
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 November 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1276502
 Alessandra Sborgia1†
Alessandra Sborgia1† Giacomo Boscia1,2
Giacomo Boscia1,2 Alfredo Niro3*†
Alfredo Niro3*† Luca Landini1
Luca Landini1 Valentina Pastore1
Valentina Pastore1 Valeria Albano1
Valeria Albano1 Marina Piepoli1
Marina Piepoli1 Rossella Donghia4
Rossella Donghia4 Stefano Dore5
Stefano Dore5 Pasquale Viggiano1
Pasquale Viggiano1 Rosa Buonamassa1
Rosa Buonamassa1 Camilla Di Pardo1
Camilla Di Pardo1 Teresa Molfetta1
Teresa Molfetta1 Eye Clinic Research Group1
Eye Clinic Research Group1 Marco Coassin6
Marco Coassin6 Roberto Dell’Omo7
Roberto Dell’Omo7 Francesco Boscia1
Francesco Boscia1 Giovanni Alessio1
Giovanni Alessio1 Giancarlo Sborgia1†
Giancarlo Sborgia1†Introduction: Inverted Internal Limiting Membrane (ILM)-flap technique demonstrated its effectiveness, in terms of anatomical closure rate and visual acuity recovery for high myopic macular holes. We evaluated macular function after a successful inverted ILM-flap for macular holes in high myopic eyes (hMMH) using microperimetry to predict visual prognosis.
Methods: A retrospective study on 23 eyes of 23 patients after surgical closure of hMMH, was performed. All patients underwent inverted ILM-flap and gas tamponade. Cataract surgery was performed in phakic eyes. Study outcomes including best-corrected visual acuity (BCVA), retinal sensitivity (RS) at central 12°, central retinal sensitivity (CRS) at central 4° and mean deviation (MD), and fixation behavior as bivariate contour ellipse area (BCEA, degrees2) measured by microperimetry, were evaluated over 6 months. A mixed-effects model was used to evaluate and compare the repeated measurements of outcomes between phakic and pseudophakic eyes. A regression model was performed to assess the relationship between BCVA at 6 months and independent variables.
Results: Overall mean BCVA improved from 0.98 ± 0.21 logMAR at baseline to 0.47 ± 0.31 logMAR at the last follow-up (p < 0.001). Over 6 months, overall sensitivity measurements improved (RS, p = 0.001; CRS, p < 0.0001; MD, p = 0.03), and the BCEA decreased in dimension, although not significantly (p ≥ 0.05). The mixed model revealed a significantly better effect of inverted ILM-flap combined with cataract surgery on BCVA and CRS in phakic eyes than inverted ILM-flap alone in pseudophakic ones. The regression model revealed a relationship of 6-month BCVA with pre-operative BCVA (β = 0.60, p = 0.02) and RS (β = −0.03, p = 0.01).
Conclusion: The inverted ILM-flap technique significantly improved visual acuity and retinal sensitivity after the hMMH closure, particularly when combined with cataract extraction. Pre-operative visual acuity and retinal sensitivity at central 12° may predict post-surgical visual acuity.
Macular hole (MH) is a known clinical finding in patients with high myopia (1), with a prevalence of 8.5% (2). The age at the onset of MH in highly myopic eyes (hMMH) significantly decreases with the increase of myopic refraction (1).
Several factors, including myopic maculopathy traction (2), epiretinal membrane, the rigidity of the internal limiting membrane (ILM) and retinal vessels, may promote the development of hMMH (3).
Jo et al. (4) recommended surgical intervention when macular traction and visual acuity impairment progresses. However, in a small percentage (6.3%) of cases, hMMH may be asymptomatic and only be revealed by optical coherence tomography (OCT) scans (5).
Surgical procedures on ILM, such as the traditional ILM peeling, autologous transplantation of ILM, and the inverted ILM-flap, have been successfully used to decrease tractional forces (6). In particular, the inverted ILM-flap demonstrated equal and sometimes greater effectiveness in terms of anatomical closure rate and visual acuity recovery when compared to other techniques (7–9).
The analysis of only visual acuity partially reveals the macular function’s complexity. In recent years, there has been an increase in the use of fundus-related microperimetry that can assess macular function by simultaneously imaging the fundus and projecting light stimuli onto a testing point. Furthermore, microperimetry revealed its more sensitive to a macular functional deficit than visual acuity (10). Using microperimetry as a functional assay helped evaluate changes in retinal function among patients with myopic maculopathy. The microperimeric analysis revealed that retinal sensitivity was strongly associated with the retinal microstructural changes according to the severity of myopic degeneration (11).
Microperimetry has provided quantitative measures of macular function, such as retinal sensitivity and fixation behavior, both before and after macular surgery. This has been observed in various conditions, including hMMH (12–17). Furthermore, pre-operative retinal sensitivity at central degrees and fixation behavior have already been demonstrated to predict post-surgical visual acuity for large idiopathic MHs (16).
We evaluated the changes in visual and microperimetric outcomes after the surgical success of the inverted ILM-flap technique for hMMH to predict visual prognosis.
We conducted a retrospective, single-center cohort study on patients who underwent a successful inverted ILM-flap approach for hMMH. All patients were referred to the Ophthalmology Clinic of the University of Bari, Italy. The study was approved by the Institutional Review Board (IRB; date: 09 January 2019, Eye Clinic, Department of Medical Science, Neuroscience and Sense Organs, University of Bari, Bari, Italy) and adhered to the tenets of the Declaration of Helsinki. All participants read and signed written informed consent.
Enrolled subjects were 18 years old or older, with high myopia, defined as axial length greater than 26 mm [measured with Zeiss IOLMaster 500® (SNR > 200)] or a myopic refractive error of ≥ − 6.0 diopters (3, 17), and a feature of closed full-thickness MH after vitrectomy with Inverted Internal Limiting Membrane (ILM)-flap technique, as revealed by OCT scans (18).
Exclusion criteria were amblyopia, corneal disease, a subcortical cataract or cataract of more than 3 nuclear sclerosis or cortical opacity, glaucoma or ocular hypertension, previous vitreoretinal surgery, diabetic retinopathy, retinal vascular disease, age-related macular degeneration, idiopathic or traumatic MHs, myopic foveoschisis with or without foveal detachment, MH complicated by a retinal detachment, and a minimum diameter of MH >1,000 μm, and the presence of a patchy chorioretinal atrophy involving the fovea [diagnosis based on spectral-domain optical coherence tomography (SD-OCT), showing light backscattering and the absence of outer retinal layers around the MH].
Each patient underwent a complete ophthalmic examination, including best corrected visual acuity (BCVA) measurement using ETDRS charts, slit lamp biomicroscopy, indirect ophthalmoscopy, OCT, and microperimetry. BCVA was recorded with Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4 meters. ETDRS values were converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. OCT was performed with Spectralis OCT (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) using Star Scans (six sections, 20, 512 A scan) and vertical and horizontal line (30, 768-A scans) scans passing through the fovea.
Retinal sensitivity and fixation behavior were evaluated by an MP-1 microperimeter (MP-1, Nidek Technologies, Padua, Italy). Retinal sensitivity was measured across a 45-point grid centered on the fovea. Sensitivity was measured using a white stimulus 0.4 degrees in diameter presented for 200 ms against a mesopic background. The threshold at each point was determined using a 4–2 staircase. The “follow-up” feature of the microperimeter was used to obtain measurements at the same retinal sites during overall visits. Mean retinal sensitivity (RS), the mean sensitivity of all 45 loci in the central 12° (1 = 300 μm), and mean central retinal sensitivity (CRS), the mean sensitivity of the central 13 loci (enclosed by a circle with a 4° diameter) were recorded. The software calculated the mean deviation (MD) after comparing the measured retinal sensitivity at the central 12° with a normative database derived from 180 healthy volunteers stratified into six age groups (19). During the sensitivity examination, fixation stability was also recorded (16). To calculate the average eye movement during fixation, BCEA (bivariate contour ellipse area) was used. This involves plotting the position of each fixation on Cartesian axes and determining the area of an ellipse that encloses a given percentage of fixation points. The value of standard deviations of horizontal and vertical eye movements during fixation were used to measure BCEA. We have analyzed BCEA for 68.2, 95.4, and 99.6% of fixation points (20). Each examination was performed before, at months 1,3, and 6 after surgery.
All surgeries were performed by the same well-experienced retinal specialist (GS) under a retrobulbar block (a mixture of 2% Lidocaine and 2% Mepivacaine).
Standard cataract phacoemulsification and intraocular lens implant were performed in phakic eyes at the time of vitrectomy. A 27-Gauge sutureless vitrectomy system was used to perform a core vitrectomy. The vitreous cortex adhering to the retinal surface was removed after injection of an ophthalmic suspension containing 4% triamcinolone acetonide (Vitreal S, Fidia Farmaceutici S.p.a., Abano Terme, Italy) to visualize the vitreous. An intraocular dye composed of soluble lutein, Brilliant Blue, and Trypan Blue (DOUBLEDYNE®, Alfa Instruments Srl, Casoria, Italy) was used to stain the ILM. The pinch and grasp technique achieved ILM peeling of at least two disk diameters around the MMH. If necessary, we adjusted the trocar size to accommodate longer forceps (Pinnacle 360° 25ga fine tip Eckardt forceps, Myopic; Synergetics, Inc., O’Fallon, MO, United States). The ILM was trimmed with the vitrector and inverted to cover the hole. A non-expansile mixture of SF6 at a concentration of 20% was injected at the end of the procedure as a tamponade, and patients were instructed to maintain a facedown position for 3 days after surgery (21).
Statistical analysis was based on all patients included in the study. No formal sample size calculation was performed. Mean and standard deviation for continuous variables and relative frequency for categorical were used. A Friedman’s test was performed on the changes in morfunctional parameters over follow-up. A categorization of the eyes according to the lens status (phakic and pseudophakic) at baseline was performed. A linear mixed model was used to evaluate repeated measurements of BCVA, RS, CRS, MD, and BCEA at each time point within each group and among the groups, and the trajectories of BCVA, RS, CRS, MD, and BCEA.
All statistical tests were performed at the p < 0.05 significance level.
The relationship between BCVA at 6 months and each independent variable was analyzed using the linear regression model. The independent variables included age, sex, lens status, axial length, macular hole size, baseline mean BCVA, RS, CRS, MD, and BCEA. A backward multiple regression model with a stepwise method was performed to assess any predictive factors associated with visual acuity 6 months after surgery (cut-off removal variable, p ≥ 0.10). Multiple regression analyses were performed on variables that correlated significantly (p < 0.05) with postoperative BCVA. The factors with a value of p < 0.05 in the multiple models were considered potential baseline predictors.
All the statistical computations were made using StataCorp, 2015, Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
A total of 26 eyes underwent an inverted ILM-flap approach for hMMH between January 2019 and November 2021. After excluding eyes with an open hMMH after surgery (1 eye), and eyes from patients not willing or able to undergo pre-operative and post-operative microperimetry testing (2 eyes), 23 eyes of 23 patients were recruited for this analysis. The age at surgery ranged from 51 to 79 years. Axial length ranged from 26.02 to 33.29 mm, and MH minimum diameter ranged from 150 μm to 709 μm (Table 1).
All phakic eyes had undergone cataract phacoemulsification and intraocular lens implant at the time of vitrectomy. In all eyes, the gas bubble was significantly reduced at the first follow-up revealing MH closure at OCT scans. All included patients had a month-6 follow-up. No ocular or systemic complications were observed.
Mean BCVA improved from 0.98 ± 0.21 logMAR at baseline to 0.47 ± 0.31 logMAR at 6 months (p < 0.0001; Table 2; Figure 1). All patients had an improvement in visual acuity ranging from 0.1 to 0.9 logMAR just after 1 month. Twenty-one patients had a higher visual acuity at 6 months than baseline; in only two eyes, baseline visual acuity remained stable at last follow-up (Supplementary Figure 1).
The mean RS increased from 11.46 ± 4.91 dB at baseline to 13.29 ± 4.40 dB at 6 months (p = 0.003). The mean CRS improved from 8.40 ± 4.76 dB at baseline to 11.24 ± 4.89 dB at the last follow-up (p = 0.002). MD changed significantly from −7.69 ± 4.64 dB to −5.99 ± 4.28 dB after 6 months from surgery (p = 0.03). The mean value of the BCEA at all different ellipses areas decreased at all time points, but not significantly (BCEA 68.2%, p = 0.18; 95.4%, p = 0.15; 99.6%, p = 0.05; Table 2; Figures 2, 3).
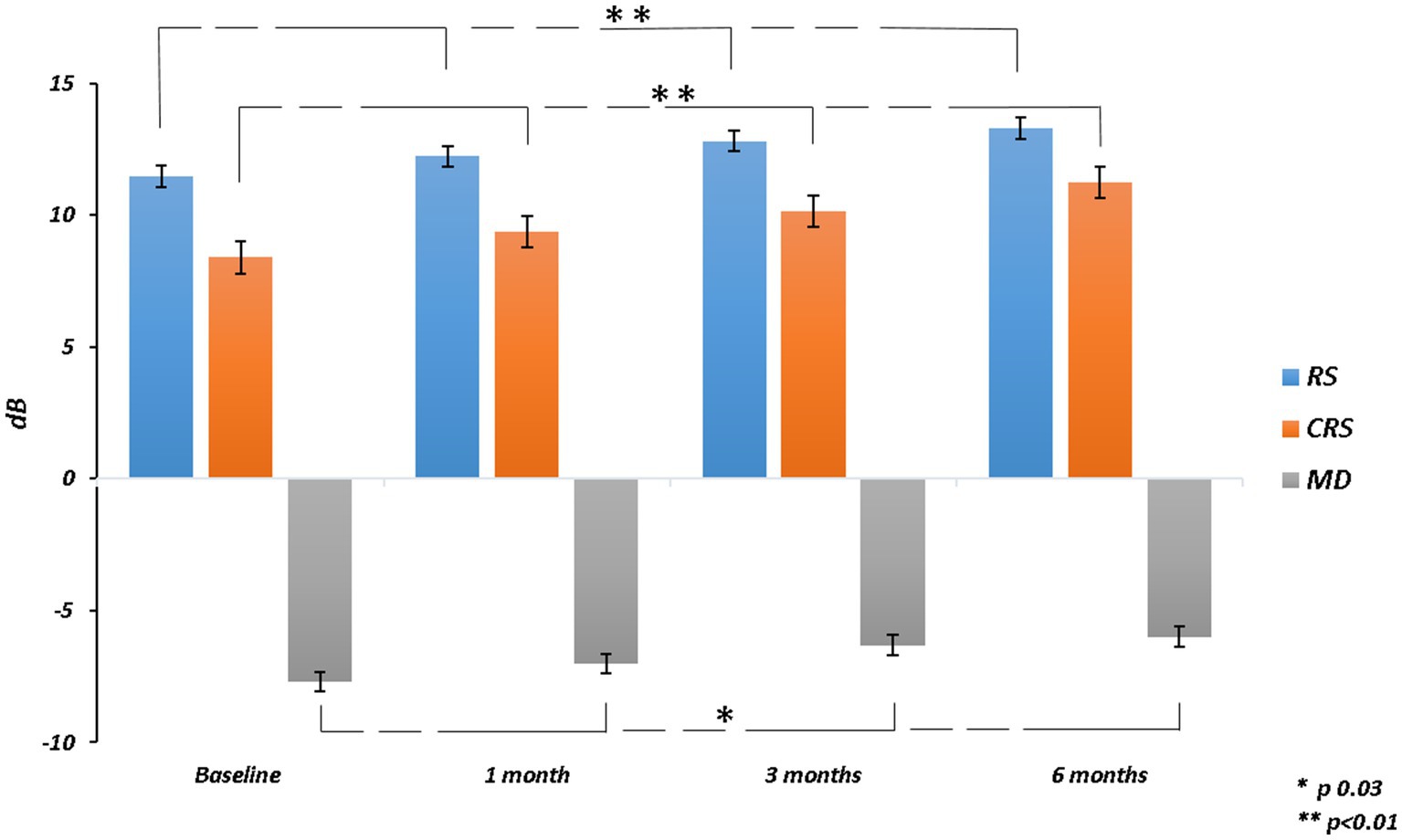
Figure 2. Retinal sensitivity (RS), central retinal sensitivity (CRS) and mean deviation (MD) changes over follow-up.

Figure 3. Bivariate contour ellipse area (BCEA) 68.2%, BCEA 95.4% and BCEA 99.6%BCEA changes over follow-up.
At the last follow-up, 8 (34.8%) patients had worse RS, 7 (30.4%) of these had worse MD than baseline, while only 3 (13.04%) of the 8 patients also had worse CRS. Only 1 (4.3%) patient showed a worsening of CRS alone (Supplementary Figure 2).
An enlargement of BCEA was reported in a few patients (BCEA 68.2%, 7 patients; BCEA 95.4%, 7 patients; BCEA 99.6%, 7 patients) at the last follow-up compared to baseline (Supplementary Figure 3). Representative cases were reported in Figure 4.

Figure 4. Microperimetric MP1 macular sensitivity interpolate maps at baseline and 6 months after surgery in some representative cases. Case 1. (Top Left) At baseline, microperimetry revealed an absolute scotoma (low values in retinal sensitivity; orange/red) within the central 4 degrees (central 13 points) surrounded by a relative ring scotoma (low-medium values in retinal sensitivity; yellow/orange). (Top Right) After 6 months from surgery, both overall and central sensitivity showed improvement despite low central values. The last BCEA showed a mild enlargement when compared to baseline. However, the majority of the fixation points (blue dots) are still clustered within the central degrees. Case 2. (Bottom Left) Results from the microperimetry test indicate a slight improvement in overall sensitivity from the initial test to the most recent follow-up. Notably, there was a marked improvement in the central scotoma. However, at the paracentral degrees, while some loci showed an increase in sensitivity, many others showed a decrease. The analysis of the BCEA revealed a narrowing of the fixation point cloud, predominantly in the outer ellipses at the last follow-up.
At baseline, pseudophakic eyes had worse functional parameters than phakic ones, but only CRS was significantly worse in pseudophakic patients. The average value of each parameter for each group showed improvement from the baseline to the final follow-up. Only BCVA significantly improved in both groups at all time points. BCEA 95.4% had a significant improvement at all visits in phakic group but only at 6 months in psuedophakic eyes. For the other outcomes, including BCEA 68.2%, RS, MD, and CRS, only in the phakic group a significant improvement was reported over follow-up.
The pseudophakic eyes had a worse mean value of all parameters than phakic ones at all follow-up visits. The mixed model revealed that the combined surgical approach in phakic eyes, involving cataract surgery and inverted ILM-flap technique, was significantly different from the only inverted ILM-flap approach in pseudophakic eyes for several outcomes. The effect of the treatment and time on BCVA and CRS was significantly different between the groups. Only the effect of the interaction between treatment and time on BCVA was significantly different (Table 3).
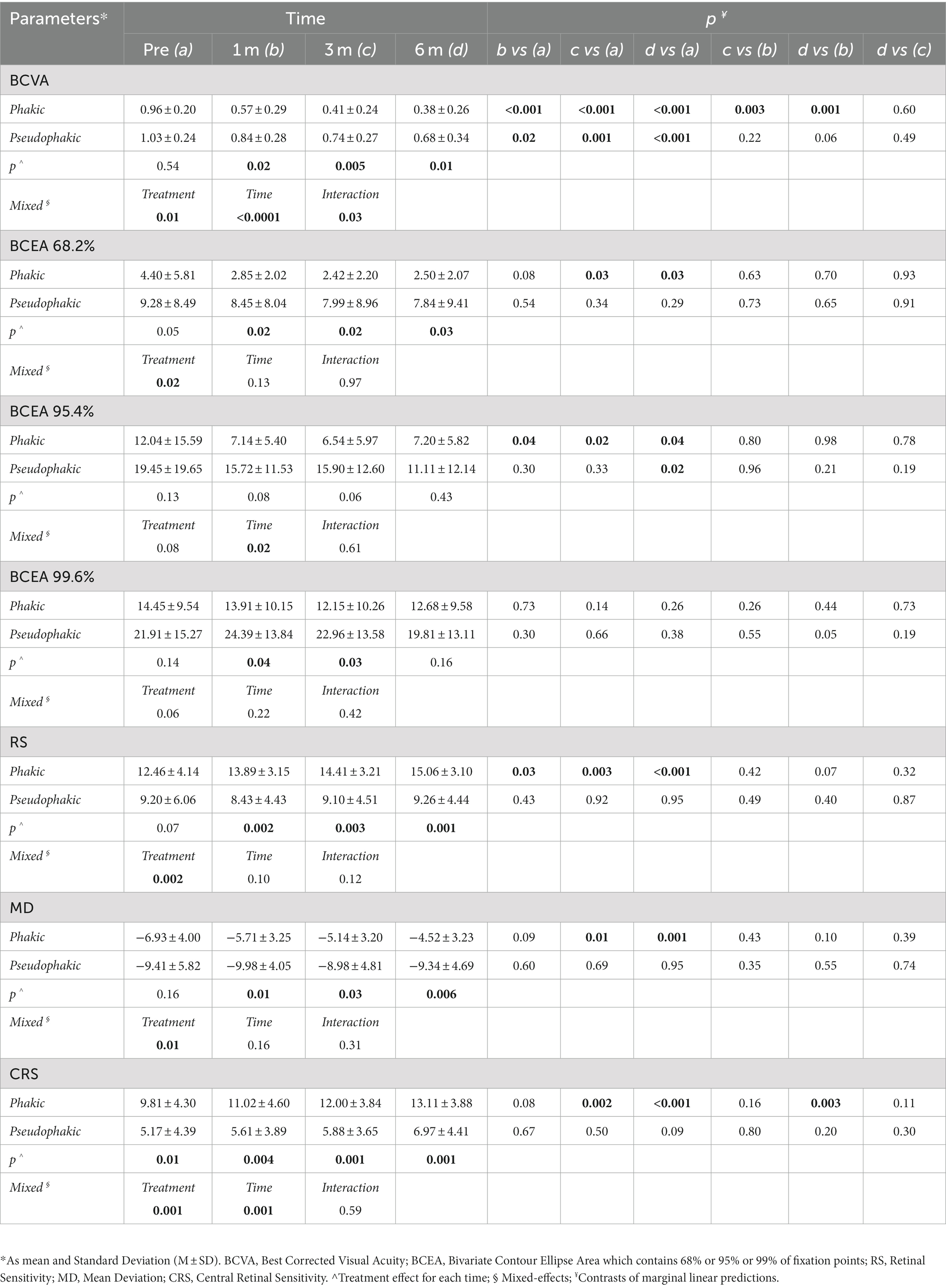
Table 3. Linear mixed model analysis to examine the effect of phakia or pseudophakia on BCVA, BCEA 68.2%, BCEA 95.4%, BCEA 99.6%, RS, MD, and CRS parameters in different times (n = 23).
The simple linear regression model showed that lens status and pre-operative functional parameters, including BCVA, RS, CRS, MD, and BCEA 68.2%, had a significant relationship with 6-month BCVA. Multiple regression models in backward with a stepwise method revealed an independent association of pre-operative BCVA and RS with final BCVA (Table 4; Figure 5).

Table 4. Linear regression model of best corrected visual acuity (BCVA) at 6 months on single variables (A). Multiple linear regression model in backward with stepwise method (B).
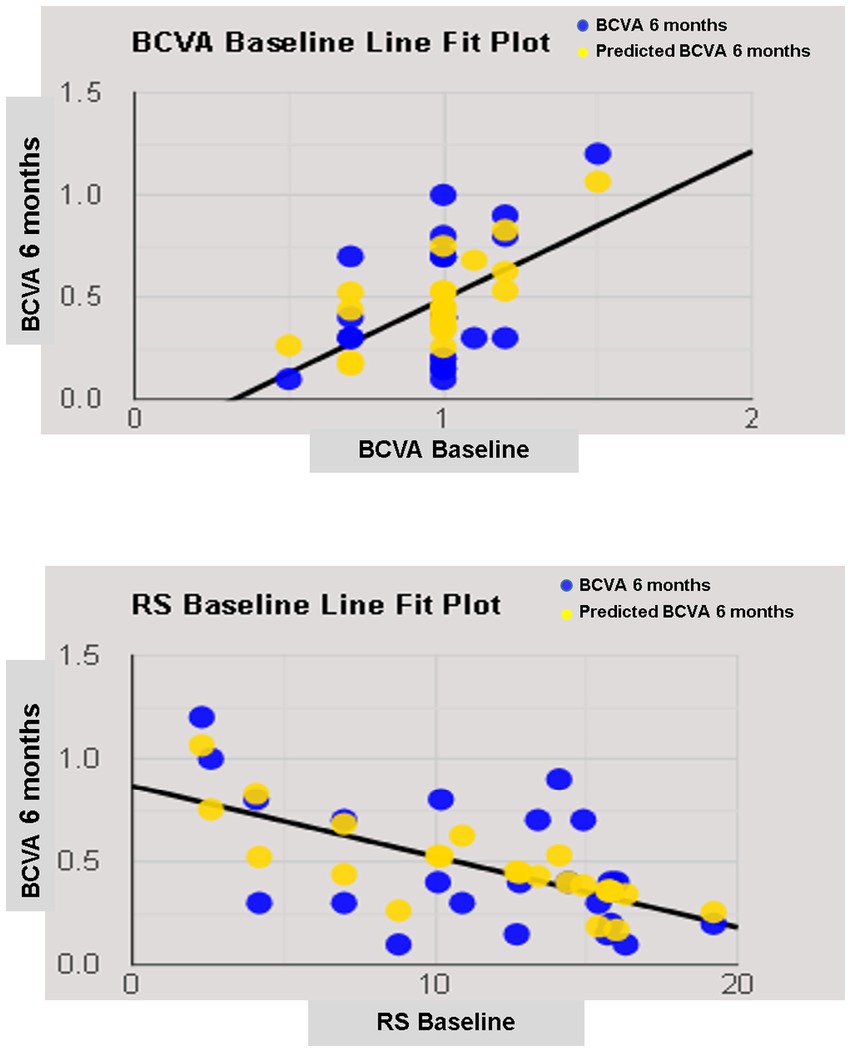
Figure 5. Line fit plot of baseline best corrected visual acuity (BCVA) and retinal sensitivity (RS) as predictive factors of 6-months BCVA.
In this study, we recorded functional outcomes, including visual acuity, retinal sensitivity, and fixation behavior before and after closure of hMMH over a six-month follow-up. In line with the previous studies (6, 21–23), we found that visual acuity and retinal sensitivity significantly improve after ILM-flap inversion. Good functional results confirmed our choice to use an inverted ILM-flap technique to cover the hole rather than fill the hole, as reported when these two surgical approaches were compared regarding recovery of visual acuity and retinal sensitivity (24). The inverted ILM-flap technique is a promising approach to repair hMMH with a high closure rate (6, 25). The flap acts as a scaffold for the activated Müller cells, promoting the hole-healing process at the macular site (26). Furthermore, the gas tamponade also provides the scaffold or creates a barrier between the retinal pigment epithelium and the fluid while enforcing further stabilization in the inverted flap (27).
After only a month following the surgery, there was an improvement in BCVA for all eyes, with an average gain of 0.32 logMAR. It is worth noting that a significant repair of the outer retinal layers and related visual gain can only be seen by the third month post-surgery (23). So, the removal of cataracts in 69.5% of the eyes alongside vitrectomy may have contributed to the early visual recovery observed. Based on the mixed model analysis, the inverted ILM-flap with cataract surgery had a significantly better effect on BCVA and CRS in phakic eyes compared to the inverted ILM-flap alone in pseudophakic ones. However, the lower pre- and post-operative retinal function observed in pseudophakic eyes can be also related to their larger MH size, which was just associated with a worse visual function (16, 28).
After 6 months, 91.3% of the 23 patients showed an improvement in their visual acuity. It is possible that the selection criteria used in the study, which included the absence of retinal detachment and schisis with hMMH and an axial length over 30 mm in only 3 cases, played a role in the higher rate of visual recovery. This has been reported previously (29–31).
The ILM-flap inversion should allow photoreceptors to assume the correct position during the hole healing and improve visual acuity (22). However, the gliosis process, promoted by the inverted flap (32), could limit or delay the restoration of outer retinal layers (30, 33) and related visual recovery (30, 31). So, the analysis of functional changes after the closure of MH could be underestimated by BCVA, and microperimetry might better analyze those changes (23, 34). Previous studies suggested the necessity of analyzing sensitivity at the parafoveal and foveal site, considering the difference in light sensitivity between different retinal sites, in part due to the “masking effect” of the fixation target during microperimetric test (35, 36) and the different age-related decline in sensitivity between different retinal sites (37). Furthermore, CRS may be more valuable than RS in providing topographic information about retinal sensitivity defects (38). At baseline, microperimetry recorded a lower CRS than RS, revealing a deep central scotoma, which corresponds to the neurosensory defect of the macular hole, surrounded by a relative scotoma around the hole (24, 39). After surgery, the mean value of all parameters of retinal sensitivity (RS, CRS, and MD) improved over 6 months. However, we observed a drop in the last CRS and RS in some patients (9/23; 39%) regardless of their visual acuity improvement. If the sensitivity improvement could be partially related to cataract extraction, as previously observed (40), the ILM peeling before flap reversal could cause paracentral scotomata leading to a reduced sensitivity at central 12° (41) due to temporary swelling of the arcuate nerve fiber layer (20, 21), and the gliosis process, promoted by the inverted ILM-flap, could negatively influence the recovery of retinal sensitivity at central 4 (16, 24, 39). Nevertheless, these surgical effects seem to regress over an extended follow-up (16, 39, 41).
Fixation stability is another functional parameter to be considered, probably more than the fixation location because the fixation site could already be naturally relocated out of the fovea (42). BCEA, as a quantitative parameter of fixation behavior, improved with a reduction in the dimension of the cloud of the fixation points at all follow-ups but failed to reach statistical significance, probably due to the small sample size. Tarita-Nistor et al. observed that patients with MH, whose fixation stability improved the most after ILM peeling, showed the best final visual acuity. In contrast, patients with poorer acuity had the slightest improvement in fixation stability (42). The abnormalities of intraretinal architectural morphology due to the macular hole by itself and its closure by ILM-flap inversion could lead to a new fixation behavior that is not always related to visual acuity, as previously suggested (42).
In linear regression analysis, anatomical parameters such as lens status and pre-operative functional parameters such as BCVA, RS, CRS, MD, and BCEA 68.2% were individually correlated with the last visual acuity. Previous papers on idiopathic MH confirmed the relationship between pre-operative lens status, visual acuity, sensitivity, and fixation stability with final visual acuity (16, 22, 28, 43–45). Unlike previous reports (43, 46), the size of the macular hole did not correlated well with postoperative visual acuity, although the small number of patients analyzed may explain this discrepancy, as previously suggested (47).
A recent paper on hMMH treated with ILM peeling confirmed a relationship between pre- and postoperative visual acuity (48). However, a regression analysis revealing functional predictive factors on visual acuity in hMMH treated with an inverted ILM-flap was not previously performed. We observed a predictive role of pre-operative visual acuity and retinal sensitivity on final visual acuity. Pre-operative visual acuity as a prognostic factor of visual recovery was well known and related to the recovery of the outer retinal layers in idiopathic MH treated with different techniques (22, 28, 45). Also, in high myopic foveoschisis with or without MH, better pre-operative BCVA is a predictor of better visual prognosis (21, 49, 50). Better pre-operative BCVA indicates more remarkable preservation of retinal neuronal function; hence, achieving better visual recovery is more likely after surgery.
On the other hand, the predictive role of retinal sensitivity at 12° confirmed previous results on idiopathic MH (16, 51). We suggested that the inverted ILM-flap not always leads to photoreceptor reconstitution at the foveal site, and retinal sensitivity at 12° is less influenced by foveal microstructure recovery after macular hole closure than sensitivity at central 4 (16).
Although the visual acuity (52), retinal sensitivity, and fixation behavior (53) were linked to the status of the retinal layers in the macular hole condition, the occurrence of functional changes with limited or unremarkable anatomical findings on structural OCT revealed that functional tests are required to solve the shortage of morphological ones. If the analysis of neuroretinal structural parameters, including the diameter of the ellipsoid zone and external limiting membrane defect, their thickness and reflectivity before and after surgery, must be standardized, especially when using image analysis software outside of the OCT device (52), also the microperimetry pays a suboptimal level of accuracy. Firstly, the microperimetric test is influenced by the patient’s clinical condition and their individual “learning effect.” Secondly, the properties of the microperimeter used, including the eye-tracker system and the “Follow-up” program, may not allow for the same level of accuracy when analyzing the fovea versus the perifovea, and may result in suboptimal overlap of each tested point between visits, respectively. Additionally, the test duration, the “ceiling effect,” and the size of the light stimulus can all affect the accuracy of the test. Larger stimuli can involve more photoreceptors that converge on a single ganglion cell (16).
It is important to note that this study has some limitations that should be taken into consideration. These include the fact that it was conducted retrospectively and with a relatively small sample size. Additionally, there was no control group included in the study, and the duration of symptoms was not analyzed. The integrity of retinal layers was not assessed, nor was its relationship with visual function. It is important to take into account the aforementioned inherent variability of the microperimetric test as a limitation when conducting studies.
To our knowledge, this is the first microperimetric analysis of functional changes after the closure of the macular hole in high myopic eyes undergoing the inverted ILM-flap technique.
The effectiveness of the inverted ILM flap technique for functional recovery has been confirmed through improved visual acuity and retinal sensitivity, particularly when combined with cataract extraction. The pre-operative visual acuity, and retinal sensitivity detectable by microperimetry, revealed their predictive role on visual acuity after 6 months from successful hMMH closure by inverted ILM-flap.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Eye Clinic, Department of Medical Science, Neuroscience and Sense Organs, University of Bari, Bari, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AS: Conceptualization, Writing – original draft. GB: Data curation, Formal analysis, Writing – review & editing. AN: Conceptualization, Formal analysis, Writing – original draft. LL: Data curation, Writing – review & editing. VP: Data curation, Writing – review & editing. VA: Data curation, Writing – review & editing. MP: Data curation, Writing – review & editing. RD: Conceptualization, Formal analysis, Validation, Writing – review & editing. SD: Data curation, Writing – review & editing. PV: Data curation, Writing – review & editing. RB: Data curation, Writing – review & editing. CP: Data curation, Writing – review & editing. TM: Data curation, Writing – review & editing. Eye Clinic Research Group: Data curation, Writing – review & editing. MC: Writing – review & editing. RD’O: Writing – review & editing. FB: Writing – review & editing. GA: Writing – review & editing. GS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Antonella Guglielmi, Giacomo Scotti, Marida Gaudiomonte, Roberto Semeraro.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the “Centro C.E.R.V.I.- Center for Low Vision Rehabilitation” of the Eye Clinic of Azienda Ospedaliero Universitaria Consorziale Policlinico of Bari, Bari, Italy, for its fundamental role in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1276502/full#supplementary-material
1. Kobayashi, H, Kobayashi, K, and Okinami, S. Macular hole and myopic refraction. Br J Ophthalmol. (2002) 86:1269–73. doi: 10.1136/bjo.86.11.1269
2. Ripandelli, G, Rossi, T, Scarinci, F, Scassa, C, Parisi, V, and Stirpe, M. Macular vitreoretinal interface abnormalities in highly myopic eyes with posterior staphyloma. Retina. (2012) 32:1531–8. doi: 10.1097/IAE.0b013e318255062c
3. Wu, LL, Ho, TC, Yang, CH, and Yang, CM. Vitreo-retinal relationship and post-operative outcome of macular hole repair in eyes with high myopia. Graefes Arch Clin Exp Ophthalmol. (2016) 254:7–14. doi: 10.1007/s00417-015-2986-2
4. Jo, Y, Ikuno, Y, and Nishida, K. Retinoschisis: a predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br J Ophthalmol. (2012) 96:197–200. doi: 10.1136/bjo.2011.203232
5. Coppé, AM, Ripandelli, G, Parisi, V, Varano, M, and Stirpe, M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology. (2005) 112:2103–9. doi: 10.1016/j.ophtha.2005.06.028
6. De Giacinto, C, Pastore, MR, Cirigliano, G, and Tognetto, D. Macular hole in myopic eyes: a narrative review of the current surgical techniques. J Ophthalmol. (2019) 2019:1–10. doi: 10.1155/2019/3230695
7. Hu, XT, Pan, QT, Zheng, JW, and Zhang, ZD. Foveal microstructure and visual outcomes of myopic macular hole surgery with or without the inverted internal limiting membrane flap technique. Br J Ophthalmol. (2019) 103:1495–502. doi: 10.1136/bjophthalmol-2018-313311
8. Bové, AM, Sabaté, S, Gómez-Resa, M, and García-Arumí, J. Anatomical and visual outcomes of inverted internal limiting membrane flap technique versus internal limiting membrane peeling in myopic macular hole without retinal detachment: a preliminary retrospective study. Retina. (2020) 40:233–40. doi: 10.1097/IAE.0000000000002368
9. Mete, M, Alfano, A, Guerriero, M, Prigione, G, Sartore, M, Polito, A, et al. Inverted internal limiting membrane flap technique versus complete internal limiting membrane removal in myopic macular hole surgery: a comparative study. Retina. (2017) 37:1923–30. doi: 10.1097/IAE.0000000000001446
10. Wu, Z, Ayton, LN, Guymer, RH, and Luu, CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. (2014) 121:1612–9. doi: 10.1016/j.ophtha.2014.02.005
11. Park, UC, Yoon, CK, Bae, K, and Lee, EK. Association of Retinal Sensitivity with Optical Coherence Tomography Microstructure in highly myopic patients. Invest Ophthalmol Vis Sci. (2022) 63:13. doi: 10.1167/iovs.63.11.13
12. Rohrschneider, K. Microperimetry in macular disease In: FG Holz and RF Spaide, editors. Medical Retina. Essentials in Ophthalmology. Berlin, Heidelberg: Springer (2007)
13. Sborgia, L, Recchimurzo, N, Niro, A, Sborgia, G, Furino, C, Boscia, F, et al. SD-OCT and microperimetric correlated changes in progressive x-linked retinoschisis after vitrectomy: a case report. Retinal cases & brief reports. (2018) 12:207–11. doi: 10.1097/ICB.0000000000000465
14. Sborgia, G, Recchimurzo, N, Sborgia, L, Niro, A, Sborgia, A, Piepoli, M, et al. Inverted internal limiting membrane-flap technique for optic disk pit maculopathy: morphologic and functional analysis. Retinal cases & brief reports. (2021) 15:31–7. doi: 10.1097/ICB.0000000000000731
15. Nam, SW, Byun, Z, Ham, DI, and Kong, M. Microperimetric evaluation for different methods of epiretinal membrane surgery. BMC Ophthalmol. (2023) 23:295. doi: 10.1186/s12886-023-03056-3
16. Sborgia, G, Niro, A, Sborgia, A, Albano, V, Tritto, T, Sborgia, L, et al. Inverted internal limiting membrane-flap technique for large macular hole: a microperimetric study. Int J Retina Vitr. (2019) 5:44. doi: 10.1186/s40942-019-0195-6
17. Shao, Q, Xia, H, Heussen, FMA, Ouyang, Y, Sun, X, and Fan, Y. Postoperative anatomical and functional outcomes of different stages of high myopia macular hole. BMC Ophthalmol. (2015) 15:93. doi: 10.1186/s12886-015-0098-8
18. Vishal, MY, Babu, N, Kohli, P, Rajendran, A, and Ramasamy, K. Retrospective study of changes in ocular coherence tomography characteristics after failed macular hole surgery and outcomes of fluid-gas exchange for persistent macular hole. Indian J Ophthalmol. (2018) 66:1130–5. doi: 10.4103/ijo.IJO_1119_17
19. Convento, E, and Barbaro, G. Technical insights in the interpretation of automatic microperimetry In: E Midena, editor. Perimetry and the fundus: An introduction to Microperimetry. Thorofare, New Jersey, USA: Slack Inc (2007). 229–37.
20. Castet, E, and Crossland, M. Quantifying eye stability during a fixation task: a review of definitions and methods. Seeing Perceiving. (2012) 25:449–69. doi: 10.1163/187847611X620955
21. Sborgia, G, Boscia, F, Niro, A, Giancipoli, E, D'Amico Ricci, G, Sborgia, A, et al. Morphologic and functional outcomes of different optical coherence tomography patterns of myopic foveoschisis after vitrectomy and inner limiting membrane peeling. Eye (Lond). (2019) 33:1768–75. doi: 10.1038/s41433-019-0490-3
22. Bleidißel, N, Friedrich, J, Klaas, J, Feucht, N, Lohmann, CP, and Maier, M. Inverted internal limiting membrane flap technique in eyes with large idiopathic full-thickness macular hole: long-term functional and morphological outcomes. Graefes Arch Clin Exp Ophthalmol. (2021) 259:1759–71. doi: 10.1007/s00417-021-05082-7
23. Chen, WC, Wang, Y, and Li, XX. Morphologic and functional evaluation before and after successful macular hole surgery using spectral-domain optical coherence tomography combined with microperimetry. Retina. (2012) 32:1733–42. doi: 10.1097/IAE.0b013e318242b81a
24. Cacciamani, A, Gelso, A, Di Nicola, M, Scarinci, F, Ripandelli, G, Costagliola, C, et al. Inverted ILM-flap techniques variants for macular hole surgery: randomized clinical trial to compare retinal sensitivity and fixation stability. Sci Rep. (2020) 10:15832. doi: 10.1038/s41598-020-72774-1
25. Chatziralli, I, Machairoudia, G, Kazantzis, D, Theodossiadis, G, and Theodossiadis, P. Inverted internal limiting membrane flap technique for myopic macular hole: a meta-analysis. Surv Ophthalmol. (2021) 66:771–80. doi: 10.1016/j.survophthal.2021.02.010
26. Shiode, Y, Morizane, Y, Matoba, R, Hirano, M, Doi, S, Toshima, S, et al. The role of inverted internal limiting membrane flap in macular hole closure. Invest Ophthalmol Vis Sci. (2017) 58:4847–55. doi: 10.1167/iovs.17-21756
27. Michalewska, Z, and Nawrocki, J. Macular hole surgery in a patient who cannot maintain facedown positioning. Case Rep Ophthalmol. (2013) 4:1–6. doi: 10.1159/000343701
28. Fallico, M, Jackson, TL, Chronopoulos, A, Hattenbach, LO, Longo, A, Bonfiglio, V, et al. Factors predicting normal visual acuity following anatomically successful macular hole surgery. Acta Ophthalmol. (2021) 99:e324–9. doi: 10.1111/aos.14575
29. Alkabes, M, Pichi, F, Nucci, P, Massaro, D, Dutra Medeiros, M, Corcostegui, B, et al. Anatomical and visual outcomes in high myopic macular hole (HM-MH) without retinal detachment: a review. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. (2014) 252:191–9. doi: 10.1007/s00417-013-2555-5
30. Xu, C, Feng, C, Han, M, He, J, Zhang, R, Yan, T, et al. Inverted internal limiting membrane flap technique for retinal detachment due to macular holes in high myopia with axial length ≥ 30 mm. Sci Rep. (2022) 12:4258. doi: 10.1038/s41598-022-08277-y
31. Theodossiadis, GP, Chatziralli, IP, and Theodossiadis, PG. Inverted internal limiting membrane insertion for macular hole–associated retinal detachment in high myopia. Am J Ophthalmol. (2016) 165:206–7. doi: 10.1016/j.ajo.2016.03.002
32. Oleñik, A, Rios, J, and Mateo, C. Inverted internal limiting membrane flap technique for macular holes in high myopia with axial length ≥30 mm. Retina. (2016) 36:1688–93. doi: 10.1097/IAE.0000000000001010
33. Hayashi, H, and Kuriyama, S. Foveal microstructure in macular holes surgically closed by inverted internal limiting membrane flap technique. Retina. (2014) 34:2444–50. doi: 10.1097/IAE.0000000000000252
34. Ozdemir, H, Karacorlu, M, Senturk, F, Karacorlu, SA, and Uysal, O. Retinal sensitivity and fixation changes 1 year after triamcinolone acetonide assisted internal limiting membrane peeling for macular hole surgery: a MP-1 microperimetric study. Acta Ophthalmol. (2010) 88:e222–7. doi: 10.1111/j.1755-3768.2010.01898.x
35. Fujiwara, A, Shiragami, C, Manabe, S, Izumibata, S, Murata, A, and Shiraga, F. Normal values of retinal sensitivity determined by macular integrity assessment. Nippon Ganka Gakkai Zasshi. (2014) 118:15–21.
36. Denniss, J, and Astle, AT. Central perimetric sensitivity estimates are directly influenced by the fixation target. Ophthalmic Physiol Opt. (2016) 36:453–8. doi: 10.1111/opo.12304
37. Sabates, FN, Vincent, RD, Koulen, P, Sabates, NR, and Gallimore, G. Normative data set identifying properties of the macula across age groups: integration of visual function and retinal structure with microperimetry and spectral-domain optical coherence tomography. Retina. (2011) 31:1294–302. doi: 10.1097/IAE.0b013e3182019be2
38. Chen, FK, Patel, PJ, Xing, W, Bunce, C, Egan, C, Tufail, AT, et al. Test–retest variability of Microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. (2009) 50:3464–72. doi: 10.1167/iovs.08-2926
39. Huang, WY, and Chen, YJ. Changes in retinal sensitivity following inverted internal limiting membrane flap technique for large macular holes. Taiwan J Ophthalmol. (2021) 11:273–9. doi: 10.4103/tjo.tjo_90_20
40. Palkovits, S, Hirnschall, N, and Georgiev, S. Effect of cataract extraction on retinal sensitivity measurements. Ophthalmic Res. (2021) 64:10–4. doi: 10.1159/000507450
41. Clark, A, Balducci, N, Pichi, F, Veronese, C, Morara, M, Torrazza, C, et al. Swelling of the arcuate nerve fiber layer after internal limiting membrane peeling. Retina. (2012) 32:1608–13. doi: 10.1097/IAE.0b013e3182437e86
42. Tarita-Nistor, L, González, EG, Mandelcorn, MS, Lillakas, L, and Steinbach, MJ. Fixation stability, fixation location, and visual acuity after successful macular hole surgery. Invest Ophthalmol Vis Sci. (2009) 50:84–9. doi: 10.1167/iovs.08-2342
43. Roth, M, Schön, N, Jürgens, L, Engineer, D, Kirchhoff, K, Guthoff, R, et al. Frequently assessed and used prognostic factors for outcome after macular hole surgery: which is better? BMC Ophthalmol. (2021) 21:398. doi: 10.1186/s12886-021-02164-2
44. Essex, RW, Hunyor, AP, Moreno-Betancur, M, Yek, JTO, Kingston, ZS, Campbell, WG, et al. The visual outcomes of macular hole surgery: a registry-based study by the Australian and new Zealand Society of Retinal Specialists. Ophthalmol Retin. (2018) 2:1143–51. doi: 10.1016/j.oret.2018.04.022
45. Steel, DH, Donachie, PHJ, Aylward, GW, Laidlaw, DA, Williamson, TH, Yorston, D, et al. Factors affecting anatomical and visual outcome after macular hole surgery: findings from a large prospective UK cohort. Eye. (2021) 35:316–25. doi: 10.1038/s41433-020-0844-x
46. Ullrich, S, Haritoglou, C, Gass, C, Schaumberger, M, Ulbig, MW, and Kampik, A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. (2002) 86:390–3. doi: 10.1136/bjo.86.4.390
47. Amari, F, Ohta, K, Kojima, H, and Yoshimura, N. Predicting visual outcome after macular hole surgery using scanning laser ophthalmoscope microperimetry. Br J Ophthalmol. (2001) 85:96–8. doi: 10.1136/bjo.85.1.96
48. Zhang, K, Yang, X, Wang, Z, Yu, Y, Liu, L, Qi, B, et al. Comparison of air versus perfluoropropane intraocular tamponade combined with vitrectomy for the treatment of macular hole in high myopia. Retina. (2023) 43:42–8. doi: 10.1097/IAE.0000000000003642
49. Lehmann, M, Devin, F, Rothschild, PR, Gaucher, D, Morin, B, Philippakis, E, et al. Preoperative factors influencing visual recovery after vitrectomy for myopic foveoschisis. Retina (Philadelphia, Pa). (2019) 39:594–600. doi: 10.1097/IAE.0000000000001992
50. Zhao, X, Wang, Y, Chen, Y, Anumiharjo, S, Wu, Y, Lian, P, et al. Factors affecting visual prognosis of myopic Foveoschisis after macular buckling. J Ophthalmol. (2022) 2022:1–7. doi: 10.1155/2022/9293347
51. Wang, Z, Qi, Y, Liang, X, Yu, Y, Chen, J, Wang, J, et al. MP-3 measurement of retinal sensitivity in macular hole area and its predictive value on visual prognosis. Int Ophthalmol. (2019) 39:1987–94. doi: 10.1007/s10792-018-1032-x
52. Yang, J, Xia, H, Liu, Y, Wang, X, Yuan, H, Hou, Q, et al. Ellipsoid zone and external limiting membrane-related parameters on spectral domain-optical coherence tomography and their relationships with visual prognosis after successful macular hole surgery. Front Med. (2021) 8:779602. doi: 10.3389/fmed.2021.779602
Keywords: high myopia, macular hole, inverted ILM-flap, microperimetry, retinal sensitivity, fixation behavior, axial length
Citation: Sborgia A, Boscia G, Niro A, Landini L, Pastore V, Albano V, Piepoli M, Donghia R, Dore S, Viggiano P, Buonamassa R, Di Pardo C, Molfetta T, Eye Clinic Research Group, Coassin M, Dell’Omo R, Boscia F, Alessio G and Sborgia G (2023) Microperimetric evaluation and predictive factors of visual recovery after successful inverted internal limiting membrane-flap technique for macular hole in high myopic eyes. Front. Med. 10:1276502. doi: 10.3389/fmed.2023.1276502
Received: 12 August 2023; Accepted: 03 November 2023;
Published: 23 November 2023.
Edited by:
Claudia Fabiani, University of Siena, ItalyReviewed by:
Jiaman Dai, University of Wyoming, United StatesCopyright © 2023 Sborgia, Boscia, Niro, Landini, Pastore, Albano, Piepoli, Donghia, Dore, Viggiano, Buonamassa, Di Pardo, Molfetta, Eye Clinic Research Group, Coassin, Dell’Omo, Boscia, Alessio and Sborgia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfredo Niro, YWxmcmVkLm5pckB0aXNjYWxpLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.