- 1Department of Thoracic Surgery, Qilu Hospital of Shandong University, Jinan, Shandong, China
- 2Department of Breast Surgery, Qilu Hospital of Shandong University, Jinan, Shandong, China
Background: The progression of early stage non-small cell lung cancer (NSCLC) is closely related to epidermal growth factor receptor (EGFR) mutation status. The purpose of this study was to systematically investigate the relationship between EGFR mutation status and demographic, imaging, and ultimately pathologic features in patients with NSCLC.
Methods: A complete literature search was conducted using the PubMed, Web of Science, EMBASE, and Cochrane Library databases to discover articles published by May 15, 2023 that were eligible. The relationship between EGFR mutation status and specific demographic, imaging, and ultimately pathologic features in patients with NSCLC was evaluated using pooled odds ratios (ORs) and their 95% confidence intervals (CIs). The standardized mean difference (SMD) with 95% CIs was the appropriate statistic to summarize standard deviations (SDs) means for continuous variables.
Results: A total of 9 studies with 1789 patients were included in this analysis. The final findings suggested that patients with a greater age, female gender, and non-smoking status would have a relatively higher incidence of EGFR mutations. Additionally, the risk of EGFR mutations increased with larger tumor diameter, tumor imaging presentation of mixed ground glass opacity (mGGO), and tumor pathological findings of minimally invasive adenocarcinoma (MIA) or invasive adenocarcinoma (IAC). Significantly, malignancies presenting as MIA are more likely to contain L858R point mutations (OR = 1.80; 95% CI: 1.04–3.13; p = 0.04) rather than exon 19 deletions (OR = 1.81; 95% CI: 0.95–3.44; p = 0.07).
Conclusion: This meta-analysis showed that imaging parameters and histological classifications of pulmonary nodules may be able to predict stage IA NSCLC genetic changes.
Introduction
Lung cancer has surpassed colonic and prostate cancers as a leading cause of cancer-related deaths globally, due to improvements in early detection and lowered average ages at diagnosis (1). Authoritative research had shown that the development of non-small cell lung cancer (NSCLC) may be influenced by the epidermal growth factor receptor (EGFR) (2). Among the frequent EGFR gene mutations, the L858R point mutations in exon 21 accounts for 40% and the exon 19 deletion mutations (19del) accounts for 45% (3, 4). Both variants have been named sensitive mutations. Additional EGFR mutations (G719X, S768I, L861Q, etc.) are classified as rare mutations. Since treatment with epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) has been demonstrated to dramatically enhance the survival of patients with NSCLC, the identification of EGFR mutations has become a crucial part of NSCLC treatment, particularly for lung adenocarcinoma (LUAD) (5–7).
Currently, EGFR-related studies are focusing on the link between EGFR mutations and NSCLC; nevertheless, this topic is fraught with controversy. The findings of studies by Yotsukura et al. Zhang and et al., which suggest that EGFR mutations are early genomic events in LUAD (8, 9), while Zhu et al. suggest that the frequency of EGFR mutations is not significantly different in minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma (IAC) (10). Also of great interest is whether or not there is a correlation between the imaging characteristics of LUAD and the frequency with which it undergoes EGFR mutations. Wei et al. found no link between EGFR and ground glass opacity (GGO) development, but Ortiz et al. found an increased probability of EGFR mutations when GGO was present in pulmonary nodules (11, 12). As a result, we aimed to perform a systematic review and meta-analysis of the literature to learn more about the correlation between EGFR mutations and the percentage of ground glass opacity and final histological classification in people with NSCLC.
Materials and methods
Protocol and ethics statement
This systematic review and meta-analysis report was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines and statements (13, 14). This protocol for a systematic review and meta-analysis has registered on the INPLASY website1 with the registration number INPLASY202320043.
Databases and search strategy
The literature review was conducted using four online databases: Pubmed, EMBASE, Cochrane Library and Web of Science until 15 May 2023. The medical keywords included in the search strategy were “lung neoplasms,” “carcinoma, non-small-cell lung,” “lung adenocarcinoma,” “carcinoma, squamous cell,” “ground glass opacity,” and “epidermal growth factor receptor.” The MeSH terminology database of the National Center for Biotechnology Information (NCBI) was queried to identify all potential expressions of these terms. For each valid combination of the two Boolean operators (“AND” and “OR”), the keywords and free words were employed. Supplementary Table S1 describes in detail the search strategy for all databases. Two authors (Jianhao Qiu and Zheng Ma) independently evaluated and cross-checked each article. In addition, we manually reviewed the reference lists of eliminated publications to ensure that we did not overlook viable, non-repetitive investigations. Any disagreements between reviewers were resolved through conversation.
Study selection and criteria
The following were the selection criteria: (1) involved adult patients who underwent pneumonectomy or histological analysis of the lung (puncture biopsy or bronchoscopic biopsy); (2) all patients were tested for EGFR mutations (detection methods included polymerase chain reaction, immunohistochemistry, and DNA sequencing); (3) involved a group of patients with EGFR mutant-positive results (mutations sites included all mutant subtypes); (4) involved a group of patients with EGFR mutant-negative results as controls; (5) at least one of the pertinent results of interest was reported (see below); (6) written in English.
The criteria for exclusion were as follows: (1) ineligible article types such as case reports, reviews, conference abstracts, non-comparative studies; (2) no outcome of interest; (3) insufficient or missing data for analysis; (4) written in a language other than English; (5) non-human participants.
Endpoints and outcome measures
The primary outcome of interest was the relationship between EGFR gene mutations and the percentage of GGO and final histological classification in patients’ tumors. Other relevant indicators included mainly demographic characteristics such as age, gender, and smoking history. In addition an analysis of the imaging size of the tumor was made. The GGO was defined as ground glass dense nodules with visible internal vessels and bronchi. If the GGO is composed of ground glass opacity only, it is defined as pure ground glass opacity (pGGO). If the GGO is a combination of ground glass opacity and solid components, it is defined as mixed ground glass opacity (mGGO). The tumor imaging size was defined as the maximum diameter of the tumor on the axial image of a certain slice on the lung window of the thoracic computed tomography (CT).
Data extraction
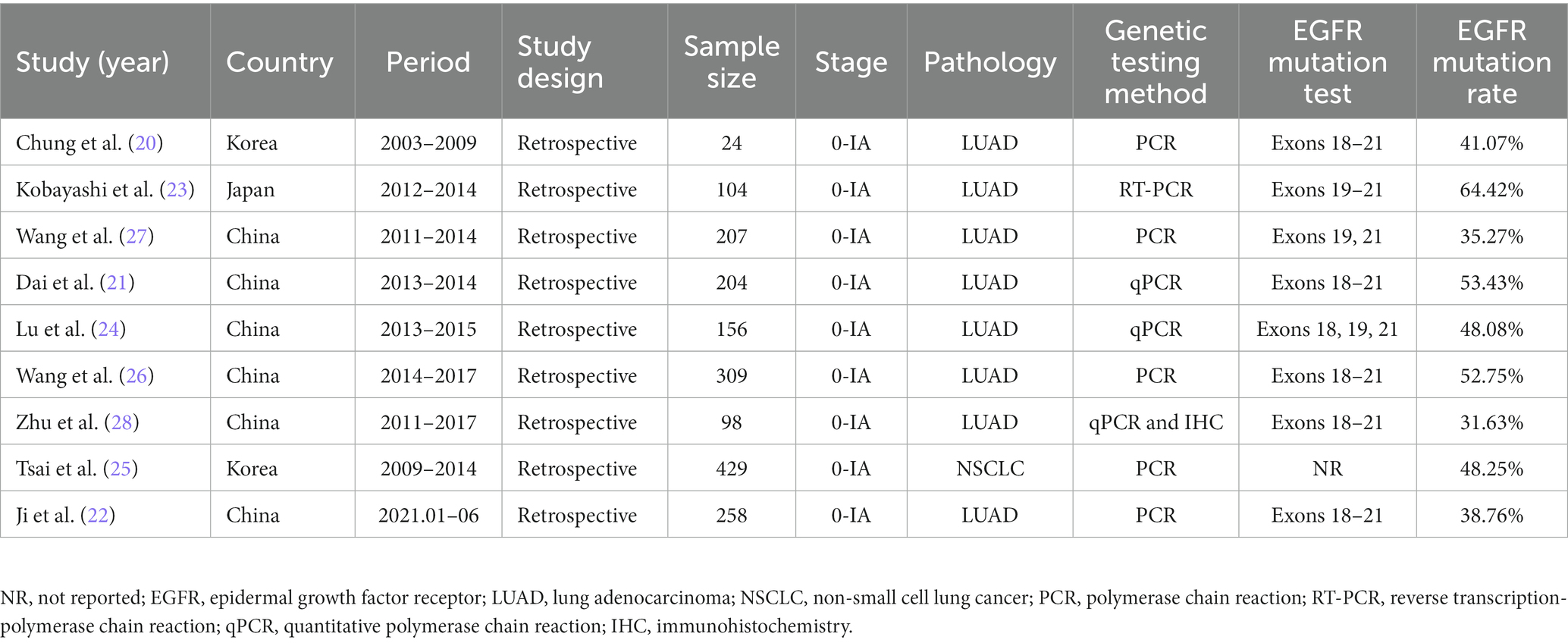
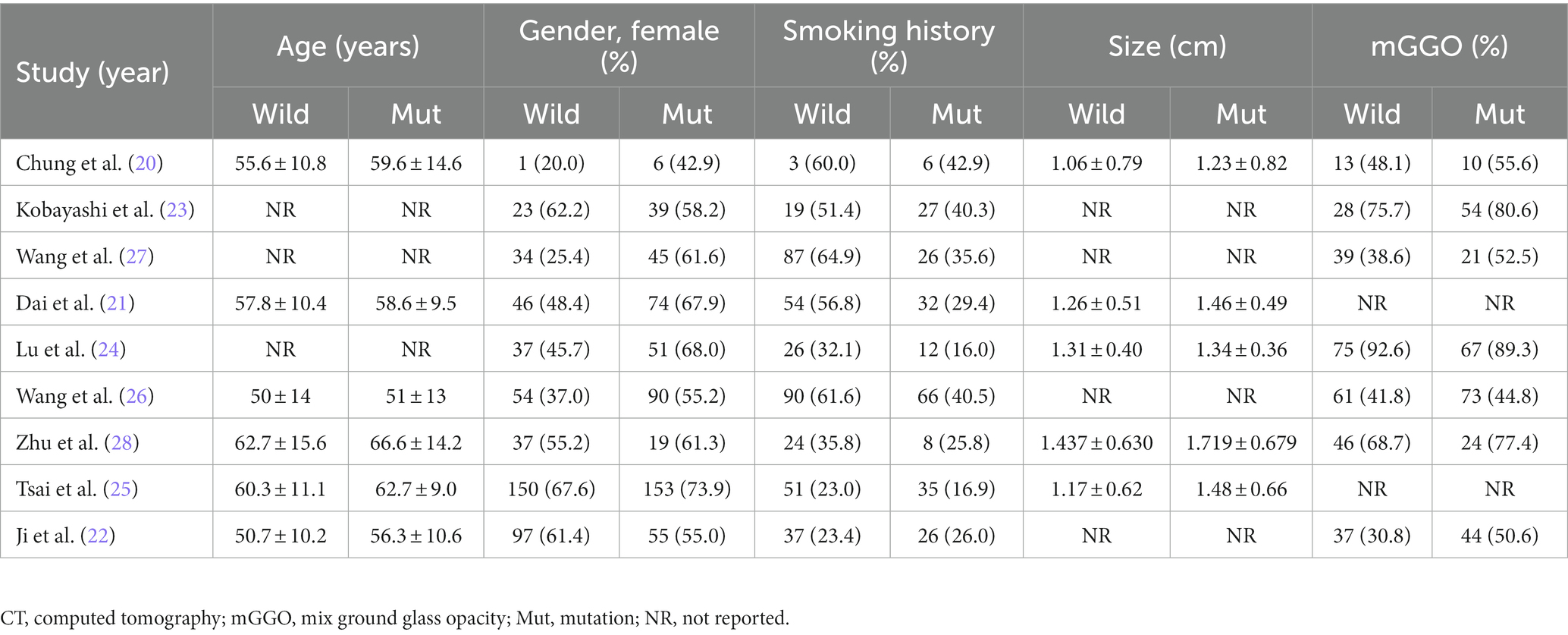
The following data were extracted from each study: (1) publication data: authors, year and country of publication; (2) experimental data: study design and period, method of EGFR gene testing and test range; (3) demographic data: number of cases, age, gender and smoking history of included samples; (4) outcome data: tumor size, proportion of ground glass components, imaging characteristics and pathological classification. Two writers (Jianhao Qiu and Zheng Ma) independently examined the relevant studies and retrieved the necessary information to fill out the predesigned forms. All disputes were settled by consensus. We did not communicate with the authors about unpublished data.
Quality of evidence
In this systematic review and meta-analysis, the quality of case–control studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) (15). Studies with a score of 6 or higher were considered eligible for further meta-analysis. The quality of each study was independently assessed by two authors (Jianhao Qiu and Zheng Ma). Any differences in quality assessment were resolved by consensus.
Statistical analysis
All statistical analyses were conducted using the Review Manager software (RevMan version 5.3; The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and the STATA 16 software package (StataCorp LLC, College Station, TX).
We calculated 95% confidence interval (CI) and odds ratio (OR) to summarize the relationship between dichotomous data and EGFR gene mutations. The standardized mean difference (SMD) with 95% CIs was the appropriate statistic to summarize standard deviations (SDs) means for continuous variables. If SDs were not supplied, we would not have included the data in the quantitative synthesis since, according to Cochrane Collaboration criteria, extrapolation of SDs is only applicable to trials with large sample sizes and normally distributed outcomes (16).
This systematic review and meta-analysis used the Cochrane Q test and the I2 statistic to quantify the degree of heterogeneity, with an I2 greater than 50% considered to be substantial (17). A two-tailed p < 0.05 was considered statistically significant. If the I2 test value was <50%, we used a fixed-effect model; nevertheless, we used a random-effect model if the I2 test value was >50% (18). Egger’s test was used to detect possible publication bias, and the presence of significant publication bias was determined if p < 0.05 for Egger’s test (19).
The stability of the pooled estimates was further examined using a sensitivity analysis, in which the effect of each study on the overall estimate could be tested by sequentially omitting individual studies.
Results
Literature search
A flow diagram outlining the search process showed Figure 1. A total of 1,740 potential articles were identified, including 356 PubMed citations, 209 Embase citations, 608 Cochrane Library citations, and 567 Web of Science citations. In addition, a manual literature search of the reference list yielded three relevant studies. A total of 9 articles were finally included in this meta-analysis after checking for duplicates and screening titles, abstracts, and full texts (20–28).

Figure 1. PRISMA flow diagram of literature retrieval. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the included studies
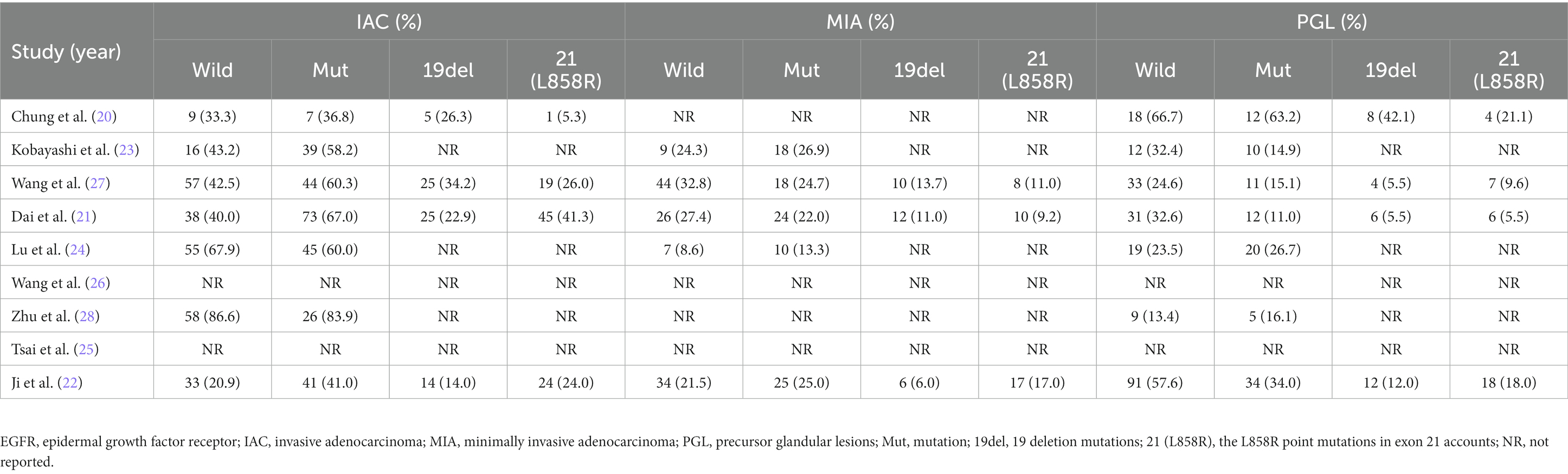
The baseline features of each research that met the inclusion criteria were summarized in Table 1, and relevant demographic, imaging, and histopathological outcomes were presented in Tables 2, 3. The 9 retrospective studies included in this meta-analysis were conducted between 2009 and 2023 in three different countries, with sample sizes ranging from 24 to 429 patients. A total of 1789 patients were finally included in this meta-analysis. The majority of patients were from China (n = 1,232; 68.87%), followed by 453 patients from Korea (25.32%), only one study was from Japan (n = 104; 5.81%). Regarding the EGFR mutations rate, the study with the highest mutations rate was from Japan (64.42%) (23). The study with the lowest mutations rate was from China (31.63%) (28). Almost all studies tested common mutation sites in the EGFR gene to varying degrees (e.g., L838R, 19del, etc.), and only one study from Korea did not specify the detailed sites of the mutations (25). The patients in the remaining 8 included literatures were all confirmed as having LUAD by postoperative pathology, and only one included literature had a postoperative pathological diagnosis of NSCLC (25).
Quality assessment
The quality assessment of the included studies is shown in Table 4. All 9 included retrospective case–control studies had a NOS score greater than 6, which is an indication that they were all of acceptable quality and no other risk of bias was found.
Older age and EGFR mutations
A total of 6 studies were included, reporting the age of 1,317 patients in relation to the EGFR mutations status of their tumors. The results of the meta-analysis showed that patients in the EGFR mutant-positive group were older compared to the EGFR mutant-negative group (SMD = 0.23; 95% CI: 0.12–0.34; p < 0.001) and a relatively low heterogeneity (I2 = 43%; p = 0.12). Using Egger’s test, no publication bias was discovered (p = 0.843) (Figure 2A).
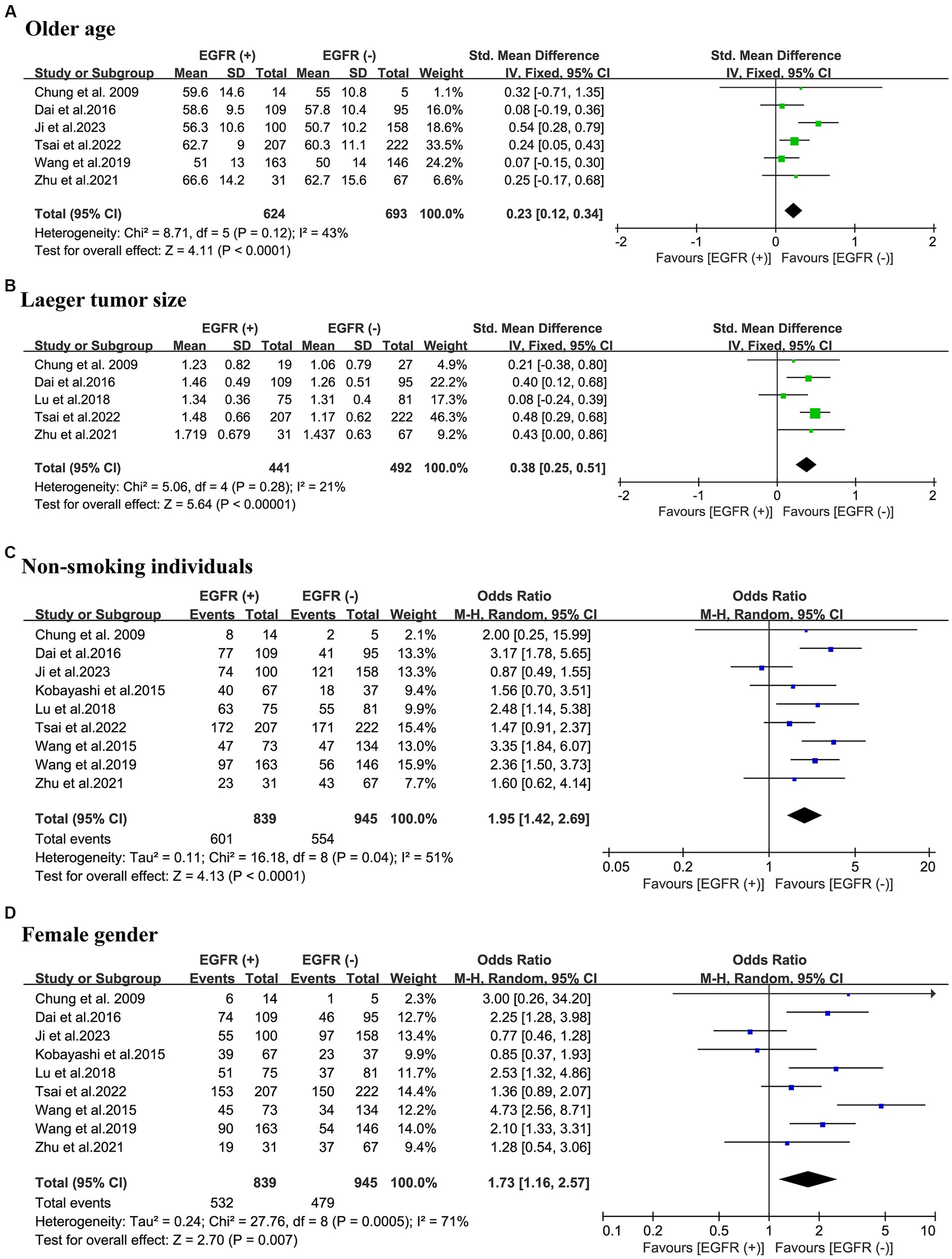
Figure 2. Meta-analysis of demographic and partial thoracic CT information of patients with NSCLC between the EGFR mutant-positive and mutant-negative groups. (A) Old age; (B) larger tumor size; (C) non-smoking individuals; (D) female gender. EGFR (+), EGFR mutations were positive; EGFR (−), EGFR mutations were negative; CT, computed tomography; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; OR, odds ratio; CI, confidence interval.
Larger tumor size and EGFR mutations
The relationship between tumor size and EGFR mutations was evaluated in a total of 933 patients from 5 studies. The meta-analysis revealed that patients with EGFR mutations exhibited larger tumor sizes (SMD = 0.38; 95% CI: 0.25–0.51; p < 0.001) and a relatively low heterogeneity (I2 = 21%; p = 0.28). The Egger’s test did not reveal any publication bias (p = 0.382). (Figure 2B).
Non-smoking individuals and EGFR mutations
The association between patients’ smoking history and EGFR mutations was examined by pooling of 1784 patients from 9 studies in 3 countries. This meta-analysis indicated that the occurrence rate of non-smoking individuals in the EGFR mutant-positive group was significantly higher than in the EGFR mutant-negative group (OR = 1.95; 95% CI: 1.42–2.69; p < 0.001). There was a high-degree of heterogeneity (I2 = 51%; p = 0.04). The Egger’s test revealed no evidence of publication bias (p = 0.964) (Figure 2C).
Female gender and EGFR mutations
To evaluate the association between patient gender and EGFR mutations, 1784 patients from 9 different studies were pooled. The meta-analysis elucidates the higher proportion of the female population in the EGFR mutant-positive group compared to the EGFR mutant-negative group (OR = 1.73; 95% CI: 1.16–2.57; p = 0.007) with a considerable heterogeneity (I2 = 71.0%; p < 0.001). No publication bias was found using Egger’s test (p = 0.803) (Figure 2D).
mGGO and EGFR mutations
Overall, 1,060 patients from 7 studies were pooled to investigate the relationship between the percentage of GGO component in their tumors and EGFR mutations. The results of the meta-analysis indicated that in the EGFR mutant-positive group compared to the EGFR mutant-negative group, there was a statistically significant increase in the incidence of pulmonary nodules as mGGO (OR = 1.43; 95% CI: 1.09–1.88; p = 0.010) with a slight heterogeneity (I2 = 0%; p = 0.44). No publication bias was found using Egger’s test (p = 0.776) (Figure 3A).
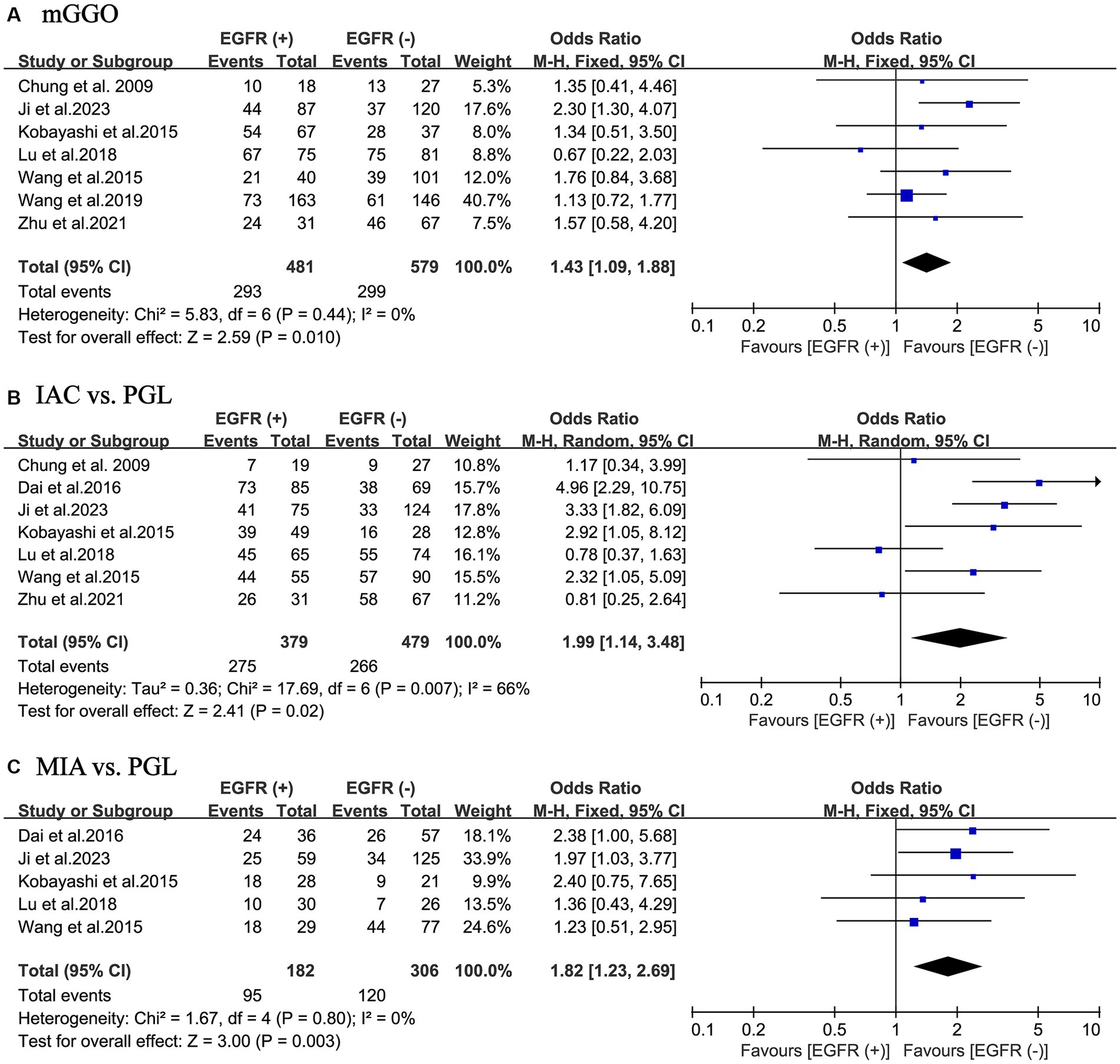
Figure 3. Meta-analysis of partial thoracic CT information and pathological outcomes of patients with NSCLC between the EGFR mutant-positive and mutant-negative groups. (A) mGGO; (B) IAC vs. PGL; (C) MIA vs. PGL. EGFR (+), EGFR mutations were positive; EGFR (−), EGFR mutations were negative; CT, computed tomography; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; mGGO, mixed ground glass opacity; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; PGL, precursor glandular lesions; OR, odds ratio; CI, confidence interval.
Histology and EGFR mutations
In total, the ultimate histological outcome of 858 patients from 7 included studies was evaluated for EGFR mutations. As shown in Figures 3B,C, the incidence of EGFR mutations is relatively high in either IAC (OR = 1.99; 95% CI: 1.14–3.48; p = 0.020) or MIA (OR = 1.82; 95% CI: 1.23–2.69; p = 0.003) relative to precursor glandular lesions (PGL). There was a considerable heterogeneity in study of IAC (I2 = 66%; p = 0.007), whereas there was a slight heterogeneity in study of MIA (I2 = 0%; p = 0.80). No publication bias was found using Egger’s test (p = 0.366 for IAC; p = 0.813 for MIA).
A more thorough analysis was done to investigate into the relationship between EGFR mutant subgroups and tumor histology. As illustrated in Figures 4A,B, there was a higher incidence of IAC in both the exon 19 deletion group (OR = 2.94; 95% CI: 1.95–4.96; p < 0.001) and in the L858R point mutation group (OR = 2.79; 95% CI: 1.28–6.04; p = 0.009) compared to the negative group. There was minor heterogeneity in study of the exon 19 deletion (I2 = 0%; p = 0.63), whereas there was a considerable heterogeneity in study of the L858R point mutation (I2 = 66%; p = 0.007). No publication bias was detected in either of the two studies using Egger’s test (p = 0.270 for IAC; p = 0.378 for MIA).
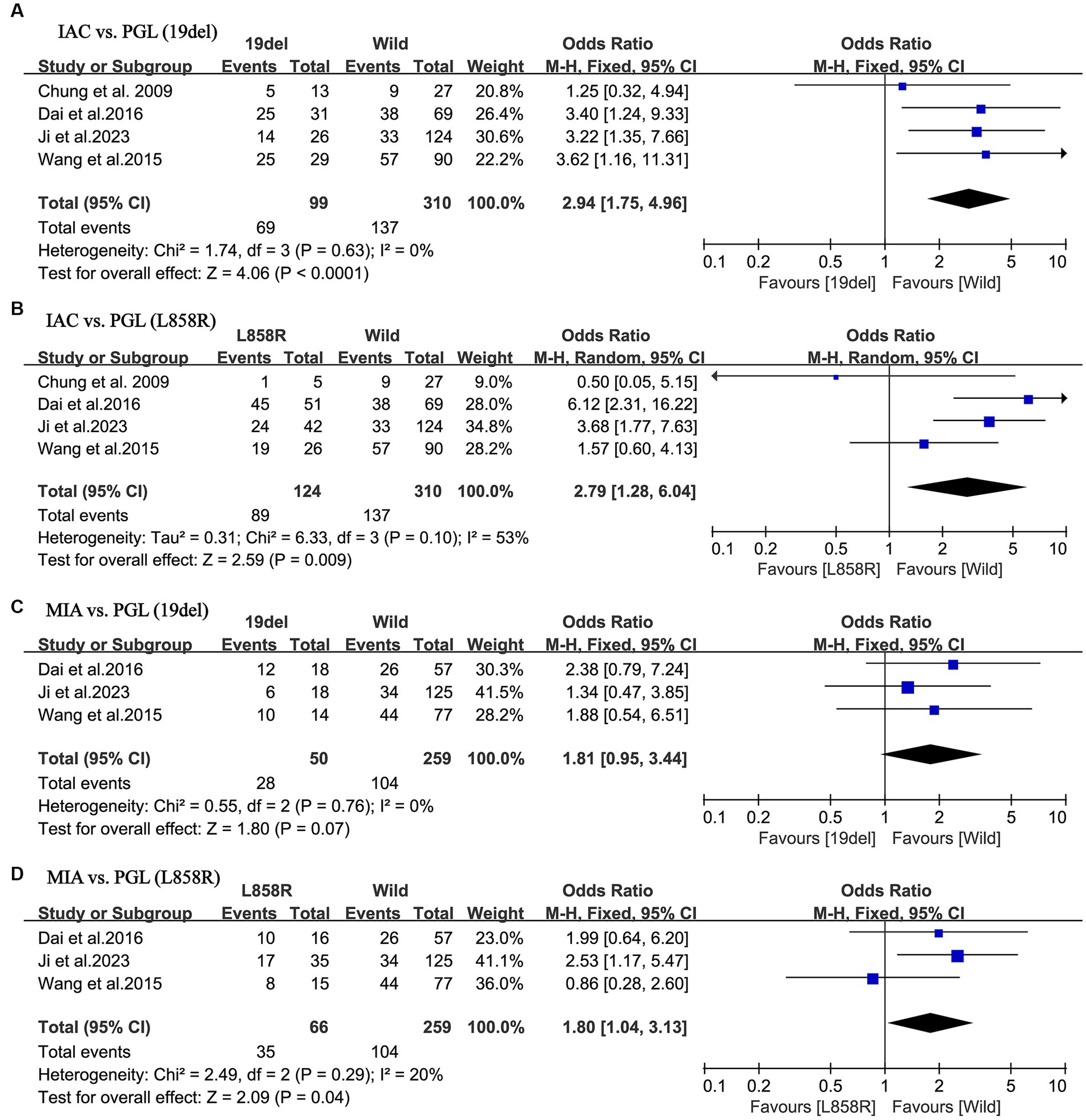
Figure 4. Meta-analysis of the pathological outcomes of patients with NSCLC between the EGFR mutant subtype groups and wild groups. (A) IAC vs. PGL (19del); (B) IAC vs. PGL (L858R); (C) MIA vs. PGL (19del); (D) MIA vs. PGL (L858R). NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; PGL, precursor glandular lesions; 19del, 19 deletion mutations; L858R, the L858R point mutations in exon 21 accounts; OR, odds ratio; CI, confidence interval.
Nevertheless, further studies on MIA demonstrated a different result. As shown in Figures 4C,D, MIA had a higher incidence in the L858R point mutation group, compared to the negative group (OR = 1.80; 95% CI: 1.04–3.13; p = 0.04). In contrast, in the exon 19 deletion group, there was no statistical difference compared to the negative group (OR = 1.81; 95% CI: 0.95–3.44; p = 0.07). Both groups of studies had low heterogeneity (I2 = 0%, p = 0.76 for the exon 19 deletion group; I2 = 20%, p = 0.29 for the L858R point mutation group). No publication bias was detected in either of the two studies using Egger’s test (p = 0.733 for IAC; p = 0.492 for MIA).
Sensitivity analysis
We performed sensitivity analyses by sequentially omitting individual studies. As shown in Supplementary Figures S1–S3, none of the pooled ORs for the remaining studies in the analysis based on each component were outside the range of estimates. In addition, there were no significant differences between the corrected pooled estimates and the original pooled estimates. The reliability of our meta-analysis was thus validated.
Discussion
A growing trend today is the identification of EGFR mutations in patients undergoing early diagnosis and therapy for NSCLC (29, 30). Although the conclusions of these investigations are still up for debate, prior researches have shown a connection between EGFR mutations and the imaging presentation of NSCLC and the ultimate histology of NSCLC. In order to draw more convincing conclusions, we conducted a systematic review and meta-analysis of 9 retrospective studies to examine the relationship between the clinical, imaging and histology characteristics of NSCLC patients and the frequency of EGFR mutations. This meta-analysis study discovered that age, tumor size, female gender, non-smoking individuals, pulmonary nodules presenting as mGGO, pulmonary nodules with histological types of MIA and IAC were significant factors influencing EGFR mutations. And further analysis confirmed that in the L858R point mutation group, there was a higher incidence of pulmonary nodules with histological type IAC or MIA. In contrast, in the exon 19 mutation group, only pulmonary nodules with IAC had a higher incidence, while pulmonary nodules with MIA were not statistically significant.
In this study, we discovered that patients who were substantially older had a higher risk of developing EGFR mutations than patients who were younger (SMD = 0.23; 95% CI: 0.12–0.34; p < 0.001). Multiple studies have shown that the risk of EGFR mutations increases with age in NSCLC patients (31, 32). Despite adjusting for patient histology, smoking status, and pathological staging, one study found an independent statistical difference between EGFR mutations and the age of the patient at diagnosis (33). In response to this conclusion, we suggest that those who are older have an increased risk of developing mutations due to changes in their own hormone levels, diminished nucleotide repair capacity, and increased exposure to carcinogenic stimuli from the environment (34–37). To validate this conclusion, additional large-scale gene sequencing with population representation is required.
The individual’s smoking history is also a significant determinant in EGFR mutations. This meta-analysis indicated that individuals without a history of smoking are more likely to have EGFR mutations (OR = 1.95; 95% CI: 1.42–2.69; p < 0.001). However, this analysis showed relatively high heterogeneity (I2 = 51%; p = 0.04). This may be attributable to variations in the statistical criteria for smoking history among the included studies. For example, the study by Wang et al. and Dai et al. defined patients who had quit smoking ≥1 year prior to the surgery were defined as former smokers (21, 27), whereas the remaining included studies did not make this distinction. In addition, we would have liked to use the smoking index, was defined as the number of cigarettes smoked per day multiplied by the number of years smoked, as a statistical measure of the extent of smoking among patients in this analysis to give more credibility. This statistical method was not utilized in any of the included studies, which prevented us from realizing our design. This can be explored further in a subsequent investigation.
Furthermore, the study revealed that the proportion of female patients in the EGFR mutant-positive group was significantly higher than in the EGFR mutant-negative group (OR = 1.73; 95% CI: 1.16–2.57; p = 0.007), meaning that women are more likely to develop EGFR mutations. Based on the findings of this study, we suggest that the estrogen level in females affects the incidence of EGFR mutations. Multiple studies have verified a positive correlation between estrogen receptors and EGFR mutations (38, 39). Additionally, the study by Mazières et al. confirmed that the expression of estrogen receptors was greater in non-smoking women than in smokers (40). This precisely explains why women who do not smoke have a higher lung cancer incidence rate. Chen et al. and Linardou et al. revealed a considerably greater probability of EGFR mutations in women in Asia, notably in East Asia (41, 42). The fact that each of the 9 studies we included was from East Asia further supports the reliability of our analysis.
For determining whether the tumor has an EGFR mutation, it’s also crucial to consider the size of the tumor and the percentage of GGO on the thoracic CT. The results of this meta-analysis revealed that the incidence of EGFR mutations was greater in pulmonary nodules exhibiting mGGO compared to those exhibiting pGGO (OR = 1.43; 95% CI: 1.09–1.88; p = 0.010). And the incidence of EGFR mutation was positively correlated with the imaging size of the tumor (SMD = 0.38; 95% CI: 0.25–0.51; p < 0.001). A study by Cai et al. in 2023 showed that pGGO and mGGO reflect the pathological development and genetic alterations of pulmonary nodules (43). This study suggests that the solid component of pGGO emerged and that the proportion of solid components progressively increased - manifesting as mGGO, which indicates progression of the tumor (i.e., pathologic findings of MIA or IAC) and an increase in the rate of EGFR mutations. Li et al. found that larger diameter tumors did reveal higher frequency and types of mutations in addition to EGFR mutations, such as ALK rearrangements, TP53 mutations, etc., in postoperative genetic testing (44).
EGFR mutations were strongly related with the histological type of the patient’s tumor. The results of the analysis indicate that the ultimate pathological outcomes of the tumors, whether MIA (OR = 1.82; 95% CI: 1.23–2.69; p = 0.003) or IAC (OR = 1.99; 95% CI: 1.14–3.48; p = 0.020), have a higher incidence of EGFR mutations than PGL. However, the meta-analysis of IAC showed a relatively high heterogeneity (I2 = 66%; p = 0.007), which might be due to subjective bias in the interpretation of IAC criteria by different institutions and different pathologists in the included studies. Several studies have shown that EGFR amplification is essential for the progression of AIS to MIA and even IAC (9, 45). Moreover, the risk of developing secondary primary lung cancer is marginally increased in MIA patients with EGFR mutations (8).
After conducting additional analysis for various subtypes of mutants, we arrived at contrasting conclusions. For tumors with pathological type IAC, both exon 19 deletion and L858R point mutation have a high incidence of mutations (OR = 2.94, 95% CI: 1.95–4.96, p < 0.001 for exon 19 deletion; OR = 2.79, 95% CI: 1.28–6.04, p = 0.009 for L858R point mutation). In contrast, tumors exhibiting MIA maintained a high mutation incidence only for the L858R point mutation (OR = 1.80; 95% CI: 1.04–3.13; p = 0.04), while losing statistical significance for the exon 19 deletion (OR = 1.81; 95% CI: 0.95–3.44; p = 0.07). It has been shown that L858R point mutations are detected more frequently in MIA with completely different tumor characteristics compared to exon 19 deletions (46). However, as only 3 papers were included in this analysis, the results are perhaps not robust enough, which still needs to be corroborated by subsequent relevant studies.
In 2004, the close link between NSCLC and EGFR mutations was identified for the first time, ushering NSCLC treatment into the period of targeted therapy. More than 80% of EGFR mutations are exon 19 deletion mutations and exon 21 L858R point mutations. According to studies, however, patients with rare EGFR mutations have a worse prognosis than those with sensitive mutations. For instance, the EGFR 20 exon insertion mutations causes a spatial site block in the structure of the EGFR protein, reducing the size of the drug-binding pocket and preventing the EGFR-TKI from binding to its target, thereby allowing the EGFR protein to remain active and the oncogenic signal to persist (47, 48). We had planned to set up further research of rare types of EGFR mutations, but unfortunately, the dearth of studies and the scarcity of data on rare mutations prevented us from implementing our plan, which may be taken into account in a future meta-analysis.
This systematic review and meta-analysis has several major advantages. First, we report for the first time that lung adenocarcinomas presenting as MIA exhibit distinct EGFR mutation features. Secondly, our study included a relevant sample size of 1789 patients whose sources included only articles with a low risk of bias and high quality, which provides a more realistic and convincing reflection of the accuracy of the final results. In addition, the sensitivity analysis performed provides evidence that all the results presented are robust. We also assessed publication bias using Egger’s test to ensure that the results are not biased by publication bias. Most importantly, the search strategy developed by our researchers was thorough to ensure that no valuable literature was missed.
This meta-analysis also has several limitations. First of all, the literature included in our study was all from East Asia, which may compromise the accuracy of the analysis of the incidence of EGFR mutations in non-Asian populations. In addition, all included studies were retrospective studies, and there may be selection bias affecting the final overall results. Furthermore, thoracic CT scan parameters, GGO ratio calculation, and EGFR mutations detection methods differed among the included studies, which may increase the heterogeneity among the studies.
Conclusion
This systematic review and meta-analysis is the most exhaustive and up-to-date examination of the literature concerning the risk factors linked with EGFR mutations. The final results proved that patients with higher age, female gender, and non-smoking individuals would have a relatively higher incidence of EGFR mutations. Also, the risk of EGFR mutations was increased with larger tumor diameter, tumor imaging presentation of mGGO, and tumor pathological findings of MIA or IAC. Importantly, tumors presenting as MIA are more likely to have the incidence of L858R point mutation. This finding is somewhat helpful in predicting and assessing the molecular pathological alterations in stage IA NSCLC. Based on the aforementioned risk factors associated with EGFR mutations, thoracic surgeons can make an initial assessment of the molecular pathology of early-stage lung nodules in order to minimize unnecessary costs to the patient, thereby reducing the patient’s financial burden. It also reduces the rate of underdiagnosis of patients with EGFR mutations, and ensures that patients with EGFR mutations will be able to detect their mutations in a timely manner and receive more rigorous postoperative follow-up.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JQ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. ZM: Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing – review & editing. RL: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. CQ: Formal analysis, Investigation, Writing – review & editing. KW: Formal analysis, Software, Writing – review & editing. BL: Data curation, Software, Writing – review & editing. YT: Conceptualization, Project administration, Supervision, Writing – review & editing. HT: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Key Research and Development Program (2021YFC2500900, 2021YFC2500904, and 2021YFC2500905) and Natural Science Foundation of Shandong Province (ZR2021LSW006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1268846/full#supplementary-material
Supplementary Figure S1 | Sensitivity analysis of demographic and partial thoracic CT information of patients with NSCLC between the EGFR positive and negative groups. (A) Old age; (B) Larger tumor size; (C) Non-smoking individuals; (D) Female gender. CT, computed tomography; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; OR, odds ratio; CI, confidence interval.
Supplementary Figure S2 | Sensitivity analysis of partial thoracic CT information and pathological outcomes of patients with NSCLC between the EGFR positive and negative groups. (A) mGGO; (B) IAC vs. PGL; (C) MIA vs. PGL. CT, computed tomography; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; mGGO, mixed ground glass opacity; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; PGL, precursor glandular lesions; OR, odds ratio; CI, confidence interval.
Supplementary Figure S3 | Sensitivity analysis of the pathological outcomes of patients with NSCLC between the EGFR mutant subtype groups and wild groups. (A) IAC vs. PGL (19del); (B) IAC vs. PGL (L858R); (C) MIA vs. PGL (19del); (D) MIA vs. PGL (L858R). NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; PGL, precursor glandular lesions; 19del, 19 deletion mutations; L858R, the L858R point mutations in exon 21 accounts; OR, odds ratio; CI, confidence interval.
Footnotes
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lynch, TJ, Bell, DW, Sordella, R, Gurubhagavatula, S, Okimoto, RA, Brannigan, BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
3. Abdallah, SM, and Hirsh, V. Irreversible tyrosine kinase inhibition of epidermal growth factor receptor with afatinib in EGFR activating mutation-positive advanced non-small-cell lung cancer. Curr Oncol. (2018) 25:S9–s17. doi: 10.3747/co.25.3732
4. da Cunha, SG, Shepherd, FA, and Tsao, MS. EGFR mutations and lung cancer. Annu Rev Pathol. (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
5. Hondelink, LM, Ernst, SM, Atmodimedjo, P, Cohen, D, Wolf, JL, Dingemans, AC, et al. Prevalence, clinical and molecular characteristics of early stage EGFR-mutated lung cancer in a real-life West-European cohort: implications for adjuvant therapy. Eur J Cancer. (2022) 181:53–61. doi: 10.1016/j.ejca.2022.12.010
6. Lemjabbar-Alaoui, H, Hassan, OU, Yang, YW, and Buchanan, P. Lung cancer: biology and treatment options. Biochim Biophys Acta. (2015) 1856:189–210. doi: 10.1016/j.bbcan.2015.08.002
7. Yoneda, K, Imanishi, N, Ichiki, Y, and Tanaka, F. Treatment of non-small cell lung cancer with EGFR−mutations. J UOEH. (2019) 41:153–63. doi: 10.7888/juoeh.41.153
8. Yotsukura, M, Asamura, H, Motoi, N, Kashima, J, Yoshida, Y, Nakagawa, K, et al. Long-term prognosis of patients with resected adenocarcinoma in situ and minimally invasive adenocarcinoma of the lung. J Thorac Oncol. (2021) 16:1312–20. doi: 10.1016/j.jtho.2021.04.007
9. Zhang, C, Zhang, J, Xu, FP, Wang, YG, Xie, Z, Su, J, et al. Genomic landscape and immune microenvironment features of preinvasive and early invasive lung adenocarcinoma. J Thorac Oncol. (2019) 14:1912–23. doi: 10.1016/j.jtho.2019.07.031
10. Zhu, J, Wang, W, Xiong, Y, Xu, S, Chen, J, Wen, M, et al. Evolution of lung adenocarcinoma from preneoplasia to invasive adenocarcinoma. Cancer Med. (2022) 12:5545–57. doi: 10.1002/cam4.5393
11. Ortiz, AFH, Camacho, TC, Vásquez, AF, Del Castillo, HV, Neira, JGA, Yepes, MM, et al. Clinical and CT patterns to predict EGFR mutation in patients with non-small cell lung cancer: a systematic literature review and meta-analysis. Eur J Radiol Open. (2022) 9:100400. doi: 10.1016/j.ejro.2022.100400
12. Wei, Z, Wang, Z, Nie, Y, Zhang, K, Shen, H, Wang, X, et al. Molecular alterations in lung adenocarcinoma with ground-glass nodules: a systematic review and meta-analysis. Front Oncol. (2021) 11:724692. doi: 10.3389/fonc.2021.724692
13. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gøtzsche, PC, Ioannidis, JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
14. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
15. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Higgins, JPT, Chandler, TJ, Cumpston, M, Li, T, Page, MJ, and Welch, VA. Cochrane handbook for systematic reviews of interventions version 6.3 Cochrane (2022). Available at: https://training.cochrane.org/handbook
17. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
18. Herrera Ortiz, A, Camacho, E, Rojas, J, Cadavid, T, Guevara, S, Rincón Cuenca, N, et al. A practical guide to perform a systematic literature review and Meta-analysis. Princ Pract Clin Res. (2021) 7:47–57. doi: 10.21801/ppcrj.2021.74.6
19. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication Bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
20. Chung, J-H, Choe, G, Jheon, S, Sung, S-W, Kim, TJ, Lee, KW, et al. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. (2009) 4:1490–5. doi: 10.1097/JTO.0b013e3181bc9731
21. Dai, J, Shi, J, Soodeen-Lalloo, AK, Zhang, P, Yang, Y, Wu, C, et al. Air bronchogram: a potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer. (2016) 98:22–8. doi: 10.1016/j.lungcan.2016.05.009
22. Ji, XY, Li, H, Chen, HH, and Lin, J. Diagnostic performance of RASSF1A and SHOX2 methylation combined with EGFR mutations for differentiation between small pulmonary nodules. J Cancer Res Clin Oncol. (2023) 149:8557–71. doi: 10.1007/s00432-023-04745-8
23. Kobayashi, Y, Mitsudomi, T, Sakao, Y, and Yatabe, Y. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol. (2015) 26:156–61. doi: 10.1093/annonc/mdu505
24. Lu, Q, Ma, Y, An, Z, Zhao, T, Xu, Z, and Chen, H. Epidermal growth factor receptor mutation accelerates radiographic progression in lung adenocarcinoma presented as a solitary ground-glass opacity. J Thorac Dis. (2018) 10:6030–9. doi: 10.21037/jtd.2018.10.19
25. Tsai, YM, Lin, KH, Kuo, YS, Lin, YC, Chien, YH, Chou, HP, et al. Clinical significance of epidermal growth factor receptor mutations in resected stage IA non-small cell lung cancer. Formosan J Surg. (2022) 55:109–15. doi: 10.4103/fjs.fjs_104_22
26. Wang, H, Guo, H, Wang, Z, Shan, B, and Lin, J. The diagnostic value of quantitative CT analysis of ground-glass volume percentage in differentiating epidermal growth factor receptor mutation and subtypes in lung adenocarcinoma. Biomed Res Int. (2019) 2019:1–8. doi: 10.1155/2019/9643836
27. Wang, T, Zhang, T, Han, X, Liu, X, Zhou, N, and Liu, Y. Impact of the international association for the study of lung cancer/American thoracic society/European respiratory society classification of stage IA adenocarcinoma of the lung: correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med. (2015) 9:2095–103. doi: 10.3892/etm.2015.2422
28. Zhu, P, Xu, XJ, Zhang, MM, and Fan, SF. High-resolution computed tomography findings independently predict epidermal growth factor receptor mutation status in ground-glass nodular lung adenocarcinoma. World J Clin Cases. (2021) 9:9792–803. doi: 10.12998/wjcc.v9.i32.9792
29. Gu, L, Huang, H, Xu, Z, Niu, X, Li, Z, Xia, L, et al. Landscape and predictive significance of the structural classification of EGFR mutations in Chinese NSCLCs: a real-world study. J Clin Med. (2022) 12:236. doi: 10.3390/jcm12010236
30. Yan, K. Osimertinib in EGFR-mutated advanced NSCLC. N Engl J Med. (2020) 382:1863. doi: 10.1056/NEJMc2001514
31. Choi, YH, Lee, JK, Kang, HJ, Lee, TS, Kim, HR, Kim, CH, et al. Association between age at diagnosis and the presence of EGFR mutations in female patients with resected non-small cell lung cancer. J Thorac Oncol. (2010) 5:1949–52. doi: 10.1097/JTO.0b013e3181f38816
32. Tanaka, K, Hida, T, Oya, Y, Yoshida, T, Shimizu, J, Mizuno, T, et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer. (2017) 123:1731–40. doi: 10.1002/cncr.30539
33. Staaf, J, Aine, M, Nacer, DF, Planck, M, and Arbajian, E. Molecular characteristics of lung adenocarcinoma with respect to patient age at diagnosis. Int J Cancer. (2023) 153:197–209. doi: 10.1002/ijc.34523
34. Lim, EH, Franklin, P, Trevenen, ML, Nieuwenhuijsen, M, Yeap, BB, Almeida, OP, et al. Exposure to low-level ambient air pollution and the relationship with lung and bladder cancer in older men, in Perth, Western Australia. Br J Cancer. (2023) 129:1500–9. doi: 10.1038/s41416-023-02411-x
35. Korsiak, J, Pinault, L, Christidis, T, Burnett, RT, Abrahamowicz, M, and Weichenthal, S. Long-term exposure to wildfires and cancer incidence in Canada: a population-based observational cohort study. Lancet Planet Health. (2022) 6:e400–9. doi: 10.1016/S2542-5196(22)00067-5
36. Gao, H, Yang, J, He, L, Wang, W, Liu, Y, Hu, Y, et al. The diagnostic potential of SHOX2 and RASSF1A DNA methylation in early lung adenocarcinoma. Front Oncol. (2022) 12:849024. doi: 10.3389/fonc.2022.849024
37. Krashin, E, Silverman, B, Steinberg, DM, Yekutieli, D, Giveon, S, Fabian, O, et al. Opposing effects of thyroid hormones on cancer risk: a population-based study. Eur J Endocrinol. (2021) 184:477–86. doi: 10.1530/EJE-20-1123
38. Kawaguchi, T, Koh, Y, Ando, M, Ito, N, Takeo, S, Adachi, H, et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan molecular epidemiology for lung Cancer study. J Clin Oncol. (2016) 34:2247–57. doi: 10.1200/JCO.2015.64.2322
39. Tani, Y, Kaneda, H, Koh, Y, Tamiya, A, Isa, S, Kubo, A, et al. The impact of estrogen receptor expression on mutational status in the evolution of non-small cell lung Cancer. Clin Lung Cancer. (2023) 24:165–74. doi: 10.1016/j.cllc.2022.12.010
40. Mazières, J, Rouquette, I, Lepage, B, Milia, J, Brouchet, L, Guibert, N, et al. Specificities of lung adenocarcinoma in women who have never smoked. J Thorac Oncol. (2013) 8:923–9. doi: 10.1097/JTO.0b013e3182904dfb
41. Chen, J, Yang, H, Teo, ASM, Amer, LB, Sherbaf, FG, Tan, CQ, et al. Genomic landscape of lung adenocarcinoma in east Asians. Nat Genet. (2020) 52:177–86. doi: 10.1038/s41588-019-0569-6
42. Linardou, H, Dahabreh, IJ, Bafaloukos, D, Kosmidis, P, and Murray, S. Somatic EGFR mutations and efficacy of tyrosine kinase inhibitors in NSCLC. Nat Rev Clin Oncol. (2009) 6:352–66. doi: 10.1038/nrclinonc.2009.62
43. Cai, Y, Chen, T, Zhang, S, Tan, M, and Wang, J. Correlation exploration among CT imaging, pathology and genotype of pulmonary ground-glass opacity. J Cell Mol Med. (2023) 27:2021–31. doi: 10.1111/jcmm.17797
44. Li, P, Liu, L, Wang, D, Yang, R, Xuan, Y, Han, Y, et al. Genomic and clinicopathological features of lung adenocarcinomas with micropapillary component. Front Oncol. (2022) 12:989349. doi: 10.3389/fonc.2022.989349
45. Inamura, K. Clinicopathological characteristics and mutations driving development of early lung adenocarcinoma: tumor initiation and progression. Int J Mol Sci. (2018) 19:1259. doi: 10.3390/ijms19041259
46. Li, M, Li, C, Ke, L, Zhan, M, and Cheng, M. Significance of the epidermal growth factor receptor mutation status and differences among molecular subgroups in surgically resected lung microinvasive adenocarcinoma. Oncol Lett. (2018) 16:7057–67. doi: 10.3892/ol.2018.9539
47. Hou, J, Li, H, Ma, S, He, Z, Yang, S, Hao, L, et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: current status and perspectives. Biomark Res. (2022) 10:21. doi: 10.1186/s40364-022-00372-6
Keywords: non-small cell lung cancer, ground glass opacity, histology, epidermal growth factor receptor, thoracic computed tomography, meta-analysis
Citation: Qiu J, Ma Z, Li R, Qu C, Wang K, Liu B, Tian Y and Tian H (2023) Distinguishing EGFR mutant subtypes in stage IA non-small cell lung cancer using the presence status of ground glass opacity and final histologic classification: a systematic review and meta-analysis. Front. Med. 10:1268846. doi: 10.3389/fmed.2023.1268846
Edited by:
Hsiao-Chi Chuang, Taipei Medical University, TaiwanReviewed by:
Nguyen Thanh Tung, Cho Ray Hospital, VietnamVincent Laiman, Gadjah Mada University, Indonesia
Copyright © 2023 Qiu, Ma, Li, Qu, Wang, Liu, Tian and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tian, dGlhbmh1aXFsQGVtYWlsLnNkdS5lZHUuY24=; Yu Tian, dGlhbnl1OTMwMzE0QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Jianhao Qiu
Jianhao Qiu Zheng Ma
Zheng Ma Rongyang Li
Rongyang Li Chenghao Qu1
Chenghao Qu1 Binyan Liu
Binyan Liu Hui Tian
Hui Tian