
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 31 October 2023
Sec. Pulmonary Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1265057
This article is part of the Research Topic Addressing Latent Tuberculosis Infection: An Essential Step in the Fight Against Tuberculosis View all 9 articles
 N. Ortiz Laza1
N. Ortiz Laza1 I. Lopez Aranaga1
I. Lopez Aranaga1 J. Toral Andres2
J. Toral Andres2 B. Toja Uriarte3
B. Toja Uriarte3 B. Santos Zorrozua4
B. Santos Zorrozua4 L. Altube Urrengoechea2
L. Altube Urrengoechea2 J. Garros Garay3
J. Garros Garay3 E. Tabernero Huguet1*
E. Tabernero Huguet1*Introduction: Contact tracing and treatment of latent tuberculosis infection (LTBI) is a key element of tuberculosis (TB) control in low TB incidence countries. A TB control and prevention program has been active in the Basque Country since 2003, including the development of the nurse case manager role and a unified electronic record. Three World Health Organization-approved LTBI regimens have been used: isoniazid for 6 months (6H), rifampicin for 4 months (4R), and isoniazid and rifampicin for 3 months (3HR). Centralized follow-up by a TB nurse case manager started in January 2016, with regular telephone follow-up, telemonitoring of blood test results, and monitoring of adherence by electronic review of drugs dispensed in pharmacies.
Objective: To estimate LTBI treatment completion and toxicity of different preventive treatment regimens in a real-world setting. Secondary objective: to investigate the adherence to different approaches to preventive treatment monitoring.
Methods: A multicentre retrospective cohort study was conducted using data collected prospectively on contacts of patients with TB in five hospitals in Biscay from 2003 to 2022.
Results: A total of 3,066 contacts with LTBI were included. The overall completion rate was 66.8%; 86.5% of patients on 3HR (n = 699) completed treatment vs. 68.3% (n = 1,260) of those on 6H (p < 0.0001). The rate of toxicity was 3.8%, without significant differences between the regimens. A total of 394 contacts were monitored by a TB nurse case manager. In these patients, the completion rate was 85% vs. 67% in those under standard care (p < 0.001). A multivariate logistic regression model identified three independent factors associated with treatment completion: being female, the 3HR regimen, and nurse telemonitoring.
Conclusion: 3HR was well tolerated and associated with a higher rate of treatment completion. Patients with nurse telemonitoring follow-up had better completion rates.
Contact tracing and treatment of latent tuberculosis infection (LTBI) are key elements of the “prevention package” of the END TB strategy. LTBI is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifest active tuberculosis (TB). LTBI treatment is particularly important in countries with a lower tuberculosis incidence, where a higher proportion of cases are due to reactivation of latent infection (1).
The Department of Health of the Basque Country, which in 2002 had a rate of 26.2 cases per 100,000 inhabitants, launched a TB control and prevention program which has been active since 2003. This program includes a TB nurse case manager in each area and a specific unified electronic record that stores data on both patients diagnosed with tuberculosis and the corresponding contact tracing. At present, the TB rate has dropped to 8.6/100,000 inhabitants in Biscay (2, 3).
Technological advances and the growing evidence of the importance of monitoring adherence to LTBI treatment led to the launch in January 2016 of centralized monitoring of the LTBI treatment by a TB nurse case manager. This was an innovation compared to the traditional standard follow-up in regular scheduled medical consultations with each specialist in the corresponding area.
Currently, three WHO-approved LTBI regimens are available in our country for the treatment of LTBI: 6 months of isoniazid (6H), 4 months of rifampicin (4R), and 3 months of isoniazid and rifampicin (3HR). These have similar efficacy and adverse effects, but shorter treatments have shown better adherence, however, there are few studies in our setting (3–6).
We undertook this study to assess the LTBI treatment completion rate in contacts in whom such treatment was recommended in our area. As secondary objectives, we aimed to investigate the results of treatment according to the different follow-up approaches and regimens, as well as to describe toxicities associated with the different preventive treatments and to analyze other factors associated with non-adherence.
This was a multicenter retrospective cohort study that used prospectively collected data on contacts of smear-positive TB patients from five hospitals in Biscay from 2003 to 2022, Santa Marina University Hospital, Galdakao University Hospital, Cruces University Hospital, San Eloy Hospital and Urduliz Hospital. Pseudo-anonymized data were extracted from the specific electronic program for regional TB control.
All contacts of people with smear-positive pulmonary or laryngeal TB in Biscay from 2003 to 2022 recorded in the electronic database of the regional TB control program. Patients diagnosed with active TB during the contact study were excluded from the analysis.
The primary variable was LTBI treatment outcome: completed [defined as taking >80% of doses of the prescribed medication regardless of treatment changes, within 12 and 18 months from the start for rifampicin-containing and isoniazid-alone therapies, respectively (7)], dropped, withdrawn due to intolerance, refused, and lost to follow-up.
Exposure variables: standard monitoring versus tele-follow-up by a nurse case manager, type of regimen: 6H, 4R, or 3HR. Other variables analyzed included age, sex, date, nationality (grouped by WHO region), degree of contact and toxicity (1).
The TB nurse case manager collected data on index cases and their contacts. Patients were studied according to the local guidelines, using a system of concentric circles representing the amount of time spent with the index case, classifying contacts as intimate (daily and more than 6 h, mostly households), regular (daily and less than 6 h) or sporadic (not daily). The latter were studied in outbreaks or if the index TB case was high risk (extensive tuberculosis, long diagnostic delay and high bacillary load).
For the diagnosis of LTBI, TST (tuberculin skin test) was used until 2012 and since then, a 2-step strategy with TST using QuantiFERON as confirmatory test similar to that described by Muñoz et al. (8).
During the first years of the study period, in all areas, these contacts were referred to the clinic where the index case was diagnosed or to their primary care center. As of January 2016, three hospitals started centralized monitoring of LTBI treatment by the corresponding TB nurse case manager, following assessment and prescription of LTBI by a pulmonologist. The nurse case manager carried out regular telephone follow-up, telemonitoring of scheduled blood test results, and monitoring of adherence through electronic review of the medication dispensed in pharmacies, as well as being available via direct channels of communication (mobile phone and WhatsApp) to address patients’ concerns and monitor possible side effects. The monitoring of LTBI treatment in the other hospitals continued in the traditional way.
The study was approved by the regional ethics committees in accordance with the Declaration of Helsinski’s guidelines for research in humans. Patient privacy and the confidentiality of personal data have been safeguarded in line with the provisions of European law (9).
Continuous variables are reported as the mean (standard deviation) for normally distributed data and otherwise as the median (interquartile range). Categorical variables are presented as frequency (percentage). When comparisons were made between two groups, Student’s t-test was used, in the case of normally-distributed continuous variables, and otherwise the Kruskall-Wallis test. Chi-squared or Fisher’s exact tests were used for the categorical data. To construct the multivariate logistic regression model, the dependent variable was the final LTBI outcome, and the independent variables were age, sex, date, degree of contact, type of follow-up, and type of regimen. Multivariate analysis was performed using a multivariate logistic regression model, including variables with p-values lower than 0.100 in the bivariate analysis as predictors. We eliminated the variables with the highest p-values one at a time until all the variables entered were significant (p-value <0.05). The Hosmer-Lemeshow goodness-of-fit test for logistic regression was used to assess the model fit. Differences were considered statistically significant when p < 0.05. All analyses were performed using R statistical software (version 4.0.1 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
A total of 17,817 persons were studied from January 2003 to December 2022 in Biscay, corresponding to 3,820 TB patients. Only 2,267 (59.3%) of them were smear or culture positive pulmonary or laryngeal TB and had contact tracing (7,8 persons per contact study on average). A contact study was also performed in children and pleural TB to try to identify the index case. Among the total sample, 132 (0.74%) new cases of TB disease were diagnosed, and 3,066 (17,2%) contacts were diagnosed with LTBI with an indication for treatment. During this long period of time the incidence of tuberculosis has decreased in our area and in parallel the total number of contacts studied, as shown in Figure 1.
The median age of contacts with an indication for LTBI treatment was 32.5 years (21.1–44.2) and 46.6% were women. The most frequent place of origin after Spain (74,8%) was the Region of the Americas (11.4%) followed by the Eastern Mediterranean Region (5.6%) (Table 1).
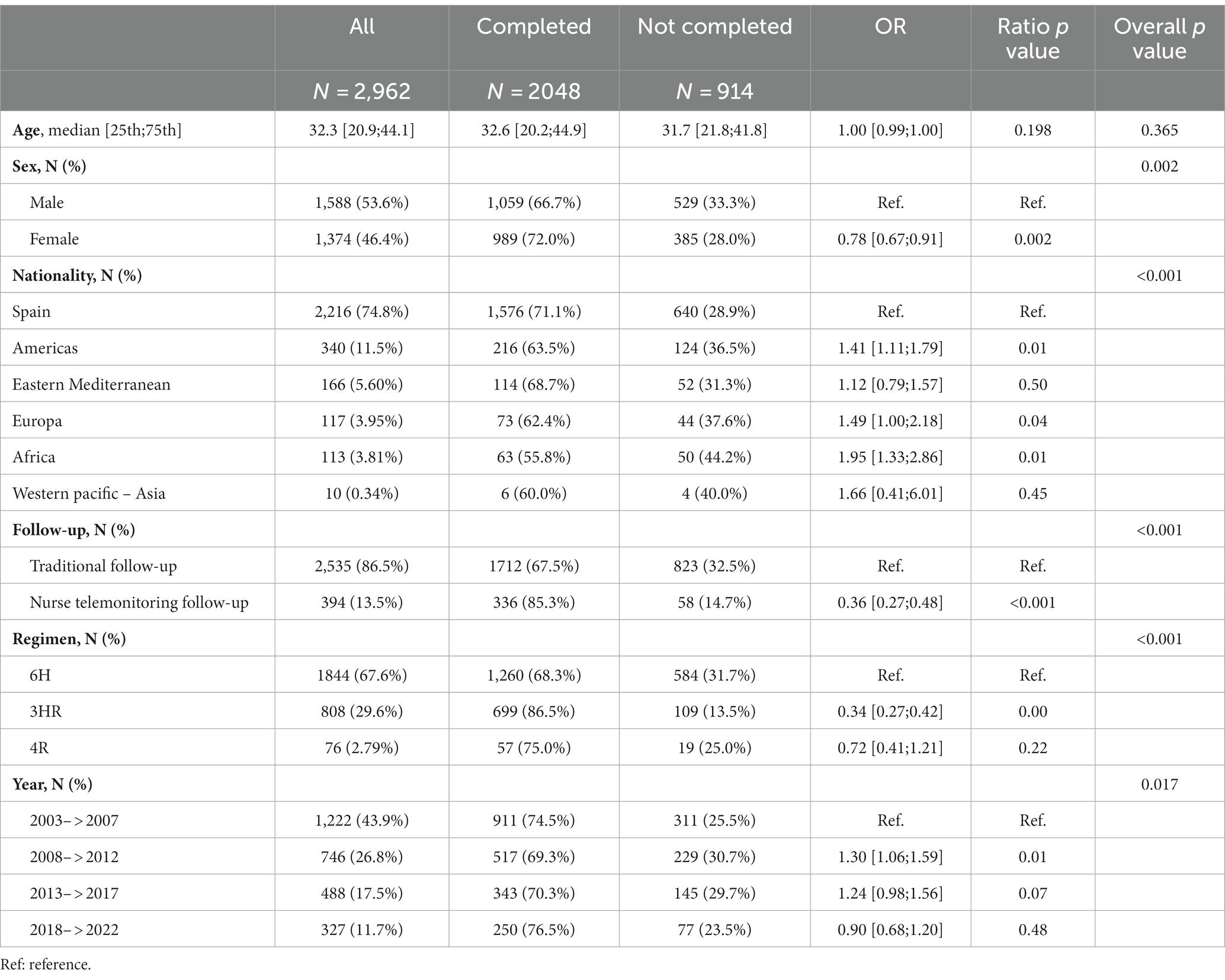
Table 1. Baseline characteristics stratified by adherence to latent tuberculosis infection treatment outcome.
Nearly half (49.5%) were household contacts, and there were no differences regarding treatment completion rate according to the degree of contact.
Overall, treatment was completed by 66.8% of the contacts with a treatment indication. A total of 115 (3.8%) of the contacts withdrew due to intolerance, with no significant differences in toxicity between the different regimens: 26 with 3HR (3.2%), 3 with 4R (3.9%), and 85 with 6H (4.6%). Hepatoxicity was the most common adverse event (91% of them). Just 6.1% dropped out of treatment, while 174 contacts (5.7%) refused treatment. Further, 304 (9.9%) of the contacts with an indication for LTBI were lost to follow-up and 104 had missing data concerning treatment outcome (the latter being excluded from the analysis).
Follow-up involved telemonitoring by the nurse case manager in 13.3% of contacts, while 86% received traditional follow-up by the corresponding pulmonology or primary care clinic. As for the regimens, only those accepted by the WHO and available in our country were taken into account for the analysis. The most frequently recommended regimen was 6H (67.6%), followed by 3HR (29.6%). There were no differences in baseline demographic characteristics among patients with the different regimens.
According to final status, in bivariate analysis, completion rates were higher in women (72%, OR: 0.78; 95% CI: 0.67–0.91), those born in Spain (71%), those who received the 3HR regimen (86.5%, OR: 0.34; 95% CI: 0.27–0.42), the 4R regimen which is also a short regimen was not statistically better probably due to small number, and contacts telemonitored by a nurse case manager (85.2%, OR: 0.36; 95% CI: 0.27–0.48) (Table 1). In the multivariate analysis, female sex (OR = 1.26; 95% CI: 1.06–1.51), the shorter regimen 3HR (OR = 2.90; 95% CI: 2.29–3.70), and tele-follow-up by a nurse case manager (OR = 1.75; 95% CI: 1.27–2.45) remained significantly associated with completing treatment (Table 2). The Hosmer-Lemeshow goodness-of-fit test for logistic regression gave a p value of 0.852.
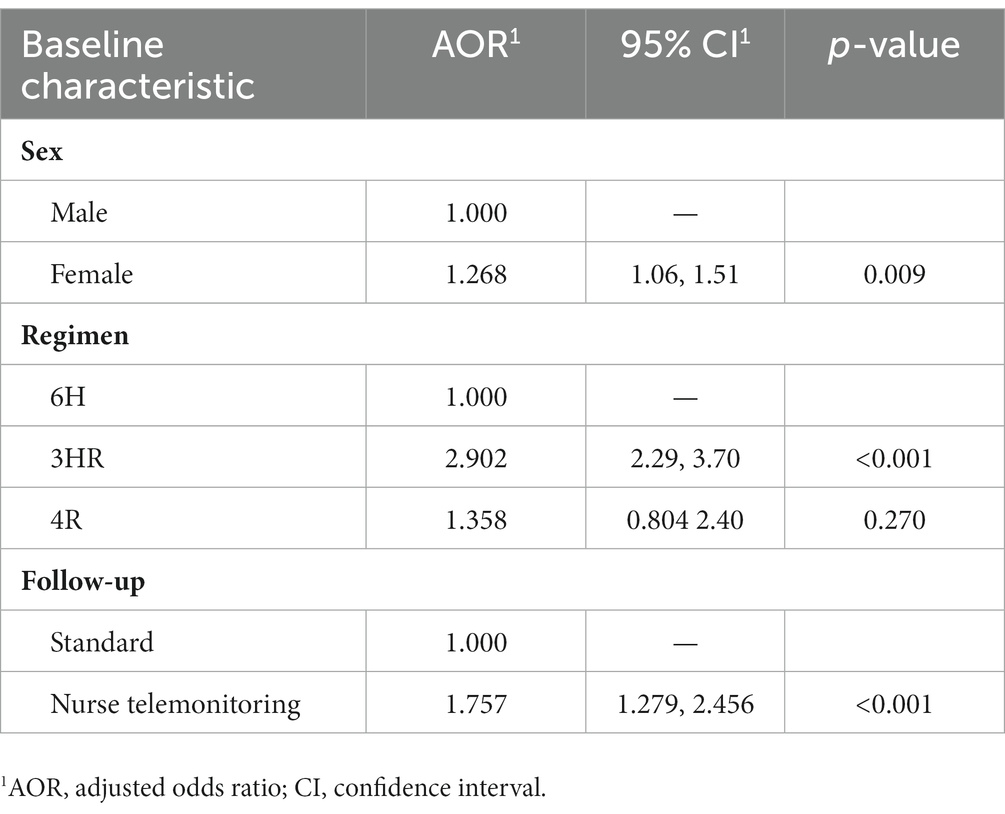
Table 2. Multivariate analysis of factors associated with completing latent tuberculosis infection treatment.
This multicenter study provides the results of real-world LTBI preventive treatment in contact studies over 20 years of the TB program in the province of Biscay.
In our study, only two-thirds (66.3%) of all patients with an indication for treatment completed the course prescribed, similar to rates found in Canada and the USA by Sullivan and Hirsch-Moverman (10, 11), and lower than those of 79% reported in Italy and 80%–89% in two multicenter studies in Spain (7, 12, 13). A systematic review of 83 studies on this topic described rates ranging from 46% to 96%, depending on the characteristics of the population studied, being much lower in homeless people than in healthcare workers (14).
Overall, 408 (13.3%) patients were lost to follow-up or had missing data on treatment outcome, comparable to results in research in North America (14.5%) (15). The rate of treatment adherence was lower among patients born outside Spain than among those born in this country in the bivariate analysis, but being foreign-born disappeared as a risk factor for non-completion of treatment in the multivariate analysis, and hence, we cannot draw definitive conclusions. The TITL treatment date also showed differences in the bivariate analysis but when included in the multivariate analysis the Hosmer and Lemeshow goodness of fit (GOF) test showed a very low value, probably because the COVID pandemic period altered the follow-up. The impact of COVID, while maintaining normal activity in our TB services, limited patient access to the healthcare system and led to a sharp decline in the number of TB cases and LTBI treatments in 2020 and 2021, as recently published in our country (16).
Analyzing our results by LTBI regimen, we found that the adherence rates are much higher with a shorter regimen, namely, 3HR, than the longer 6H, reaching as high as 86% vs. 68%. This has been widely described in previous studies, also in Spain by Jimenez-Fuentes, and is in line with current WHO and CDC recommendations (5, 6, 17–19). According to a meta-analysis some years ago, short regimens offer similar results in terms of protection with similar rates of adverse effects but much better adherence and lower costs (20). The use of these short regimens based on rifampicin is associated with 20%–40% higher adherence rates (21, 22) without significantly increasing the risk of rifamycin resistance (23). Further, there was no difference in toxicity between the different regimens, ranging from 3.2% with 3HR to 4.5% with 6H, similar to rates described in previous studies (18, 21).
Finally, one of the main findings of this study was that tele-follow-up by a nurse case manager significantly improved the rate of LTBI treatment completion, 85% versus 67% in the standard follow-up, with a statistically significant difference that was maintained in the multivariate analysis. This follow-up by a nurse with expertise in TB management allowed for comprehensive and individualized management (24). The tele-follow-up protocol included health education, and provision of oral and written information on latent TB infection and the indicated treatment and its possible side effects. The patients had easy access to a clinician with specialized training in TB for support, via direct channels of communication, for both addressing concerns and monitoring possible side effects, avoiding unnecessary travel for patients and reducing patient volume at healthcare centers. Some previous studies have highlighted that treatment non-completion was strongly associated not only with adverse effects but also with the inconvenience of clinic and pharmacy schedules (14, 17). On the other hand, technological developments have not only facilitated regular communication between patient and nurse case manager but also enabled close monitoring through the medical record and electronic review of the collection of medication from pharmacies. A recent review on the effects of digital health technologies in LTBI, suggests that they are at least equivalent to current practice (25). In addition, a study on immigrants in Israel showed that LTBI treatment supervised by an expert nurse with reduced physician follow-up was safe and proved to be cheaper than standard monitoring (9).
The main limitation of this study was the missing data. This was an analysis of data collected over 20 years, and many missing records could not be recovered. Nonetheless, since there was a large sample size, we believe that this will have had a minimal impact on the results. Another limitation is that confounding factors such as comorbidities, substance use, and socioeconomic status, identified in the literature as associated with low adherence, have not been analyzed (26). On the other hand, due to the long study period, the diagnostic methods have not been uniform, in particular, with very little use of QuantiFERON for the diagnosis of latent tuberculosis infection in the early years of the study. The same applies to the different treatment regimens, with the 3HR regimen only being widely used after 2012 and the 4R regimen after 2018. Lastly, we measured adherence based on self-report of missed doses and pharmacy records but did not collect empty boxes.
These results have clinical implication and show other aspects that could be improved in the management of TB contacts in our setting. In our study, non-Spaniards had worse treatment completion rates, so having cultural mediators and community health workers could help to improve completion rates as has been shown in asylum seekers in Sweden (27).
The use of rifapentine which is not available in our country at present, has shown higher treatment completion rates and could simplify treatment adherence (25, 28, 29).
In conclusion, to move towards TB elimination in low-incidence countries, efforts should focus on improving the results of contact tracing and completion rates of indicated LTBI treatment. Adherence is better with the short rifamycin-based regimens with no apparent difference in toxicity.
This study suggests that the role of the nurse case manager with TB expertise, telemonitoring and electronic review of the collection of medication from pharmacies may be key, especially in low-incidence countries, to improve preventive treatment adherence.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Biomedical Research Ethics Committee of Euskadi PI2023097. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
NO: Data curation, Investigation, Writing – original draft. IL: Conceptualization, Methodology, Writing – review & editing. JT: Data curation, Writing – review & editing. BT: Writing – review & editing. BS: Formal analysis, Writing – review & editing. LA: Data curation, Investigation, Writing – review & editing. JG: Data curation, Investigation, Writing – review & editing. ET: Methodology, Writing – original draft, Writing – review & editing, Investigation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Concha Castells for her support for this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Global tuberculosis report 2022. Geneva: World Health Organization; (2022). Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.who.int/publications/i/item/9789240061729
2. Informe epidemiológico sobre la situación de la tuberculosis en España. Año (2021). Centro Nacional de Epidemiología. Instituto de Salud Carlos III. Available at: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/archivos%20A-Z/Tuberculosis/RENAVE_informe_Vigilancia%20TB_%202021.pdf
3. Aristegui, J, Baonza, I, Basterretxea, M, Cabeza, I, Garcia, MA, Korta, J, et al. Programa de tuberculosis de la Comunidad Autónoma del País Vasco. Eusko Jaurlaritzaren Argitalpen Zerbitzu Nagusia Servicio Central de Publicaciones del Gobierno Vasco Donostia-San Sebastián 1, 01010 Vitoria-Gasteiz; (2013). Available at: https://www.euskadi.eus/contenidos/informacion/vigilancia_protocolos/es_def/adjuntos/Tuberculosis_cas.pdf
4. Rosales-Klintz, S, Bruchfeld, J, Haas, W, Heldal, E, Houben, RMGJ, van Kessel, F, et al. Guidance for programmatic management of latent tuberculosis infection in the European Union/European economic area. Eur Respir J. (2019) 53:1802077. doi: 10.1183/13993003.02077-2018
5. WHO operational handbook on tuberculosis. Module 1: Prevention – tuberculosis preventive treatment. Geneva: World Health Organization; (2020). Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.who.int/publications/i/item/9789240002906
6. Jiménez-Fuentes, MA, de Souza-Galvao, ML, Mila Augé, C, Solsona Peiró, J, and Altet-Gómez, MN. Rifampicin plus isoniazid for the prevention of tuberculosis in an immigrant population. Int J Tuberc Lung Dis. (2013) 17:326–32. doi: 10.5588/ijtld.12.0510
7. Villa, S, Ferrarese, M, Sotgiu, G, Castellotti, PF, Saderi, L, Grecchi, C, et al. Latent tuberculosis infection treatment completion while shifting prescription from isoniazid-only to rifampicin-containing regimens: a two-decade experience in Milan, Italy. J Clin Med. (2019) 9:101. doi: 10.3390/jcm9010101
8. Muñoz, L, Santin, M, Alcaide, F, Ruíz-Serrano, MJ, Gijón, P, Bermúdez, E, et al. QuantiFERON-TB gold in-tube as a confirmatory test for tuberculin skin test in tuberculosis contact tracing: a noninferiority clinical trial. Clin Infect Dis. (2018) 66:396–403. doi: 10.1093/cid/cix745
9. General data protection regulation 2016/679: European Union; (2016) Available at: http://data.europa.eu/eli/reg/2016/679/oj
10. Sullivan, K, Pease, C, Zwerling, A, Mallick, R, van Dyk, D, Mulpuru, S, et al. Seven-year retrospective study understanding the latent TB infection treatment cascade of care among adults in a low incidence country. BMC Public Health. (2021) 21:964. doi: 10.1186/s12889-021-10733-9
11. Hirsch-Moverman, Y, Colson, PW, Bethel, J, Franks, J, and El-Sadr, WM. Can a peer-based intervention impact adherence to the treatment of latent tuberculous infection? Int J Tuberc Lung Dis. (2013) 17:1178–85. doi: 10.5588/ijtld.12.0823
12. Anibarro, L, Casas, S, Paz-Esquete, J, Gonzalez, L, Pena, A, Guerra, MR, et al. Treatment completion in latent tuberculosis infection at specialist tuberculosis units in Spain. Int J Tuberc Lung Dis. (2010) 14:701–7.20487607.
13. Gullón Blanco, JA, Rodrigo Sanz, T, Álvarez Navascues, F, Tabernero Huguet, E, Sabría Mestres, J, and García-García, JM. Estudio de contactos de pacientes con tuberculosis: organización y prevalencia de la infección tuberculosa latente. Arch Bronconeumol. (2021) 57:509–11. doi: 10.1016/j.arbres.2020.12.021
14. Iqbal, SA, Isenhour, CJ, Mazurek, G, Langer, AJ, Chang, MH, and Truman, BI. Factors associated with latent tuberculosis infection treatment failure among patients with commercial health insurance—United States, 2005-2016. J Public Health Manag Pract. (2021) 27:E151–61. doi: 10.1097/PHH.0000000000001077
15. Hirsch-Moverman, Y, Shrestha-Kuwahara, R, Bethel, J, Blumberg, HM, Venkatappa, TK, Horsburgh, CR, et al. Latent tuberculous infection in the United States and Canada: who completes treatment and why?. Tuberculosis epidemiologic studies consortium (TBESC). Int J Tuberc Lung Dis. (2015) 19:31–8. doi: 10.5588/ijtld.14.0373
16. Godoy, P, Parrón, I, Barrabeig, I, Caylà, JA, Clotet, L, Follia, N, et al. Impact of the COVID-19 pandemic on contact tracing of patients with pulmonary tuberculosis. Eur J Pub Health. (2022) 32:643–7. doi: 10.1093/eurpub/ckac031
17. Sandgren, A, Vonk Noordegraaf-Schouten, M, Van Kessel, F, Stuurman, A, Oordt-Speets, A, and Van Der Werf, MJ. Initiation and completion rates for latent tuberculosis infection treatment: a systematic review. BMC Infect Dis. (2016) 16:204. doi: 10.1186/s12879-016-1550-y
18. Fiske, CT, Yan, FX, Hirsch-Moverman, Y, Sterling, TR, and Reichler, MR. Tuberculosis epidemiologic studies consortium task order 2 team. Risk factors for treatment default in close contacts with latent tuberculous infection. Int J Tuberc Lung Dis. (2014) 18:421–7. doi: 10.5588/ijtld.13.0688
19. Sterling, TR, Njie, G, Zenner, D, Cohn, DL, Reves, R, Ahmed, A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. (2020) 69:1–11. doi: 10.15585/mmwr.rr6901a1
20. Zenner, D, Beer, N, Harris, RJ, Lipman, MC, Stagg, HR, and Van Der Werf, MJ. Treatment of latent tuberculosis infection: an updated network Meta-analysis. Ann Intern Med. (2017) 167:248. doi: 10.7326/M17-0609
21. Macaraig, MM, Jalees, M, Lam, C, and Burzynski, J. Improved treatment completion with shorter treatment regimens for latent tuberculous infection. Int J Tuberc Lung Dis. (2018) 22:1344–9. doi: 10.5588/ijtld.18.0035
22. Alsdurf, H, Hill, PC, Matteelli, A, Getahun, H, and Menzies, D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. (2016) 16:1269–78. doi: 10.1016/S1473-3099(16)30216-X
23. Den Boon, S, Matteelli, A, and Getahun, H. Rifampicin resistance after treatment for latent tuberculous infection: a systematic review and meta-analysis. Int J Tuberc Lung Dis. (2016) 20:1065–71. doi: 10.5588/ijtld.15.0908
24. Bishara, H, Ore, L, Vinitsky, O, Bshara, H, Armaly, N, and Weiler-Ravell, D. Cost of nurse-managed latent tuberculous infection treatment among hard-to-reach immigrants in Israel. Int J Tuberc Lung Dis. (2015) 19:799–804. doi: 10.5588/ijtld.14.0674
25. Wong, YJ, Ng, KY, and Lee, SWH. Digital health use in latent tuberculosis infection care: a systematic review. Int J Med Inform. (2022) 159:104687. doi: 10.1016/j.ijmedinf.2022.104687
26. Moro, RN, Sterling, TR, Saukkonen, J, Vernon, A, Horsburgh, CR, Chaisson, RE, et al. Factors associated with non-completion of follow-up: 33-month latent tuberculous infection treatment trial. Int J Tuberc Lung Dis. (2017) 21:286–96. doi: 10.5588/ijtld.16.0469
27. Olsson, O, Winqvist, N, Olsson, M, Olsson, P, and Björkman, P. High rate of latent tuberculosis treatment completion in immigrants seeking asylum in Sweden. Infect Dis. (2018) 50:678–86. doi: 10.1080/23744235.2018.1459046
28. Sterling, TR, Villarino, ME, Borisov, AS, Shang, N, Gordin, F, Bliven-Sizemore, E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. (2011) 365:2155–66. doi: 10.1056/NEJMoa1104875
29. Harries, AD, Kumar, AMV, Satyanarayana, S, Takarinda, KC, Timire, C, and Dlodlo, RA. Treatment for latent tuberculosis infection in low- and middle-income countries: progress and challenges with implementation and scale-up. Expert Rev Respir Med. (2020) 14:195–208. doi: 10.1080/17476348.2020.1694907
Keywords: latent tuberculosis infection, treatment, adherence, nurse case manager, telemonitoring
Citation: Ortiz Laza N, Lopez Aranaga I, Toral Andres J, Toja Uriarte B, Santos Zorrozua B, Altube Urrengoechea L, Garros Garay J and Tabernero Huguet E (2023) Latent tuberculosis infection treatment completion in Biscay: differences between regimens and monitoring approaches. Front. Med. 10:1265057. doi: 10.3389/fmed.2023.1265057
Received: 21 July 2023; Accepted: 17 October 2023;
Published: 31 October 2023.
Edited by:
Luis Anibarro, Unidad Tuberculosis. Enfermedades Infecciosas, Medicina Interna. Complexo hospitalario universitario Pontevedra, SpainReviewed by:
Clemax Couto Sant’Anna, Federal University of Rio de Janeiro, BrazilCopyright © 2023 Ortiz Laza, Lopez Aranaga, Toral Andres, Toja Uriarte, Santos Zorrozua, Altube Urrengoechea, Garros Garay and Tabernero Huguet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Tabernero Huguet, ZXZhdGFiZXJuYUB5YWhvby5lcw==
†ORCID: E. Tabernero Huguet http://orcid.org/0000-0001-7255-9290
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.