, and Khot, U. Operational efficiency and productivity improvement initiatives in a large cardiac catheterization laboratory. JACC Cardiovasc Interv. (2018) 11:329–38. doi: 10.1016/j.jcin.2017.09.025
32. Rempakos, A, Kostantinis, S, Simsek, B, Karacsonyi, J, Choi, JW, and Poommipanit, P. Outcomes of chronic Total occlusion percutaneous coronary intervention after a previous failed attempt. Am J Cardiol. (2023) 193:61–9. doi: 10.1016/j.amjcard.2023.01.045
33. Miura, K, Tanaka, H, Kishi, K, Muramatsu, T, Okada, H, and Oikawa, Y. Impact of timing and treatment strategy on coronary perforation during percutaneous coronary intervention for chronic total occlusion. Am J Cardiol. (2022) 172:26–34. doi: 10.1016/j.amjcard.2022.02.019
34. Francone, M, Budde, RPJ, Bremerich, J, Dacher, JN, Loewe, C, and Wolf, F. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol. (2020) 30:2627–50. doi: 10.1007/s00330-019-06357-8
35. Williams, PD, and Awan, M. Stent selection for percutaneous coronary intervention. Contin Cardiol Educ. (2017) 3. doi: 10.1002/cce2.54
36. Hoffmann, C, Hossaini, S, Cousins, S, Blencowe, N, McNair, AGK, and Blazeby, JM. Reporting modifications in surgical innovation: a systematic scoping review protocol. Int J Surg Protoc. (2021) 25:250–6. doi: 10.29337/ijsp.167
37. Audisio, K, Lia, H, Robinson, NB, Rahouma, M, Soletti, G Jr, and Cancelli, G. Impact of the COVID-19 pandemic on non-COVID-19 clinical trials. J Cardiovasc Dev Dis. (2022) 9:19. doi: 10.3390/jcdd9010019
38. Hazra, A, and Bondarenko, I. Clinical trials can adapt for refugees. Science. (2023) 380:592. doi: 10.1126/science.adh1190
39. European Medicines Agency. (2022). Points to consider on implications of coronavirus disease (COVID-19) on methodological aspects of ongoing clinical trials. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/points-consider-implications-coronavirus-disease-covid-19-methodological-aspects-ongoing-clinical_en-0.pdf (Accessed May, 2023)
40. Lee, JK, Grace, KA, Foster, TG, Crawley, MJ, Erowele, GI, and Sun, HJ. How should we measure medication adherence in clinical trials and practice? Ther Clin Risk Manag. (2007) 3:685–90.
41. Graham, L, Wright, J, Walwyn, R, Russell, AM, Bryant, L, and Farrin, A. Measurement of adherence in a randomised controlled trial of a complex intervention: supported self-management for adults with learning disability and type 2 diabetes. BMC Med Res Methodol. (2016) 16:132. doi: 10.1186/s12874-016-0236-x
42. Borrelli, B. The assessment, monitoring, and enhancement of treatment Fidelity in public health clinical trials. J Public Health Dent. (2011) 71:S52–63. doi: 10.1111/j.1752-7325.2011.00233.x
43. Ferorelli, D, Benevento, M, Vimercati, L, Spagnolo, L, De Maria, L, and Caputi, A. Improving healthcare Workers' adherence to surgical safety checklist: the impact of a short training. Front Public Health. (2022) 9:732707. doi: 10.3389/fpubh.2021.732707
44. Lindsay, AC, Bishop, J, Harron, K, Davies, S, and Haxby, E. Use of a safe procedure checklist in the cardiac catheterisation laboratory. BMJ Open Qual. (2018) 7:e000074. doi: 10.1136/bmjoq-2017-000074
45. Bergs, J, Hellings, J, Cleemput, I, Zurel, Ö, De Troyer, V, and Van Hiel, M. Systematic review and meta-analysis of the effect of the World Health Organization surgical safety checklist on postoperative complications. Br J Surg. (2014) 101:150–8. doi: 10.1002/bjs.9381
46. Smilowitz, NR, Ferguson, JJ, and Weisz, G. Controversies surrounding authorship of manuscripts by industry employees: academic and industry perspectives. EuroIntervention. (2018) 13:1967–74. doi: 10.4244/EIJ-D-16-00918
47. Goldacre, B, DeVito, NJ, Heneghan, C, Irving, F, Bacon, S, and Fleminger, J. Compliance with requirement to report results on the EU clinical trials register: cohort study and web resource. BMJ. (2018) 362:k3218. doi: 10.1136/bmj.k3218
48. Gazendam, AM, Slawaska-Eng, D, Nucci, N, Bhatt, O, and Ghert, M. The impact of industry funding on randomized controlled trials of biologic therapies. Medicines (Basel). (2022) 9:18. doi: 10.3390/medicines9030018
49. Lundh, A, Lexchin, J, Mintzes, B, Schroll, JB, and Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. (2017) 2:MR000033. doi: 10.1002/14651858.MR000033.pub3
50. The patenting versus publishing dilemma. Nat Commun. (2023) 14:1562. doi: 10.1038/s41467-023-37243-z
51. National Academy of Sciences, National Academy of engineering (US) and Institute of Medicine (US) committee on science, engineering, and public policy. On being a scientist: A guide to responsible conduct in research. 3rd ed. Washington, DC: National Academies Press (US) (2009).
52. Clinicaltrials.gov. FDAAA 801 and the Final Rule. (2023). Available at: https://clinicaltrials.gov/ct2/manage-recs/fdaaa (Accessed May, 2023)
53. U.S. National Library of Medicine. ClinicalTrials.gov. (2023). Available at: https://classic.clinicaltrials.gov/ct2/home (Accessed May, 2023)
Keywords: cardiology, TIDieR checklist, ClinicalTrials.gov, quality of reporting, non-pharmaceutic interventions
Citation: Lišnić V, Ashraf H, Viđak M and Marušić A (2023) Completeness of intervention description in invasive cardiology trials: an observational study of ClinicalTrials.gov registry and corresponding publications. Front. Med. 10:1276847. doi: 10.3389/fmed.2023.1276847
Edited by:
Lise Aagaard, Independent Researcher, Copenhagen, DenmarkReviewed by:
Tony Tse, National Institutes of Health (NIH), United StatesPerrine Janiaud, University Hospital of Basel, Switzerland
Copyright © 2023 Lišnić, Ashraf, Viđak and Marušić. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marin Viđak, bWFyaW4udmlkamFrQGdtYWlsLmNvbQ==
†These authors share senior authorship
 Viktoria Lišnić
Viktoria Lišnić Hishaam Ashraf
Hishaam Ashraf Marin Viđak
Marin Viđak Ana Marušić3†
Ana Marušić3†