
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 16 November 2023
Sec. Hematology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1267512
 Bingbing Wen1†
Bingbing Wen1† Yuming Zhang2†
Yuming Zhang2† Haiqing Lin3†
Haiqing Lin3† Jin Lou1
Jin Lou1 Chuangqing Tu4
Chuangqing Tu4 Yirong Jiang5
Yirong Jiang5 Xiaolian Liu6
Xiaolian Liu6 Yan Chen7
Yan Chen7 Huiqing He8
Huiqing He8 Zelin Liu9
Zelin Liu9 Xiaoling Xie10
Xiaoling Xie10 Wangxiang Huang11*
Wangxiang Huang11* Liping Pang12*
Liping Pang12* Xin Du1*
Xin Du1*Introduction: Early stable deep molecular response (DMR) to nilotinib is associated with goal of treatment-free remission (TFR) in patients with chronic-phase chronic myeloid leukemia (CML-CP). It is important to early distinguish between patients who can achieve a DMR and those who are fit for TFR.
Methods: We performed a multicenter study to explore the early cumulative MR4.5 rate at 18 months with nilotinib in patients with newly diagnosed CML-CP (ND-CML-CP) in China. Of the 29 institutes, 106 patients with ND-CML-CP received nilotinib (300 mg BID).
Results and discussion: The cumulative MR4.5 rate of nilotinib treatment at 18 months was 69.8% (74/106). The cumulative MMR and MR4.0 rates for nilotinib at 18 months were 94.3% (100/106) and 84.9% (90/106), respectively. Patients with an ultra-early molecular response (u-EMR) at 6 weeks were not significantly different in obtaining DMR or MMR by 24 months compared with those without u-EMR (p = 0.7584 and p = 0.9543, respectively). Our study demonstrated that nilotinib treatment in patients with ND-CML-CP contributed to obtain high early MR4.5.
Chronic myeloid leukemia (CML) is characterized by the presence of a BCR::ABL1 fusion gene on the Philadelphia chromosome (1). The BCR::ABL1 fusion gene produces the BCR::ABL1 tyrosine kinase, which leads to leukemia cell proliferation (2–4). Three tyrosine kinase inhibitors (TKIs) have been used for the frontline treatment of CML-CP in China including imatinib, nilotinib and dasatinib according to the NCCN guidelines (5, 6). Imatinib was first approved for therapy of newly diagnosed CML-CP (ND-CML-CP) and had efficacy superior to that of interferon-α plus cytarabine (7). Nilotinib and dasatinib, second-generation TKIs, had also been approved as therapies (8, 9). In vitro, nilotinib exhibited greater selectivity for ABL kinase and had a higher level of inhibitory compared to imatinib (10, 11). The recommended dose of nilotinib is 300 mg twice daily (BID) for patients with ND-CML-CP (12). Frontline nilotinib (300 mg BID) was reported to be associated with a higher rate of deep molecular response (DMR) than imatinib (MR4.5 by 2 years, nilotinib vs. imatinib: 26% vs. 10%; p < 0.0001) in the ENESTnd study (13). In addition, 54 and 61.0% of patients in the nilotinib arm (300 mg BID) achieved MR4.5 compared with 31 and 39.2% of patients in the imatinib arm after 5 years and 10 years of follow-up, respectively (13, 14).
Treatment-free remission (TFR) has recently become the new goal for patients with CML-CP. DMR serves as a milestone in TFR, and has been described in the European LeukemiaNet (ELN) and LALNET recommendations and NCCN guidelines (15–17). Patients with achievement of early stable DMR can acquire a chance to discontinue medication for TFR. Therefore, it is important to early distinguish between patients who can achieve of the DMR and those who are fit for TFR. However, the cumulative MR4.5 rate of nilotinib was not yet detected in the ENESTchina study with a 12-month follow-up, and the early cumulative incidence of the MR4.5 rate of nilotinib by 18 months was also not investigated in previous studies. In addition, early molecular response (EMR) may be as the first milestone in the treatment of CML-CP and a new marker for long-term outcomes such as in progression-free survival (PFS) or overall survival (OS) (17–19). Masahiro et al. showed that patients who achieved EMR at 3 or 6 months may have higher rate of DMR by 36 months and better PFS than those without EMR at 3 or 6 months with second-generation TKIs treatments (20). Therefore, we explored the relationship between ultra-early molecular response (u-EMR) including international scale (IS) decreased by more than 10% from baseline at 6 weeks and the cumulative incidence of MR4.5 rate in this study.
We designed a phase IV, prospective, multicenter study to detect the early cumulative incidence of MR4.5 rate by 18 months with nilotinib based on EUTOS long-term survival scores (ELTS) score in patients with ND-CML-CP in China. Because the cumulative MR4.5 rate of nilotinib was not yet detected in the ENESTchina study with a 12-month follow-up, these clinical data will be an important supplement for the efficacy of MR4.5 in real world ND-CML-CP patients in China. The cumulative incidences of MR4.0 and MMR, u-EMR, PFS and OS were also evaluated in this trial.
This phase IV, multicenter, single-arm, prospective study was conducted in China. The key eligibility criteria included age of 18 years or above, confirmed ND-CML-CP (positive BCR::ABL1 mRNA or positive Philadelphia chromosome) within 6 months of study registration, allowed a cytoredutive therapy with hydroxyurea before starting nilotinib therapy, no accelerated phase (AP) or blast phase (BP) criteria, and an Eastern Cooperative Oncology Group performance-status (ECOG) score less than 2 were eligible for inclusion. Patients with T315I mutations in BCR::ABL1 or those who had previously received treatment with any TKI treatment were excluded. Patients with a history of severe heart or lung disease were excluded from this trial.
This study was approved by the institutional review boards of Shenzhen Second People’s Hospital and other participating institutions. This study was adhered to the ethical principles of the Declaration of Helsinki. Written informed consents was obtained from all patients prior to the study procedures. This study was registered in the Clinical Trial prs. Gov Registry (NCT03942094).
Each patient in this trial received nilotinib 300 mg BID until disease progression. The primary endpoint was the cumulative MR4.5 rate at 18 months. The secondary endpoints were cumulative MR4.5; molecular response 4.0 (MR4.0); major molecular response (MMR) at 3, 6, 9, 12, and 24 months; ultra-early molecular response (u-EMR); PFS; and OS.
MMR was defined as BCR::ABL1 IS (BCR::ABL11/ABL1 ratio on the International Scale [IS]) ≤ 0.1%. MR4.0 was defined as BCR::ABL1 IS ≤0.01%. MR4.5 was defined as BCR::ABL1 IS ≤0.0032% (5). We defined the ultra-early molecular response (u-EMR) of nilotinib, including international scale (IS) decreased by more than 10% from baseline at 6 weeks, considering that previous studies defined an early molecular response (EMR) as BCR::ABL1 transcript level ≤ 10% according to the International Scale after 3 months of therapy (18, 21, 22). PFS was defined as disease progression to AP/BP or loss of response. OS was defined as the time from day 0 to the last follow-up visit or death. Adverse events owing to TKIs were determined according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
The clinical data cut-off date was September 30, 2022. Population analyses were performed using the intention-to-treat method. Data are presented as medians. The cumulative MMR, MR4.0, MR4.5, PFS, and OS were assessed using the Kaplan–Meier method, patient background, achievement of u-EMR, etc., using the log-rank test. All statistical analyses were performed using Stata software version 14.1.
Between July 2019 and September 2022, 106 patients newly diagnosed with CML-CP were enrolled from 29 Chinese institutions. The median age was 36 years (range, 18–76 years), and 68 patients (64.2%) were men. The median time from diagnosis to nilotinib treatment was 11 days (range, 0–84 days). The numbers of patients with low, intermediate and high ELTS scores were 72 (67.9%), 21 (19.8%), and 11 (10.4%), respectively. 5 (4.7%) patients had chromosomal abnormalities in addition to the Philadelphia chromosome, and 31 (29.2%) had a large spleen size ≥10 cm below the costal margin. The median hemoglobin (Hb), platelet count (PLT), and white-cell count (WBC) in the peripheral blood at the time of CML diagnosis were 108 g/L (range, 56–384 g/L), 451 × 109/L (range, 73–3,444 × 109/L) and 122.4 × 109/L (range, 8.0–525.1 × 109/L), respectively. Clinical characteristics and the key baseline values are summarized in Table 1.
The cumulative MR4.5 rate of nilotinib treatment at 18 months was 69.8% (74/106). The median time for patients to achieve MR4.5 was 6 months (range, 3–24) months. The cumulative MMR and MR4.0 rates for nilotinib at 18 months were 94.3% (100/106) and 84.9% (90/106), respectively (Figure 1). Additionally, u-EMR was achieved at 6 weeks (BCR::ABL1 IS≤10% or IS decreased ≥ half of the IS at diagnosis) by 40.4% (21/54) and 84.4% (38/45) of patients, respectively. However, patients with u-EMR at 6 weeks did not significantly differ in achieving DMR or MMR at 24 months compared to those without u-EMR (p = 0.7584 and p = 0.9543, respectively, Figure 2). The cumulative rates of PFS and OS were 98.1% (104/106) and 100% (106/106), respectively. The median followed-up period was 27 months (range, 18–42 months), and the median PFS and OS in this trial were both not reached (Figure 3).

Figure 1. Cumulative incidence of responses of nilotinib with major molecular response (MMR), molecular response 4 (MR4.0) and molecular response 4.5 (MR4.5).
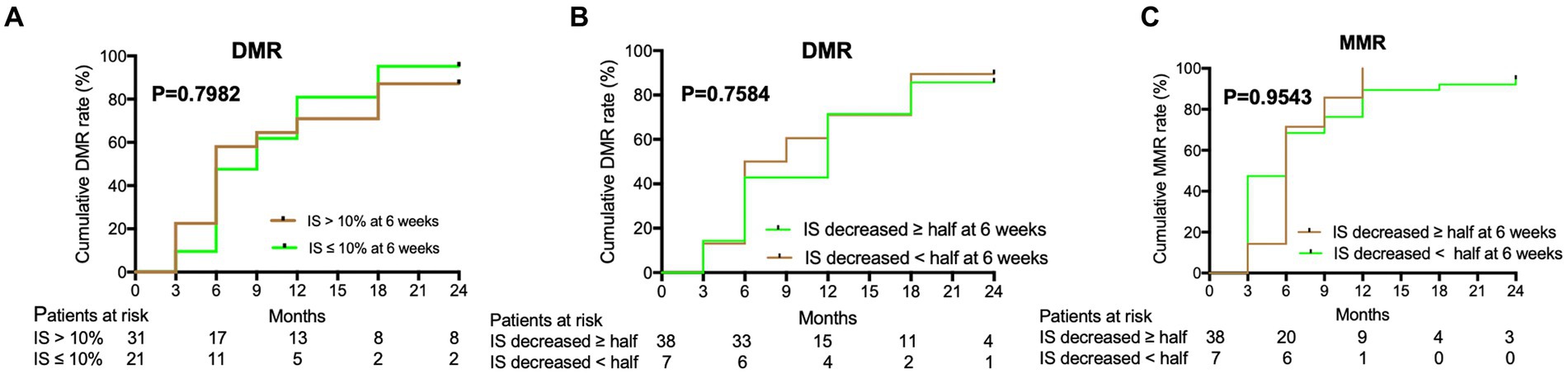
Figure 2. Deep molecular response (DMR) and major molecular response (MMR) were predicted by the decreased rate of IS of nilotinib at 6 weeks. (A) DMR of nilotinib was predicted by IS decreased >10% or IS decreased ≤ 10% of IS in baseline level at 6 weeks. (B) DMR of nilotinib was predicted by IS decreased ≥ half of IS or IS decreased < half of IS in baseline level at 6 weeks. (C) MMR of nilotinib was predicted by IS decreased ≥ half of IS or IS decreased < half of IS in baseline level at 6 weeks.
In the univariate analysis of subgroups, patients with intermediate or high ELTS scores tended to achieve a lower cumulative response of MR4.5 by 18 months (HR = 0.462, p = 0.020; HR = 0.387, p = 0.042, respectively). Similarly, patients with a spleen size <10 cm below the costal margin tended to achieve a higher cumulative response of MR4.5 by 18 months, than those with a spleen size ≥10 cm below the costal margin (HR = 1.927, p = 0.021). However, there were no significant difference using multivariate analysis for nilotinib with cumulative response of MR4.5 before 18 months treatment (Table 2).
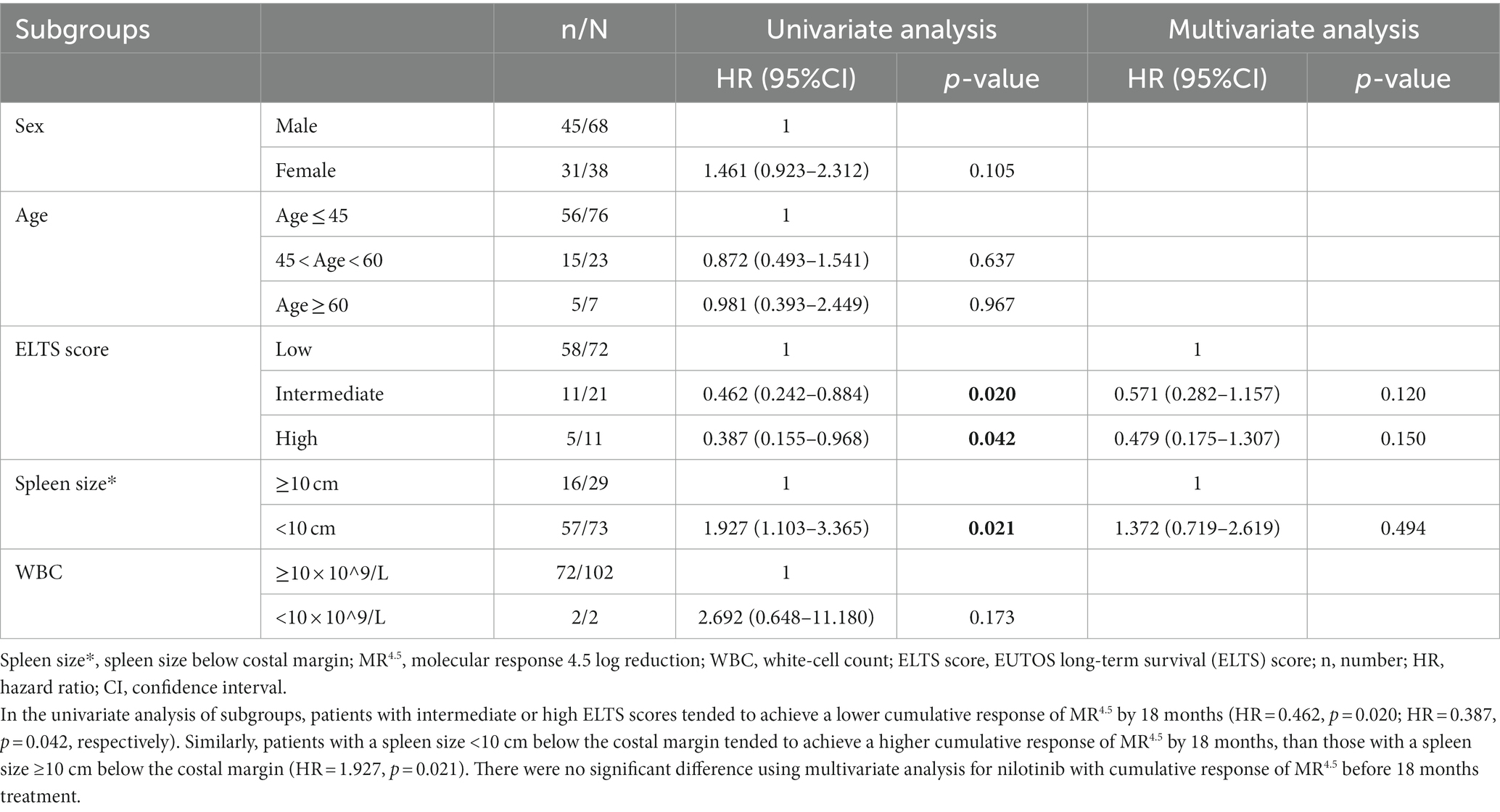
Table 2. Univariate and multivariate analysis for nilotinib with cumulative response of MR4.5 before 18 months treatment.
As for TFR, in two bigger centres, 28 patients reached the condition of TFR and only 4 patients try to discontinue medication for TFR as required by themselves. RT-qPCR of BCR::ABL1 1 in 4 patients above were till negative for 6 months.
The most common hematological adverse events (AEs) were thrombocytopenia (46% [grade 3–4, 2%]), anemia (30% [grade 3–4, 4%]), leukopenia (14% [grade 3–4, 4%]), and neutropenia (13% [grade 3–4, 11%]). The most common non-hematologic AEs were rash (70%), myalgia (48%), fatigue (41%), dry eyes (37.6%), and itching (36.8%). Most patients with non-hematologic AEs were at grade 1–2 and could recover later. Besides, one patient gave up the treatment of nilotinib because of coronary heart disease. Moreover, one patient discontinued nilotinib treatment owing to high blood glucose and lipid levels (Table 3).
Early stable DMR of nilotinib is associated with the goal of achieving TFR in patients with CML-CP. Therefore, it is important to early distinguish between patients who can achieve a DMR and those who are fit for TFR. Our prospective and multicenter study investigated the early cumulative MR4.5 rate by 18 months with nilotinib in patients with ND-CML-CP. The results of this trial showed that treatment with nilotinib contributed to a high rate of early MR4.5 for patients with ND-CML-CP in the real world.
Across 29 institutes in China, the cumulative MR4.5 rate by 18 months was 69.8% for patients with ND-CML-CP in this study, which was higher than the MR4.5 achieved in 24-month follow-up of the ENEST1st trial (50%) (23) and 24-month follow-up of the N-Road study (45.7%) (24) and in the Michihide’s trial with a median observation period of 3.4 years (50%) (25). In addition, the overall MR4.5 rates in treatments of imatinib 400 mg daily, imatinib 800 mg daily, dasatinib 100 mg daily, and nilotinib 800 mg daily groups after a long follow-up period were 57, 74, 71, and 71%, respectively (26). However, the cumulative MR4.5 rate has not yet been reported in the ENESTchina study with a 12-month follow-up. Frontline nilotinib 800 mg daily with a median follow-up of 78.3 months and frontline dasatinib 100 mg daily with a median follow-up of 6.5 years achieved MR4.5 in 75 and 79.5% of patients with ND-CML-CP, respectively (27, 28). In the future, it will be important to perform a study of frontline nilotnib 300 mg daily in patients with ND-CML-CP with long-term follow-up in China.
Similarly, for patients with ND-CML-CP, the cumulative MMR and MR4.0 rates of nilotinib at 18 months in this trial were higher than those in the ENEST1st and N-Road studies, both with a 24-month follow-up (94.3% vs. 80.4% vs. 82.2 and 84.9% vs. 55.2% vs. 58.3%, respectively) (23, 24). In addition, the cumulative MMR rate of nilotinib was 52.2% in the ENESTchina study with a 12-month follow-up (29). The highest MR4.5, MR4.0, and MMR rates in our study may be related to a high proportion of low ELTS scores (67.9%, 72/106), young population of CML-CP and a small body surface area (BSA) in Asian patients. Our findings suggest that nilotinib can achieve a high early DMR in patients with ND-CML-CP during TKI treatment.
DMR serves as a milestone in the process of achieving TFR, and has been described in the ELN and LALNET recommendations and NCCN guidelines (15–17). As TFR will be the new goal of CML-CP treatment in the future (30), u-EMR at 6 weeks may predict the early achievement of DMR in ND-CML-CP treatment. In our study, 84.4% of the patients with ND-CML-CP achieved u-EMR at 6 weeks, indicating that nilotinib could quickly reduce the tumor load. This may be because nilotinib has high binding affinity, high selectivity for ABL kinase, and high inhibitory activity (10, 11). Besides, Masahiro et al. reported that 87.0% (328/377) of patients with ND-CML-CP treated with nilotinib achieved EMR at 3 months (BCR::ABL1 IS <10%), which indicated a significantly superior PFS compared with those without EMR after a 5-year follow-up (p < 0.0001) (20). In our study, patients with u-EMR at 6 weeks were not significantly different in achieving DMR or MMR by 24 months compared to those without u-EMR. Long-term follow-up with nilotinib should be performed to identify the relationship between the u-EMR and DMR or PFS.
No patient showed exacerbation of AP/BC, and the reasons may be that nilotinib improves the poor prognosis of intermediate or high-risk patients based on Euro score, as presented in the ENESTnd trial and DASISION trials (14, 31). The cumulative rates of PFS and OS in our study were 98.1 and 100%, respectively, which are similar to the results of TARGET system study, with 94.1% PFS and 97.1% OS after a 5-year follow-up (20). Univariate analysis of the subgroups showed that patients with intermediate or high ELTS scores tended to achieve a lower cumulative response to MR4.5 by 18 months. Similarly, patients with a splenic size <10 cm below the costal margin tended to achieve a higher cumulative response of MR4.5 by 18 months, than those with a splenic size ≥10 cm below the costal margin. The may be because the intermediate or high ELTS score and larger spleen size indicate relatively high tumor loads for ND-CML-CP patients.
This study has some limitations. It was difficult to analyze the subgroups because of the small number of participants. Additionally, this study was designed as a single-arm clinical trial that lacked of comparison with other TKI treatments. Therefore, the generalizability of our results is limited. Long-term follow-up with nilotinib and comparison with other TKI treatments are required in the future studies in China.
This prospective multicenter study demonstrated that treatment with nilotinib in patients with newly diagnosed CML-CP contributed to a high early molecular response 4.5 (MR4.5).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Shenzhen Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BW: Writing – original draft. YZ: Data curation, Writing – review & editing. HL: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. CT: Data curation, Writing – review & editing. YJ: Data curation, Writing – review & editing. XL: Writing – review & editing. YC: Data curation, Writing – review & editing. HH: Data curation, Writing – review & editing. ZL: Data curation, Writing – review & editing. XX: Data curation, Writing – review & editing. WH: Data curation, Writing – review & editing. LP: Data curation, Writing – review & editing. XD: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Shenzhen Key Medical Discipline (No. SZXK008).
The authors thank the patients who participated in this study and their families, as well as investigators and staffs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Soverini, S, de Benedittis, C, Mancini, M, and Martinelli, G. Mutations in the BCR-ABL1 kinase domain and elsewhere in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2015) 15:S120–8. doi: 10.1016/j.clml.2015.02.035
2. Garcia-Manero, G, Faderl, S, O'Brien, S, Cortes, J, Talpaz, M, and Kantarjian, HM. Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer. (2003) 98:437–57. doi: 10.1002/cncr.11520
3. Jemal, A, Tiwari, RC, Murray, T, Ghafoor, A, Samuels, A, Ward, E, et al. Cancer statistics, 2004. CA Cancer J Clin. (2004) 54:8–29. doi: 10.3322/canjclin.54.1.8
4. Redaelli, A, Bell, C, Casagrande, J, Stephens, J, Botteman, M, Laskin, B, et al. Clinical and epidemiologic burden of chronic myelogenous leukemia. Expert Rev Anticancer Ther. (2004) 4:85–96. doi: 10.1586/14737140.4.1.85
5. Baccarani, M, Deininger, MW, Rosti, G, Hochhaus, A, Soverini, S, Apperley, JF, et al. European leukemia net recommendations for the management of chronic myeloid leukemia: 2013. Blood. (2013) 122:872–84. doi: 10.1182/blood-2013-05-501569
6. Chronic Myeloid Leukemia. (2023). NCCN clinical practice guidelines in oncology. Version 1.2024 — August 1, 2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
7. Druker, BJ, Guilhot, F, O'Brien, SG, Gathmann, I, Kantarjian, H, Gattermann, N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. (2006) 355:2408–17. doi: 10.1056/NEJMoa062867
8. Kantarjian, H, Shah, NP, Hochhaus, A, Cortes, J, Shah, S, Ayala, M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. (2010) 362:2260–70. doi: 10.1056/NEJMoa1002315
9. Saglio, G, Kim, DW, Issaragrisil, S, le Coutre, P, Etienne, G, Lobo, C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. (2010) 362:2251–9. doi: 10.1056/NEJMoa0912614
10. Golemovic, M, Verstovsek, S, Giles, F, Cortes, J, Manshouri, T, Manley, PW, et al. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin Cancer Res. (2005) 11:4941–7. doi: 10.1158/1078-0432.CCR-04-2601
11. O'Hare, T, Walters, DK, Stoffregen, EP, Jia, T, Manley, PW, Mestan, J̈, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. (2005) 65:4500–5. doi: 10.1158/0008-5472.CAN-05-0259
12. Jabbour, E, and Kantarjian, H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am J Hematol. (2022) 97:1236–56. doi: 10.1002/ajh.26642
13. Hochhaus, A, Saglio, G, Hughes, TP, Larson, RA, Kim, DW, Issaragrisil, S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. (2016) 30:1044–54. doi: 10.1038/leu.2016.5
14. Kantarjian, HM, Hughes, TP, Larson, RA, Kim, DW, Issaragrisil, S, le Coutre, P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. (2021) 35:440–53. doi: 10.1038/s41375-020-01111-2
15. Pavlovsky, C, Abello Polo, V, Pagnano, K, Varela, AI, Agudelo, C, Bianchini, M, et al. Treatment-free remission in patients with chronic myeloid leukemia: recommendations of the LALNET expert panel. Blood Adv. (2021) 5:4855–63. doi: 10.1182/bloodadvances.2020003235
16. Chronic Myeloid Leukemia. (2022). NCCN clinical practice guidelines in oncology. Version 1.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
17. Jain, P, Kantarjian, H, Nazha, A, O’Brien, S, Jabbour, E, Romo, CG, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. (2013) 121:4867–74. doi: 10.1182/blood-2013-03-490128
18. Hanfstein, B, Müller, MC, Hehlmann, R, Erben, P, Lauseker, M, Fabarius, A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. (2012) 26:2096–102. doi: 10.1038/leu.2012.85
19. Jabbour, E, Kantarjian, HM, Saglio, G, Steegmann, JL, Shah, NP, Boqué, C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. (2014) 123:494–500. doi: 10.1182/blood-2013-06-511592
20. Kizaki, M, Takahashi, N, Iriyama, N, Okamoto, S, Ono, T, Usui, N, et al. Efficacy and safety of tyrosine kinase inhibitors for newly diagnosed chronic-phase chronic myeloid leukemia over a 5-year period: results from the Japanese registry obtained by the new TARGET system. Int J Hematol. (2019) 109:426–39. doi: 10.1007/s12185-019-02613-1
21. Branford, S, Yeung, DT, Ross, DM, Prime, JA, Field, CR, Altamura, HK, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. (2013) 121:3818–24. doi: 10.1182/blood-2012-10-462291
22. Marin, D, Ibrahim, AR, Lucas, C, Gerrard, G, Wang, L, Szydlo, RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol Off J Am Soc Clin Oncol. (2012) 30:232–8. doi: 10.1200/JCO.2011.38.6565
23. Hochhaus, A, Mahon, FX, le Coutre, P, Petrov, L, Janssen, JJWM, Cross, NCP, et al. Nilotinib first-line therapy in patients with Philadelphia chromosome-negative/BCR-ABL-positive chronic myeloid leukemia in chronic phase: ENEST1st sub-analysis. J Cancer Res Clin Oncol. (2017) 143:1225–33. doi: 10.1007/s00432-017-2359-9
24. Nishiwaki, K, Sugimoto, KJ, Tamaki, S, Hisatake, J, Yokoyama, H, Igarashi, T, et al. Optimal treatment strategy with nilotinib for patients with newly diagnosed chronic-phase chronic myeloid leukemia based on early achievement of deep molecular response (MR (4.5)): the phase 2, multicenter N-road study. Cancer Med. (2020) 9:3742–51. doi: 10.1002/cam4.3034
25. Tokuhira, M, Kimura, Y, Sugimoto, K, Nakazato, T, Ishikawa, M, Fujioka, I, et al. Efficacy and safety of nilotinib therapy in patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Med Oncol. (2018) 35:38. doi: 10.1007/s12032-018-1093-8
26. Jabbour, E, and Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. (2020) 95:691–709. doi: 10.1002/ajh.25792
27. Masarova, L, Cortes, JE, Patel, KP, O’Brien, S, Nogueras-Gonzalez, GM, Konopleva, M, et al. Long-term results of a phase 2 trial of nilotinib 400 mg twice daily in newly diagnosed patients with chronic-phase chronic myeloid leukemia. Cancer. (2020) 126:1448–59. doi: 10.1002/cncr.32623
28. Maiti, A, Cortes, JE, Patel, KP, Masarova, L, Borthakur, G, Ravandi, F, et al. Long-term results of frontline dasatinib in chronic myeloid leukemia. Cancer. (2020) 126:1502–11. doi: 10.1002/cncr.32627
29. Wang, J, Shen, ZX, Saglio, G, Jin, J, Huang, H, Hu, Y, et al. Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina. Blood. (2015) 125:2771–8. doi: 10.1182/blood-2014-09-601674
30. Hughes, TP, and Ross, DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. (2016) 128:17–23. doi: 10.1182/blood-2016-01-694265
31. Cortes, JE, Saglio, G, Kantarjian, HM, Baccarani, M, Mayer, J, Boqué, C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-Naïve chronic myeloid leukemia patients trial. J Clin Oncol Off J Am Soc Clin Oncol. (2016) 34:2333–40. doi: 10.1200/JCO.2015.64.8899
Keywords: 18 months, deep molecular response, nilotinib, newly diagnosed, chronic-phase chronic myeloid leukemia
Citation: Wen B, Zhang Y, Lin H, Lou J, Tu C, Jiang Y, Liu X, Chen Y, He H, Liu Z, Xie X, Huang W, Pang L and Du X (2023) 18 months follow-up of deep molecular response 4.5 (MR4.5) with nilotinib in patients with newly diagnosed chronic-phase chronic myeloid leukemia: a prospective, multi-center study in China. Front. Med. 10:1267512. doi: 10.3389/fmed.2023.1267512
Received: 26 July 2023; Accepted: 25 October 2023;
Published: 16 November 2023.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Georg-Nikolaus Franke, University Hospital Leipzig, GermanyCopyright © 2023 Wen, Zhang, Lin, Lou, Tu, Jiang, Liu, Chen, He, Liu, Xie, Huang, Pang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangxiang Huang, aHd4MTk3MDExQDE2My5jb20=; Liping Pang, cGxwMjU3NjIwMDJAMTYzLmNvbQ==; Xin Du, ZHV4aW5nekBtZWRtYWlsLmNvbS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.